Benign Cutaneous Neoplasms with Syndromic Associations
Abstract
1. Introduction
2. Hair Follicle Tumors
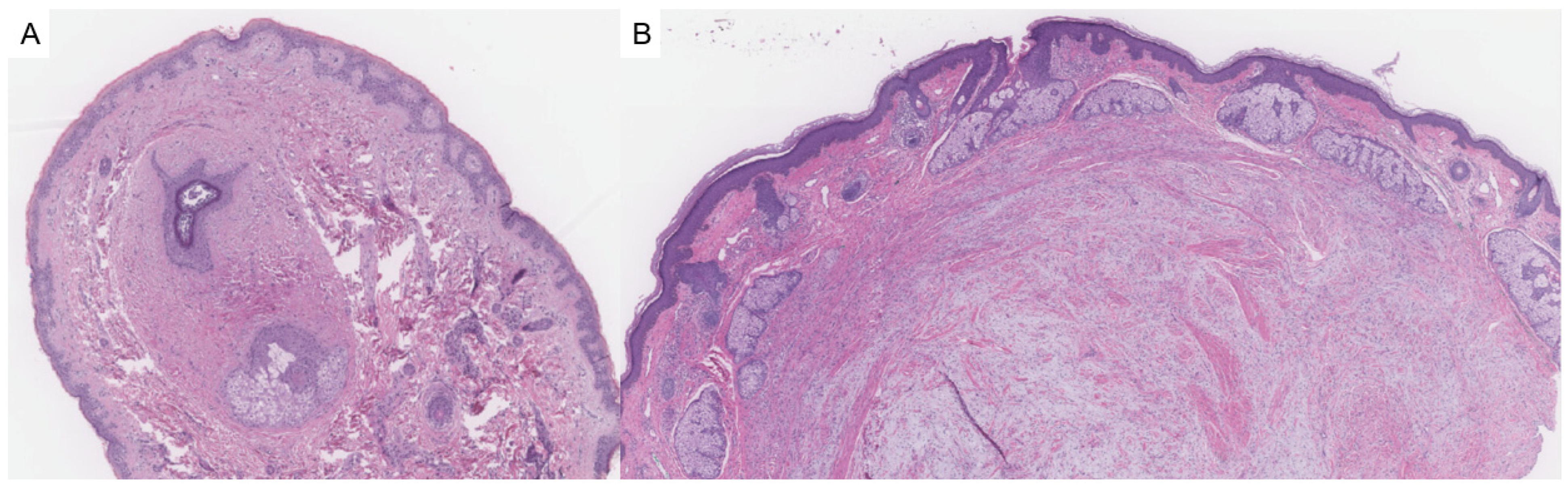
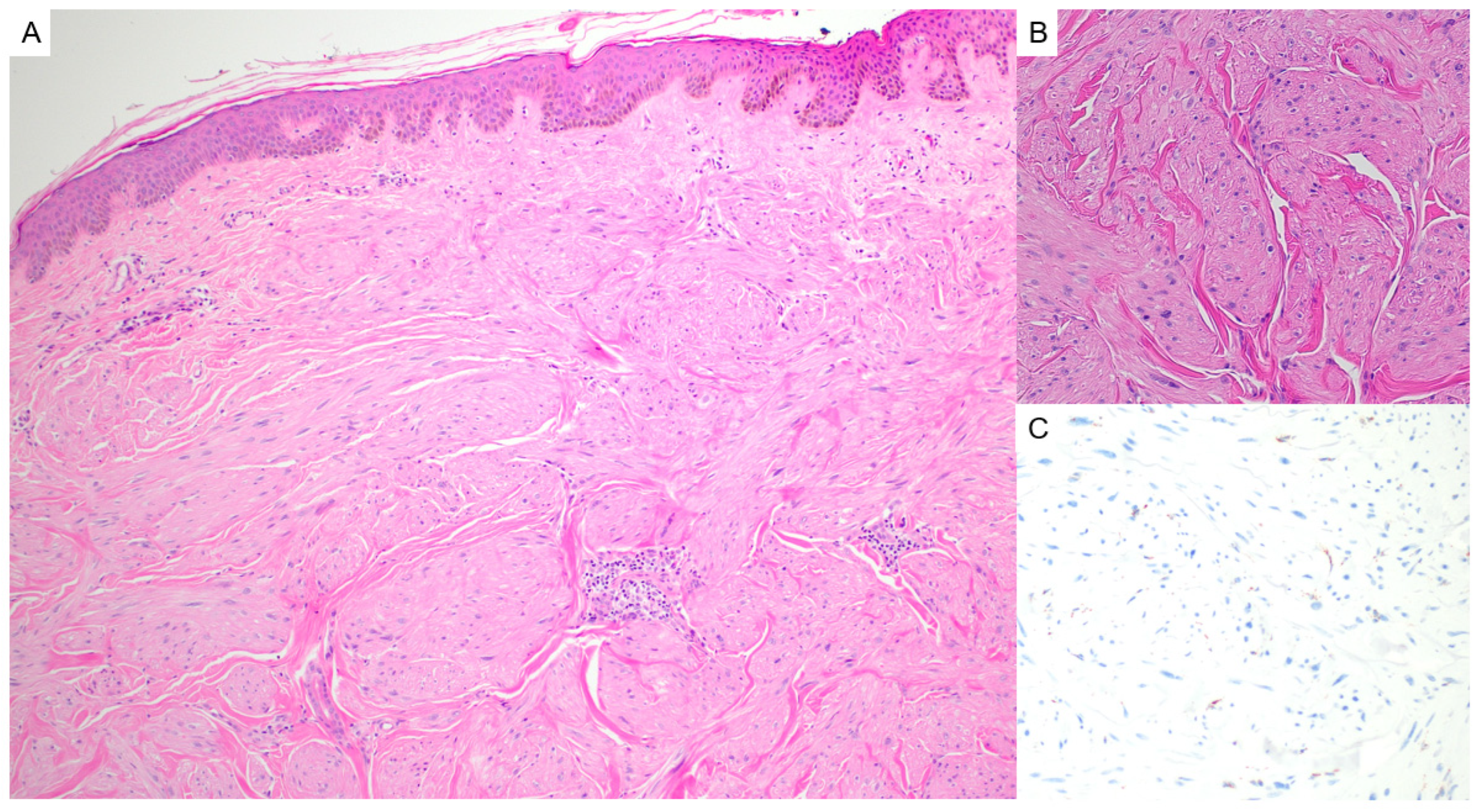
2.1. Fibrofolliculoma/Trichodiscoma
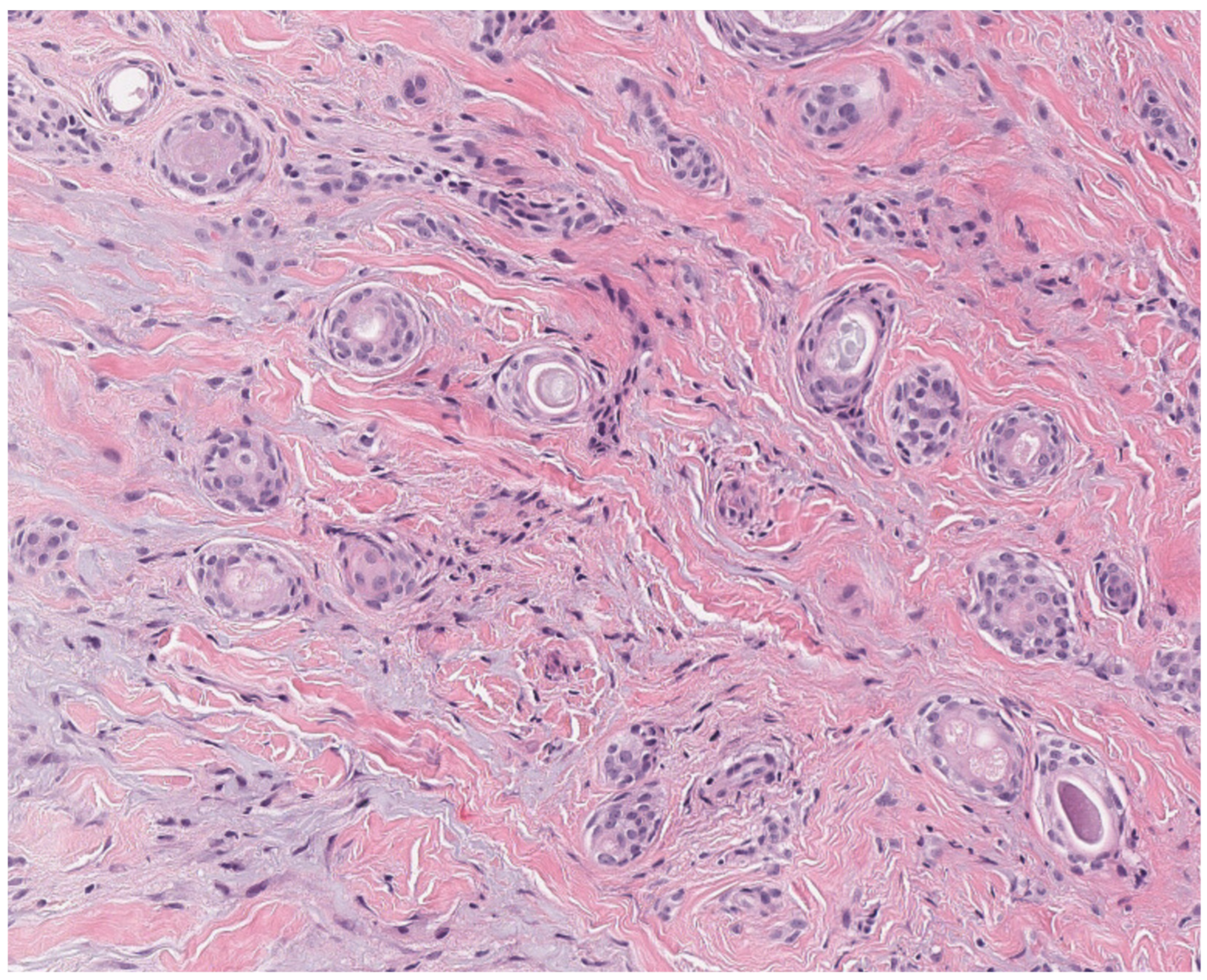
2.2. Tricholemmoma
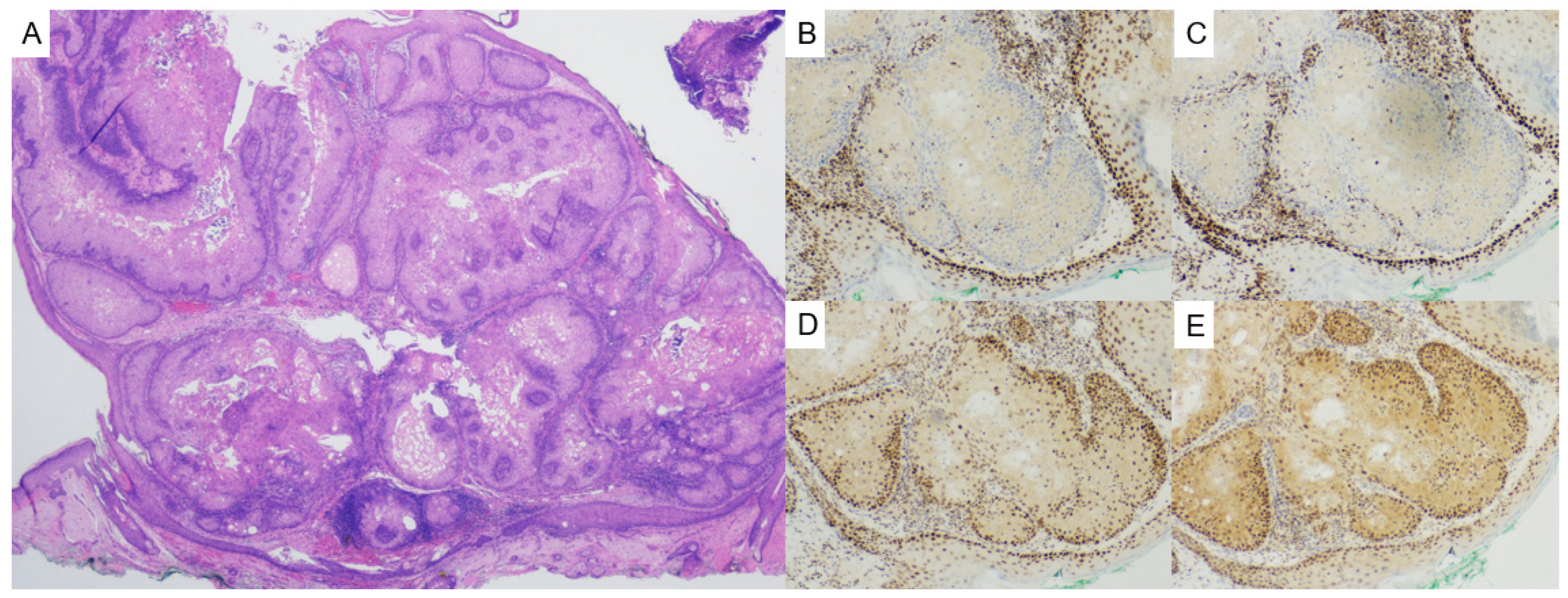
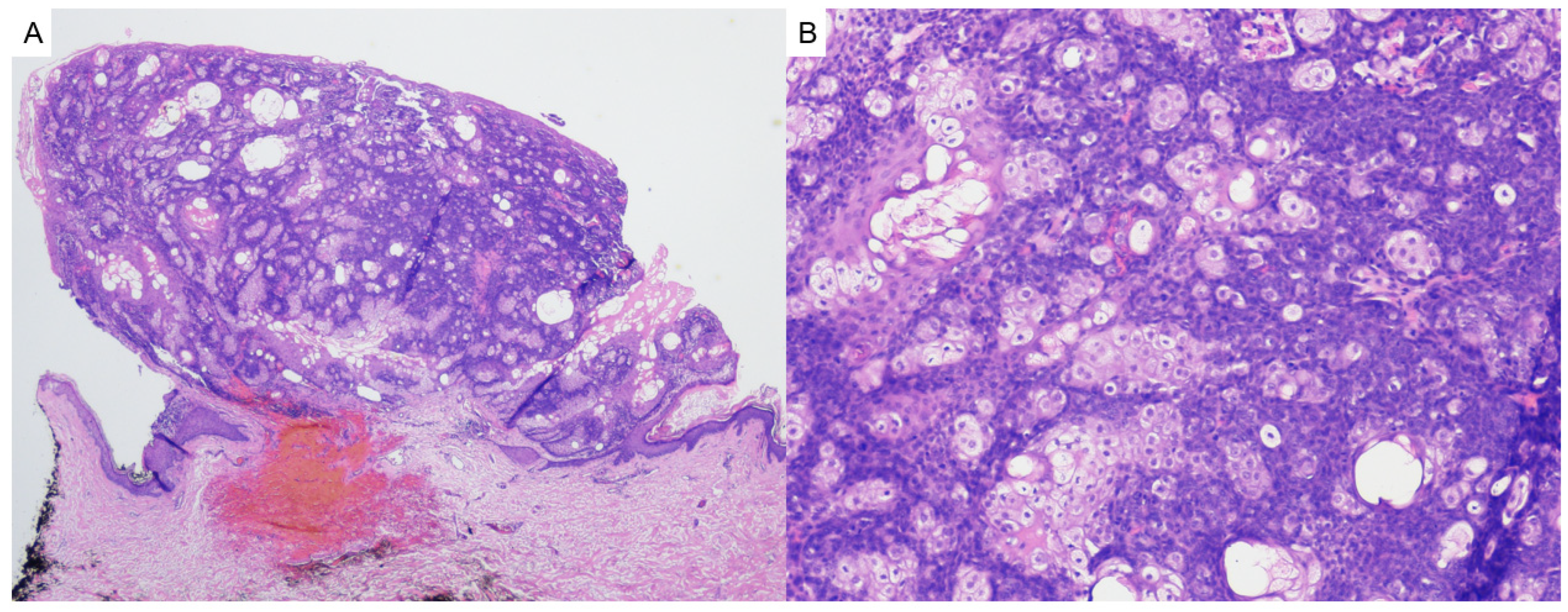
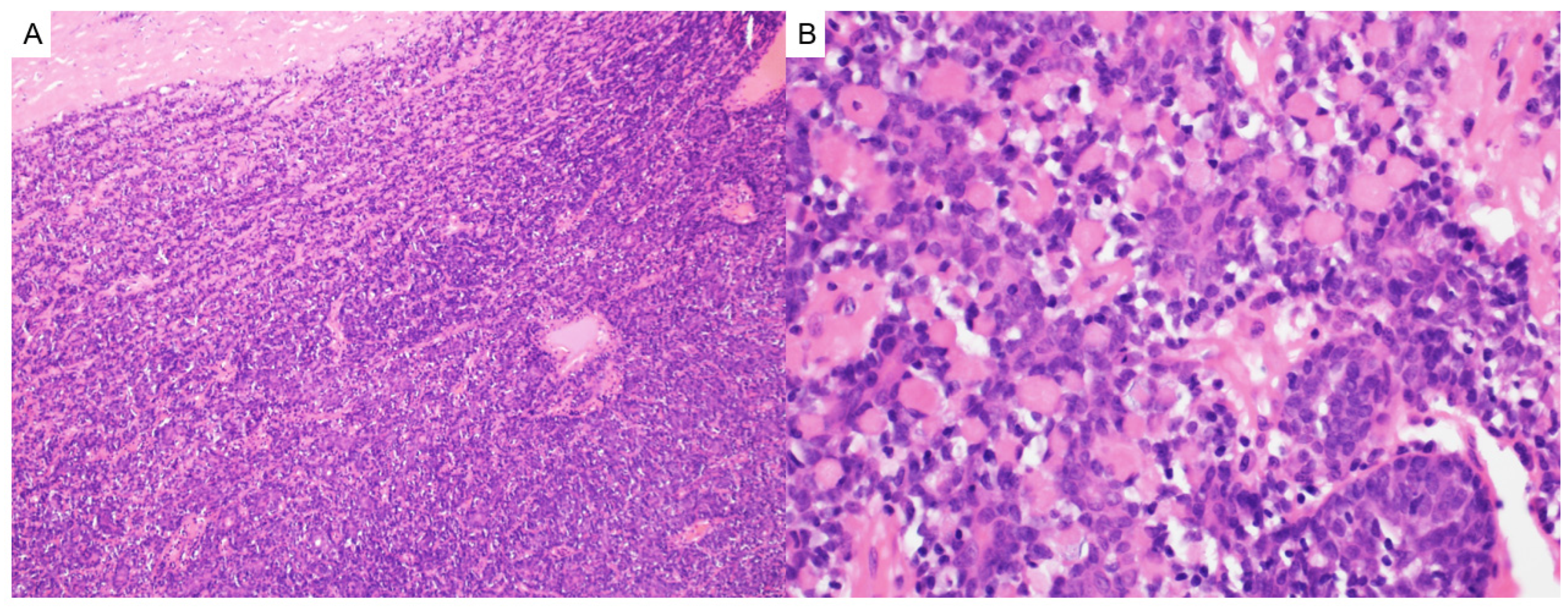
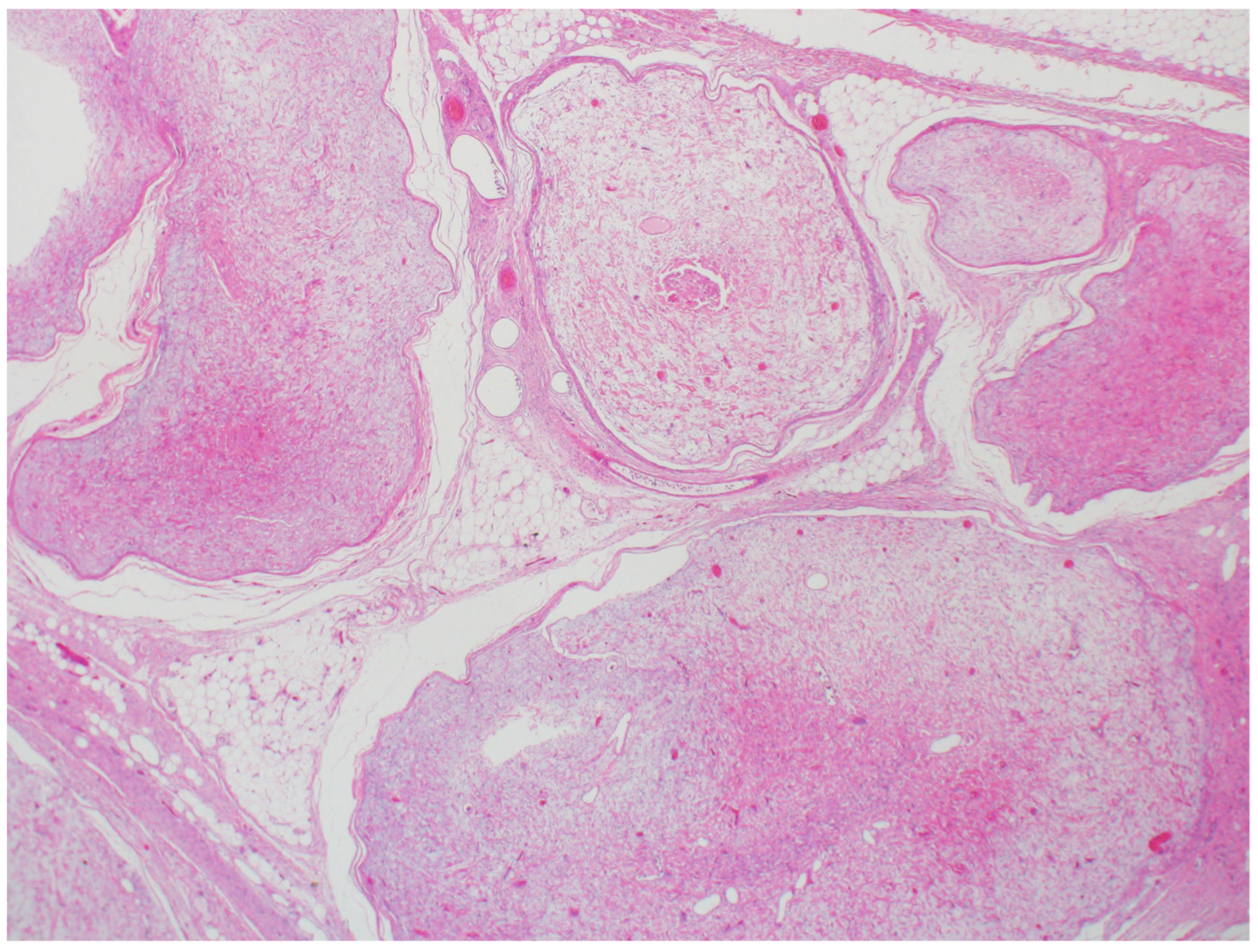
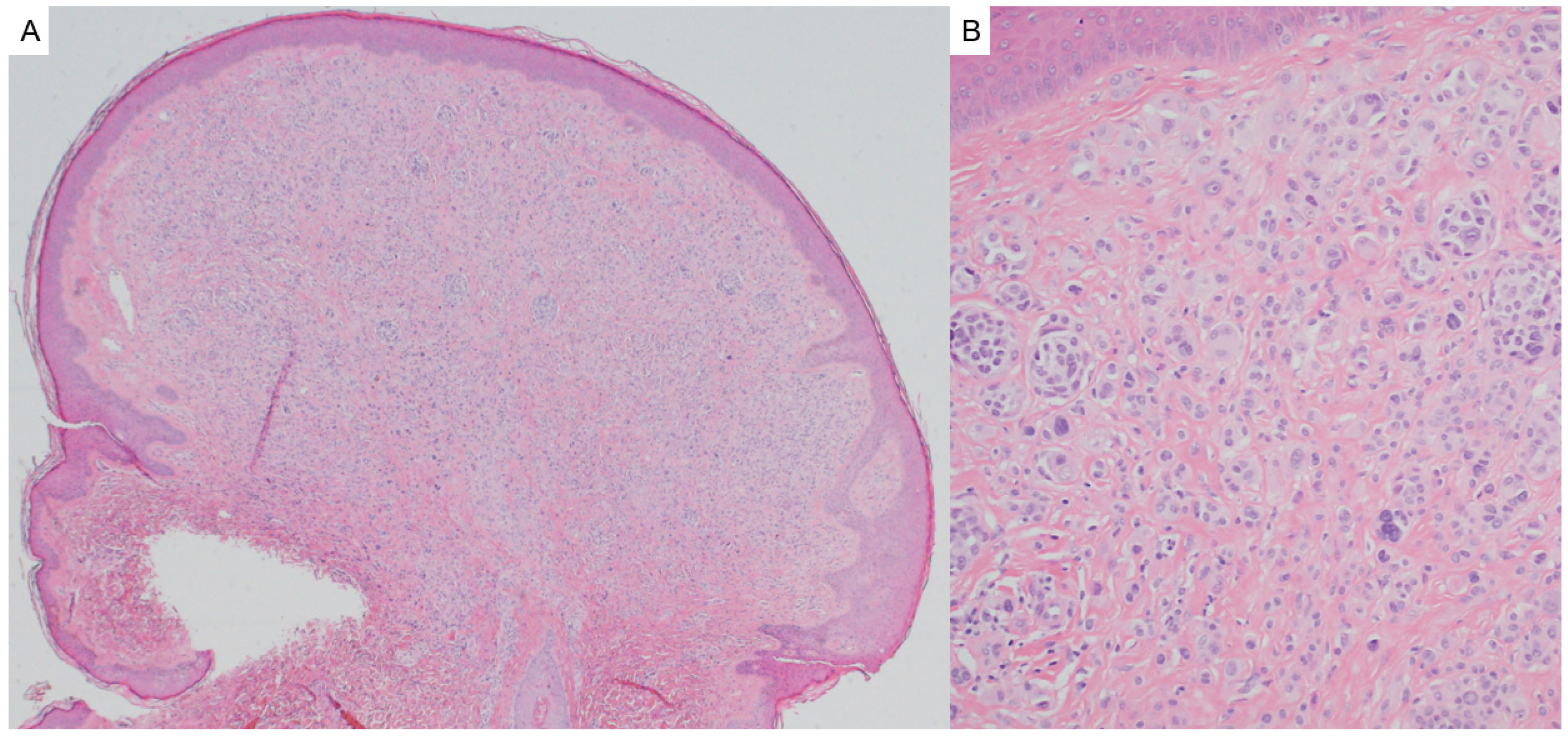
