Abstract
We present a Caputo fractional order mathematical model that describes the cellular infection of the Hepatitis B virus and the immune response of the body with Holling type II functional response. We study the existence of unique positive solutions and the local and global stability of virus-free and endemic equilibria. Finally, we present numerical results using the Adam-type predictor–corrector iterative scheme.
1. Introduction
Hepatitis B is a known killer infectious disease caused by the Hepatitis B virus (HBV), which attacks the liver cells (Hepatocyte), resulting in changes in the antigen structure of the surface of the liver cells, thus, leading to attack and destruction by a mechanism called ‘self-mediated immune damage’.
Although “recovered” patients have lifetime protective immunity, tiny levels of HBV DNA can still be discovered occasionally. These traces of HBV DNA are infectious and stimulate HBV-specific B- and T-cell responses, which control viremia [1]. However, HBV replication is strongly suppressed by the antiviral effects of inflammatory cytokines generated by HBV-specific T lymphocytes when they detect the antigen in the infected or transgenic liver, as shown in chimpanzee [2] and transgenic mouse [3].
Hepatitis B infection is in two phases; acute and chronic. The acute stage is the earliest stage of the infection whereby the body can recover by its immune system within six months. The acute infection stage might be asymptotic and characterized by viral replication. Loss of appetite, joint and muscle discomfort, low-grade fever, and potential stomach aches are all signs of an acute infection. Recovery from acute HBV infection means that the virus is no longer in the bloodstream, but inactive in the liver. However, if one uses immune-suppressing drugs, the virus in your liver might be reactivated in the future. HBV replicates only within the host cell since it lacks the enzymes necessary for protein and nucleic acid synthesis [4]. Chronic HBV infection occurs when the virus is still detectable six months after the infection, indicating that the virus has not been fully cleared by the immune system and is still present in the patient’s blood and liver. The majority of patients with chronic infections are asymptomatic. HBV is transmitted from an infected person to an uninfected person through sexual transmission, child-birth relation, misuse of contaminated needles such as syringes, exchange of infected body fluids such as blood, saliva, menstrual, seminal, and vaginal fluid [5].
It is also well-known that infected people do not always show symptoms in the early stages, although some may experience an acute illness that lasts several weeks, including Jaundice (yellowing of the skin and eye), dark urine, and extreme fatigue, nausea, vomiting, and some abdominal pains. Despite the above symptoms mentioned, symptoms exhibited by patients also depend on the status of the immune system of the patient, the age, and general health of the patient [4].
Due to the severity of hepatitis B infection, it has intrigued many scientists and researchers to investigate the dynamics of HBV. In [6], a spatio-temporal dynamics of a fractional model for HBV infection with cellular immunity was proposed. A time-fractional diffusion model for HBV infection with capsids and Cytotoxic T lymphocytes (CTL) immune response and were able to show that the diffusion and the order of fractional derivatives in the sense of Caputo do not affect the stability of the equilibria, but they can affect the time to arrive at the equilibria. When the order of the fractional derivative increases, for example, the model’s solutions converge quickly to the equilibrium points.
In [7], the authors looked at a fractional-order HBV immunological model’s global analysis and simulation. Using the Lyapunov function to prove the model’s stability, they derived basic reproduction numbers and presented their relationship with the corresponding equilibria. Studying the hepatitis B virus, the authors in [8] incorporated the immune response and took into account both cytolytic and noncytolytic effect pathways. The dynamics of immune response to hepatitis B, which takes into account contributions from innate and adaptive immune responses, as well as cytokines, has also been studied, see, [9].
In this article, we consider an HBV model with a Holling type 2 functional response rate and three (3) compartments, i.e., healthy hepatocytes (H), infected hepatocytes (I), and the body’s immune response (R). We study the existence and uniqueness of the solutions and the equilibrium states, i.e., virus-free and endemic equilibrium state. When the virus present in the liver cells exceeds the cytokines, the virus will overcome the cytokines and multiply in the body, resulting in an endemic state where the virus comes to stay in the body. When cytokines outnumber the viruses in the body, the viruses are cleared from the body, and the person is virus-free.
The rest of the paper is organized as follows; Section 2 presents preliminary results for fractional calculus. We present the fractional order model and the analysis in Section 3. In Section 4, we present the local and global stability analysis. The numerical results and simulations are presented in Section 5. Finally, we present the conclusions in Section 6.
2. Mathematical Preliminary on Fractional Calculus
In this section, we present useful results from the theory of fractional calculus; see, for example, the monographs [10,11] for further details.
Definition 1
([11]). The fractional order integral of the function of order is defined in the sense of Riemann–Liouville as where Then fractional integral of order v for a function g in the sense of Riemann–Liouville is defined as
where and is the Euler gamma function.
Definition 2
([11]). For a function the Caputo fractional-order derivative of order α is defined by
where and denotes the smallest integer that is less or equal to
Lemma 1.
Let and Then
In particular, if then
The generalized mean value theorem for the Caputo fractional order is given by
Lemma 2.
