Abstract
Introduction: Scarlet fever is an acute infectious disease that is characterized by development of characteristic pin-point exanthema in a patient with signs of streptococcal pharyngitis and tonsillitis. Nowadays, due to effective antibacterial treatment, severe complicated cases of scarlet fever are occasionally rare. Because of immunity particularities, patients with Down syndrome are predisposed to bacterial respiratory diseases (and scarlet fever among them), that usually have prolonged complicated course. Case report: A clinical case of scarlet fever in a patient with Down syndrome was analyzed. The disease had a severe course with a specific skin syndrome and was complicated by pneumonia. Hospitalization and adequate treatment of the patient led to his complete recovery despite late initiation of antibacterial medicine. Discussion: A long severe complicated course of the disease in the presented case was caused by combination of the late initiation of antibacterial treatment and the patient’s personal particularities of reactivity. Conclusions: Patients with Down syndrome should be adequately monitored by family doctors in case of pharyngitis with early testing for group A hemolytic Streptococcus infection and administration of the correct antibacterial treatment, if the test is positive.
Introduction
Scarlet fever is an acute infectious disease that is characterized by development of characteristic pin-point exanthema in a patient with signs of streptococcal pharyngitis. For many years, in the era of antibiotics the incidence of scarlet fever was declining. However, a recent increase in the number of cases has been seen. During the past decade, there have been reports of major outbreaks in several countries. Countries of the Pacific Region, Western and Eastern Europe had a sudden increase in the incidence of this disease [,,].
Scarlet fever belongs to seasonal diseases (winter morbidity is typical) with periodic epidemic increase every 5–7 years possibly due to the accumulation of type-specific herd immunity among the susceptible population in the community. Scarlet fever generally has a higher incidence in males than in females, and the majority of cases occur in children aged 3–9 years, predominantly urban residents [].
Case report
Nowadays, severe complicated cases of scarlet fever are occasionally rare. We want to present one such case in a patient with Down syndrome from our practice.
A boy of 9 years and 10 months entered the infectious department on the 5th day of his disease. On admission he complained of sore throat, rash, and malaise; he refused to eat and drink.
Present history: the disease had started 5 days earlier with high grade fever, up to 40 °С. The next day, he was examined by a family doctor and an acute tonsillitis was diagnosed. Local antiseptic spray and acetaminophen were given. The patient was febrile for 4 days. A pin-point rash appeared on the lower extremities and in the inguinal skin folds on the 4th day. The next day, the rash spread all over the body and changed its appearance, the temperature became normal; but he was very weak, thus he was hospitalized.
Past history: the boy was the 4th child in his family and was a patient with Down syndrome.
On admission: the patient’s general condition was severe. He was conscious, but crying, afraid of examination. Vital signs: heart rate 96 bmp, respiratory rate 28 per minute, blood pressure 110/60 mmHg.
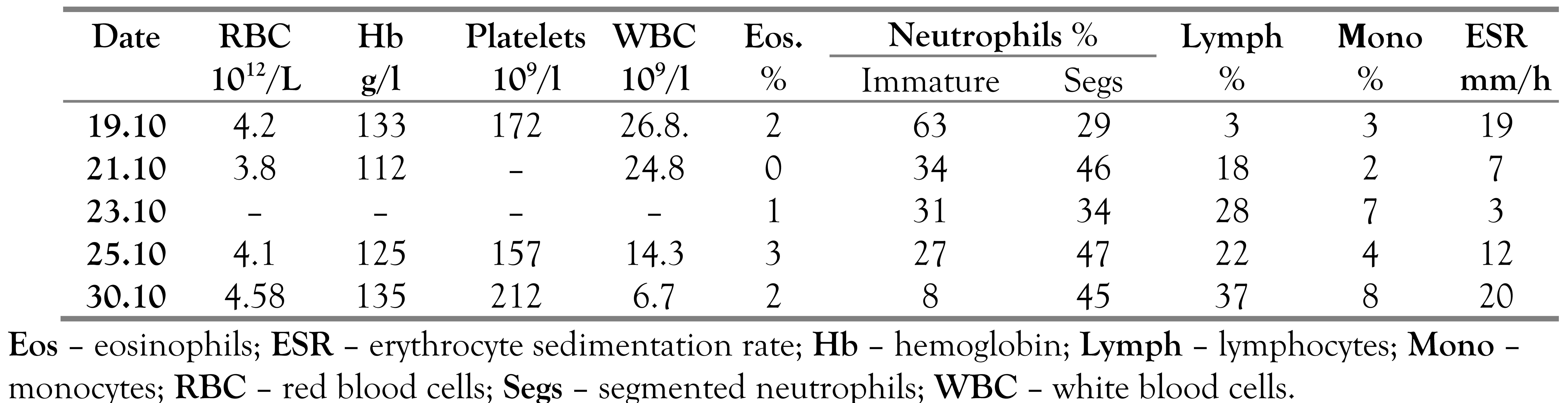
The skin was dry, edematous on the trunk. Facial erythema with perioral pallor, hyperemia of the neck, trunk and limbs with cyanotic tint were noticed. Small vesicles (miliary exanthema) were located in periumbilical region, on the lateral surfaces of the trunk, lumbar region, and inner surfaces of the thighs (Figure 1).
