Abstract
There are several publications on heterogeneous catalytic ozonation; however, their conclusions and the comparisons between them are not always consistent due to the variety of applied experimental conditions and the different solid materials used as catalysts. This review attempts to limit the major influencing factors in order to reach more vigorous conclusions. Particularly, it highlights two specific factors/parameters as the most important for the evaluation and comparison of heterogeneous catalytic ozonation processes, i.e., (1) the pH value of the solution and (2) the initial concentration of the (micro-)pollutants. Based on these, the role of Point of Zero Charge (PZC), which concerns the respective solid materials/catalysts in the decomposition of ozone towards the production of oxidative radicals, is highlighted. The discussed observations indicate that for the pH range 6–8 and when the initial organic pollutants’ concentrations are around 1 mg/L (or even lower, i.e., micropollutant), then heterogeneous catalytic ozonation follows a radical mechanism, whereas the applied solid materials show their highest catalytic activity under their neutral charge. Furthermore, carbons are considered as a rather controversial group of catalysts for this process due to their possible instability under intense ozone oxidizing conditions.
1. Introduction
Chemical oxidation processes are used as common alternatives of biological treatment processes, especially for the removal of refractory from biodegradation compounds. The aim of chemical oxidation processes is faster and better mineralization of emerging contaminants; ozonation is among these processes. However, the basic disadvantage of single ozonation is that it rarely leads to total mineralization. Instead, saturated organic compounds, such as aldehydes or short-chain carboxylic acids, can be formed by the partial oxidation of the original substances [1]. In addition, ozonation can be characterized as a relatively costly process in comparison with other alternative treatment methods. However, it presents specific advantages, as well as certain disadvantages, compared to the other available Advanced Oxidation Processes (AOPs) dealing with the removal of micropollutants (e.g., Fenton) [2]. These disadvantages may be overcome by adding an appropriate catalyst, and the modified process is then called “catalytic ozonation”. The main advantages of catalytic ozonation over the conventional process are relevant with more efficient ozone consumption and faster process throughput due to the higher rates of mineralization and higher removal efficiency of oxidized compounds [3].
Catalytic ozonation belongs to the Advanced Oxidation Processes (AOPs) because it is expected to enhance the production of hydroxyl radicals (considered as strongly oxidant agents). According to the specific type of catalyst added to the oxidation system, the catalytic ozonation can be divided into homogeneous and heterogeneous catalytic ozonation when transition metal ions or solid materials are used as catalysts, respectively [4]. Heterogeneous catalytic ozonation is advantageous over homogenous catalytic ozonation due to the easier separation of the catalyst (and its potential reuse) from the aqueous solution in which the oxidation reaction takes place [5].
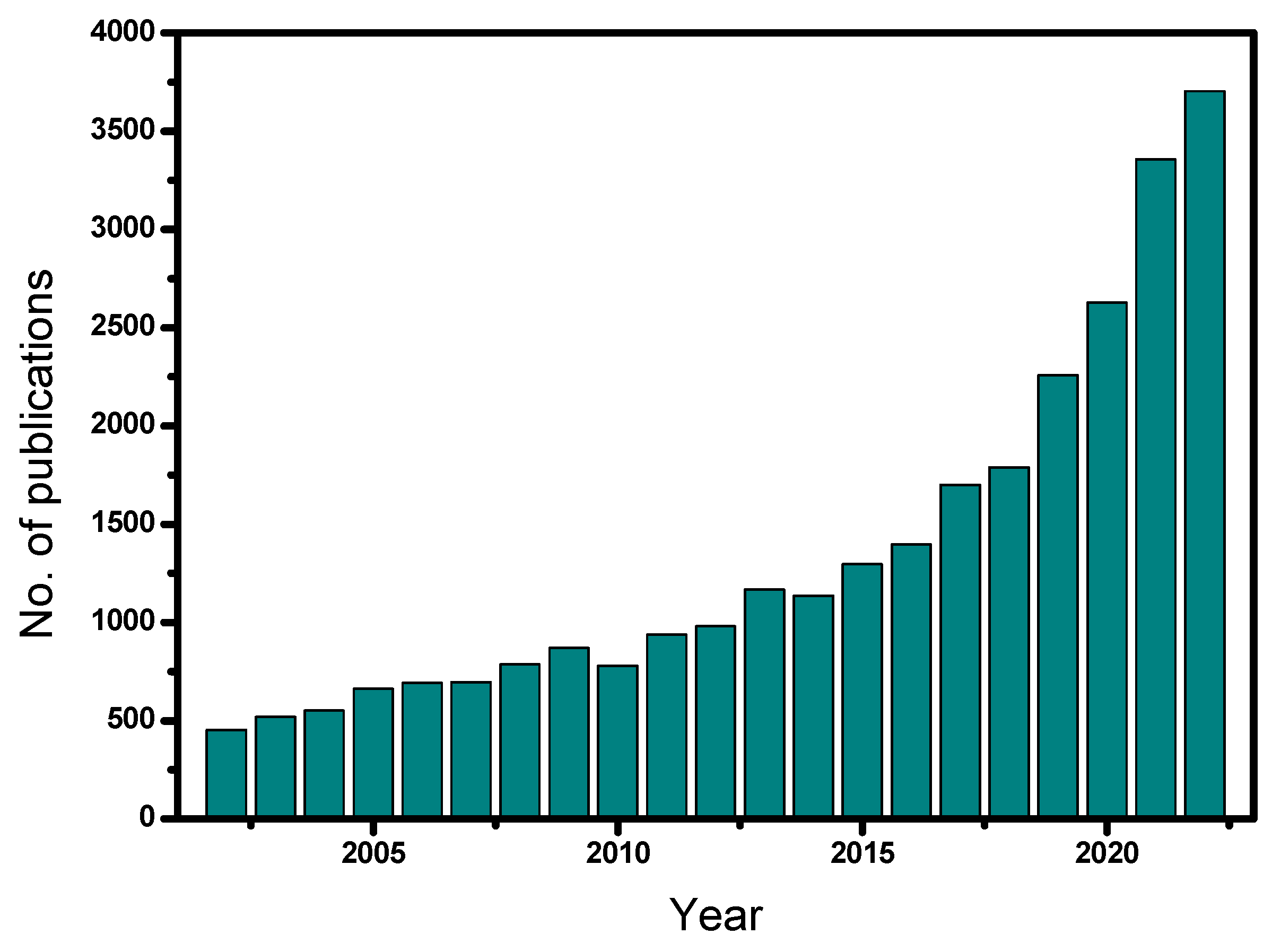
The frequency of the studies that have been published in the field of heterogeneous catalytic ozonation over the last 20 years (2002–2022) with the use of different solid catalysts is shown in Figure 1. Note that most of these studies are based on batch experiments. The relevant literature was collected from Science Direct, Scopus, MDPI, and PubMed databases using relevant keywords, such as heterogeneous catalytic ozonation, advanced oxidation processes, catalysts, micropollutants, etc. From Figure 1, it becomes clear that heterogeneous catalytic ozonation is a relatively recent investigated process, which began to be studied systematically during the last ten years. Of the approximately 30,000 studies, concerning this process, 24,000 were published from 2010 onwards. Most of these studies are related to the production of an efficient catalyst or to the modification of commercial materials, usually by depositing proper metals into their structure, whereas a lower number of studies refer to the investigation of the reaction mechanism(s).
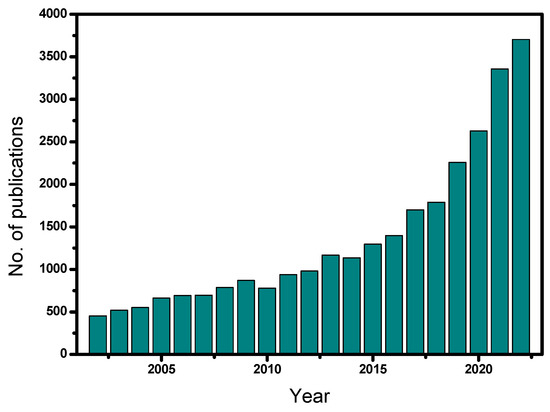
Figure 1.
Published scientific studies on heterogeneous catalytic ozonation for the period 2002–2022.
In heterogeneous catalytic ozonation, the applied catalyst is in solid form and the reaction takes place either in the bulk (aqueous) solution or onto its surface. When the combination of ozone with a solid material is considered as a catalytic ozonation process, then the efficiency of ozonation in the presence of the solid must be greater than the sum of the removals caused by the adsorption of the pollutant and by the effect of single ozonation under the same pH value [6]. However, the highest catalytic activity is noticed when the difference between single and catalytic ozonation is the highest. The key to heterogeneous catalytic ozonation efficiency is to find the appropriate (solid) material that can act as an effective catalyst [7]. The catalytic effect takes place when one of the following conditions is met [4]:
- Ozone is adsorbed on the surface of the catalyst.
- The organic molecule is adsorbed on the surface of the catalyst.
- Ozone and the organic molecule are both adsorbed on the surface of the catalyst and then interact.
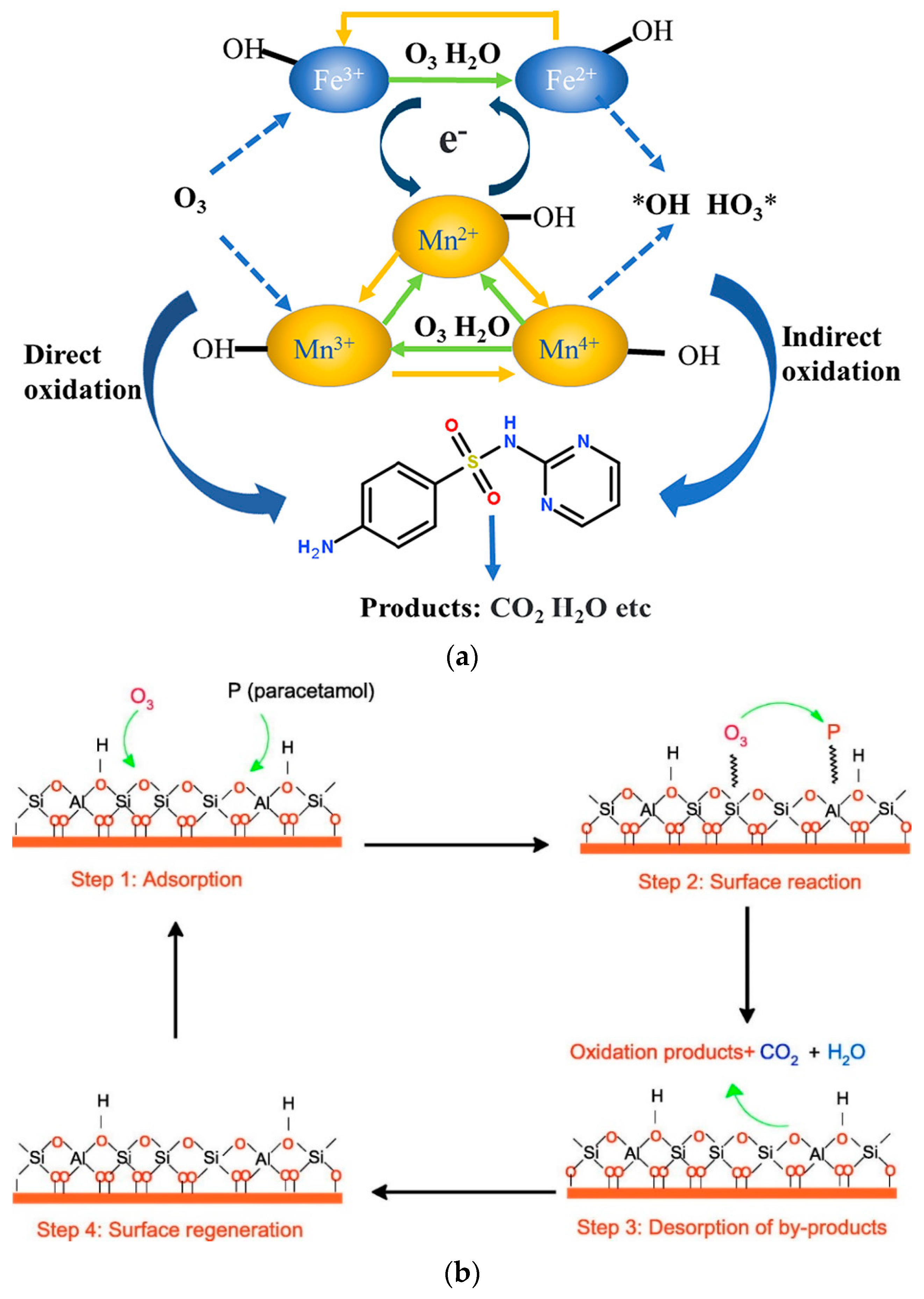
Radical [8] and non-radical [9,10] mechanisms are also reported, although the most common is the radical mechanism. In nearly all cases occurring through the application of radical mechanisms, the predominant species are the hydroxyl radicals; however, there are certain studies in which the hydroxyl radicals are not responsible for the removal of micropollutants, while the superoxide radicals [11,12] or the single oxygen atoms [11,13] are the main oxidizing species. Superoxide radicals are less powerful oxidizing agents than the hydroxyl radicals. However, they can form singlet oxygen atoms, which are more powerful and selective oxidizing species, such as hydroxyl-free radicals and hydrogen peroxide [14]. More information about the mechanism of these radical species produced during the AOPs processes can be found in Gottschalk et al. [15] and Rayroth et al. [16]. The non-radical mechanism can take place via two main specific routes: (1) surface complexes formed by the adsorption of ozone and pollutant on the solid catalyst surface and (2) adsorbed oxygen species onto the solid surface by the dissociation of O3 that are in contact with the catalyst active sites [13]. Examples of these two main mechanisms are presented in Figure 2.
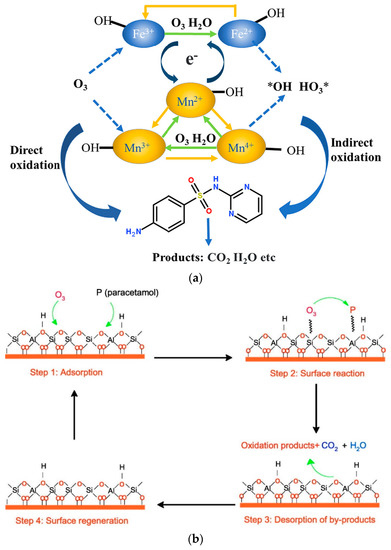
Figure 2.
Examples of (a) radical [8] and (b) non-radical mechanism [10] for the removal of micropollutants during heterogeneous catalytic ozonation.
Recently, Inchaurrondo & Font [17] published a review regarding the use of natural (catalytic) materials in the ozonation of organic pollutants. This research concludes that despite the large number of studies concerning the catalytic ozonation mechanism(s), there is still a lack of understanding. However, this is not something new; already in 2013, Nawrocki published a relevant paper on the controversial issues observed between the heterogeneous catalytic ozonation literature studies [4], reporting seven relevant cases and in particular: (1) lack of proper pH control, (2) adsorption of substrate onto the catalyst surface, (3) adsorption of ozonation products, (4) realistic catalyst-organic substrate ratio, (5) purity of the catalyst, (6) “one run” catalyst, and (7) natural water conditions. Overall, this review summarizes the studies on heterogeneous catalytic ozonation at that time, attempting to limit the aforementioned controversial factors while trying to reach valid conclusions. The literature studies included in the current review use mostly stable catalysts (except for some specific cases, as mentioned in the text) in realistic ratios with the examined organic compounds and under stable pH values. The evaluation of these studies used as main indicator their efficiency with respect to adsorption, single ozonation, and/or catalytic ozonation. Furthermore, the present review introduces two more factors that are considered as most important for the evaluation and comparison of the heterogeneous catalytic ozonation processes, i.e., (1) the pH value of (aqueous) medium and (2) the (small) initial concentration of examined organic compounds. Moreover, the studies presented in this review are based on simulated experimental conditions, as the relevant ones conducted with natural water conditions are rarely reported.
