Mechanical Behaviour of Dental Luting Cements: Static, Dynamic, and Finite Element Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Mechanical Test Protocols
2.3. Protocol of the Finite Element Analysis
2.4. Statistical Evaulation
3. Results
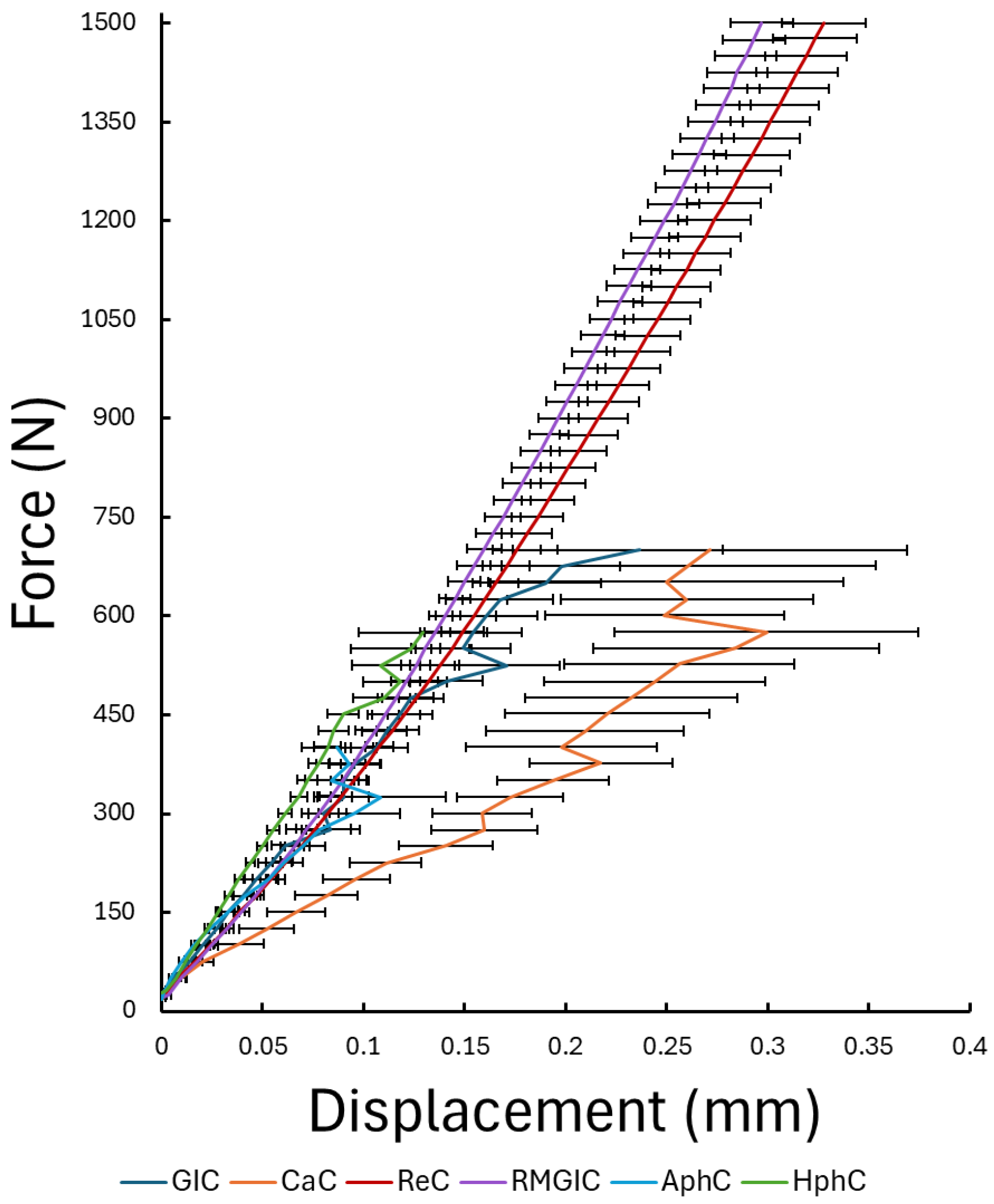
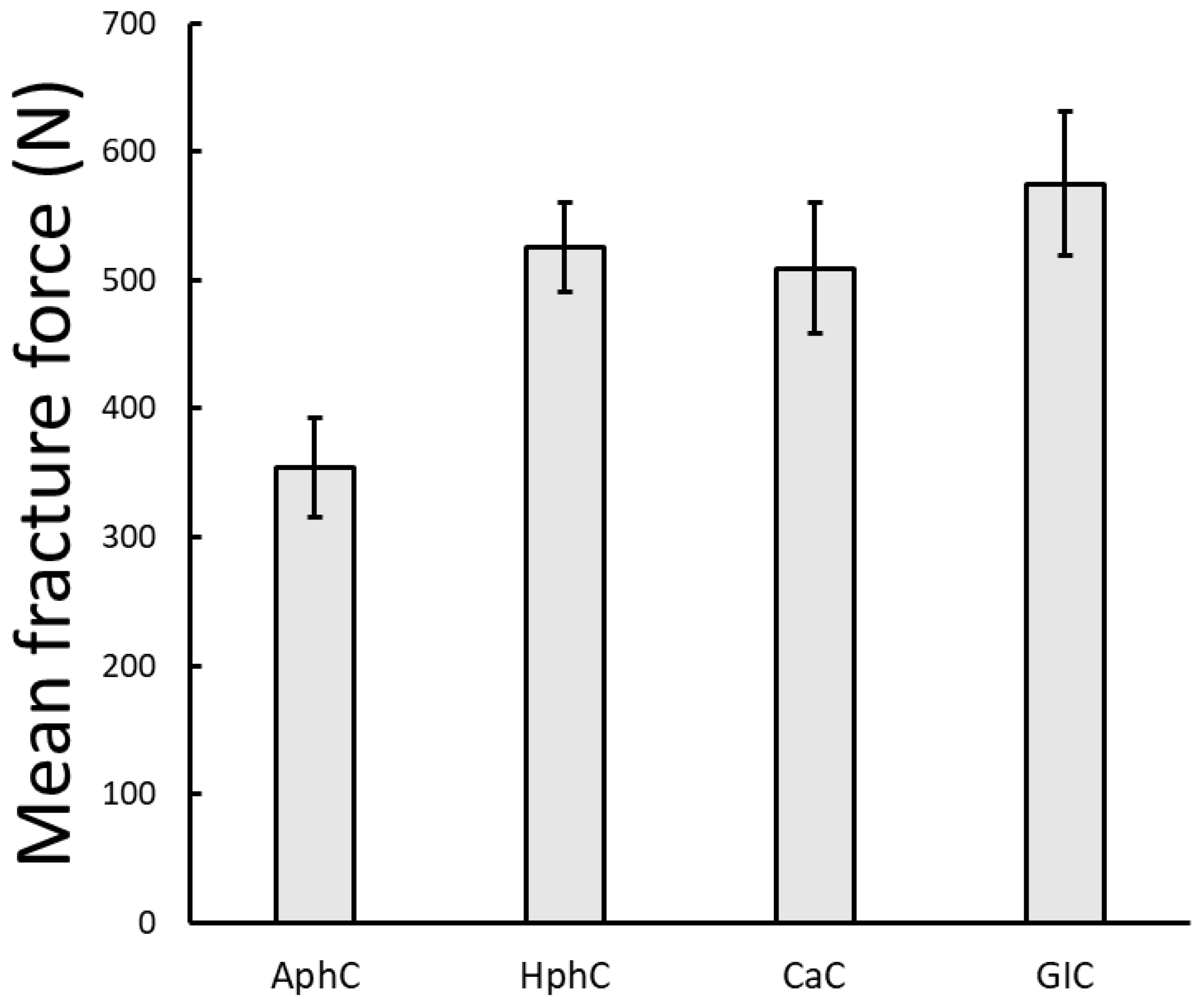
3.1. Results of the Static Loading Tests
3.2. Results of the Dynamic Loading Tests
3.3. Results of Finite Element Analysis
4. Discussion
4.1. Static Load Performance
4.2. Fatigue Behaviour and Clinical Relevance
4.3. Finite Element Analysis
4.4. Limitations
5. Conclusions
- Resin-based (ReC) and resin-modified glass ionomer (RMGIC) cements exhibited superior mechanical strength and fatigue resistance, showing no fractures even at maximum test conditions. Their performance indicates excellent long-term reliability, making them the materials of choice for high-stress clinical situations such as full-coverage crowns, long-span bridges, or implant-supported restorations.
