Abstract
Background: “Dimocarpus longan Lour” is a tropical and subtropical evergreen tree species mainly found in China, India, and Thailand; this plant, found naturally in Bangladesh, even locally, is used as “kaviraj” medication for treating different diseases, such as gastrointestinal disorders, wounds, fever, snake bites, menstrual problem, chickenpox, bone fractures, neurological disorders, and reproductive health. Different parts of this plant, especially juice pulp, pericarp, seeds, leaves, and flowers, contain a diverse group of botanical phytocompounds, and nutrient components which are directly related to alleviating numerous diseases. This literature-based review provides the most up-to-date data on the ethnomedicinal usages, phytochemical profiling, and bio-pharmacological effects of D. longan Lour based on published scientific articles. Methodology: A literature-based review was conducted by collecting information from various published papers in reputable journals and cited organizations. ChemDraw, a commercial software package, used to draw the chemical structure of the phytochemicals. Results: Various phytochemicals such as flavonoids, tannins, and polyphenols were collected from the various sections of the plant, and other compounds like vitamins and minerals were also obtained from this plant. As a treating agent, this plant displayed many biologicals activities, such as anti-proliferative, antioxidant, anti-cancer, anti-tyrosinase, radical scavenging activity, anti-inflammatory activity, anti-microbial, activation of osteoblast differentiation, anti-fungal, immunomodulatory, probiotic, anti-aging, anti-diabetic, obesity, neurological issues, and suppressive effect on macrophages cells. Different plant parts have displayed better activity in different disease conditions. Still, the compounds, such as gallic acid, ellagic acid, corilagin acid, quercetin, 4-O-methyl gallic acid, and (-)-epicatechin showed better activity in the biological system. Gallic acid, corilagin, and ellagic acid strongly exhibited anti-cancer activity in the HepG2, A549, and SGC 7901 cancer cell lines. Additionally, 4-O-methyl gallic acid and (-)-epicatechin have displayed outstanding antioxidant activity as well as anti-cancer activity. Conclusion: This plant species can be considered an alternative source of medication for some diseases as it contains a potential group of chemical constituents.
1. Introduction
The Chinese word “longan”, meaning “dragon-eye”, conveys an accurate description of the fruit details after removing the fruit’s skin [1]. Dimocarpus longan Lour, or simply longan, is the well-known tropical and subtropical tree species of the Sapindaceae family under the Dimocarpus genus growing mainly in South Asian countries. However, China, Thailand, India, and more recently Vietnam, cultivated this plant only for commercial purposes [2]. As well as developing Asian countries, the tree can be found in Central and South American countries, southern African countries, and Australia [3]. However, depending on the climate and soil conditions, the evergreen longan tree is approximately 20 m in height, has mild green leaves, unisexual/bisexual flowers, and heart-shaped fruits, i.e., 22–36 mm in diameter and weight of 6–19 g [2]. Here, the edible fresh fruits that have outstanding importance like thin pericarp, soft pulp covering the seed, and hence, the aril is sweeter; of late, the whole fruit has diverse nutrient components that are directly related to medicinal usages [4]. From ancient times, various parts of this plant as pulp, pericarp, seed, leaves, and flowers have health benefits due to it containing fundamental bioactive components, which protect the body from different disorders, namely, insomnia, amnesia, nerve pain, fever, snake bites, gastrointestinal disorders, cuts and wounds, and menstrual problems [5,6,7].
Generally, Dimocarpus longan is a plentiful source of excellent botanical compounds from different parts (pulp, pericarp, seed, leaves, flowers); among them, the pulp is the rudimentary source of nutritional value with excellent ions (i.e., K, Mg, P, Fe, Ca), and most of them have diverse biological functions for human health [8]. Some research output data provided the active amino acid contents within the pulp part of the longan fruit juice [9,10]. However, several research findings reported that bioactive phytochemicals from the pulp part of the Lour are phenolic as well as saccharides, both poly- and mono-saccharides [11,12,13,14,15]. Additionally, in recent years, various scientific reports validated that few potential health effective compounds were extracted from the pericarp [16,17,18,19,20,21,22]. However, their brownish black seeds are also major sources of botanical phytocompounds [18,20,23,24,25,26], furthermore, leaves and flowers produce the major botanical phytoconstituents (ethyl gallate, astragalin, luteolin, gentisic acid, epicatechin, proanthocyanidin) that have potential health benefits and most interestingly, all of them belong to either the polyphenol, flavonoid, or both, groups [27,28].
Aforementioned botanical bioactive compounds have found numerous biological activity by in vivo and in vitro model analysis, for example, longan leaf extracts have antiproliferative activity against cancer cell lines, the pericarp extracts including 4-O-methylgallic acid and (-)-epicatechin also have potent antioxidant capability and provide health benefits [21]. Additionally, polysaccharides derived from the pulp of the D. longan plant effectively affect hepatoma cells (one kind of cancer cell) and must be followed in a dose-dependent manner [29]. Furthermore, the glucans (1-3)-β-D-glucan and (1-6)-α-D-glucan have potent anti-cancer activity, as the experiment conducted by the Iteku Bekomo Jeff and colleagues [30] showed. It is important to mention that extracted longan pulp polysaccharides (LP I–IV) directly inhibited the proliferation of HeLa, A549, and HepG2 cancer cell lines at different concentrations ranging from 5.6 to 16.8 percent, 8.3 to 23.2 percent, and 4.7 to 29.5 percent, respectively, and most importantly, LP III inhibited the A549 and HepG2 cells more strongly than other pulp crude extracts [31]. Moreover, three phenolic compounds (gallic acid, corilagin, and ellagic acid) exhibited significant anti-cancer activity in the SGC 7901, HepG2, as well as A549 cancer cell lines [19]; at the same time, flower and seed extracts of the longan plant possessed strong anti-cancer potential on several cancer cell lines via mediating the cancer modulatory pathways [32]. Consequently, inflammation and inflammation-mediated diseases are minimized via mediating the H2O extract of longan pericarp [33]. Longan leaf extract and specific extracted chemical components possess significant activity towards the HCV (hepatitis-C virus) and influenza virus infection, respectively [34,35]; lipopolysaccharide (LP-1, 2) derived from the longan pulp possesses immunomodulatory activity [12]. Here, ellagic acid showed the most potent anti-fungal activity, longan seeds have better anti-fungal activity against opportunistic yeast, for example, Candida species and Cryptococcus neoformans [36]. According to the findings of several research studies, the leaf extract has anti-aging properties that are dose-dependent [37,38], on the other hand, research outcomes demonstrated that the extracted compounds from the longan fruit and water extract of the longan flower have been shown to have strong neuroprotective effect through enhancing the survival of immature neurons [39,40]. The seed extract of the longan plant have also shown strong anti-diabetic and anti-hyperglycemic effect in both in vitro and in vivo research models by inhibiting glucosidase activity [41]. Moreover, longan polyphenol (quercetin) inhibits tyrosinase activity [42]; longan flower water extract directly ameliorates hyperlipidemic effects and obesity following the regulation of SREBP-1c with FAS gene expression molecular mechanisms [43,44]. D. longan fruit extract directly involves the activation of Erk-1/2 (extracellular signal regulated kinase-1/2) enzyme-dependent-RUNX-2 (runt related transcription factor-2) factors and initiates the differentiation of osteoblasts along with strong activity towards osteoporosis issues [14].
The current review provides more advanced information on the ethnomedicinal uses, taxonomical details, phytochemical profiling, and pharmacological effects of D. longan Lour based on published scientific reports and databases.
