Abstract
Aim: The present paper aims to systematize data concerning the prevalence and risk of dental erosion (DE) in adult patients with gastroesophageal reflux disease (GERD) compared to controls. Materials and methods: Core electronic databases, i.e., MEDLINE/PubMed, EMBASE, Cochrane, Google Scholar, and the Russian Science Citation Index (RSCI), were searched for studies assessing the prevalence and risk of DE in adult GERD patients with publication dates ranging from 1 January 1985 to 20 January 2022. Publications with detailed descriptive statistics (the total sample size of patients with GERD, the total sample size of controls (if available), the number of patients with DE in the sample of GERD patients, the number of patients with DE in the controls (if available)) were selected for the final analysis. Results: The final analysis included 28 studies involving 4379 people (2309 GERD patients and 2070 control subjects). The pooled prevalence of DE was 51.524% (95 CI: 39.742–63.221) in GERD patients and 21.351% (95 CI: 9.234–36.807) in controls. An association was found between the presence of DE and GERD using the random-effects model (OR 5.000, 95% CI: 2.995–8.345; I2 = 79.78%) compared with controls. When analyzing studies that only used validated instrumental methods for diagnosing GERD, alongside validated DE criteria (studies that did not specify the methodologies used were excluded), a significant association between the presence of DE and GERD was revealed (OR 5.586, 95% CI: 2.311–13.503; I2 = 85.14%). Conclusion: The meta-analysis demonstrated that DE is quite often associated with GERD and is observed in about half of patients with this extremely common disease of the upper gastrointestinal tract.
1. Introduction
Gastroesophageal reflux disease (GERD) is one of the most common gastrointestinal disorders, which is caused by a dysfunction of the motor-evacuation function of the gastroesophageal zone leading to spontaneous and regularly repeated retrograde reflux of the gastric and duodenal liquids into the esophagus [1,2]. According to a recent meta-analysis by Nirwan JS et al. in 2020—which summarized the results of 102 studies—the global prevalence of GERD is 13.98% (95% CI: 12.47–15.56) [3].
A characteristic feature of GERD is a chronic, recurrent pattern of symptoms that has a significant negative impact on the patient’s quality of life [2,4]. The classic clinical manifestations of the disease are heartburn, belching, and regurgitation; however, in some cases, GERD may be characterized by complex atypical symptoms, also referred to as extraesophageal syndromes [5,6]. In the largest prospective multicenter cohort study, i.e., ProGERD (n = 6215), atypical symptoms were detected in 32.8% of patients with heartburn [7]. According to the global Montreal Consensus (2006), a cough, laryngitis, bronchial asthma, and erosion of dental hard tissues of reflux etiology are extraesophageal syndromes that are significantly associated with GERD [8].
Dental erosion (DE) refers to non-carious lesions of the hard tissues of the tooth (mainly enamel and, in some cases, dentin) that are induced by a chemical reaction involving acids and that lead to demineralization processes independently of a bacterial factor [9,10]. DE leads to aesthetic defects and, in the case of prolonged progression, dentin exposure and the development of hypersensitivity, which has a negative impact on the quality of life [11,12]. According to the latest review, the average global incidence of DE among the adult population is 20–45% [13]. Moreover, on the epidemiological level, there has been an increase in the frequency of DE in all age groups, which may indicate an increasing influence of risk factors for this pathology in the population [14,15]. The genesis of DE is multifactorial and may be related to external acidifying factors (diet and lifestyle) and internal factors (chronic reflux of gastric contents into the oral cavity; recurrent vomiting) (Table 1) [10,13]. GERD is the most common trigger of DE, which is a result of the retrograde reflux of acidic gastric contents into the oral cavity [5,6,16,17,18]. According to several early systematic reviews, the incidence of DE in adult GERD patients is 32.5–38.96% [19,20]. Furthermore, various studies have noted that the higher the severity of erosive damage to the hard tissues of the teeth in GERD patients compared to controls [6,21]. To date, a large number of published studies on the prevalence of DE in patients with GERD have accumulated around the world, requiring systematization to objectify the global prevalence. The present paper aims to systematize data concerning the prevalence and risk of dental erosion (DE) in adult patients with gastroesophageal reflux disease (GERD) compared to controls.

Table 1.
Factors leading to the development of DE.