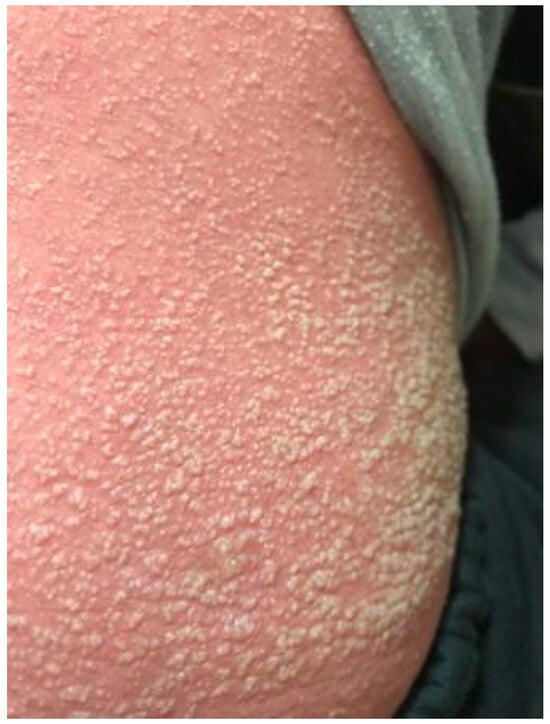
Figure 1.
Reddened skin and miliary exanthema in a patient with severe scarlet fever on admission.
Lips were dry with cracks, especially in the corners of the mouth. An unpleasant odor from the oral cavity was present. The visible oral mucosa was reddish. The tongue had a folded appearance; it was coated with whitish-yellowish exudate. Palatine tonsils were reddened with whitish exudates on them (Figure 2). Anterior cervical lymphatic nodes 1.5–2 cm in diameter were painful, elastic, and movable on palpation. Meningeal symptoms were negative. Chest percussion revealed resonant sound on admission that on the 8th day of illness was shortened below the left scapula. Breathing was harsh on admission and was weakened on the 8th day of illness correspondingly. Heart borders and heart tones were normal. The abdomen was soft and painless, with liver (+1 cm) soft and elastic on palpation. Urination was regular, but constipation was present for 2 days.
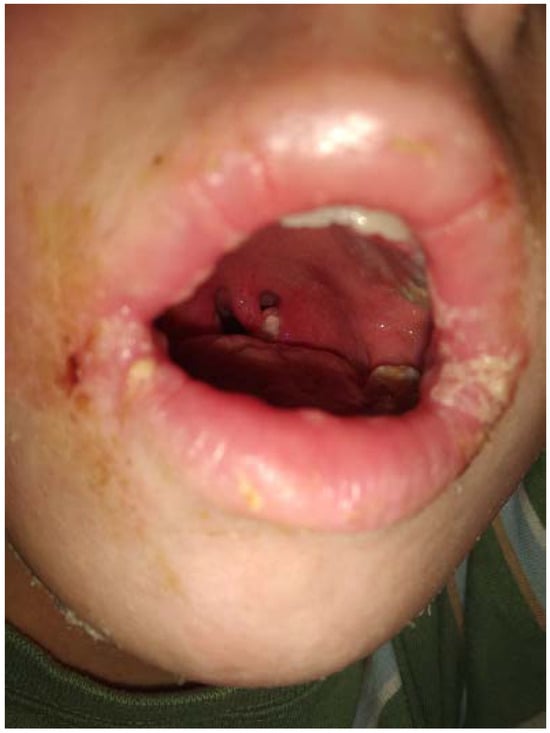
Figure 2.
