Abstract
The continuous rising of the cancer patient death rate undoubtedly shows the pressure to find more potent and efficient drugs than those in clinical use. These agents only treat a narrow range of cancer conditions with limited success and are associated with serious side effects caused by the lack of selectivity. In this frame, innovative syntheses approaches can decisively contribute to the success of “smart compounds” that might be only selective and/or active towards the cancer cells, sparing the healthy ones. In this scope, ruthenium chemistry is a rising field for the search of proficient metallodrugs by the use of macromolecular ruthenium complexes (dendrimers and dendronized polymers, coordination-cage and protein conjugates, nanoparticles and polymer-“ruthenium-cyclopentadienyl” conjugates) that can take advantage of the singularities of tumor cells (vs. healthy cells).
1. Introduction
There has been a growing awareness that nanotechnology applied to medicine has considerable potential to improve the treatment of several diseases. Specifically, in cancer therapy, the polymer-metal complex of oxaliplatin has been approved for the treatment of malignant tumors, including colorectal cancer, in 2003 [1].
The literature concerning macromolecules for drug delivery applications is mainly dedicated to platinum drugs [2,3,4], with some reports in initial study phases using copper [5], palladium [6], gold [7], tungsten [8] and ruthenium (which will be the focus of this review). Most of the approaches to the development of macromolecular drugs are based on the EPR (enhanced permeation and retention) effect, which was first identified by Maeda et al. in 1986 [9], and states that macromolecules selectively accumulate in tumors relative to healthy tissues, due to their defective vessel vascular structure and decreased lymphatic drainage. This passive targeting results, thus, in the passive accumulation of macromolecules in solid tumors, increasing the therapeutic index, while preventing the undesirable side effects generated by free drugs [10].This finding was a landmark in the anticancer nanomedicine field (the drug concentration in tumor can be 10 to 100 times higher than that in the blood) [11,12].
However, the Food and Drug Administration (FDA) has only approved 11 nano-therapeutics for cancer therapy so far [2,3,4]. One of the reasons for this situation is certainly related to the problems encountered in the development of new covalently bound macromolecule-drug conjugates, such as multi-step preparation, complicated and poor reproducibility synthesis, which often cause an inevitable loss of drug activity. It is thus of upmost importance to develop newer and simpler strategies for conjugate drugs with carriers without using such long processes. This problem can be partially overcome by using a one-step coordination strategy, as with some of the examples fully exposed in this review.
We will mostly focus on the syntheses of macromolecular ruthenium complexes (dendrimers and dendronized polymers, coordination-cage and protein conjugates, nanoparticles and polymer-“ruthenium-cyclopentadienyl” conjugates) to be used as chemotherapeutic agents in cancer treatment. Nowadays, ruthenium complexes are established alternatives to Pt‐based drugs in cancer therapy, showing different mechanisms of action and spectrums of activity and possessing the potential to overcome platinum-resistance, as well as lower toxicity [13,14,15,16,17,18]. There are not yet any commercially available ruthenium drugs, even though there are two important examples that have completed Phase I clinical trials, namely KP1019 [19] ([HInd][trans-RuIIICl4(Ind)2]; Ind = indazole) and NAMI-A [20] ([HIm][trans-RuIIICl4(DMSO)Im], Im = imidazole, DMSO = dimethylsulfoxide). Ruthenium is now a clear candidate for the search for new chemotherapeutics, since complexes bearing this metal core present several properties that make them attractive within this area, such as multiple oxidation states (II, III and IV) accessible under physiological conditions, favorable ligand-exchange kinetics with low toxicity, antitumor activity either in vitro as in vivo, as well as antimetastatic and intrinsic angiostatic activity. In this review, we will discuss the rationale behind the syntheses of these macromolecular ruthenium-based drugs and the coordination to metal strategies. We will finally discuss the best synthesis routes in order to shorten the gap between the huge number of papers published annually and the few compounds proceeding to clinical trials.
2. Multinuclear Approaches
The idea behind the multinuclearity in metal-conjugates is the increase of the cytotoxicity of a drug by increasing the number of metal centers. In this frame, dendrimers, coordination-cages conjugates, coordinate polymers or the coordination of a drug to a biomolecule are emerging fields in metal-based drugs, due to their multimeric scaffolds.
2.1. Ruthenium-Based Dendrimers
Dendrimers are synthetic, highly-branched macromolecules that arise from a central core and present a well-defined architecture, which can be easily tunable to present different molecular weights and sizes and can be straightforwardly functionalizable with the molecules of interest.
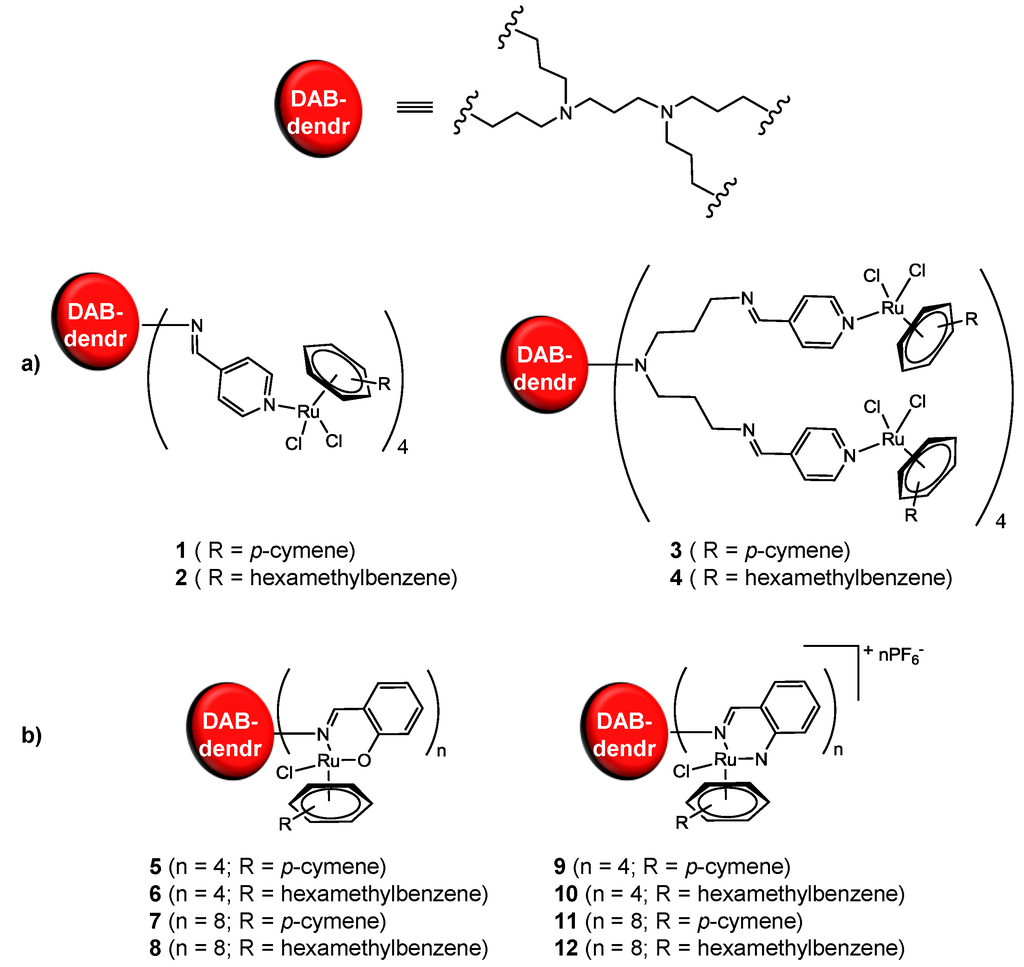
Scheme 1.
(a) Tetra- and octanuclear arene ruthenium dendritic systems [21]; (b) Tetra- and octa-nuclear chelating neutral (N,O) and cationic (N,N) ruthenium(II) metallodendrimers [22].
A series of first- and second-generation monodentate (N-donor) ruthenium(II)-arene (arene = p-cymene or hexamethylbenzene) metallodendrimers based on poly(propyleneimine) dendritic scaffolds was synthesized in order to exploit the EPR effect [21]. Dinuclear arene ruthenium complexes, [Ru(arene)Cl2]2, react with the dendritic scaffolds by stirring at room temperature in CH2Cl2 to yield the neutral tetranuclear and octanuclear ruthenium metallodendrimers (Scheme 1a) [21]. The yellow-orange products are isolated as air-stable solids in high yields (79%–98%) [21]. The complexes are soluble in most organic solvents [21]. The 1H NMR spectra of all the compounds show broadened peaks upon complexation of the multinuclear ruthenium moieties [21]. Evidence of the coordination of the aromatic nitrogen atom to the ruthenium metal was observed through a deshielding in the doublet assigned to aromatic protons on the carbon adjacent to the pyridyl nitrogen atom [21]. This deshielding is attributed to the electron-withdrawing effects of the coordinating metal [21]. The ruthenium functionalized dendrimers were precipitated with the inclusion of solvent molecules, trapped between the dendritic arms (confirmed by elemental analysis) [21].
A second series of metallodendrimers, containing tetranuclear and octanuclear chelating neutral (N,O) and cationic (N,N) first- and second-generation ruthenium(II) arene metallodendrimers based on poly(propyleneimine) dendritic scaffolds, was also synthesized from dinuclear arene ruthenium precursors, [Ru(arene)2Cl2]2 (arene = p-cymene, hexamethylbenzene), by reactions with salicylaldimine and iminopyridyl dendritic ligands in ethanol at room temperature (Scheme 1b) [22]. The N,N cationic complexes are isolated as hexafluorophosphate salts. These compounds are air-stable, the neutral complexes being soluble in most polar organic solvents and the cationic salts soluble in dimethylsulfoxide, acetone and acetonitrile [22]. The 1H NMR spectra of all the complexes is in agreement with the proposed structures, and the infrared spectra show shifts in the (C=N)imine absorption band (~1650 cm−1) to lower wavenumbers (~1620 cm−1), supporting the coordination of the imine nitrogen to the ruthenium [22]. MALDI-TOF (Matrix Assisted Laser Desorption Ionization-Time of Flight) studies confirmed that all of the dendrimer end-groups were functionalized with ruthenium(II) arene moieties [22].