2. Materials and Methods
2.1. Study Sources and Search
A search was carried out in MEDLINE/PubMed, EMBASE, Cochrane, Google Scholar, and the Russian Science Citation Index (RSCI) for studies published between 1 January 1985 and 20 January 2022 (inclusive) based on the analysis of titles and abstracts of entries within these databases. The following keyword combinations were used to search the MEDLINE/PubMed database: “dental erosion [Title/Abstract] OR dental erosions [Title/Abstract] OR acid erosions [Title/Abstract] OR erosive toothwear [Title/Abstract] AND reflux [Title/Abstract]”. The corresponding terms in English were used for searching in the Google Scholar and RSCI database.
2.2. Study Selection
The criteria for the meta-analysis were as follows: relevant publications in peer-reviewed periodicals in English or Russian; publications with detailed descriptive statistics, which allowed the resulting data to be included in a meta-analysis; studies in the adult population of patients with GERD. Studies conducted on specific patient populations (diseases and conditions that may affect the objectivity and comparability of data) were excluded from the analysis. In cases of duplicated results in two publications (from different or the same electronic database), one was selected for the final analysis. The methodological quality of each of the included studies was assessed using the Newcastle–Ottawa Scale (NOS).
2.3. Data Extraction
Two investigators (D.N.A. and F.S.S.) independently extracted data using standardized forms. The year of publication, country, methodology for diagnosing GERD, criteria for diagnosing DE, the total sample size of patients with GERD, the total sample size of controls (if available), the number of patients with DE in the sample of patients with GERD, and the number of patients with DE in the sample of controls (if available) were analyzed. Any disagreements were resolved by discussion until reaching a consensus.
2.4. Statistical Analysis
Statistical data processing was carried out using the specialized software MedCalc 20.023 (MedCalc Software, Ostend, Belgium) in Microsoft Windows 11 (Microsoft, Redmond, WA, USA). The results are presented as the pooled frequency of DE in GERD patients/controls and a 95% confidence interval (95% CI). Heterogeneity between different studies was assessed using Cochrane’s Q test and I2 test. Significant heterogeneity was noted for results at p < 0.05 and I2 > 50. The probability of a publication error was estimated by constructing a funnel plot and calculations according to the Begg–Mazumdar correlation test and Egger’s test.
3. Results
3.1. Search Results
A search of the electronic databases returned 243 scientific papers for further analysis. Of these, 157 studies were excluded because they were not original clinical studies (83 reviews and systematic reviews; 31 experimental studies; 33 clinical observations; 10 other irrelevant studies). The 86 remaining studies were analyzed in detail for compliance with the inclusion criteria, which led to the exclusion of 58 studies (Figure 1). Finally, the remaining 28 original studies were considered eligible and included in the final meta-analysis (Table 2) [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48].
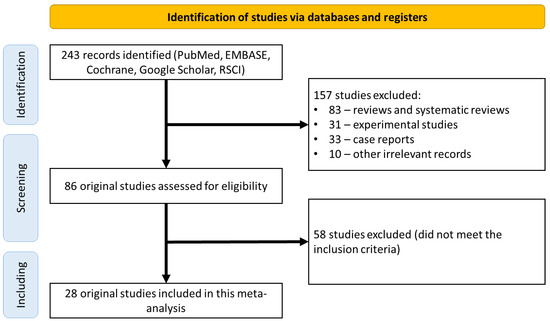
Figure 1.
CONSORT diagram detailing the study selection strategy.

Table 2.
Characteristics of selected studies.
3.2. Description of the Studies
The final analysis included 28 studies involving 4379 people (2309 patients with GERD and 2070 healthy subjects) performed in Brazil (n = 2) [24,39], the UK (n = 1) [27], Denmark (n = 1) [25], India (n = 5) [37,38,43,46,47], Iran (n = 1) [35], Spain (n = 2) [21,29], Italy (n = 1) [30], China (n = 2) [40,42], Mexico (n = 1) [36], Nigeria (n = 1) [26], Pakistan (n = 1) [44], Russia (n = 3) [28,41,48], Romania (n = 1) [47], Serbia (n = 1) [31], USA (n = 1) [33], Finland (n = 2) [22,23], France (n = 1) [47], and Japan (n = 1) [32]. The control population was represented in 21 studies [21,24,25,26,27,29,30,31,32,33,34,35,36,38,39,40,41,42,43,45,47]. In most studies, validated instrumental examination methods were used to diagnose GERD (n = 17) [21,22,23,24,25,26,27,28,30,32,34,35,36,38,42,43,44], and dental examination was used to diagnose DE using validated Eccles and Jenkins criteria (n = 7) [21,22,23,24,28,29,31], Smith and Knight (n = 6) [26,27,30,32,39,42], Basic Erosive Wear Examination (BEWE) (n = 5) [34,40,43,46,47], and the Lussi index (n = 2) [25,45]. The NOS assessment identified eight studies with a low risk of bias (scores of 7 or more) [21,25,27,30,39,40,42,43].