2.3. Pilomatricoma
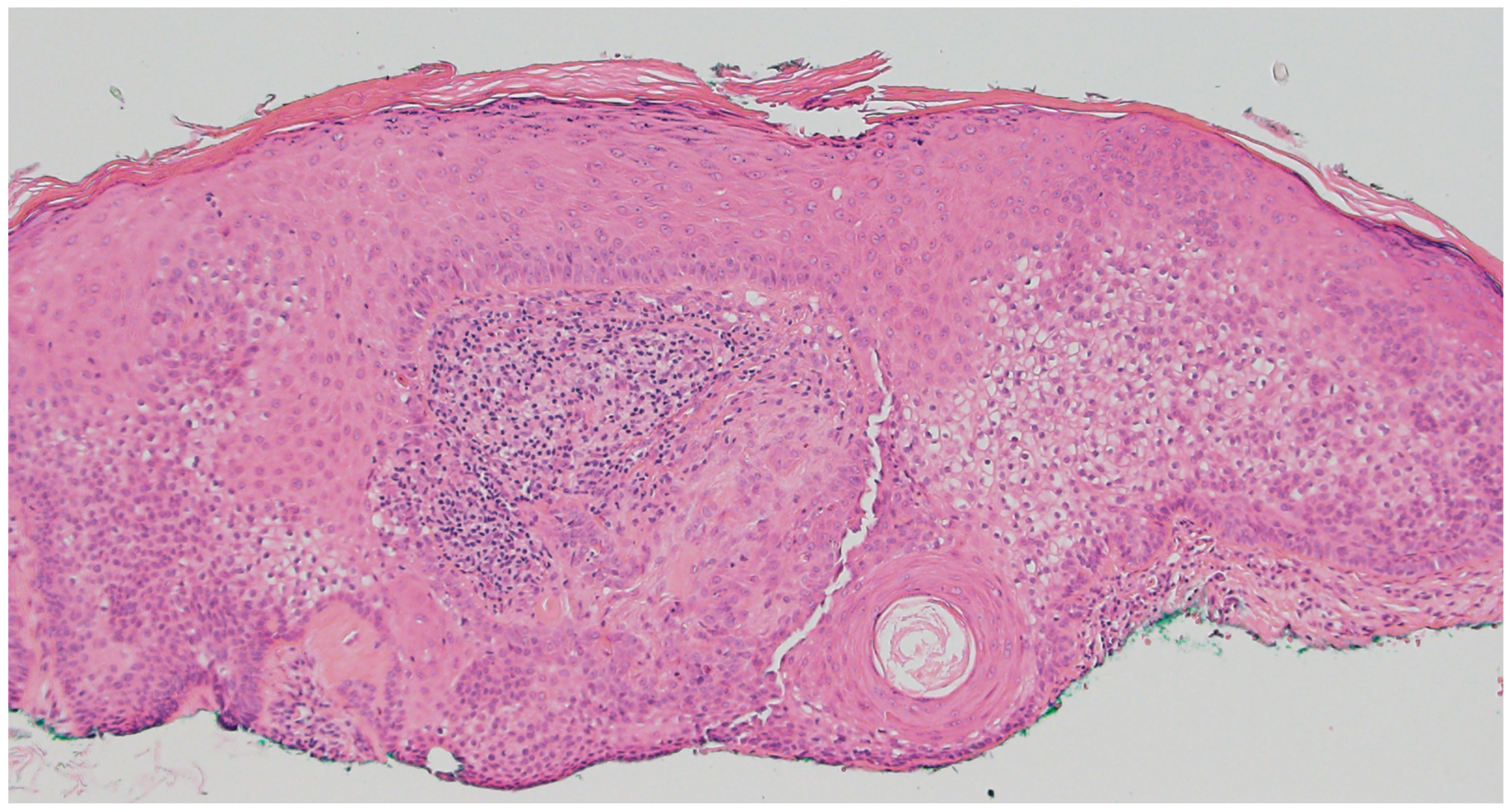
2.4. Trichoepithelioma
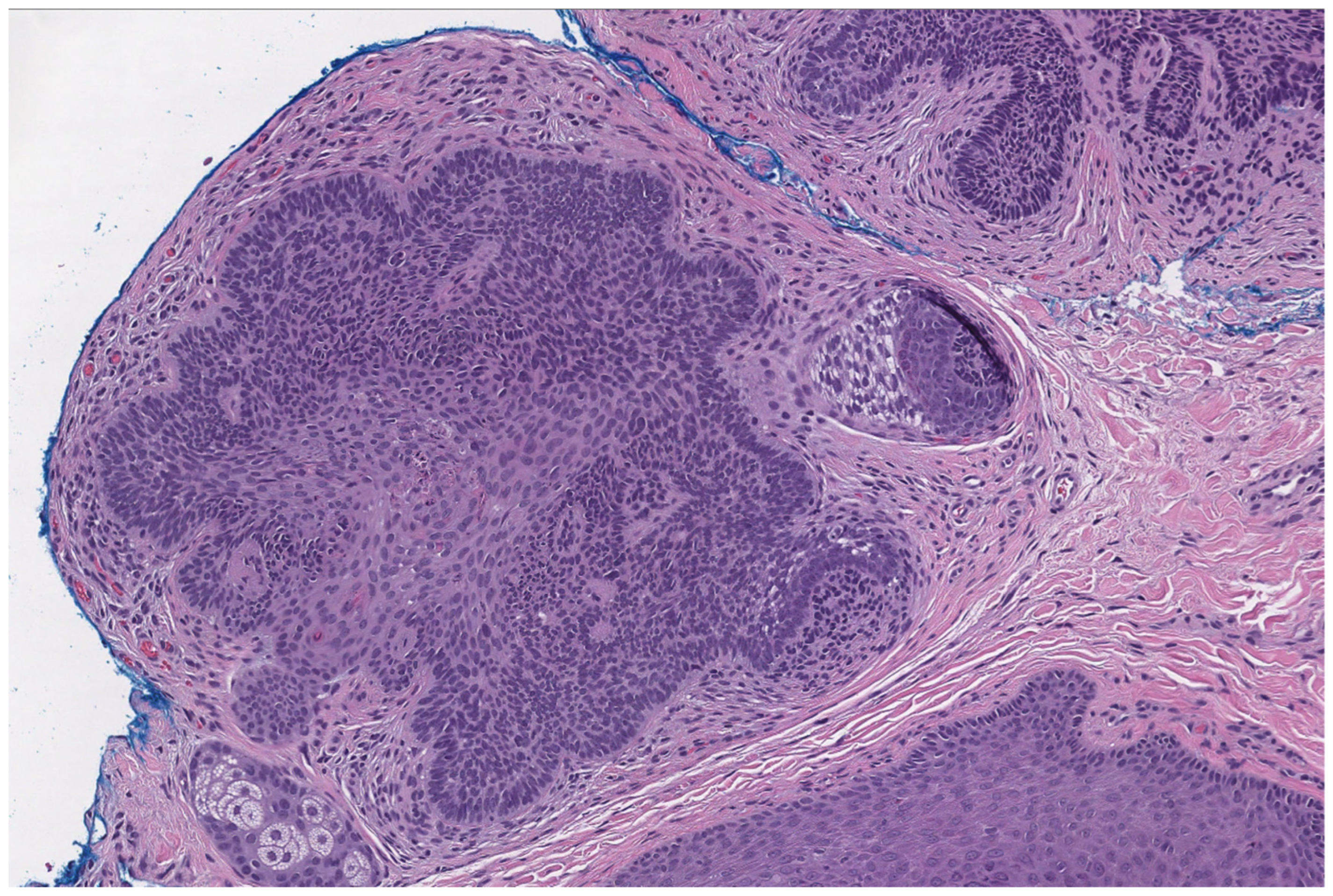
2.5. Basaloid Follicular Hamartoma
3. Sebaceous Tumors
3.1. Sebaceous Adenoma/Sebaceous Epithelioma
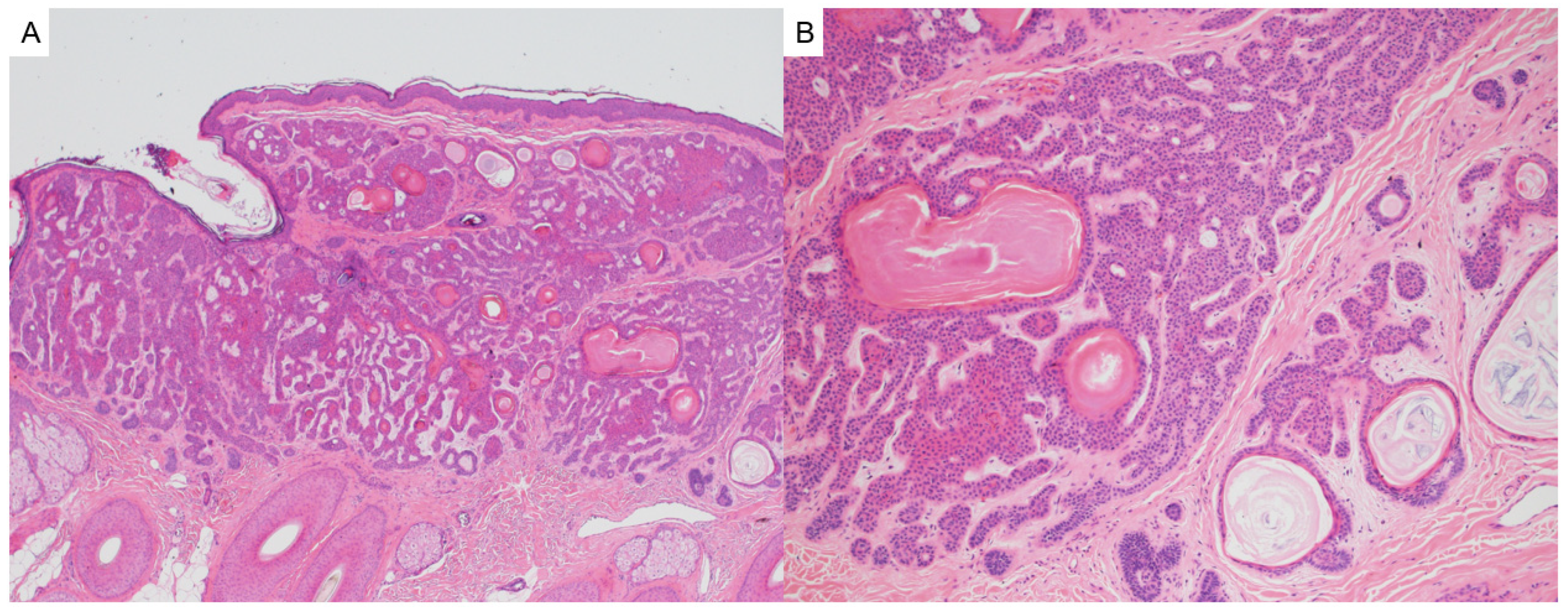
3.2. Steatocystoma
4. Sweat Gland Tumors
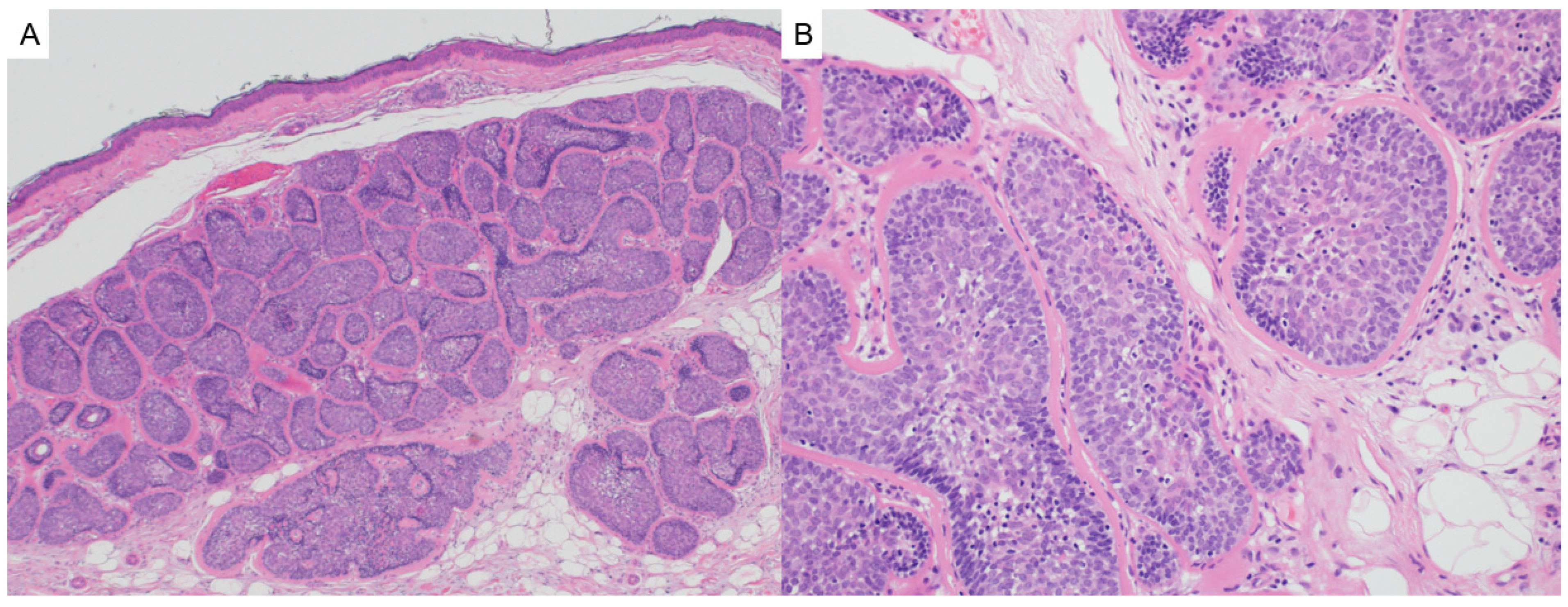
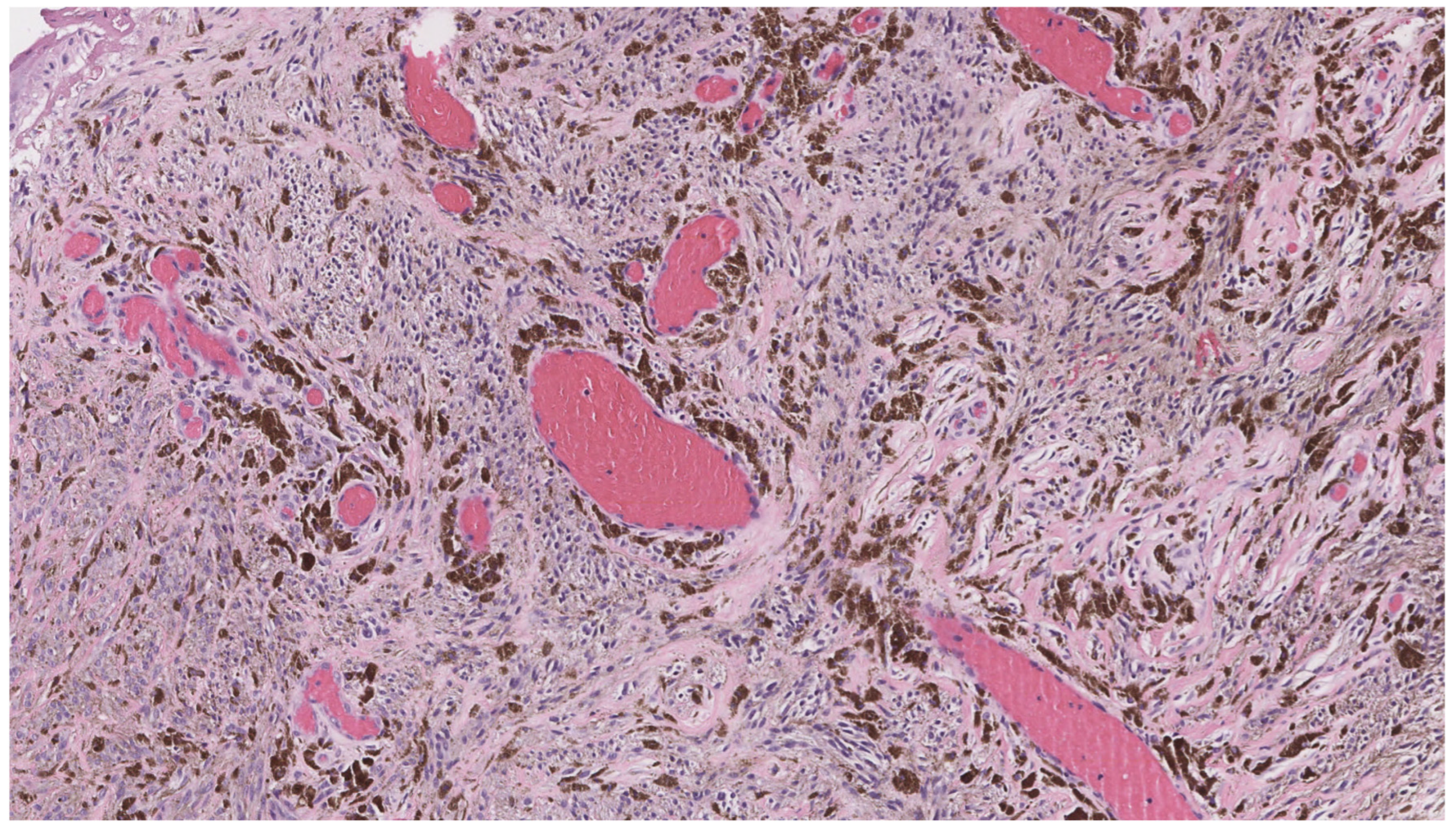
4.1. Cylindroma/Spiradenoma
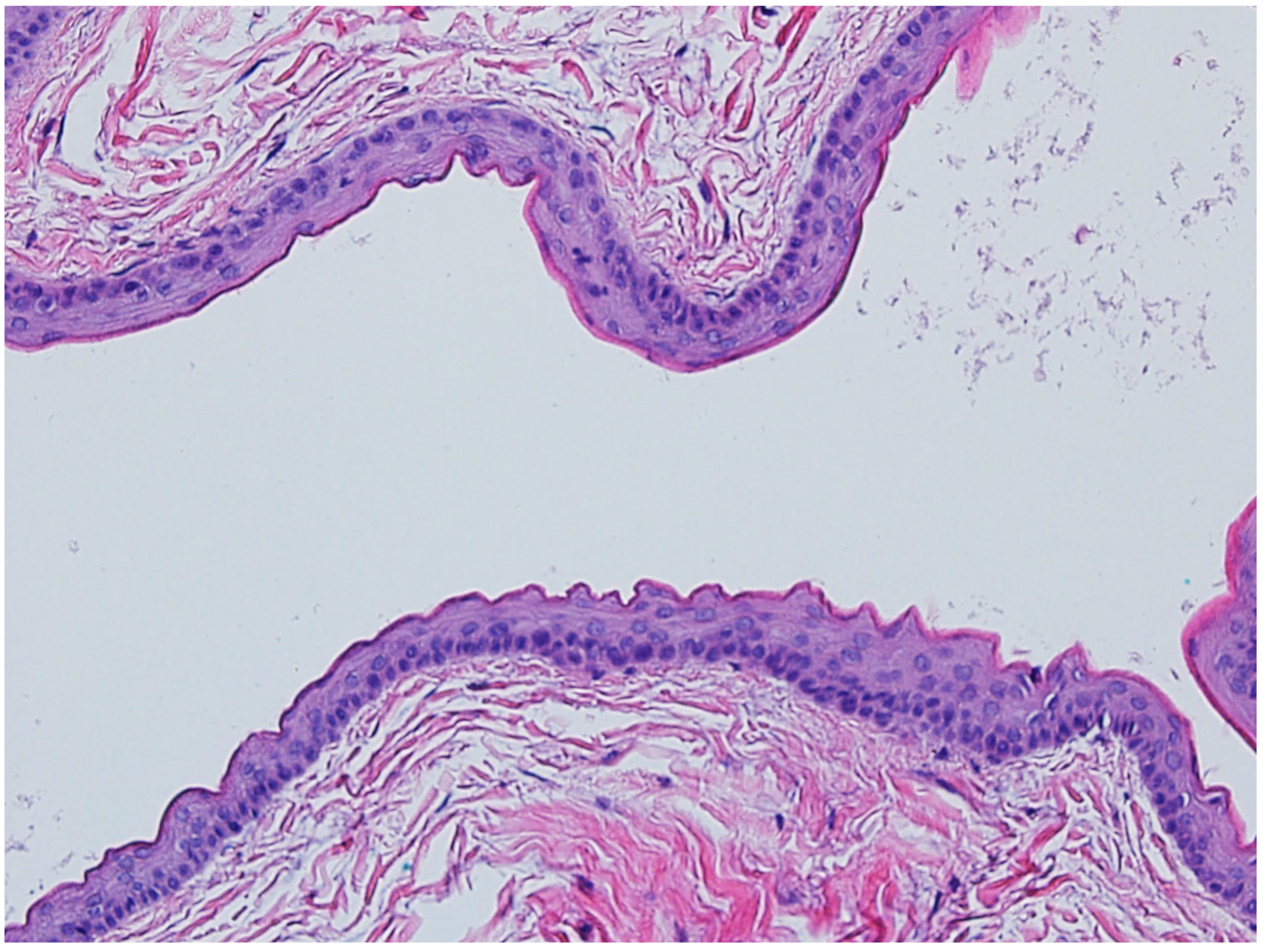
4.2. Syringoma
5. Neural Tumors
Neurofibromas
6. Smooth Muscle Tumors
Leiomyomas
7. Vascular Tumors
7.1. Angiokeratoma Corporis Diffusum
7.2. Other Vascular Anomalies
8. Adipocytic Tumors
Lipomas
9. Fibrohistiocytic/Fibrovascular Tumors
9.1. Superficial Angiomyxoma
9.2. Facial Angiofibromas/Acral Fibrokeratomas
10. Melanocytic Tumors
10.1. BAPomas
10.2. Pigmented Epithelioid Melanocytoma
10.3. Dysplastic/Atypical Nevi
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gruber, V.; Hofmann-Wellenhof, R.; Wolf, P.; Hofmann-Wellenhof, E.L.; Schmidt, H.; Berghold, A.; Wedrich, A. Common Benign Melanocytic and Non-Melanocytic Skin Tumors among the Elderly: Results of the Graz Study on Health and Aging. Dermatology 2023, 239, 379–386. [Google Scholar] [CrossRef]
- Tidman, A.S. Be vigilant for skin manifestations of inherited cancer syndromes. Practitioner 2017, 261, 23–27. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29023082 (accessed on 10 June 2025).