2. Research Methodology
In this current review, all of the significant data were collected and analyzed, as well as summarized, from diverse areas of the Dimocarpus longan plant, including botanical description, ethnomedicinal purposes, bioactive phytoconstituents, pharmacological activities through searching PubMed, Google Scholar, Scopus, Willy online sources, ScienceDirect, ResearchGate, SpringerLink, Web of Science, and several patent offices (as-USPTO, CIPO, WIPO). However, all of the published work on Dimocarpus longan was cited in this investigation, which is published in English along with distinct keywords, are used for searching information such as D. longan and D. longan Lour, botanical description, active phytochemicals of longan, scientific classification, anti-cancer, anti-microbial and D. longan, anti-inflammation, and longan plant parts were used. All references listed in the collected articles were also examined to identify further relevant papers. All chemical compound structures were drawn via the ChemDraw tool.
3. Results and Discussion
3.1. Scientific Classification of Longan and Geographical Description
Taxonomical details of longan tree here—[9]
Kingdom—Plantae
Division—Angiospermea (flowering plant)
Class—Eudicots
Subclass—Rosidea
Order—Sapindales
Family—Sapindaceae
Genus—Dimocarpus
Species—Dimocarpus longan Lour
China is the original birthplace of the longan tree, but it is widespread in all parts of South Asian countries. All tropical and sub-tropical countries produce this tree, but it is mainly propagated in China and Thailand [45]. It is noteworthy that different countries commercially cultivate this tree, for example, China, Thailand, India, and Vietnam [46]. Counter-wise, Crane et al. (2005) [3] reported that the tree was found in Taiwan, Myanmar, Cambodia, Laos, Australia, Kenya, some Central and South American countries, and southern African countries. In Bangladesh, the district of Barisal is most famous for cultivating the Dimocarpus longan Lour and it is known locally as “Kath litchi or Ashphal”, which is used as an edible fruit as well as for medicinal purposes (mainly used as an antidote) [47].
3.2. Complete Botanical Description of D. longan Tree
The longan is a very gracious, vertical, and static tree with 20 m height and diameter depending on climate and soil conditions. The orbicular shape at the top of the tree grows with uneven and mercurial peel [48]. Evergreen leaves of the longan tree are dilated with 6–9 leaflets per pair of spare and paripinnate leaves. The leaves are up to 30 cm (12 inches) long and 3.5–5 cm wide with deep margins and stingless tops of the leaves. However, the longan tree forms shiny leaves with dark green on the upper sides and on the lower base the leaves have a mild green color. Leaves are usually smooth but now and then they have a woolly texture [49]. A longan tree usually forms one shoot per year, but sometimes it produces more than one flurry of the shoot and the tree shoots over summer or autumn. Moreover, flowers are small, just 5–6 petalled and the tree produces both unisexual and bisexual (hermaphroditic) flowers [50]. The petals of these flowers are yellow-brown with the tree bearing flowers towards the end of winter. Besides, the female flower conveys a carpellate ovary; flowers are 4–18 inches (10–45 cm) long, held on the panicle in bunch form [3]. Dimocarpus longan fruits are small and drupaceous fruit of 22–36 mm in diameter and weight of 6–19 g. Heart-shaped longan fruits contain only one seed, and fruits are mostly yellowish to light brown with mellifluous carriage peel. The edible portion is robust with fettle white-peel; furthermore, 350 fruits may be carried by panicles and flowering to harvest is from 140–190 days [3]. Hence, mature longan fruit contains one seed inside the fruit; basically, the seed is orbicular with black or brown color with a rounded white spot that has the appearance of a dragon’s eye [51]. Various parts of this part are graphically represented under the Figure 1.

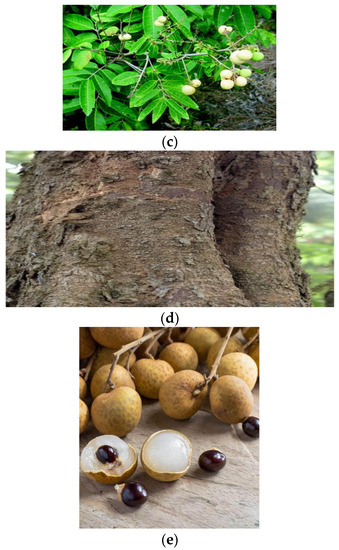
Figure 1.
Diverse parts of the D. longan plant, namely, (a) complete fruits, (b) flowers, (c) leaves, (d) trunk, and (e) seeds.
3.3. Ethnobotanical Usages of Longan
In China, the pulp of the longan Lour fruit showed diverse effective health biological functions, for instance, flourishing blood metabolism, calming and relaxing nerves, alleviating insomnia, restraining amnesia, enhancing longevity, relieving nerve pain, curing nerve swelling, and medicating palpitations [14]. Additionally, for a long time, the longan plant was used to treat fatigue diseases. The phytochemical constituents of the flowers and seeds of longan decrease the pain associated with urinary disorders. However, the flower, root, pulp, and pericarp have antioxidant, anti-glycation, anti-tyrosinase, anti-fungal, anti-microbial, and anti-cancerous activities. For these reasons, these parts are used in medication for diabetes, cancer, fungal, microbial infections, etc. [14,49]. In Bangladesh, the local “kaviraj” in Barisal use it for different diseases, such as gastrointestinal disorders, cuts and wounds, fever, snake bites, menstrual problems, chickenpox, bone fractures, cattle disorders, and so on; it is more prevalently used as the antidote for poison [47]. In Tabgail, longan is used locally to treat neurological disorders and reproductive health [52].
3.4. Nutrient Components and Phytochemicals Profiling of Dimocarpus longan
3.4.1. Nutrient Components of the Fruits
Carbohydrates (12–23%), potassium (196.5 mg/100 g), ascorbic acid (43.12–163.7 mg/100 g), and water (about 80%) are all contained in fresh longan pulp [8]. Despite not having the maximum polysaccharide content, the fruit pulp is the edible portion widely used in traditional medicine [53]. Fresh longan fruit is rich in nutritional components and free amino acids [54]. Dietary compositions and amino acid compositions are illustrated in Table 1 and Table 2, respectively. Fresh longan fruit pulp contains potassium (266 mg/100 g), which maintains the proper functioning of nerves and muscles of humans [14]. Additional minerals, including iron (Fe), calcium (Ca), phosphorus (P), and magnesium (Mg), are abundant in longan fruit pulp. longan fruit pulp is rich in vitamins such as vit-C (ascorbic acid), riboflavin, thiamin, and niacin (Table 1). Furthermore, water, protein, ash, carbohydrate, and fiber are available in the Lour. fruit pulp. Fresh longan fruit pulp contains seven essential amino acids, and most importantly, few free amino acids, namely, glutamic acid (Glu), alanine (Ala), aspartic acid (Asp), valine (Val), and leucine (Leu) are found (Table 2).

Table 1.
Nutritional content per 100 g of fresh D. longan Lour fruit pulp (acquired from the USDA National Nutrient Database [14].

Table 2.
Amino acid (aa) composition per 100 g of fresh D. longan Lour fruit pulp (acquired from the USDA National Nutrient Database (https://fdc.nal.usda.gov/fdc-app.html#/food-details/169089/nutrients; accessed on 10 September 2021).