3.3. Prevalence of DE in GERD Patients
The pooled prevalence of DE in GERD patients and controls was 51.524% (95 CI: 39.742–63.221) and 21.351% (95 CI: 9.234–36.807), respectively (Figure 2). In the analysis, a random-effects model was used, as there was significant heterogeneity between both groups (I2GERD = 96.95%, I2control = 98.21%; p < 0.0001). Sub-analysis of the data showed that the pooled prevalence of DE in GERD patients was 46.497% (95 CI: 30.125–63.266) in Europe, 65.644% (95 CI: 45.560–83.170) in Asia, and 41.902% (95 CI: 11.019–76.927) in America (Figure 3).
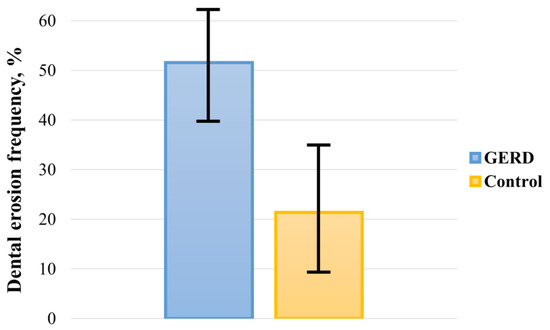
Figure 2.
Pooled frequency of DE in patients with GERD and controls.
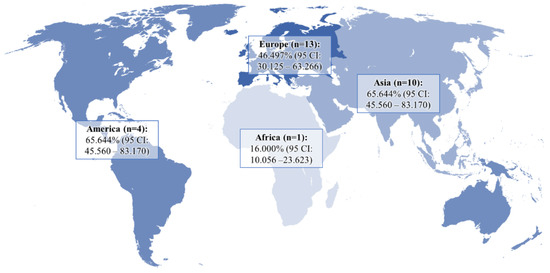
Figure 3.
Pooled frequency of DE in patients with GERD in different regions of the world.
3.4. Risk of DE in GERD Patients
Compared with controls, there was a significant association between the presence of DE and GERD according to the fixed effects model (OR 4.384, 95% CI: 3.607–5.329). However, given the high heterogeneity of the results of the included studies (I2 = 79.78%, 95% CI: 69.82–86.46), the risk was recalculated using a random-effects model (OR 5.000, 95% CI: 2.995–8.345) (Figure 4). When analyzing studies that used only validated instrumental methods for diagnosing GERD, alongside validated DE criteria (studies that did not specify methodologies were excluded), a significant association between the presence of DE and GERD was also revealed (OR 5.586, 95% CI: 2.311–13.503; I2 = 85.14%). The probability of publication bias was assessed by constructing a funnel plot and based on calculations of the Begg–Mazumdar test and the Egger’s test. A visual analysis of the funnel-shaped scattering diagram (Figure 5) did not reveal any significant asymmetry. In addition, the results of the Begg–Mazumdar test (p > 0.05) and the Egger’s test (p > 0.05) allowed for the presence of significant publication bias to be excluded.
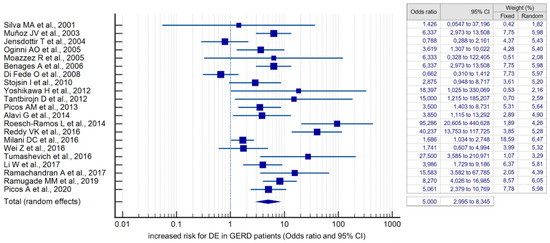
Figure 4.
Forest plot showing the cumulative risk (OR) of DE in GERD patients [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48].
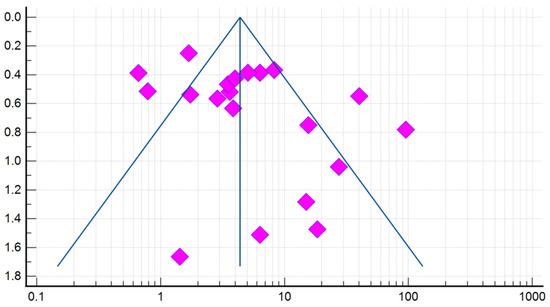
Figure 5.
A funnel plot estimating the likelihood of a publication bias when calculating the risk (OR) of DE in patients with GERD.
4. Discussion
GERD is a widespread acid-dependent disease that develops when the motor function of the upper gastrointestinal tract is impaired [1]. Approximately one-third of patients with GERD present with atypical extraesophageal symptoms [6,7]. DE is the most common dental manifestation of GERD and is caused by persistent retrograde reflux of acidic gastric contents into the oral cavity [16,17,49]. These pathological changes in the hard tissues of the teeth are more often localized on the vestibular (buccal), occlusal, and lingual surfaces of the teeth [6].
