Growth Factors Released from Advanced Platelet-Rich Fibrin in the Presence of Calcium-Based Silicate Materials and Their Impact on the Viability and Migration of Stem Cells of Apical Papilla
Abstract
:1. Introduction
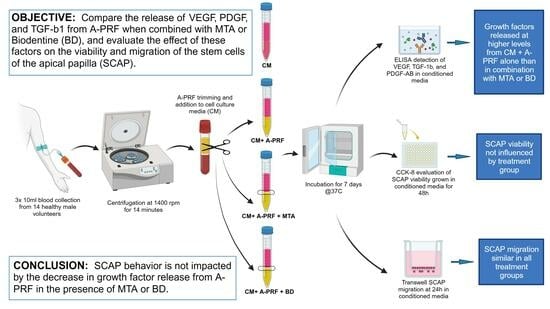
2. Materials and Methods
2.1. Subjects and Sample Collection
2.2. A-PRF Preparation and Media Conditioning
2.3. Growth Factor Quantification
2.4. Cell Culture and Reagent Preparation
2.5. Cell Viability Assay
2.6. Cell Migration Assay
2.7. Statistical Analysis
3. Results
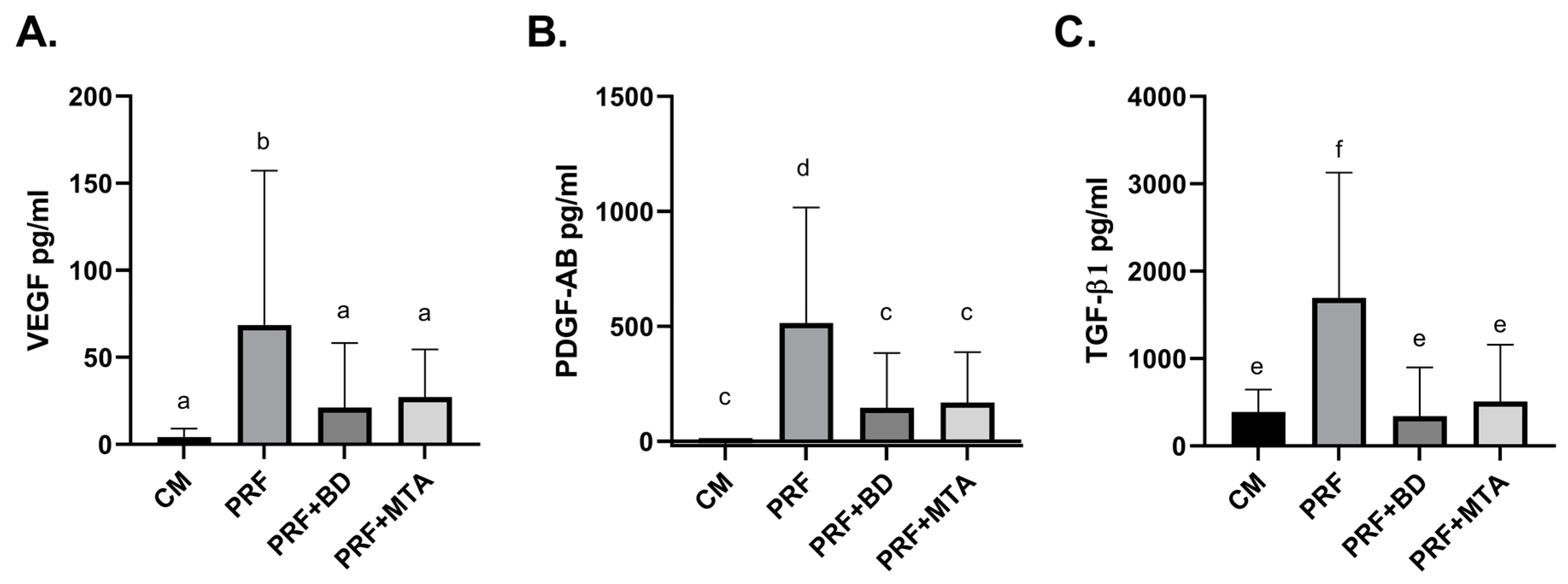
3.1. Release of Growth Factors
3.2. Correlation of Growth Factors with A-PRF Weight
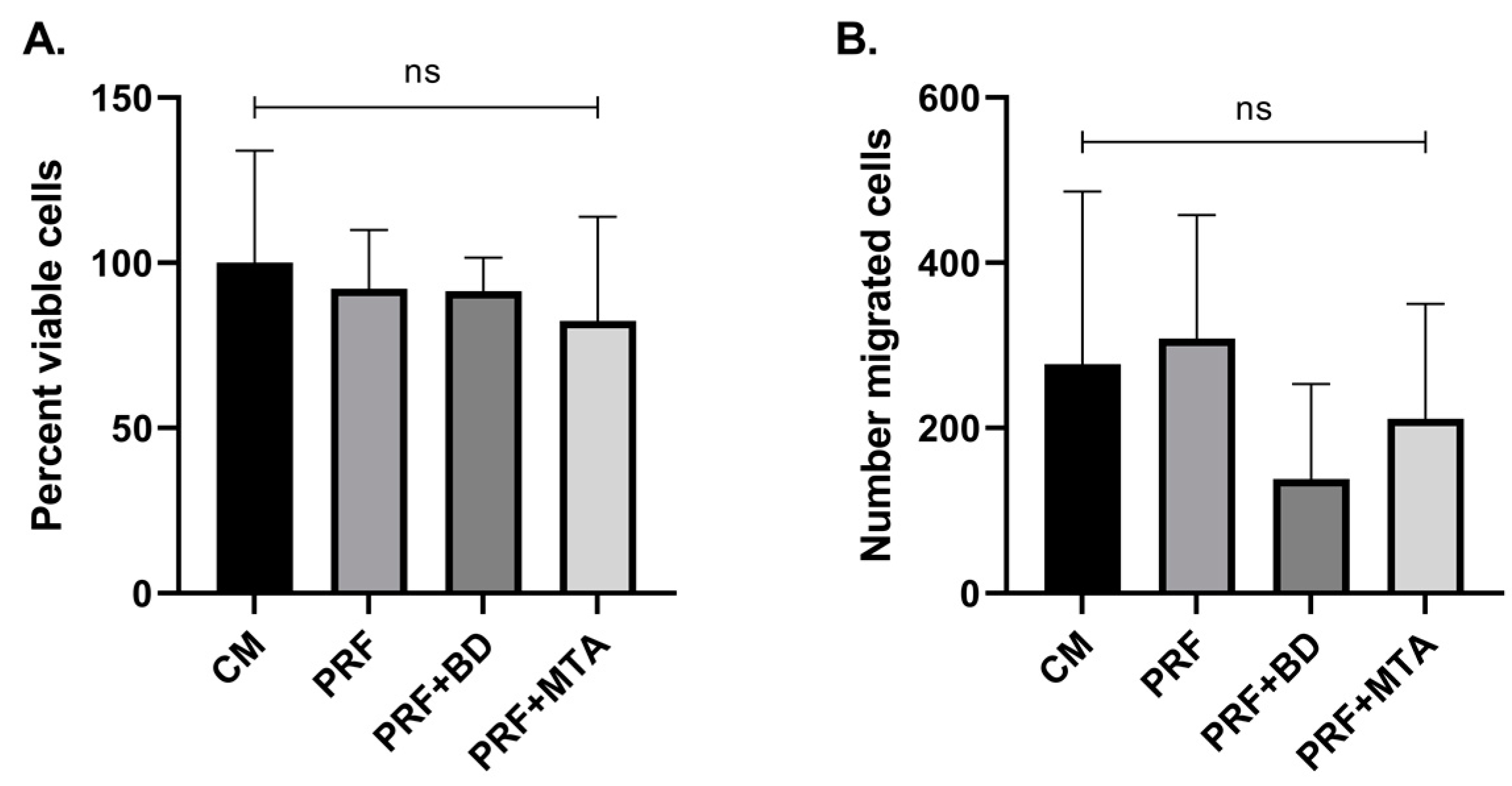
3.3. SCAP Viability and Migration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cvek, M. Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta-percha. A retrospective clinical study. Dent. Traumatol. 1992, 8, 45–55. [Google Scholar] [CrossRef]
- Wilkinson, K.L.; Beeson, T.J.; Kirkpatrick, T.C. Fracture resistance of simulated immature teeth filled with resilon, gutta-percha, or composite. J. Endod. 2007, 33, 480–483. [Google Scholar] [CrossRef]
- Murray, P.E. Platelet-Rich Plasma and Platelet-Rich Fibrin Can Induce Apical Closure More Frequently Than Blood-Clot Revascularization for the Regeneration of Immature Permanent Teeth: A Meta-Analysis of Clinical Efficacy. Front. Bioeng. Biotechnol. 2018, 6, 139. [Google Scholar] [CrossRef]
- Murray, P.E.; Garcia-Godoy, F.; Hargreaves, K.M. Regenerative endodontics: A review of current status and a call for action. J. Endod. 2007, 33, 377–390. [Google Scholar] [CrossRef]
- Banchs, F.; Trope, M. Revascularization of immature permanent teeth with apical periodontitis: New treatment protocol? J. Endod. 2004, 30, 196–200. [Google Scholar] [CrossRef]
- Nakashima, M.; Akamine, A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J. Endod. 2005, 31, 711–718. [Google Scholar] [CrossRef]
- Brizuela, C.; Huang, G.T.; Diogenes, A.; Botero, T.; Khoury, M. The Four Pillars for Successful Regenerative Therapy in Endodontics: Stem Cells, Biomaterials, Growth Factors, and Their Synergistic Interactions. Stem Cells Int. 2022, 2022, 1580842. [Google Scholar] [CrossRef]
- Albuquerque, M.T.; Valera, M.C.; Nakashima, M.; Nör, J.E.; Bottino, M.C. Tissue-engineering-based strategies for regenerative endodontics. J. Dent. Res. 2014, 93, 1222–1231. [Google Scholar] [CrossRef]