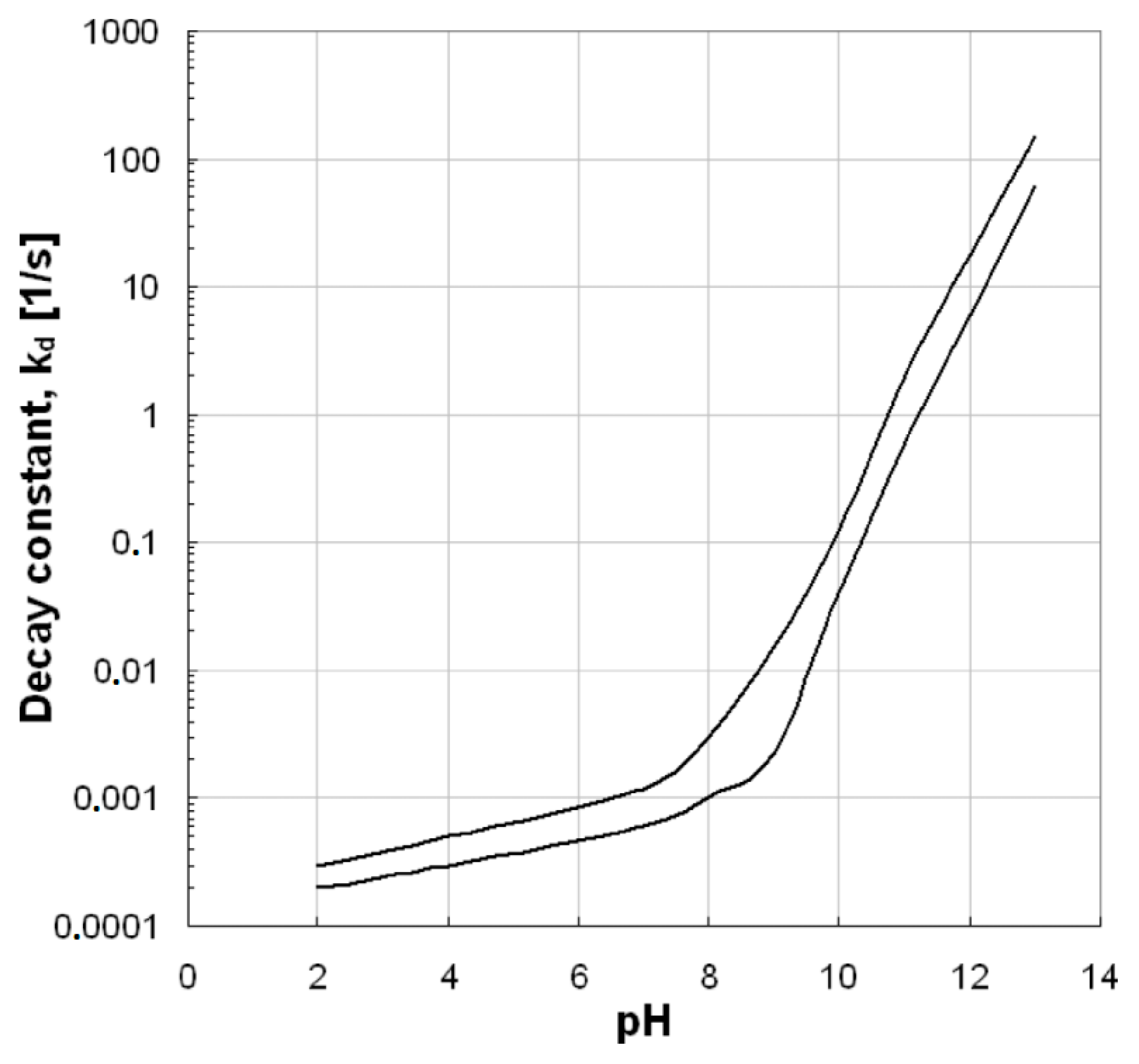
The pH value is a very important factor for ozonation processes because it can highly affect the decomposition of ozone, as well as the surface properties of used solid materials/catalysts (e.g., the point-of-zero charge/PZC) and the charge of the examined organic compounds. Additionally, it affects the electrostatic interactions between the pollutant and the catalyst surface [8,18]. As Figure 3 shows, the increase of pH value can lead to increased ozone decomposition, which subsequently leads to the production of more hydroxyl radicals. The hydroxyl radicals are more powerful oxidizing agents than the ozone and, therefore, when they predominate in an oxidation reaction, the process efficiency becomes higher. In contrast, the stability of ozone molecules at the acidic pH values is higher and, hence, it is more difficult to be decomposed. However, the effect provoked by the pH value mainly depends on the specific type of applied AOP. As Aziz et al. [19] showed, the single ozonation process under acidic pH values presents lower efficiency with respect to the removal of micropollutants, while the Fenton process is more effective for these pH values. In the present study, the removal of micropollutants from common aqueous matrixes is evaluated and, therefore, the pH values of respective studies are in the range of those usually encountered in natural waters (i.e., 6–8). These observations can affect not only the efficiency, but also the mechanism of the catalytic ozonation process. More information about the effect of pH value on ozone decomposition and the subsequent production of hydroxyl radicals can be found in the aforementioned reference [19].
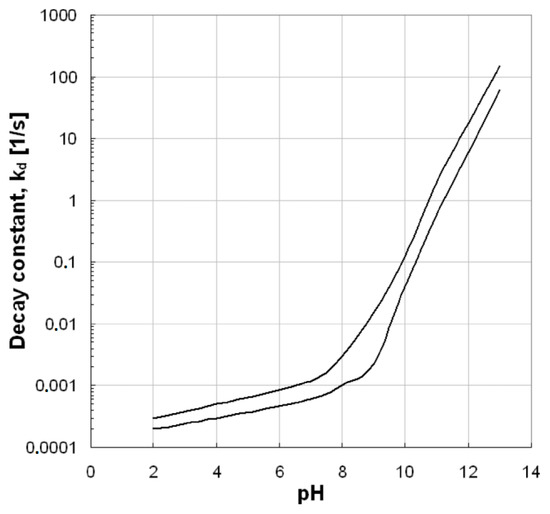
Figure 3.
Ozone decomposition constant as a function of pH value [20].
The pH of the (aqueous) matrix solution should be constant throughout the oxidation reaction in order to properly evaluate (and compare) the ozonation process with other alternatives. Su et al. [21] used Fe3O4@SiO2@MgO material as a catalyst and compared its performance with the efficiency of separate FeO4@SiO2 and Fe3O4 solid compounds. The three catalysts have PZC values equal to 9.6, 7.16, and 7.01, respectively. The pH of the solution was not buffered, but it was adjusted before the initiation of the oxidation reaction. They observed that the optimal catalyst was Fe3O4@SiO2@MgO. However, the presence of MgO in its structure possibly could increase the solution pH (towards alkaline values), unlike the other two catalysts, thus, promoting the decomposition of ozone and the higher production of hydroxyl radicals. Nevertheless, under real (not simulated) conditions, the solution pH can present certain buffering capacity, and, therefore, its pH value is not expected to change significantly by the addition of solid catalysts [22]. In this study, the efficiency of the catalytic process by using these three solids cannot be compared.
There are numerous studies that have shown that during catalytic ozonation, the mineralization of pollutants increases, while the removal of pollutants presents lower or equal efficiency, as compared to the application of single ozonation. Moreover, there are certain cases where the catalytic ozonation process proved to be suitable for the reduction of DOC in wastewater, yet the removal of the pollutants content was not considered as sufficient [23,24,25,26]. The micropollutants commonly occur in low concentrations (few μg/L) and are not easily degraded by the presence of ozone molecules, as they can be attacked/oxidized with smaller possibilities. The main by-product of ozone decomposition, i.e., the hydroxyl radicals, are more powerful oxidizing agents than the ozone molecule, but they present shorter lifetime (duration of only some seconds) [27]. These two factors, overall, can reduce the efficiency of catalytic ozonation in terms of micropollutants removal. For example, Liu et al. [26] used the composite material Zn-CNTs for the removal of 4-chloro-3-methyl-phenol. The zero-valent form of zinc (Zn0) is a strong reducing agent, and when it reacts with oxygen, it can produce H2O2, which is expected to enhance synergistically the catalytic ozonation process. The production of H2O2 was even higher for the Zn-CNTs/O3 system because it can be produced both by ozone decomposition and by the reaction between Zn and O2 in the system. However, this catalytic combination showed lower efficiency than the case of single ozonation, probably because the oxygen atoms occupied the most active sites of the catalyst (to produce H2O2) and they were unavailable for the required oxidation purpose. Simultaneously, the competition between the H2O2 and the examined organic compound with the ozone molecules reduced the effect of the Zn presence. However, even though the removal of the pollutant was not improved by the addition of this catalyst, the total mineralization of the studied oxidation system was enhanced.
Thus, due to the large variety of experimental conditions during the performance of catalytic ozonation experiments, it is important for the proper evaluation of catalytic ozonation mechanisms that the relevant factors be limited and properly controlled. Catalytic ozonation is a promising method for the removal of emerging contaminants (micropollutants) from contaminated aqueous matrixes. This review focuses on the ability of this process to be applied during water treatment operations mainly for the removal of organic pollutants and not for mineralization. Therefore, the only studies performed for the common natural water pH range 6–8, where the pH value is controlled and remains relatively constant throughout the reaction, will be evaluated. Another important factor included in this review is the (small) initial concentration of examined micropollutants, which should be equal to or lower than 1 mg/L in order to simulate better real environmental conditions.
2. Catalyst Categories
The major categories of solids commonly used as catalysts in the heterogeneous catalytic ozonation process for the degradation of organic pollutants in aquatic solutions are [4]:
- Metal oxides, such as MnO2, Fe3O4 or Al2O3.
- Metals, such as Fe, Mn, Co, Cu, and Ce deposited onto different substrates, such as Al2O3, MCM-41 or SBA-15.
- Minerals, such as zeolites, perovskites, cordierite, and ceramic honeycomb.
- Carbons, such as AC, GO, and CNTs.
Among them, the category of carbons is the most controversial. There are studies showing that carbons are not stable under the strong oxidizing conditions created by the presence of ozone; thus, they do not present the usually required for long-term stability during the application of ozonation processes. Valdés et al. [28] reported that the exposure of activated carbons to ozone can lead to the modification of their surface properties and to different textural characteristics, where the following equations can possibly take place [29]:
HO− + C* → C*-HO−
C*-HO− + O3 → C*-O3− + HO•
In the 1st reaction (Equation (1)), the hydroxide ions (HO−) are adsorbed onto the surface of solid material, and then (Equation (2)) the ozone reacts with the adsorbed hydroxide ion, producing hydroxyl radicals. These reactions eventually can lead to the further decomposition of C*-O3− product; thus, the carbon adsorption centers are occupied by the hydroxyl ions, reducing their overall adsorption capacity. As a result, although the hydroxyl radicals are produced from these reactions, most of the adsorption centers in the solids (carbons) surface are occupied, and the reaction of ozone (according to Equation (2)) with the substrate is restricted [29]. Razumovskii et al. [30] has also reported that exposure to ozone can decrease the surface reactivity that may protect the surface of carbons from the destructive (oxidation) action of ozone.
Additionally, ozone treatment has as a consequence the transformation of surface alkaline carbon sites towards acidic ones and, thus, the generation of new acidic sites in the system, causing the reduction of the PZC value. Valdes et al. [28] reported that after 120 min of ozone exposure, the PZC of carbon was reduced from 8.8 to 2; the same observations were reported also by other researchers [31,32]. Ozone can affect also the structural characteristics of carbons by reducing their surface area because the pore walls are destroyed by the ozonation of carbons [28,33]. Furthermore, when considering catalytic activity, Sanchez-Polo & Rivera-Utrilla [33] reported that the decrease of alkaline (oxygenated) surface groups has led to the reduction of H2O2 production, thereby decreasing the process efficiency overall.
3. Factor 1: The pH Value of the Medium
Table 1, Table 2, Table 3 and Table 4 present the relevant studies as published in the literature. The examined materials presenting catalytic action in the pH range 6–8 can be divided according to the aforementioned major categories of catalytic materials. The catalysts described in the next paragraphs are stable under the strong oxidizing conditions of ozone, except for certain cases, as noted in the text.
3.1. Metal Oxides
Various metal oxides have shown sufficient catalytic action during the ozonation processes, such as MnO2, TiO2, Fe2O3, etc. [4]. The surface of metal oxides in water has a layer of hydroxyl groups, whose formation follows two main paths. In the first, the unsaturated metal ions and the oxygen atoms of surface oxide lattice can strongly adsorb water molecules and decompose them into H+ and HO− species, resulting in the formation of surface hydroxyl groups. In the second, during the preparation/synthesis of metal oxides, hydroxyl groups can be formed, being integrated into the solid. Overall, these hydroxyl groups are considered as the main active sites for the adsorption and decomposition of ozone molecules. These groups can be negatively, positively, or neutrally charged, depending upon the solution pH; the adsorption and decomposition of ozone also depends upon the respective charge of sites [34].
Fe3O4 material is a low cost, non-toxic solid that can be easily (surface) modified. In addition, Fe3O4- and Fe3O4-based materials can be easily separated from the aqueous phase by the employment of a simple magnetic process [35], which is among their main advantages. Zhang et al. [35] used Fe3O4-MnO2 hybrid magnetic composite for the removal of bisphenol A (BPA) from aqueous solutions. When there was only this material in the solution, about 11.2% of BPA was removed after 30 min of treatment. The results indicate that the adsorption process influences, but only slightly, the removal efficiency of BPA. The adsorption effect of Fe3O4-MnO2 might be due to its higher surface area, when compared to the respective raw materials. Meanwhile, the removal efficiency of BPA by applying catalytic ozonation can reach up to 97% for the same reaction time and with the same Fe3O4-MnO2/O3 system, which was superior when compared to the application of single ozonation (72%). Under the same conditions, the removal of BPA by another oxidation system (Fe3O4/O3) was 81.21%, proving that the applied magnetic material (Fe3O4) itself can present certain catalytic activity. However, the BPA has a rather high reaction rate constant with ozone (~105 M−1s−1) and it can be easily removed even by the application of single ozonation, as reported by other researchers [36].
Zhang et al. [35] studied also the effect of different pH values on the catalytic action of this magnetic material (Fe3O4-MnO2) and found a certain improvement, as the solution pH increased from 5 to 9. BPA has two dissociation constants, i.e., the first dissociation constant is at 9.6, while the second is at 10.2. Therefore, at pH values <9.6, the neutral molecule of BPA is the predominant species, and this organic compound cannot be influenced within the examined pH range. Thus, in this case, the increased removal efficiency of micropollutants at higher pH values was mainly due to the higher rates of ozone self-decomposition or due to the enhancement of ozone decomposition by the presence of catalyst. In this study, although the charge of organic compound was considered, the PZC of the used solid material was not further measured or discussed.
In a relevant published study, the M-Fe2O4 spinel ferrites, where M represents transition metal ions, have been used as catalysts also in heterogeneous catalytic ozonation [37]. Several materials, such as NiFe2O4 [38], CuFe2O4 [39], and Mn0.95Bi0.05Fe2O4 [40], showed catalytic activity for the pH range 6–8. However, none of these materials presented significant adsorption capacity; therefore, the removal of micropollutants in the presence of these materials can be depicted as the oxidative reactions between the pollutants and the produced hydroxyl radicals and not the synergistic effect of adsorption and ozonation processes. Yan et al. [41] used α-Fe0.9Mn0.1OOH solid for the removal of iohexol from water solutions. The adsorption capacity of this material was less than 3%; therefore, in this case, the removal of pollutant was also mainly due to the improved oxidation conditions. The solid material presented catalytic activity and removed 95% of the micropollutant after 20 min of oxidation reaction time at pH 7. According to the researchers, the catalytic action was based on the surface oxygen vacancies, which enhances the decomposition of ozone for the production of hydroxyl radicals.
Zhao et al. [42] used magnetic Mn-doped ZnFe2O4 ferro-spinel as catalyst for the removal of di-n-butyl phthalate (DBP). Mn-Zn ferrite is a soft magnetic ferrite material with the advantages of high magnetization, strong stability, and small losses during application, facilitating its removal from water. Then, 36% of DBP was removed by the application of single ozonation after 30 min of treatment. However, the removal of DBP was enhanced by the simultaneous presence of ozone and catalyst, and the ZnFe2O4/O3 was able to degrade DBP by 50.5%, whereas the presence of Mn-ZnFe2O4 could increase the removal up to 91.7% within 30 min treatment time. Both catalysts showed negligible adsorption capacity, which confirms the DBP removal was attributed to the oxidation and not to adsorption process. The pH study showed that while in single ozonation an increase of pH can led to the increase of process efficiency, in the catalytic ozonation this does not happen. An improvement of DBP removal was obtained at the pH range 5–6.8, and when the solution pH further increased, the efficiency of DBP removal decreased. The PZC of this catalyst was 6.8 and, thus, the highest catalytic activity was obtained at the pH value where the catalyst surface was neutrally charged.
The α-FeOOH (goethite) material is considered as the thermodynamically stable Fe(III) oxide and is frequently used in wastewater treatment processes due to the high density of hydroxyl groups [43]. Figure 4 shows a schematic representation of ozone reaction with the respective surface hydroxyl groups, resulting in the improved production of hydroxyl radicals and, hence, to the increase of ozonation catalytic activity. Li et al. [44] used goethite to remove nitrobenzene at the neutral pH value. The presence of this solid increased the removal of the examined pollutant when compared to the application of single ozonation (i.e., without the presence), although not presenting any significant adsorption capacity (being <3%). Nevertheless, the researchers did not provide any specific explanations about the reasons of the catalytic action since their research is based mainly on the preparation of this catalyst. However, and despite its advantages, this material shows certain drawbacks also, such as the lack of active reaction sites, the high resistance to mass transfer due to larger particle sizes, and the lower specific surface area in comparison with that of natural goethite.
Nanoscale natural catalysts could overcome these drawbacks. Pelalak et al. [45] used a natural nanoscale goethite to remove sulfasalazine and observed that the greater catalytic activity occurred at pH 7, where the surface hydroxyl groups were found to be neutrally charged. These sites were the active ozone adsorption sites onto the surface of the material. When goethite was treated with nitrogen plasma, it presented more hydroxyl groups, resulting in even greater performance. Sui et al. [46] also used α-FeOOH solid as a catalyst, but for the removal of oxalic acid. The difference with the other studies is that in this case, both the ozone decomposition and the hydroxyl radicals production promoted when the surface was neutrally or positively charged, i.e., at neutral or acidic pH values, respectively (PZC = 7.2).
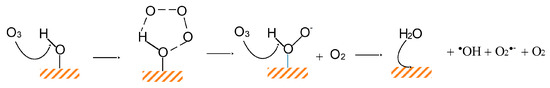
Figure 4.
Schematic representation of the ozone reaction with the hydroxyl groups of metal oxide surface [47].
Figure 4.
Schematic representation of the ozone reaction with the hydroxyl groups of metal oxide surface [47].