3.4.2. Phytochemical Profiling
A vast amount of potential bioactive phytoconstituents have also been isolated from different parts of the longan Lour tree and all compounds are reported within Table 3. Moreover, pulp is the chief source of the major phytocompounds, for instance, protocatechuic acid, vanillic acid, caffeic acid, 4-methylcatechol, p-Coumaric acid, ferulic acid, syringic acid, chlorogenic acid, quinic acid, narirutin, naringin, rhoifolin, hesperidin, phthalic acid, methyl hesperidin, naringenin, phlorizin, gallic acid, epicatechin, (-)-epicatechin, isoquercitrin, and coumarin [11,14,15,55]. On the contrary, pericarp accommodates many compounds, including protocatechuic acid, ellagic acid, ethyl gallate, gallic acid, corilagin, isoscopoletin, brevifolin, 4-O-methylgallic acid, proanthocyanidin trimer (a type), (-)-epicatechin, quercetin, proanthocyanidins c1, methyl gallate, methyl brevifolin carboxylate, and rutin [16,17,18,19,20,21,22]. Furthermore, the D. longan seed contains corilagin, gallic acid, ellagic acid, 3′-o-methyl-ellagic acid 4′-o-β-d glucopyranoside, ethyl gallate, geraniin, (s)-flavogallonic acid, as well as isomallotinic acid [18,20,23,24,25,26]. Furthermore, diverse research findings indicated that the leaves and flowers of longan Lour consist of several botanical phytochemicals, namely, ethyl gallate, astragalin, luteolin, kaempferol, quercetin, gentisic acid, epicatechin, and proanthocyanidin [27,28]; of note, most of the chemical compounds belong to either the polyphenol, flavonoid, or both groups. The chemical structure of all compounds is represented in Figure 2.

Table 3.
Tabular representation of extracted bioactive phytochemicals from the different parts of the D. longan Lour plant.
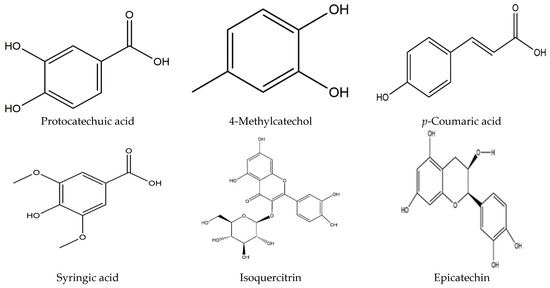
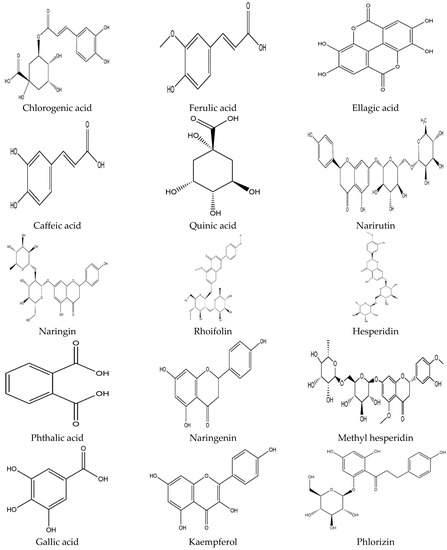
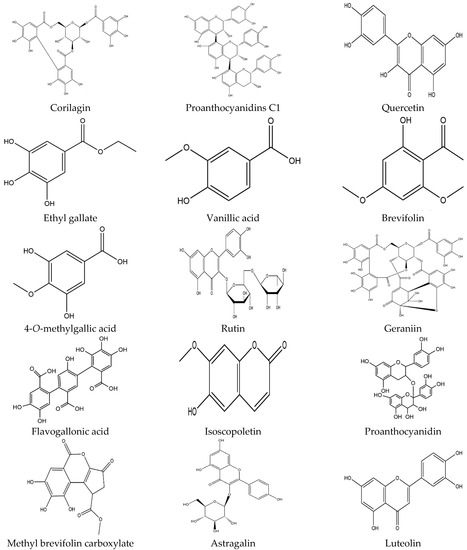
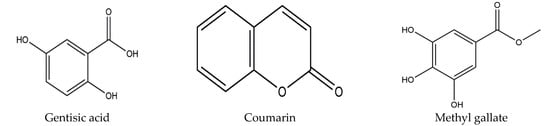
Figure 2.
Diagrammatic representation of different chemical structures.
3.5. Pharmacological Activities of Dimocarpus longan
The pharmacological investigation of the D. longan Lour are as follows and the data are summarized in Table 4.

Table 4.
Potential pharmacological activities of “Dimocarpus longan” plant.
3.5.1. Antiproliferative, Antioxidant Activity and Anticancer Activity
The excessive, abnormal, and uncontrolled growth of the body’s tissue cells are characteristics of cancer. Cancer cells (invasive) infiltrate and continue to expand the surrounding tissue (metastasis). Secondary metabolites are plant-derived compounds with bioactivity that can inhibit cancer cell proliferation [56]. The antiproliferative activity of D. longan leaf extracts against cell lines derived from cancer was studied in a controlled environment and in in vivo research models. The research study also established that longan leaf ethanol extract possessed significant antiproliferative activity against cancer-derived cell lines. Table 4 also shows the significant pharmacological activities of isolated compounds of longan. The highest antiproliferative activity was obtained by extracting WEHI-164 at 600 μg/mL and 57.45 percent by 500 μg/mL of ethanol at THP-1 at 44.93 percent [57].
Antioxidants are the chemical substances that can improve shelf-life by delaying the oxidation process when incorporated into cellular components, namely, DNA/RNA, protein, and lipid molecules, which are one of the main reasons for foodstuff degradation during production and storage [58]. Accordingly, bioactive compounds, particularly from plant sources, have become more critical in recent years [59]. Many plant-derived bioactive compounds, and crude vegetable and fruit extracts were known to positively affect the free radicals in biological systems as significant antioxidant compounds [60,61]. The pericarp of the longan fruit is densely packed with bioactive substances such as phenolic compounds, polyphenols, hydrolyzable tannins, and polysaccharides. Those compounds had considerable antioxidant activity in different models of antioxidants, including 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging, radical scavenging activity of superoxide anions, total antioxidative capacity, and inhibitory lipid peroxidation activity [22]. Phenolic compounds of longan plant parts have long been thought to possess significant antioxidant and free radical scavenging properties, due to its ability to suppress the enzymes responsible for the production of reactive oxygen species (ROS) and to reduce rapidly oxidized ROS [62,63]. To further investigate the findings of various research, Fu et al. discovered that longan possessed a ferric reducing antioxidant power (FRAP) value of 8.61 ± 0.44 µmol Fe (II)/g and a total phenolic value of 5.88 ± 0.34 µmol Trolox/g. The study also revealed a strong interaction (R2 = 0.8416) among the FRAP value and total phenolic content [59,64]. Several crops of longan have been studied for their antioxidant potential, and the cellular antioxidant activity (CAA) scores ranged from 0.49 to 6.71 mol quercetin equivalents (QE)/100 g of fruit with an average value of 2.76 mol QE/100 g of fruit. According to CAA values, the antioxidant activity of longan fruit appears to be dominated by phenolics and flavonoids [11]. The FRAP value of longan plant seed was also greater compared to the longan peel and pulp [65], in which the pulp has the lowest FRAP value within the three components. In addition, 4-O-methyl gallic acid and (-)-epicatechin also have antioxidant capabilities and health benefits extracted from the pericarp [22].