The development of DE within GERD occurs stage by stage. Initially, under the influence of repeated acid attacks, there is a gradual degradation of the tooth pellicle, which serves to protect the tooth hard tissue from the effects of acids [5,49]. The loss of the pellicle leads to direct contact of hydrochloric acid refluxate with the enamel surface and initiation of its demineralization at pH < 5.5 due to the dissolution of hydroxyapatite crystals (Figure 6) [49,50]. Deep DE leads to the opening of dentinal tubules and the development of hypersensitivity [5]. Saliva, which contains bicarbonates, antimicrobial substances, calcium, and phosphates, is the main protective element that can halt demineralization and promote the mineralization of dental hard tissues [50,51]. However, in GERD patients, hyposalivation is often observed, especially in obese individuals, which is also important in DE genesis [32,51].
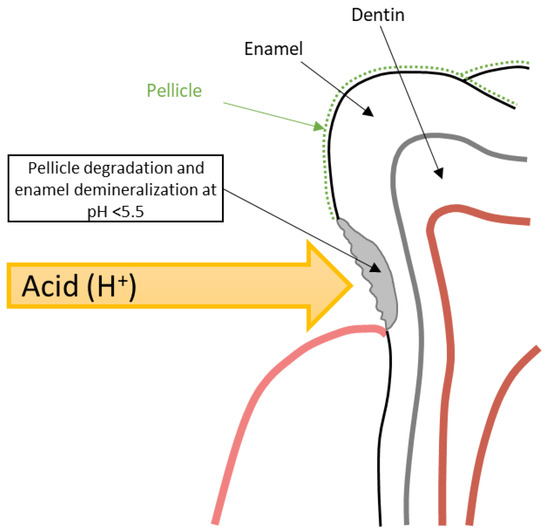
Figure 6.
Schematic model of DE formation in GERD patients.
In the studies conducted to date, the frequency of DE in GERD patients varies widely from 3.226% to 95.604% [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48]. Through the pooling of the results of the 28 selected studies in the present meta-analysis, the pooled incidence of DE in GERD patients was determined as 51.524% (95 CI: 39.742–63.221). Moreover, compared with healthy subjects, GERD significantly increases the risk of developing DE with an OR of 5.000 (95% CI: 2.995–8.345). The data obtained are consistent with the latest systematic reviews indicating that GERD is a significant risk factor for DE [18,20,52]. In consideration of this fact, lifestyle and diet changes can be recommended for GERD patients to prevent DE (sleeping with the head of the bed raised; exclusion of excessive consumption of carbonated drinks, drinks with a low pH, sour fruits, and certain drugs). In addition to the implementation of careful individual oral hygiene (the use of rinsing agents with neutral pH), remineralizing therapy at home with the use of remineralizing gels, and regular examinations by a dentist [6,53]. Oral care products can help prevent (or at least reduce DE). There is good evidence that hydroxyapatite-containing (calcium phosphate) products are working well [54,55].
In the case of hyposalivation, it is advisable to use saliva substitutes in addition to stimulating natural salivation through the consumption of sugar-free chewing gum and specialized lozenges containing xylitol [53]. As part of DE prevention, periodic use of antacids and alginates after reflux episodes is possible. According to the latest recommendations, antisecretory therapy using proton pump inhibitors (PPIs) is the first-line therapy for the induction and maintenance of clinical remission of GERD [56,57]. With the dental manifestation of GERD, empirical observations indicate it is reasonable to use PPI therapy twice a day for three months to prevent further damage [5,6]. In a randomized controlled trial using optical coherence tomography in GERD patients with associated DE, it was shown that PPI therapy (esomeprazole 20 mg twice a day) reduces the demineralization of dental hard tissue compared with a placebo [58]. In another longitudinal non-comparative study with a follow-up period of 1 year, the use of PPIs helped in halting the progression of DE in 74% of GERD patients [59].
There are several limitations of our study. First, the studies included in the meta-analysis are characterized by significant heterogeneity in both the methods used to diagnose GERD and the criteria for diagnosing DE. Secondly, in certain studies, subjective diagnostic tools were used to diagnose GERD, e.g., questionnaires, rather than objective instrumental diagnostic methods. In addition, the limitation of this study is that the protocol of systematic review was not registered in the PROSPERO registry. However, in terms of the number of studies assessed, this meta-analysis is by far the largest to evaluate the prevalence and risk of DE in adult patients with GERD by summarizing relevant results.