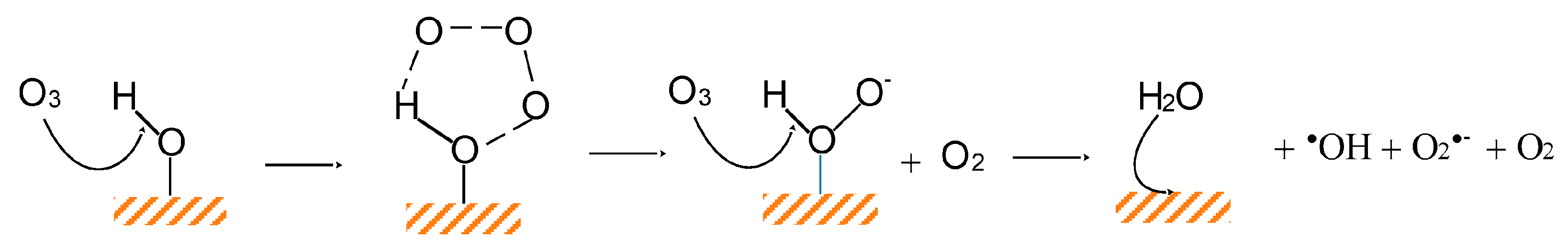
Another metal oxide evaluated as catalyst in the heterogeneous catalytic ozonation is magnesium nano-oxide (nano-MgO). Wang et al. [7] and Zhu et al. [48] used this material to remove effectively phenol or quinoline, respectively. Magnesium oxide is a non-toxic, eco-friendly, and relatively low-cost compound. Nanomaterials have a higher specific surface area, making their active sites more accessible. The two MgO materials present different PZC values. The first was more alkaline with PZC = 10.5, while the other had PZC = 7.2, noting that the effect of the adsorption process was negligible for both cases. The solid materials showed higher catalytic activity when their surface was neutrally charged (i.e., when pH = PZC). At pH values higher than 7, the HO− content in the aqueous phase increased, which primarily determines the decomposition of ozone and the production of hydroxyl radicals. The results of these studies suggest that the surface of metal oxides can affect the catalytic ozonation process to a higher degree than the presence of HO−.
Dai et al. [49] used zinc oxide and showed that the addition of ZnAl2O4 had positive results with regard to the removal of 5-sulfosalicilic acid. The catalytic ozonation in this study occurred via the production of hydroxyl radicals, which was enhanced by the presence of the catalyst in the ozonation system. The solid material was slightly positively charged at the experimental pH 7 as its respective PZC value was 8.
Among the metal oxides, manganese oxide has received appreciable attention due to its catalytic performance, the possibility of multivalent Mn species (+2, +3, +4), and the easier availability [50]. However, in the relevant literature, its action has been tested mainly at the acidic pH values [51,52]. Nawaz et al. [11] used 6 different phases of this material (α-, β-, γ-, δ-, ε- and λ-) to evaluate their catalytic activities for the removal of 4-nitrophenol at pH 7. All 6 materials showed good catalytic action, but the highest removal rates for the case of 4-nitrophenol was observed by the presence of α-MnO2. The α-MnO2 showed the lowest PZC value, equal to 2.6. On the other hand, the examined organic compound had pKa equal to 7.2, i.e., it was slightly higher than the respective solution pH at 7. Therefore, the catalyst was strongly negatively charged, while the organic compound was slightly positively charged, and, as a result, the electrostatic interactions were rather weak and the adsorption of this micropollutant into the catalyst surface was almost negligible. The removal of this pollutant was based on the catalytic oxidation, but the main radical species in this case were the superoxide radicals and the singlet oxygen, rather than the hydroxyl radicals, as observed in other studies. However, and despite its catalytic activity, the greatest disadvantage of α-MnO2, making it rather unsuitable to be used as a catalyst in the ozonation systems, was its instability under the oxidative conditions of ozone. He et al. [53] also used α-MnO2 for the removal of metoprolol. As in the previous research, the PZC value of this material was very low (2.1). Therefore, the material presented its highest catalytic activity at pH 3, where its surface was almost neutrally charged, as well as at pH 10, where the self-decomposition of ozone towards the production of hydroxyl radicals was (anyway) very high. However, this material showed catalytic activity also at pH 7, removing metoprolol by 99.6% after 30 min of oxidation reaction time. As reported by Benner & Ternes [54], metoprolol is an organic molecule, presenting pH-depending reaction rate constant with ozone. The pKa of metoprolol is 9.7 and the molecule consists of the aromatic ring and the secondary amine, noting that the ozone reactivity for these two sites is different. The amine moiety is active when the molecule is deprotonated, and the reaction rate constant with ozone is then equal to 8.6 × 105 M−1s−1. In the protonated form, the molecule has a better chance to be attacked by ozone in the aromatic ring, with the respective reaction rate constant being 330 M−1s−1. Therefore, at different pH values, the charge of organic molecules can greatly affect the efficiency of ozonation because the ease of reacting with ozone molecules changes. In the study of He et al. [53], the optimum pH value was 3, at which metoprolol was completely protonated; thus, the only reactive site was the aromatic ring. Under this condition, the reaction rate constant of metoprolol with ozone was the lowest, proving that the neutral surface charge of the catalyst can enhance to a great extent the contact of ozone with the catalyst surface and its subsequent decomposition towards the formation of hydroxyl radicals.

Table 1.
Metal oxides as catalysts in the heterogeneous catalytic ozonation process for the removal of micropollutants at pH values 6–8.
Table 1.
Metal oxides as catalysts in the heterogeneous catalytic ozonation process for the removal of micropollutants at pH values 6–8.
| Catalyst | PZC | Micropollutant | Ccat. (g/L) | CO3 (mg/L) | Cmicr. (mg/L) | pH | Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Goethite (α-FeOOH) | / | Nitrobenzene | 0.1 | 25 | 100 | 7.0 | SAP < 3% (40 min) SOP = 74.8% (40 min) COP = 94.3% (40 min) | [44] |
| α-MnO2 | 2.1 | Metoprolol | 0.2 | 10 | 7.0 | SAP = 10% (30 min) SOP = 65% (30 min) COP = 99.62% (30 min) | [53] | |
| α-Fe0.9Mn0.1OOH | / | Iohexol | 0.1 | 0.8 | 1 | 7.0 | SAP < 3% (20 min) SOP = 45% (20 min) COP = 95% (20 min) | [41] |
| Naturals–Goethite | 7.2 | Sulfasalazine | 1.5 | 5 | 10 | 7.0 | SAP = 6.52% (40 min) SOP = 61.44% (40 min) COP = 75.64% (40 min) | [45] |
| PTG-N2 (goethite treated with nitrogen plasma) | 6.9 | SAP = 9.07% (40 min) SOP = 61.44% (40 min) COP = 96.05% (40 min) | ||||||
| Fe3O4 | / | BPA | 0.1 | 50 | 7.0 | SOP = 72% (30 min) COP = 81.2% (30 min) | [35] | |
| Fe3O4-MnO2 | / | SAP = 11.2% (30 min) SOP = 72% (30 min) COP = 97% (30 min) | ||||||
| ZnFe2O4 | 6.9 | DBP | 0.01 | 0.5 | 7.0 | SAP = ng SOP = 36% (30 min) COP = 50.5% (30 min) | [42] | |
| Mn-ZnFe2O4 | 6.8 | SAP = ng SOP = 36% (30 min) COP = 91.7% (30 min) | ||||||
| ZnAl2O4 | 8 | 5-sulfosalicilic acid | 0.2 | 500 | 7.0 | SAP = 2% (60 min) SOP = 49.4% (60 min) COP = 64.8% (60 min) | [49] | |
| Fe3O4@SiO2@TiO2 | 6.2 | Catechol | 0.2 | 1000 | 8.0 | SOP = 51% (30 min) COP = 70.5% (30 min) | [55] | |
| CuFe2O4MNPs | / | DMAC | 30 | 4.6 | 200 | 6.7 | SOP = 55.4% (120 min) COP = 95.4% (120 min) | [39] |
| Mn3O4 | / | Phenol | 0.04 | 100 | 6.8 | SOP = 57.3% (10 min) COP = 59.8% (10 min) | [7] | |
| Fe2O3 | / | SOP = 57.3% (10 min) COP = 62.2% (10 min) | ||||||
| ZnO | / | SOP = 57.3% (10 min) COP = 68.3% (10 min) | ||||||
| MgO | 10.5 | SAP < 5% (10 min) SOP = 57.3% (10 min) COP = 80.1% (10 min) | ||||||
| α-MnO2 | 2.6 | 4-nitrophenol | 0.1 | 20 | 25 | 7.0 | SAP = 3% (90 min) SOP = 50% (45 min) COP = 99.3% (45 min) | [11] |
| β-MnO2 | 7.3 | SOP = 50% (45 min) COP = 86.4% (45 min) | ||||||
| γ-MnO2 | 3.7 | SOP = 50% (45 min) COP = 93.3% (45 min) | ||||||
| δ-MnO2 | 3.5 | SOP = 50% (45 min) COP = 96.6% (45 min) | ||||||
| ε-MnO2 | 4.1 | SOP = 50% (45 min) COP = 89.8% (45 min) | ||||||
| λ-MnO2 | 5.4 | SOP = 50% (45 min) COP = 90.0% (45 min) | ||||||
| MgO | 7.2 | Quinolone | 0.2 | 2 | 20 | 6.8 | SAP = 1% (15 min) SOP = 53.8% (15 min) COP = 90.7% (15 min) | [48] |
| Mn0.95Bi0.05Fe2O4 | / | DBP | 0.01 | 0.3 | 0.05 | 6.9 | SAP = 4% (60 min) SOP = 33% (60 min) COP = 69% (60 min) | [40] |
| NiFe2O4 | / | Phenol | 1 | 300 | 6.5 | SAP = 0% (60 min) SOP = 38.9% (60 min) COP = 55.2% (60 min) | [38] | |
| Goethite (α-FeOOH) | 7.2 | Oxalic acid | 15 | 1.1 | 0.9 | 7.0 | SAP = 10% (30 min) SOP = 22% (30 min) COP = 54% (30 min) | [46] |
Although Fe3O4 material shows good catalytic activity, its core can be easily damaged by the outer (oxidative) atmosphere [21]; hence, some researchers used silica to cover and protect the sensitive surface. Kermani et al. [55] used it with the addition of TiO2. TiO2 has shown good efficiency for the removal of organic compounds, especially when combined with ozone, and it is used most frequently in photocatalytic processes. The disadvantage of TiO2 is its difficulty to be subsequently separated from aqueous solutions, i.e., after its use [56], a factor, however, limited by the co-presence of Fe3O4. The catalytic activity of Fe3O4@SiO2@TiO2 solid was evaluated by considering the removal of catechol. The pH study showed that when the pH increased, this could lead to the improvement of catechol removal. The optimum pH was 8 because at this pH value, a synergistic effect between the adsorption and the ozonation processes can take place. The PZC value of the catalyst is 6.18, while the pKa of catechol is 9.45. Therefore, at pH 8, the catalyst is negatively charged, while the organic compound was positively charged, thus, favoring its adsorption [55].
3.2. Metals Deposited on Suitable Substrates
Bai et al. [23] used as substrate fibrous silica nanospheres (denoted as Fe-KCC-1), aiming to take advantage of the greater specific surface area of this material (SBET = 465 m2/g) due to the noticed improved thermal and hydrothermal stabilities. They deposited iron onto this structure because there are several studies showing that iron can present catalytic activity in ozonation systems. Although the adsorption rates of examined micropollutants do not seem to be very high in this case (<15%), they are still considered as satisfactory enough for the application of catalytic ozonation process. The increase of pH value resulted in an increase of micropollutant removal. The difference in the removal rates between the single and the catalytic ozonation processes was higher for the acidic than for the neutral pH values, while for the alkaline pH values the removal did not show any substantial difference as it was >95%. The PZC value of this solid catalyst was 4.30 and, therefore, it was almost neutrally charged at the examined acidic pH values. Nevertheless, over the entire pH range (3–11) this material presented sufficient catalytic activity, as the respective removal rates were higher than those obtained by the sum of single ozonation and adsorption sub-processes.
Alumina is the most frequently used metal oxide as a common substrate for the deposition of several metals, such as Cu, Mn, Fe, Ni, and Ti, to improve the catalytic activity of ozonation systems. Moreover, it can act as a catalyst without any preliminary modification procedure. Peng et al. [57] and Bing et al. [58] deposited onto its structure Ni and Ti, respectively, taking advantage of the larger surface area of this material in comparison with considering also the respective Lewis active sites. At pH 7 these hybrid materials showed the greater catalytic activity, both in terms of pollutants removal and of system mineralization. Although by increasing the pH value, more hydroxyl radicals will be produced, the important role of PZC value in the heterogeneous catalytic ozonation process was also observed during these studies. The ozonation systems present a limit at which the oxidation efficiency is the highest. The PZC values of Ni/Al2O3 and of γ-Ti/Al2O3 composite materials were near 8 and 7.3, respectively. In the first case, the adsorption did not particularly influence the catalytic activity and the optimum pH value was 8, at which the hydroxyl groups of the solid surface were neutrally charged. This charge promoted to a great extent the decomposition of ozone towards the production of hydroxyl radicals when compared to the respective protonated forms.
On the other hand, in the O3/γ-Ti/Al2O3 system applied to remove ibuprofen by combining the adsorption and the ozonation processes, the optimum pH value was 7. By increasing the pH value from 3 to 7, the efficiency of the process increased, since in this pH range the solid material/catalyst was positively charged; therefore, the ionized ibuprofen forms could be adsorbed on the surface of the catalyst due to electrostatic interactions. However, at even higher pH values, the adsorption process was not favored. This observation is important because the major oxidizing species in this system were the singlet oxygen and the superoxide radicals and not the (previously addressed) hydroxyl radicals. In contrast, Yan et al. [59], using the Cu-O-Mn/γ-Al2O3 material as catalyst, did not observe any decrease of removal rate for the examined micropollutants by increasing the pH value. Their probe organic compound was effectively removed both at pH 7, where the surface of catalyst was neutrally charged, as well as at pH 10. They have attributed this observation to the larger production of hydroxyl radicals occurring at the alkaline pH values. However, they did not consider the difference of efficiency between the catalytic and the single ozonation processes under the same pH value. Considering that, the catalytic ozonation process efficiency was higher at the pH value where the surface of the material was neutrally charged, i.e., at pH 7, since the removal of micropollutants at this pH was enhanced when compared to single ozonation.
Another catalyst used in the heterogeneous catalytic ozonation process is iron silicate (IS). This material was selected due to its higher density of surface hydroxyl groups and stable structure. Yuan et al. [60] deposited α-Fe2O3 onto this structure, whereas Liu et al. [61] deposited FeOOH. The adsorption capacity of these materials was very small; therefore, it did not present any significant contribution to the removal rate results. As a result, the removal of pollutants by applying these systems was based mainly on the adsorption of ozone onto the surface of these solid catalysts and the subsequent production of hydroxyl radicals.
Chen et al. [62] used the Fe-Cu/MCM-41 bimetallic material for the removal of oxalic acid and compared its efficiency with that of MCM-41, Fe/MCM-41, and Cu/MCM-41 materials. The addition of these metals to the MCM-41 structure can result in the reduction of solids’ specific surface areas and to the increase of PZC value from 2 to 6.1. The metals in the structure of these materials can enhance the degradation of examined organic compounds through their ability to change the oxidation states. The bimetallic mesoporous materials have been found to improve catalytic activity and to prevent nanoparticles’ aggregation. The MCM-41 material presents lack of catalytic activity, but provides an effective substrate for the deposition of proper metals. The acidity of the catalyst is directly related to the catalytic activity. The electron transfer is enhanced by the presence of these metals (Fe, Cu) in the structure of material. Up to the pH value 6.1, which was the PZC value of Fe-Cu/MCM-41 material, the adsorption rate was around 10%, while at even higher values the adsorption capacity of this solid decreased due to the development of negative surface charges. The removal efficiency of catalytic ozonation increased with the increase of pH value, but the highest catalytic action was presented at pH 4, which was the highest difference between the single and catalytic ozonation reported. However, it must be noted that the pH of the respective oxidation reactions was not kept stable throughout the process, as it was adjusted only at the beginning of experiments. MCM-41 material as substrate was also examined by Tang et al. [63] and Sui et al. [64], who used as catalysts Co-Mn/MCM-41 and MnOx-/MCM-41 materials, respectively. Tang et al. [63] found that the optimum pH value for the removal of DMP was 5.6, which was also the PZC of the used solid/catalyst. In contrast, Sui et al. [64] showed that the examined material exhibited catalytic activity at a pH value near 7; however, they did not perform any further study regarding the pH influence on the efficiency of catalytic ozonation.
Another catalyst used for the removal of chloronitrobenzene was the ZCSP (zinc-copper silicate polymer) [65]. The co-existence of silicon, zinc, and copper has increased the stability of the catalyst and, in addition, the presence of zinc-copper oxide contributed to the higher density of this material regarding the surface hydroxyl groups. Its adsorption capacity was about 10% after 15 min of contact time, and the presence of catalyst in the system almost doubled the removal of the examined pollutant, as compared to the application of single ozonation. The major oxidizing species in this case were the hydroxyl radicals, although several other species could be also produced and participate in this reaction.
Other silicon-containing material used for the removal of sulfamethoxazole is FMSO (Iron Manganese Silicate Oxide) [34]. In this study, it was also proved that when the catalyst is neutrally charged (in this case the PZC = 6.7), then the adsorption of ozone onto the catalyst surface is favored and the respective mass transfer at the aquatic solution is enhanced. At the pH value 7, the adsorption process of pollutant was not favored because the surface of the solid becomes neutrally charged, as only 1.8% of sulfamethoxazole has been adsorbed. However, since the major oxidizing species in this ozonation process were the hydroxyl radicals, whose formation was enhanced, the material presented catalytic activity, as the removal of pollutant increased by 69% when compared to the single ozonation and for the same pH value.