The cytotoxicity of Metallodendrimers 1–12 was evaluated against A2780 human ovarian cancer cells after an incubation period of 72 h using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay (Table 1) [21,22]. The complexes showed moderate anti-proliferative activity (between 20–50 µM per metallodendrimer), with the exception of 9, which was not cytotoxic (IC50 > 200 µM). As expected, there is a correlation between the nuclearity of the dendritic compound and its cytotoxicity, i.e., monoruthenium compounds have only modest cytotoxicity, whereas the tetranuclear and octanuclear compounds present increasing cytotoxicities (Table 1).

Table 1.
IC50 of ruthenium Metallodendrimers 1–12 on A2780 human ovarian cancer cells after 72 h of exposure.
| Compound | IC50 (µM) per metallodendrimer | IC50 (µM) mononuclear Ru-derivative |
|---|---|---|
| 1 a | 43 | ≈100 |
| 2 a | 40 | |
| 3 a | 21 | |
| 4 a | 20 | |
| 5 b | 50 | 20–50 |
| 6 b | 27 | |
| 7 b | 22 | |
| 8 b | 10 | |
| 9 b | >200 | >200 |
| 10 b | 32 | |
| 11 b | 23 | |
| 12 b | 4 |
IC50 cisplatin in the same experimental conditions: a 1.6 µM [21]; b 1.5 µM [22].
Replacing p-cymene by hexamethylbenzene enhances the cytotoxicity of the metallodendrimers by a factor of two in the case of neutral Compounds 5–8 and by a factor of six for cationic Compounds 9–12. In the case of Metallodendrimers 1–4, this change does not affect the cytotoxicity.
More recently, a new class of air-stable cationic zero generation ruthenium-based metallodendrimers prepared using nitrile-functionalized poly(alkylidenamine) has been synthesized under the basis of the recognized activity of ruthenium compounds as anticancer drugs and the known stability of these dendrimers at physiological temperature [23]. Metallodendrimers 13–16 (Scheme 2) were synthesized by peripherally functionalization of the corresponding dendrimers with the ruthenium moieties, [Ru(η5-C5H5)(PPh3)2]+ or [RuCl(dppe)2]+[23]. For the synthesis of 13 and 15, solutions of the corresponding core with [Ru(η5-C5H5)(PPh3)2Cl] (4.5 molar ratio) and TlPF6 (4.5 molar ratio) in methanol were stirred at room temperature for approximately one day [23]. The resulting mixture was filtered, the precipitate rapidly extracted with CH2Cl2 and the solvent evaporated, affording the tetrakis-ruthenium dendrimers, 13 or 15, in the form of yellow products [23]. In the synthesis of 14 and 16, the five coordinate cis-[RuCl(dppe)2][PF6] complex (4.5 molar ratio) was stirred in 1,2-dichloroethane at 90 °C, for about two days [23]. After work-up, the compounds were dissolved in the minimum amount of CH2Cl2 and purified by re-precipitation with Et2O, giving pure yellowish Complexes 14 or 16 [23]. All the metallodendrimers were isolated in reasonably good yields (57%–74%). Spectroscopic (UV-Vis, IR, NMR) and mass spectrometry techniques confirmed the total functionalization of the ligand cores with the respective metal complex moieties [23]. Time degradation studies of the new metallodendrimers by NMR spectroscopy in DMSO-d6 at 37 °C showed different behaviors along time between the metallodendrimers functionalized with [Ru(η5-C5H5)(PPh3)2]+ or [RuCl(dppe)2]+ [23]. While Metallodendrimers 13 and 15, functionalized with [Ru(η5-C5H5)(PPh3)2]+, are unstable at physiological temperature, Metallodendrimers 14 and 16, functionalized with [RuCl(dppe)2]+, present a higher stability in DMSO. Compound 16 does not show signals of degradation with time [23]. Currently, the cytotoxic properties of Metallodendrimers 13–16 are being studied and attempts to improve their solubility and stability in aqueous media are being made [23].
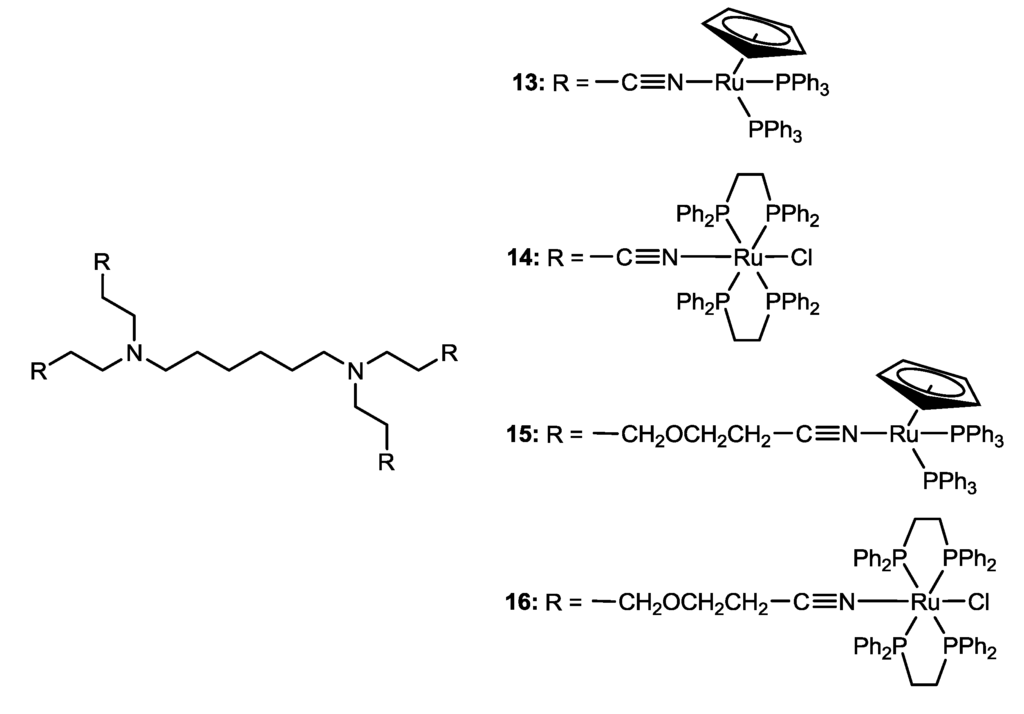
Scheme 2.
Metallodendrimers 13–16 [23].
2.2. Ruthenium-Based Coordination-Cage Conjugates
Some research groups have been developing several ruthenium coordination-cages (metallaprisms, metallarectangles, metallacycles) for application in cancer chemotherapy [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38].
The reaction of dinuclear arene ruthenium complexes [Ru2(arene)2(OO  OO)2Cl2] (arene = p-cymene, hexamethylbenzene; OO
OO)2Cl2] (arene = p-cymene, hexamethylbenzene; OO  OO = 2,5-dihydroxy-1,4-benzoquinonato; 2,5-dichloro-1,4-benzoquinonato) with pyrazine or bipyridine linkers (N
OO = 2,5-dihydroxy-1,4-benzoquinonato; 2,5-dichloro-1,4-benzoquinonato) with pyrazine or bipyridine linkers (N  N = 4,4'-bipyridine; 1,2-bis(4-pyridyl)ethylene) in methanol, at room temperature, using AgCF3SO3 as a halide scavenger, afford the synthesis of the water soluble tetranuclear metallacyclic cations of general formula [Ru4(arene)4(N
N = 4,4'-bipyridine; 1,2-bis(4-pyridyl)ethylene) in methanol, at room temperature, using AgCF3SO3 as a halide scavenger, afford the synthesis of the water soluble tetranuclear metallacyclic cations of general formula [Ru4(arene)4(N  N)2(OO
N)2(OO  OO)2]4+ (Scheme 3, Complexes 17–26) [24,25,39]. The larger rectangles, incorporating the 1,2-bis(4-pyridyl)-ethylene linker, are ca. five times more cytotoxic (IC50 £ 6 µM) than the 4,4'-bipyridine-containg cations (IC50 ³ 30 µM) for the A2780 human ovarian cancer cells (Table 2) [24]. The authors suggested that these variations could result from the different sized cavities, different flexibilities and different packing arrangements (observed from the X-ray diffraction of [Ru4(hexamethylbenzene)4(4,4'-bipyridine)2(2,5-dihydroxy-1,4-benzo-quinonato)2]4+19 and [Ru4(hexamethylbenzene)4(1,2-bis(4-pyridyl)ethylene)2(2,5-di-hydroxy-1,4-benzoquinonato)2]4+23) [24]. In each case, the hexamethylbenzene complexes exhibit lower IC50 than their p-cymene analogues, probably due to the greater lipophilicity of the second [24].
OO)2]4+ (Scheme 3, Complexes 17–26) [24,25,39]. The larger rectangles, incorporating the 1,2-bis(4-pyridyl)-ethylene linker, are ca. five times more cytotoxic (IC50 £ 6 µM) than the 4,4'-bipyridine-containg cations (IC50 ³ 30 µM) for the A2780 human ovarian cancer cells (Table 2) [24]. The authors suggested that these variations could result from the different sized cavities, different flexibilities and different packing arrangements (observed from the X-ray diffraction of [Ru4(hexamethylbenzene)4(4,4'-bipyridine)2(2,5-dihydroxy-1,4-benzo-quinonato)2]4+19 and [Ru4(hexamethylbenzene)4(1,2-bis(4-pyridyl)ethylene)2(2,5-di-hydroxy-1,4-benzoquinonato)2]4+23) [24]. In each case, the hexamethylbenzene complexes exhibit lower IC50 than their p-cymene analogues, probably due to the greater lipophilicity of the second [24].