- Shen, Z.; Hoffman, J.D.; Hao, F.; Pier, E. More than just skin deep: Faciocutaneous clues to genetic syndromes with malignancies. Oncologist 2012, 17, 930–936. [Google Scholar] [CrossRef]
- Schmidt, L.S.; Linehan, W.M. Molecular genetics and clinical features of Birt-Hogg-Dubé syndrome. Nat. Rev. Urol. 2015, 12, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Misago, N.; Kimura, T.; Narisawa, Y. Fibrofolliculoma/trichodiscoma and fibrous papule (perifollicular fibroma/angiofibroma): A revaluation of the histopathological and immunohistochemical features. J. Cutan. Pathol. 2009, 36, 943–951. [Google Scholar] [CrossRef]
- Kutzner, H.; Requena, L.; Rütten, A.; Mentzel, T. Spindle cell predominant trichodiscoma: A fibrofolliculoma/trichodiscoma variant considered formerly to be a neurofollicular hamartoma: A clinicopathological and immunohistochemical analysis of 17 cases. Am. J. Dermatopathol. 2006, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Scalvenzi, M.; Argenziano, G.; Sammarco, E.; Delfino, M. Hereditary multiple fibrofolliculomas, trichodiscomas and acrochordons: Syndrome of Birt-Hogg-Dubè. J. Eur. Acad. Dermatol. Venereol. 1998, 11, 45–47. Available online: http://www.ncbi.nlm.nih.gov/pubmed/9731965 (accessed on 23 May 2025). [CrossRef] [PubMed]
- Wong, T.Y.; Suster, S.; Cheek, R.F.; Mihm, M.C. Benign cutaneous adnexal tumors with combined folliculosebaceous, apocrine, and eccrine differentiation. Clinicopathologic and immunohistochemical study of eight cases. Am. J. Dermatopathol. 1996, 18, 124–136. [Google Scholar] [CrossRef]
- Gustafson, S.; Zbuk, K.M.; Scacheri, C.; Eng, C. Cowden syndrome. Semin. Oncol. 2007, 34, 428–434. [Google Scholar] [CrossRef]
- Kaptan, M.A.; Kattampallil, J.; Rosendahl, C. Trichilemmoma in continuity with pigmented basal cell carcinoma; with dermatoscopy and dermatopathology. Dermatol. Pract. Concept. 2015, 5, 57–59. [Google Scholar] [CrossRef]
- Tardío, J.C. CD34-reactive tumors of the skin. An updated review of an ever-growing list of lesions. J. Cutan. Pathol. 2009, 36, 89–102. [Google Scholar] [CrossRef]
- Ung, T.; Tan, J.H.; Mudhar, H. Three patients with desmoplastic tricholemmoma with an incidental histological surprise impacting on management. Br. J. Ophthalmol. 2012, 96, 461–462. [Google Scholar] [CrossRef]
- Romano, C.; Schepis, C. PTEN gene: A model for genetic diseases in dermatology. Sci. World J. 2012, 2012, 252457. [Google Scholar] [CrossRef]
- Al-Zaid, T.; Ditelberg, J.S.; Prieto, V.G.; Lev, D.; Luthra, R.; Davies, M.A.; Diwan, A.H.; Wang, W.; Lazar, A.J. Trichilemmomas show loss of PTEN in Cowden syndrome but only rarely in sporadic tumors. J. Cutan. Pathol. 2012, 39, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Hobert, J.A.; Eng, C. PTEN hamartoma tumor syndrome: An overview. Genet. Med. 2009, 11, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Núñez, A.; Nájera Botello, L.; Romero Maté, A.; Martínez-Sánchez, C.; Busquets, M.U.; Komáromy, A.C.; Martínez, J.B. Retrospective study of pilomatricoma: 261 tumors in 239 patients. Actas Dermo-Sifiliográficas 2014, 105, 699–705. [Google Scholar] [CrossRef]
- Neema, S.; Kashif, A.W.; Vasudevan, B. Dermoscopy of Pilomatrixoma. Indian Dermatol. Online J. 2023, 14, 450–451. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.D. Pilomatricoma. Ear Nose Throat J. 2012, 91, 18–20. [Google Scholar] [CrossRef]
- Di Brizzi, E.V.; Piccolo, V.; Moscarella, E.; Pellerone, S.; Argenziano, G. Dermoscopy of pilomatricoma: Retrospective descriptive study on 35 paediatric patients. J. Eur. Acad. Dermatol. Venereol. 2025, 39, e141–e143. [Google Scholar] [CrossRef]
- Alnaqshanbandi, S.M.; McAfee, J.L.; Ko, J.S.; Billings, S.D.; Ronen, S. Role of Immunohistochemistry in the Diagnosis of Pilomatrical Tumors. Am. J. Surg. Pathol. 2024, 48, 1543–1550. [Google Scholar] [CrossRef]
- Pujol, R.M.; Casanova, J.M.; Egido, R.; Pujol, J.; de Moragas, J.M. Multiple familial pilomatricomas: A cutaneous marker for Gardner syndrome? Pediatr. Dermatol. 1995, 12, 331–335. [Google Scholar] [CrossRef]
- Ciriacks, K.; Knabel, D.; Waite, M.B. Syndromes associated with multiple pilomatricomas: When should clinicians be concerned? Pediatr. Dermatol. 2020, 37, 9–17. [Google Scholar] [CrossRef]
- Xin, T.Y.; Saniasiaya, J.; Kulasegarah, J.; Fan, C.S. Commonly Misdiagnosed Facial Lesion: Pilomatricoma. Acta Medica 2023, 66, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tan, X.; Yang, X.; Hu, H.; Lin, K.; Wang, C.; Fu, H.; Zhang, J. Retrosynthetic analysis via deep learning to improve pilomatricoma diagnoses. Comput. Biol. Med. 2024, 182, 109152. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, F.P.; Martins, A.I.; Costa e Silva, J.; Teixeira, N.; Ramos, J.N. Multiple Trichoepithelioma Syndrome: A Case Report. Cureus 2023, 15, e42930. [Google Scholar] [CrossRef] [PubMed]
- Nagy, N.; Dubois, A.; Szell, M.; Rajan, N. Genetic Testing in CYLD Cutaneous Syndrome: An Update. Appl. Clin. Genet. 2021, 14, 427–444. [Google Scholar] [CrossRef]
- Kazakov, D.V. Brooke-Spiegler Syndrome and Phenotypic Variants: An Update. Head Neck Pathol. 2016, 10, 125–130. [Google Scholar] [CrossRef]
- Mohammadi, A.A.; Seyed Jafari, S.M. Trichoepithelioma: A rare but crucial dermatologic issue. World J. Plast. Surg. 2014, 3, 142–145. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25489539 (accessed on 5 August 2025).
- Rahman, J.; Tahir, M.; Arekemase, H.; Murtazaliev, S.; Sonawane, S. Desmoplastic Trichoepithelioma: Histopathologic and Immunohistochemical Criteria for Differentiation of a Rare Benign Hair Follicle Tumor From Other Cutaneous Adnexal Tumors. Cureus 2020, 12, e9703. [Google Scholar] [CrossRef]
- Gill, P.; Naugler, C.; Abi Daoud, M.S. Utility of Ber-EP4 and MOC-31 in Basaloid Skin Tumor Detection. Appl. Immunohistochem. Mol. Morphol. 2019, 27, 584–588. [Google Scholar] [CrossRef]
- Dubois, A.; Rajan, N. CYLD Cutaneous Syndrome; University of Washington: Seattle, WA, USA, 1993. Available online: https://pubmed.ncbi.nlm.nih.gov/24838222/ (accessed on 5 August 2025).
- Barbieux, S.; Jouenne, F.; Machet, M.-C.; Fraitag, S.; Macagno, N.; Battistella, M.; Cribier, B.; Sohier, P.; Laurent-Roussel, S.; Carlotti, A.; et al. Re-evaluation of the concept of basaloid follicular hamartoma associated with naevoid basal cell carcinoma syndrome: A morphological, immunohistochemical and molecular study. Pathology 2025, 57, 49–56. [Google Scholar] [CrossRef]
- Lee, D.A.; Grossman, M.E.; Schneiderman, P.; Celebi, J.T. Genetics of skin appendage neoplasms and related syndromes. J. Med. Genet. 2005, 42, 811–819. [Google Scholar] [CrossRef]
- Mills, O.; Thomas, L.B. Basaloid follicular hamartoma. Arch. Pathol. Lab. Med. 2010, 134, 1215–1219. [Google Scholar] [CrossRef]
- Jih, D.M.; Shapiro, M.; James, W.D.; Levin, M.; Gelfand, J.; Williams, P.T.; Oakey, R.J.; Fakharzadeh, S.; Seykora, J.T. Familial basaloid follicular hamartoma: Lesional characterization and review of the literature. Am. J. Dermatopathol. 2003, 25, 130–137. [Google Scholar] [CrossRef]
- Honarpisheh, H.; Glusac, E.J.; Ko, C.J. Cytokeratin 20 expression in basaloid follicular hamartoma and infundibulocystic basal cell carcinoma. J. Cutan. Pathol. 2014, 41, 916–921. [Google Scholar] [CrossRef]
- Gumaste, P.; Ortiz, A.E.; Patel, A.; Baron, J.; Harris, R.; Barr, R. Generalized basaloid follicular hamartoma syndrome: A case report and review of the literature. Am. J. Dermatopathol. 2015, 37, e37–e40. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, I.; Vakirlis, E.; Sotiriou, E.; Bakirtzi, K.; Lallas, A.; Ioannides, D. Sebaceous Neoplasms. Diagnostics 2023, 13, 1676. [Google Scholar] [CrossRef]
- Eisen, D.B.; Michael, D.J. Sebaceous lesions and their associated syndromes: Part I. J. Am. Acad. Dermatol. 2009, 61, 549–560, quiz 561–562. [Google Scholar] [CrossRef]
- Cohen, P.R.; Kurzrock, R. Germline Testing of Mismatch Repair Genes Is Needed in the Initial Evaluation of Patients with Muir-Torre Syndrome-Associated Cutaneous Sebaceous Neoplasms: A Case Series. Cureus 2023, 15, e33975. [Google Scholar] [CrossRef] [PubMed]
- Gallon, R.; Holt, G.; Alfailakawi, W.; Husain, A.; Jones, C.; Sowter, P.; Santibanez-Koref, M.; Jackson, M.S.; Burn, J.; Cook, S.; et al. Novel microsatellite instability test of sebaceous tumours to facilitate low-cost universal screening for Lynch syndrome. Clin. Exp. Dermatol. 2025, 50, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Task Force/Committee Members; Vidal, C.I.; Sutton, A.; Armbrect, E.A.; Lee, J.B.; Litzner, B.R.; Hurley, M.Y.; Panel, R.; Alam, M.; Duncan, L.M.; et al. Muir-Torre syndrome appropriate use criteria: Effect of patient age on appropriate use scores. J. Cutan. Pathol. 2019, 46, 484–489. [Google Scholar] [CrossRef]
- Mintsoulis, D.; Beecker, J. Muir-Torre syndrome. CMAJ 2016, 188, E95. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Oh, C.; Mangold, E.; Egan, C.A. Muir-Torre syndrome: Diagnostic and screening guidelines. Australas. J. Dermatol. 2006, 47, 266–269. [Google Scholar] [CrossRef]
- Dores, G.M.; Curtis, R.E.; Toro, J.R.; Devesa, S.S.; Fraumeni, J.F. Incidence of cutaneous sebaceous carcinoma and risk of associated neoplasms: Insight into Muir-Torre syndrome. Cancer 2008, 113, 3372–3381. [Google Scholar] [CrossRef]
- Wang, J.; Qi, X.; Zhang, K.; Zhang, W. A case report of Muir-Torre syndrome (MTS) in a Chinese patient. BMC Ophthalmol. 2025, 25, 218. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, L.; Fu, X.; Yu, G.; Liu, H.; Zhang, F. Mutation analysis of the KRT17 gene in steatocystoma multiplex and a brief literature review. Clin. Exp. Dermatol. 2020, 45, 132–134. [Google Scholar] [CrossRef]
- Brownstein, M.H. The genodermatopathology of adnexal tumors. J. Cutan. Pathol. 1984, 11, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Georgakopoulos, J.R.; Ighani, A.; Yeung, J. Numerous asymptomatic dermal cysts: Diagnosis and treatment of steatocystoma multiplex. Can. Fam. Physician 2018, 64, 892–899. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30541803 (accessed on 5 August 2025). [PubMed]
- Palaniappan, V.; Karthikeyan, K. Steatocystoma Multiplex. Indian Dermatol. Online J. 2024, 15, 105–112. [Google Scholar] [CrossRef]
- Smith, F.J.; Hansen, C.D.; Hull, P.R.; Kaspar, R.L.; McLean, W.H.I.; O’Toole, E.; Sprecher, E. Pachyonychia Congenita; University of Washington: Seattle, WA, USA, 1993. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1280/ (accessed on 5 August 2025).
- Mohiuddin, W.; Laun, J.; Cruse, W. Brooke-Spiegler Syndrome. Eplasty 2018, 18, ic14. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30093932 (accessed on 23 May 2025).
