Abstract
Epithelioid fibrous histiocytoma (EFH) is a type of uncommon skin tumor mostly harboring Anaplastic Lymphoma Kinase (ALK) gene rearrangement, with different fusion partners reported. Whether this tumor is a separate entity or has a relationship with conventional fibrous histiocytomas is still a matter of debate. Benign course is the rule after complete surgical excision. A rare subtype of EFH with fusiform cells has been described, with specific fusion partners. Inflammatory myofibroblastic tumor (IMT) is a type of soft tissue tumor rarer than EFH, and it can display distant metastases. Some cases of primary cutaneous IMT included two with Cysteinyl-tRNA Synthetase 1 (CARS)-ALK rearrangement. IMT can have the same fusion partners as EFH, such as DCTN1, TMP3 or EML4 genes. We report the case of a 42-year-old woman presenting EFH with fusiform morphology harboring CARS-ALK fusion and discuss similarities and differences with IMT.
1. Introduction
Epithelioid histiocytous fibroma (EFH) is recognized by the World Human Organization classification of skin tumors by a distinct type of fibro-histiocytic proliferation from fibrous histiocytoma, harboring specific clinical and histopathological features. Almost all EFHs have an expression of Anaplastic Lymphoma Kinase (ALK) protein evidenced by immunohistochemistry, which is associated with an ALK gene fusion with another partner. Some authors have individualized a particular subtype of spindle cell epithelioid fibrous histiocytoma with specific fusion partners. We report yet another novel fusion partner of ALK in EFH, namely the Cysteinyl-tRNA Synthetase 1 (CARS) gene. CARS-ALK rearrangement was first identified in a metastasis from an inflammatory myofibroblastic tumor (IMT) [1]. Our case report of a novel CARS-ALK rearrangement in a spindle cell EFH histologically close to a conventional fibrous histiocytoma raises discussion about the differential diagnosis between fibrous histiocytoma, EFH and IMT (Table 1).

Table 1.
Comparison chart for the differential diagnosis of fibrous histiocytoma, epithelioid fibrous histiocytoma and inflammatory myofibroblastic tumor.
2. Case Report
We have detected a CARS-ALK rearrangement in an EFH that concerned a healthy 42-year-old woman presenting with a nodular lesion of the forearm, which clinically resembled a benign fibrous histiocytoma. Histologically, the lesion consisted of a slightly raised, relatively well-circumscribed, unencapsulated dermal nodule, composed of spindled to dendritic cells, arranged in a whorled fashion (Figure 1). Architecture was close to a conventional fibrous histiocytoma (dermal nodule with fibro-histiocytic cells, epidermal hyperplasia). However, cells were plumper than usual, and not associated with a typical coarse collagen at the periphery. We then made the hypothesis of an EFH. Although the tumor cells were not epithelioid, as typically seen in EFH, there was a diffuse cytoplasmic and granular immunoreactivity for the ALK protein, as well as for factor XIIIa and CD68, but not for smooth muscle actin, consistent with the diagnosis of EFH. The overexpression of the ALK protein correlated nicely with an ALK gene rearrangement detected by fluorescence in situ hybridization (using the LSI-Vysis ALK Dual Color Break Apart Rearrangement (Abbott, Chicago, IL, USA.) probe). Upon next generation sequencing, using the FusionPlex® Lung Archer® (Archer, Boulder, CO, USA) panel, the fusion transcript between exon 17 of the CARS gene (NM_001751.5, breakpoint chr11:3033425) and exon 20 of the ALK gene (NM_004304.4, breakpoint chr2:29446394) was identified. According to this configuration, most of the regions of CARS and the catalytic domain of ALK are retained (Figure 2). The tumor did not recur after ten months of follow-up.
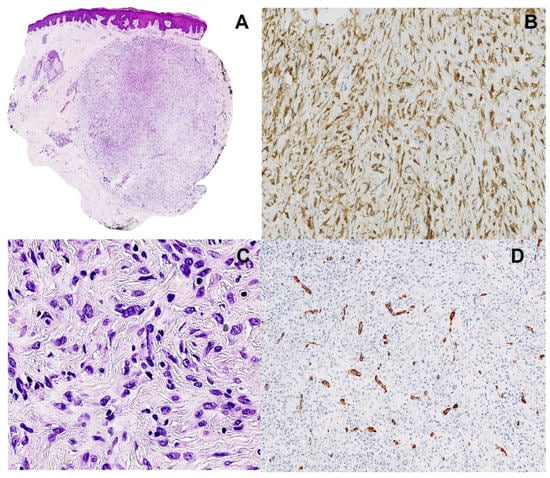
Figure 1.