 OO)2Cl2] (arene = p-cymene, hexamethylbenzene; OO
OO)2Cl2] (arene = p-cymene, hexamethylbenzene; OO  OO = 2,5-dihydroxy-1,4-benzoquinonato; 2,5-dichloro-1,4-benzoquinonato) with pyrazine or bipyridine linkers (N
OO = 2,5-dihydroxy-1,4-benzoquinonato; 2,5-dichloro-1,4-benzoquinonato) with pyrazine or bipyridine linkers (N  N = 4,4'-bipyridine; 1,2-bis(4-pyridyl)ethylene) in methanol, at room temperature, using AgCF3SO3 as a halide scavenger, afford the synthesis of the water soluble tetranuclear metallacyclic cations of general formula [Ru4(arene)4(N
N = 4,4'-bipyridine; 1,2-bis(4-pyridyl)ethylene) in methanol, at room temperature, using AgCF3SO3 as a halide scavenger, afford the synthesis of the water soluble tetranuclear metallacyclic cations of general formula [Ru4(arene)4(N  N)2(OO
N)2(OO  OO)2]4+ (Scheme 3, Complexes 17–26) [24,25,39]. The larger rectangles, incorporating the 1,2-bis(4-pyridyl)-ethylene linker, are ca. five times more cytotoxic (IC50 £ 6 µM) than the 4,4'-bipyridine-containg cations (IC50 ³ 30 µM) for the A2780 human ovarian cancer cells (Table 2) [24]. The authors suggested that these variations could result from the different sized cavities, different flexibilities and different packing arrangements (observed from the X-ray diffraction of [Ru4(hexamethylbenzene)4(4,4'-bipyridine)2(2,5-dihydroxy-1,4-benzo-quinonato)2]4+19 and [Ru4(hexamethylbenzene)4(1,2-bis(4-pyridyl)ethylene)2(2,5-di-hydroxy-1,4-benzoquinonato)2]4+23) [24]. In each case, the hexamethylbenzene complexes exhibit lower IC50 than their p-cymene analogues, probably due to the greater lipophilicity of the second [24].
OO)2]4+ (Scheme 3, Complexes 17–26) [24,25,39]. The larger rectangles, incorporating the 1,2-bis(4-pyridyl)-ethylene linker, are ca. five times more cytotoxic (IC50 £ 6 µM) than the 4,4'-bipyridine-containg cations (IC50 ³ 30 µM) for the A2780 human ovarian cancer cells (Table 2) [24]. The authors suggested that these variations could result from the different sized cavities, different flexibilities and different packing arrangements (observed from the X-ray diffraction of [Ru4(hexamethylbenzene)4(4,4'-bipyridine)2(2,5-dihydroxy-1,4-benzo-quinonato)2]4+19 and [Ru4(hexamethylbenzene)4(1,2-bis(4-pyridyl)ethylene)2(2,5-di-hydroxy-1,4-benzoquinonato)2]4+23) [24]. In each case, the hexamethylbenzene complexes exhibit lower IC50 than their p-cymene analogues, probably due to the greater lipophilicity of the second [24].Cationic tetra- and hexa-nuclear opened metalla-assemblies incorporating 5,15-bis(4-pyridyl)-10,20-diphenylporphyrin (Scheme 3, Complexes 25–26) or 5,10,15-tris(4-pyridyl)-20-phenylporphyrin (Scheme 4, Complexes 30–31) panels and dinuclear arene ruthenium clips [Ru2(p-cymene)2(OO  OO)2]2+ (OO
OO)2]2+ (OO  OO) = oxalate, 2,5-dioxydo-1,4-benzoquinonato, dobq) have been synthesized in the presence of AgCF3SO3 (the synthesis details are ambiguous) [25]. The compounds are sparingly soluble in water and stable in deuterated water at 60 °C for 48 h (NMR studies) [25]. All the complexes are cytotoxic against A2780 human ovarian cancer cells, the complexes with the dobq ligand (26 and 31) being more cytotoxic than the oxalate derivatives (25 and 30); this feature shows the importance of the spacer in the cytotoxic activity [25].
OO) = oxalate, 2,5-dioxydo-1,4-benzoquinonato, dobq) have been synthesized in the presence of AgCF3SO3 (the synthesis details are ambiguous) [25]. The compounds are sparingly soluble in water and stable in deuterated water at 60 °C for 48 h (NMR studies) [25]. All the complexes are cytotoxic against A2780 human ovarian cancer cells, the complexes with the dobq ligand (26 and 31) being more cytotoxic than the oxalate derivatives (25 and 30); this feature shows the importance of the spacer in the cytotoxic activity [25].
 OO)2]2+ (OO
OO)2]2+ (OO  OO) = oxalate, 2,5-dioxydo-1,4-benzoquinonato, dobq) have been synthesized in the presence of AgCF3SO3 (the synthesis details are ambiguous) [25]. The compounds are sparingly soluble in water and stable in deuterated water at 60 °C for 48 h (NMR studies) [25]. All the complexes are cytotoxic against A2780 human ovarian cancer cells, the complexes with the dobq ligand (26 and 31) being more cytotoxic than the oxalate derivatives (25 and 30); this feature shows the importance of the spacer in the cytotoxic activity [25].
OO) = oxalate, 2,5-dioxydo-1,4-benzoquinonato, dobq) have been synthesized in the presence of AgCF3SO3 (the synthesis details are ambiguous) [25]. The compounds are sparingly soluble in water and stable in deuterated water at 60 °C for 48 h (NMR studies) [25]. All the complexes are cytotoxic against A2780 human ovarian cancer cells, the complexes with the dobq ligand (26 and 31) being more cytotoxic than the oxalate derivatives (25 and 30); this feature shows the importance of the spacer in the cytotoxic activity [25].A solution the N,N'-di(4-pyridyl)-1,4,5,8-naphthalenetetracarboxydiimide donor ligand in CH3NO2 was added dropwise to a CH3OH solution containing equimolar amounts of [Ru2(µ4-C2O4)(MeOH)2(η6-p-iPrC6H4Me)2][O3SCF3]2, [Ru2(dobq)(MeOH)2(η6-p-iPrC6H4Me)2][O3SCF3]2 or [Ru2(donq)(H2O)2(η6-p-iPrC6H4Me)2][O3SCF3]2. The mixture was stirred for 48 h at 60 °C, filtered and the solvent evaporated to dryness. Pure compounds were isolated after washing the products with diethyl ether (yield ≈ 90%) [33].UV-Vis absorption spectra presented the expected π→π* transition bands, corresponding to the extended aromatic systems of the dipyridyl ligands [33]. The X-ray analysis of the compound bearing the [Ru2(dobq)(MeOH)2(η6-p-iPrC6H4Me)2][O3SCF3]2 moiety proved the rectangle nature of this family of compounds [33]. All these compounds were tested against gastric (AGS) and colon (HCT-15) human cancer cells [33]. While compounds bearing the µ4-C2O4 and dobq spacers were found to be poorly active against these cancer cell lines, Compound 27 (Scheme 3), bearing the donq linker, proved to be better than cisplatin for the AGS cells and with comparable IC50 values for the HCT-15 cell line [33]. These results emphasize the importance of the spacer.
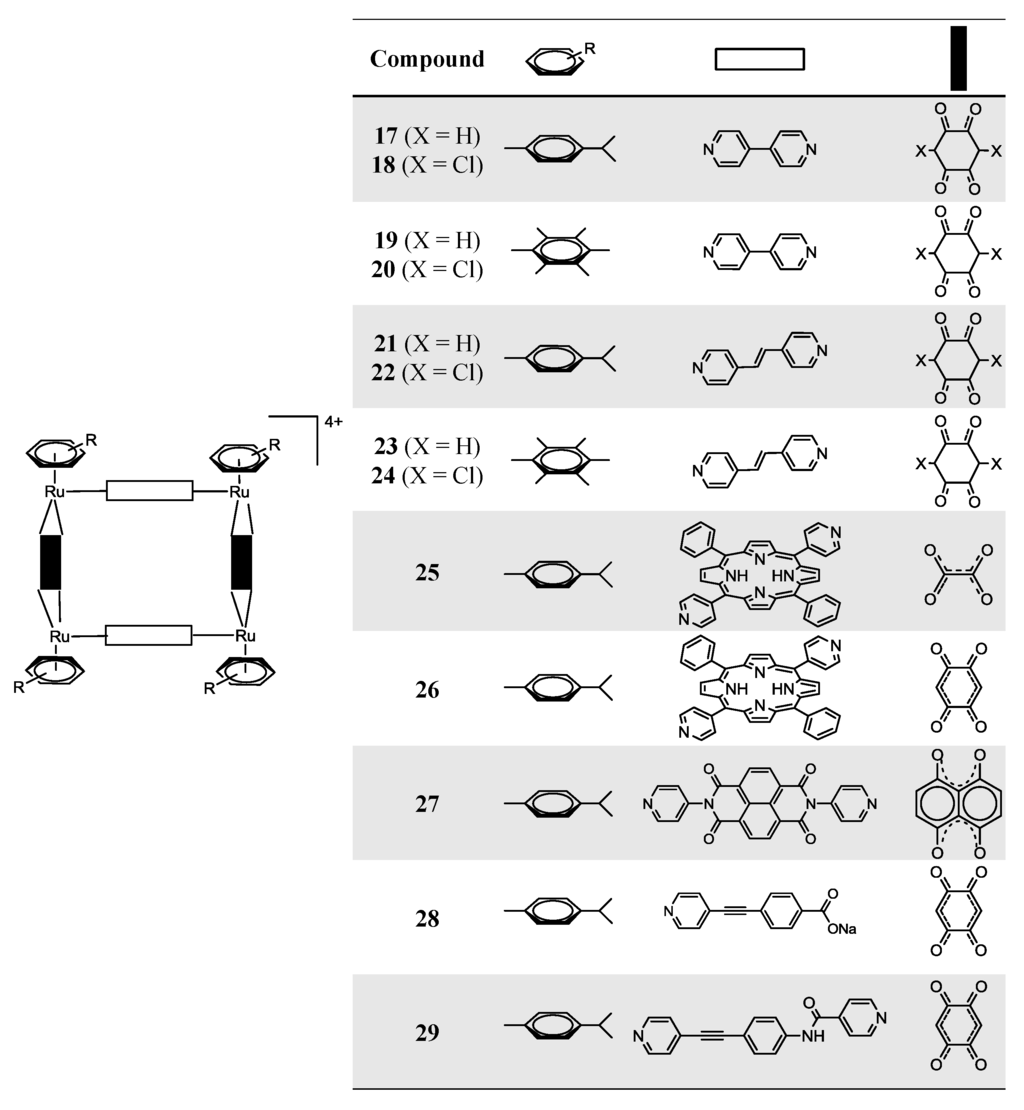
Scheme 3.
Metallarectangles 17–29 [24,25,33,34,35].