It is now known that cancer is the number one health threat to the general population, and thus we need to prevent and treat it by using potential strategies [66]. It is increasingly a preventable disease because cancer develops progressively slowly and takes many years to become a life-threatening condition [67,68,69]. A pure polysaccharide (LPS1) derived from the pulp of the longan plant has a dose-dependent manner for the significant effect on hepatoma cells, most likely due to the immunomodulatory activities of (1–6)-α-D-glucan [29]. The research study by Iteku Bekomo Jeff and colleagues demonstrated that the anti-cancer activity of the glucans (1-3)-β-D-glucan and (1–6)-α-D-glucan were confirmed [30]. In the in vitro studies, a new water-soluble polysaccharide derived from the longan pulp (LP1) demonstrated a significant anti-tumor effect on the SKOV3 and HO8910 cancer cell lines, with the antiproliferative percentages of 40 percent at a concentration of 40 mg/L and 50 percent at a concentration of 320 mg/L, respectively, at different concentrations [12]. Four extracted longan polysaccharides (LP I–IV) and refined longan pulp polysaccharides inhibited the proliferation of A549, HeLa, and HepG2 cancer cell lines at different concentrations ranging from 5.6 to 16.8 percent, 8.3 to 23.2 percent, and 4.7 to 29.5 percent, respectively, and LP III inhibited A549 and HepG2 cells more strongly than refined or crude longan pulp polysaccharides [31]. The insights of the factors associated with polysaccharide anti-tumor function were in the following order: water solubility > chain conformation > average molar masses (Mw) [70]. According to cancer epidemiological studies, enhancing the consumption rate of phenolic contents is associated with a lower risk of cancer formation [71,72,73,74]. Three phenolic compounds exhibited significant anti-cancer activity in the HepG2, A549, and SGC 7901 cancer cell lines: gallic acid, corilagin, and ellagic acid [19]. Chih-Cheng Lin et al. (2012) [32] noted that the extracted compounds of longan flower and seed possessed strong anti-cancer potential on several cancer cell lines through inhibiting the cancer modulatory pathways.
3.5.2. Anti-Inflammatory Properties
Inflammation has been defined as the tissue’s localized protective response to injury or infection, manifested by pain, redness, and swelling. The inflammatory process involves several physiological systems with a central role in the immune system. Several molecules and signaling pathways are upregulated in damaged areas as a result of inflammation. The inducing features of nitric oxide synthase (iNOS) and cyclooxygenase-2 are these pro-inflammatory enzymes (COX-2). Increased levels of nitric oxide (NO) and prostaglandins (PGs) are caused by the genes iNOS and COX-2, respectively [75]. Most strong evidence for NO’s role as a mediating role of the inflammatory response has come from studies on an animal rheumatoid model, human osteoarthritis, and rheumatoid arthritis, among other sources [76].
Additionally, to cope with the increase in oxidative stress and inflammation that occur during injury, tissues contain antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx). In recent times, it was demonstrated that dysfunctional cellular antioxidant mechanisms contribute to the development of a number of adverse and cancerous diseases in organisms [77]. There is evidence that the critical roles played by antioxidant enzymes in the inflammation pathway defend the organisms from oxidative stress [78]. The suppression of NO and tumor necrosis factor (TNF) as well as the enhancement of antioxidant enzyme activities, such as catalase, superoxide dismutase, and glutathione peroxidase, have shown that the water extract of longan pericarp (WLP) has anti-inflammatory properties [33].
3.5.3. Immunomodulatory Activities
Polysaccharides derived from a variety of natural sources have been shown to possess immunomodulating properties [14,79]. LPD2, an effective polysaccharide derived from longan pulp, demonstrated a significant effect on the upregulation of macrophages phagocytic effect as the multiplication of splenic lymphocytes through the toll-like receptor 2 (TLR2) and 4 (TLR4) facilitated myeloid differentiation factor 88/interleukin receptor-associated kinases (MDF88/ILRK) signaling pathway and the tumor necrosis factor receptor-associated factor 6 (TRAF6) signaling pathway [12,80,81,82]. The major reason why LPD2 is the stronger immunomodulatory substance is higher molecular weight, acetyl groups, and (1–4)-β-Glc. LP1 and LP1-S were shown to significantly raise the pinocytic effect of murine macrophages and development of nitric oxide (NO), interleukin 6 (IL 6), interleukin (IL-1), and tumor necrosis factor-alpha (TNF-alpha) in vitro, according to experimental research [83]. Cytokines released during the immune response by the helper T-lymphocyte play a significant role in controlling the existence of the reaction. For instance, type 1 helper T-cells (Th1 cells) release interferon (IFN- γ) and interleukin-2 (IL-2) to modulate cell-mediated immunity [84]. IFN- is a multifunctional cytokine that has immunomodulatory effects on a variety of immune cells. IFN- has been shown in mammals as a marker of cellular immunity in infected organisms [85]. Consequently, the IFN-α detection can be made to preliminarily evaluate T cell activation’s extent [86]. The water-soluble polysaccharide (LP1) extracted from Dimocarpus longan pulp has shown solid immunomodulatory activities. The research studies have significantly demonstrated that the LP1 have effectively regulated the expression of the cytokine interferon-γ (IFN-γ) and enhanced the activity of murine macrophages and the B- and T-lymphocyte production [12].
3.5.4. Prebiotic Activities
Prebiotics are the functional foodstuffs categorized as edible products that have to be measured by their health benefit by their intake in the bloodstream, and by the component’s main activity [87]. The non-digestible carbon-hydrates, such as resistant starch, galacto-oligosaccharides (GOS), fructo-oligosaccharides (FOS), and various oligosaccharides that produce carbohydrates fermentable by advantageous colon microorganisms are prebiotics that are obtained from natural sources such as vegetables, rootstock, fruit, milk, or honey [88,89]. The research studies noted that the longan pulp polysaccharides showed intense prebiotic activity on several probiotic bacterial strains. The superfine grinding-assisted enzymatic treatments (LP-SE) of longan pulp polysaccharides exhibited the most important prebiotic activities with great potential in the use of functional food and medical industries [90]. The polysaccharides from the pulp of longan had more significant effects on Lactobacillus plantarum, Lactobacillus bulgaricus, Lactobacillus fermentum, and Leuconostoc mesenteroides than LP-H (longan pulp polysaccharide extracted using warm water) and LP-S (longan pulp polysaccharide extracted using superfine grinding) [90]. Longan cellulose with a degree of hydrolysis of 21% demonstrated a greater prebiotic significance and growth level of bacteria for Lactobacillus acidophilus and Bifidobacterium lactis [91].
3.5.5. Anti-Microbial Activities
Plant extracts and phytochemicals, both of which have been shown to have anti-microbial properties, can become very useful in therapeutic approaches [92]. Several studies in various countries have been carried out to demonstrate this efficiency [93]. Due to the production of compounds in the plant secondary metabolic pathways, several species of plants have been used for their anti-microbial properties. These products are characterized by essential ingredients, such as phenolic content found in essential oils known as tannin [94,95]. The anti-microbial properties of longan Lour seed extracts were examined using disc diffusion methods, and the minimum inhibitory concentration was determined. The DL-P01-SI01 (Dimocarpus longan: crude methanolic extract; fractions: DL-P01, aquation; ethyl acetate subfractions) fraction demonstrated the highest activity against Staphylococcus aureus and methicillin-resistant S. aureus at an MIC of 64 mg/mL, attributed to the phenolic compounds [24]. Apriyanto et al. (2015) [34] reported that the longan tree leaf extract possesses activity towards the hepatitis-C virus and minimizing of death rate. Anti-influenza activity has also been noted by the chemical components from the parasitic plant on Dimocarpus longan Lour [35].
3.5.6. Anti-Fungal Activities
Intermittent fungi cause severe disease and mortality in patients with weak immune conditions [96]. Candida can be found in the normal flora of the mouth, skin, intestines, and vaginal area. Candida albicans is one of the Candida species found in the oral cavity and is responsible for most oral candidal infections [97]. Cryptococcus neoformans is a yeast-like encapsulated fungus that causes central nervous system and pulmonary problems in immunocompromised people and is an opportunistic fungal infection in both plants and animals [98,99]. The results of many studies have shown that longan seeds have anti-fungal activity against opportunistic yeast (Candida species and Cryptococcus neoformans). Ellagic acid showed the most potent anti-fungal activity, followed by corilagin and gallic acid, respectively, from all the extracted longan compounds. Candida krusei and some Candida albicans clinical strains were more efficiently suppressed by ellagic acid than Candida parapsilosis and Candida neoformans [36]. The significant pharmacological activities of isolated compounds of longan are represented in Table 4.
