Abstract
While the advent of immune-checkpoint inhibitors has revolutionized cancer therapy, immune-related adverse effects (irAEs) have also been on the rise. Cutaneous toxicities are among the most common irAEs, especially in the context of programmed cell death protein-1 (PD-1) inhibitors like pembrolizumab. Herein, we report a case of anti-PD-1-induced lichen planus pemphigoides (LPP)—a rare autoimmune blistering disorder with characteristics of both lichen planus and bullous pemphigoid. To our knowledge, this is the first reported case of LPP following anti-PD-1 therapy for metastatic adrenocortical cancer. Recognizing that LPP is within the spectrum of irAEs is important, especially as the indications for immunotherapy grow to include rarer malignancies like adrenocortical cancer. In addition to our case presentation, we also provide a comprehensive review of the literature surrounding immunotherapy-induced LPP—highlighting key characteristics towards the early recognition and clinical management of this cutaneous irAE.
1. Introduction
Immune-checkpoint inhibitors (ICIs), including those targeting programmed cell death protein-1 (PD-1) receptors on T cells, are revolutionizing the treatment of solid organ and hematologic malignancies. Blocking the inhibitory signals of cytotoxic T cells, ICIs enable the upregulation of the antitumor immune response—harnessing the power of the lymphocytic system against cancer cells. Pembrolizumab is one such anti-PD-1 agent, with a growing list of approved indications including non-small-cell lung cancers, metastatic melanoma, and head and neck squamous cell cancer []. While ICIs hold immense potential in their direct clinical benefit, these inhibitors may also non-specifically activate the immune system, resulting in a subtype of side effects called immune-related adverse events (irAEs). Cutaneous toxicities are among the most common irAEs, occurring in 30–40% of patients treated with PD-1 inhibitors []. Maculopapular rash is the most commonly reported side effect, while vitiligo, lichenoid reactions, psoriasiform eruptions, and pruritus are also frequently observed [,]. Additionally, there have been well-established associations between ICIs with lichenoid dermatitis and autoimmune blistering disorders like bullous pemphigoid (BP) []. Far less frequently reported is lichen planus pemphigoides (LPP)—the unique crossover between lichen planus (LP) and BP.
LPP is a very rare autoimmune dermatosis which develops in the context of autoantibodies against BP180, a structural protein in the hemidesmosomes at the dermal–epidermal junction []. The estimated prevalence of this disorder is approximately 1 per million patients []. This subepidermal blistering disorder presents as pruritic, violaceous papules over distal extremities. It is then followed by bullous lesions on either lichenoid or normal skin [,]. Ultimately, the presence of autoantibodies at the dermal–epidermal junction, such as those against BP180 and BP230, is critical to distinguishing the diagnosis of LPP from other similar disorders like bullous LP []. Immunofluorescence and enzyme-linked immunosorbent assay studies can provide valuable information, especially in the workup of patients presenting with atypical irAEs.
To date, relatively few cases of anti-PD-1 associated LPP have been reported. Herein, we report a patient who presented with LPP following pembrolizumab treatment for metastatic adrenocortical cancer (ACC). This is the first case, to our knowledge, that describes the presentation of LPP in the context of ACC—an exceedingly rare malignancy for which immunotherapy has only recently been indicated for. In addition to our case report of anti-PD-1-induced LPP, we also provide a summary of clinical, histopathologic, and immunofluorescence features of cases previously reported in the literature.
2. Case Report
A 46-year-old woman presented with a 3-week history of painful and pruritic blisters. Her pertinent past medical history includes an 8-month history of metastatic adrenocortical carcinoma, for which she was initially treated with six cycles of etoposide, doxorubicin, and cisplatin plus oral mitotane chemotherapy that was complicated by neuropathy. She was subsequently treated with four cycles of pembrolizumab, a PD-1 inhibitor. Hives and angioedema developed 11 days after the first dose of pembrolizumab, which resolved with oral antihistamines. She went on to receive three additional doses; however, pembrolizumab was discontinued after the fourth dose, as her metastatic disease had progressed. One week after her final infusion of pembrolizumab, the patient simultaneously developed tense bulla and pruritic plaques of the trunk and extremities. The physical exam was notable for diffuse lichenoid plaques and tense bullae on the chest, abdomen, and extremities with the greatest disease burden affecting the lower extremities (Figure 1). The exam was negative for oral mucosal findings like Wickham striae. A biopsy taken from the right thigh demonstrated subepidermal separation with a patchy dermal lymphocytic infiltrate, basilar dyskeratosis, necrosis, and presence of rare eosinophils (Figure 2A). Direct immunofluorescence (DIF) exhibited linear deposition of IgG and C3 along the basement-membrane zone (Figure 2B). The collective clinical, histopathologic, and immunofluorescence findings were consistent with a diagnosis of LPP. The patient was treated with topical and oral steroids, and the condition improved over a 3-month follow-up period without sequelae.