For let and Then it holds
3. Fractional Order Model Derivation and Analysis
We consider a liver consisting of Healthy Hepatocyte Infected hepatocytes , and immune response-dependent cytotoxic cells We assume a logistic growth of healthy hepatocytes, see, e.g., [12,13]. Healthy Hepatocytes of the liver are known to have half-lives of more than six months; see, [14]. In the presence of the Hepatitis B virus, we assume that the rate at which hepatocytes become infected is proportional to Given as the contact rate and assuming that infected hepatocytes die at a rate the dynamic model for the infected hepatocytes and the immune response can be described as
with the initial conditions and where and are the rates of deactivation of virons and cytotoxic cells, respectively. Let be the rate of production of cytotoxic cells, and be the baseline degradation rate. We note that the immune response of the cytotoxic cells to the viral invasion was included via a positive feedback mechanism, with a response term which is a saturating function of the amount of virus present, the full description of the parameters can be seen in Table 1. Following [15,16], the fractional order model of the mathematical model (4) is given by Caputo derivativeas follows
subject to the initial conditions and where denotes Caputo fractional derivative with the order of the derivative

Table 1.
The parameters involved in the model equation are described in detail (5).
3.1. Qualitative Properties
Here, we study the well-posedness of the fractional order model. By means of a Banach contraction mapping, we the existence and uniqueness of the solutions.
Let and where,
The dynamical system (5) can be written as,
which is equivalent to (6). The fractional model has integral representation,
As a result, we show that the fractional model is bounded and positive as long as positive initial conditions are provided. By using the integral representation (8), we present an analysis of the model. We consider a Banach space, of all continuous functions from to i.e., endowed with the norm,
Moreover, . Let us consider a well-defined operator such that
3.2. Positivity and Boundedness of Solution
The model (5) will be biologically meaningful given that the solution of the system with non-negative initial conditions will remain non-negative for any given time . This is true for this model, given that the theorem below is satisfied.
Theorem 1.
Let then for the solution of system is bounded and remains positive for
Proof.
Let us consider the trajectory of solution along H-axis where and Given,
By using and applying the beta-gamma relations, we obtain,
Similar arguments as above yields
where and yielding non-negative invariance on all axes. Considering a positive solution in the plane such that for some we have and and In the plane, Thus, we obtain by the mean value theorem for the Caputo-fractional order derivative Lemma 2 which is a contradiction to our earlier statement. □
For the boundedness of the solution, we show that the model and solution are continuous and exist within a given closed interval or region .
Theorem 2.
The model (5) has solutions bounded within the invariant region given by
3.3. Existence and Uniqueness of the Solution
We demonstrate that there is a solution to the model (5), i.e., the model is mathematically and biologically consistent. To prove this, we use the lemma below.
Lemma 3.
Let . The function defined above satisfies ≤ for some .
Proof.
and R are all dependent on t and from the first component of see (6) we observe that,
where Grouping constant terms and simplifying (13) we obtain,
where with and Similarly, we do same for second equation in (6) and we get,
where with and finally, we can express,
where with and Finally, we obtain,
and □
Next, we show that the solution to the model problem exists and is unique using the Banach contraction mapping and follows the technique from [17].
Theorem 3.
Let the result of Lemma 3 hold and let If then there exist a unique solution of model (5) on .
Proof.
By Banach contraction mapping, we will show that is both a self-map and a contraction. By definition, and let and a close convex set . In the case of the self-map property, it is sufficient to demonstrate that so let then
where Thus and is a self-map. Next, we show that is a contraction.
If then is a contraction mapping, and thus, there exists a unique fixed point on via Banach contraction principle. □
The existence of the solution follows from Schauder’s fixed point theorem.
Lemma 4.
Let such that
such that Then, the operator is completely continuous.
Proof.
For all we obtain
Thus, is uniformly bounded. Next, we show that is equicontinuous. Let such that and then
Thus, is equicontinuous. It is completely continuous as a result of the Arzelá-Ascoli theorem. □
4. Equilibrium Points and Basic Reproduction Number
The model (5) has a virus-free equilibrium (VFE) obtained by setting the right-hand side of (5) to zero. Using the next-generation matrix method, the new virus terms and the remaining transfer terms are given by
The effective basic reproduction number is given by
where denotes the spectral radius, and Next, we consider other equilibrium points, . Here, we consider the case where there are no healthy Hepatocytes, i.e., we equate the right-hand side of (5) to zero, and assume in the model (5) yielding
From (17), we have By substituting and simplifying, we have
The final equilibrium point is given by Again, we set the right-hand side of (5) to zero yielding,
The second equation of (5) yields
Since is non-zero, we have
Finally, the last equation of (5) yields
4.1. Local Stability Analysis
Here, we present the local stability analysis of the model.
4.1.1. Local Stability of VFE Point
At this stage, we assume there is no viral infection of the Hepatocytes, i.e., the person is virus free. Following [18], we present the local stability of the virus-free equilibrium in the following theorem.