5. Conclusions
Present meta-analysis demonstrates that DE is quite often associated with GERD and observed in about half of patients with this extremely common disease of the upper gastrointestinal tract. Given this association, it is advisable to more actively identify patients at a high risk of DE among patients with GERD and refer them to a dentist for the timely prevention and correction of this dental pathological process.
Author Contributions
The concept and design of the study—O.O.Y., I.V.M. and N.I.K.; collection and processing of material—D.N.A. and F.S.S.; statistical data processing—D.N.A. and F.S.S.; writing the text—D.N.A., S.V.L., F.S.S. and M.N.B.; editing—O.O.Y., I.V.M., N.I.K., S.V.L., P.A.B. and K.Y.Z. All authors made a significant contribution to the preparation of the work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
MedCalc Database for statistical analysis: https://cloud.mail.ru/public/Y3aV/WRppBDEgM (accessed on 27 June 2022) and PRISMA statement: https://cloud.mail.ru/public/5AZG/bKGRzL4f9 (accessed on 27 June 2022).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maret-Ouda, J.; Markar, S.R.; Lagergren, J. Gastroesophageal Reflux Disease: A Review. JAMA 2020, 324, 2536–2547. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, K.H.; Meining, A. Current Insights in the Pathophysiology of Gastroesophageal Reflux Disease. Chirurgia 2021, 116, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Nirwan, J.S.; Hasan, S.S.; Babar, Z.U.; Conway, B.R.; Ghori, M.U. Global Prevalence and Risk Factors of Gastro-oesophageal Reflux Disease (GORD): Systematic Review with Meta-analysis. Sci. Rep. 2020, 10, 5814. [Google Scholar] [CrossRef] [PubMed]
- Chatila, A.T.; Nguyen, M.T.T.; Krill, T.; Roark, R.; Bilal, M.; Reep, G. Natural history, pathophysiology and evaluation of gastroesophageal reflux disease. Dis. Mon. 2020, 66, 100848. [Google Scholar] [CrossRef] [PubMed]
- Ghisa, M.; Della Coletta, M.; Barbuscio, I.; Marabotto, E.; Barberio, B.; Frazzoni, M.; De Bortoli, N.; Zentilin, P.; Tolone, S.; Ottonello, A.; et al. Updates in the field of non-esophageal gastroesophageal reflux disorder. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, M.; Lupi, G.; Cicerchia, F.; Ferro, A.; Barutta, F.; Beccuti, G.; Gruden, G.; Pellicano, R. Extra-Esophageal Presentation of Gastroesophageal Reflux Disease: 2020 Update. J. Clin. Med. 2020, 9, 2559. [Google Scholar] [CrossRef]
- Jaspersen, D.; Kulig, M.; Labenz, J.; Leodolter, A.; Lind, T.; Meyer-Sabellek, W.; Vieth, M.; Willich, S.N.; Lindner, D.; Stolte, M.; et al. Prevalence of extra-oesophageal manifestations in gastro-oesophageal reflux disease: An analysis based on the ProGERD Study. Aliment. Pharmacol. Ther. 2003, 17, 1515–1520. [Google Scholar] [CrossRef]
- Vakil, N.; van Zanten, S.V.; Kahrilas, P.; Dent, J.; Jones, R.; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: A global evidence-based consensus. Am. J. Gastroenterol. 2006, 101, 1900–1920. [Google Scholar] [CrossRef]
- Schlueter, N.; Amaechi, B.T.; Bartlett, D.; Buzalaf, M.A.R.; Carvalho, T.S.; Ganss, C.; Hara, A.T.; Huysmans, M.D.N.J.M.; Lussi, A.; Moazzez, R.; et al. Terminology of Erosive Tooth Wear: Consensus Report of a Workshop Organized by the ORCA and the Cariology Research Group of the IADR. Caries Res. 2020, 54, 2–6. [Google Scholar] [CrossRef]
- Warreth, A.; Abuhijleh, E.; Almaghribi, M.A.; Mahwal, G.; Ashawish, A. Tooth surface loss: A review of literature. Saudi Dent. J. 2020, 32, 53–60. [Google Scholar] [CrossRef]
- Twetman, S. The evidence base for professional and self-care prevention—Caries, erosion and sensitivity. BMC Oral Health 2015, 15, S4. [Google Scholar] [CrossRef] [PubMed]