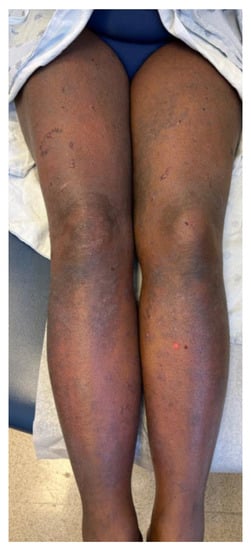
Figure 1.
Clinical characteristics of patient included tense bulla and pruritic plaques disproportionately affecting the lower extremities. Physical exam was also notable for lichenoid plaques and tense bullae on the chest, abdomen, and extremities.
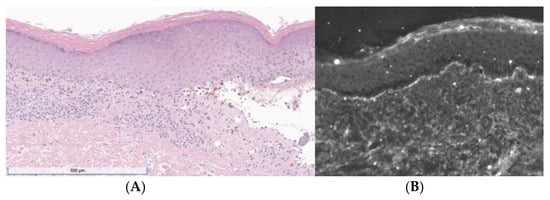
Figure 2.
(A) H&E showing band-like lymphocytic infiltrate with vacuolization of the basal layer, necrotic keratinocytes, pigment incontinence, and a subepidermal split. (B) DIF was positive for linear deposition of IgG (depicted) and C3.
3. Discussion
LPP, an uncommon autoimmune blistering disorder displaying characteristics of both LP and BP, is a rare manifestation of anti-PD-1 cutaneous toxicity. The diagnosis requires careful clinical correlation with histopathologic features, such as the presence of autoantibodies at the dermal–epidermal junction. Given the rarity of this irAE, we present our case report in conjunction with a summary of the literature on the topic. A comprehensive review of English-language medical literature was conducted using PubMed, identifying articles with terms including “lichen planus pemphigoides”, “LPP”, “PD-1 inhibitor”, “pembrolizumab”, “nivolumab”, “tislelizumab”, “PD-L1 inhibitor”, “atezolizumab”, or “durvalumab”. A total of 20 cases, including this one, are summarized from reports published between April 2017 and April 2023.
Clinical features—such as patient demographics, immunotherapy course, and LPP presentation—are summarized in Table 1 and Table 2. The mean onset age of LPP was 65.2 years, ranging between 12 and 84 years old. Among the 20 reported cases, there was a slight predominance of affected females (60%, 12/20) versus males (40%, 8/20)—mirroring the higher prevalence of LP and autoimmune blistering diseases among women [,]. Six (30%) patients identified as White, 4 (20%) identified as Black, 5 (25%) identified as Asian, and 5 (25%) did not report race. The majority (45%, 9/20) had underlying lung cancer, followed by melanoma (15%, 3/20) and urothelial cancer (15%, 3/20). Less common indications included breast cancer (5%, 1/20), Merkel cell carcinoma (5%, 1/20), renal cell carcinoma (5%, 1/20), hepatocellular carcinoma (5%, 1/20), and our case presentation of ACC (5%, 1/20). The majority of these malignancies were treated with pembrolizumab (55%, 11/20). Nivolumab (35%, 7/20) was another commonly used anti-PD-1 agent, with the remaining cases utilizing tislelizumab (5%, 1/20) or atezolizumab (5%, 1/20). The use of adjuvant therapy such as chemotherapy was noted in 20% (4/20) of patients, along with 10% (2/20) on targeted tyrosine kinase inhibitors like sitravatinib. Other medications utilized by patients were reported in five cases, with only one report of statin use and no use of angiotensin-converting enzyme (ACE) inhibitors. This is relevant given that LPP has been associated with ACE inhibitors, antituberculosis medications, and statins [].