- Neema, S.; Sandhu, S.; Kashif, A.W. Dermoscopy of Cylindroma. Indian Dermatol. Online J. 2022, 13, 818–819. [Google Scholar] [CrossRef]
- Jordão, C.; de Magalhães, T.C.; Cuzzi, T.; Ramos-e-Silva, M. Cylindroma: An update. Int. J. Dermatol. 2015, 54, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Cassarino, D.S.; Su, A.; Robbins, B.A.; Altree-Tacha, D.; Ra, S. SOX10 immunohistochemistry in sweat ductal/glandular neoplasms. J. Cutan. Pathol. 2017, 44, 544–547. [Google Scholar] [CrossRef]
- Macagno, N.; Sohier, P.; Kervarrec, T.; Pissaloux, D.; Jullie, M.-L.; Cribier, B.; Battistella, M. Recent Advances on Immunohistochemistry and Molecular Biology for the Diagnosis of Adnexal Sweat Gland Tumors. Cancers 2022, 14, 476. [Google Scholar] [CrossRef]
- Lobos, P.; Lobos, C.; Baksai, K. Reflectance confocal microscopy of spiradenoma. JAAD Case Rep. 2022, 24, 105–107. [Google Scholar] [CrossRef]
- Layegh, P.; Sharifi-Sistani, N.; Abadian, M.; Moghiman, T. Brooke-Spiegler syndrome. Indian J. Dermatol. Venereol. Leprol. 2008, 74, 632–634. [Google Scholar] [CrossRef]
- Lee, A.Y.; Kawashima, M.; Nakagawa, H.; Ishibashi, Y. Generalized eruptive syringoma. J. Am. Acad. Dermatol. 1991, 25, 570–571. [Google Scholar] [CrossRef]
- Lei, H.; Wang, Z.; Ma, X.; Zhang, Z.; Feng, Y.; Zheng, Y. Eruptive syringomas: Summary of ninety cases and a brief literature review. J. Cosmet. Dermatol. 2023, 22, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Schepis, C.; Siragusa, M.; Palazzo, R.; Batolo, D.; Romano, C. Perforating milia-like idiopathic calcinosis cutis and periorbital syringomas in a girl with Down syndrome. Pediatr. Dermatol. 1994, 11, 258–260. [Google Scholar] [CrossRef]
- Lam, M.; Lu, J.D.; Elhadad, L.; Sibbald, C.; Alhusayen, R. Common Dermatologic Disorders in Down Syndrome: Systematic Review. JMIR Dermatol. 2022, 5, e33391. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, A.; Rytina, E.; Batchelor, J.; Ha, T. Nicolau Balus syndrome with microcystic adnexal carcinoma. J. Am. Acad. Dermatol. 2013, 68, AB56. [Google Scholar] [CrossRef]
- Williams, K.; Shinkai, K. Evaluation and management of the patient with multiple syringomas: A systematic review of the literature. J. Am. Acad. Dermatol. 2016, 74, 1234–1240.e9. [Google Scholar] [CrossRef]
- Marzano, A.V.; Fiorani, R.; Girgenti, V.; Crosti, C.; Alessi, E. Familial syringoma: Report of two cases with a published work review and the unique association with steatocystoma multiplex. J. Dermatol. 2009, 36, 154–158. [Google Scholar] [CrossRef]
- Schepis, C.; Siragusa, M.; Palazzo, R.; Ragusa, R.M.; Massi, G.; Fabrizi, G. Palpebral syringomas and Down’s syndrome. Dermatology 1994, 189, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Feingold, M. Syringomas in Down syndrome. Am. J. Dis. Child. 1991, 145, 966–967. [Google Scholar] [CrossRef]
- Rabin, K.R.; Whitlock, J.A. Malignancy in children with trisomy 21. Oncologist 2009, 14, 164–173. [Google Scholar] [CrossRef]
- Schaller, J.; Rytina, E.; Rütten, A.; Hendricks, C.; Ha, T.; Requena, L. Sweat duct proliferation associated with aggregates of elastic tissue and atrophodermia vermiculata: A simulator of microcystic adnexal carcinoma. Report of two cases. J. Cutan. Pathol. 2010, 37, 1002–1009. [Google Scholar] [CrossRef]
- Ly, I.; Romo, C.G.; Gottesman, S.; Kelly, K.M.; Kornacki, D.; York, Z.; Lee, S.Y.; Rhodes, S.D.; Staedtke, V.; Steensma, M.R.; et al. Target Product Profile for Cutaneous Neurofibromas: Clinical Trials to Prevent, Arrest, or Regress Cutaneous Neurofibromas. J. Invest. Dermatol. 2023, 143, 1388–1396. [Google Scholar] [CrossRef]
- Gerber, P.A.; Antal, A.S.; Neumann, N.J.; Homey, B.; Matuschek, C.; Peiper, M.; Budach, W.; Bölke, E. Neurofibromatosis. Eur. J. Med. Res. 2009, 14, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Ortonne, N.; Wolkenstein, P.; Blakeley, J.O.; Korf, B.; Plotkin, S.R.; Riccardi, V.M.; Miller, D.C.; Huson, S.; Peltonen, J.; Rosenberg, A.; et al. Cutaneous neurofibromas: Current clinical and pathologic issues. Neurology. 2018, 91 (Suppl. 1), S5–S13. [Google Scholar] [CrossRef]
- Poplausky, D.; Young, J.N.; Tai, H.; Rivera-Oyola, R.; Gulati, N.; Brown, R.M. Dermatologic Manifestations of Neurofibromatosis Type 1 and Emerging Treatments. Cancers 2023, 15, 2770. [Google Scholar] [CrossRef] [PubMed]
- Duman, N.; Elmas, M. Dermoscopy of cutaneous neurofibromas associated with neurofibromatosis type 1. J. Am. Acad. Dermatol. 2015, 73, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Ly, K.I.; Blakeley, J.O. The Diagnosis and Management of Neurofibromatosis Type 1. Med. Clin. N. Am. 2019, 103, 1035–1054. [Google Scholar] [CrossRef]
- Phillips, T.G.; Persia, O.R.; Jimenez Lopez, J.A. Neurofibromatosis type 1: More than skin deep. J. Fam. Pract. 2020, 69, 401–405. Available online: http://www.ncbi.nlm.nih.gov/pubmed/33175920 (accessed on 23 May 2025). [CrossRef]
- Marcoval, J.; Llobera-Ris, C.; Moreno-Vílchez, C.; Penín, R.M. Cutaneous Leiomyoma: A Clinical Study of 152 Patients. Dermatology 2022, 238, 587–593. [Google Scholar] [CrossRef]
- Malik, K.; Patel, P.; Chen, J.; Khachemoune, A. Leiomyoma cutis: A focused review on presentation, management, and association with malignancy. Am. J. Clin. Dermatol. 2015, 16, 35–46. [Google Scholar] [CrossRef]
- Dilek, N.; Yüksel, D.; Sehitoğlu, I.; Saral, Y. Cutaneous leiomyoma in a child: A case report. Oncol. Lett. 2013, 5, 1163–1164. [Google Scholar] [CrossRef]
- McCarthy, A.J.; Chetty, R. Benign Smooth Muscle Tumors (Leiomyomas) of Deep Somatic Soft Tissue. Sarcoma 2018, 2018, 2071394. [Google Scholar] [CrossRef] [PubMed]
- Valcarcel-Jimenez, L.; Frezza, C. Fumarate hydratase (FH) and cancer: A paradigm of oncometabolism. Br. J. Cancer 2023, 129, 1546–1557. [Google Scholar] [CrossRef]
- Buelow, B.; Cohen, J.; Nagymanyoki, Z.; Frizzell, N.; Joseph, N.M.; McCalmont, T.; Garg, K. Immunohistochemistry for 2-Succinocysteine (2SC) and Fumarate Hydratase (FH) in Cutaneous Leiomyomas May Aid in Identification of Patients with HLRCC (Hereditary Leiomyomatosis and Renal Cell Carcinoma Syndrome). Am. J. Surg. Pathol. 2016, 40, 982–988. [Google Scholar] [CrossRef]
- Hampel, H.; Bennett, R.L.; Buchanan, A.; Pearlman, R.; Wiesner, G.L.; Guideline Development Group of the American College of Medical Genetics and Genomics Professional Practice and Guidelines Committee and of the National Society of Genetic Counselors Practice Guidelines Committee. A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: Referral indications for cancer predisposition assessment. Genet. Med. 2015, 17, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Al-Chaer, R.N.; Folkmann, M.; Mårtensson, N.L.; Feldt-Rasmussen, U.; Mogensen, M. Cutaneous manifestations of Fabry disease: A systematic review. J. Dermatol. 2025, 52, 571–582. [Google Scholar] [CrossRef]
- Mehta, A.; Hughes, D.A. Fabry Disease; University of Washington: Seattle, WA, USA, 1993. Available online: https://pubmed.ncbi.nlm.nih.gov/20301469/ (accessed on 23 May 2025).
- Suzuki, N.; Konohana, I.; Fukushige, T.; Kanzaki, T. Beta-mannosidosis with angiokeratoma corporis diffusum. J. Dermatol. 2004, 31, 931–935. [Google Scholar] [CrossRef]
- Kanitakis, J.; Allombert, C.; Doebelin, B.; Deroo-Berger, M.; Grande, S.; Blanc, S.; Claudy, A. Fucosidosis with angiokeratoma. Immunohistochemical & electronmicroscopic study of a new case and literature review. J. Cutan. Pathol. 2005, 32, 506–511. [Google Scholar] [CrossRef]
- Harzer, K.; Beck-Wödl, S.; Haack, T.B. Angiokeratoma corporis diffusum with severe acroparesthesia, an endothelial abnormality, and inconspicuous genetic findings. J. Cutan. Pathol. 2022, 49, 293–298. [Google Scholar] [CrossRef]
- Goldenberg, D.C.; Vikkula, M.; Penington, A.; Blei, F.; Schultze-Kool, L.; Wassef, M.; Frieden, I.J.; the ISSVA Vascular Anomalies Classification Group. Updated Classification of Vascular Anomalies. A living document from the International Society for the Study of Vascular Anomalies Classification Group. J. Vasc. Anomalies 2025, 6, e113. [Google Scholar] [CrossRef]
- Yehia, L.; Eng, C. PTEN Hamartoma Tumor Syndrome; University of Washington: Seattle, WA, USA, 1993. Available online: https://pubmed.ncbi.nlm.nih.gov/20301661/ (accessed on 5 August 2025).