- West, N.; Seong, J.; Davies, M. Dentine hypersensitivity. Monogr. Oral Sci. 2014, 25, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Schlueter, N.; Luka, B. Erosive tooth wear—A review on global prevalence and on its prevalence in risk groups. Br. Dent. J. 2018, 224, 364–370. [Google Scholar] [CrossRef]
- Jaeggi, T.; Lussi, A. Prevalence, incidence and distribution of erosion. Monogr. Oral Sci. 2014, 25, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Martignon, S.; Bartlett, D.; Manton, D.J.; Martinez-Mier, E.A.; Splieth, C.; Avila, V. Epidemiology of Erosive Tooth Wear, Dental Fluorosis and Molar Incisor Hypomineralization in the American Continent. Caries Res. 2021, 55, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Marsicano, J.A.; de Moura-Grec, P.G.; Bonato, R.C.; Sales-Peres, M.C.; Sales-Peres, A.; Sales-Peres, S.H. Gastroesophageal reflux, dental erosion, and halitosis in epidemiological surveys: A systematic review. Eur. J. Gastroenterol. Hepatol. 2013, 25, 135–141. [Google Scholar] [CrossRef]
- Lee, R.J.; Aminian, A.; Brunton, P. Dental complications of gastro-oesophageal reflux disease: Guidance for physicians. Intern. Med. J. 2017, 47, 619–623. [Google Scholar] [CrossRef]
- Ortiz, A.C.; Fideles, S.O.M.; Pomini, K.T.; Buchaim, R.L. Updates in association of gastroesophageal reflux disease and dental erosion: Systematic review. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 1037–1046. [Google Scholar] [CrossRef]
- Pace, F.; Pallotta, S.; Tonini, M.; Vakil, N.; Bianchi Porro, G. Systematic review: Gastro-oesophageal reflux disease and dental lesions. Aliment. Pharmacol. Ther. 2008, 27, 1179–1186. [Google Scholar] [CrossRef]
- Picos, A.; Badea, M.E.; Dumitrascu, D.L. Dental erosion in gastro-esophageal reflux disease. A systematic review. Clujul Med. 2018, 91, 387–390. [Google Scholar] [CrossRef]
- Muñoz, J.V.; Herreros, B.; Sanchiz, V.; Amoros, C.; Hernandez, V.; Pascual, I.; Mora, F.; Minguez, M.; Bagan, J.V.; Benages, A. Dental and periodontal lesions in patients with gastro-oesophageal reflux disease. Dig. Liver Dis. 2003, 35, 461–467. [Google Scholar] [CrossRef]
- Järvinen, V.; Meurman, J.H.; Hyvärinen, H.; Rytömaa, I.; Murtomaa, H. Dental erosion and upper gastrointestinal disorders. Oral Surg. Oral Med. Oral Pathol. 1988, 65, 298–303. [Google Scholar] [CrossRef]
- Meurman, J.H.; Toskala, J.; Nuutinen, P.; Klemetti, E. Oral and dental manifestations in gastroesophageal reflux disease. Oral Surg. Oral Med. Oral Pathol. 1994, 78, 583–589. [Google Scholar] [CrossRef]
- Silva, M.A.; Damante, J.H.; Stipp, A.C.; Tolentino, M.M.; Carlotto, P.R.; Fleury, R.N. Gastroesophageal reflux disease: New oral findings. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 91, 301–310. [Google Scholar] [CrossRef]
- Jensdottir, T.; Arnadottir, I.B.; Thorsdottir, I.; Bardow, A.; Gudmundsson, K.; Theodors, A.; Holbrook, W.P. Relationship between dental erosion, soft drink consumption, and gastroesophageal reflux among Icelanders. Clin. Oral Investig. 2004, 8, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Oginni, A.O.; Agbakwuru, E.A.; Ndububa, D.A. The prevalence of dental erosion in Nigerian patients with gastro-oesophageal reflux disease. BMC Oral Health 2005, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Moazzez, R.; Anggiansah, A.; Bartlett, D.W. The association of acidic reflux above the upper oesophageal sphincter with palatal tooth wear. Caries Res. 2005, 39, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Maev, I.V.; Barer, G.M.; Busarova, G.A.; Pustovoit, E.V.; Polikanova, E.N.; Burkov, S.G.; Yurenev, G.L. Dental manifestations of gastroesophageal reflux disease. Klin. Med. 2005, 11, 33–38. [Google Scholar]