Liu et al. [66] used as catalyst a combination of iron and manganese oxides (MnxOy/γ-Fe2O3), which previously reported studies have shown can present catalytic activity. The use of this hybrid catalyst resulted in the increase of SMA removal, as compared to the application of single ozonation. However, the removal rate was already very high and, thus, the variations of pH values did not substantially influence it. Nevertheless, certain differences in the performance with respect to the solution pH were still observed regarding the total mineralization achieved by the process, which increased with the increase of pH value due to the increase of hydroxyl radicals’ production. β-MnO2 was examined as a substrate for the deposition of CuO by Ke et al. [50]. This catalyst was used to remove oxalic acid at pH 6. The Cu1MnT material presented a PZC value equal to 3.49 and, therefore, it was negatively charged at the examined experimental pH value. Its adsorption capacity was less than 2% due to the electrostatic repulsion between the negatively charged catalyst surface and the oxalate ions (C2O42−). The efficiency of single ozonation (3.2%) was similar to that of adsorption (very small), but the addition of this catalyst enhanced significantly the removal of oxalic acid (up to 87.5%) after 30 min of reaction/oxidation time. The addition of raw MnO2 can remove the pollutant by only 12.5%; therefore, the higher efficiency of dopped catalyst can be attributed to the co-presence of Cu. The good coordination potential and the multivalent properties of Cu can increase the active surface sites and promote electron transfer. Additionally, the pH study revealed that at pH 3, the material was neutrally charged, and this catalyst showed its higher catalytic activity when assisted by the (simultaneous) adsorption process. Furthermore, it showed higher catalytic performance also at pH around 6.
Other interesting materials used as substrate in the heterogeneous catalytic ozonation process are the Metal Organic Frameworks (MOFs). MOFs contain large numbers of electric-rich hydroxyl groups on their surface, as well as Lewis’s acid sites, which are coordinatively unsaturated, formed during the preparation/synthesis routes, and act as both adsorption and catalytic sites. Furthermore, the adsorption capacity of MOFs is higher than that of simple metal oxides due to their improved pore structures and larger surface areas [67]. Ye et al. [68] used three-dimensional Co/Ni bimetallic organic frameworks for the removal of atrazine at pH 7. The adsorption capacity of Ni/MOF, Co/MOF, and Co/Ni-MOF materials was 9.6%, 13.1%, and 9.9%, respectively. The prepared MOFs can also adsorb atrazine and achieve rapidly the respective equilibrium stage. Because atrazine is an oxidizing-resistant organic compound, it can only be removed by 47.8% during the application of single ozonation and after 10 min reaction time. However, the addition of these solids enhanced the removal of the examined pollutant, and the efficiency of catalytic process was 75.5%, 67%, and 93.9% with the O3/Ni/MOF, O3/Co/MOF, and O3/Co/Ni/MOF systems, respectively. As previously reported, the pH value of a solution impacts not only the surface charge of catalysts, but also the chemical form of the respective pollutant [69]. Ye et al. [68] found that the highest adsorption capacity presented by the bimetallic MOF at pH 7, and it was equal to 13.5% after 10 min contact time, whereas the highest rate of pollutant during the catalytic ozonation was found at pH 9 (95.7%). The pKa of atrazine is 1.64, therefore, it exists mainly in the non-protonated form for the pH range 3–9. At the lower pH values, the Zeta-Potential of this catalyst approached zero and the functional groups on the surface were close to neutral state, which was beneficial for the adsorption of atrazine. In addition, the adsorption was enhanced due to the promotion of the van-der-Waals interaction forces and to the hydrogen bonds between the surface oxygen-containing functional groups and the highly electronegative N atoms of triazine ring [70]. Although the adsorption capacity of this material was analyzed throughout the pH range, no results were reported for the single ozonation for the various pH values, and, therefore, it is not possible to define at which pH value the catalyst presents (comparatively) its highest catalytic activity. In this study, it is referred that “the improvement of atrazine removal especially marked in the pH range from 3.0 to 5.0”.

Table 2.
Metals deposited onto substrates and the composite materials used as catalysts during the heterogeneous catalytic ozonation process (at the pH range 6–8).
Table 2.
Metals deposited onto substrates and the composite materials used as catalysts during the heterogeneous catalytic ozonation process (at the pH range 6–8).
| Catalyst | PZC | Micropollutant | Ccat. (g/L) | CO3 (mg/L) | Cmicr. (mg/L) | pH | Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| β-MnO2 | / | Oxalic acid | 0.5 | 50 | 6 | SAP < 2% (30 min) SOP = 3.2% (30 min) COP = 12.5% (30 min) | [50] | |
| Cu1MnT | 3.49 | SAP < 2% (30 min) SOP = 3.2% (30 min) COP = 87.5% (30 min) | ||||||
| Fe3O4 | / | Acetaminophen | 0.08 | 25 | 7 | SAP < 10% (10 min) SOP = 38.8% (10 min) COP = 49% (10 min) | [71] | |
| Ce-UiO-66 | / | SAP < 10% (10 min) SOP = 38.8% (10 min) COP = 72.1% (10 min) | ||||||
| Fe3O4@Ce-UiO-66 | / | SAP < 10% (10 min) SOP = 38.8% (10 min) COP = 87.4% (10 min) | ||||||
| MnxOy/γ-Fe2O3 | 7.2 | Sulfamethazine | 0.3 | 6 | 20 | 7 | SAP < 2% (60min) SOP = 70% (10 min) COP = 95% (10 min) | [66] |
| Cu-O-Mn-γ-Al2O3 | 7.9 | Polyvinyl alcohol | 0.15 | 20 | 7 | SAP < 3% (10 min) SOP = 36% (10 min) COP = 73.7% (10 min) | [59] | |
| Cu/γ-Al2O3 | / | SOP = 36% (10 min) COP = 58.6% (10 min) | ||||||
| Mn/γ-Al2O3 | / | SOP = 36% (10 min) COP = 56.6% (10 min) | ||||||
| KCC-1 | ~4.0 | Sulfamethazine | 0.3 | 15 | 20 | 7 | SAP < 15% (15 min) SOP = 86% (10 min) COP = 90% (10 min) | [23] |
| Fe-KCC-1 | 4.3 | SAP < 15% SOP = 86% (10 min) COP = 96% (10 min) | ||||||
| Ni-MOF | / | Atrazine | 0.5 | 8 | 10 | 7 | SAP = 9.6% (20 min) SOP = 47.8% (20 min) COP = 75.5% (20 min) | [68] |
| Co-MOF | / | SAP = 13.1% (20 min) SOP = 47.8% (20 min) COP = 67% (20 min) | ||||||
| Co/Ni-MOF | / | SAP = 9.9% (20 min) SOP = 47.8% (20 min) COP = 93.9% (20 min) | ||||||
| Ni/Al2O3 (5.78% wt) | / | Succinic acid | 10 | 200 | 7 | SOP = 41.2% (90 min) COP = 90.4% (60 min) | [57] | |
| FeO3Si | / | p-chlorobenzene | 0.1 | 0.9 | 0.1 | 7 | SAP < 3.2% (30 min) SOP = 49.2% (30 min) COP = 82.8% (30 min) | [72] |
| γ-Al2O3 | / | Ibuprofen | 1.5 | 30 | 10 | 7 | SOP = 80% (10 min) COP = 85% (10 min) | [58] |
| TiO2/γ-Al2O3 | / | SOP = 80% (10 min) COP = 91% (10 min) | ||||||
| γ-Ti-Al2O3 | 5.9 | SOP = 80% (10 min) COP = 100% (10 min) | ||||||
| MCM-41 | 2.8 | Oxalic acid | 1 | 1.67 | 10 | 6 | SOP = 16.5% (60 min) COP = 15% (60 min) | [62] |
| Fe-MCM-41 | 5.1 | SOP = 16.5% (60 min) COP = 23.4% (60 min) | ||||||
| Cu-MCM-41 | 6.0 | SOP = 16.5% (60 min) COP = 69.7% (60 min) | ||||||
| Fe-Cu-MCM-41 | 6.1 | SOP = 16.5% (60 min) COP = 95% (60 min) | ||||||
| IS (Iron Silicate) | / | p-CNB | 0.5 | 0.6 | 0.1 | 7 | SAP = 4.0% (15 min) SOP = 56.7% (15 min) | [61] |
| IS-FeOOH | / | SAP = 3.3 (15 min) SOP = 56.7% (15 min) COP = 99.8% (15 min) | ||||||
| ZCSP | / | p-CNB | 0.3 | 0.6 | 0.1 | 7 | SAP = 10% (15 min) SOP = 45% (15 min) COP = 99.3% (15 min) | [65] |
| FMSO | / | Sulfamethoxazole | 1 | 9.05 | 25.3 | 7 | SAP = 1.8% (20 min) SOP = 73% (60 min) COP = 90.6% (60 min) | [34] |
| MCM-41 | / | DEHP | 1 | 83 | 10 | 7 | SAP = 9% (15 min) SOP = 87% (15 min) COP = 92% (15 min) | [63] |
| Co-MCM-41 | / | SAP = 10% (15 min) SOP = 87% (15 min) COP = 97% (15 min) | ||||||
| Mn-MCM-41 | / | SAP = 8% (15 min) SOP = 87% (15 min) COP = 94% (15 min) | ||||||
| Co-Mn-MCM-41 | 4.9 | SAP = 4% (15 min) SOP = 87% (15 min) COP = 99.7% (15 min) | ||||||
| MCM-41 | 2.5 | Nitrobenzene | 1 | 0.98 | 0.12 | 6.91 | SAP = 38.8% (10 min) SOP = 20.1% (10 min) COP = 43.9% (10 min) | [64] |
| MnOx/MCM-41 (1.05%wt Mn) | 2.8 | SAP = 36.7% (10 min) SOP = 20.1% (10 min) COP = 88.9% (10 min) |
Mohebali et al. [71] used a specific MOF of UiO-66-architecture as the substrate and then synthesized a magnetic one to be easily separable from the solution and doped it with Ce, producing the Fe3O4@Ce-UiO-66 nanocomposite. Their target compound was acetaminophen, 38.8% of which was degraded by the application of single ozonation. Τhe addition of Fe3O4, Ce-UiO-66 and Fe3O4@Ce-UiO-66 materials enhanced the degradation of the examined micropollutant and removal rates of 49%, 72.1%, and 87.4%, obtained respectively (at pH 7), noting also that the adsorption capacity of all examined solids was <10%. Additionally, the pH study showed that the highest rate of Fe3O4@Ce-UiO-66 catalytic activity presented at the neutral pH values. However, the PZC of this solid was not determined and, therefore, the effect of surface charge on its catalytic activity cannot be evaluated.
3.3. Minerals
Among the major materials in this category that are frequently used as catalysts in the heterogeneous catalytic ozonation process are the zeolites. Several types of them have been reported so far in the relevant literature. There are studies showing that the zeolites do not actually enhance the production of hydroxyl radicals; in this case the removal of pollutants is based on the oxidation reactions with molecular ozone [10,73]. In the case that this material can act as catalyst to improve the removal of a pollutant, such as zeolite A [10], the oxidation efficiency has been maximized at pH 7 due to the enhancement of contact between ozone and pollutant, i.e., between PZC = 6.4 < pH < pKa = 9.38. Nevertheless, when the ZSM-5 material was used as catalyst, whereby the removal of ibuprofen was based upon the oxidation reaction by ozone and the adsorption capacity of this material was negligible, then the optimum treatment pH value was 3. At this pH value, ozone is more stable, and it does not decompose in great extent towards the formation of hydroxyl radicals. Another type of zeolite used in the catalytic ozonation process is the synthetic clinoptilolite, applied in the form of nano-size particles for the removal of nalidixic acid [74]. Its efficiency was compared with that of natural clinoptilolite [75], which was found to be less reactive than the nano-clinoptilolite due to different structure. The reduction of particle sizes (leading to nanostructures) can result in the increase of the specific surface area of this material and, consequently, to higher adsorption and ozone decomposition rates. The adsorption capacity of both materials for this micropollutant is very low; therefore, the pollutant degradation was based on the oxidation by hydroxyl radicals and the removal rate increased by increasing the respective pH value. The material showed higher catalytic activity at pH 7, which can further be increased with the increase of pH value up to 9. However, in this study, there is no comparison between the single and the catalytic ozonation processes for the various examined pH values and, hence, it was not possible to determine the respective highest catalytic activity.
Pumice has also been used as catalyst in several studies, either as raw material or after its preliminary modification. The respective PZC of this material was between 6 and 7 for all examined cases [76,77,78]. In these studies, the pumice showed the greatest catalytic activity also at pH values around 7, where it was almost neutrally charged, thus favoring the adsorption of ozone onto its surface and, therefore, the higher production of hydroxyl radicals. In these studies, the adsorption capacity of catalysts was very low, and the adsorption process did not contribute to the overall removal of organic pollutant.
Zhao et al. [79] used cordierite for the removal of nitrobenzene, and copper was deposited onto its surface to enhance its catalytic action. The addition of copper to the cordierite structure increased the adsorption capacity of the resulting solid only by 2.5%, i.e., it was rather negligible for the modified material. Nitrobenzene is an organic compound that cannot be practically removed by oxidation/reaction with molecular ozone [80]. The addition of Cu-cordierite to the ozonation system increased the removal efficiency, as this material presents a larger surface area and higher PZC and surface density of hydroxyl groups. The PZC of this solid was 6.94, and its highest catalytic activity presented in the pH value 7, at which it was neutrally charged.
In addition, ceramic honeycomb was used to remove nitrobenzene after depositing Cu and Mn metals onto its surface to enhance catalytic activity [81]. Although ceramic honeycomb exhibits catalytic activity for the degradation of organic compounds in aqueous solutions, the high ozone consumption and the low treatment efficiency are considered as the two main disadvantages for practical applications. To overcome these problems, ceramic honeycomb should be suitably modified. The adsorption capacity of this catalyst was low (2%), but with proper metal deposition, it can be further decreased. The presence of metals on the surface of substrates can generally increase in great extent the density of surface hydroxyl groups, which is directly related to the production of hydroxyl radicals and, subsequently, to the overall efficiency of the ozonation system. Similar results were also observed in the study of Hou et al. [82], who used ceramic honeycomb as a substrate for the deposition of Mn/Fe/K metals.

Table 3.
Minerals used as catalysts in the heterogeneous catalytic ozonation process at the pH range 6–8.
Table 3.
Minerals used as catalysts in the heterogeneous catalytic ozonation process at the pH range 6–8.
| Catalyst | PZC | Micropollutant | Ccat. (g/L) | CO3 (mg/L) | Cmicr. (mg/L) | pH | Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ceramic honeycomb | / | 4-Meq | cylinders | 1.1 | 50 | 6.8 | SAP < 3% (30 min) SOP = 54.9% (30 min) COP = 59.6% (30 min) | [83] |
| Fluorinated ceramic honeycomb | / | SAP < 3% (30 min) SOP = 54.9% (30 min) COP = 77.8% (30 min) | ||||||
| Zeolite A | 6.4 | Paracetamol | 1 | 0.98 | 0.12 | 6.9 | SAP = 4.78% (60 min) SOP = 76.8% (60 min) COP = 90.7% (60 min) | [10] |
| FSO/PMC | 7.2 | Diclofenac | 0.8 | 5.52 | 29.6 | 7.0 | SAP = 7.3% (20 min) SOP = 100% (8 min) COP = 100% (6 min) | [77] |
| Clinoptilolite | / | Nalidixic acid | 6 | 20 | 7.0 | SAP = 10.8% (60 min) SOP = 43% (60 min) COP = 73.8% (60 min) | [74] | |
| Natural clinoptilolite | / | Nalidixic acid | 2 | 20 | 7.0 | SAP = 12% (60 min) SOP = 43% (60 min) COP = 60% (60 min) | [75] | |
| Nano-clinoptilolite | / | SAP = 20% (60 min) SOP = 43% (60 min) COP = 91.1% (60 min) | ||||||
| Pumice | 6.1 | p-CNB | 0.5 | 0.9 | 0.1 | 6.0 | SAP < 5.5% (15 min) SOP = 40.8% (15 min) COP = 73% (15 min) | [78] |
| Fe/Pumice (6.1% wt) | 6.4 | SAP < 5.5% (15 min) SOP = 40.8% (15 min) COP = 9038% (15 min) | ||||||
| Z25H (SiO2/Al2O3 = 25) | 5.0 | Ibuprofen | 15 | 6.67 | 20 | 7.2 | SAP = 10% (30 min) SOP = 42% (30 min) COP = 78% (30 min) | [73] |
| Z900Na (SiO2/Al2O3 = 900) | 9.2 | SAP = 5% (30 min) SOP = 42% (30 min) COP = 68% (30 min) | ||||||
| Z25Na (SiO2/Al2O3 = 25) | 9.3 | SAP = 10% (30 min) SOP = 42% (30 min) COP = 70% (30 min) | ||||||
| Pumice | / | p-CNB | 1 | 0.6 | 0.1 | 6.9 | SAP = 3.9% (20 min) SOP = 52% (20 min) COP = 88% (20 min) | [76] |
| Perovskite (Co) | / | Diclofenac | 0.1 | 20 | 30 | 7.0 | SOP = 42% (2 min) COP = 40% (2 min) | [84] |
| Perovskite (Cu) | / | SOP = 42% (2 min) COP = 49% (2 min) | ||||||
| Cordierite | 6.6 | Nitrobenzene | 5 pieces | 1.0 | 0.05 | 6.9 | SAP = 2.0% (20 min) SOP = 31% (20 min) COP = 50% (20 min) | [79] |
| Cu-cordierite | 6.8 | SAP = 2.5% (20 min) SOP = 31% (20 min) COP = 77.9% (20 min) | ||||||
| Ceramic honeycomb | / | Nitrobenzene | 58.3 | 1.0 | 0.05 | 6.87 | SAP = 2% (10 min) SOP = 37% (10 min) COP = 58% (10 min) | [81] |
| Modified ceramic honeycomb (1% wt Mn– 0.5% wt Cu) | / | SAP = 1% (10 min) SOP = 37% (10 min) COP = 83% (10 min) | ||||||
| Ceramic honeycomb | / | Benzophenone | 10 | 6.9 | SOP = 48.3% (120 min) COP = 68.8% (120 min) | [82] | ||
| Mn-Fe-K-ceramic honeycomb | / | SOP = 48.3% (120 min) COP = 81.4% (120 min) |
However, the metal-loaded catalysts may present the risk of subsequent heavy metal pollution of treated waters (after their respective leaching), as well as the relatively easier catalyst deactivation due to the loss of metal components during ozonation. A strategy to eliminate this possibility is the development of metal-free catalysts. Pan et al. [83] synthesized a fluorinated ceramic honeycomb for the removal of 4-methylquinoline (4-Meq), and its catalytic activity was compared to the raw one. The adsorption capacity of both solid materials was less than 3%. The 4-Meq was removed by 54.9% by the application of single ozonation, whereas in the presence of honeycomb, the removal reached 59.6%. The addition of fluorinated honeycomb can further enhance the removal of this organic pollutant, and the process efficiency was increased up to 77.8%, which was 1.4 times higher than that of single ozonation. The scientists reported that the active sites, resulting from the fluorination procedure are the main factors for improvement of the catalytic activity of honeycomb rather than the contribution of the surface area or of the adsorption process. Fluorinated honeycomb can enhance the production of hydroxyl radicals as the major oxidizing species. However, also in this study, the surface charge of examined solid materials was not reported and, hence, its influence on catalytic activity cannot be evaluated.