- In contrast, conventional cements, such as glass ionomer (GIC), polycarboxylate (CaC), and zinc phosphate cements (AphC, HphC) displayed lower compressive and cyclic load resistance. Among these, GIC showed relatively better static performance, while zinc phosphate cement exhibited the lowest mechanical strength. Despite their limitations, these traditional materials may still be suitable for low-stress, short-span, or temporary applications where chemical adhesion or fluoride release are desired advantages.
- Finite element analysis confirmed that resin-based and resin-modified cements distribute stress more evenly under load, reducing the risk of crack initiation and explaining their enhanced mechanical durability. The agreement between experimental and computational results validates the applied testing methodology.
- Clinically, understanding the mechanical and structural behaviour of luting agents is crucial for selecting the most appropriate material and ensuring the longevity of fixed restorations. Future studies should address long-term degradation under thermocycling and humidity to further approximate the intraoral environment.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AphC | Adhesor Zinc Phosphate Cement |
| HphC | Harvard Zinc Phosphate Cement |
| CaC | Harvard Polycarboxylate Cement |
| GIC | Glass Ionomer Cement |
| RMGIC | Reinforced Glass Ionomer Luting Cement |
| ReC | Self-Adhesive Resin Cement |
References
- Shillingburg, H.T.; Sather, D.A.; Stone, S.E. Cementation and Bonding. In Fundamentals of Fixed Prosthodontics, 4th ed.; Quintessence Publishing: Chicago, IL, USA, 2012. [Google Scholar]
- McCabe, J.F.; Walls, A. Cements Based on Phosphoric Acid. In Applied Dental Materials, 9th ed.; Blackwell Publishing: Oxford, UK; Ames, IA, USA, 2008. [Google Scholar]
- de la Macorra, J.C.; Pradíes, G. Conventional and Adhesive Luting Cements. Clin. Oral Investig. 2002, 6, 198–204. [Google Scholar] [CrossRef]
- Lad, P.P.; Kamath, M.; Tarale, K.; Kusugal, P.B. Practical Clinical Considerations of Luting Cements: A Review. J. Int. Oral Health 2014, 6, 116–120. [Google Scholar]
- Hill, E.; Lott, J. A Clinically Focused Discussion of Luting Materials. Aust. Dent. J. 2011, 56 (Suppl. S1), 67–76. [Google Scholar] [CrossRef]
- Radhakrishnan, L.; Nandakumar, K.; Jassim, A.; Mohamed Ali, K.P.A. Effect of three different liquid medias in the sorption and solubility of luting cements: An in vitro study. Cureus 2023, 15, 47311. [Google Scholar] [CrossRef]
- Labban, N.; AlSheikh, R.; Lund, M.; Matis, B.A.; Moore, B.K.; Cochran, M.A.; Platt, J.A. Evaluation of the water sorption and solubility behavior of different polymeric luting materials. Polymers 2021, 13, 2851. [Google Scholar] [CrossRef] [PubMed]
- Heboyan, A.; Vardanyan, A.; Karobari, M.I.; Marya, A.; Avagyan, T.; Tebyaniyan, H.; Mustafa, M.; Rokaya, D.; Avetisyan, A. Dental Luting Cements: An Updated Comprehensive Review. Molecules 2023, 28, 1619. [Google Scholar] [CrossRef] [PubMed]
- Vishnu, G.; Jeevanandan, G. Evaluation of Microleakage Using Different Luting Cements in Kedo Zirconia Crowns: An In Vitro Assessment. Cureus 2024, 16, 66237. [Google Scholar] [CrossRef]
- Lia, Z.C.; White, S.N. Mechanical properties of dental luting cements. J. Prosthet. Dent. 1999, 81, 597–609. [Google Scholar] [CrossRef]