Theorem 4.
The virus-free equilibrium point of the fractional model is locally asymptotically stable (LAS) if , and unstable if .
Proof.
The local stability analysis of the VFE is determined by using the eigenvalues of the Jacobian of (5) given by
Evaluating the Jacobian matrix at the VFE point yields,
The eigenvalues of the Jacobian matrix determine an equilibrium’s local stability where the eigenvalues are () and .
The virus-free equilibrium is locally stable, given that all the eigenvalues are negative. Considering Equation (23) we have,
4.1.2. Local Stability of Virus Endemic Point
In this section, we investigate the stability of the Virus Endemic Equilibrium Point(VEE) to see if a small change around it will cause a drastic change or effect on the model or will cause it to return to its original state. In this model, we consider two-state endemic points and investigate their stability. We prove this by showing that by the theorem below.
Theorem 5.
The VEE of the system (5) is locally asymptotically stable whenever and unstable whenever
Proof.
We proceed by substituting into (22), to obtain
The characteristic equation of matrix is where
with
If , and then and are all non-negative. By the Routh–Hurwitz criterion [19], we can conclude that the endemic equilibrium point is locally asymptotically stable. □
Theorem 6.
If , then is unstable and stable when and a unique endemic equilibrium exist and are locally asymptotically stable in the interior of Ω with
Proof.
We also evaluate the Jacobian matrix (22) at the endemic state . We have that
The characteristic equation of matrix is where
with
If , and which implies that and are all non-negative. Then by Routh–Hurwitz criterion [19], the endemic equilibrium point is locally asymptotically stable. □
4.2. Global Stability Analysis
To obtain the global stability of the equilibrium points, we use the Ulam–Hyers stability criteria. We show that the fractional model (5) is both stable and generalized stable in the sense of Ulam–Hyers. We say is a solution of
if and only if there exists satisfying,
- 1.
- ,
- 2.
Note that any function satisfying (2) also satisfies the integral inequality given,
Definition 3.
Definition 4.
The fractional order model (5) and (7) is said to be generalized Ulam–Hyers stability if there exists a continuous function with , such that, for each solution of (25), there exist a solution of the model (5) such that,
The stability of the fractional-order model is now presented as follows;
Theorem 7.
Proof.
Let X be a unique solution of (7) guaranteed by theorem(3.3) see (3), satisfies (25) and recalling the expression for (8), (26) we have for we have that,
From the above, we obtain , where We conclude that the proposed problem (5) is both Ulam–Hyers and generalized Ulam–Hyers stable since such that □
4.2.1. Global Stability of VFE Point
Using a Lyapunov function, we demonstrate that the virus-free equilibrium point is globally asymptotically stable.
Theorem 8.
The virus free equilibrium, is globally asymptotically stable if
Proof.
Consider the Lyapunov function of the Goh–Volterra type,
where The solution of the system (5) is determined by the derivative of as follows
By evaluating the above expression at the , we obtain,
where we have assumed that at and Therefore, if Furthermore, by the LaSalle’s invariance principle, the virus-free equilibrium point is globally asymptotically stable. □
4.2.2. Global Stability of Virus EE Point
Finally, we investigate the global stability of the endemic equilibrium points.
Theorem 9.
The virus EE, is globally asymptotically stable if
Proof.
We consider a Lyapunov function,
where By differentiating and evaluating at we obtain
where
and
If then provided . Hence, is negative semi-definite, and by the LaSalle’s invariance principle [20], the endemic equilibrium point, is globally asymptotically stable. □
We prove the global stability of the second endemic equilibrium point.
Theorem 10.
The virus EE, is globally asymptotically stable if
Proof.
Again, we consider the Goh–Volterra type lyapunov function,
where It follows that By differentiating and evaluating at we obtain
where
and
If then provided . Then by LaSalle’s invariance principle [20], the endemic equilibrium point, , is globally asymptotically stable. □
5. Numerical Results and Simulation
In this section, we present the numerical simulation to explain the dynamics of the Caputo fractional order nonlinear HBV mathematical model using the Adams-type predictor–corrector iterative scheme, see for example, [21,22] to solve the fractional-order differential equations. Using an equivalent integral formulation of our fractional model (7), we consider a uniform time discretization of given by where denote the step size. For any given approximation the next approximate is derived via the Adams-type predictor–corrector iterative scheme as follows;
Predictor:
Corrector:
with
and
5.1. Example 1
In this example, we assume parameter values as follows We consider the initial conditions with a time span of 0 to 20 days and varying values of the fractional order are reported in Figure 1, Figure 2 and Figure 3. Finally in Figure 4 and Figure 5, we present the dynamics of the model for and Here, we obtain a basic reporduction number This example demonstrates the case where the healthy hepatocytes are growing slower than the infected hepatocytes, which activates the growth of more immune response cytotoxic cells, as can be seen in Figure 3 and Figure 4.