- Lindhurst, M.J.; Sapp, J.C.; Teer, J.K.; Johnston, J.J.; Finn, E.M.; Peters, K.; Turner, J.; Cannons, J.L.; Bick, D.; Blakemore, L.; et al. A mosaic activating mutation in AKT1 associated with the Proteus syndrome. N. Engl. J. Med. 2011, 365, 611–619. [Google Scholar] [CrossRef]
- Mejía Granados, D.M.; de Baptista, M.B.; Bonadia, L.C.; Bertuzzo, C.S.; Steiner, C.E. Clinical and Molecular Investigation of Familial Multiple Lipomatosis: Variants in the HMGA2 Gene. Clin. Cosmet. Investig. Dermatol. 2020, 13, 1–10. [Google Scholar] [CrossRef]
- Calonje, E.; Guerin, D.; McCormick, D.; Fletcher, C.D. Superficial angiomyxoma: Clinicopathologic analysis of a series of distinctive but poorly recognized cutaneous tumors with tendency for recurrence. Am. J. Surg. Pathol. 1999, 23, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Neumann, N.M.; LeBoit, P.E.; Cohen, J.N. Superficial Angiomyxomas Frequently Demonstrate Loss of Protein Kinase A Regulatory Subunit 1 Alpha Expression: Immunohistochemical Analysis of 29 Cases and Cutaneous Myxoid Neoplasms with Histopathologic Overlap. Am. J. Surg. Pathol. 2022, 46, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, F.; Krakowski, A.C.; Lian, C.G.; Nazarian, R.M.; Maleszewski, J.J. Sporadic superficial angiomyxomas demonstrate loss of PRKAR1A expression. Histopathology 2022, 80, 1001–1003. [Google Scholar] [CrossRef]
- Wilkes, D.; McDermott, D.A.; Basson, C.T. Clinical phenotypes and molecular genetic mechanisms of Carney complex. Lancet Oncol. 2005, 6, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Hasbani, D.M.; Crino, P.B. Tuberous sclerosis complex. Handb. Clin. Neurol. 2018, 148, 813–822. [Google Scholar] [CrossRef]
- Ebrahimi-Fakhari, D.; Meyer, S.; Vogt, T.; Pföhler, C.; Müller, C.S.L. Dermatological manifestations of tuberous sclerosis complex (TSC). J. Dtsch. Dermatol. Ges. 2017, 15, 695–700. [Google Scholar] [CrossRef]
- Liebman, J.J.; Nigro, L.C.; Matthews, M.S. Koenen tumors in tuberous sclerosis: A review and clinical considerations for treatment. Ann. Plast. Surg. 2014, 73, 721–722. [Google Scholar] [CrossRef]
- Göktay, F.; Altan, Z.M.; Haras, Z.B.; Güneş, P.; Yaşar, Ş.; Aytekin, S.; Haneke, E. Multibranched acquired periungual fibrokeratomas with confounding histopathologic findings resembling papillomavirus infection: A report of two cases. J. Cutan. Pathol. 2015, 42, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Henske, E.P.; Jóźwiak, S.; Kingswood, J.C.; Sampson, J.R.; Thiele, E.A. Tuberous sclerosis complex. Nat. Rev. Dis. Prim. 2016, 2, 16035. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Papneja, A.; Hyrcza, M.; Al-Habeeb, A.; Ghazarian, D. Gene of the month: BAP1. J. Clin. Pathol. 2016, 69, 750–753. [Google Scholar] [CrossRef]
- Wiesner, T.; Obenauf, A.C.; Murali, R.; Fried, I.; Griewank, K.G.; Ulz, P.; Windpassinger, C.; Wackernagel, W.; Loy, S.; Wolf, I.; et al. Germline mutations in BAP1 predispose to melanocytic tumors. Nat. Genet. 2011, 43, 1018–1021. [Google Scholar] [CrossRef]
- Garfield, E.M.; Walton, K.E.; Quan, V.L.; VandenBoom, T.; Zhang, B.; Kong, B.Y.; Isales, M.C.; Panah, E.; Kim, G.; Gerami, P. Histomorphologic spectrum of germline-related and sporadic BAP1-inactivated melanocytic tumors. J. Am. Acad. Dermatol. 2018, 79, 525–534. [Google Scholar] [CrossRef]
- Zhang, A.J.; Rush, P.S.; Tsao, H.; Duncan, L.M. BRCA1-associated protein (BAP1)-inactivated melanocytic tumors. J. Cutan. Pathol. 2019, 46, 965–972. [Google Scholar] [CrossRef]
- Walpole, S.; Pritchard, A.L.; Cebulla, C.M.; Pilarski, R.; Stautberg, M.; Davidorf, F.H.; De La Fouchardière, A.; Cabaret, O.; Golmard, L.; Stoppa-Lyonnet, D.; et al. Comprehensive Study of the Clinical Phenotype of Germline BAP1 Variant-Carrying Families Worldwide. J. Natl. Cancer Inst. 2018, 110, 1328–1341. [Google Scholar] [CrossRef]
- Isales, M.C.; Mohan, L.S.; Quan, V.L.; Garfield, E.M.; Zhang, B.; Shi, K.; Arva, N.; Beaubier, N.; Yazdan, P.; White, K.; et al. Distinct Genomic Patterns in Pigmented Epithelioid Melanocytoma: A Molecular and Histologic Analysis of 16 Cases. Am. J. Surg. Pathol. 2019, 43, 480–488. [Google Scholar] [CrossRef]
- Mandal, R.V.; Murali, R.; Lundquist, K.F.; Ragsdale, B.D.; Heenan, P.; McCarthy, S.W.; Mihm, M.C.; Scolyer, R.A.; Zembowicz, A. Pigmented epithelioid melanocytoma: Favorable outcome after 5-year follow-up. Am. J. Surg. Pathol. 2009, 33, 1778–1782. [Google Scholar] [CrossRef]
- Bax, M.J.; Brown, M.D.; Rothberg, P.G.; Laughlin, T.S.; Scott, G.A. Pigmented epithelioid melanocytoma (animal-type melanoma): An institutional experience. J. Am. Acad. Dermatol. 2017, 77, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Zembowicz, A.; Knoepp, S.M.; Bei, T.; Stergiopoulos, S.; Eng, C.; Mihm, M.C.; Stratakis, C.A.M. Loss of expression of protein kinase a regulatory subunit 1alpha in pigmented epithelioid melanocytoma but not in melanoma or other melanocytic lesions. Am. J. Surg. Pathol. 2007, 31, 1764–1775. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.N.; Joseph, N.M.; North, J.P.; Onodera, C.; Zembowicz, A.; LeBoit, P.E. Genomic Analysis of Pigmented Epithelioid Melanocytomas Reveals Recurrent Alterations in PRKAR1A, and PRKCA Genes. Am. J. Surg. Pathol. 2017, 41, 1333–1346. [Google Scholar] [CrossRef]
- Bouys, L.; Bertherat, J. Management of Endocrine Disease: Carney complex: Clinical and genetic update 20 years after the identification of the CNC1 (PRKAR1A) gene. Eur. J. Endocrinol. 2021, 184, R99–R109. [Google Scholar] [CrossRef] [PubMed]
- Drozdowski, R.; Spaccarelli, N.; Peters, M.S.; Grant-Kels, J.M. Dysplastic nevus part I: Historical perspective, classification, and epidemiology. J. Am. Acad. Dermatol. 2023, 88, 1–10. [Google Scholar] [CrossRef]
- Shea, C.R.; Prieto, V.G.; Shachaf, C.M.; Florell, S.R. Grading Melanocytic Dysplasia: Updated Histopathologic Criteria. J. Cutan. Pathol. 2024. [Google Scholar] [CrossRef]