The synthesis of asymmetrical metallarectangles has also been tested [34,35]. In the first case, a solution of ambidentate donor sodium 4-(pyridin-4-ylethynyl)benzoate in MeOH was added dropwise to a solution of ruthenium acceptor [Ru2(donq)(H2O)2(η6-p-iPrC6H4Me)2][O3SCF3]2 in MeOH in a 1:1 molar ratio [34]. The final product was treated with diethyl ether, affording the sea-green Compound 28 (Scheme 3) [34]. Due to the asymmetry of the ambidentate donor, the formation of two isomers is possible (Scheme 5). This feature was followed by NMR spectroscopy; the four protons of the donq ligand are distinct if we have one or another isomer: for the head-to-tail isomer (A), two protons are oriented towards the Ru–N centers and the other two protons are oriented towards the Ru–O centers (on the same clip), thus making them chemically different; in the case of the head-to-head isomer (B), all the donq protons of a given clip have the same neighborhood, thus being equivalent [34]. In this frame, the head-to-head isomer presents two singlets in the 1H NMR, while the head-to-tail isomer presents two sets of doublets [34]. In the particular case of Compound 28, the 1H-NMR showed the predominance of the head-to-head isomer [34]. This can be justified in terms of ring strain, as it was observed by the solid-state structure of Compound 28 [34]. The authors describe that the Ru–Ru–N angle in the head-to-tail isomer is 78.37°, while the Ru–Ru–O angle is 96.85° [34]. In this frame, the presence of two pyridyl or two carboxylate groups on the same clip would give unfitting angularities and eventually lead to the terminal ligand-ends being too close or too far apart, thus making the coordination with the second clip unfavorable [34].The in vitro anticancer activity of this compound was tested against lung (A549), gastric (AGS), colon (HCT-15) and liver (SK-hep-1) human cancer cell lines. The compound is active for all these cancer cell lines, in particular for the AGS human gastric cancer cell line. When the donq linker is replaced by dobq, a non-cytotoxic compound is obtained (IC50 in all tested cancer cell lines >200 µM) [34].
A second asymmetrical molecular rectangle was obtained by suspension of N-(4-(pyridine-4-ylenthynyl)phenyl)-isonicotinamide and [Ru2(donq)(H2O)2(η6-p-iPrC6H4Me)2][O3SCF3]2 in CH2Cl2/CH3OH (1:1) for 6 h at room temperature [35]. The crude product (obtained after evaporation of the solvent) was dissolved in acetone and recrystallized by slow diffusion of diethyl ether, resulting in the green crystalline solid, 29 (Scheme 3) [35]. Once again, due to the linker asymmetry, the formation of two isomers might occur. In this case, however, it seems that there is not a preferred one (data from 1H NMR show the signals of both isomers) [35]. The IC50 values determined for colorectal (Colo320), lung (A549 and H1299) and breast (MCF7) human cancer cell lines reveal very low IC50 values (0.1–10.18 µM), placing this compound among the best ruthenium-macromolecular compounds tested for in vitro anticancer activity [35].
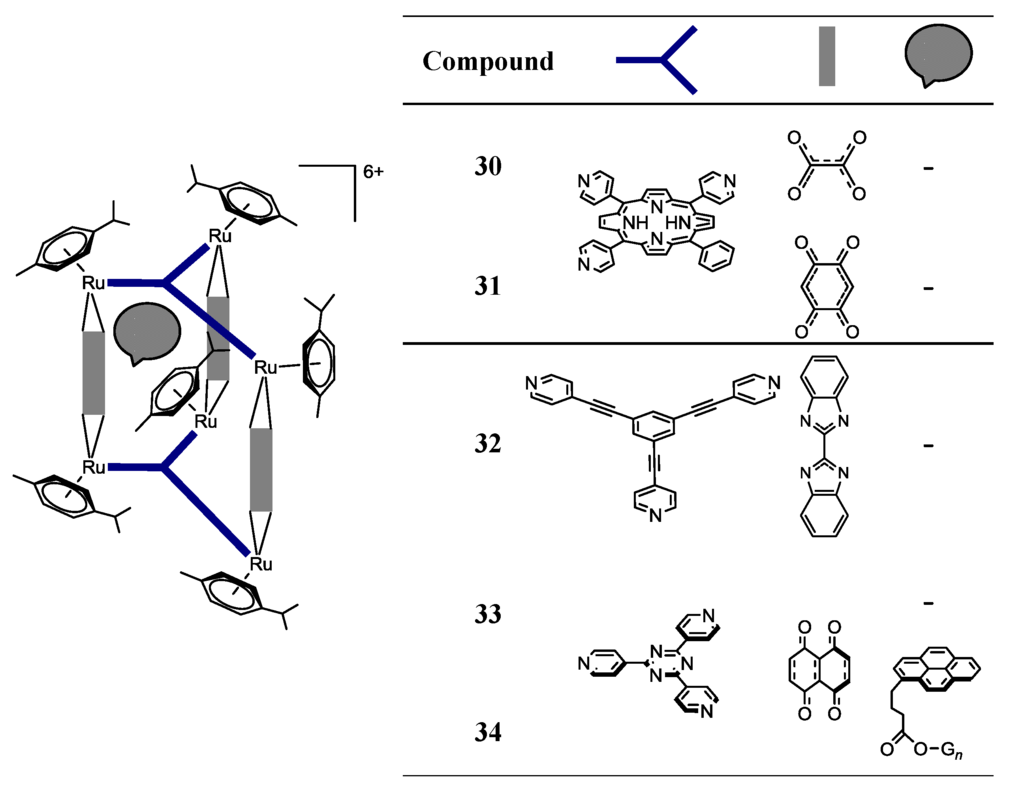
Scheme 4.
Structure of the Metalla-assemblies 30–34 [25,26,37].
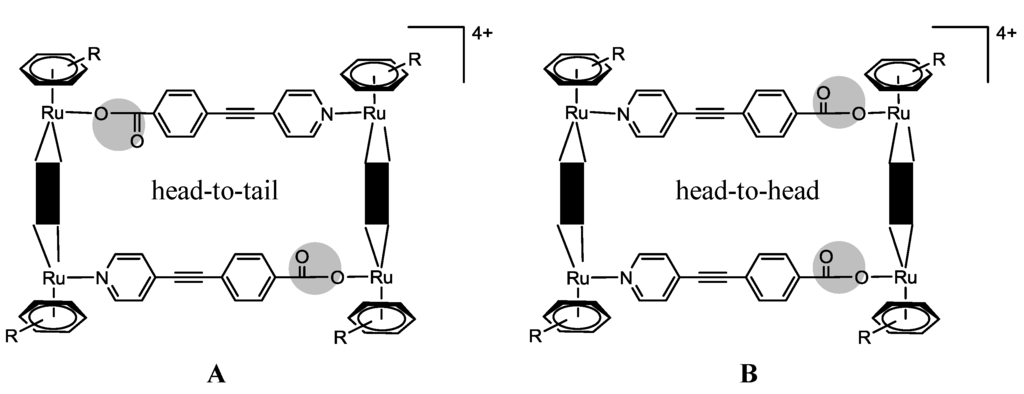
Scheme 5.
Possible isomers of Compound 28.
Self-assembly of the 5,10,15,20-tetra(4-pyridyl)porphyrin (tpp-H2) tetradentate panel with the dinuclear p-cymene ruthenium clip, [Ru2(p-cymene)2(OO  OO)Cl2] (OO
OO)Cl2] (OO  OO = oxalato; dobq), affords the cationic organometallic cube, [Ru8(p-cymene)8(tpp-H2)2(OO
OO = oxalato; dobq), affords the cationic organometallic cube, [Ru8(p-cymene)8(tpp-H2)2(OO  OO)4]8+ [26]. In addition, the reaction of the dinuclear arene ruthenium dobq clips, [Ru2(indane)2(dobq)Cl2] and [Ru2(nonylbenzene)2(dobq)Cl2], in MeOH for 48 h at reflux temperature, with tpp-H2 in the presence of AgCF3SO3, affords the corresponding cationic cubes, [Ru8(indane)8(tpp-H2)2(dobq)4]8+ and [Ru8(nonylbenzene)8(tpp-H2)2(dobq)4]8+, respectively [26]. However, these octanuclear ruthenium compounds are poorly soluble in H2O and show decreased cytotoxic activity compared with their hexanuclear homologues, showing, in this case, that there is not a direct correlation between the number of ruthenium centers vs. cytotoxicity.
OO)4]8+ [26]. In addition, the reaction of the dinuclear arene ruthenium dobq clips, [Ru2(indane)2(dobq)Cl2] and [Ru2(nonylbenzene)2(dobq)Cl2], in MeOH for 48 h at reflux temperature, with tpp-H2 in the presence of AgCF3SO3, affords the corresponding cationic cubes, [Ru8(indane)8(tpp-H2)2(dobq)4]8+ and [Ru8(nonylbenzene)8(tpp-H2)2(dobq)4]8+, respectively [26]. However, these octanuclear ruthenium compounds are poorly soluble in H2O and show decreased cytotoxic activity compared with their hexanuclear homologues, showing, in this case, that there is not a direct correlation between the number of ruthenium centers vs. cytotoxicity.
 OO)Cl2] (OO
OO)Cl2] (OO  OO = oxalato; dobq), affords the cationic organometallic cube, [Ru8(p-cymene)8(tpp-H2)2(OO
OO = oxalato; dobq), affords the cationic organometallic cube, [Ru8(p-cymene)8(tpp-H2)2(OO  OO)4]8+ [26]. In addition, the reaction of the dinuclear arene ruthenium dobq clips, [Ru2(indane)2(dobq)Cl2] and [Ru2(nonylbenzene)2(dobq)Cl2], in MeOH for 48 h at reflux temperature, with tpp-H2 in the presence of AgCF3SO3, affords the corresponding cationic cubes, [Ru8(indane)8(tpp-H2)2(dobq)4]8+ and [Ru8(nonylbenzene)8(tpp-H2)2(dobq)4]8+, respectively [26]. However, these octanuclear ruthenium compounds are poorly soluble in H2O and show decreased cytotoxic activity compared with their hexanuclear homologues, showing, in this case, that there is not a direct correlation between the number of ruthenium centers vs. cytotoxicity.