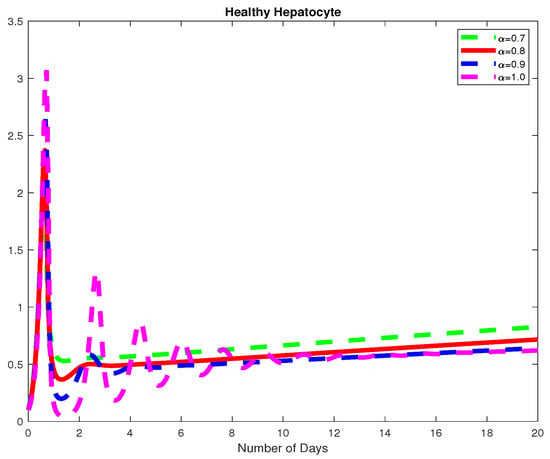
Figure 1.
The dynamics of the healthy hepatocytes, for varying fractional order .
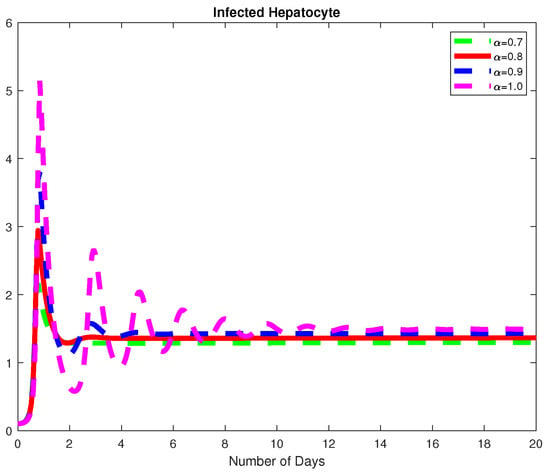
Figure 2.
The dynamics of the Infected hepatocytes, for varying fractional order .
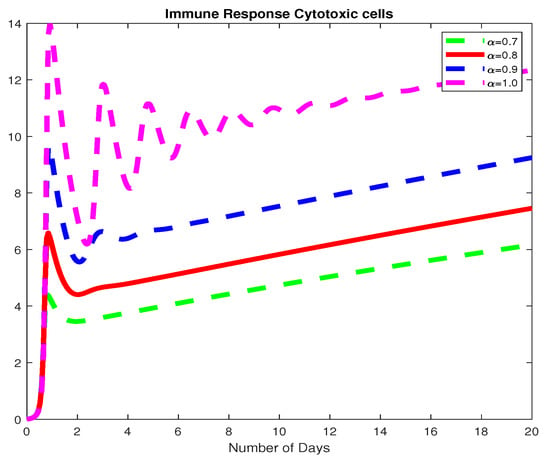
Figure 3.
The dynamics of the immune response cytotoxic cells, for varying fractional order .
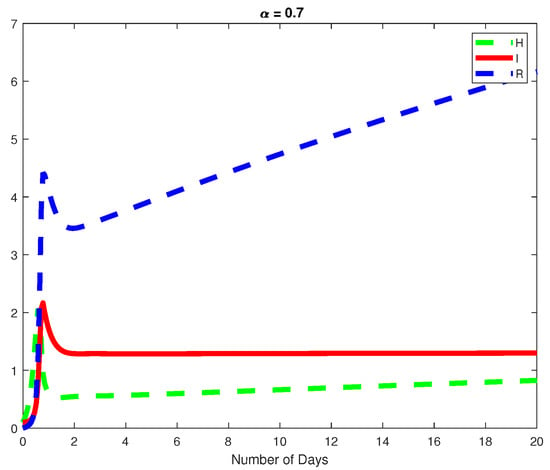
Figure 4.
For the same fractional order, we plot the dynamics of the Healthy and Infected Hepatocyte as well as the Immune response cytotoxic cells for .
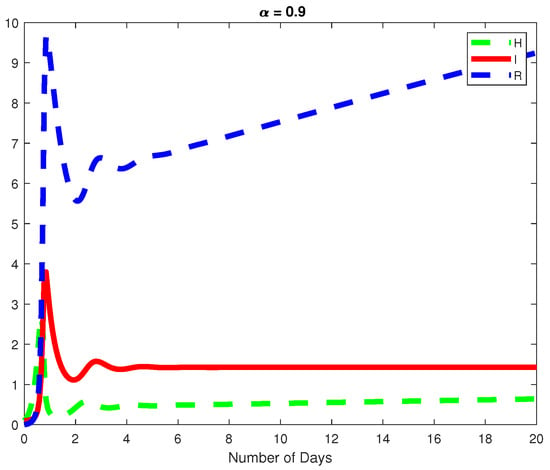
Figure 5.
For the same fractional order, we plot the dynamics of the Healthy and Infected Hepatocyte as well as the Immune response cytotoxic cells for .