3.4. Carbons
Carbons widely appear in the relevant literature as catalysts for the heterogenous catalytic ozonation process. Although they are mostly used as substrates for the deposition of metals or metal oxides, they can also be applied as catalysts due to their higher specific surface area and improved adsorption capacity against most organic micropollutants. However, in several cases, it has been proved that their specific structural and chemical surface properties can also affect the ozonation process [28]. Table 4 includes the relevant literature survey of different carbons that have been applied as catalysts in the catalytic ozonation process for the removal of micropollutants and in the pH range 6–8.
The multi-walled carbon nanotubes (MWCNTs) have been used quite often recently in several studies because they present better results than common activated carbons. MWCNTs contain more mesopores, which favor the diffusion of reagents in the catalyst surface from the aqueous solutions, than micropores. The main disadvantage of this catalyst is its difficult separation from the solution after treatment. However, the addition/deposition of magnetic Fe3O4 can facilitate the separation. Magnetic Fe3O4 also contains the Fe2+/Fe3+ pair in its octahedral structure, which can further facilitate electron transfer and hydroxyl radical generation [85,86,87].
Bai et al. [85], Huang et al. [86], and Bai et al. [87] used Fe3O4/MWCNTs material as catalyst for the removal of SMA, BPA, and phthalic acid, respectively; however, these studies showed conflicting results. In all three studies, the catalyst presented moderate adsorption capacity (about 20%). Bai et al. [85] observed that at pH 4 and 4.7, the solid material presented its highest catalytic activity. This was attributed to the correlation between the PZC of this material (equal to 3.2) and the pKa of the examined organic compounds, which was higher than 5. Therefore, the contacts between the chemicals/agents could be favored by the opposite charge. In contrast, Huang et al. [86] claimed that the decomposition of ozone is enhanced by the bonding of surface oxygen groups of the catalyst surface with BPA molecules via the creation of hydrogen bonds, resulting in increased removal efficiency. Through this mechanism, the removal of micropollutants increased with the increase of pH value. However, the influence of the pH value during adsorption or single ozonation processes was not evaluated.
Graphene oxide has also been used in several studies for the removal of micropollutants by the application of catalytic ozonation, applied usually as substrate for the deposition of metal oxides onto its structure [24,88,89]. The graphene oxide was examined mainly due to its good electrical and mechanical properties. However, when used as raw material, it did not present any catalytic activity, meaning that either graphene oxide does not enhance the production of hydroxyl radicals or it acts as an inhibitor, presenting a higher reaction rate constant with the hydroxyl radicals and, hence, scavenging them. The addition of metals to the graphene structure seems to increase substantially its catalytic activity, as well as the removal of micropollutants, by following a radical mechanism, in which the contribution of the adsorption process was almost negligible (in the relevant studies that the contribution of adsorption was evaluated). The main disadvantage of most studies in this case was the absence of the catalyst stability evaluation [24,88]. Therefore, the stability of carbon-based catalysts under the oxidizing conditions of ozone is (at least) questionable.
Liu et al. [90] used several oxides, such as MnOx, FeOx, CrOx, CoOx, NiOx, and ZnO, deposited onto graphite tο remove diethyl phthalate (DEP); among them, ZnO was found to be the most effective. During the single ozonation process, the increase of pH value also entailed the increase of micropollutant removal; however, in the case of catalytic ozonation, this did not occur. Higher removal rates were observed at pH 5.8, while removal was slightly improved when the pH value increased up to 9. The increase of pH value did not influence the structure of DEP, as it is not an ionizable compound, but it did strongly influence the charge of the catalyst surface. The PZC of this solid was 7, thus at pH 5.8 it was positively charged. The highest catalytic activity was presented at pH 5.8; however, the process efficiency was not evaluated at pH 7, at which the catalyst was neutrally charged. DEP removal was based on the oxidative reactions in the bulk solution, while the catalyst showed moderate adsorption capacity (about 12%).
Lu et al. [91] used FMSACs materials (ferromagnetic sludge-based activated carbons) to remove p-CBA. The presence of iron in the structure of this activated carbon was found to enhance the catalytic activity and the adsorption ability (with optimal content 2.3% wt.), while the presence of higher amounts of Fe on its structure reduced the removal of p-CBA. There are two possible reasons for this observation: (1) the overloading of material with iron can led to the decrease of catalyst surface area and (2) the excess of iron oxide on the surface of the catalyst can probably reduce the density of active sites by distorting its original structure. The process in this case has followed the radical mechanism, and the catalyst, due to its magnetic nature, can be easily separated from the solution subsequently after the performed treatment.
The decomposition of ozone onto the AC surface depends on the structural and chemical properties of carbons. Faria et al. [25] studied two different types of carbons (i.e., AC and ACHNO3) to evaluate the influence of their properties for the efficiency of heterogeneous catalytic ozonation. The carbons have similar specific surface areas, but different PZC values. The PZC of these solids was 8.5 and 3, respectively. At the alkaline pH values, the removal of aniline was reduced as the CO2 transforms to CO32− and HCO3−, which are well known radical inhibitors’ agents (scavengers), in the aqueous solution. Therefore, although the ozone decomposition was higher at pH 9, this was not reflected in the higher removal of aniline, and, thus, the removals at pH values 7 and 9 were similar. The AC presented higher catalytic activity because it had higher adsorption capacity as compared to the ACHNO3 for the neutral pH values, as well as higher ability to decompose ozone and to create hydroxyl radicals.
Huang et al. [92] also studied activated carbon as catalyst for the heterogeneous catalytic ozonation with iron deposited on its structure. The carbon, except of catalytic action, also presented a quite large adsorption capacity (26%). The synergistic effect between the adsorption and the oxidation processes increased the removal rates during the application of catalytic ozonation. The deposition of metals onto the carbon surface further increased the process efficiency as the respective surface-active sites increased, although its adsorption capacity decreased. Therefore, in this case, the high removal rates were mainly due to the enhanced production of hydroxyl radicals. The PZC of this solid was 8.5, and the optimum pH of the process was 8, while the further increase of pH decreased the process efficiency. At the pH value 8, the surface was almost entirely neutrally charged, and it was more active at this point. However, the stability of the solid material was not evaluated.
Dadban Shahamat et al. [93] and Farzadkia et al. [94] also used activated carbons as catalysts, depositing Fe3O4 onto their surfaces. The resulting magnetic properties made the subsequent separation from the bulk solution after treatment easier. The addition of Fe3O4 to the structure of AC resulted in the reduction of the specific surface area, pore volume and PZC value, but this did not influence its catalytic action. The PZC of these materials was 7.7. In the first study, the optimum pH was 6 because the micropollutant had pKa equal to 4.11, while in the second study, the optimum pH was 8 because the examined phenol was an alkaline compound with pKa equal to 9.9. However, in the study of Farzadkia et al. [94], there was a lack of adsorption and stability experiments; hence, it did not prove whether the material can be used as catalyst in the ozonation process.
Activated carbon as substrate for the deposition of metal oxides was also examined by Liu et al. [8]. They evaluated the catalytic activity of AC, FeyOz/AC, and MnxFeyOz/AC for the removal of sulfamerazine (SMZ). The performance of the catalytic systems was higher when compared to the application of single ozonation and adsorption as separate processes. The best catalyst was the MnxFeyOz-10 material, where SMZ removal reached 90.5% within 8 min of reaction time. The adsorption capacity of this catalyst was 14.3%, and the performance of single ozonation was 64.8%. Furthermore, they observed that the solution pH could greatly influence the catalytic ozonation process because it affects the surface properties of the catalyst, the properties of the examined organic compound, and the decomposition of ozone, as other researchers have also previously reported [68]. In the research of Liu et al. [8], the optimum pH was 10 due to: (1) the higher number of hydroxyl radicals produced at this pH value; (2) the reaction of ozone with the active sites of the catalyst surface due to its positive charge (PZC = 5.27); and (3) the hydrolysis of SMZ (with pKa = 2.29) at pH values higher than the pKa value. The SMZ molecule interacted with the catalyst by forming the SMZ-MnxFeyOz/AC complex, and the oxidizing species subsequently degraded the adsorbed SMZ. However, in this research, the rate of single ozonation for different pH values was not evaluated and, for this reason, the mentioned highest catalytic activity at pH 10 can be disputed. As previously reported, for a system to be characterized as catalytic, it should present greater removal of pollutants than the sum of adsorption and of single ozonation processes’ efficiencies [17]. At pH 10, the self-decomposition of ozone is accelerated in large extent and, thus, even the single ozonation process presents high efficiency.
Tian et al. [95] examined various types of carbons (i.e., AC, MWCNTs, coconut biochar, coal dust aggregates) for the removal of 1.4-dioxane. Coconut biochar and the coal dust aggregates presented the highest adsorption capacity, but all of the studied materials showed good catalytic action. However, due to the higher cost of MWCNTs, this catalyst was not used for further studies. On the contrary, although activated carbon is considered a low-cost material, it was rather unstable in the oxidizing conditions of ozone because its adsorption capacity and its catalytic activity were reduced.
Biochar was also used for the removal of atrazine by Tian et al. [96]. The catalytic activity of this solid material was evaluated after depositing Mn or Fe metals onto its surface. The researchers found that the catalytic activity of raw biochar was not intensive, as by its presence, the removal of atrazine reached 58.6%, while the rates of adsorption and of single ozonation were 6.5% and 48%, respectively, after 30 min reaction time. The addition of modified biochar enhanced the micropollutants’ removal, and the process efficiency was 83% and even up to 100% due to the presence of MnOx/biochar and FeOx/biochar, respectively. The adsorption capacity of both catalysts was less than 10%. The catalytic activity of biochar was enhanced by the increase of Lewis acidic sites and by the improved electron transfer caused by the presence of transition metal ions. However, the biochar was not stable under the oxidizing conditions created by the presence of ozone.
Another interesting carbon material is graphite carbon nitride (g-C3N4), which has shown increased popularity, especially in photocatalytic reaction studies. It has also been applied in the heterogeneous catalytic ozonation process due to improved chemical stability, good intrinsic electron mobility, relatively lower cost, and easier availability [97]. Furthermore, it can facilitate the uniform dispersion of active components and provide more effective sites, acting as a stable catalyst support, because of its graphene-similar structure [98]. Therefore, several researchers have combined it with metal oxides to improve its catalytic activity. Liu et al. [99] used modified (by copper oxide) g-C3N4 for the removal of oxalic acid. The removal efficiencies of oxalic acid were 2%, 56%, and 91% for single ozonation, O3/CuO, and O3/CuO-gC3O4 systems, respectively, after 15 min reaction time at pH 6. The relevant pH study showed that the catalytic ozonation removal efficiency was almost stable and about 97% in a pH range of 4–9. The PZC of these materials was determined to be 4.86; therefore, its surface hydroxyl groups were mostly in the deprotonated form when pH > 4.86. Zhao et al. [81] reported that the negative-charged surface had a strong reactivity towards ozone to generate more hydroxyl radicals, promoting the degradation of oxalic acid. When the pH value increased further, the decomposition of ozone accelerated, and more radicals were produced. However, this explanation is in contrast with most of the presented research in the current review. Adsorption experiments have not been conducted on this research, thus there is a possibility that the catalytic ozonation efficiency is a result (at least partly) of the adsorption process rather than oxidation.

Table 4.
Carbons used as catalysts in the heterogeneous catalytic ozonation process at the pH range 6–8.
Table 4.
Carbons used as catalysts in the heterogeneous catalytic ozonation process at the pH range 6–8.
| Catalyst | PZC | Micropollutant | Ccat. (g/L) | CO3 (mg/L) | Cmicr. (mg/L) | pH | Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| AC | / | Sulfamerazine | 0.05 | 10 | 6.1 | SAP = 5.5% (8 min) SOP = 64.8% (8 min) COP = 75.8% (8 min) | [8] | |
| FeyOz/AC | / | SAP = 0.9% (8 min) SOP = 64.8% (8 min) COP = 81.2% (8 min) | ||||||
| MnxFeyOz/AC | 5.26 | SAP = 14.3% (8 min) SOP = 64.8% (8 min) COP = 90.5% (8 min) | ||||||
| Biochar | / | Atrazine | 0.02 | 25 | 1 | 7.0 | SAP = 6.5% (30 min) SOP = 48% (30 min) COP = 58% (30 min) | [96] |
| MnOx/biochar | / | SAP < 10% (30 min) SOP = 48% (30 min) COP = 83% (30 min) | ||||||
| FeOx/biochar | / | SAP < 10% (30 min) SOP = 48% (30 min) COP = 100% (30 min) | ||||||
| CuO | / | Oxalic acid | 0.05 | 50 | 6.0 | SOP = 2% (15 min) COP = 56% (15 min) | [99] | |
| CuO-g-C3N4 | 4.86 | 0.5 | SOP = 2% (15 min) COP = 91% (15 min) | |||||
| g-C3N4 | 4.95 | Atrazine | 0.5 | 50 | 2 | 6.0 | SAP < 5% (5 min) SOP = 63.15% (5 min) COP = 56.83% (5 min) | [60] |
| O@g-C3N4 | 4.39 | SAP < 5% (5 min) SOP = 63.15% (5 min) COP = 92.91% (5 min) | ||||||
| Fe3O4/MWCNTs | 3.2 | Sulfamethazine | 0.5 | 50 | 20 | 7.0 | SAP = 6% (6 min) SOP = 90% (6 min) COP = 90% (6 min) | [85] |
| Graphite | 4.5 | Diethyl phthalate | 0.1 | 0.67 | 6.2 | SAP = 12% (60 min) SOP = 50% (10 min) COP = 56% (10 min) | [90] | |
| Zn-Graphite (3.5% wt) | 7.0 | SOP = 50% (10 min) COP = 94% (10 min) | ||||||
| Zn-CNTs | / | 4-chloro-3- methyl-phenol | 2 | 50 | 50 | 6.0 | SAP = 26.75% (60 min) SOP = 90% (10 min) COP = 92% (10 min) | [26] |
| GO | / | p-CBA | 0.03 | 4 | 0.078 | 7 | SAP = ng SOP = 20% (5 min) COP = 40% (5 min) | [24] |
| GO/TiO2 | / | SAP = ng SOP = 20% (5 min) COP = 76% (5 min) | ||||||
| GO/Fe3O4 | / | SAP = ng SOP = 20% (5 min) COP = 80% (5 min) | ||||||
| GO/TiO2/Fe3O4 | / | SAP = ng SOP = 20% (5 min) COP = 95% (5 min) | ||||||
| FMSACs (2.3% wt) | / | p-CBA | 0.04 | 1 | 20 | 6.0 | SAP = 10% (40 min) SOP = 42% (40 min) COP = 80% (40 min) | [91] |
| rGO-MnFe2O4 | / | DBP | 0.01 | 1 | 0.5 | 7.0 | SAP = 30% (60 min) SOP = 32% (60 min) COP = 85% (60 min) | [88] |
| nOG (non-oxidized) | / | p-CBA | 0.025 | 1 | 0.16 | 7.0 | SOP = 15% (5 min) COP = 42% (5 min) | [100] |
| Fe3O4 | / | BPA | 0.5 | 3 | 50 | 7.0 | SAP = 1% (40 min) SOP = 50% (40 min) COP = 77% (40 min) | [86] |
| MWCNTs | / | SAP = 42% (40 min) SOP = 50% (40 min) COP = 90% (40 min) | ||||||
| Fe3O4/MWCNTs | / | SAP = 40% (40 min) SOP = 50% (40 min) COP = 91% (40 min) | ||||||
| Fe3O4-MWCNTs | 3.2 | DEHP | 0.3 | 6 | 20 | 6.8 | SAP = 40% (5 min) SOP = 70% (30 min) COP = 96% (30 min) | [87] |
| AC | / | 1.4-dioxane | 0.4 | 27 | 50 | 7.0 | SAP = 4.2% (30 min) SOP = 9.2% (30 min) COP = 82.8% (30 min) | [95] |
| MWCNTs | / | SAP = 1% (30 min) SOP = 9.2% (30 min) COP = 45% (30 min) | ||||||
| Biocarbon (coconut) | / | SAP = 21% (30 min) SOP = 9.2% (30 min) COP = 42% (30 min) | ||||||
| Coal dust aggregates | / | SAP = 14% (30 min) SOP = 9.2% (30 min) COP = 27% (30 min) | ||||||
| Fe3O4/AC | 7.7 | 2.4-dinitrophenol | 2 | 167 | 500 | 6.0 | SAP = 7% (15 min) SOP = 51% (15 min) COP = 86% (15 min) | [93] |
| AC | / | DBP | 0.01 | 0.15 | 2 | 6.0 | SAP = 26% (60 min) SOP = 38% (60 min) COP = 58% (60 min) | [92] |
| Fe/AC (15% wt) | / | SAP = 13% (60 min) SOP = 38% (60 min) COP = 63% (60 min) | ||||||
| Fe3O4/AC | 7.7 | Phenol | 0.5 | 167 | 100 | 6.0 | SOP = 51% (5 min) COP = 79.9% (5 min) | [94] |
| Norit GAC | 8.5 | Aniline | 0.5 | 50 | 102 | 7.0 | SAP = 50% (60 min) SOP = 100% (20 min) COP = 100% (15 min) | [25] |
| ACHNO3 | 3 | SAP = 35% (60 min) SOP = (100% (20 min) COP = 100% (15 min) |
Yuan et al. [60] also used graphitic carbon nitride, modified with oxygen (O@g-C3N4), as an eco-friendly catalyst for the removal of atrazine. The PZC of this material was 4.39, i.e., lower than that of simple g-C3N4 (with PZC = 4.95), meaning that the surface properties were also modified and the electron density was re-arranged. As in the previous research, the catalyst showed high removal efficiency over a wide pH range (3–9). The presence of O@g-C3N4 promoted the degradation of atrazine at these pH values, and more than 75% degraded within only 3 min of reaction time. The difference with the previous research is based on two factors. Firstly, in this study, the ozonation efficiency was not stable for the pH range 3–9, and it increased with the increase of pH value. Therefore, the catalyst showed its highest catalytic action at the acidic pH values for which it was neutrally charged. Secondly, the adsorption capacity of the catalyst was defined, and it was less than 4% under all of the examined pH conditions. Therefore, atrazine was mainly subjected to degradation during this process rather than adsorption. Moreover, by using this catalyst, H2O2 could be produced during ozonation, which may have led (synergistic) to the production of more hydroxyl radicals. The catalyst was stable within 50 min of the tested reaction time, which is not considered a reliable timeframe to assess the stability of this material.