- Ong, T.K.; Lim, G.S.; Singh, M.; Fial, A.V. Quantitative Assessment of Root Development after Regenerative Endodontic Therapy: A Systematic Review and Meta-Analysis. J. Endod. 2020, 46, 1856–1866.e2. [Google Scholar] [CrossRef]
- Pietruszka, P.; Chruścicka, I.; Duś-Ilnicka, I.; Paradowska-Stolarz, A. PRP and PRF-Subgroups and Divisions When Used in Dentistry. J. Pers. Med. 2021, 11, 944. [Google Scholar] [CrossRef]
- Joshi, S.R.; Palekar, A.U.; Pendyala, G.S.; Mopagar, V.; Padmawar, N.; Shah, P. Clinical Success of Platelet-rich Fibrin and Mineral Trioxide Aggregate (MTA) or MTA-like Agents in Healing of Periapical Lesion in Nonsurgically Treated Pulpless Immature Permanent Teeth: A Systematic Review. J. Int. Soc. Prev. Community Dent. 2020, 10, 379–383. [Google Scholar] [CrossRef]
- Jung, I.Y.; Lee, S.J.; Hargreaves, K.M. Biologically based treatment of immature permanent teeth with pulpal necrosis: A case series. J. Endod. 2008, 34, 876–887. [Google Scholar] [CrossRef]
- Nosrat, A.; Seifi, A.; Asgary, S. Regenerative endodontic treatment (revascularization) for necrotic immature permanent molars: A review and report of two cases with a new biomaterial. J. Endod. 2011, 37, 562–567. [Google Scholar] [CrossRef]
- Alghofaily, M.; Torabinejad, M.; Nosrat, A. Regenerative Endodontic Treatment Using Periapical Blood or Circulating Blood as Scaffold: A Volumetric Analysis. J. Endod. 2022, 48, 625–631. [Google Scholar] [CrossRef]
- Bi, J.; Liu, Y.; Liu, X.M.; Lei, S.; Chen, X. Platelet-rich Fibrin Improves the Osteo-/Odontogenic Differentiation of Stem Cells from Apical Papilla via the Extracellular Signal-regulated Protein Kinase Signaling Pathway. J. Endod. 2020, 46, 648–654. [Google Scholar] [CrossRef]
- Dhurat, R.; Sukesh, M. Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author’s Perspective. J. Cutan. Aesthet. Surg. 2014, 7, 189–197. [Google Scholar] [CrossRef]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e56–e60. [Google Scholar] [CrossRef]
- Choukroun, J.; Ghanaati, S. Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients’ own inflammatory cells, platelets and growth factors: The first introduction to the low speed centrifugation concept. Eur. J. Trauma. Emerg. Surg. 2018, 44, 87–95. [Google Scholar] [CrossRef]
- Ghanaati, S.; Booms, P.; Orlowska, A.; Kubesch, A.; Lorenz, J.; Rutkowski, J.; Landes, C.; Sader, R.; Kirkpatrick, C.; Choukroun, J. Advanced platelet-rich fibrin: A new concept for cell-based tissue engineering by means of inflammatory cells. J. Oral Implantol. 2014, 40, 679–689. [Google Scholar] [CrossRef]