5.2. Example 2
In this example, we consider the parameters values Here, we demonstrate the case where which means that the virus will die out after a number of days, see Figure 6, Figure 7 and Figure 8. Again we see in Figure 9 and Figure 10, the dynamics of the model for varying parameters and This example demonstrates the case where the healthy hepatocytes are growing faster than the infected hepatocytes. This activates a minimal growth of immune response cytotoxic cells, as can be seen in Figure 9 and Figure 10.
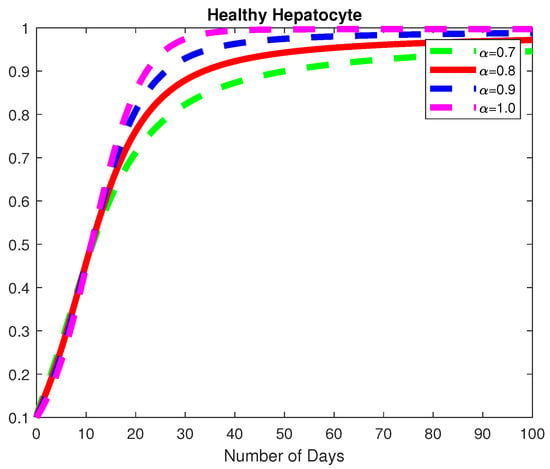
Figure 6.
The dynamics of the healthy hepatocytes, for varying fractional order .
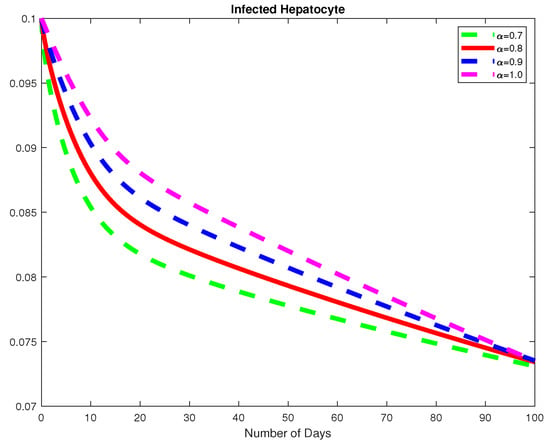
Figure 7.
The dynamics of the infected hepatocytes, for varying fractional order .
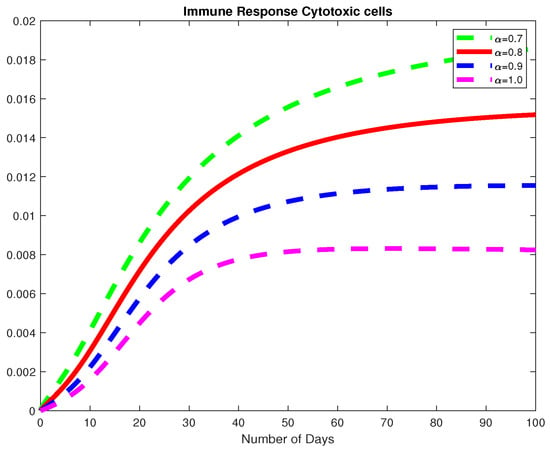
Figure 8.
The dynamics of the immune response cytotoxic cells, for varying fractional order .
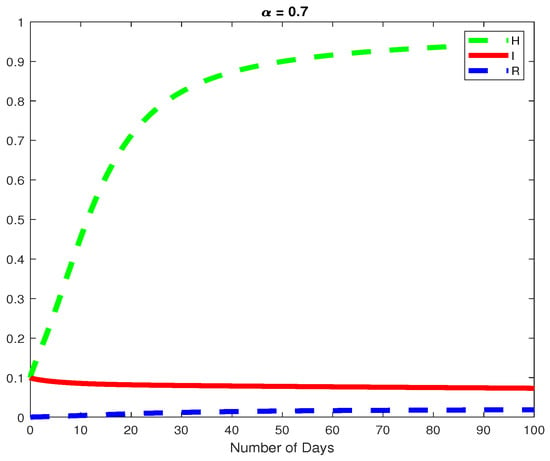
Figure 9.
The dynamics of the Healthy and Infected Hepatocyte and the Immune resonse cytotoxic cells for .
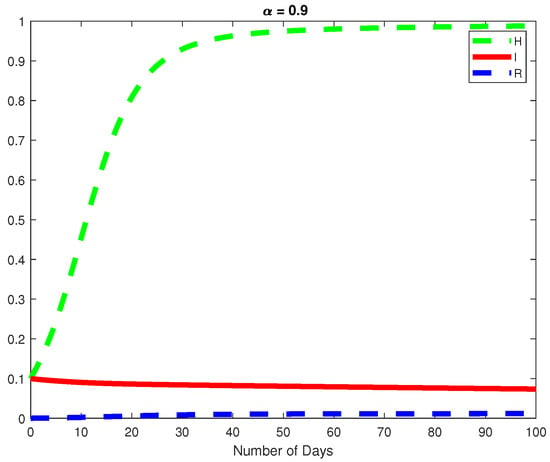
Figure 10.