Table 1.
Patient demographics and clinical characteristics of LPP presentation.

Table 2.
Temporal characterization of LPP onset and clinical management.
In our case and review of the literature, the presentation of LPP following immunotherapy ubiquitously included the formation of erythematous papules and plaques on the extremities. Associated pruritus was observed in 35% (7/20) of cases, and a delayed blister presentation was reported in 4 cases, ranging from 3–26 weeks after the first observation of lichenoid plaques. In addition to the upper and lower extremities, another common site of LPP was the trunk (40%, 9/20), back (25%, 5/20), and scalp (5%, 1/20). Mucosal involvement, most often presenting as reticulated white patches, was observed in 45% (9/20) of patients. Nail-bed involvement, noted in 10% (2/20) of cases, was far less common.
The average time to LPP onset from ICI commencement was 21.25 weeks, with a range of 1–79 weeks. Our patient’s presentation of LPP arose one week after the final dose of pembrolizumab, corresponding to the 12-week mark since initiation. Other associated irAEs were noted in 35% (7/20) of patients, ranging from dermatologic manifestations like hives, angioedema, joint swelling, bullous tinea, and hand–foot syndrome to more systemic irAEs like pneumonitis and heart failure. For 6 (30%) patients, ICI was discontinued prior to the onset of LPP due to either disease progression (5/20, 25%) or earlier irAE (1/20, 5%). The average time between ICI cessation and LPP diagnosis was 17.2 weeks, ranging between 1–52 weeks. This latency period, also observed in our patient, is of particular note as cutaneous toxicities can present even after cessation of immunotherapy. Among patients who were actively on immunotherapy when LPP presented (70%, 14/20), all but two (10%) had subsequent discontinuation of ICI. The majority of patients were treated with either oral or topical corticosteroids (90%, 18/20), with the remaining cases (10%, 2/20) managed with antibiotics. In the case of severe cutaneous AEs, current guidelines recommend immunotherapy withdrawal and treatment with systemic steroids [].
Histopathologic and immunofluorescence findings are highlighted in Table 3. Band-like lichenoid dermatitis was identified in all cases, often accompanied by basal-layer vacuolization (55%, 11/20), hyperkeratosis (55%, 11/20), lymphocytic infiltrate at the dermal–epidermal junction (45%, 9/20), and dyskeratotic keratinocytes (45%, 9/20). Subepidermal blisters were reported in 15 cases (75%) and eosinophilic infiltrate was observed in 10 cases (50%). DIF revealed linear C3 or IgG deposition for 95% of patients (18/19), with 68% (13/19) presenting with both C3 and IgG deposition. Four cases (21%) reported only a positive C3 deposition, and one case (5%) was positive for a linear IgG deposition at the dermal–epidermal junction. One case had negative DIF findings, but indirect immunofluorescence (IIF) demonstrated a weak linear IgG deposition at the basement-membrane zone on monkey esophagus. Another case revealed a DIF finding of fibrinogen along the basement-membrane zone. This finding, while not frequently reported in the reviewed cases of LPP, has been independently associated with both LP and BP [,].

Table 3.
Histopathologic and immunofluorescence features of LPP cases.
IIF was not performed for the majority of cases (75%, 15/20), but among those that did, 4 cases (80%) were positive for linear IgG on salt-split skin. Serum enzyme-linked immunosorbent assay (ELISA) was performed in 70% of cases (14/20), while 10% (2/20) did not conduct this assay and 20% (4/20) did not report. Among those that performed ELISA, 86% (12/14) were positive for elevated anti-BP180 titers. Anti-BP230 titer was also elevated in one of these cases (8%, 1/12), and negative in 2 (16%, 2/12). The remainder of cases with positive BP180 antibodies (75%, 9/12) did not report serum anti-BP230 titers. Two patients (10%, 2/20) revealed negative anti-BP180 and negative anti-BP230 titers. In one of these cases, however, an immunoblotting revealed IgG antibodies specific to C-terminus of BP180 []. The second patient with negative BP180 and BP230 antibodies had a unique presentation of follicular immunobullous dermatosis, as opposed to the subepidermal bullae at the nonfollicular dermal–epidermal junction observed in the other reported LPP cases []. The authors proposed the role of a novel antibody mediating the presentation of LPP at the hair follicle—suggesting a unique target at the perifollicular basement membrane that triggered a lichenoid reaction [].