OO)4]8+ [26]. In addition, the reaction of the dinuclear arene ruthenium dobq clips, [Ru2(indane)2(dobq)Cl2] and [Ru2(nonylbenzene)2(dobq)Cl2], in MeOH for 48 h at reflux temperature, with tpp-H2 in the presence of AgCF3SO3, affords the corresponding cationic cubes, [Ru8(indane)8(tpp-H2)2(dobq)4]8+ and [Ru8(nonylbenzene)8(tpp-H2)2(dobq)4]8+, respectively [26]. However, these octanuclear ruthenium compounds are poorly soluble in H2O and show decreased cytotoxic activity compared with their hexanuclear homologues, showing, in this case, that there is not a direct correlation between the number of ruthenium centers vs. cytotoxicity.The reaction of the [Ru2(bis-benzimidazole)(MeOH)2(η6-p-iPrC6H4Me)2][O3SCF3]2 clip with 1,3,5-tris-(4-pyridylethynyl)-benzene in a 3:2 molar ratio results in self-assembled Metalla-prism 32 (Scheme 4) [37]. This compound was found to inhibit the proliferation of colon (Colo320), lung (A549 and H1299) and breast (MCF7) human cancer cell lines at low concentrations [37].

Table 2.
IC50 of the ruthenium coordination-cage conjugates, 17–26, 30–31 and 33–34, on A2780 human ovarian cancer cells after 72 h of exposure.
| Compound | IC50 (µM) per coordination-cage |
|---|---|
| 17 a | 66 |
| 18 a | 43 |
| 19 a | 27 |
| 20 a | 33 |
| 21 a | 6 |
| 22 a | 29 |
| 23 a | 4 |
| 24 a | 23 |
| 25 b | 11 |
| 26 b | 5.6 |
| 30 b | 3.1 |
| 31 b | 2.1 |
| 33 | 3.1 |
| 34 | 2.4 |
IC50 cisplatin in the same experimental conditions: a 2 µM [24]; b 1.6 µM [25].
Arene-ruthenium metallacages were used to encapsulate lipophilic pyrenyl functionalized poly(benzylether) dendrimers (Scheme 4, 33 vs. 34) [27,28,31]. The host-guest systems, 33, were prepared using a two-step strategy. Firstly, the dinuclear complex, [Ru2(p-cymene)2(donq)Cl2] (donq = 5,8-dioxydo-1,4-naphthoquinonato), was reacted with AgCF3SO3 in MeOH at room temperature affording the dinuclear intermediate. Then, 0.66 equivalents of 2,4,6-tris(4-pyridyl)-1,3,5-triazine (tpt) and 0.33 equivalents of the guest molecule were added, and the solution was stirred at 60 °C for 24 h to obtain the corresponding inclusion compounds. The resulting hexacationic host-guest systems are obtained in a good yield (80%) as triflate salts, [34][CF3SO3]6. Both Metallacage 33 and the host-guest system, 34, exhibit a similar cytotoxicity on the A2780 cell line (Table 2). However, these results did not clarify whether the guest is released or not after cellular internalization into the cancer cells. The cytotoxicity seemed to be inversely related with the size of the encapsulated dendrimer in the majority of cases, i.e., smaller dendrimers lead to lower cytotoxicities. Replacement of donq by doaq (5,8-dioxido-1,4-anthraquinonato) or dotq (6,11-dioxido-5,12-naphthacenedionato) led to less cytotoxic compounds [30].
2.3. Ruthenium(II)-Coordinate Polymers
RAPTA-C, [RuCl2(p-cymene)(PTA)] (PTA = 1,3,5-triaza-7-phosphaadamantane), was bounded to poly(2-chloroethyl methacrylate)(PCEMA) and poly(2-chloroethyl methacrylate-co-N-(2-hydroxypropyl) methacrylamide) (P(HPMA-CEMA)) to generate water-soluble macromolecular drugs [40]. Two strategies for the synthesis of the RAPTA-C-polymer conjugate were employed using the nitrogen groups of PTA as a site for alkylation: (a) the synthesis of the complex and direct conjugation to the polymer; or (b) attachment of PTA to the polymer and subsequent complexation with the dimer, to give the polymer-RAPTA-C conjugate [40]. The high temperature needed in the direct reaction (a) of RAPTA-C with the polymer led to the loss of the p-cymene ligand, invalidating this procedure [40]. In the two-step reaction (b), despite the fact that only 50% of the iodated copolymer had reacted (see Scheme 6), this method was chosen as the preferred pathway for the subsequent synthesis of the water-soluble polymer, P(HPMA172-IEMA44-(RAPTA-C-EMA)44), 35 (Scheme 6) [40]. One should note that these macromolecular ruthenium complexes were only synthesized in an NMR experiment using DMSO-d6 for seven days and were then recovered by dialysis [40]. The cytotoxicity of the RAPTA-C-copolymer conjugate was measured on the ovarian cancer cell line, OVCAR-3, revealing high IC50 values (>300 µM) [40].
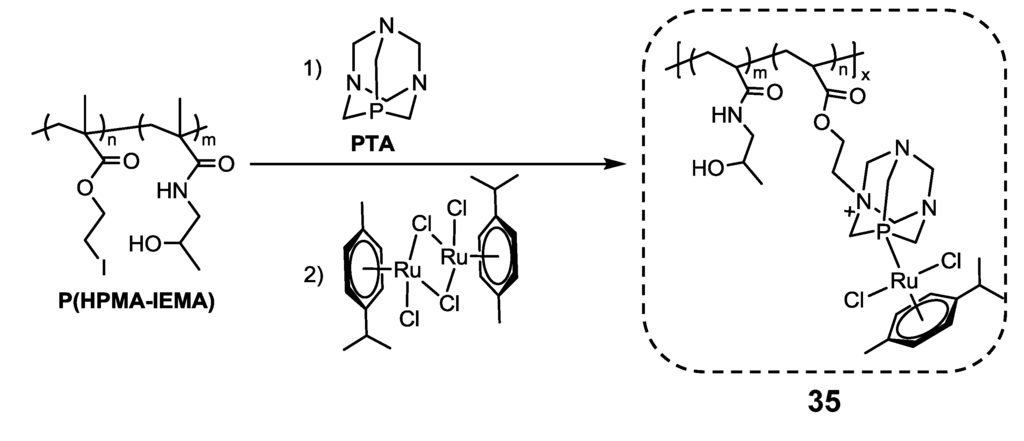
Scheme 6.
Synthesis of complex copolymer-RAPTA-C ([RuCl2(p-cymene)(PTA)] (PTA = 1,3,5-triaza-7-phosphaadamantane))conjugate (35) in DMSO-d6[40].
2.4. Ruthenium(II)-HSA Conjugates
Organoruthenium complexes of the general formula [Ru(η6-arene)Cl(L)]Cl, where arene is 4-formylphenoxyacetyl-η6-benzylamide and L is a cyclin-dependent kinase (Cdk) inhibitor, [3-(1H-benzimidazol-2-yl)-1H-pyrazolo[3,4-b]pyridines or indolo[3,2-d]benzazepines, were conjugated to recombinant human serum albumin (rHSA) to exploit the EPR effect (Scheme 7) [41]. The conjugation of the ruthenium moiety to modified rHSA was carried out via hydrazine bond formation according to a previous reported procedure [42]. Briefly, purified rHSA is shaken with 10 equivalents a solution of succinyl HCl terephthalic hydrazine in dimethylformamide (DMF) for 16 h at room temperature (the DMF volume did not exceed 5% (v/v)) [41]. The reaction mixture is then ultrafiltered against the conjugation buffer (100 mM MES, 2-(N-morpholino)ethanesulfonic acid, 0.9% NaCl, pH 6.0), and the concentration determined using the Bradford assay [41]. The modified protein solution is added to solutions of several complexes in order to achieve a 3:1 metal/protein ratio and shaken for 6 h at room temperature [41]. Afterwards, the protein mixture solution is desalted and restored in PBS (phosphate buffered saline) [41]. MALDI-TOF-MS analysis showed that the obtained samples correspond most likely to the presence of about two bound ruthenium moieties per protein [41].
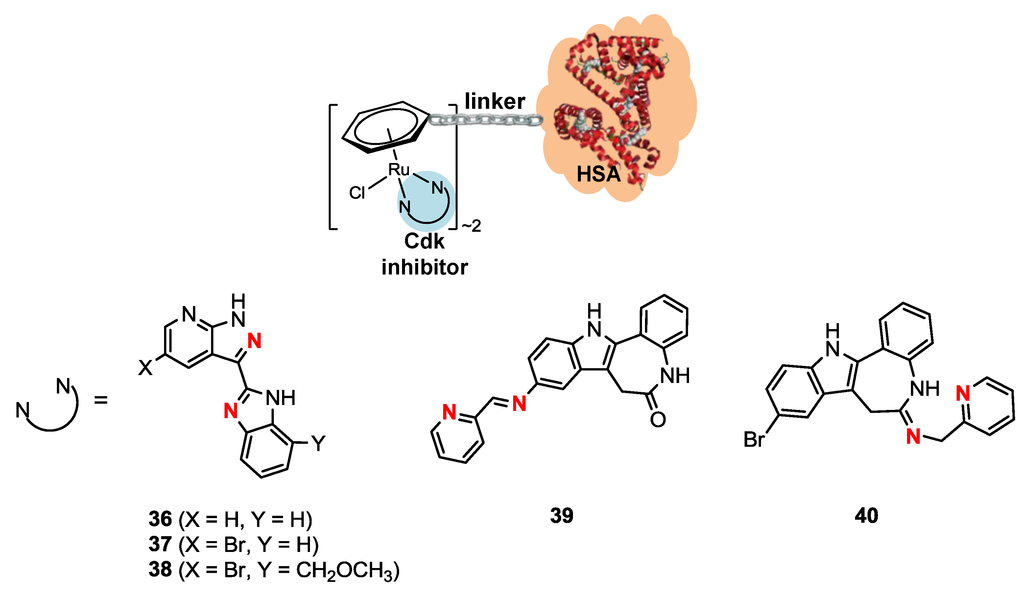
Scheme 7.
Ruthenium-recombinant human serum albumin (rHSA) conjugates 36–40 [41]. Coordinating nitrogen in bold.

Table 3.