The dynamics of the Healthy and Infected Hepatocyte and the Immune response cytotoxic cells for .
5.3. Example 3
Next, we study the parameters and to understand the dynamics of the model with respect to the basic reproduction number, Using the parameter values from Example 2 at a fractional order of we choose varying values for Here, we note that the basic viral reproduction number for Thus, if the infection rate of the normal cells is less than the natural death rate of the HB virus; then the HB virus will finally die out as depicted in Figure 11, Figure 12 and Figure 13. We also notice that, for the Healthy Hepatocytes grow whiles the for the Healthy Hepatocytes diminishes.
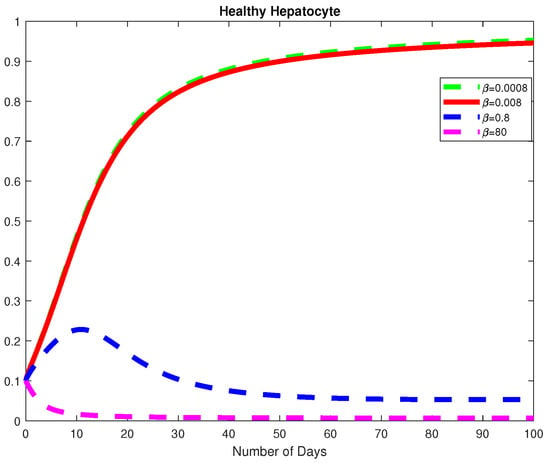
Figure 11.
The dynamics of the Healthy Hepatocytes for varying values of .
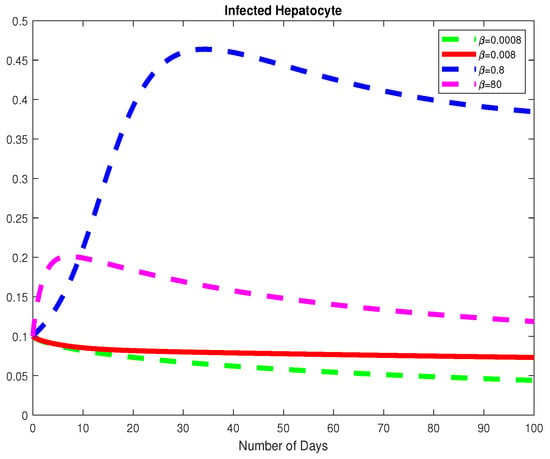
Figure 12.
The dynamics of the Infected Hepatocytes for varying values of .
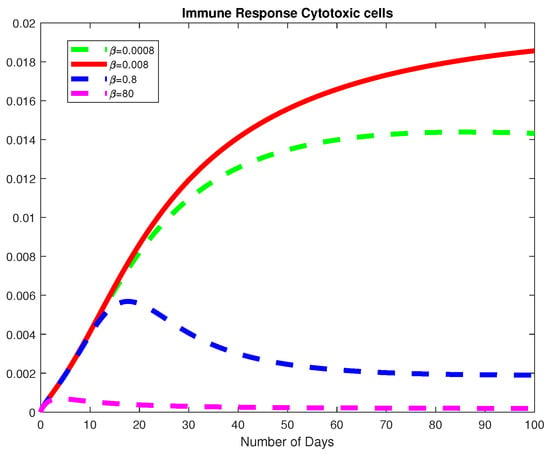
Figure 13.
The dynamics of the Immune Response Cytotoxic cells for varying values of .
5.4. Example 4
In this example, we consider all the parameter values in Example 2 except for for a fixed fractional order We used varying values of We obtained the basic viral reproduction number for all the values except at which means that the virus will be removed whenever the natural death rate of the virus is less than the rate of infection of healthy hepatocytes, see Figure 14, Figure 15 and Figure 16. In Figure 17, we notice that when the Healthy Hepatocytes are high, the infected Hepatocytes are low and lead to a low amount of the immune response cytotoxic cells.
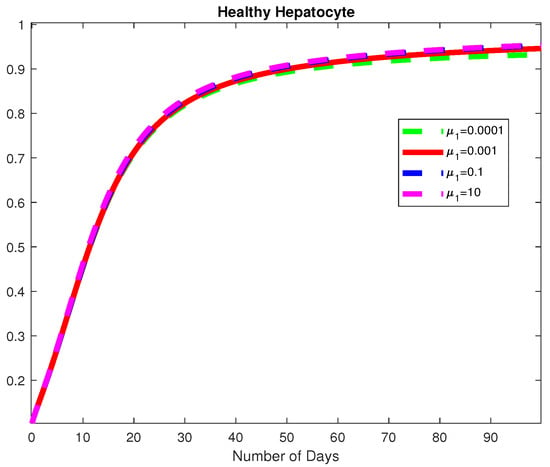
Figure 14.
The dynamics of the Healthy Hepatocytes for varying values of .