One of our recent studies [32] showed that a neutral-charged catalyst presenting moderate/high wettability and moderate adsorption capacity against the examined micropollutants can improve the contact of ozone with the catalyst surface and the subsequent production of hydroxyl radicals. However, the adsorption capacity, although it appears to improve the overall process efficiency, does not present any specific correlation with the catalytic activity, and it is not considered to be a particularly essential factor for this case.
In this review, various carbon-based materials from all of the aforementioned categories have been presented. However, the AC and the MWCNTs materials proved to be rather unstable under the oxidizing conditions of ozone. Most of the reviewed studies showed that ozone decomposition depends on surface hydroxyl groups. Indeed, Wang et al. [101] showed that there is a linear correlation between the PZC and the quantity of hydroxyl groups in the surface of materials, supporting the role of surface charge during the application of the catalytic ozonation process.
The ozone molecule, due to its structure, presents both nucleophilic and electrophilic properties. The (electrophilic) hydrogen atoms and the (nucleophilic) oxygen atoms of hydroxyl groups can simultaneously react with the ozone molecules by forming a ring when the surfaces of the hydroxyl groups of solids are neutrally charged. This ring is very unstable, and it can be easily decomposed towards the formation of superoxide radicals, resulting in the enhancement of their production. When the hydroxyl groups are positively or negatively charged, the interaction with ozone molecules is reduced and mass transfer is hindered [34]. This was also proved in one of our previous studies [32], where the production of hydroxyl radicals in the absence of micropollutants at pH values 7 and 8 was measured. As Figure 5 shows, the production of hydroxyl radicals was higher when the solid material was almost neutrally charged.
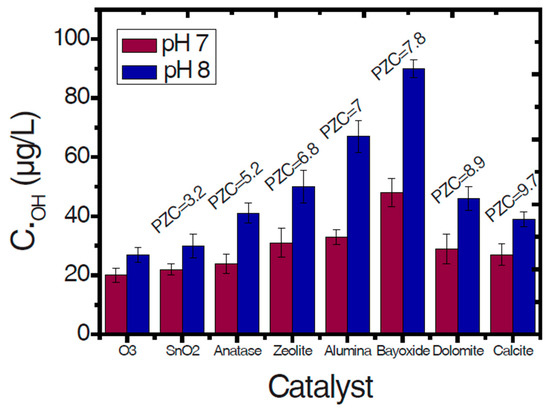
Figure 5.
Production of hydroxyl radicals with the use of several solid materials as catalysts, presenting different PZC values at pH values 7 and 8.
4. Factor 2: The Initial Micropollutants’ Concentrations
Only in a few published studies can the initial concentration of examined micropollutants be compared with real/natural conditions. Micropollutants have received this name due to their low concentrations (in the range of ng/L up to several μg/L) commonly occurring in nature [102]. However, in most relevant studies, the examined concentrations are substantially higher (to be easier analyzed); thus, they cannot be considered micropollutants anymore. Furthermore, when the initial concentrations of studied micropollutants are too high, even when 95% removal rate can be obtained by the application of a treatment process, their residual concentrations would still be higher than that existing in real conditions in natural waters. For example, Tang et al. [63] used Co-Mn/MCM-41 material as catalyst for the removal of dimethyl phthalate (DMP) with an initial concentration of 10 mg/L. After 15 min of the process, the removal of the organic compound reached 99.7%; however, the residual concentration of this pollutant in the bulk solution was still 30 μg/L, which could be the initial concentration of several micropollutants under real conditions.
Table 5 shows the materials that present catalytic activity for the pH range 6–8, where the initial concentration of examined micropollutants was not higher than 1 mg/L. The recorded efficiencies are not referred to as % removal, as in the previous Tables, but as the residual concentrations of organic pollutants (in μg/L).
In the study of Yuan et al. [72], the presence of α-Fe3O4 catalytic (enhanced) ozonation process removed p-chlorobenzene by 62%, i.e., after 30 min reaction time, the concentration of this micropollutant in the bulk solution was still 38 μg/L. Mn0.95Bi0.05Fe2O4 material was used for the removal of DBP by Ren et al. [40]. Τhe initial concentration of DBP in this case was 0.05 mg/L, while after 60 min oxidation/reaction time, its concentration was still 15.5 μg/L (i.e., 69% removal). Another catalyst used for DBP removal was Mn/ZnFe2O4 [42]. In this case, the initial concentration of micropollutant was 0.5 mg/L, whereas the residual concentration was 41.5 μg/L, i.e., 91.7% removal after 30 min reaction time. The use of goethite to remove oxalic acid resulted in one of the highest residual concentrations (414 μg/L), even when compared to its initial concentration (900 μg/L) [46]. Oxalic acid is a pollutant that usually presents higher removal rates at acidic pH values, where it can react directly with ozone molecules. The reaction rate constant of oxalic acid with hydroxyl radicals is 4.7 × 107 M−1s−1, which is significantly lower when compared to that of other micropollutants [103]. The highest efficiency was showed when using Fe0.9Mn0.1OOH as catalyst, as examined by Yan et al. [41] for the removal of iohexol. Its removal during heterogeneous catalytic ozonation reached 95%, which means its residual concentration was 50 μg/L after 20 min of this oxidation process.
Yuan et al. [72] deposited α-Fe3O4 material onto IS structure and the removal of p-chloronitrobenzene was enhanced, reaching 82.8%, i.e., 17.2 μg/L residual concentration after 30 min application of the oxidation process. Liu et al. [61] also used iron silicate to remove p-chlorobenzene following the deposition of FeOOH upon its structure. The use of this material further improved the removal of the examined micropollutant and shortened the reaction time, as the residual concentration after 15 min was 0.2 μg/L. The p-chlorobenzene was also used as a probe compound by Liu et al. [65] by the addition of ZCSP as catalyst. This catalyst also greatly enhanced the removal of micropollutants up to 99.3% within 15 min (leaving, however, 0.7 μg/L as residual concentration). Another material that has been used for the removal of nitrobenzene by the application of heterogeneous catalytic ozonation was MnOx/MCM-41 [64]. This material presented high adsorption capacity, enhancing the catalytic effect. In this case, the residual concentration of micropollutant in the bulk solution was 13.4 μg/L.
Various materials belong to the category of minerals and present catalytic activity for the selected pH range (6–8), while the initial concentration of examined micropollutants was ≤1 mg/L. In most cases, the minerals were used as substrates for the deposition of proper metals onto their surface structure. Pumice was used as a raw material in one of these studies [76], showing a catalytic effect for the enhanced removal of p-chlorobenzene. The oxidation efficiency 88% was reached, i.e., the residual concentration of this pollutant was 12 μg/L after 20 min of reaction time. When pumice was used as a substrate for the deposition of iron onto its structure [78], the catalytic action was enhanced and the removal of p-chlorobenzene reached 90.8% (i.e., leaving 9.2 μg/L residual concentration) after 15 min of oxidation time. Zhao et al. [79] used Cu-cordierite to remove nitrobenzene and reduced its concentration up to 77.9% (11 μg/L residual concentration). Nitrobenzene, examined as a probe compound for the evaluation of O3/Mn-Cu/ceramic honeycomb material (used as catalyst), was applied by Zhao et al. [81]. The residual concentration of this micropollutant after 10 min reaction time was 8.5 μg/L. Nitrobenzene is an ozone-resistant compound with kO3 equal to 1.6 M−1s−1 [104]. This means that the aforementioned materials can greatly accelerate the decomposition of ozone and the production of hydroxyl radicals (•OH).

Table 5.
Literature studies regarding the removal of micropollutants with initial concentrations ≤1 mg/L by the application of heterogeneous catalytic ozonation.
Table 5.
Literature studies regarding the removal of micropollutants with initial concentrations ≤1 mg/L by the application of heterogeneous catalytic ozonation.
| Catalyst | Micropollutant | Initial Micropollutant Concentration (μg/L) | pH | Residual Micropollutant Concentration (μg/L) | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| SAP | SOP | COP | ||||||
| Metal Oxides | ZnFe2O4 | DBP | 500 | 7 | ng | 320 (30 min) | 247.5 (30 min) | [42] |
| Mn-ZnFe2O4 | 41.5 (30 min) | |||||||
| α-Fe0.9Mn0.1OOH | Iohexole | 1000 | 7 | 970 (20 min) | 550 (20 min) | 50 (20 min) | [41] | |
| α-Fe2O3 | p-CNB | 100 | 7 | <96.8% (30 min) | 50.8 (30 min) | 38 (30 min) | [72] | |
| Mn0.95Bi0.05Fe2O4 | DBP | 50 | 6.9 | 48 (60 min) | 33.5 (60 min) | 15.5 (60 min) | [40] | |
| Goethite (α-FeOOH) | Oxalic acid | 900 | 7 | 810 (30 min) | 702 (30 min) | 414 (30 min) | [46] | |
| Metals on substrates | α-Fe2O3-IS (FeO3Si) | p-CNB | 100 | 7 | <96.8 (30 min) | 50.8 (30 min) | 17.2 (30 min) | [72] |
| IS-FeOOH | p-CNB | 100 | 7 | 96.7 (15 min) | 43.3 (15 min) | 0.2 (15 min) | [61] | |
| ZCSP | p-CNB | 100 | 7 | 90 (15 min) | 55 (15 min) | 0.7 (15 min) | [65] | |
| MnOx/MCM-41 (1.05% wt) | Nitrobenzene | 120 | 6.91 | 76 (10 min) | 95.9 (10 min) | 13.4 (10 min) | [64] | |
| Minerals | Zeolite A | Paracetamol | 120 | 6.91 | 114.3 (60 min) | 27.8 (60 min) | 11.2 (60 min) | [10] |
| Fe/Pumice (6.1% wt) | p-chlorobenzene | 100 | 6 | <94.5 (15 min) | 59.2 (15 min) | 9.2 (15 min) | [79] | |
| Pumice | p-chlorobenzene | 100 | 6.86 | 96.1 (20 min) | 48 (20 min) | 12 (20 min) | [77] | |
| Cu-cordierite (2% wt) | Nitrobenzene | 50 | 6.9 | 48.8 (20 min) | 34.5 (20 min) | 11 (20 min) | [80] | |
| Modified ceramic honeycomb (1% wt Mn, 0.5% wt Cu) | Nitrobenzene | 50 | 6.87 | 49.5 (10 min) | 31.5 (10 min) | 8.5 (10 min) | [82] | |
| Carbons | Biochar | Atrazine | 1000 | 7.0 | 935 (30 min) | 520 (30 min) | 420 (30 min) | [96] |
| MnOx/biochar | >900 (30 min) | 170 (30 min) | ||||||
| FeOx/biochar | >900 (30 min) | 0 (30 min) | ||||||
| GO/TiO2/Fe3O4 | p-CBA | 80 | 7 | ng | 64 (5 min) | 4 (5 min) | [24] | |
| Graphite | DEP | 670 | 6.2 | 589.6 (60 min) | 335 (10 min) | 294.8 (10 min) | [90] | |
| Zn-Graphite (3.5% wt) | / | 40.2 (10 min) | ||||||
| rGO-MnFe2O4 (5% wt) | DBP | 500 | 7 | 350 (60 min) | 340 (60 min) | 75 (60 min) | [88] | |
| nGO (non-oxidized) | p-CBA | 160 | 7 | ng | 136 (5 min) | 92.8 (5 min) | [100] | |
| oGO | ng | 136 (5 min) | 59.2 (5 min) | |||||
Ikhlaq et al. [10] used zeolite A as catalyst for the removal of paracetamol. The initial concentration in this case was 120 μg/L, and after 60 min treatment time, 90.7% of this pollutant was removed, i.e., the residual concentration was 11.2 μg/L. However, the process temperature was 15 °C, meaning that the ozone dissolution in water and its self-decomposition rate was not optimized [105], as at common ambient temperatures (around 20 °C) its removal rate would probably be even higher.
Most studies published in the relevant literature considering carbon materials with catalytic activity for the pH range 6–8 and for initial concentrations of micropollutants lower than 1 mg/L used graphene oxide as catalyst. The adsorption capacity, as found in the case of rGO-MnFe2O4, reached 30% after 60 min. In this case, the removal of the DBP micropollutant through adsorption was similar to the ozonation removal rate (32%). The degradation of the micropollutant was greatly enhanced by the combination of ozone and the catalyst, and it reached 85%, i.e., the residual concentration of micropollutant was 75 μg/L [88]. The peroxidized form of GO showed even higher catalytic activity than the non-oxidized form, reaching 63% of p-chlorobenzoic acid removal. The initial concentration was 160 μg/L, while after 5 min of reaction time the concentration reduced to 59.2 μg/L [100]. The highest removal rates were with the application of GO/TiO2/Fe3O4 catalyst, which removed 95% of p-chlorobenzene after 5 min. The residual concentration was 4 μg/L, which was one of the lowest detected in the relevant literature. However, this material cannot be used widely as a catalyst until it is proven to be stable enough under the strong oxidizing conditions created by the presence of ozone [24]. Another carbon material that has been used under similar conditions was graphite. The initial DEP concentration was 0.67 mg/L, while the residual concentration by using Zn-graphite as catalyst and after 10 min oxidation time was decreased to 40.2 μg/L. The raw graphite did not show any particular catalytic activity, and after the same duration of oxidation reaction, the residual concentration of this pollutant was higher (294.8 μg/L) [90].
Biochar also showed sufficient catalytic action for low initial pollutant concentrations, but only after the deposition of a proper metal oxide on its surface structure. An optimized catalyst was found to be FeOx-biochar, in the presence of which the residual concentration of atrazine was under the analytical detection limit; in the study of Tian et al. [96], 100% removal was reported. This study can be considered as the most efficient among all those reported in this review, not only because of the negligible residual concentration of examined micropollutant, but also because it represents the relatively highest initial concentration. Additionally, the probe compound is atrazine, which is one of the most ozone-resistant organic compounds, presenting a reaction rate constant equal to 6 M−1s−1 [106]. Therefore, more hydroxyl radicals have to be produced for the efficient degradation of this micropollutant. However, the main disadvantage of this catalyst is its instability under the highly oxidizing conditions created by the presence of ozone, proving that the carbons generally constitute a rather controversial group of catalysts with regard to their application in the heterogeneous catalytic ozonation treatment process.
This literature survey showed that almost all of the relevant research conducted at the pH range 6–8 followed a mechanism based upon the formation of radicals, and although several radical species were detected, the hydroxyl radicals were still the predominant oxidative species. Additionally, two possible mechanisms were reported related to the PZC value of the examined solid materials. Catalytic ozonation shows its highest catalytic activity when: (1) the solution pH is equal to the PZC of the used solid/catalyst when the adsorption process is not involved in the removal procedure of the examined micropollutants and (2) the PZC is higher than the solution pH when adsorption is part of the catalytic ozonation mechanism and the pKa of the examined organic compounds (micropollutants) is lower than the solution pH. Consequently, in the latter case, the catalyst and the pollutant are oppositely charged.
When the initial concentration of micropollutants is considered, the results of the relevant studies converge more. For initial concentrations of micropollutants lower than 1 mg/L, the adsorption process seems to have no particular influence on the efficiency of catalytic ozonation, and the major mechanism pathway is the oxidative reaction caused by the presence of radical species (mostly hydroxyl). In this case, only the 1st proposed mechanism takes place, i.e., the catalytic action is enhanced by the neutrally charged surface of the solid catalyst. It is important for the further development of the catalytic ozonation process, especially considering full-scale applications, that the applied experimental conditions efficiently simulate real existing conditions in natural waters as closely as possible.
5. Limitations & Future Perspectives
Although the catalytic ozonation process can overcome some single ozonation disadvantages and presents higher micropollutants removal efficiencies, it has still its own limitations. The main ones are the relative increase of respective process costs, as well as the possible leaching of unwanted constituents from the used solid catalysts, leading potentially to the inactivation of catalysts after several application cycles and to the possible production of undesirable by-products. Several suggestions regarding the reduction of process costs have been reported in the literature, e.g., combination with biological treatment, applied mostly for the case of by-products minimization [2]. However, the major trend to address on this issue is the use of lower costs and highly efficient solid catalysts. The use of proper catalysts can increase the efficiency of ozone decomposition for the production of more oxidative radicals/species and, hence, to save expenses [17]. Nevertheless, the preparation of catalysts can also be a costly procedure, although the use of simple and controllable methods can reduce this cost and improve the stability of the used catalyst [107]; thus, the respective preparation and modification techniques should be further developed. Another way is the use of cheap and widely available natural solid materials, such as specific minerals [17].