3.5.7. Neuroprotective Activities
Human brain synaptic vesicles contain the neurotrophin, brain-derived neurotrophic factor (BDNF), composed and deposited in the synaptic lesions to respond to endogenous or exogenous transmissions. Integration with the trkB receptor or the p75NTR receptor reveals its characteristics by interacting with the tropomyosin-related tyrosine kinase receptor B (trkB) or the p75 neurotrophin binding site p75NTR [100,101]. BDNF, which assists in neuronal transmission and memory incorporation, is an important component in producing and maintaining long-term memory synaptic transmission [102,103]. Neurogenesis appears to occur repetitively all throughout adulthood in two areas of the adult brain, known as the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus (DG) of the brainstem [104,105,106].
Additional findings have shown that brain-derived neurotrophic factor (BDNF) is necessary to preserve neuronal cells throughout development and neurogenesis [107,108]. In general, it seems that BDNF cascades and neurogenesis are a memory development procedure. In recent years, a growing number of studies have focused on neuroprotective strategies involving dietary supplements for the therapeutic interventions of central nervous system neurodegenerative disorders [109,110,111]. In the mice research model, the extracted compounds from the longan fruit part have shown a strong neuroprotective effect through enhancing the survival of immature neurons [39]. The research study by Anya Maan-Yuh Lin and colleagues reported that the water extract of longan flower possessed a potent neuroprotective effect in the brain rat model developed with the MPP+-induced neurotoxicity [40].
3.5.8. Anti-Aging Activities
Ageing is characterized by progressive disintegration of cells, a significant risk factor for developing a wide variety of degenerative diseases, including cardiovascular disease, neurodegenerative disease [112], and even skin ageing [113]. Many other research studies also reported that the phytochemical component of longan leaves showed potential anti-ageing characteristics. The longan leaves hydroethanolic extract (HE) demonstrated radical activity in the experimentation of DPPH and hydrogen peroxide with IC50 values of 30.03 ± 7.64 and 71.40 ± 15.30 μg/mL, respectively. Moreover, it showed inhibition of lipid peroxidation with IC50 of 537.01 ± 42.32 µg/mL. The HE was found to inhibit hyaluronidase and collagenase with IC50 of 234.80, 21.52 and 314.44 62.14 g/mL, respectively. The extract also showed inhibition of MMP-2 and MMP-9 that is more potent than gallic acid by zymography at 1.0 mg/mL [38].
3.5.9. Anti-Diabetic Effect and Anti-Hyper Glycemic Effects
Diabetes mellitus (DM) was one of the world’s leading causes of death. This figure is expected to reach 438 million by 2030 when misdiagnosed cases of diabetes are also included [114]. Subsequent studies have shown that hyperlipidemia and oxidative stress each play an important role in developing diabetes, with each increasing the risk of abnormalities [115]. As a result, there are many oral hypoglycemic medication therapies for the management of diabetes, such as biguanides and sulfonylureas, but these medications can produce severe side effects [116]. The research study by Ya-Yuan Tang and colleagues reported that the polyphenols and alkaloids from extracted by-products of the longan fruits possessed a strong anti-diabetic effect in vitro [18]. The pericarp extract of the longan plant revealed the potent anti-diabetic with anti-hyperglycemic activity in the mouse model by enhancing the gene expression associated with the production of insulin [17]. Moreover, the seed extract also showed strong anti-diabetic and anti-hyperglycemic effect on both the in vitro and in vivo research models by inhibiting the glucosidase activity [41].
3.5.10. Anti-Tyrosinase Properties
Browning of crude fruits, vegetables and beverages is a serious problem in the food processing industry and one of the major causes of postharvest quality loss during collection and management [117,118]. Browning of fruits and vegetables due to enzymatic action is primarily due to the oxidation of endogenous phenolic compounds [119]. The phenolic oxidation is known to be caused by an enzyme that is known as tyrosinase (monophenol, o-diphenol: oxygen oxidoreductase; EC 1.14.18.1). It is widespread in microorganisms, animals, and plants and is also responsible not only for plant browning but also animal melanization [120].
It has been demonstrated that the longan pericarp extract has anti-tyrosinase activity. When looking at ultra-high-pressure-induced extraction of 500 MPa and traditional extractions, the longan pericarp extract from the ultra-high-pressure-induced extraction exhibited the greatest proportion of anti-tyrosinase property, 23.6 ± 1.2% at the concentration of 100 g/mL, when compared to traditional extraction [121]. The mechanism of action of some tyrosinase inhibitors is via hydrophilic groups that attach with the active site of an enzyme, causing steric hindrance or altered conformation [122]. According to the study by Rout and Banerjee, the ultrasonication of polysaccharides from longan fruit pericarp (PLFP) inhibited tyrosinase activity non-competitively [123,124]. A wide variety of tests conducted on fresh and processed longan seed extracts revealed tyrosinase inhibition, and the IC50 values for fresh and processed extracts were 2.9 and 3.2 mg/mL, comparatively [23]. The polyphenols of longan have also been shown to have tyrosinase inhibitory activity. In their study, Guan et al. discovered that the inhibitory effect of longan polyphenol extract on tyrosinase activity was dose-dependent. The inhibitory impact also resulted in a high sample concentration rate. It is conceivable that ellagic acid, gallic acid, corilagin, and ethyl gallate are responsible for inhibitory activity. Such compound holds various hydroxyl groups that are structurally similar to the substrate and have the potential to attach to the copper ion active site of tyrosinase, removing active oxygen and inhibiting tyrosinase enzyme expression [14]. The results of inhibitory activity studies confirmed that longan polyphenols inhibited tyrosine diphenolase in a reversible and competitive manner. As a result, the combined effect of longan polyphenols and substances with enzymes does not create an irreversible change in the cognitive shape of the enzyme. It uses longan polyphenols as a highly competitive aid to copper ions that inhibits tyrosinase formulation in the catalyzed reaction, which ultimately reduces the level of tyrosinase in the reaction mixture. Kubo et al. also characterized the methodology by which quercetin inhibits tyrosinase activity; they discovered that quercetin inhibits tyrosinase activity compared to active tyrosinase centers [42].
3.5.11. Miscellaneous Activities
Obesity is regarded as serious health condition that leads to the manifestation of diverse health problems, including cardiovascular disease, diabetes mellitus, hypertension, fatty liver, some cancers, mental health problems, and so on, and thereby this is a life-threatening problem [125], as these conditions increase the lipid levels of our bodies. Here, the longan flower water extract directly ameliorates the hyperlipidemic effects and obesity with such effective activity showed by the polyphenol compounds. The following mechanisms act as control of the expression level of hepatic PPAR-alpha gene, regulation of SREBP-1c with FAS gene expression, reducing the exogenous lipid absorption, and the large amount of the fecal TG (triglyceride) output. Additionally, the total biological process occurred within the in vitro rat model in a dose-dependent manner [43,44].