IC50 of the different ruthenium-rHSA Conjugates 36–40 on A2780 human ovarian cancer cells after 72 h exposure.
| Compound | IC50 (µM) per compound |
|---|---|
| 36 | >200 |
| 36-rHSA | 45 |
| 37 | >200 |
| 37-rHSA | 43 |
| 38 | >200 |
| 38-rHSA | 46 |
| 39 | >100 |
| 39-rHSA | 49 |
| 40 | 85 |
| 40-rHSA | 26 |
The high molecular weight compounds (36-rHSA to 40-rHSA), together with their non-protein complexes (36–40) were evaluated in vitro in an ovarian carcinoma cell line (A2780). From Table 3, one can observe that the complexes alone are not cytotoxic. When coordinated with rHSA, there is an increase in cytotoxicity. One should not neglect that the cyclin-dependent kinase (Cdk) inhibitor ligands are much more cytotoxic for CH1 (human ovarian carcinoma), SW480 (human colon carcinoma) and A549 (human non-small cell lung carcinoma) cell lines than their corresponding ruthenium Complexes 36–40. Unfortunately, data for the A2780 cancer cell line is not provided.
3. Mononuclear Approaches
3.1. Ruthenium Nanoparticles
Nanoparticles find increasing applications in medicinal chemistry as drug delivery agents, medicinal imaging tools or as diagnostic agents. In addition, nanoparticles can also benefit from the enhanced permeability and retention effect and can be tunable to present specific properties.
Ruthenium(0) nanoparticles stabilized by a long-chain N-ligand derived from isonicotinic acid (L) have been prepared by the solvent-free reduction of [Ru(η6-C6H6)(L)Cl2] in a magnetically stirred stainless-steel autoclave with H2 (50 bar) at 100 °C for 64 h (41) [43]. The mean particle size was found to be 8.5 nm (established by transmission electron microscopy, TEM), which is relatively large. Smaller ruthenium nanoparticles stabilized by the isonicotinic ester ligand L were obtained by reducing [Ru(η6-arene)(H2O)3]SO4 in ethanol in the presence of one equivalent of L in a magnetically stirred stainless-steel autoclave under 50 bar pressure of H2 at 100 °C for 14 h (Table 4; 42: arene = C6H6; 43: arene = p-MeC6H4Pri; 44 arene = C6Me6) [43].
The in vitro cytotoxicity of the L-stabilized Ru nanoparticles, 41–44, and their corresponding small molecules (i.e., complexes of the general formula [Ru(η6-arene)(L)Cl2]) havebeen studied in the A2780 ovarian cancer cell line using the MTT assay. While the small molecules exhibit a good to moderate cytotoxicity, the nanoparticles exhibit only moderate cytotoxicity in the studied ovarian cancer cell line, with the exception of thep-cymene derived system, 43, which was unusually inactive (Table 4). For 41, 42 and 44, neither the nanoparticles size nor the nature of the ligands in the precursor complex appear to have an effect on cytotoxicity, since all three compounds exhibit similar IC50 values (29–39 µM). It is plausible to think that the in vitro activity of the complexes and nanoparticles is mainly due to the isonicotinic ester ligand L, since it presents, itself, a high cytotoxicity (IC50 of L in A2780 after 72 h of exposure = 5 µM).

Table 4.
IC50 of the ruthenium (0) nanoparticles, 41–44, on A2780 human ovarian cancer cells after 72 h of exposure.
| Compound | Ru nanoparticles | Mean size (nm) | IC50 (µM) |
|---|---|---|---|
| 41 |  | 8.5 | 29 |
| 42 | 2.8 | 34 | |
| 43 | 2.3 | >200 | |
| 44 | 2.2 | 39 |
3.2. Polymer-“Ruthenium-Cyclopentadienyl” Conjugate
RuIICp (Cp = cyclopentadienyl) low molecular weight drugs [44,45,46,47,48,49,50,51,52] are currently promising candidates in the search fornew chemotherapeutics, due to the excellent cytotoxic results against human colon adenocarcinoma (LoVo and HT29), pancreatic (Mia PaCa), promyelocytic leukemia (HL-60), breast (MDAMB231 and MCF7), prostate (PC3) and ovarian (A2780 and A2780cisR) cancer cell lines (IC50 values in the nano- to micro-molar range) [44,45,46,47,48,49,50,51,52]. Importantly, the [CpRu(P)(bpy)]+ family (P = phosphane coligand, bpy = bipyridine) presents very good stability in an aqueous environment. This feature of these complexes prompted the search fornew polymer-metal complexes using the same organometallic core as potential anticancer agents.
Conceptually, polymer conjugates share several features with other macromolecular approaches (liposomes, dendrimers, nanotubes and nanoparticles), but they have the added benefit of the synthetic chemical versatility that allows the tailoring of the molecular weight and also the adding of biomimetic features [53]. In this frame, the unprecedented synthesis of 45 (Scheme 8), and its preliminary in vitro results have been recently published [54]. [RuCp(P)(bpyPLA)]+ (RuPMC; Cp = η5-C5H5, P = triphenylphosphane and bpyPLA = 2,2'-bipyridine-4,4'-D-glucose end-capped polylactide) has been synthesized in a good yield by halide abstraction from [Ru(η5-C5H5)(PPh3)2Cl] with silver AgCF3SO3, under reflux for 3 h in CH2Cl2 in the presence of the bpyPLA macroligand. The molecular weight of the bpyPLA macroligand can be easily tuned by playing with the monomer/initiator ratio.
A degradation study, by UV-visible spectroscopy, of the RuPMC performed in order to infer the polymer hydrolysis at physiological and at tumor cell pH (pH = 7.4 and 5, respectively) showed that RuPMC is stable over a period of at least 72 h in an aqueous environment at physiologic pH, while at acidic pH, some degradation of the PLA is observed. Such behavior suggests a pH-dependent degradation, which is important considering drug delivery, since the measured pH of most solid tumors range from pH 5.7 to pH 7.2, while in blood it remains well-buffered and constant at pH 7.4 [55]. Accordingly, this feature of the polymer degradation discards the need for a biodegradable linker and provides the opportunity for site-specific drug delivery, mainly within endosomal/lysosomal compartments, where the pH approaches 4.5–6.0 [56].
This polymer-“ruthenium-cyclopentadienyl” conjugate 45 is cytotoxic against human MCF7 and MDAMB231 breast and A2780 ovarian adenocarcinoma, revealing IC50 values in the micromolar range (IC50 = 3.9, 3.8 and 1.6 µM, respectively). ICP-MS (inductively coupled plasma mass spectrometry) studies showed that the Ru-polymer conjugate enters the MCF7 estrogen receptor positive cancer cells and is retained ca. 50% in the nucleus, foreseeing its application as a therapeutic agent in, for example, hormone-responsive cancers. On the contrary, its Ru-precursor (TM34, [RuII(η5-C5H5)(bipy)(PPh3)]+, in Scheme 8) is mainly found in the membrane (ca. 80%), forecasting different mechanisms of cellular uptake and of cell death for these two compounds bearing the same cytotoxic fragment.
Direct comparison of the IC50 values between RuPMC and its low molecular weight parent drug, TM34, reveals a decrease on the cytotoxicity of RuPMC (3.9 vs. 0.29 μM for MCF7). However, one should not neglect the potential effect that the prolonged plasma half-life of the RuPMC could have on the improvement of the chemotherapeutic efficacy, allowing a positive final outcome, as has been described for many platinum-related compounds [57,58,59,60]. This new RuPMC seems to be a viable candidate for the intended drug-delivery application, yet further studies are needed to prove its higher in vivo accumulation in cancer cells.
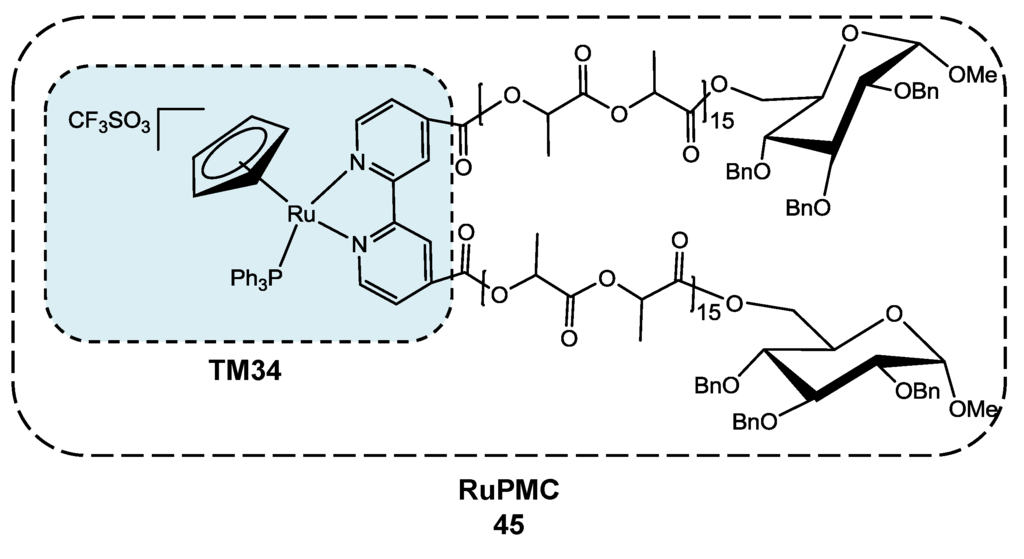
Scheme 8.
D-Glucose end-capped polylactide ruthenium-cyclopentadienyl (RuPMC, 45) and TM34.
4. Conclusions
Regardless of the advances in the area of macromolecular compounds, there is still the need to develop high molecular weight and biodegradable carriers that can better exploit EPR-mediated tumor targeting. There is an urgency to move away from heterogeneous carriers towards better defined structures. In this frame, several strategies are being developed, which can be seen as a step forward to this end. An approach that has attracted much attention lately is a synergic effect between the EPR effect and the introduction of increasing nuclearity, which is expected to strengthen the cytotoxicity, while also raising the selectivity towards the cancer cells (sparing the healthy ones). Metallodendrimers have thus appeared as a promising option, since they combine the features of monodisperse nanoscale geometry with high end-group density at their surface. Furthermore, other supramolecular assemblies, like ruthenium-based coordination-cage conjugates and ruthenium-rHSA conjugates, showed cytotoxicity against several cancer cell lines. However, it seems that there is not always a direct correlation between the nuclearity and the cytotoxicity of the compounds, possibly due to solubility issues or to over-positively charged complexes that might originate retention at the cell membrane. Indeed, the only reported case of a conjugate bearing approximately forty four ruthenium centers per molecule (P(HPMA172-IEMA44-(RAPTA-C-EMA)44) showed no benefit in terms of cytotoxicity towards the ovarian cancer cell line, OVACAR-3 (IC50 > 300 µM), compared to RAPTA-C (IC50 ≈ 200 µM).