Another important factor for further consideration is the stability of used catalysts. In the presented literature studies, most experiments were conducted in batch mode and the stability tests do not actually reflect the real operational treatment conditions. The main disadvantage in these cases is the duration of the respective experiments. In most cases, the leaching of metals and the subsequent inactivation of catalysts were examined within the extensive duration of some hours [50,83,96], whereas the duration of heterogeneous catalytic ozonation experiments is usually far less. Therefore, the way these experiments were conducted should be revised accordingly and more pilot-scale applications should be considered/studied. Except for the inactivation of catalysts, the leaching behavior of constituents during the ozonation reactions can potentially lead to secondary pollution problems. This important issue of pollution should be carefully addressed, especially for living organisms [108,109].
Another source of secondary pollution is the formation of intermediate oxidation products, which can be produced, e.g., by the incomplete mineralization of target organic compounds, and their toxicity may be even higher than the initial ones [110]. New ways to enhance the mineralization of oxidation systems (and, therefore, the inactivation of possibly hazardous intermediate compounds) should be examined, such as combination with other commonly applied techniques, e.g., biological treatment. The scientific/technical developments regarding these issues are expected to lead towards the implementation of heterogeneous catalytic ozonation processes for full-scale applications as an effective economical and environmentally friendly final polishing water treatment process, removing the micropollutants content effectively.
Author Contributions
Conceptualization, S.P. and A.Z.; investigation, S.P.; resources, A.Z.; writing—original draft preparation, S.P.; writing—review and editing, A.Z. and M.M.; visualization, S.P.; supervision, A.Z. and M.M.; project administration, A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 4-Meq | 4-methylquinoline | IS | Iron Silicate |
| AC | Activated Carbon | KCC | Fibrous Silicon Nanospheres |
| α-FeOOH | Goethite | kO3 | Rate constant of ozone |
| AOPs | Advanced Oxidation Processes | MCM-41 | Mobile Composition of Matter No. 41 |
| BPA | Bisphenol A | MOFs | Metal Organic Frameworks |
| CNTs | Carbon Nanotubes | MWCNTs | Multiwalled Carbon Nanotubes |
| Ccat. | Catalyst concentration | ng | Negligible |
| Cmicr. | Micropollutant concentration | •OH | Hydroxyl radicals |
| CO3 | Ozone concentration | p-CBA | p-chlorobenzoic acid |
| COP | Catalytic Ozonation Process | PZC | Point of Zero Charge |
| DBP | di-n-butyl phthalate | SAP | Single Adsorption Process |
| DEHP | Di(2-ethylhexyl) phthalate | SBA-15 | Santa Barbara Amorphous-15 |
| DEP | Diethyl phthalate | SMA | Sulfamethazine |
| DMAC | Dimethylacetamide | SMZ | Sulfamerazine |
| DMP DOC | Dimethyl phthalateDissolved Organic Matter | SOP | Single Ozonation Process |
| FMSACs | Ferromagnetic sludge-based activated carbons | T | Temperature |
| FMSO | Iron Manganese Silicate Oxide | ZCSP | Zinc Copper Silicate Polymer |
| GO | Graphene Oxide | ZSM-5 | Zeolite Socony Mobil-5 |
References
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed]
- Mousset, E.; Loh, W.H.; Lim, W.S.; Jarry, L.; Wang, Z.; Lefebvre, O. Cost Comparison of Advanced Oxidation Processes for Wastewater Treatment Using Accumulated Oxygen-Equivalent Criteria. Water Res. 2021, 200, 117234. [Google Scholar] [CrossRef] [PubMed]
- Faria, P.C.C.; Monteiro, D.C.M.; Órfão, J.J.M.; Pereira, M.F.R. Cerium, Manganese and Cobalt Oxides as Catalysts for the Ozonation of Selected Organic Compounds. Chemosphere 2009, 74, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, J. Catalytic Ozonation in Water: Controversies and Questions. Discussion Paper. Appl. Catal. B 2013, 142–143, 465–471. [Google Scholar] [CrossRef]
- Liotta, L.F.; Gruttadauria, M.; di Carlo, G.; Perrini, G.; Librando, V. Heterogeneous Catalytic Degradation of Phenolic Substrates: Catalysts Activity. J. Hazard. Mater. 2009, 162, 588–606. [Google Scholar] [CrossRef]
- Nawrocki, J.; Fijołek, L. Catalytic Ozonation—Effect of Carbon Contaminants on the Process of Ozone Decomposition. Appl. Catal. B 2013, 142–143, 307–314. [Google Scholar] [CrossRef]
- Wang, B.; Xiong, X.; Ren, H.; Huang, Z. Preparation of MgO Nanocrystals and Catalytic Mechanism on Phenol Ozonation. RSC Adv. 2017, 7, 43464–43473. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, W.; Yang, Z.; Yang, Y.; Li, H. Efficient Ozone Catalysis by Manganese Iron Oxides/Activated Carbon for Sulfamerazine Degradation. J. Water Process Eng. 2022, 49, 103050. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Brown, D.R.; Kasprzyk-Hordern, B. Mechanisms of Catalytic Ozonation on Alumina and Zeolites in Water: Formation of Hydroxyl Radicals. Appl. Catal. B 2012, 123–124, 94–106. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Waheed, S.; Joya, K.S. Catalytic Ozonation of Paracetamol on Zeolite A: Non-Radical Mechanism. Catal. Commun. 2018, 112, 15–20. [Google Scholar] [CrossRef]
- Nawaz, F.; Cao, H.; Xie, Y.; Xiao, J.; Chen, Y.; Ghazi, Z.A. Selection of Active Phase of MnO2 for Catalytic Ozonation of 4-Nitrophenol. Chemosphere 2017, 168, 1457–1466. [Google Scholar] [CrossRef]
- Nawaz, F.; Xie, Y.; Cao, H.; Xiao, J.; Wang, Y.; Zhang, X.; Li, M.; Duan, F. Catalytic Ozonation of 4-Nitrophenol over an Mesoporous α-MnO2 with Resistance to Leaching. Catal. Today 2015, 258, 595–601. [Google Scholar] [CrossRef]
- Yu, G.; Wang, Y.; Cao, H.; Zhao, H.; Xie, Y. Reactive Oxygen Species and Catalytic Active Sites in Heterogeneous Catalytic Ozonation for Water Purification. Environ. Sci. Technol. 2020, 54, 5931–5946. [Google Scholar] [CrossRef]
- Zada, A.; Khan, M.; Khan, M.A.; Khan, Q.; Habibi-Yangjeh, A.; Dang, A.; Maqbool, M. Review on the Hazardous Applications and Photodegradation Mechanisms of Chlorophenols over Different Photocatalysts. Environ. Res. 2021, 195, 110742. [Google Scholar] [CrossRef]
- Gottschalk, C.; Libra, J.A.; Saupe, A. Ozonation of Water and Waste Water: A Practical Guide to Understanding Ozone and Its Applications, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Rayaroth, M.P.; Aravindakumar, C.T.; Shah, N.S.; Boczkaj, G. Advanced Oxidation Processes (AOPs) Based Wastewater Treatment—Unexpected Nitration Side Reactions—A Serious Environmental Issue: A Review. Chem. Eng. J. 2022, 430, 133002. [Google Scholar] [CrossRef]
- Inchaurrondo, N.S.; Font, J. Clay, Zeolite and Oxide Minerals: Natural Catalytic Materials for the Ozonation of Organic Pollutants. Molecules 2022, 27, 2151. [Google Scholar] [CrossRef]
- Iqbal, J.; Shah, N.S.; Khan, Z.U.H.; Rizwan, M.; Murtaza, B.; Jamil, F.; Shah, A.; Ullah, A.; Nazzal, Y.; Howari, F.; et al. Visible Light Driven Doped CeO2 for the Treatment of Pharmaceuticals in Wastewater: A Review. J. Water Process Eng. 2022, 49, 103130. [Google Scholar] [CrossRef]
- Hama Aziz, K.H. Application of Different Advanced Oxidation Processes for the Removal of Chloroacetic Acids Using a Planar Falling Film Reactor. Chemosphere 2019, 228, 377–383. [Google Scholar] [CrossRef]
- Gardoni, D.; Vailati, A.; Canziani, R. Decay of Ozone in Water: A Review. Ozone Sci. Eng. 2012, 34, 233. [Google Scholar] [CrossRef]
- Su, W.; Li, Y.; Hong, X.; Lin, K.Y.A.; Tong, S. Catalytic Ozonation of N, N-Dimethylacetamide in Aqueous Solution by Fe3O4@SiO2@MgO Composite: Optimization, Degradation Pathways and Mechanism. J. Taiwan Inst. Chem. Eng. 2022, 135, 104380. [Google Scholar] [CrossRef]
- Weiner, F.R.; Matthews, A.R. Measurement of Water Quality. In Environmental Engineering; Butterworth Heinemann: Oxford, UK, 2003. [Google Scholar]
- Bai, Z.; Wang, J.; Yang, Q. Iron Doped Fibrous-Structured Silica Nanospheres as Efficient Catalyst for Catalytic Ozonation of Sulfamethazine. Environ. Sci. Pollut. Res. 2018, 25, 10090–10101. [Google Scholar] [CrossRef]
- Jothinathan, L.; Hu, J. Kinetic Evaluation of Graphene Oxide Based Heterogenous Catalytic Ozonation for the Removal of Ibuprofen. Water Res. 2018, 134, 63–73. [Google Scholar] [CrossRef]
- Faria, P.C.C.; Órfão, J.J.M.; Pereira, M.F.R. Ozonation of Aniline Promoted by Activated Carbon. Chemosphere 2007, 67, 809–815. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, A.; Liu, Y.; Wang, J. Enhanced Degradation and Mineralization of 4-Chloro-3-Methyl Phenol by Zn-CNTs/O3 System. Chemosphere 2018, 191, 54–63. [Google Scholar] [CrossRef]
- Maezono, T.; Tokumura, M.; Sekine, M.; Kawase, Y. Hydroxyl Radical Concentration Profile in Photo-Fenton Oxidation Process: Generation and Consumption of Hydroxyl Radicals during the Discoloration of Azo-Dye Orange II. Chemosphere 2011, 82, 1422–1430. [Google Scholar] [CrossRef]
- Valdés, H.; Sánchez-Polo, M.; Rivera-Utrilla, J.; Zaror, C.A. Effect of Ozone Treatment on Surface Properties of Activated Carbon. Langmuir 2002, 18, 2111–2116. [Google Scholar] [CrossRef]
- Morales-lara, F.; Pe, M.J.; Altmajer-vaz, D.; Garc, M.; Melguizo, M.; Lo, F.J. Functionalization of Multiwall Carbon Nanotubes by Ozone at Basic PH. Comparison with Oxygen Plasma and Ozone in Gas Phase. J. Phys. Chem. 2013, 117, 11647–11655. [Google Scholar] [CrossRef]
- Razumovskii, S.D.; Gorshenev, V.N.; Kovarskii, A.L.; Kuznetsov, A.M.; Shchegolikhin, A.N. Carbon Nanostructure Reactivity: Reactions of Graphite Powders with Ozone. Fuller. Nanotub. Carbon Nanostruct. 2007, 15, 53–63. [Google Scholar] [CrossRef]
- Álvarez, P.M.; García-Araya, J.F.; Beltrán, F.J.; Masa, F.J.; Medina, F. Ozonation of Activated Carbons: Effect on the Adsorption of Selected Phenolic Compounds from Aqueous Solutions. J. Colloid. Interface Sci. 2005, 283, 503–512. [Google Scholar] [CrossRef]
- Psaltou, S.; Kaprara, E.; Triantafyllidis, K.; Mitrakas, M.; Zouboulis, A. Heterogeneous Catalytic Ozonation: The Significant Contribution of PZC Value and Wettability of the Catalysts. J. Environ. Chem. Eng. 2021, 9, 106173. [Google Scholar] [CrossRef]
- Sánchez-Polo, M.; Rivera-Utrilla, J. Effect of the Ozone-Carbon Reaction on the Catalytic Activity of Activated Carbon during the Degradation of 1,3,6-Naphthalenetrisulphonic Acid with Ozone. Carbon 2003, 41, 303–307. [Google Scholar] [CrossRef]
- Gao, G.; Kang, J.; Shen, J.; Chen, Z.; Chu, W. Heterogeneous Catalytic Ozonation of Sulfamethoxazole in Aqueous Solution over Composite Iron–Manganese Silicate Oxide. Ozone Sci. Eng. 2016, 39, 24–32. [Google Scholar] [CrossRef]
- Zhang, H.; He, Y.; Lai, L.; Yao, G.; Lai, B. Catalytic Ozonation of Bisphenol A in Aqueous Solution by Fe3O4–MnO2 Magnetic Composites: Performance, Transformation Pathways and Mechanism. Sep. Purif. Technol. 2020, 245, 116449. [Google Scholar] [CrossRef]
- Garoma, T.; Matsumoto, S. Ozonation of Aqueous Solution Containing Bisphenol A: Effect of Operational Parameters. J. Hazard. Mater. 2009, 167, 1185–1191. [Google Scholar] [CrossRef]
- Shih, K.; White, T.; Leckie, J.O. Nickel Stabilization Efficiency of Aluminate and Ferrite Spinels and Their Leaching Behavior. Environ. Sci. Technol. 2006, 40, 5520–5526. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, Y.; Wang, G.; Jiang, P.; Zhang, J.; Wu, L.; Li, K. Novel Magnetically Separable Nanomaterials for Heterogeneous Catalytic Ozonation of Phenol Pollutant: NiFe2O4 and Their Performances. Chem. Eng. J. 2013, 219, 295–302. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, F.; Zhang, Y.; Pan, Z.; Lai, B. Catalytic Ozonation of N, N-Dimethylacetamide (DMAC) in Aqueous Solution Using Nanoscaled Magnetic CuFe2O4. Sep. Purif. Technol. 2018, 193, 368–377. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, Y.; Zeng, T.; Feng, J.; Ma, J.; Mitch, W.A. Influence of Bi-Doping on Mn1−XBixFe2O4 Catalytic Ozonation of Di-n-Butyl Phthalate. Chem. Eng. J. 2016, 283, 622–630. [Google Scholar] [CrossRef]
- Yan, P.; Shen, J.; Zhou, Y.; Yuan, L.; Kang, J.; Wang, S.; Chen, Z. Interface Mechanism of Catalytic Ozonation in an α-Fe0.9Mn0.1OOH Aqueous Suspension for the Removal of Iohexol. Appl. Catal. B 2020, 277, 119055. [Google Scholar] [CrossRef]
- Zhao, Y.; An, H.; Dong, G.; Feng, J.; Ren, Y.; Wei, T. Elevated Removal of Di-n-Butyl Phthalate by Catalytic Ozonation over Magnetic Mn-Doped Ferrospinel ZnFe2O4 Materials: Efficiency and Mechanism. Appl. Surf. Sci. 2020, 505, 144476. [Google Scholar] [CrossRef]
- Liu, H.; Chen, T.; Frost, R.L. An Overview of the Role of Goethite Surfaces in the Environment. Chemosphere 2014, 103, 1–11. [Google Scholar] [CrossRef]
- Li, P.; Du, L.; Jing, J.; Ding, X.; Shao, S.; Jiao, W.; Liu, Y. Preparation of FeOOH Nanoparticles Using an Impinging Stream-Rotating Packed Bed and Their Catalytic Activity for Ozonation of Nitrobenzene. J. Taiwan Inst. Chem. Eng. 2021, 127, 102–108. [Google Scholar] [CrossRef]
- Pelalak, R.; Alizadeh, R.; Ghareshabani, E. Enhanced Heterogeneous Catalytic Ozonation of Pharmaceutical Pollutants Using a Novel Nanostructure of Iron-Based Mineral Prepared via Plasma Technology: A Comparative Study. J. Hazard. Mater. 2020, 392, 122269. [Google Scholar] [CrossRef]
- Sui, M.; Sheng, L.; Lu, K.; Tian, F. FeOOH Catalytic Ozonation of Oxalic Acid and the Effect of Phosphate Binding on Its Catalytic Activity. Appl. Catal. B 2010, 96, 94–100. [Google Scholar] [CrossRef]
- Psaltou, S.; Zouboulis, A. Catalytic Ozonation and Membrane Contactors—A Review Concerning Fouling Occurrence and Pollutant Removal. Water 2020, 12, 2964. [Google Scholar] [CrossRef]
- Zhu, H.; Ma, W.; Han, H.; Han, Y.; Ma, W. Catalytic Ozonation of Quinoline Using Nano-MgO: Efficacy, Pathways, Mechanisms and Its Application to Real Biologically Pretreated Coal Gasification Wastewater. Chem. Eng. J. 2017, 327, 91–99. [Google Scholar] [CrossRef]
- Dai, Q.; Zhang, Z.; Zhan, T.; Hu, Z.T.; Chen, J. Catalytic Ozonation for the Degradation of 5-Sulfosalicylic Acid with Spinel-Type ZnAl2O4 Prepared by Hydrothermal, Sol-Gel, and Coprecipitation Methods: A Comparison Study. ACS Omega 2018, 3, 6506–6512. [Google Scholar] [CrossRef]