Pathological findings. (A). Silhouette of the lesion showing epidermal hyperplasia, dermal nodule with endophytic growth (magnification ×12.5). (B). Cytoplasmic staining with ALK1 antibody (immunohistochemistry), highlighting the dendritic shape of the cells (magnification ×200). (C). Dendritic to epithelioid cells, with ovoid vesicular nuclei and tiny nucleoli, arranged in a whorled fashion (hematoxylin and eosin, magnification ×200). (D). Smooth muscle actin immunohistochemistry, showing small vessels within the tumor, without staining of the tumor cells (magnification ×200).
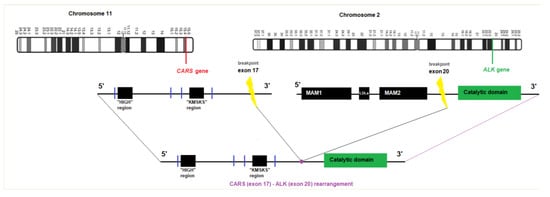
Figure 2.
Graphical view of the CARS-ALK fusion transcript.
3. Discussion and Conclusions
CARS-ALK fusions are described in IMT [2], even in skin locations [3,4]. However, to our knowledge, this is the first case to report a CARS-ALK fusion in EFH. CARS is a gene located in chromosome 11, encoding a class 1 aminoacyl-tRNA synthetase. This gene is one of several located near the imprinted gene domain altered in Beckwith-Wiedemann syndrome, Wilms tumor and other cancers. ALK encodes a receptor tyrosine kinase, which belongs to the insulin receptor superfamily, with an intracellular kinase domain. CARS-ALK fusion participates in two reactions: ligand-independent dimerization and autophosphorylation of ALK fusion. In IMT, the chimeric fused genes are likely to contribute to the neoplastic transformation by providing an active promoter, leading to overexpression of the ALK fusion product with preserved C-terminal regions, harboring the receptor tyrosine kinase activity and mediating the homo-oligomerization of the chimeric product, leading to activation of the ALK gene signaling pathway [5]. By analogy, a similar mechanism might play a role in EFH. The morphological findings in our case closely resemble those of both cases with CLTC-ALK fusions described by Georgantzoglou et al., who have identified the CLTC gene as a novel fusion partner of the ALK gene in two cases of EFH [6]. Indeed, both types of EFH were associated with predominantly fusiform to dendritic cells, instead of epithelioid cells, arranged in a whorled fashion, showing no exophytic growth or epidermal collarette and lacking a prominent capillary component. These morphological features are reminiscent of the spindle cell variant of epithelioid cell histiocytofibroma [7], which has been reported to present ALK fusions with DCTN1, TMP3 and EML4 genes [8]. Furthermore, these fusions have also been identified in IMT [9,10,11]. IMT and EFH can both show cytoplasmic expression of ALK and factor XIIIa, but, unlike IMT and some classical benign fibrous histiocytomas, EFH does not express smooth muscle actin [12]. Unlike IMT, EFH has no distant metastatic potential. Still, striking similarities can be found between both entities, as IMT can also harbor epithelioid cell morphology and express CD30, as seen in EFH, or may have few inflammatory cells [3,13,14]. Inversely, EFH may show a prominent inflammatory infiltrate, reminiscent of IMT. As molecular pathology can better classify tumors with poor cell differentiation identifying recurrent fusion abnormalities, clinical context and morphology are still important to discriminate tumors with different potential when they share the same fusion genes. Whether these similarities in morphology and molecular pathology represent a true biological relationship between EFH and IMT, defining a spectrum within these two entities, remains subject of future study.
Author Contributions
Conceptualization: L.-P.S. and E.S.; methodology: L.-P.S., A.-K.D.R. and A.-F.D.; formal analysis and investigation: L.-P.S., A.-K.D.R. and A.-F.D.; writing—original draft preparation: L.-P.S. and A.-K.D.R.; writing—review and editing: L.-P.S. and A.-K.D.R.; funding acquisition: L.-P.S., A.-K.D.R., E.S. and S.E.; resources: L.-P.S., A.-K.D.R. and L.L.; supervision: L.-P.S., A.-K.D.R. and L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We acknowledge Jonathan Vanderveken for his help.
Conflicts of Interest
The authors have no relevant financial or non-financial interests to disclose.