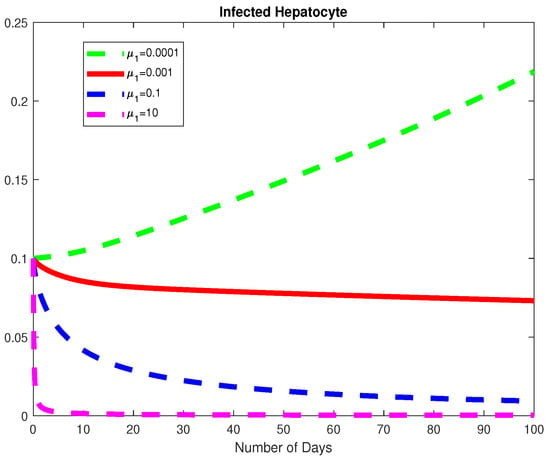
Figure 15.
The dynamics of the Infected Hepatocytes for varying values of .
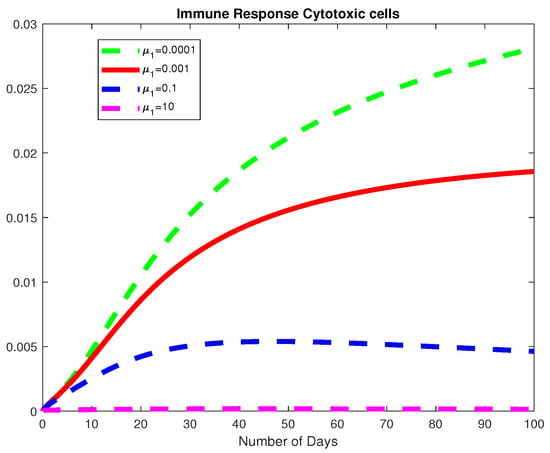
Figure 16.
The dynamics of the Immune response cytotoxic cells for varying values of .
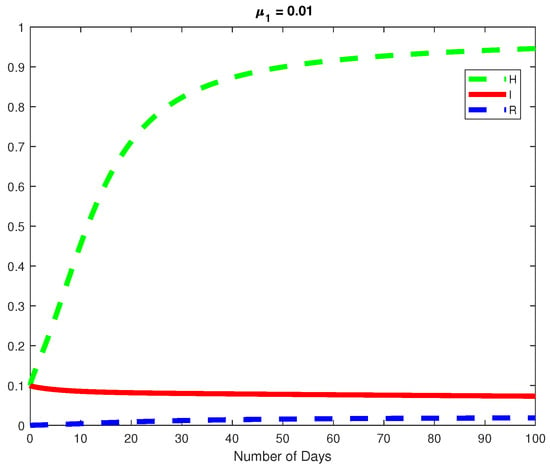
Figure 17.
The dynamics of the model for at a fractional order of .
Analyzing the impact of K in the model, we realize that, in both endemic and disease-free states, a significant increase in the value of K does not change the state of stability, for instance, keeping all parameters constant in the case of endemic with , when , and when . we also analyzed , which affects the rate at which Healthy hepatocytes become infected; thus, an increase in always causes the infected hepatocytes to increase, indicating a positive relationship between and I. An increase in in an endemic condition indicates that the virus will eventually affect all the healthy hepatocytes, and once there are no healthy hepatocytes left to rely on, the virus will eventually go extinct see (Figure 10). Taking , when and , . similarly when , . The maximum value can take in the endemic case is and a minimum value of . In the case of disease free when , takes values . Thus, when , and when , .
6. Conclusions
We presented the theoretical and numerical simulations for Caputo fractional order model for Hepatitis B cellular viral infection. We presented the existence and uniqueness as well as the boundedness of the model. We showed that the virus-free equilibrium (VFE) is locally asymptotically stable when else is unstable, i.e., replicating and becomes persistent in the liver. By means of Routh–Hurwitz, we established local stability. Using Ulam–Hyers stability, we showed that the fractional model is globally asymptotically stable. Subsequently, we presented the global stability results for the virus-free and endemic equilibrium points. Finally, using a predictor–corrector iterative scheme, several numerical simulations were presented for different fractional orders of the derivative, which corresponds to the analytical results predicted.
Author Contributions
M.O.O.: Conceptualization, methodology, formal analysis, writing—original draft, writing—review & editing; E.N.W.: writing—original draft, writing—review & editing. A.L.S.: writing—original draft, writing—review & editing; E.K.E.: writing—original draft, writing—review & editing; E.O.: writing—original draft, writing—review & editing; S.E.M.: conceptualization, methodology, formal analysis, writing—original draft, writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
S.E.M. was supported by a grant from UNESCO-TWAS and the Swedish International Development Cooperation Agency (SIDA). The views expressed herein do not necessarily represent those of UNESCO-TWAS, SIDA, or its Board of Governors.