- El Bagdadi, K.; Kubesch, A.; Yu, X.; Al-Maawi, S.; Orlowska, A.; Dias, A.; Booms, P.; Dohle, E.; Sader, R.; Kirkpatrick, C.J.; et al. Reduction of relative centrifugal forces increases growth factor release within solid platelet-rich-fibrin (PRF)-based matrices: A proof of concept of LSCC (low speed centrifugation concept). Eur. J. Trauma. Emerg. Surg. 2019, 45, 467–479. [Google Scholar] [CrossRef]
- Kobayashi, E.; Fluckiger, L.; Fujioka-Kobayashi, M.; Sawada, K.; Sculean, A.; Schaller, B.; Miron, R.J. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin. Oral Investig. 2016, 20, 2353–2360. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.; Sonoyama, W.; Liu, Y.; Liu, H.; Wang, S.; Shi, S. The hidden treasure in apical papilla: The potential role in pulp/dentin regeneration and bioroot engineering. J. Endod. 2008, 34, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.Y.; Wang, S.; et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE 2006, 1, e79. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Fan, W.; Deng, Q.; He, H.; Huang, F. Stem Cells from the Apical Papilla: A Promising Source for Stem Cell-Based Therapy. Biomed. Res. Int. 2019, 2019, 6104738. [Google Scholar] [CrossRef] [PubMed]
- Bakopoulou, A.; Leyhausen, G.; Volk, J.; Tsiftsoglou, A.; Garefis, P.; Koidis, P.; Geurtsen, W. Effects of HEMA and TEDGMA on the in vitro odontogenic differentiation potential of human pulp stem/progenitor cells derived from deciduous teeth. Dent. Mater. 2011, 27, 608–617. [Google Scholar] [CrossRef]
- Huang, G.T.; Yamaza, T.; Shea, L.D.; Djouad, F.; Kuhn, N.Z.; Tuan, R.S.; Shi, S. Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng. Part. A 2010, 16, 605–615. [Google Scholar] [CrossRef]
- Bakhtiar, H.; Esmaeili, S.; Fakhr Tabatabayi, S.; Ellini, M.R.; Nekoofar, M.H.; Dummer, P.M. Second-generation Platelet Concentrate (Platelet-rich Fibrin) as a Scaffold in Regenerative Endodontics: A Case Series. J. Endod. 2017, 43, 401–408. [Google Scholar] [CrossRef]
- Jayadevan, V.; Gehlot, P.M.; Manjunath, V.; Madhunapantula, S.V.; Lakshmikanth, J.S. A comparative evaluation of Advanced Platelet-Rich Fibrin (A-PRF) and Platelet-Rich Fibrin (PRF) as a Scaffold in Regenerative Endodontic Treatment of Traumatized Immature Non-vital permanent anterior teeth: A Prospective clinical study. J. Clin. Exp. Dent. 2021, 13, e463–e472. [Google Scholar] [CrossRef]
- Malkondu, O.; Karapinar Kazandag, M.; Kazazoglu, E. A review on biodentine, a contemporary dentine replacement and repair material. Biomed. Res. Int. 2014, 2014, 160951. [Google Scholar] [CrossRef]