Oral hyperemia, tonsillitis and cracks on the lips in a patient with severe scarlet fever.
Laboratory tests: a complete blood count (CBC) showed severe leukocytosis (26.8*109/L) and left shift of leukocyte formula (immature neutrophils—63%, segmented neutrophils—29%), elevated erythrocyte sedimentation rate (19 mm/h) on admission and was practically normal on discharge (Table 1).

Table 1.
Laboratory parameters.
Urinalyses showed mild proteinuria and leukocyturia on admission, further results (3 and 10 days later) were normal. Blood biochemistry on admission showed elevated C-reactive protein (11.6 mg/L) and increase in ASLO titer (250 and 880 IU in 2 weeks), other parameters were within the reference range.
Rapid streptotest was positive and throat culture was positive for Streptococcus pyogenes.
Chest X-ray on the 8th day of illness was done because of a weakened breathing and a shortened percussion sound. It demonstrated confluent focal infiltration in the left lung near the dome of the diaphragm. The left costo-diaphragmatic sinus was shaded. The dome of the diafragm on the left was covert. The shadow of the heart was not changed.
ECG demonstrated an incomplete blockage of the right branch of the His bundle without overload of the right ventricle, moderate metabolic changes in the myocardium, interpreted as a toxin-induced complication.
Hence, scarlet fever, typical severe form complicated with left-sided focal pneumonia was diagnosed in a patient with Down syndrome.
Etiologic therapy with IV cefazolin 1000 mg 2 t.d. for 14 days, pathogenetic therapy with intravenous prednisolone 1.5 mg/kg and IV fluids for 2 days, and symptomatic therapy with antihistamines to decrease itching were administered.
In dynamics: body temperature was normal through all the period of hospital treatment; there were no new lesions; the patient’s tongue remained coated with the previous folded appearance; massive desquamation of the epidermis started in the periumbilical region, the inner surfaces of the thighs 2 days after admission and progressed all over the body through the next two weeks (Figure 3). Signs of pharyngitis resolved in 6 days, oral cracks healed in 10 days. The patient was successfully discharged home on the 16th day of admission (20th day of the disease) with normal CBC, C reactive protein, urinalysis, ECG and chest X-ray results.
Figure 3.
Desquamation on the right hand in a patient with severe scarlet fever, 11th day of hospital treatment.
Discussion
Scarlet fever is caused by a limited number of Streptococcus pyogenes lineages that belong to group A hemolytic Streptococcus (GAS), and is associated with the exotoxin genes Ssa, SpeA and SpeC [].
The rash that occurs in scarlet fever is a delayed-type hypersensitivity to an exotoxin; it occurs in patients with a previous exposure to S. pyogenes. This is consistent with scarlet fever being more prevalent in certain geographic areas with regions with more cases of GAS infections. Elsewhere, loss of the immunologic memory on the basis of organism hyperreactivity can lead to recurrent cases of scarlet fever [].
The immunological status of children with Down syndrome (DS) has some particularities. Compared to children without DS, it is characterized by a significant decrease in the absolute number of T-lymphocytes (CD3+) and subpopulations (CD4+, CD8+), absolute number of B-lymphocytes (CD20+) and general IgG antibodies. The number of activated cells (CD25+), natural killers (CD16+) is increased, and IgE is significantly increased, predisposing them to humoral IgE-dependent reactions and allergic manifestations []. The numbers of blood memory B-cells are reduced, with impaired molecular maturation of IgA and IgM, which play an important role in mucosal immunity.
But these are not the only important factors. Individuals with Down syndrome are characterized by some upper and lower airway abnormalities (specific craniofacial features, adenotonsillar hypertrophy, airway malacia and hypotonia), altered mucus secretion, gastroesophageal reflux, chronic aspiration []. These non-immunological factors contribute to upper and lower respiratory tract infections, pneumonia among them, that could be one more reason of severe complicated course of scarlet fever except for the late administration of an antibacterial therapy.