References
- Debelenko, L.V.; Arthur, D.C.; Pack, S.; Helman, L.J.; Schrump, D.S.; Tsokos, M. Identification of CARS-ALK Fusion in Primary and Metastatic Lesions of an Inflammatory Myofibroblastic Tumor. Lab. Investig. 2003, 83, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Cools, J.; Wlodarska, I.; Somers, R.; Mentens, N.; Pedeutour, F.; Maes, B.; De Wolf-Peeters, C.; Pauwels, P.; Hagemeijer, A.; Marynen, P. Identification of novel fusion partners of ALK, the anaplastic lymphoma kinase, in anaplastic large-cell lymphoma and inflammatory myofibroblastic tumor. Genes Chromosom. Cancer 2002, 34, 354–362. [Google Scholar] [CrossRef]
- Kanatani, Y.; Ogawa, K.; Shinkuma, S.; Mitsui, Y.; Miyagawa, F.; Ando, J.; Kuwahara, M.; Takeda, M.; Fujii, T.; Fukumoto, T.; et al. An unusual case of inflammatory myofibroblastic tumor harboring ALK-CARS fusion with few inflammatory cells: A potential diagnostic pitfall. J. Dermatol. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- McCollum, K.J.; Jour, G.; Al-Rohil, R.N. Cutaneous inflammatory myofibroblastic tumor with CARS-ALK fusion: Case report and literature review. J. Cutan. Pathol. 2022. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.W.; Xue, L.; Ma, Z.; Kinney, M.C. Alk+ CD30+ lymphomas: A distinct molecular genetic subtype of non-Hodgkin’s lymphoma. Br. J. Haematol. 2001, 113, 275–295. [Google Scholar] [CrossRef] [PubMed]
- Georgantzoglou, N.; Green, D.; Winnick, K.N.; Sumegi, J.; Charville, G.W.; Bridge, J.A.; Linos, K. Molecular investigation of ALK-rearranged epithelioid fibrous histiocytomas identifies CLTC as a novel fusion partner and evidence of fusion-independent transcription activation. Genes Chromosom. Cancer 2022, 61, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Kazlouskaya, V.; Ho, J.; Jedrych, J.; Karunamurthy, A.; Kazlouskaya, V. Spindle cell variant of epithelioid cell histiocytoma (spindle cell histiocytoma) with ALK gene fusions: Cases series and review of the literature. J. Cutan. Pathol. 2020, 48, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Kazakov, D.V.; Kyrpychova, L.; Martinek, P.; Grossmann, P.; Steiner, P.; Vanecek, T.; Pavlovsky, M.; Bencik, V.; Michal, M. ALK Gene Fusions in Epithelioid Fibrous Histiocytoma: A Study of 14 Cases, with New Histopathological Findings. Am. J. Dermatopathol. 2018, 40, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Vidrine, D.W.; Berry, J.F.; Garbuzov, A.; Falcon, C.; Tubbs, R.S.; Bui, C.J. DCTN1-ALK gene fusion in inflammatory myofibroblastic tumor (IMT) of the CNS. Child’s Nerv. Syst. 2021, 37, 2147–2151. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.; Perez-Atayde, A.; Hibbard, M.K.; Rubin, B.P.; Cin, P.D.; Pinkus, J.L.; Pinkus, G.S.; Xiao, S.; Yi, E.S.; Fletcher, C.D.; et al. TPM3-ALK and TPM4-ALK Oncogenes in Inflammatory Myofibroblastic Tumors. Am. J. Pathol. 2000, 157, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; He, X.; Cui, L.; Qiu, Y.; Li, Y.; Chen, H.; Zhang, H. Case Report: Early Distant Metastatic Inflammatory Myofibroblastic Tumor Harboring EML4-ALK Fusion Gene: Study of Two Typical Cases and Review of Literature. Front. Med. 2022, 9, 826705. [Google Scholar]
- Collins, K.; Ramalingam, P.; Euscher, E.D.; Reques Llanos, A.; García, A.; Malpica, A. Uterine Inflammatory Myofibroblastic Neoplasms with Aggressive Behavior, Including an Epithelioid Inflammatory Myofibroblastic Sarcoma: A Clinicopathologic Study of 9 Cases. Am. J. Surg. Pathol. 2022, 46, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Szablewski, V.; Laurent-Roussel, S.; Rethers, L.; Rommel, A.; Vaneechout, P.; Camboni, A.; Willocz, P.; Copie-Bergman, C.; Ortonne, N. Atypical fibrous histiocytoma of the skin with CD30 and p80/ALK1 positivity and ALK gene rearrangement. J. Cutan. Pathol. 2014, 41, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.A.; Mariño-Enriquez, A.; Fletcher, C.D.M.; Hornick, J. ALK rearrangement and overexpression in epithelioid fibrous histiocytoma. Mod. Pathol. 2015, 28, 904–912. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).