- Benages, A.; Muñoz, J.V.; Sanchiz, V.; Mora, F.; Mínguez, M. Dental erosion as extraoesophageal manifestation of gastro-oesophageal reflux. Gut 2006, 55, 1050–1051. [Google Scholar] [CrossRef][Green Version]
- Di Fede, O.; Di Liberto, C.; Occhipinti, G.; Vigneri, S.; Lo Russo, L.; Fedele, S.; Lo Muzio, L.; Campisi, G. Oral manifestations in patients with gastro-oesophageal reflux disease: A single-center case-control study. J. Oral Pathol. Med. 2008, 37, 336–340. [Google Scholar] [CrossRef]
- Stojsin, I.; Brkanić, T.; Slavoljub, Z. Reflux disease as an etiological factor of dental erosion. Srp. Arh. Celok. Lek. 2010, 138, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, H.; Furuta, K.; Ueno, M.; Egawa, M.; Yoshino, A.; Kondo, S.; Nariai, Y.; Ishibashi, H.; Kinoshita, Y.; Sekine, J. Oral symptoms including dental erosion in gastroesophageal reflux disease are associated with decreased salivary flow volume and swallowing function. J. Gastroenterol. 2012, 47, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Tantbirojn, D.; Pintado, M.R.; Versluis, A.; Dunn, C.; Delong, R. Quantitative analysis of tooth surface loss associated with gastroesophageal reflux disease: A longitudinal clinical study. J. Am. Dent. Assoc. 2012, 143, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Picos, A.M.; Poenar, S.; Opris, A.; Chira, A.; Bud, M.; Berar, A.; Picos, A.; Dumitrascu, D.L. Prevalence of dental erosions in GERD: A pilot study. Clujul Med. 2013, 86, 344–346. [Google Scholar] [PubMed]
- Alavi, G.; Alavi, A.; Saberfiroozi, M.; Sarbazi, A.; Motamedi, M.; Hamedani, S. Dental Erosion in Patients with Gastroesophageal Reflux Disease (GERD) in a Sample of Patients Referred to the Motahari Clinic, Shiraz, Iran. J. Dent. 2014, 15, 33–38. [Google Scholar]
- Roesch-Ramos, L.; Roesch-Dietlen, F.; Remes-Troche, J.M.; Romero-Sierra, G.; Mata-Tovar, C.J.; Azamar-Jácome, A.A.; Barranca-Enríquez, A. Dental erosion, an extraesophageal manifestation of gastroesophageal reflux disease. The experience of a center for digestive physiology in Southeastern Mexico. Rev. Esp. Enferm. Dig. 2014, 106, 92–97. [Google Scholar] [CrossRef]
- Vinesh, E.; Masthan, K.; Kumar, M.S.; Jeyapriya, S.M.; Babu, A.; Thinakaran, M. A Clinicopathologic Study of Oral Changes in Gastroesophageal Reflux Disease, Gastritis, and Ulcerative Colitis. J. Contemp. Dent. Pract. 2016, 17, 943–947. [Google Scholar] [CrossRef]
- Reddy, V.K.; Poddar, P.; Mohammad, S.; Saha, S. Association between dental erosion and possible risk factors: A hospital-based study in gastroesophageal reflux disease patients. J. Indian Assoc. Public Health Dent. 2016, 14, 154–159. Available online: https://www.jiaphd.org/text.asp?2016/14/2/154/183814 (accessed on 19 January 2022).
- Milani, D.C.; Venturini, A.P.; Callegari-Jacques, S.M.; Fornari, F. Gastro-oesophageal reflux disease and dental erosions in adults: Influence of acidified food intake and impact on quality of life. Eur. J. Gastroenterol. Hepatol. 2016, 28, 797–801. [Google Scholar] [CrossRef]
- Wei, Z.; Du, Y.; Zhang, J.; Tai, B.; Du, M.; Jiang, H. Prevalence and Indicators of Tooth Wear among Chinese Adults. PLoS ONE 2016, 11, e0162181. [Google Scholar] [CrossRef]
- Tumashevich, O.O.; Rumyantsev, V.A.; Galochkina, A.B. Dental Syndrome in Gastroesophageal Reflux Disease. Exp. Clin. Gastroenterol. 2016. Available online: https://www.nogr.org/jour/article/view/61/61 (accessed on 19 January 2022).