Although children with DS have bacterial infections more frequently, viral infections in this population are usually complicated by secondary bacterial diseases. The incidence of severe GAS infection is significantly higher in them compared with children without DS. Children with DS exhibit prolonged illness, with longer mean duration of hospitalization and need additional treatment with a higher percentage of patients who need intensive care to overcome the same infection compared to children without DS [,]. Furthermore, the allergic component of their disease could be much more severe than in the general population, as was seen in our patient.
The incubation period of scarlet fever lasts 2 to 7 days. Typically, scarlet fever is associated with an acute pharyngitis. Pharyngitis may present with reddened swollen tonsils (with or without purulent exudates) and bright red pharynx with initial pin-point enanthema on the soft palate.
Skin rash usually appears on the 1st or 2nd day of the disease. It is blanching, pin-point and feels like sandpaper. The trunk, underarms, and groin are affected first, and then it spreads to the extremities. Pastia lines are found in the neck, antecubital, and groin skin folds. Usually, the palms and soles and circumoral area are spared. Miliary (as 1 mm vesicles with whitish-yellowish exudate), papular (on knees and elbows mainly), and hemorrhagic rash (as petechial lesions in skin folds) are other variants of exanthema in scarlet fever usually seen simultaneously with typical sandpaper lesions. Both miliary and hemorrhagic ones are characteristic for severe cases []. After the initial rash begins to resolve, a period of desquamation can occur and last up to two weeks. Typical pin-point exanthema had late appearance (on the 4th day of the disease) in our patient, and progressed to miliary exanthema on the base of totally reddened skin with future total complete desquamation of epidermis, confirming the severe course of his disease.
The “strawberry tongue” begins with a white coating of the tongue with hyperplastic papillae for 2–3 days of illness. As the white coating resolves, the papules remain, giving the appearance of a strawberry since 4th day of the disease. All these are induced by Spe (SpeA mainly). But, the “strawberry tongue” was not present in the described case.
The diagnosis of scarlet fever is based on typical clinical picture. The “gold standard” for laboratory confirmation is a positive throat culture. Faster and more convenient rapid antigen tests are highly specific (95% or higher), so a positive result provides an immediate diagnosis and obviates the need for culture. In children and adolescents, a negative rapid test should be confirmed with a throat culture, as the sensitivity of the culture method is higher than that of the rapid antigen tests []. Positive rapid test in our patient was confirmed with positive throat swab: S. pyogenes was obtained.
Serologic assays for antibodies to S. pyogenes antigens, such as SLO or DNase B, are useful for retrospective diagnosis of an antecedent S. pyogenes infection in cases of suspected acute rheumatic fever or post-streptococcal glomerulonephritis, but these tests are not useful for the acute diagnosis of S. pyogenes pharyngitis, as a rise in specific antibodies begins only 7 to 14 days after the onset of infection, reaching maximum levels at 3 to 4 weeks (ASLO titer in our patient had significant growth through 2 weeks) []. Complete blood count shows leukocytosis with left shift of the leukocyte formula that corresponded to the severe course of the disease in our patient.
The early antibacterial treatment of scarlet fever is important, both to address symptoms and to prevent further spread of infection. Beta lactam antibiotics are the preferred treatment for GAS infection due to their clinical efficacy, safety in children, and low cost []. Penicillin or amoxicillin in adequate doses and regimens still represent the first-line treatment; they seem to clinically outperform cephalosporins and macrolides in the treatment of GAS infections. If the affected person has an allergy to penicillin, a first-generation cephalosporin or clindamycin can be used []. Cephalosporin of the 1st generation was given to our patient as it was one of the only available group of parenteral beta lactams in the hospital.
Treatment with intravenous immunoglobulin (IVIG) is recommended to patients who have severe course of scarlet fever or complicated with toxic shock syndrome because of specific antitoxic immunity absence []. Symptomatic treatment includes paracetamol or ibuprofen for temperature control and fluid replacement.
For the majority of patients treated promptly, the outcome is excellent. After the diagnosis is made and treatment is initiated, the patient can return to normal activity 24 h after the fever has resolved. Recovery is usually complete in 3–6 days but the skin symptoms may take 14–21 days to subside []. A long severe complicated course of the disease in the presented case was caused by combination of late initiation of antibacterial treatment and personal particularities of the immune system reactivity. Despite late hospitalization, initiation of antibacterial and supportive treatment in adequate doses led to his complete recovery 3 weeks after the first signs of scarlet fever had appeared.