- Rosenstiel, S.F.; Land, M.F.; Crispin, B.J. Dental Luting Agents: A Review of the Current Literature. J. Prosthet. Dent. 1998, 80, 280–301. [Google Scholar] [CrossRef] [PubMed]
- White, S.N.; Yu, Z.; Tom, J.F.M.D.; Sangsurasak, S. In Vivo Microleakage of Luting Cements for Cast Crowns. J. Prosthet. Dent. 1994, 71, 333–338. [Google Scholar] [CrossRef]
- Osborne, J.W.; Wolff, M.S. The Effect of Powder/Liquid Ratio on the In Vivo Solubility of Polycarboxylate Cement. J. Prosthet. Dent. 1991, 66, 49–51. [Google Scholar] [CrossRef]
- Pameijer, C.H. A Review of Luting Agents. Int. J. Dent. 2012, 2012, 752861. [Google Scholar] [CrossRef]
- Pameijer, C.H. Biocompatibility of luting cements for dental applications. Biocomp. Dent. Mat. 2017, 77–94. [Google Scholar] [CrossRef]
- Attar, N.; Tam, L.E.; McComb, D. Mechanical and Physical Properties of Contemporary Dental Luting Agents. J. Prosthet. Dent. 2003, 89, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Heboyan, A.G.; Vardanyan, A.R.; Avetisyan, A.A. Cement Selection in Dental Practice. World Sci. News 2019, 126, 65–71. [Google Scholar] [CrossRef]
- Miotti, L.; Follak, A.; Montagner, A.; Pozzobon, R.; da Silveira, B.; Susin, A. Is Conventional Resin Cement Adhesive Performance to Dentin Better Than Self-Adhesive? A Systematic Review and Meta-Analysis of Laboratory Studies. Oper. Dent. 2020, 45, 484–495. [Google Scholar] [CrossRef]
- Chen, S.; Öhman, C.; Jefferies, S.R.; Gray, H.; Xia, W.; Engqvist, H. Compressive Fatigue Limit of Four Types of Dental Restorative Materials. J. Mech. Behav. Biomed. Mater. 2016, 61, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Arola, D. Fatigue testing of biomaterials and their interfaces. Dent. Mater. 2017, 33, 367–381. [Google Scholar] [CrossRef]
- Körtvélyessy, G.; Szabó, Á.L.; Pelsőczi-Kovács, I.; Tarjányi, T.; Tóth, Z.; Kárpáti, K.; Matusovits, D.; Hangyási, B.D.; Baráth, Z. Different Conical Angle Connection of Implant and Abutment Behavior: A Static and Dynamic Load Test and Finite Element Analysis Study. Materials 2023, 16, 1988. [Google Scholar] [CrossRef]
- di Lauro, A.E.; Ciaramella, S.; Tribst, J.P.M.; Aliberti, A.; Ausiello, P. Comparison of Bulk Polymeric Resin Composite and Hybrid Glass Ionomer Cement in Adhesive Class I Dental Restorations: A 3D Finite Element Analysis. Polymers 2024, 16, 2525. [Google Scholar] [CrossRef]
- Łępicka, M.; Niewczas, A.M.; Rodziewicz, M.U.; Pikuła, K.; Kordos, P.; Gredes, T.; Kurzydłowski, K.J. The Influence of Hydrothermal Fatigue on the Clinically Relevant Functional Properties of Conventional Glass-Ionomer Cements. Sci. Rep. 2023, 13, 8738. [Google Scholar] [CrossRef] [PubMed]
- Ganeshapooban, D.; Shahid, S.; Stefanova, V.; Todorova, V. Comparison of the compressive strength of conventional and resin-modified glass ionomer cements. J. IMAB 2024, 30, 5840–5844. [Google Scholar] [CrossRef]
- Hertz, H. On Hardness. Verh. Ver. Beförderung Gewerbe Fleisses 1882, 61, 410. [Google Scholar]
- Hertz, H. On the Contact of Elastic Solids. J. Reine Angew. Math. 1881, 92, 156–171. [Google Scholar]
- Fischer-Cripps, A.C. Predicting Hertzian Fracture. J. Mater. Sci. 1997, 32, 1277–1285. [Google Scholar] [CrossRef]
- Fischer-Cripps, A.C. Introduction to Contact Mechanics, 2nd ed.; Springer: Boston, MA, USA, 2007. [Google Scholar] [CrossRef]
- Chemani, B.; Chemani, H. Physico-Chemical and Mechanical Analysis of Dental Biomaterial “Cement Orthophosphate Zinc”. Procedia Eng. 2012, 42, 1396–1401. [Google Scholar] [CrossRef]