- Siddiqui, F.S.; Puckett, Y.; Dunn, C. Familial Atypical Multiple Mole and Melanoma Syndrome; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/pubmed/26892865 (accessed on 16 September 2025).
- Soura, E.; Eliades, P.J.; Shannon, K.; Stratigos, A.J.; Tsao, H. Hereditary melanoma: Update on syndromes and management: Genetics of familial atypical multiple mole melanoma syndrome. J. Am. Acad. Dermatol. 2016, 74, 395–407, quiz 408–410. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.T.; Fusaro, R.M.; Pester, J.; Lynch, J.F. Familial atypical multiple mole melanoma (FAMMM) syndrome: Genetic heterogeneity and malignant melanoma. Br. J. Cancer 1980, 42, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Aslan Kayiran, M.; Karadağ, A.S.; Küçük, Y.; Çobanoğlu Şimşek, B.; Erdemir, V.A.; Akdeniz, N. Are clinicians successful in diagnosing cutaneous adnexal tumors? a retrospective, clinicopathological study. Turk. J. Med. Sci. 2020, 50, 832–843. [Google Scholar] [CrossRef]
- Brown, S.; Brennan, P.; Rajan, N. Inherited skin tumour syndromes. Clin. Med. 2017, 17, 562–567. [Google Scholar] [CrossRef]
- Cook, S.; Bajwa, D.; Hollestein, L.; Husain, A.; Rajan, N. A 5-year retrospective review of skin adnexal tumours received at a tertiary dermatopathology service: Implications for linked genetic diagnoses. Br. J. Dermatol. 2022, 186, 167–173. [Google Scholar] [CrossRef]
- Ponti, G.; Pellacani, G.; Seidenari, S.; Pollio, A.; Muscatello, U.; Tomasi, A. Cancer-associated genodermatoses: Skin neoplasms as clues to hereditary tumor syndromes. Crit. Rev. Oncol. Hematol. 2013, 85, 239–256. [Google Scholar] [CrossRef] [PubMed]


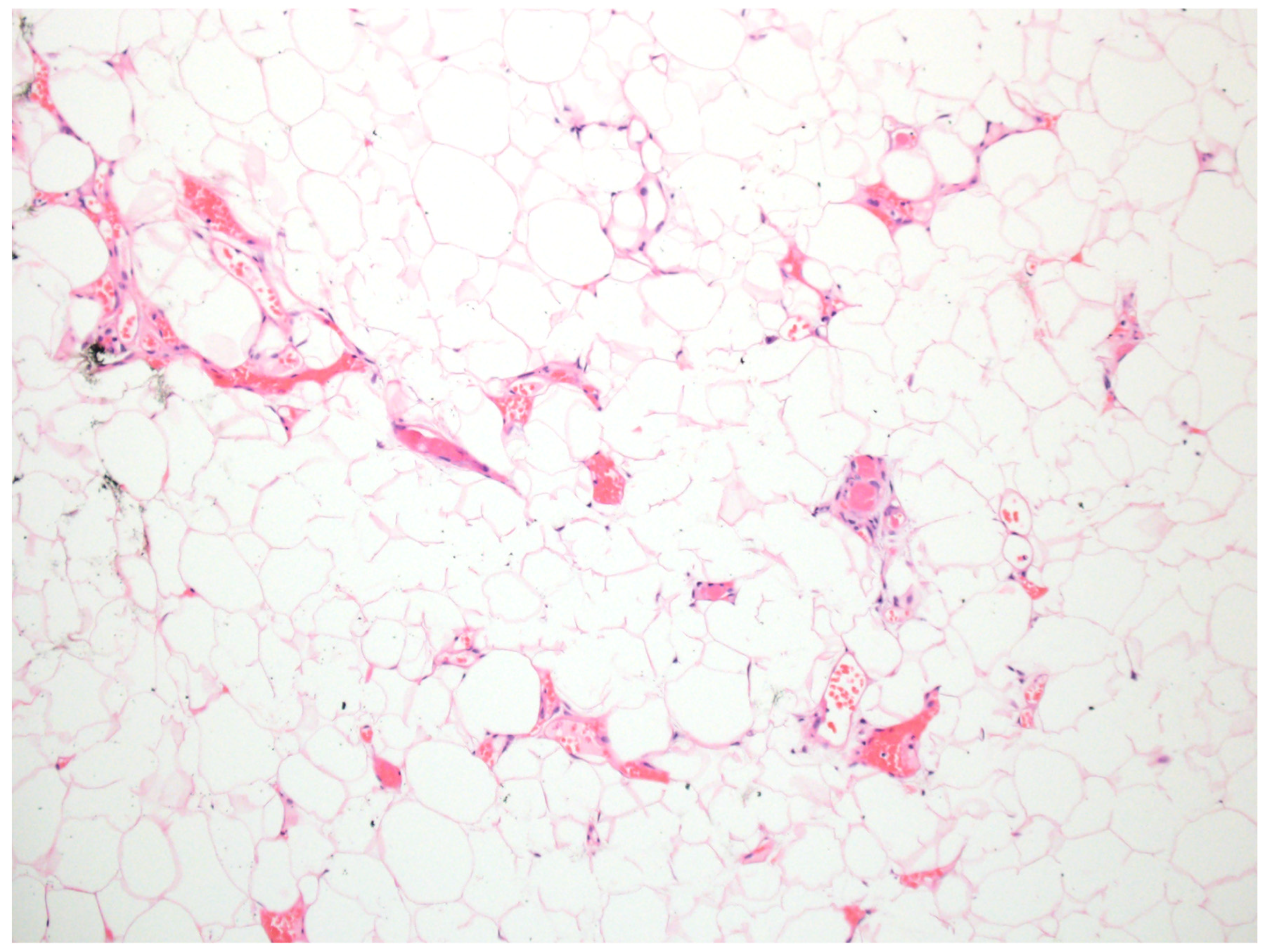
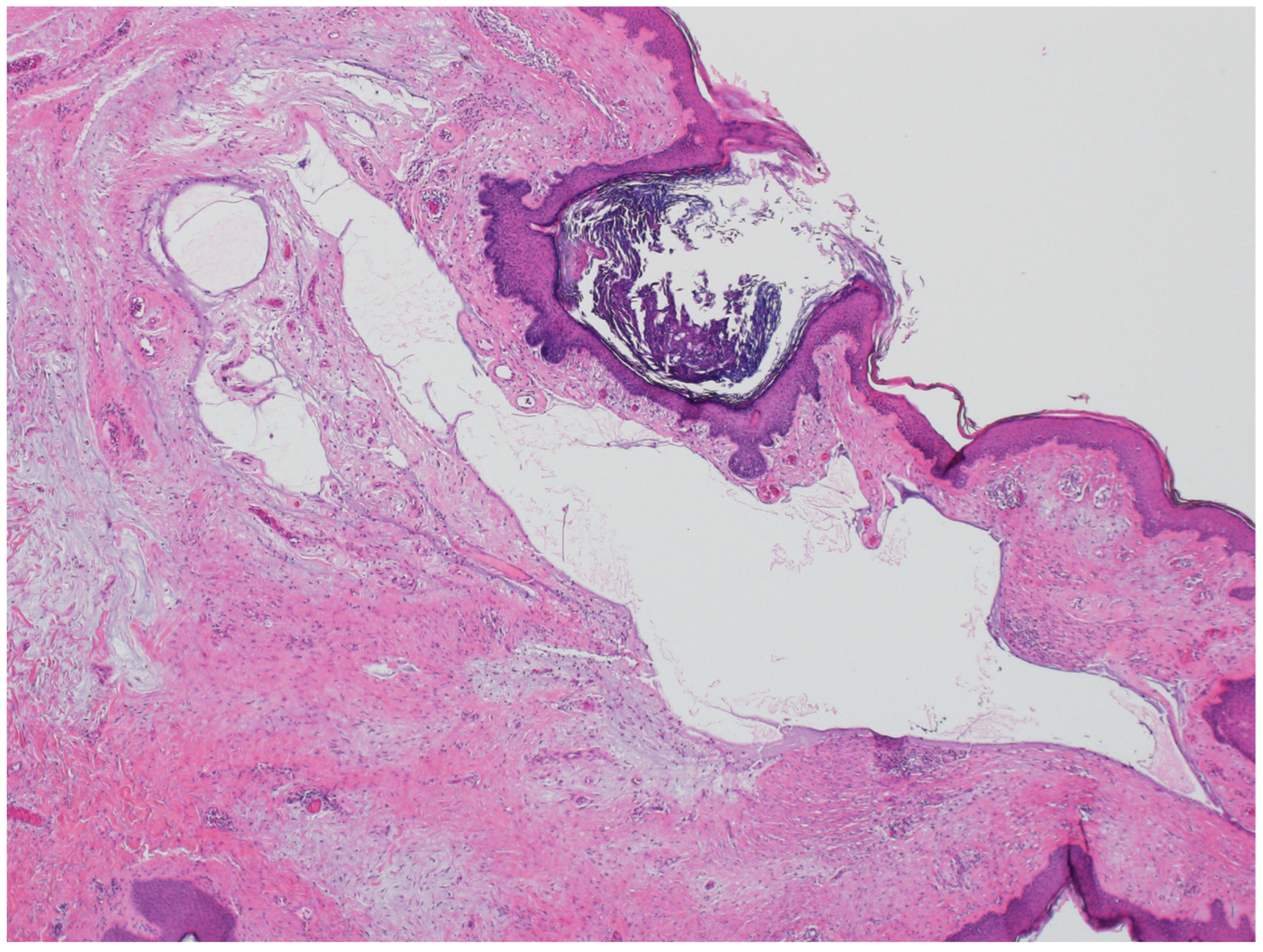
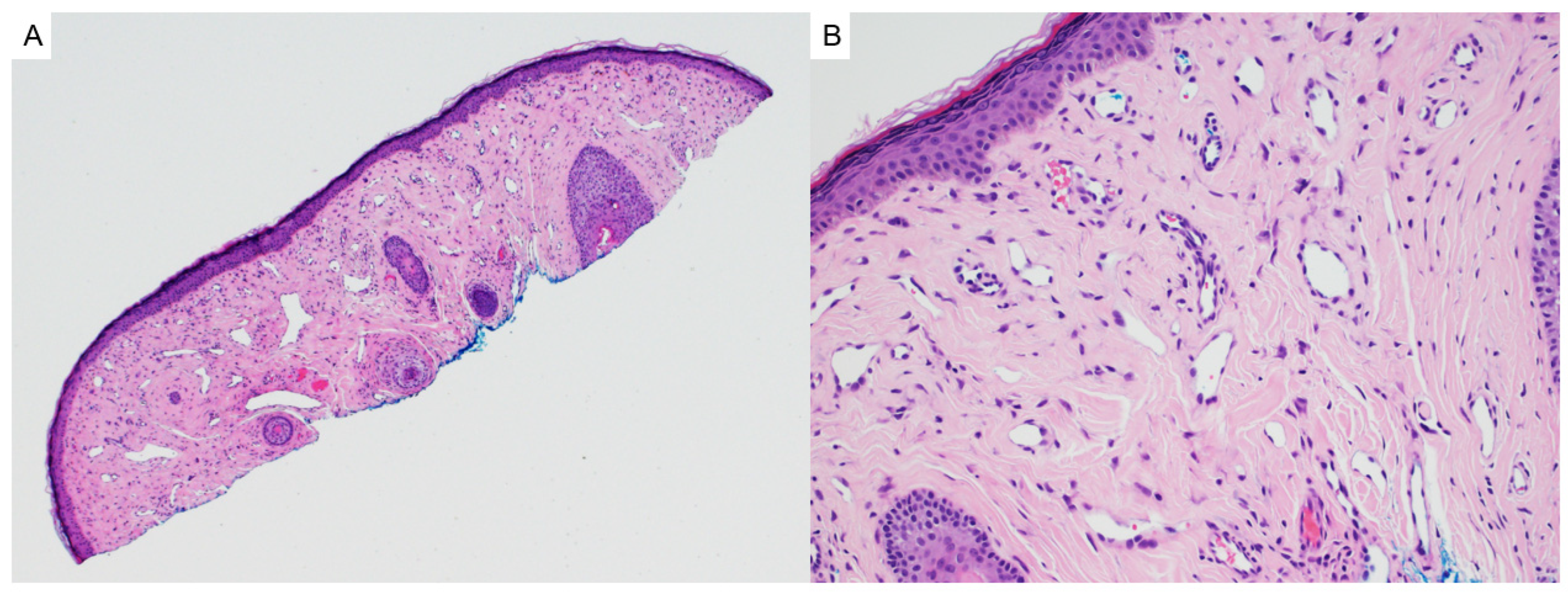
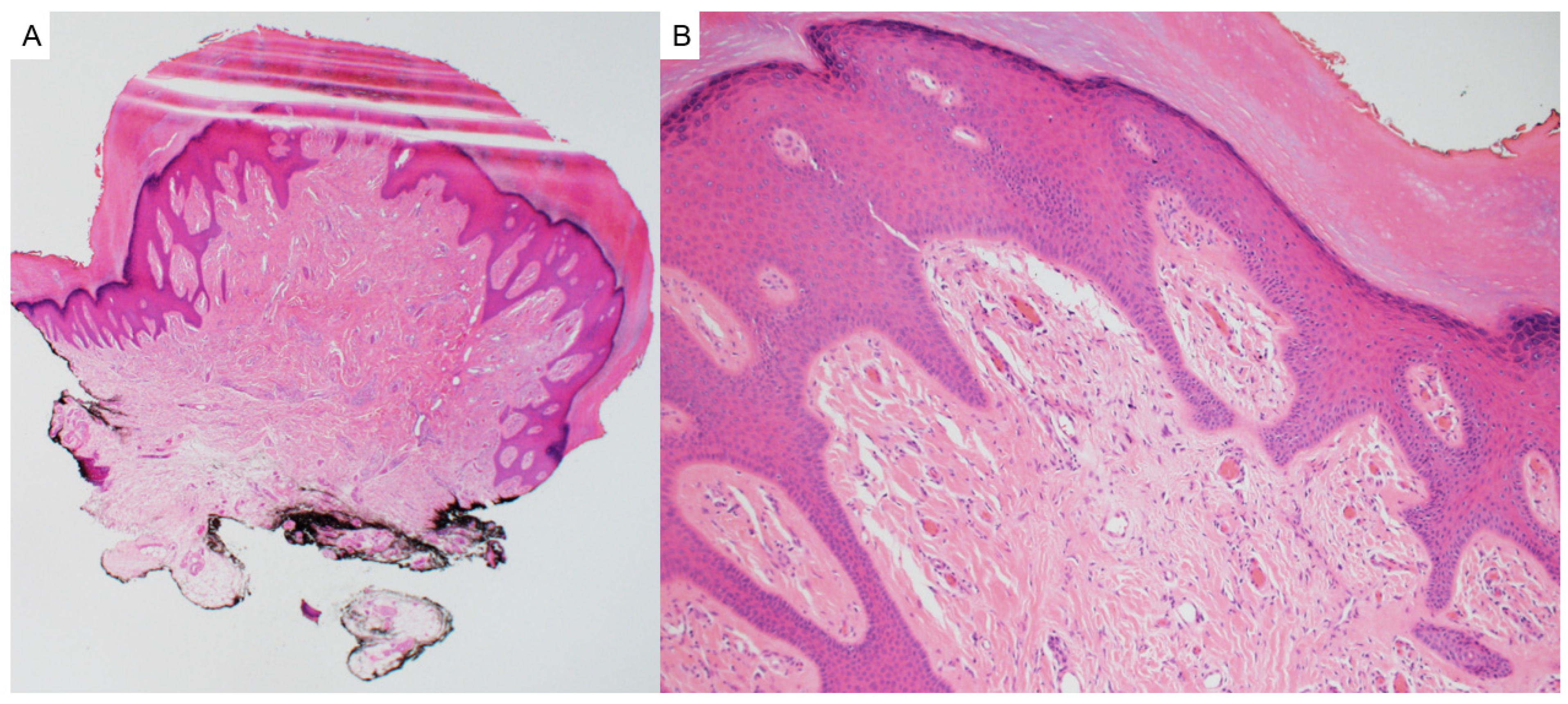
| Entity | Associated Syndrome(s) | OMIM # | Syndrome Associated Gene(s) | Other Syndrome Associated Skin Lesions | Syndrome Associated Malignancy |
|---|---|---|---|---|---|
| Fibrofolliculoma/Trichodiscoma | Birt–Hogg–Dubé syndrome | 135150 | FLCN | Facial angiofibromas, acrochordons | Renal cell carcinoma |
| Tricholemmoma | PTEN Hamartoma Tumor Syndrome (e.g., Cowden, Bannayan–Riley–Ruvalcaba, Proteus) | 153480 (Cowden) 158350 (Bannayan–Riley–Ruvalcaba), 176920 (Proteus) | PTEN | Acral keratoses, lipomas, milia, mucocutaneous papillomas | Breast, thyroid, endometrial cancers |
| Pilomatricoma | Gardner’s Syndrome | 175100 | APC | Epidermoid cysts, fibromas | Colon cancer |
| Basaloid follicular hamartoma | Nevoid basal cell carcinoma syndrome/Gorlin syndrome | 109400 | PTCH1, SUFU | Basal cell carcinoma, palmar or plantar pits | Medulloblastoma |
| Sebaceous adenoma/epithelioma | Muir–Torre syndrome | 158320 | MSH2, MLH1, MSH6, PMS2 | Sebaceous carcinoma, keratoacanthoma | Colorectal, genitourinary, endometrial, gastric, pancreatic, and breast carcinomas |
| Steatocystoma | Steatocystoma multiplex and pachyonychia congenita type 2 | 184500 (steatocystoma multiplex), 167210 (pachyonychia congenita type 2) | KRT17 | Severe nail abnormalities, painful palmoplantar keratoderma, vellus hair cysts, follicular keratoses in pachyonychia congenita type 2 | - |
| Cylindroma/Spiradenoma/Spiradenocylindroma/Trichoepitheliomas | CYLD cutaneous syndrome, including: Brooke–Spiegler syndrome, familial cylindromatosis, multiple familial trichoepitheliomas | 605041 (Brooke–Spiegler syndrome) 132700 (familial cylindromatosis) 601606 (multiple familial trichoepitheliomas) | CYLD | Basal cell carcinomas and malignant neoplasms arising from spiradenoma, cylindroma, or spiradenocylindroma | Salivary gland tumors |
| Syringoma | Down syndrome, rare others | 190685 | Trisomy 21 | Inflammatory dermatoses (Down syndrome) | Leukemia (Down syndrome) |
| Neurofibroma | Neurofibromatosis Type 1 | 162200 | NF1 | Café-au-lait macules, axillary/inguinal freckling, malignant peripheral nerve sheath tumor | Malignant peripheral nerve sheath tumor, breast cancer |
| Leiomyoma | Hereditary leiomyomatosis and renal cell cancer (Reed syndrome) | 605839 | FH | - | Type 2 papillary renal cell carcinoma |
| Angiokeratoma corporis diffusum | Anderson–Fabry disease | 301500 | GLA (α-galactosidase A) | - | - |
| Lipoma | Cowden and Proteus syndromes | 153480 (Cowden), 176920 (Proteus) | PTEN (Cowden), AKT1 (Proteus) | - | Breast, thyroid, endometrial cancers (Cowden) |
| Superficial angiomyxoma | Carney complex | 160980 | PRKAR1A | Spotty skin pigmentation | Melanocytic tumors, endocrine neoplasms |
| Facial angiofibromas/Acral fibrokeratomas | Tuberous sclerosis complex | 191100 (TSC1), 613254 (TSC2) | TSC1, TSC2 | - | - |
| BAPoma | BAP1 tumor predisposition syndrome | 614327 | BAP1 | Cutaneous melanoma | Uveal melanoma, mesothelioma, renal cell carcinoma |
| Pigmented epithelioid melanocytoma | Carney complex (in some cases) | 160980 | PRKAR1A | Spotty skin pigmentation | Melanocytic tumors, endocrine neoplasms |
| Dysplastic/atypical melanocytic nevi | Familial Atypical Multiple Mole and Melanoma Syndrome | 155600 | CDKN2A, CDK4, ARF | Melanoma | Internal malignancies, especially pancreatic carcinoma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the European Society of Dermatopathology. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lider, S.; Mandap, C.; Gill, P. Benign Cutaneous Neoplasms with Syndromic Associations. Dermatopathology 2025, 12, 34. https://doi.org/10.3390/dermatopathology12040034
Lider S, Mandap C, Gill P. Benign Cutaneous Neoplasms with Syndromic Associations. Dermatopathology. 2025; 12(4):34. https://doi.org/10.3390/dermatopathology12040034
Chicago/Turabian StyleLider, Sean, Chanel Mandap, and Pavandeep Gill. 2025. "Benign Cutaneous Neoplasms with Syndromic Associations" Dermatopathology 12, no. 4: 34. https://doi.org/10.3390/dermatopathology12040034
APA StyleLider, S., Mandap, C., & Gill, P. (2025). Benign Cutaneous Neoplasms with Syndromic Associations. Dermatopathology, 12(4), 34. https://doi.org/10.3390/dermatopathology12040034