Conclusions
Scarlet fever, that nowadays has mild to moderate course due to an adequate antibacterial therapy in immunocompetent children, could be rather severe in patients with DS, because of their immune system particularities. The presented case was not an exception. The disease had a severe course with characteristic severe skin syndrome and was complicated by pneumonia. Hence, patients with DS should be adequately monitored by family doctors in case of pharyngitis, with early testing for GAS infection and administration of the correct antibacterial treatment, if the test is positive.
Author Contributions
HP was responsible for design of the case report, writing the article, critical revision of the article. IH was responsible for design of case report, collection of data, data analysis and interpretation, writing the article; IS was responsible for data analysis and interpretation, critical revision of the article. All authors contributed to, read and approved the final manuscript.
Funding
None to declare.
Informed Consent Statement
Written informed consent was obtained from the parents for the publication of this case report and the associated images.
Conflicts of Interest
All authors—none to declare.
References
- Yung, C.F.; Thoon, K.C. A 12 year outbreak of scarlet fever in Singapore. Lancet Infect. Dis. 2018, 18, 942. [Google Scholar] [CrossRef] [PubMed]
- Brockmann, S.O.; Eichner, L.; Eichner, M. Constantly high incidence of scarlet fever in Germany. Lancet Infect. Dis. 2018, 18, 499–500. [Google Scholar] [CrossRef] [PubMed]
- Staszewska-Jakubik, E.; Czarkowski, M.P.; Kondej, B. Scarlet fever in Poland in 2014. Przegl. Epidemiol. 2016, 70, 195–202. [Google Scholar] [PubMed]
- Silva-Costa, C.; Carriço, J.A.; Ramirez, M.; Melo-Cristino, J. Scarlet fever is caused by a limited number of Streptococcus pyogenes lineages and is associated with the exotoxin genes ssa, speA and speC. Pediatr. Infect. Dis. J. 2014, 33, 306–310. [Google Scholar] [CrossRef] [PubMed]
- de Dios Javierre, B.; García Ventura, M.; Arrudi Moreno, M.; García Vera, C. [Recurrent scarlet fever: A common entity]. An. Pediatr. (Barc). 2017, 87, 232–233. [Google Scholar] [CrossRef] [PubMed]
- Schoch, J.; Rohrer, T.R.; Kaestner, M.; et al. Quantitative, phenotypical, and functional characterization of cellular immunity in children and adolescents with Down syndrome. J. Infect. Dis. 2017, 215, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Pandit, C.; Fitzgerald, D.A. Respiratory problems in children with Down syndrome. J. Paediatr. Child Health 2012, 48, E147–E152. [Google Scholar] [CrossRef] [PubMed]
- Megged, O.; Schlesinger, Y. Down syndrome and streptococcus group A disease in hospitalized children. Acta Paediatr. 2010, 99, 796–797. [Google Scholar] [CrossRef] [PubMed]
- Verstegen, R.H.J.; Driessen, G.J.; Bartol, S.J.W.; et al. Defective B-cell memory in patients with Down syndrome. J. Allergy Clin. Immunol. 2014, 134, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Khaertynov, K.S.; Anokhin, V.A.; Abilmazhganova, L.M.; Ismagilova, M.I. [The case of toxic shock syndrome in a patient with scarlet fever]. Vestn. Sovrem. Klin. Medicini. 2013, 6, 32–35. [Google Scholar] [CrossRef]
- Wessels, M.R. Pharyngitis and scarlet fever. In Streptococcus pyogenes: Basic Biology to Clinical Manifestations [Internet]; 10 February 2016 [Updated 25 March, 2016]; Ferretti, J.J., Stevens, D.L., Fischetti, V.A., Eds.; University of Oklahoma Health Sciences Center: Oklahoma City, OK, USA, 2016. [Google Scholar]
- Shulman, S.T.; Bisno, A.L.; Clegg, H.W.; et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2012, 55, e86–e102. [Google Scholar] [CrossRef] [PubMed]
© GERMS 2020.