- Nowicka, A.; Wilk, G.; Lipski, M.; Kolecki, J.; Buczkowska-Radlinska, J. Tomographic Evaluation of Reparative Dentin Formation after Direct Pulp Capping with Ca(OH)2, MTA, Biodentine, and Dentin Bonding System in Human Teeth. J. Endod. 2015, 41, 1234–1240. [Google Scholar] [CrossRef]
- Torabinejad, M.; Hong, C.U.; Pitt Ford, T.R.; Kettering, J.D. Antibacterial effects of some root end filling materials. J. Endod. 1995, 21, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, C.; Lung, C.Y.K.; Schembri Wismayer, P.; Arias-Moliz, M.T.; Camilleri, J. The Relationship of Surface Characteristics and Antimicrobial Performance of Pulp Capping Materials. J. Endod. 2018, 44, 1115–1120. [Google Scholar] [CrossRef]
- Ballal, N.V.; Narkedamalli, R.; Ruparel, N.B.; Shenoy, P.A.; Bhat, V.R.; Belle, V.S. Effect of Maleic Acid Root Conditioning on Release of Transforming Growth Factor Beta 1 from Infected Root Canal Dentin. J. Endod. 2022, 48, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Sedgley, C.M.; Botero, T.M. Dental stem cells and their sources. Dent. Clin. N. Am. 2012, 56, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Wongwatanasanti, N.; Jantarat, J.; Sritanaudomchai, H.; Hargreaves, K.M. Effect of Bioceramic Materials on Proliferation and Odontoblast Differentiation of Human Stem Cells from the Apical Papilla. J. Endod. 2018, 44, 1270–1275. [Google Scholar] [CrossRef]
- Mullaguri, H.; Suresh, N.; Surendran, S.; Velmurugan, N.; Chitra, S. Role of pH Changes on Transforming Growth Factor-beta1 Release and on the Fibrin Architecture of Platelet-rich Fibrin When Layered with Biodentine, Glass Ionomer Cement, and Intermediate Restorative Material. J. Endod. 2016, 42, 766–770. [Google Scholar] [CrossRef]
- Ruparel, N.B.; de Almeida, J.F.; Henry, M.A.; Diogenes, A. Characterization of a stem cell of apical papilla cell line: Effect of passage on cellular phenotype. J. Endod. 2013, 39, 357–363. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Hong, S.; Chen, W.; Jiang, B. A Comparative Evaluation of Concentrated Growth Factor and Platelet-rich Fibrin on the Proliferation, Migration, and Differentiation of Human Stem Cells of the Apical Papilla. J. Endod. 2018, 44, 977–983. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Hernandez, M.; Choukroun, J. Platelet-Rich Fibrin and Soft Tissue Wound Healing: A Systematic Review. Tissue Eng. Part. B Rev. 2017, 23, 83–99. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Yoshioka, T.; Sugaya, H.; Gosho, M.; Aoto, K.; Kanamori, A.; Yamazaki, M. Growth factor levels in leukocyte-poor platelet-rich plasma and correlations with donor age, gender, and platelets in the Japanese population. J. Exp. Orthop. 2019, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Schär, M.O.; Diaz-Romero, J.; Kohl, S.; Zumstein, M.A.; Nesic, D. Platelet-rich concentrates differentially release growth factors and induce cell migration in vitro. Clin. Orthop. Relat. Res. 2015, 473, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Peters, O.A.; Galicia, J.; Arias, A.; Tolar, M.; Ng, E.; Shin, S.J. Effects of two calcium silicate cements on cell viability, angiogenic growth factor release and related gene expression in stem cells from the apical papilla. Int. Endod. J. 2016, 49, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Grech, L.; Mallia, B.; Camilleri, J. Characterization of set Intermediate Restorative Material, Biodentine, Bioaggregate and a prototype calcium silicate cement for use as root-end filling materials. Int. Endod. J. 2013, 46, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Janini, A.C.P.; Pelepenko, L.E.; Boldieri, J.M.; Dos Santos, V.A.B.; da Silva, N.A.; Raimundo, I.M.; Gomes, B.P.F.A.; Marciano, M.A. Biocompatibility analysis in subcutaneous tissue and physico-chemical analysis of pre-mixed calcium silicate-based sealers. Clin. Oral Investig. 2023, 27, 2221–2234. [Google Scholar] [CrossRef]
- Marciano, M.A.; Pelepenko, L.E.; Francati, T.M.; Antunes, T.B.M.; Janini, A.C.P.; Rohwedder, J.J.R.; Shelton, R.M.; Camilleri, J. Bismuth release from endodontic materials: In vivo analysis using Wistar rats. Sci. Rep. 2023, 13, 9738. [Google Scholar] [CrossRef]
- Strauss, F.J.; Nasirzade, J.; Kargarpoor, Z.; Stahli, A.; Gruber, R. Effect of platelet-rich fibrin on cell proliferation, migration, differentiation, inflammation, and osteoclastogenesis: A systematic review of in vitro studies. Clin. Oral Investig. 2020, 24, 569–584. [Google Scholar] [CrossRef]
- Beitzel, K.; McCarthy, M.B.; Cote, M.P.; Russell, R.P.; Apostolakos, J.; Ramos, D.M.; Kumbar, S.G.; Imhoff, A.B.; Arciero, R.A.; Mazzocca, A.D. Properties of biologic scaffolds and their response to mesenchymal stem cells. Arthroscopy 2014, 30, 289–298. [Google Scholar] [CrossRef]
- Giusti, I.; D’Ascenzo, S.; Manco, A.; Di Stefano, G.; Di Francesco, M.; Rughetti, A.; Dal Mas, A.; Properzi, G.; Calvisi, V.; Dolo, V. Platelet concentration in platelet-rich plasma affects tenocyte behavior in vitro. Biomed. Res. Int. 2014, 2014, 630870. [Google Scholar] [CrossRef]