The mechanism by which PD-1 inhibitors induce LPP is not fully understood. Clinically, the majority of LPP cases follow a timeline where lichenoid skin lesions precede the formation of tense blisters. Thus, it has been suggested that lichenoid inflammation itself may promote an autoimmune response against subepidermal proteins [,]. One hypothesis is that the increased cytotoxic CD8+ T cells from PD-1 inhibition renders extensive apoptosis of the basal epidermis, thereby exposing various antigens of the dermal–epidermal junction to autoreactive T cells []. This antigen presentation may enable the formation of autoantibodies, leading to the classic presentation of subepidermal blisters seen in LPP []. Details surrounding this hypothesized mechanism remain elusive given the low frequency of LPP.
To our knowledge, this is the first reported case of LPP associated with pembrolizumab treatment for metastatic adrenocortical cancer (ACC). ACC is a very aggressive and rare endocrine malignancy with an estimated incidence of 0.5 to 2 cases per million individuals [,]. Given the poor prognosis of ACC, efforts are being made to utilize immunotherapy as salvage therapy. Our patient was also started on pembrolizumab upon the failure of first-line mitotane therapy. Unfortunately, the anti-PD-1 salvage therapy was discontinued as the metastatic disease progressed.
Interestingly, multiple studies have shown an association between cutaneous toxicities and superior clinical outcomes. The development of dermatological AEs has been correlated with increased tumor response rate, progression-free survival, and overall survival [,]. It is hypothesized that the presentation of irAEs can serve as a surrogate marker for ICI efficacy []. However, the majority of these studies have focused on more common cutaneous AEs such as maculopapular rash, vitiligo, and hypopigmentation. There is currently no assessment of the relationship between LPP development and treatment outcome.
Since the first FDA approval of PD-1 inhibitors in 2014, the clinical development of such agents has gained much traction. There are currently 5 PD-1 inhibitors and 3 PD-L1 inhibitors on the market, with many more under ongoing investigation. Additionally, the indications for these immunotherapy drugs continue to expand as clinical trials include broader sets of neoplasms like ACC. For instance, just over the past decade, the indications for pembrolizumab have grown from advanced melanoma to numerous solid tumors including lung cancer, urothelial cancer, cervical cancer, esophageal cancer, renal cell carcinoma, and triple-negative breast cancer, to name a few (Figure 3). Additionally, the efficacy of pembrolizumab extends to hematologic malignancies and tissue-agnostic tumors based on specific molecular phenotypes. As the number and indications of anti-PD-1 agents continue to grow in the foreseeable future, it becomes more likely to see rare immune-related toxicities like LPP. As of June 2020, for instance, there were only 10 reported cases of anti-PD-1-induced LPP compared to the 20 patients presented in this review. The steep rise in reported cutaneous irAEs further underscores the careful surveillance and awareness needed as immunotherapy use becomes more widespread.
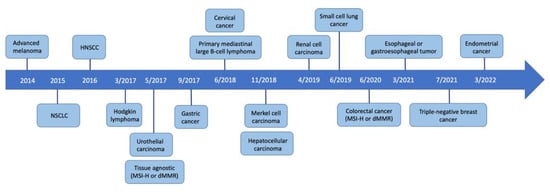
Figure 3.
Timeline of FDA-approved indications for pembrolizumab.
4. Conclusions
In summary, we report the first known case of LPP following anti-PD-1 therapy for metastatic ACC. While LPP is rare independently, its presentation with an orphan malignancy like ACC makes this case all the more unique. We also provide a summary of clinical, histopathologic, and immunofluorescence findings of 20 immunotherapy-induced LPP cases in the literature. Clinical presentation includes erythematous papules and plaques favoring the extremities and trunk. The delayed presentation of bullae after plaque formation was observed, in addition to mucosal involvement in about half the cases. Histopathology of LPP presented with a band-like lichenoid dermatitis with subepidermal bullae, often accompanied by vacuolization or hyperkeratosis. DIF revealed linear C3 or IgG, while IIF, when performed, often demonstrated linear IgG along the basement-membrane zone. Serum anti-BP180 levels were also elevated in the majority of cases. Recognizing that LPP is within the spectrum of cutaneous AEs is important as the early recognition and prompt treatment of such irAEs is critical for patient safety. This case highlights key features of LPP that can help broaden our understanding of the growing number of cutaneous toxicities in the context of PD-1 inhibitors.