- Wang, Y.; Darvell, B.W. Effect of Elastic Modulus Mismatch on Failure Behaviour of Glass Ionomer Cement under Hertzian Indentation. Dent. Mater. 2012, 28, 279–286. [Google Scholar] [CrossRef]
- Cattani-Lorente, M.-A.; Dupuis, V.; Payan, J.; Moya, F.; Meyer, J.-M. Effect of Water on the Physical Properties of Resin-Modified Glass Ionomer Cements. Dent. Mater. 1999, 15, 71–78. [Google Scholar] [CrossRef]
- Furuichi, T.; Takamizawa, T.; Tsujimoto, A.; Miyazaki, M.; Barkmeier, W.; Latta, M. Mechanical Properties and Sliding-Impact Wear Resistance of Self-Adhesive Resin Cements. Oper. Dent. 2016, 41, E83–E92. [Google Scholar] [CrossRef] [PubMed]
- Molnár, J.; Fráter, M.; Sáry, T.; Braunitzer, G.; Vallittu, P.K.; Lassila, L.; Garoushi, S. Fatigue Performance of Endodontically Treated Molars Restored with Different Dentin Replacement Materials. Dent. Mater. 2022, 38, e83–e93. [Google Scholar] [CrossRef] [PubMed]
- White, S.N.; Yu, Z. Compressive and Diametral Tensile Strengths of Current Adhesive Luting Agents. J. Prosthet. Dent. 1993, 69, 568–572. [Google Scholar] [CrossRef]
- Diaz-Arnold, A.M.; Vargas, M.A.; Haselton, D.R. Current Status of Luting Agents for Fixed Prosthodontics. J. Prosthet. Dent. 1999, 81, 135–141. [Google Scholar] [CrossRef]
- Tavangar, M.S.; Jafarpur, D.; Bagheri, R. Evaluation of Compressive Strength and Sorption/Solubility of Four Luting Cements. J. Dent. Biomater. 2017, 4, 387–393. [Google Scholar]
- Nicholson, J.W.; McKenzie, M.A.; Goodridge, R.; Wilson, A.D. Variations in the Compressive Strength of Dental Cements Stored in Ionic or Acidic Solutions. J. Mater. Sci. Mater. Med. 2001, 12, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, L.A.; Kerby, R.E.; Seghi, R.; Berlin, J.S.; Lee, J.S. Fracture Toughness of Resin-Based Luting Cements. J. Prosthet. Dent. 2000, 83, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Yli-Urpo, H.; Lassila, L.V.J.; Närhi, T.; Vallittu, P.K. Compressive Strength and Surface Characterization of Glass Ionomer Cements Modified by Particles of Bioactive Glass. Dent. Mater. 2005, 21, 201–209. [Google Scholar] [CrossRef]
- Battancs, E.; Fráter, M.; Sáry, T.; Gál, E.; Braunitzer, G.; Szabó, P.B.; Garoushi, S. Fracture Behavior and Integrity of Different Direct Restorative Materials to Restore Noncarious Cervical Lesions. Polymers 2021, 13, 4170. [Google Scholar] [CrossRef]
- Fráter, M.; Sáry, T.; Molnár, J.; Braunitzer, G.; Lassila, L.; Vallittu, P.K.; Garoushi, S. Fatigue Performance of Endodontically Treated Premolars Restored with Direct and Indirect Cuspal Coverage Restorations Utilizing Fiber-Reinforced Cores. Clin. Oral Investig. 2022, 26, 3501–3513. [Google Scholar] [CrossRef]
- Assaf, J.; Hardan, L.; Kassis, C.; Bourgi, R.; Devoto, W.; Amm, E.; Moussa, C.; Sawicki, J.; Lukomska-Szymanska, M. Influence of Resin Cement Thickness and Elastic Modulus on the Stress Distribution of Zirconium Dioxide Inlay-Bridge: 3D Finite Element Analysis. Polymers 2021, 13, 3863. [Google Scholar] [CrossRef]
- Gönder, H.Y.; Fidancıoğlu, Y.D.; Fidan, M.; Mohammadi, R.; Karabekiroğlu, S. Comparison of Resin Cement’s Different Thicknesses and Poisson’s Ratios on the Stress Distribution of Class II Amalgam Restoration Using Finite Element Analysis. Appl. Sci. 2023, 13, 4125. [Google Scholar] [CrossRef]