A study by Zhu et al., 2016 [13], demonstrated that the polysaccharides of D. longan pulp could significantly promote the upregulation of sox9, aggrecan, and collagen II gene expression, consequently, synthesis of the CAM (cartilage extracellular matrix) protein as well as chondrocyte act as an excellent activity towards osteoporosis; the experiment was conducted within an in vitro model animal. On the other hand, D. longan fruit extract directly involves the activation of Erk-1/2 (extracellular signal regulated kinase-1/2) enzyme-dependent-RUNX-2 (Runt related transcription factor-2) factors via following the phosphorylation mechanism along with initiating the differentiation of osteoblasts. Longan fruit extract also represses the mRNA expression of osteoclast and thereby inhibits the differentiation of osteoclast, mediates the osteoporosis disease severity, and decreases the TRAP (tartrate resistant acid phosphatase) protein-mediated multinucleated cells in the RAW264.7 cells [14]. Additionally, NF-κB (nuclear factor-kappa B) pathway downregulation, NFATc1 (nuclear factor of activated T-cells c1) suppression through the longan Lour fruit extract efficiently involves the suppression of osteoclast differentiation in vitro. Additionally, the administration of Lour fruit extract in vivo experiment model ovariectomized rats and zebrafish enhanced their mineral contents in bone, minimizing bone disorder risk [126,127]. Longan Lour flower H2O extract attenuates the serological TG (triglyceride), disaggregates the lipid moiety, and downregulates the MMP-2,9 (matrix metalloproteinases-2,9) gene expressions, thereby protecting the hepatic cells; in vitro hypercaloric-dietary rat model study [28].
4. Concluding Remarks
Nowadays, natural food products are given more attention by people to combat diseases, including cardiovascular diseases, immune dysfunctions, and cancer insurgencies. Additionally, consumers are turning to compounds derived from medicinal plants to treat a wide range of conditions, including malignancy, due to the lower risk of complications and lower cost of these biomolecules. It was recently found that researchers from pharmaceutical sectors and medication are searching for natural compounds as medicinal agents since synthetic compounds show substantial side effects to the patients’ bodies. For this reason, this review was conducted to explore natural phytochemicals that offer therapeutic activities; as a model plant, Dimocarpus longan Lour was reviewed, and it significantly exhibited a diverse group of chemical compounds. As a source of flavonoid and phenolic components, this plant displayed different biological activities, and more interestingly, it showed strong anti-cancer and anti-diabetic activities.
Consequently, this review article has demonstrated that the compounds derived from Dimocarpus longan Lour will be used as a complementary and alternative medicine to treat many different types of diseases. They can also serve as possible sources of phyto-therapeutic lead molecules. However, according to previously published research, the pharmacokinetic evidence for this promising, highly nutritious medicinal plant and its derivative products is insufficient in this case. Therefore, more research on these natural compounds is highly required, especially on their toxicogenetical profiles. Therefore, more research is needed to discover the specific disease controlling and toxicological mechanisms and their pharmacokinetics properties.
Author Contributions
D.D. and P.B. contributed equally to the conceptualization and study design; P.P., P.B., D.D., A.S.M.S., M.A.I., R.H. participated equally in data collection, writing, and draft preparation; R.H., M.S. and A.A.M. drew all chemical structures and visualizations; M.A.R., M.N.H. and D.D. performed reviewing and editing; M.A.R., M.N.H. and B.K. visualized and supervised; B.K., funded the project. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2020R1I1A2066868), the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1A5A2019413), a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HF20C0116), and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HF20C0038).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lin, Y.; Lai, Z.; Tian, Q.; Lin, L.; Lai, R.; Yang, M.; Zhang, D.; Chen, Y.; Zhang, Z. Endogenous target mimics down-regulate miR160 mediation of ARF10, -16, and -17 cleavage during somatic embryogenesis in Dimocarpus longan Lour. Front. Plant Sci. 2015, 6, 956. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Z.; Joyce, D.C.; Ketsa, S. Postharvest biology and handling of longan fruit (Dimocarpus longan Lour.). Postharvest Biol. Technol. 2002, 26, 241–252. [Google Scholar] [CrossRef]
- Crane, J.H.; Balerdi, C.F.; Sargent, S.A.; Maguire, I. Longan Growing in the Florida Home Landscape; Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 2005. [Google Scholar]
- Pham, V.; Herrero, M.; Hormaza, J. Fruiting pattern in longan, Dimocarpus longan: From pollination to aril development. Ann. Appl. Biol. 2016, 169, 357–368. [Google Scholar] [CrossRef]
- Lim, T. Dimocarpus longan subsp. malesianus var. malesianus. In Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2013; pp. 33–38. [Google Scholar]
- Mei, Z.Q.; Fu, S.Y.; Yu, H.Q.; Yang, L.Q.; Duan, C.G.; Liu, X.Y.; Gong, S.; Fu, J.J. Genetic characterization and authentication of Dimocarpus longan Lour. using an improved RAPD technique. Genet. Mol. Res. GMR 2014, 13, 1447–1455. [Google Scholar] [CrossRef]
- Hussain, H.; Hamdan, N.; Sim, E.U.-H. Anticancer and antimicrobial peptides from medicinal plants of Borneo island in Sarawak. Adv. Tradit. Med. 2021, 21, 189–197. [Google Scholar] [CrossRef]
- Yang, B.; Jiang, Y.; Shi, J.; Chen, F.; Ashraf, M. Extraction and pharmacological properties of bioactive compounds from longan (Dimocarpus longan Lour.) fruit—A review. Food Res. Int. 2011, 44, 1837–1842. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q.J. Modern pharmacological actions of longan fruits and their usages in traditional herbal remedies. J. Med. Plants Stud. 2019, 7, 179–185. [Google Scholar]
- Xu, X.; Chen, X.; Chen, Y.; Zhang, Q.; Su, L.; Chen, X.; Chen, Y.; Zhang, Z.; Lin, Y.; Lai, Z. Genome-wide identification of miRNAs and their targets during early somatic embryogenesis in Dimocarpus longan Lour. Sci. Rep. 2020, 10, 4626. [Google Scholar] [CrossRef]
- Zhang, R.; Khan, S.A.; Lin, Y.; Guo, D.; Pan, X.; Liu, L.; Wei, Z.; Zhang, Y.; Deng, Y.; Zhang, M. Phenolic profiles and cellular antioxidant activity of longan pulp of 24 representative Chinese cultivars. Int. J. Food Prop. 2018, 21, 746–759. [Google Scholar] [CrossRef]