Establishing structure-activity relationships is of primordial importance, since small changes in the chemical structure might dictate significant cytotoxicity differences. This is the case of coordination-cages, where both linkers and arene ligands have a strong influence on the cytotoxicity, probably due to the different size cavities, flexibilities and packing, as well as different lipophilicities.
Some of the problems encountered in the development of new covalently bound metal-conjugates lie on the loss of drug activity. This is the case of the reported ruthenium nanoparticles, where the macromolecular drugs lead to a marked decrease in the cytotoxic properties of the low molecular weight compounds. It is thus imperative to develop simpler strategies for the coordination of drugs to the carriers. One good example was observed on the one-step coordination strategy in ruthenium cyclopentadienyl derivatives, where the cytotoxicity of the final polymer-ruthenium conjugate was maintained in the low micromolar range.
Ruthenium-conjugates seem to be a promising alternative, although many studies must still be done. Most of the cytotoxic studies were performed mainly over one cancer cell line (namely, human ovarian A2780), and there is still the need to present in vivo studies in order to have a proof of concept, i.e., if these new macromolecular compounds are indeed better than their low molecular weight parental drugs by the so-called EPR effect. Furthermore, studies revealing the stability and speciation of these metal-conjugates in an aqueous environment and blood are mandatory.
In the chemical point of view, creating carriers that degrade under acidic conditions to trigger the drug release, by the slightly acidic tumor environment, is seen as a good strategy, already tested with good results in platinum drugs. Furthermore, this effect can also be achieved after the internalization by cancer cells, resulting in the accumulation of the polymer in the acidic endosomes and lysosomes. Finally, it is also expected that receptor-targeting ligands will lead to improved tumor targeting through the EPR effect. In this frame, innovative chemical reactions leading to “smart drugs” are powerful tools for the search of new chemotherapeutics presenting chemical diversity and original architectures.
Acknowledgments
The authors thank the Portuguese Foundation for Science and Technology (FCT) within the scope of projects PEst-OE/QUI/UI536/2011, PTDC-QUI-QUI-118077-2010 and FCT Investigator Programme.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chau, I.; Cunningham, D. Oxaliplatin for colorectal cancer in the united states: better late than never. J. Clin. Oncol. 2003, 21, 2049–2051. [Google Scholar] [CrossRef]
- Vicent, M.J.; Duncan, R. Polymer conjugates: Nanosized medicines for treating cancer. Trends Biotechnol. 2006, 24, 39–47. [Google Scholar] [CrossRef]
- Vinditto, V.J.; Szoka, F.C., Jr. Cancer nanomedicines: So many papers and so few drugs! Adv. Drug Deliv. Rev. 2013, 65, 80–88. [Google Scholar] [CrossRef]
- Wang, R.; Billone, P.S.; Mullett, W.M.J. Nanomedicine in action: An overview of cancer nanomedicine on the market and in clinical trials. Nanomaterials 2013, 2013, 629681. [Google Scholar]
- Zhao, X.; Loo, A.C.J.; Lee, P.P.-F.; Tan, T.T.Y.; Chu, C.K. Synthesis and cytotoxic activities of chloropyridylimineplatinum(II) and chloropyridyliminecopper(II) surface-functionalized poly(amido-amine) dendrimers. J. Inorg. Biochem. 2010, 104, 105–110. [Google Scholar] [CrossRef]
- Ahamad, T.; Mapolie, S.F.; Alshehri, S.M. Synthesis and characterization of polyamide metallodendrimers and their anti-bacterial and anti-tumor activities. Med. Chem. Res. 2012, 21, 2023–2031. [Google Scholar] [CrossRef]
- Robilotto, T.J.; Alt, D.S.; von Recum, H.A.; Gray, T.G. Cytotoxic gold(I)-bearing dendrimers from alkyne precursors. Dalton Trans. 2011, 40, 8083–8085. [Google Scholar] [CrossRef]
- Hurley, A.L.; Mohler, D.L. Organometallic photonucleases: Synthesis and DNA-cleavage studies of cyclopentadienyl metal-substituted dendrimers designed to increase double-strand scission. Org. Lett. 2000, 2, 2745–2748. [Google Scholar] [CrossRef]
- Maeda, H.; Matsumura, Y. A new concept for macromoecular therapeutics in cancer chemotherapy: mechanisms of tumoritropic accumulation of proteins and the antitumor agent smancs. Crit. Rev. Ther. Drug. Carrier Syst. 1986, 46, 6387–6392. [Google Scholar]
- Kopecek, J.; Kopeckova, P.; Minko, T.; Lu, Z.R. HPMA copolymer-anticancer drug conjugates: Design, activity, and mechanism of action. Eur. J. Pharm. Biopharm. 2000, 50, 61–81. [Google Scholar] [CrossRef]
- Maeda, H.; Bharate, G.Y.; Daruwalla, J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur. J. Pharm. Biopharm. 2009, 71, 409–419. [Google Scholar]
- Duncan, R.; Dimitrijevic, S.; Evagorou, E.G. The role of polymer conjugates in the diagnosis and treatment of cancer. STP Pharma Sci. 1996, 6, 237–263. [Google Scholar]
- Ang, W.H.; Dyson, P.J. Classical and non-classical ruthenium-based anticancer drugs: Towards targeted chemotherapy. Eur. J. Inorg. Chem. 2006, 20, 4003–4018. [Google Scholar]
- Levina, A.; Mitra, A.; Lay, P.A. Recent developments in ruthenium anticancer drugs. Metallomics 2009, 1, 458–470. [Google Scholar] [CrossRef]
- Bruijnincx, P.C.A.; Sadler, P.J. New trends for metal complexes with anticancer activity. Curr. Opin. Chem. Biol. 2008, 12, 197–206. [Google Scholar] [CrossRef]
- Süss-Fink, G. Arene ruthenium complexes as anticancer agents. Dalton Trans. 2010, 39, 1673–1688. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Emadi, A. Ruthenium-based chemotherapeutics: Are they ready for prime time? Cancer Chemother. Pharmacol. 2010, 66, 1–9. [Google Scholar] [CrossRef]
- Bergamo, A.; Gaiddon, C.; Schellens, J.H.M.; Beijnen, J.H.; Sava, G. Approaching tumour therapy beyond platinum drugs: Status of the art and perspectives of ruthenium drug candidates. J. Inorg. Biochem. 2012, 106, 90–99. [Google Scholar] [CrossRef]
- Hartinger, C.G.; Jakupec, M.A.; Zorbas-Seifrieda, S.; Groessl, M.; Egger, A.; Berger, W.; Zorbas, H.; Dyson, P.J.; Keppler, B.K. KP1019, a new redox-active anticancer agent—Preclinical development and results of a clinical phase I study in tumor patients. Chem. Biodivers. 2008, 5, 2140–2155. [Google Scholar] [CrossRef]
- Alessio, E.; Mestroni, G.; Bergamo, A.; Sava, G.; Alessio, E.; Mestroni, G.; Bergamo, A.; Sava, G. Ruthenium antimetastatic agents. Curr. Topics Med. Chem. 2004, 4, 1525–1535. [Google Scholar] [CrossRef]
- Govender, P.; Antonels, N.C.; Mattsson, J.; Renfrew, A.K.; Dyson, P.J.; Moss, J.R.; Therrien, B.; Smith, G.S. Anticancer activity of multinuclear arene ruthenium complexes coordinated to dendritic polypyridyl scaffolds. J. Organomet. Chem. 2009, 694, 3470–3476. [Google Scholar] [CrossRef]
- Govender, P.; Renfrew, A.K.; Clavel, C.M.; Dyson, P.J.; Therrien, B.; Smith, G.S. Antiproliferative activity of chelating N,O- and N,N-ruthenium(II) arene functionalised poly(propyleneimine) dendrimer scaffolds. Dalton Trans. 2011, 40, 1158–1167. [Google Scholar] [CrossRef]
- Rodrigues, J.; Jardim, M.G.; Figueira, J.; Gouveia, M.; Tomás, H.; Rissanen, K. Poly(alkylidenamines) dendrimers as scaffolds for the preparation of low-generation ruthenium based metallodendrimers. New J. Chem. 2011, 35, 1938–1943. [Google Scholar] [CrossRef]
- Mattsson, J.; Govindaswamy, P.; Renfrew, A.K.; Dyson, P.J.; Štěpnička, P.; Süss-Fink, G.; Therrien, B. Synthesis, molecular structure, and anticancer activity of cationic arene ruthenium metallarectangles. Organometallics 2009, 28, 4350–4357. [Google Scholar] [CrossRef]
- Barry, N.P.E.; Zava, O.; Furrer, J.; Dyson, P.J.; Therrien, B. Anticancer activity of opened arene ruthenium metalla-assemblies. Dalton Trans. 2010, 39, 5272–5277. [Google Scholar] [CrossRef]
- Barry, N.P.E.; Zava, O.; Dyson, P.J.; Therrien, B. Synthesis, characterization and anticancer activity of porphyrin-containing organometallic cubes. Aust. J. Chem. 2010, 63, 1529–1537. [Google Scholar] [CrossRef]
- Pitto-Barry, A.; Barry, N.P.E.; Zava, O.; Deschenaux, R.; Therrien, B. Encapsulation of pyrene-functionalized poly(benzyl ether) dendrons into a water-soluble organometallic cage. Chem. Asian J. 2011, 6, 1595–1603. [Google Scholar] [CrossRef]