- Ke, L.; Liu, J.; Sun, L.; Pan, F.; Yuan, X.; Xia, D. A Non-Specific Surface Area Dominated Catalytic Ozonation with CuO Modified β-MnO2 in Efficient Oxalic Acid Degradation. J. Water Process Eng. 2022, 46, 102535. [Google Scholar] [CrossRef]
- Tan, X.; Wan, Y.; Huang, Y.; He, C.; Zhang, Z.; He, Z.; Hu, L.; Zeng, J.; Shu, D. Three-Dimensional MnO2 Porous Hollow Microspheres for Enhanced Activity as Ozonation Catalysts in Degradation of Bisphenol, A.J. Hazard. Mater. 2017, 321, 162–172. [Google Scholar] [CrossRef]
- Tong, S.; Liu, W.; Leng, W.; Zhang, Q. Characteristics of MnO2 Catalytic Ozonation of Sulfosalicylic Acid and Propionic Acid in Water. Chemosphere 2003, 50, 1359–1364. [Google Scholar] [CrossRef]
- He, Y.; Wang, L.; Chen, Z.; Shen, B.; Wei, J.; Zeng, P.; Wen, X. Catalytic Ozonation for Metoprolol and Ibuprofen Removal over Different MnO2 Nanocrystals: Efficiency, Transformation and Mechanism. Sci. Total Environ. 2021, 785, 147328. [Google Scholar] [CrossRef]
- Benner, J.; Ternes, T.A. Ozonation of Metoprolol: Elucidation of Oxidation Pathways and Major Oxidation Products. Environ. Sci. Technol. 2009, 43, 5472–5480. [Google Scholar] [CrossRef]
- Kermani, M.; Kakavandi, B.; Farzadkia, M.; Esrafili, A.; Jokandan, S.F.; Shahsavani, A. Catalytic Ozonation of High Concentrations of Catechol over TiO2@Fe3O4magnetic Core-Shell Nanocatalyst: Optimization, Toxicity and Degradation Pathway Studies. J. Clean. Prod. 2018, 192, 597–607. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Peng, J.; Lai, L.; Jiang, X.; Jiang, W.; Lai, B. Catalytic Ozonation of Succinic Acid in Aqueous Solution Using the Catalyst of Ni/Al2O3 Prepared by Electroless Plating-Calcination Method. Sep. Purif. Technol. 2018, 195, 138–148. [Google Scholar] [CrossRef]
- Bing, J.; Hu, C.; Zhang, L. Enhanced Mineralization of Pharmaceuticals by Surface Oxidation over Mesoporous γ-Ti-Al2O3 Suspension with Ozone. Appl. Catal. B 2017, 202, 118–126. [Google Scholar] [CrossRef]
- Yan, Z.; Zhu, J.; Hua, X.; Liang, D.; Dong, D.; Guo, Z.; Zheng, N.; Zhang, L. Catalytic Ozonation for the Degradation of Polyvinyl Alcohol in Aqueous Solution Using Catalyst Based on Copper and Manganese. J. Clean. Prod. 2020, 272, 122856. [Google Scholar] [CrossRef]
- Yuan, L.; Shen, J.; Yan, P.; Zhang, J.; Wang, Z.; Zhao, S.; Chen, Z. Catalytic Ozonation of 4-Chloronitrobenzene by Goethite and Fe2+ -Modified Goethite with Low Defects: A Comparative Study. J. Hazard. Mater. 2019, 365, 744–750. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Gong, W.; Chen, Z.; Liu, H.; Bu, Y.; Zhang, Y. Heterogeneous Catalytic Ozonation of P-Chloronitrobenzene (PCNB) in Water with Iron Silicate Doped Hydroxylation Iron as Catalyst. Catal. Commun. 2017, 89, 81–85. [Google Scholar] [CrossRef]
- Chen, W.; Li, X.; Liu, M.; Li, L. Effective Catalytic Ozonation for Oxalic Acid Degradation with Bimetallic Fe-Cu-MCM-41: Operation Parameters and Mechanism. J. Chem. Technol. Biotechnol. 2017, 92, 2862–2869. [Google Scholar] [CrossRef]
- Tang, Y.; Pan, Z.; Li, L. PH-Insusceptible Cobalt-Manganese Immobilizing Mesoporous Siliceous MCM-41 Catalyst for Ozonation of Dimethyl Phthalate. J. Colloid. Interface Sci. 2017, 508, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Sui, M.; Liu, J.; Sheng, L. Mesoporous Material Supported Manganese Oxides (MnOx/MCM-41) Catalytic Ozonation of Nitrobenzene in Water. Appl. Catal. B 2011, 106, 195–203. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Gong, W.; Dou, Y.; Wang, S.; Wang, W. Structural Characterizations of Zinc-Copper Silicate Polymer (ZCSP) and Its Mechanisms of Ozonation for Removal of p-Chloronitrobenzene in Aqueous Solution. Sep. Purif. Technol. 2017, 172, 251–257. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Fang, Y.; Yang, Z. Heterogeneous Catalytic Ozonation of Sulfamethazine in Aqueous Solution Using Maghemite-Supported Manganese Oxides. Sep. Purif. Technol. 2021, 274, 118945. [Google Scholar] [CrossRef]
- Gholipour-Ranjbar, H.; Soleimani, M.; Naderi, H.R. Application of Ni/Co-Based Metal-Organic Frameworks (MOFs) as an Advanced Electrode Material for Supercapacitors. New J. Chem. 2016, 40, 9187–9193. [Google Scholar] [CrossRef]
- Ye, G.; Luo, P.; Zhao, Y.; Qiu, G.; Hu, Y.; Preis, S.; Wei, C. Three-Dimensional Co/Ni Bimetallic Organic Frameworks for High-Efficient Catalytic Ozonation of Atrazine: Mechanism, Effect Parameters, and Degradation Pathways Analysis. Chemosphere 2020, 253, 126767. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, J.; Sun, Z.; Zhai, X. Mechanism of Influence of Initial PH on the Degradation of Nitrobenzene in Aqueous Solution by Ceramic Honeycomb Catalytic Ozonation. Environ. Sci. Technol. 2008, 42, 4002–4007. [Google Scholar] [CrossRef]
- Sawhney, B.L.; Singh, S.S. Sorption of Atrazine by Al-And Ca-Saturated Smectite. Clays Clay Miner. 1997, 45, 333–338. [Google Scholar] [CrossRef]
- Mohebali, H.; Moussavi, G.; Karimi, M.; Giannakis, S. Catalytic Ozonation of Acetaminophen with a Magnetic, Cerium-Based Metal-Organic Framework as a Novel, Easily-Separable Nanocomposite. Chem. Eng. J. 2022, 434, 134614. [Google Scholar] [CrossRef]
- Yuan, L.; Shen, J.; Yan, P.; Chen, Z. Interface Mechanisms of Catalytic Ozonation with Amorphous Iron Silicate for Removal of 4-Chloronitrobenzene in Aqueous Solution. Environ. Sci. Technol. 2018, 52, 1429–1434. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Brown, D.R.; Kasprzyk-Hordern, B. Catalytic Ozonation for the Removal of Organic Contaminants in Water on ZSM-5 Zeolites. Appl. Catal. B 2014, 154–155, 110–122. [Google Scholar] [CrossRef]
- Khataee, A.; Rad, T.S.; Fathinia, M. The Role of Clinoptilolite Nanosheets in Catalytic Ozonation Process: Insights into the Degradation Mechanism, Kinetics and the Toxicity. J. Taiwan Inst. Chem. Eng. 2017, 77, 205–215. [Google Scholar] [CrossRef]
- Khataee, A.; Rad, T.S.; Fathinia, M.; Joo, S.W. Production of Clinoptilolite Nanorods by Glow Discharge Plasma Technique for Heterogeneous Catalytic Ozonation of Nalidixic Acid. RSC Adv. 2016, 6, 20858–20866. [Google Scholar] [CrossRef]
- Yuan, L.; Shen, J.; Chen, Z.; Liu, Y. Pumice-Catalyzed Ozonation Degradation of p-Chloronitrobenzene in Aqueous Solution. Appl. Catal. B 2012, 117–118, 414–419. [Google Scholar] [CrossRef]
- Gao, G.; Shen, J.; Chu, W.; Chen, Z.; Yuan, L. Mechanism of Enhanced Diclofenac Mineralization by Catalytic Ozonation over Iron Silicate-Loaded Pumice. Sep. Purif. Technol. 2017, 173, 55–62. [Google Scholar] [CrossRef]
- Yuan, L.; Shen, J.; Chen, Z.; Guan, X. Role of Fe/Pumice Composition and Structure in Promoting Ozonation Reactions. Appl. Catal. B 2016, 180, 707–714. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Z.; Man, J.; Liu, H. Enhancement Mechanism of Heterogeneous Catalytic Ozonation by Cordierite-Supported Copper for the Degradation of Nitrobenzene in Aqueous Solution. Environ. Sci. Technol. 2009, 43, 2047–2053. [Google Scholar] [CrossRef]
- Jiao, W.; Yang, P.; Gao, W.; Qiao, J.; Liu, Y. Apparent Kinetics of the Ozone Oxidation of Nitrobenzene in Aqueous Solution Enhanced by High Gravity Technology. Chem. Eng. Process. Process Intensif. 2019, 146, 107690. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, J.; Sun, Z.; Liu, H. Mechanism of Heterogeneous Catalytic Ozonation of Nitrobenzene in Aqueous Solution with Modified Ceramic Honeycomb. Appl. Catal. B 2009, 89, 326–334. [Google Scholar] [CrossRef]
- Hou, Y.J.; Ma, J.; Sun, Z.Z.; Yu, Y.H.; Zhao, L. Degradation of Benzophenone in Aqueous Solution by Mn-Fe-K Modified Ceramic Honeycomb-Catalyzed Ozonation. J. Environ. Sci. 2006, 18, 1065–1072. [Google Scholar] [CrossRef]
- Pan, J.; Qian, M.; Li, Y.; Wang, H.; Guan, B. Catalytic Ozonation of Aqueous 4-Methylquinoline by Fluorinated Ceramic Honeycomb. Chemosphere 2022, 307, 135678. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, F.J.; Pocostales, P.; Alvarez, P.; Garcia-Araya, J.F.; Gimeno, O. Perovskite Catalytic Ozonation of Some Pharmaceutical Compounds in Water. Ozone Sci. Eng. 2010, 32, 230–237. [Google Scholar] [CrossRef]
- Bai, Z.; Yang, Q.; Wang, J. Catalytic Ozonation of Sulfamethazine Antibiotics Using Fe3O4/Multiwalled Carbon Nanotubes. Environ. Process Sustain. Energy 2018, 37, 678–685. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, W.; Hu, L.; Zeng, J.; He, C.; Tan, X.; He, Z.; Zhang, Q.; Shu, D. Combined Adsorption and Catalytic Ozonation for Removal of Endocrine Disrupting Compounds over MWCNTs/Fe3O4composites. Catal. Today 2017, 297, 143–150. [Google Scholar] [CrossRef]
- Bai, Z.; Yang, Q.; Wang, J. Catalytic Ozonation of Dimethyl Phthalate Using Fe3O4/Multi-Wall Carbon Nanotubes. Environ. Technol. 2016, 38, 2048–2057. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, H.; An, H.; Zhao, Y.; Feng, J.; Xue, L.; Luan, T.; Fan, Z. Catalytic Ozonation of Di-n-Butyl Phthalate Degradation Using Manganese Ferrite/Reduced Graphene Oxide Nanofiber as Catalyst in the Water. J. Colloid. Interface Sci. 2018, 526, 347–355. [Google Scholar] [CrossRef]
- Li, G.; Lu, Y.; Lu, C.; Zhu, M.; Zhai, C.; Du, Y.; Yang, P. Efficient Catalytic Ozonation of Bisphenol-A over Reduced Graphene Oxide Modified Sea Urchin-like α-MnO2 Architectures. J. Hazard. Mater. 2015, 294, 201–208. [Google Scholar] [CrossRef]
- Liu, Z.; Tu, J.; Wang, Q.; Cui, Y.; Zhang, L.; Wu, X. Catalytic Ozonation of Diethyl Phthalate in Aqueous Solution Using Graphite Supported Zinc Oxide. Sep. Purif. Technol. 2018, 200, 51–58. [Google Scholar] [CrossRef]
- Lu, S.; Liu, Y.; Feng, L.; Sun, Z.; Zhang, L. Characterization of Ferromagnetic Sludge-Based Activated Carbon and Its Application in Catalytic Ozonation of p-Chlorobenzoic Acid. Environ. Sci. Pollut. Res. 2018, 25, 5086–5094. [Google Scholar] [CrossRef]
- Huang, Y.; Cui, C.; Zhang, D.; Li, L.; Pan, D. Heterogeneous Catalytic Ozonation of Dibutyl Phthalate in Aqueous Solution in the Presence of Iron-Loaded Activated Carbon. Chemosphere 2015, 119, 295–301. [Google Scholar] [CrossRef]
- Dadban Shahamat, Y.; Sadeghi, M.; Shahryari, A.; Okhovat, N.; Bahrami Asl, F.; Baneshi, M.M. Heterogeneous Catalytic Ozonation of 2,4-Dinitrophenol in Aqueous Solution by Magnetic Carbonaceous Nanocomposite: Catalytic Activity and Mechanism. Desalination Water Treat 2015, 57, 20447–20456. [Google Scholar] [CrossRef]
- Farzadkia, M.; Dadban Shahamat, Y.; Nasseri, S.; Mahvi, A.H.; Gholami, M.; Shahryari, A. Catalytic Ozonation of Phenolic Wastewater: Identification and Toxicity of Intermediates. J. Eng. 2014, 2014, 520929. [Google Scholar] [CrossRef]
- Tian, G.P.; Wu, Q.Y.; Li, A.; Wang, W.L.; Hu, H.Y. Promoted Ozonation for the Decomposition of 1,4-Dioxane by Activated Carbon. Water Sci. Technol. Water Supply 2017, 17, 613–620. [Google Scholar] [CrossRef]
- Tian, S.Q.; Qi, J.Y.; Wang, Y.P.; Liu, Y.L.; Wang, L.; Ma, J. Heterogeneous Catalytic Ozonation of Atrazine with Mn-Loaded and Fe-Loaded Biochar. Water Res. 2021, 193, 116860. [Google Scholar] [CrossRef]
- Zada, A.; Ali, N.; Subhan, F.; Anwar, N.; Ali Shah, M.I.; Ateeq, M.; Hussain, Z.; Zaman, K.; Khan, M. Suitable Energy Platform Significantly Improves Charge Separation of G-C3N4 for CO2 Reduction and Pollutant Oxidation under Visible-Light. Prog. Nat. Sci. Mater. Int. 2019, 29, 138–144. [Google Scholar] [CrossRef]
- Oh, W.; Chang, V.W.C.; Hu, Z.T.; Goei, R.; Lim, T.T. Enhancing the Catalytic Activity of G-C3N4 through Me Doping (Me = Cu, Co and Fe) for Selective Sulfathiazole Degradation via Redox-Based Advanced Oxidation Process. Chem. Eng. J. 2017, 323, 260–269. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; He, S.; Sun, L.; Yuan, X.; Xia, D. Heterogeneous Catalytic Ozonation of Oxalic Acid with an Effective Catalyst Based on Copper Oxide Modified G-C3N4. Sep. Purif. Technol. 2020, 234, 16120. [Google Scholar] [CrossRef]
- Ahn, Y.; Oh, H.; Yoon, Y.; Park, W.K.; Yang, W.S.; Kang, J.W. Effect of Graphene Oxidation Degree on the Catalytic Activity of Graphene for Ozone Catalysis. J. Environ. Chem. Eng. 2017, 5, 3882–3894. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Z.; Chai, B.; Cheng, S.; Lu, X.; Bai, X. Heterogeneous Catalytic Ozonation of Natural Organic Matter with Goethite, Cerium Oxide and Magnesium Oxide. RSC Adv. 2016, 6, 14730–14740. [Google Scholar] [CrossRef]
- Kim, M.K.; Zoh, K.D. Occurrence and Removals of Micropollutants in Water Environment. Environ. Eng. Res. 2016, 21, 319–332. [Google Scholar] [CrossRef]
- Agudelo, E.A.; Cardona, G.S.A. Selection of Catalysts for Use in a Heterogeneous Catalytic Ozonation System. Ozone Sci. Eng. 2020, 42, 146–156. [Google Scholar] [CrossRef]
- Jiao, W.; Shao, S.; Yang, P.; Gao, K.; Liu, Y. Kinetics and Mechanism of Nitrobenzene Degradation by Hydroxyl Radicals-Based Ozonation Process Enhanced by High Gravity Technology. Front. Chem. Sci. Eng. 2021, 15, 1197–1205. [Google Scholar] [CrossRef]
- Elovitz, M.S.; von Gunten, U.; Kaiser, H.P. Hydroxyl Radical/Ozone Ratios during Ozonation Processes. II. The Effect of Temperature, PH, Alkalinity, and DOM Properties. Ozone Sci. Eng. 2000, 22, 123–150. [Google Scholar] [CrossRef]
- Acero, J.L.; Stemmler, K.; von Gunten, U. Degradation Kinetics of Atrazine and Its Degradation Products with Ozone and OH Radicals: A Predictive Tool for Drinking Water Treatment. Environ. Sci. Technol. 2000, 34, 591–597. [Google Scholar] [CrossRef]
- Wang, W.; Yao, H.; Yue, L. Supported-Catalyst CuO/AC with Reduced Cost and Enhanced Activity for the Degradation of Heavy Oil Refinery Wastewater by Catalytic Ozonation Process. Environ. Sci. Pollut. Res. 2020, 27, 7199–7210. [Google Scholar] [CrossRef]
- Pines, D.S.; Reckhow, D.A. Effect of Dissolved Cobalt (II) on the Ozonation of Oxalic Acid. Environ. Sci. Technol. 2002, 36, 4046–4051. [Google Scholar] [CrossRef]
- Krewski, D.; Yokel, R.A.; Nieboer, E.; Borchelt, D.; Cohen, J.; Harry, J.; Kacew, S.; Lindsay, J.; Mahfouz, A.M.; Rondeau, V.; et al. Human Health Risk Assessment for Aluminium, Aluminium Oxide, and Aluminium Hydroxide. J. Toxicol. Environ. Health 2007, 10, 1–269. [Google Scholar] [CrossRef]
- Gomes, J.F.; Frasson, D.; Pereira, J.L.; Gonçalves, F.J.M.; Castro, L.M.; Quinta-Ferreira, R.M.; Martins, R.C. Ecotoxicity Variation through Parabens Degradation by Single and Catalytic Ozonation Using Volcanic Rock. Chem. Eng. J. 2019, 360, 30–37. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).