- Meng, F.-Y.; Ning, Y.-L.; Qi, J.; He, Z.; Jie, J.; Lin, J.-J.; Huang, Y.-J.; Li, F.-S.; Li, M.S. Structure and antitumor and immunomodulatory activities of a water-soluble polysaccharide from Dimocarpus longan pulp. Int. J. Mol. Sci. 2014, 15, 5140–5162. [Google Scholar] [CrossRef]
- Zhu, S.; Zhou, B.; Liu, Q.; Wu, H.; Zheng, L. Effect of Longan polysaccharides on proliferation and phenotype maintenance in rabbit articular chondrocytes in vitro. Med Biol. Eng. Comput. 2016, 54, 607–617. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, S.; Ho, C.-T.; Bai, N.; Wellness, H. Phytochemical constituents and biological activities of longan (Dimocarpus longan Lour.) fruit: A review. Food Sci. Hum. Wellness 2020, 9, 95–102. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, X.; Li, W.; Tan, S.; Zheng, Q. Phenolic content, antioxidant capacity, and α-amylase and α-glucosidase inhibitory activities of Dimocarpus longan Lour. Food Sci. Biotechnol. 2020, 29, 683–692. [Google Scholar] [CrossRef]
- Bai, X.; Pan, R.; Li, M.; Li, X.; Zhang, H. HPLC Profile of Longan (cv. Shixia) pericarp-sourced phenolics and their antioxidant and cytotoxic effects. Molecules 2019, 24, 619. [Google Scholar] [CrossRef]
- Li, L.; Xu, J.; Mu, Y.; Han, L.; Liu, R.; Cai, Y.; Huang, X. Chemical characterization and anti-hyperglycaemic effects of polyphenol enriched longan (Dimocarpus longan Lour.) pericarp extracts. J. Funct. Foods 2015, 13, 314–322. [Google Scholar] [CrossRef]
- Tang, Y.Y.; He, X.M.; Sun, J.; Li, C.B.; Li, L.; Sheng, J.F.; Xin, M.; Li, Z.C.; Zheng, F.J.; Liu, G.M.; et al. Polyphenols and alkaloids in byproducts of longan fruits (Dimocarpus Longan Lour.) and their bioactivities. Molecules 2019, 24, 1186. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.N.; Hao, J.; Shi, J.; Liu, T.; Li, J.; Wei, X.; Qiu, S.; Xue, S.; Jiang, Y. Antioxidant and anticancer activities of high pressure-assisted extract of longan (Dimocarpus longan Lour.) fruit pericarp. Innov. Food Sci. Emerg. Technol. 2009, 10, 413–419. [Google Scholar] [CrossRef]
- Zhu, X.R.; Wang, H.; Sun, J.; Yang, B.; Duan, X.W.; Jiang, Y.M. Pericarp and seed of litchi and longan fruits: Constituent, extraction, bioactive activity, and potential utilization. J. Zhejiang Univ. Sci. B 2019, 20, 503–512. [Google Scholar] [CrossRef]
- Sun, J.; Shi, J.; Jiang, Y.; Xue, S.J.; Wei, X. Identification of two polyphenolic compounds with antioxidant activities in longan pericarp tissues. J. Agric. Food Chem. 2007, 55, 5864–5868. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, W.; Liu, Y.; Zhan, R.; Chen, Y. Two new flavonol glycosides from Dimocarpus longan leaves. Nat. Prod. Res. 2015, 29, 163–168. [Google Scholar] [CrossRef]
- Rangkadilok, N.; Sitthimonchai, S.; Worasuttayangkurn, L.; Mahidol, C.; Ruchirawat, M.; Satayavivad, J. Evaluation of free radical scavenging and antityrosinase activities of standardized longan fruit extract. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2007, 45, 328–336. [Google Scholar] [CrossRef]
- Tseng, H.C.; Wu, W.T.; Huang, H.S.; Wu, M.C. Antimicrobial activities of various fractions of longan (Dimocarpus longan Lour. Fen Ke) seed extract. Int. J. Food Sci. Nutr. 2014, 65, 589–593. [Google Scholar] [CrossRef]
- Soong, Y.Y.; Barlow, P.J. Isolation and structure elucidation of phenolic compounds from longan (Dimocarpus longan Lour.) seed by high-performance liquid chromatography-electrospray ionization mass spectrometry. J. Chromatogr. A 2005, 1085, 270–277. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Xu, Y.-J.; Ge, Z.-Z.; Zhu, W.; Xu, Z.; Li, C.-M. Structural elucidation and antioxidant activity evaluation of key phenolic compounds isolated from longan (Dimocarpus longan Lour.) seeds. J. Funct. Foods 2015, 17, 872–880. [Google Scholar] [CrossRef]
- Mai, J.; Liang, J.; Liu, X.; Tan, L.; Xu, H.; Li, Y.; Zhou, Y.; Yang, C.; Xin, C. Simultaneous determination of 5 components in the leaves of Dimocarpus longan by Quantitative Analysis of Multicomponents by Single Marker (QAMS) based on UPLC and HPLC. J. Anal. Methods Chem. 2020, 2020, 3950609. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-W.; Yang, D.-J.; Chang, Y.-Y.; Hsu, C.-L.; Tseng, J.-K.; Chang, M.-H.; Wang, M.; Chen, Y.-C. Polyphenol-rich longan (Dimocarpus longan Lour.)-flower-water-extract attenuates nonalcoholic fatty liver via decreasing lipid peroxidation and downregulating matrix metalloproteinases-2 and-9. Food Res. Int. 2012, 45, 444–449. [Google Scholar] [CrossRef]
- Zhu, Q.; Jiang, Y.; Lin, S.; Wen, L.; Wu, D.; Zhao, M.; Chen, F.; Jia, Y.; Yang, B. Structural identification of (1→6)-α-d-glucan, a key responsible for the health benefits of longan, and evaluation of anticancer activity. Biomacromolecules 2013, 14, 1999–2003. [Google Scholar] [CrossRef] [PubMed]
- Jeff, I.B.; Yuan, X.; Sun, L.; Kassim, R.M.; Foday, A.D.; Zhou, Y. Purification and in vitro anti-proliferative effect of novel neutral polysaccharides from Lentinus edodes. Int. J. Biol. Macromol. 2013, 52, 99–106. [Google Scholar] [CrossRef]
- Yi, Y.; Huang, F.; Zhang, M.W.; Zhang, R.F.; Deng, Y.Y.; Wei, Z.C.; He, J.R. Solution properties and in vitro anti-tumor activities of polysaccharides from longan pulp. Molecules 2013, 18, 11601–11613. [Google Scholar] [CrossRef]
- Lin, C.C.; Chung, Y.C.; Hsu, C.P. Potential roles of longan flower and seed extracts for anti-cancer. World J. Exp. Med. 2012, 2, 78–85. [Google Scholar] [CrossRef]
- Huang, G.J.; Wang, B.S.; Lin, W.C.; Huang, S.S.; Lee, C.Y.; Yen, M.T.; Huang, M.H. Antioxidant and anti-inflammatory properties of longan (Dimocarpus longan Lour.) pericarp. Evid. Based Complementary Altern. Med. ECAM 2012, 2012, 709483. [Google Scholar] [CrossRef]
- Apriyanto, D.R.; Aoki, C.; Hartati, S.; Hanafi, M.; Kardono, L.B.; Arsianti, A.; Louisa, M.; Sudiro, T.M.; Dewi, B.E.; Sudarmono, P.; et al. Anti-hepatitis C virus activity of a crude extract from longan (Dimocarpus longan Lour.) leaves. Jpn. J. Infect. Dis. 2016, 69, 213–220. [Google Scholar] [CrossRef]
- Cheng, J.C.; Liaw, C.C.; Lin, M.K.; Chen, C.J.; Chao, C.L.; Chao, C.H.; Kuo, Y.H.; Chiu, Y.P.; Peng, Y.S.; Huang, H.C. Anti-influenza virus activity and chemical components from the parasitic plant Cuscuta japonica choisy on Dimocarpus longans Lour. Molecules 2020, 25, 4427. [Google Scholar] [CrossRef]
- Rangkadilok, N.; Tongchusak, S.; Boonhok, R.; Chaiyaroj, S.C.; Junyaprasert, V.B.; Buajeeb, W.; Akanimanee, J.; Raksasuk, T.; Suddhasthira, T.; Satayavivad, J. In vitro antifungal activities of longan (Dimocarpus longan Lour.) seed extract. Fitoterapia 2012, 83, 545–553. [Google Scholar] [CrossRef]
- Puspita, R.; Bintang, M.; Priosoeryanto, B.P. Antiproliferative activity of longan (Dimocarpus longan Lour.) leaf extracts. J. Appl. Pharm. Sci. 2019, 9, 102–106. [Google Scholar]