- Pitto-Barry, A.; Barry, N.P.E.; Zava, O.; Deschenaux, R.; Dyson, P.J.; Therrien, B. Double targeting of tumours with pyrenyl-modified dendrimers encapsulated in an arene-ruthenium metallaprism. Chem. Eur. J. 2011, 17, 1966–1971. [Google Scholar] [CrossRef]
- Barry, N.P.E.; Edafe, F.; Therrien, B. Anticancer activity of tetracationic arene ruthenium metalla-cycles. Dalton Trans. 2011, 40, 7172–7180. [Google Scholar] [CrossRef]
- Barry, N.P.E.; Zava, O.; Dyson, P.J.; Therrien, B. Excellent correlation between drug release and portal size in metalla-cage drug-delivery systems. Chem. Eur. J. 2011, 17, 9669–9667. [Google Scholar] [CrossRef]
- Pitto-Barry, A.; Zava, O.; Dyson, P.J.; Deschenaux, R.; Therrien, B. Enhancement of cytotoxicity by combining pyrenyl-dendrimers and arene ruthenium metallacages. Inorg. Chem. 2012, 51, 7119–7124. [Google Scholar] [CrossRef]
- Furrer, M.A.; Garci, A.; Denoyelle-Di-Muro, E.; Trouillas, P.; Giannini, F.; Furrer, J.; Clavel, C.M.; Dyson, P.J.; Süss-Fink, G.; Therrien, B. Synthesis, characterisation and in vitro anticancer activity of hexanuclear thiolato-bridged arene ruthenium metalla-prisms. Chem. Eur. J. 2013, 19, 3198–3203. [Google Scholar] [CrossRef]
- Dubey, A.; Min, J.W.; Koo, H.J.; Kim, H.; Cook, T.R.; Kang, S.C.; Stang, P.J.; Chi, K.-W. Anticancer potency and multi-resistant studies of self-assembled arene-ruthenium metallarectangles. Chem. Eur. J. 2013, 19, 11622–11628. [Google Scholar] [CrossRef]
- Jung, H.; Dubey, A.; Koo, H.J.; Vajpayee, V.; Cook, T.R.; Kim, H.; Kang, S.C.; Stang, P.J.; Chi, K.-W. Self-assembly of ambidentate pyridyl-carboxylate ligands with octahedral ruthenium metal centers: Self-selection for a single-linkage isomer and anticancer-potency studies. Chem. Eur. J. 2013, 19, 6709–6717. [Google Scholar] [CrossRef]
- Mishra, A.; Jung, H.; Park, J.W.; Kim, H.K.; Kim, H.; Stang, P.J.; Chi, K.-W. Anticancer activity of self-assembled molecular rectangles via arene-ruthenium acceptors and a new unsymmetrical amide ligand. Organometallics 2012, 31, 3519–3526. [Google Scholar] [CrossRef]
- Vajpayee, V.; Lee, S.; Kim, S.-H.; Kang, S.C.; Cook, T.R.; Kim, H.; Kim, D.W.; Verma, S.; Lah, M.S.; Kim, I.S.; et al. Self-assembled metala-rectangles bearing azodipyridyl ligands: Synthesis, characterization and antitumor activity. Dalton Trans. 2013, 42, 466–475. [Google Scholar] [CrossRef]
- Vajpayee, V.; mi Lee, S.; Park, J.W.; Dubey, A.; Kim, H.; Cook, T.R.; Stang, P.J.; Chi, K.-W. Growth inhibitory activity of a bis-benzimidazole-bridged arene ruthenium metalla-rectangle and-prism. Organometallics 2013, 32, 1563–1566. [Google Scholar] [CrossRef]
- Cook, T.R.; Vajpayee, V.; Lee, M.H.; Stang, P.J.; Chi, K.-W. Biomedical and biochemical applications of self-assembled metallacycles and metallacages. Acc. Chem. Res. 2013, 46, 2464–2474. [Google Scholar] [CrossRef]
- Han, Y.-F.; Jia, W.-G.; Lin, Y.-J.; Jin, G.-X. Stepwise formation of molecular rectangles of half-sandwich rhodium and ruthenium complexes containing bridging chloranilate ligands. Organometallics 2008, 27, 5002–5008. [Google Scholar] [CrossRef]
- Blunden, B.M.; Thomas, D.S.; Stenzel, M.H. Macromolecular ruthenium complexes as anti-cancer agents. Polym. Chem. 2012, 3, 2964. [Google Scholar] [CrossRef]
- Stepanenko, I.N.; Casini, A.; Edafe, F.; Novak, M.S.; Arion, V.B.; Dyson, P.J.; Jakupec, M.A.; Keppler, B.K. Conjugation of organoruthenium(II) 3-(1H-benzimidazol-2-yl)pyrazolo[3,4-b]pyridines and indolo[3,2-d]benzazepines to recombinant human serum albumin: A strategy to enhance cytotoxicity in cancer cells. Inorg. Chem. 2011, 50, 12669–12679. [Google Scholar] [CrossRef]
- Warnecke, A.; Fichtner, I.; Garmann, D.; Jaehde, U.; Kratz, F. Synthesis and biological activity of water-soluble maleimide derivatives of the anticancer drug carboplatin designed as albumin-binding prodrugs. Bioconjugate Chem. 2004, 15, 1349–1359. [Google Scholar] [CrossRef]
- Süss-Fink, G.; Khan, F.-A.; Juillerat-Jeanneret, L.; Dyson, P.J.; Renfrew, A.K. Synthesis and anticancer activity of long-chain isonicotinic ester ligand-containing arene ruthenium complexes and nanoparticles. J. Clus. Sci. 2010, 21, 313–324. [Google Scholar] [CrossRef]
- Garcia, M.H.; Morais, T.S.; Florindo, P.; Piedade, M.F.M.; Moreno, V.; Ciudad, C.; Noe, V. Inhibition of cancer cell growth by ruthenium(II) cycplopentadienyl derivative complexes with heteroaromatic ligands, journal of inorganic biochemistry. J. Inorg. Biochem. 2009, 103(3), 354–361. [Google Scholar]
- Garcia, M.H.; Valente, A.; Florindo, P.; Morais, T.S.; Piedade, M.F.M.; Duarte, M.T.; Moreno, V. New ruthenium(II) mixed metallocene derived complexes: Synthesis, characterization by X-ray diffraction studies and evaluation on DNA interaction by atomic force microcopy. Inorg. Chim. Acta 2010, 363, 3765–3775. [Google Scholar] [CrossRef]
- Moreno, V.; Lorenzo, J.; Avilés, F.X.; Garcia, M.H.; Ribeiro, J.; Florindo, P.; Robalo, M.P. Studies of the antiproliferative activity of ruthenium(II) cyclopentadienyl derived complexes with nitrogen coordinated ligands. Bioinorg. Chem. Appl. 2010. [Google Scholar] [CrossRef]
- Moreno, V.; Font-Bardia, M.; Calvet, T.; Lorenzo, J.; Avilés, F.X.; Garcia, M.H.; Morais, T.S.; Valente, A.; Robalo, M.P. DNA interaction and cytotocixity studies of new ruthenium(II) cyclopentadienyl derivative complexes containing heteroaromatic ligands. J. Inorg. Biochem. 2011, 105(2), 241–249. [Google Scholar]
- Tomaz, A.I.; Jakusch, T.; Morais, T.S.; Marques, F.; Almeida, R.F.M.; de Mendes, F.; Enyedy, E.A.; Santos, I.; Pessoa, J.C.; Kiss, T.; et al. [RuII(η5-C5H5)(bipy)(PPh3)]+ a promising large spectrum antitumor agent: cytotoxic activity and interaction with human serum albumin. J. Inorg. Biochem. 2012, 117, 261–269. [Google Scholar]
- Morais, T.S.; Silva, T.J.L.; Marques, F.; Robalo, M.P.; Avecilla, F.; Madeira, P.J.A.; Mendes, P.J.G.; Santos, I.; Garcia, M.H. Synthesis of organometallic ruthenium(II) complexes with strong activity against several human cancer cell lines. J. Inorg. Biochem. 2012, 114, 65–74. [Google Scholar] [CrossRef]
- Morais, T.S.; Santos, F.; Côrte-Real, L.; Marques, F.; Robalo, M.P.; Madeira, P.J.A.; Garcia, M.H. Biological activity and cellular uptake of [RuII(η5-C5H5)(PPh3)(Me2bpy)][CF3SO3]. J. Inorg. Biochem. 2013, 122, 8–17. [Google Scholar] [CrossRef]
- Morais, T.S.; Santos, F.C.; Jorge, T.F.; Côrte-Real, L.; Madeira, P.J.A.; Marques, F.; Robalo, M.P.; Matos, A.; Santos, I.; Garcia, M.H. New water-soluble ruthenium(II) cytotoxic complex: Biological activity and cellular distribution. J. Inorg. Biochem. 2013, 130, 1–14. [Google Scholar]
- Côrte-Real, L.; Matos, A.P.; Alho, I.; Morais, T.S.; Tomaz, A.I.; Garcia, M.H.; Santos, I.; Bicho, M.P.; Marques, F. Cellular uptake mechanisms of an antitumor ruthenium compound: The endossomal/lysosomal system as a target for anticancer metal-based drugs. Microsc.Microanal. 2013, 19, 1122–1130. [Google Scholar] [CrossRef]
- Duncan, R. Designing polymer conjugates as lysosomotropic nanomedicines. Biochem. Soc. Trans. 2007, 35, 56–60. [Google Scholar] [CrossRef]
- Valente, A.; Garcia, M.H.; Marques, F.; Miao, Y.; Rousseau, C.; Zinck, P. First polymer “ruthenium-cyclopentadienyl” complex as potential anticancer agent. J. Inorg. Biochem. 2013, 127, 79–81. [Google Scholar] [CrossRef]
- Tannock, I.F.; Rotin, D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989, 49, 4373–4384. [Google Scholar]
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef]
- Cabral, H.; Nishiyama, N.; Okazaki, S.; Koyama, H.; Kataoka, K. Preparation and biological properties of dichloro(1,2-diaminocyclohexane)platinum(II) (DACHPt)-loaded polymeric micelles. J. Control. Rel. 2005, 101, 223–232. [Google Scholar] [CrossRef]
- Uchino, H.; Matsumura, Y.; Negishi, T.; Hayashi, F.; Honda, T.; Nishiyama, N.; Kataoka, K.; Naito, S.; Kakizoe, T. Cisplatin-incorporating polymeric micelles (NC-6004) can reduce nephrotoxicity and neurotoxicity of cisplatin in rats. Br. J. Cancer 2005, 93, 678–687. [Google Scholar] [CrossRef]
- Nishiyama, N.; Kataoka, K. Preparation and characterization of size-controlled polymeric micelle containing cis-dichlorodiammineplatinum(II) in the core. J. Control. Rel. 2001, 74, 83–94. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, Y.-S.; Park, K.; Lee, S.; Nam, H.Y.; Min, K.H.; Lo, H.G.; Park, J.H.; Choi, K.; Jeong, S.Y.; et al. Antitumor efficacy of cisplatin-loaded glycol chitosan nanoparticles in tumor-bearing mice. J. Control. Rel. 2008, 127, 41–49. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).