- Sanz, J.L.; Forner, L.; Almudever, A.; Guerrero-Girones, J.; Llena, C. Viability and Stimulation of Human Stem Cells from the Apical Papilla (hSCAPs) Induced by Silicate-Based Materials for Their Potential Use in Regenerative Endodontics: A Systematic Review. Materials 2020, 13, 974. [Google Scholar] [CrossRef]
- Gomes-Cornélio, A.L.; Rodrigues, E.M.; Salles, L.P.; Mestieri, L.B.; Faria, G.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M. Bioactivity of MTA Plus, Biodentine and an experimental calcium silicate-based cement on human osteoblast-like cells. Int. Endod. J. 2017, 50, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Gomes Cornélio, A.L.; Salles, L.P.; Campos da Paz, M.; Cirelli, J.A.; Guerreiro-Tanomaru, J.M.; Tanomaru Filho, M. Cytotoxicity of Portland cement with different radiopacifying agents: A cell death study. J. Endod. 2011, 37, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Metlerska, J.; Fagogeni, I.; Nowicka, A. Efficacy of Autologous Platelet Concentrates in Regenerative Endodontic Treatment: A Systematic Review of Human Studies. J. Endod. 2019, 45, 20–30.e1. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smoczer, C.; Yuth, K.R.; Askar, M.A.; Young, L.A.; Paurazas, S.B. Growth Factors Released from Advanced Platelet-Rich Fibrin in the Presence of Calcium-Based Silicate Materials and Their Impact on the Viability and Migration of Stem Cells of Apical Papilla. Dent. J. 2023, 11, 220. https://doi.org/10.3390/dj11090220
Smoczer C, Yuth KR, Askar MA, Young LA, Paurazas SB. Growth Factors Released from Advanced Platelet-Rich Fibrin in the Presence of Calcium-Based Silicate Materials and Their Impact on the Viability and Migration of Stem Cells of Apical Papilla. Dentistry Journal. 2023; 11(9):220. https://doi.org/10.3390/dj11090220
Chicago/Turabian StyleSmoczer, Cristine, Kenneth R. Yuth, Mazin A. Askar, Laura A. Young, and Susan B. Paurazas. 2023. "Growth Factors Released from Advanced Platelet-Rich Fibrin in the Presence of Calcium-Based Silicate Materials and Their Impact on the Viability and Migration of Stem Cells of Apical Papilla" Dentistry Journal 11, no. 9: 220. https://doi.org/10.3390/dj11090220
APA StyleSmoczer, C., Yuth, K. R., Askar, M. A., Young, L. A., & Paurazas, S. B. (2023). Growth Factors Released from Advanced Platelet-Rich Fibrin in the Presence of Calcium-Based Silicate Materials and Their Impact on the Viability and Migration of Stem Cells of Apical Papilla. Dentistry Journal, 11(9), 220. https://doi.org/10.3390/dj11090220