- Petrauskienė, S.; Saldūnaitė-Mikučionienė, K.; Narbutaitė, J. Survival Analysis of Glass Ionomer Cement and Resin-Based Sealant Retention: A 10-Year Follow-Up Study. Medicina 2024, 60, 756. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, C.-T.; Li, L.; Zhou, Y.-X.; Zhao, K.; Mu, Y.-D.; Wang, X.-D. The Effect of Viscoelastic Behavior of Resin-Based Dental Materials on the Resin-Dentin Shear Bond Strength. J. Dent. 2025, 160, 105926. [Google Scholar] [CrossRef] [PubMed]
- Maravic, T.; Mazzitelli, C.; Mayer-Santos, E.; Mancuso, E.; Gracis, S.; Breschi, L.; Fuzzi, M. Current Trends for Cementation in Prosthodontics: Part 1—The Substrate. Polymers 2025, 17, 566. [Google Scholar] [CrossRef] [PubMed]








| Material | Density (kg/m3) | Modulus of Elasticity (GPa) | Poisson’s Ratio |
|---|---|---|---|
| AhpC | 3250 | 13.5 | 0.3 |
| HphC | 3250 | 13.5 | 0.3 |
| CaC | 2570 | 16 | 0.3 |
| GIC | 1880 | 7.7 | 0.3 |
| RMGIC | 1880 | 9.9 | 0.3 |
| ReC | 1700 | 7.4 | 0.3 |
| Loading head | 7850 | 220 | 0.3 |
| p Values | GIC | HphC | CaC | AhpC |
|---|---|---|---|---|
| GIC | 1 | 0.872 | 0.744 | 0.009 * |
| HphC | 1 | 0.994 | 0.059 | |
| CaC | 1 | 0.103 | ||
| AhpC | 1 |
| HphC | AphC | ReC | CaC | GIC | RMGIC | |
|---|---|---|---|---|---|---|
| Survival cycle number | 4567 (4785 ± 341) | 4507 (4303 ± 207) | 10,500 (9489 ± 706) | 4505 (4663 ± 273) | 5295 (5399 ± 206) | 9025 (9070 ± 291) |
| HphC | AphC | ReC | CaC | GIC | RMGIC | |
|---|---|---|---|---|---|---|
| HphC | 1 | 0.965 | <0.001 * | 0.99 | 0.829 | <0.001 * |
| AphC | 1 | <0.001 * | 0.987 | 0.417 | <0.001 * | |
| ReC | 1 | <0.001 * | <0.001 * | 0.97 | ||
| CaC | 1 | 0.606 | <0.001 * | |||
| GIC | 1 | <0.001 * | ||||
| RMGIC | 1 |
| Phase 1 | Phase 2 | Phase 3 | Phase 4 | Phase 5 | Phase 6 | Phase 7 | No Fracture | |
|---|---|---|---|---|---|---|---|---|
| HphC | 0 | 1 | 1 | 6 | 3 | 0 | 0 | 0 |
| AphC | 0 | 0 | 1 | 6 | 0 | 0 | 0 | 0 |
| ReC | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 8 |
| CaC | 0 | 1 | 1 | 13 | 0 | 1 | 0 | 0 |
| GIC | 0 | 0 | 0 | 8 | 5 | 0 | 0 | 0 |
| RMGIC | 0 | 0 | 0 | 0 | 0 | 3 | 6 | 2 |
| AphC | HphC | CaC | GIC | RMGIC | ReC | |
|---|---|---|---|---|---|---|
| AphC | 1 | 0.452 | 0.725 | 0.030 * | <0.001 * | 0.004 * |
| HphC | 1 | 0.540 | 0.285 | <0.001 * | 0.002 * | |
| CaC | 1 | 0.030 * | <0.001 * | <0.001 * | ||
| GIC | 1 | <0.001 * | 0.002 * | |||
| RMGIC | 1 | 0.096 | ||||
| ReC | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarjányi, T.; Mészáros, C.; Kiss, R.A.; Tóth, Z.; Pelsőczi, I. Mechanical Behaviour of Dental Luting Cements: Static, Dynamic, and Finite Element Studies. Dent. J. 2025, 13, 601. https://doi.org/10.3390/dj13120601
Tarjányi T, Mészáros C, Kiss RA, Tóth Z, Pelsőczi I. Mechanical Behaviour of Dental Luting Cements: Static, Dynamic, and Finite Element Studies. Dentistry Journal. 2025; 13(12):601. https://doi.org/10.3390/dj13120601
Chicago/Turabian StyleTarjányi, Tamás, Csongor Mészáros, Rebeka Anna Kiss, Zsolt Tóth, and István Pelsőczi. 2025. "Mechanical Behaviour of Dental Luting Cements: Static, Dynamic, and Finite Element Studies" Dentistry Journal 13, no. 12: 601. https://doi.org/10.3390/dj13120601
APA StyleTarjányi, T., Mészáros, C., Kiss, R. A., Tóth, Z., & Pelsőczi, I. (2025). Mechanical Behaviour of Dental Luting Cements: Static, Dynamic, and Finite Element Studies. Dentistry Journal, 13(12), 601. https://doi.org/10.3390/dj13120601