Acknowledgments
Authors express appreciation to Hilda Moore (Cape Coast Teaching Hospital) for the insight on Hepatitis B.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rehermann, B.; Ferrari, C.; Pasquinelli, C.; Chisari, F.V. The hepatitis B virus persists for decades after patients’ recovery from acute viral hepatitis despite active maintenance of a cytotoxic T–lymphocyte response. Nat. Med. 1996, 2, 1104–1108. [Google Scholar]
- Wieland, S.F.; Spangenberg, H.C.; Thimme, R.; Purcell, R.H.; Chisari, F.V. Expansion and contraction of the hepatitis B virus transcriptional template in infected chimpanzees. Proc. Natl. Acad. Sci. USA 2004, 101, 2129–2134. [Google Scholar] [CrossRef]
- Guidotti, L.G.; Ishikawa, T.; Hobbs, M.V.; Matzke, B.; Schreiber, R.; Chisari, F.V. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity 1996, 4, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Reuter, T.Q.; Gomes-Gouvea, M.; Chuffi, S.; Duque, U.H.; Carvalho, J.A.; Perini, W.; Queiroz, M.M.; Segal, I.M.; Azevedo, R.S.; Pinho, J.R.R. Hepatitis B virus genotypes and subgenotypes and the natural history and epidemiology of hepatitis B. Ann. Hepatol. 2022, 27, 100574. [Google Scholar] [PubMed]
- Khan, T.; Jung, I.H.; Khan, A.; Zaman, G. Classification and sensitivity analysis of the transmission dynamic of hepatitis B. Theor. Biol. Med. Model. 2017, 14, 1–17. [Google Scholar]
- Bachraoui, M.; Ichou, M.A.; Hattaf, K.; Yousfi, N. Spatiotemporal dynamics of a fractional model for hepatitis B virus infection with cellular immunity. Math. Model. Nat. Phenom. 2021, 16, 5. [Google Scholar]
- Yang, X.; Su, Y.; Yang, L.; Zhuo, X. Global analysis and simulation of a fractional order HBV immune model. Chaos Solitons Fractals 2022, 154, 111648. [Google Scholar] [CrossRef]
- Khatun, Z.; Islam, M.S.; Ghosh, U. Mathematical modeling of hepatitis B virus infection incorporating immune responses. Sensors Int. 2020, 1, 100017. [Google Scholar]
- Fatehi Chenar, F.; Kyrychko, Y.; Blyuss, K. Mathematical model of immune response to hepatitis B. J. Theor. Biol. 2018, 447, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Kilbas, A.A.; Srivastava, H.M.; Trujillo, J.J. Theory and Applications of Fractional Differential Equations; Elsevier: Amsterdam, The Netherlands, 2006; Volume 204. [Google Scholar]
- Podlubny, I. Fractional Differential Equations: An Introduction to Fractional Derivatives, Fractional Differential Equations, to Methods of Their Solution and Some of Their Applications; Academic Press: San Diego, CA, USA, 1999. [Google Scholar]
- Komarova, N.L.; Barnes, E.; Klenerman, P.; Wodarz, D. Boosting immunity by antiviral drug therapy: A simple relationship among timing, efficacy, and success. Proc. Natl. Acad. Sci. USA 2003, 100, 1855–1860. [Google Scholar] [PubMed]
- Eikenberry, S. A tumor cord model for doxorubicin delivery and dose optimization in solid tumors. Theor. Biol. Med Model. 2009, 6, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.M.; Perelson, A.S. Hepatitis B virus viral dynamics: Effects of drug dose and baseline alanine aminotransferase. J. Hepatol. 2002, 37, 277–279. [Google Scholar] [PubMed]
- Diethelm, K. A fractional calculus based model for the simulation of an outbreak of dengue fever. Nonlinear Dyn. 2013, 71, 613–619. [Google Scholar] [CrossRef]
- Sardar, T.; Rana, S.; Bhattacharya, S.; Al-Khaled, K.; Chattopadhyay, J. A generic model for a single strain mosquito-transmitted disease with memory on the host and the vector. Math. Biosci. 2015, 263, 18–36. [Google Scholar] [CrossRef] [PubMed]
- Lin, W. Global existence theory and chaos control of fractional differential equations. J. Math. Anal. Appl. 2007, 332, 709–726. [Google Scholar] [CrossRef]
- Li, M.Y. An Introduction to Mathematical Modeling of Infectious Diseases; Springer: Berlin/Heidelberg, Germany, 2018; Volume 2. [Google Scholar]
- Harianto, J. Local stability analysis of an SVIR epidemic model. CAUCHY 2017, 5, 20–28. [Google Scholar] [CrossRef]
- Adda, P.; Bichara, D. Global stability for SIR and SIRS models with differential mortality. arXiv 2011, arXiv:1112.2662. [Google Scholar]
- Diethelm, K.; Ford, N.J.; Freed, A.D. Detailed error analysis for a fractional Adams method. Numer. Algorithms 2004, 36, 31–52. [Google Scholar]
- Diethelm, K.; Ford, N.J.; Freed, A.D. A predictor-corrector approach for the numerical solution of fractional differential equations. Nonlinear Dyn. 2002, 29, 3–22. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).