Author Contributions
Conceptualization, V.M., M.C.M. and J.C.S.; writing—original draft preparation, V.M.; writing—review and editing, M.C.M. and J.C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to deidentified patient information, informed consent, and publication of a case report as opposed to original investigation.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gong, J.; Chehrazi-Raffle, A.; Reddi, S.; Salgia, R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. J. Immunother. Cancer 2018, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, O.; ElHalawani, H.; Fouad, M. Risk of cutaneous toxicities in patients with solid tumors treated with immune checkpoint inhibitors: A meta-analysis. Future Oncol. 2015, 11, 2471–2484. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Hübner, F.; Langan, E.A.; Recke, A. Lichen Planus Pemphigoides: From Lichenoid Inflammation to Autoantibody-Mediated Blistering. Front. Immunol. 2019, 10, 01389. [Google Scholar] [CrossRef]
- Weston, G.; Payette, M. Update on lichen planus and its clinical variants. Int. J. Women’s Dermatol. 2015, 1, 140–149. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Murrell, D.F. Autoimmune blistering diseases in females: A review. Int. J. Women’s Dermatol. 2015, 1, 4–12. [Google Scholar] [CrossRef]
- Kyriakis, K.P.; Terzoudi, S.; Palamaras, I.; Michailides, C.; Emmanuelidis, S.; Pagana, G. Sex and age distribution of patients with lichen planus. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 625–626. [Google Scholar] [CrossRef]
- Wat, M.; Mollanazar, N.K.; Ellebrecht, C.T.; Forrestel, A.; Elenitsas, R.; Chu, E.Y. Lichen-planus-pemphigoides-like reaction to PD-1 checkpoint blockade. J. Cutan. Pathol. 2022, 49, 978–987. [Google Scholar] [CrossRef]
- Kwon, C.W.; Murthy, R.K.; Kudchadkar, R.; Stoff, B.K. Pembrolizumab-induced lichen planus pemphigoides in a patient with metastatic Merkel cell carcinoma. JAAD Case Rep. 2020, 6, 1045–1047. [Google Scholar] [CrossRef]
- Okada, H.; Kamiya, K.; Murata, S.; Sugihara, T.; Sato, A.; Maekawa, T.; Komine, M.; Ohtsuki, M. Case of lichen planus pemphigoides after pembrolizumab therapy for advanced urothelial carcinoma. J. Dermatol. 2020, 47, e321–e322. [Google Scholar] [CrossRef]
- Sugawara, A.; Koga, H.; Abe, T.; Ishii, N.; Nakama, T. Lichen planus-like lesion preceding bullous pemphigoid development after programmed cell death protein-1 inhibitor treatment. J. Dermatol. 2021, 48, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Senoo, H.; Kawakami, Y.; Yokoyama, E.; Yamasaki, O.; Morizane, S. Atezolizumab-induced lichen planus pemphigoides in a patient with metastatic non-small-cell lung cancer. J. Dermatol. 2020, 47, e121–e122. [Google Scholar] [CrossRef] [PubMed]
- Schmidgen, M.I.; Butsch, F.; Schadmand-Fischer, S.; Steinbrink, K.; Grabbe, S.; Weidenthaler-Barth, B.; Loquai, C. Pembrolizumab-induced lichen planus pemphigoides in a patient with metastatic melanoma. J. Dtsch. Dermatol. Ges. 2017, 15, 742–745. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Fujimura, T.; Mizuashi, M.; Aiba, S. Lichen planus pemphigoides developing from patient with non-small-cell lung cancer treated with nivolumab. J. Dermatol. 2019, 46, e374–e375. [Google Scholar] [CrossRef]