- Li, W.; Liu, J.; Chen, S.; Wang, Y.; Zhang, Z. Prevalence of dental erosion among people with gastroesophageal reflux disease in China. J. Prosthet. Dent. 2017, 117, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.; Raja Khan, S.I.; Vaitheeswaran, N. Incidence and Pattern of Dental Erosion in Gastroesophageal Reflux Disease Patients. J. Pharm. Bioallied Sci. 2017, 9, S138–S141. [Google Scholar] [CrossRef] [PubMed]
- Warsi, I.; Ahmed, J.; Younus, A.; Rasheed, A.; Akhtar, T.S.; Ain, Q.U.; Khurshid, Z. Risk factors associated with oral manifestations and oral health impact of gastro-oesophageal reflux disease: A multicentre, cross-sectional study in Pakistan. BMJ Open 2019, 9, e021458. [Google Scholar] [CrossRef]
- Ramugade, M.M.; Sayed, A.; Sapkale, K.D.; Sonkurla, S. Evaluation of Nexus of Dental Erosion and Gastro-esophageal Reflux Disease: A Hospital-based Cross-sectional Study. J. Clin. Diagn. Res. 2019, 13, ZC17–ZC20. [Google Scholar] [CrossRef]
- Jacob, S.; Babu, A.; Sasidharan Latha, S.; Vivekanandan Glorine, S.J.; Surendran, L.; Gopinathan, A.S. Independent Variables of Dental Erosion among Tertiary Care Hospital Patients of a Developing Country. J. Int. Soc. Prev. Community Dent. 2019, 9, 612–618. [Google Scholar] [CrossRef]
- Picos, A.; Lasserre, J.F.; Chisnoiu, A.M.; Berar, A.M.; d’Incau, E.; Picos, A.M.; Chira, A.; des Varannes, S.B.; Dumitrascu, D.L. Factors associated with dental erosions in gastroesophageal reflux disease: A cross-sectional study in patients with heartburn. Med. Pharm. Rep. 2020, 93, 23–29. [Google Scholar] [CrossRef]
- Smirnova, T.A.; Novozhilova, O.A.; Kochubeinik, A.V.; Smirnov, D.V. Dental mask for gastroesophageal reflux disease. Dent. Everyone 2021, 2, 18–23. [Google Scholar] [CrossRef]
- Ranjitkar, S.; Kaidonis, J.A.; Smales, R.J. Gastroesophageal reflux disease and tooth erosion. Int. J. Dent. 2012, 2012, 479850. [Google Scholar] [CrossRef]
- Featherstone, J.D.B.; Lussi, A. Understanding the chemistry of dental erosion. Monogr. Oral Sci. 2006, 20, 66–76. [Google Scholar] [CrossRef]
- Ranjitkar, S.; Smales, R.J.; Kaidonis, J.A. Oral manifestations of gastroesophageal reflux disease. J. Gastroenterol. Hepatol. 2012, 27, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Chiesa-Estomba, C.M.; Calvo Henriquez, C.; Mouawad, F.; Ristagno, C.; Barillari, M.R.; Schindler, A.; Nacci, A.; Bouland, C.; Laino, L.; et al. Laryngopharyngeal reflux, gastroesophageal reflux and dental disorders: A systematic review. PLoS ONE 2020, 15, e0237581. [Google Scholar] [CrossRef] [PubMed]
- Marshall, T.A. Dietary assessment and counseling for dental erosion. J. Am. Dent. Assoc. 2018, 149, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Min, J.H.; Kwon, H.K.; Kim, B.I. Prevention of dental erosion of a sports drink by nano-sized hydroxyapatite in situ study. Int. J. Paediatr. Dent. 2015, 25, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Amaechi, B.T.; Fabritius, H.O.; Enax, J. Overview of Calcium Phosphates used in Biomimetic Oral Care. Open Dent. J. 2018, 12, 406–423. [Google Scholar] [CrossRef]
- Chapelle, N.; Ben Ghezala, I.; Barkun, A.; Bardou, M. The pharmacotherapeutic management of gastroesophageal reflux disease (GERD). Expert Opin. Pharmacother. 2021, 22, 219–227. [Google Scholar] [CrossRef]
- Hunt, R.; Armstrong, D.; Katelaris, P.; Afihene, M.; Bane, A.; Bhatia, S.; Chen, M.H.; Choi, M.G.; Melo, A.C.; Fock, K.M.; et al. World Gastroenterology Organisation Global Guidelines: GERD Global Perspective on Gastroesophageal Reflux Disease. J. Clin. Gastroenterol. 2017, 51, 467–478. [Google Scholar] [CrossRef]
- Wilder-Smith, C.H.; Wilder-Smith, P.; Kawakami-Wong, H.; Voronets, J.; Osann, K.; Lussi, A. Quantification of dental erosions in patients with GERD using optical coherence tomography before and after double-blind, randomized treatment with esomeprazole or placebo. Am. J. Gastroenterol. 2009, 104, 2788–2795. [Google Scholar] [CrossRef]
- Wilder-Smith, C.H.; Materna, A.; Martig, L.; Lussi, A. Longitudinal study of gastroesophageal reflux and erosive tooth wear. BMC Gastroenterol. 2017, 17, 113. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).