- Kerkemeyer, K.L.S.; Lai, F.Y.X.; Mar, A. Lichen planus pemphigoides during therapy with tislelizumab and sitravatinib in a patient with metastatic lung cancer. Australas. J. Dermatol. 2020, 61, 180–182. [Google Scholar] [CrossRef]
- Strickley, J.D.; Vence, L.M.; Burton, S.K.; Callen, J.P. Nivolumab-induced lichen planus pemphigoides. Cutis 2019, 103, 224–226. [Google Scholar]
- Shah, R.R.; Bhate, C.; Hernandez, A.; Ho, C.H. Lichen planus pemphigoides: A unique form of bullous and lichenoid eruptions secondary to nivolumab. Dermatol. Ther. 2022, 35, e15432. [Google Scholar] [CrossRef]
- Liu, S.S.; Howard, T.; Fattah, Y.H.; Adams, A.; Hanly, A.J.; Karai, L.J. Lichen Planopilaris Pemphigoides: A Novel Bullous Dermatosis Due to Programmed Cell Death Protein-1 Inhibitor Therapy. Am. J. Dermatopathol. 2023, 45, 246–249. [Google Scholar] [CrossRef]
- Ee, S.; Liang, M.W.; Tee, S.I.; Wang, D.Y. Lichen planus pemphigoides after pembrolizumab immunotherapy in an older man. Ann. Acad. Med. Singap. 2022, 51, 804–806. [Google Scholar] [CrossRef]
- Mueller, K.A.; Cordisco, M.R.; Scott, G.A.; Plovanich, M.E. A case of severe nivolumab-induced lichen planus pemphigoides in a child with metastatic spitzoid melanoma. Pediatr. Dermatol. 2023, 40, 154–156. [Google Scholar] [CrossRef]
- Yoshida, S.; Shiraishi, K.; Yatsuzuka, K.; Mori, H.; Koga, H.; Ishii, N.; Sayama, K. Lichen planus pemphigoides with antibodies against the BP180 C-terminal domain induced by pembrolizumab in a melanoma patient. J. Dermatol. 2021, 48, e449–e451. [Google Scholar] [CrossRef] [PubMed]
- Boyle, M.M.; Ashi, S.; Puiu, T.; Reimer, D.; Sokumbi, O.; Soltani, K.; Onajin, O. Lichen Planus Pemphigoides Associated with PD-1 and PD-L1 Inhibitors: A Case Series and Review of the Literature. Am. J. Dermatopathol. 2022, 44, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Buajeeb, W.; Okuma, N.; Thanakun, S.; Laothumthut, T. Direct Immunofluorescence in Oral Lichen Planus. J. Clin. Diagn. Res. 2015, 9, ZC34–ZC37. [Google Scholar] [CrossRef]
- Provost, T.T.; Tomasi, T.B. Immunopathology of bullous pemphigoid. Basement membrane deposition of IgE, alternate pathway components and fibrin. Clin. Exp. Immunol. 1974, 18, 193–200. [Google Scholar] [PubMed]
- Rzepecki, A.K.; Cheng, H.; McLellan, B.N. Cutaneous toxicity as a predictive biomarker for clinical outcome in patients receiving anticancer therapy. J. Am. Acad. Dermatol. 2018, 79, 545–555. [Google Scholar] [CrossRef]
- Shipman, A.R.; Cooper, S.; Wojnarowska, F. Autoreactivity to bullous pemphigoid 180: Is this the link between subepidermal blistering diseases and oral lichen planus? Clin. Exp. Dermatol. 2011, 36, 267–269. [Google Scholar] [CrossRef]
- Raj, N.; Zheng, Y.; Kelly, V.; Katz, S.S.; Chou, J.; Do, R.K.G.; Capanu, M.; Zamarin, D.; Saltz, L.B.; Ariyan, C.E.; et al. PD-1 Blockade in Advanced Adrenocortical Carcinoma. J. Clin. Oncol. 2020, 38, 71–80. [Google Scholar] [CrossRef]
- Else, T.; Kim, A.C.; Sabolch, A.; Raymond, V.M.; Kandathil, A.; Caoili, E.M.; Jolly, S.; Miller, B.S.; Giordano, T.J.; Hammer, G.D. Adrenocortical Carcinoma. Endocr. Rev. 2014, 35, 282–326. [Google Scholar] [CrossRef]
- Sanlorenzo, M.; Vujic, I.; Daud, A.; Algazi, A.; Gubens, M.; Luna, S.A.; Lin, K.; Quaglino, P.; Rappersberger, K.; Ortiz-Urda, S. Pembrolizumab Cutaneous Adverse Events and Their Association with Disease Progression. JAMA Dermatol. 2015, 151, 1206–1212. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).