- Doungsaard, P.; Chansakaow, S.; Sirithunyalug, J.; Shang-Chian, L.; Wei-Chao, L.; Chia-Hua, L.; Kuan-Ha, L.; Leelapornpisid, P. In vitro biological activities of the anti-aging potential of Dimocarpus longan leaf extracts. CMU J. Nat. Sci. 2020, 19, 235–251. [Google Scholar] [CrossRef]
- Park, S.J.; Park, D.H.; Kim, D.H.; Lee, S.; Yoon, B.H.; Jung, W.Y.; Lee, K.T.; Cheong, J.H.; Ryu, J.H. The memory-enhancing effects of Euphoria longan fruit extract in mice. J. Ethnopharmacol. 2010, 128, 160–165. [Google Scholar] [CrossRef]
- Lin, A.M.; Wu, L.Y.; Hung, K.C.; Huang, H.J.; Lei, Y.P.; Lu, W.C.; Hwang, L.S. Neuroprotective effects of longan (Dimocarpus longan Lour.) flower water extract on MPP+-induced neurotoxicity in rat brain. J. Agric. Food Chem. 2012, 60, 9188–9194. [Google Scholar] [CrossRef]
- Rakariyatham, K.; Zhou, D.; Rakariyatham, N.; Shahidi, F. Sapindaceae (Dimocarpus longan and Nephelium lappaceum) seed and peel by-products: Potential sources for phenolic compounds and use as functional ingredients in food and health applications. J. Funct. Foods 2020, 67, 103846. [Google Scholar] [CrossRef]
- Kubo, I.; Kinst-Hori, I.; Chaudhuri, S.K.; Kubo, Y.; Sánchez, Y.; Ogura, T. Flavonols from Heterotheca inuloides: Tyrosinase inhibitory activity and structural criteria. Bioorganic Med. Chem. 2000, 8, 1749–1755. [Google Scholar] [CrossRef]
- Yang, D.J.; Chang, Y.Y.; Hsu, C.L.; Liu, C.W.; Lin, Y.L.; Lin, Y.H.; Liu, K.C.; Chen, Y.C. Antiobesity and hypolipidemic effects of polyphenol-rich longan (Dimocarpus longans Lour.) flower water extract in hypercaloric-dietary rats. J. Agric. Food Chem. 2010, 58, 2020–2027. [Google Scholar] [CrossRef]
- Patra, S.; Nithya, S.; Srinithya, B.; Meenakshi, S.M. Review of medicinal plants for anti-obesity activity. Transl. Biomed. 2015, 6. [Google Scholar] [CrossRef]
- Lai, Z.; Chen, C.; Zeng, L.; Chen, Z. Somatic embryogenesis in longan [Dimocarpus longan Lour.]. In Somatic Embryogenesis in Woody Plants; Springer: Dordrecht, The Netherlands, 2000; pp. 415–431. [Google Scholar]
- Wang, B.; Tan, H.W.; Fang, W.; Meinhardt, L.W.; Mischke, S.; Matsumoto, T.; Zhang, D. Developing single nucleotide polymorphism (SNP) markers from transcriptome sequences for identification of longan (Dimocarpus longan) germplasm. Hortic. Res. 2015, 2, 14065. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.R.; Das, S.; Alam, M.; Rahman, A. Documentation of wild edible minor fruits used by the local people of Barishal, Bangladesh with emphasis on traditional medicinal values. J. Bio-Sci. 2019, 27, 69–81. [Google Scholar] [CrossRef]
- Menzel, C.M.; Waite, G.K. Litchi and Longan: Botany, Production and Uses; CABI Publishing: Wallingford, UK, 2005. [Google Scholar]
- Subhadrabandhu, S.; Stern, R.A. Taxonomy, Botany and Plant Development; CABI Publishing: Wallingford, UK, 2005; pp. 25–34. [Google Scholar]
- Pham, V.; Herrero, M.; Hormaza, J.I. Phenological growth stages of longan (Dimocarpus longan) according to the BBCH scale. Sci. Hortic. 2015, 189, 201–207. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, E.; Xu, L.; Li, Z.; Wang, Z.; Li, C. Comparison on characterization of longan (Dimocarpus longan Lour.) polyphenoloxidase using endogenous and exogenous substrates. J. Agric. Food Chem. 2010, 58, 10195–10201. [Google Scholar] [CrossRef]
- Haque, M.; Bari, L.; Hasan, M.; Sultana, M.; Reza, S. A survey on medicinal plants used by the folk medicinal practitioners in Tangail Sadar Upazilla, Tangail, Bangladesh. J. Environ. Sci. Nat. Resour. 2014, 7, 35–39. [Google Scholar] [CrossRef][Green Version]
- Okuyama, E.; Ebihara, H.; Takeuchi, H.; Yamazaki, M. Adenosine, the anxiolytic-like principle of the Arillus of Euphoria longana. Planta Med. 1999, 65, 115–119. [Google Scholar] [CrossRef]
- Yang, E.; Sim, K. Characterisation of nutritional, physiochemical, and mineral compositions of aril and seed of longan fruit (Dimocarpus longan L.). Int. Food Res. J. 2021, 28, 91–101. [Google Scholar]
- Khan, S.A.; Liu, L.; Lai, T.; Zhang, R.; Wei, Z.; Xiao, J.; Deng, Y.; Zhang, M. Phenolic profile, free amino acids composition and antioxidant potential of dried longan fermented by lactic acid bacteria. J. Food Sci. Technol. 2018, 55, 4782–4791. [Google Scholar] [CrossRef]
- Ayoola, G.; Coker, H.; Adesegun, S.; Adepoju-Bello, A.; Obaweya, K.; Ezennia, E.C.; Atangbayila, T. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop. J. Pharm. Res. 2008, 7, 1019–1024. [Google Scholar]
- Priosoeryanto, B.P.; Tateyama, S.; Yamaguchi, R.; Uchida, K. Antiproliferation and colony-forming inhibition activities of recombinant feline interferon (rFeIFN) on various cells in vitro. Can. J. Vet. Res. Rev. Can. Rech. Vet. 1995, 59, 67–69. [Google Scholar]
- Rahman, M.A.; Cho, Y.; Nam, G.; Rhim, H. Antioxidant compound, oxyresveratrol, inhibits APP production through the AMPK/ULK1/mTOR-mediated autophagy pathway in mouse cortical astrocytes. Antioxidants 2021, 10, 408. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Rahman, M.A.; Sohag, A.A.M.; Uddin, M.J.; Dash, R.; Sikder, M.H.; Rahman, M.S.; Timalsina, B.; Munni, Y.A.; Sarker, P.P.; et al. Black cumin (Nigella sativa L.): A comprehensive review on phytochemistry, health benefits, molecular pharmacology, and safety. Nutrients 2021, 13, 1784. [Google Scholar] [CrossRef]
- Prasad, K.N.; Divakar, S.; Shivamurthy, G.R.; Aradhya, S.M. Isolation of a free radical-scavenging antioxidant from water spinach (Ipomoea aquatica Forsk). J. Sci. Food Agric. 2005, 85, 1461–1468. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hannan, M.A.; Dash, R.; Rahman, M.H.; Islam, R.; Uddin, M.J.; Sohag, A.A.M.; Rahman, M.H.; Rhim, H. Phytochemicals as a complement to cancer chemotherapy: Pharmacological modulation of the autophagy-apoptosis pathway. Front. Pharmacol. 2021, 12, 639628. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.P.; Hopia, A.I.; Heinonen, M. Berry phenolics and their antioxidant activity. J. Agric. Food Chem. 2001, 49, 4076–4082. [Google Scholar] [CrossRef]
- Robards, K.; Prenzler, P.D.; Tucker, G.; Swatsitang, P.; Glover, W.J.F.C. Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 1999, 66, 401–436. [Google Scholar] [CrossRef]
- Zhang, R.; Zeng, Q.; Deng, Y.; Zhang, M.; Wei, Z.; Zhang, Y.; Tang, X. Phenolic profiles and antioxidant activity of litchi pulp of different cultivars cultivated in Southern China. Food Chem. 2013, 136, 1169–1176. [Google Scholar] [CrossRef]
- Guo, C.; Yang, J.; Wei, J.; Li, Y.; Xu, J.; Jiang, Y. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr. Res. 2003, 23, 1719–1726. [Google Scholar] [CrossRef]
- Dey, D.; Quispe, C.; Hossain, R.; Jain, D.; Ahmed Khan, R.; Janmeda, P.; Islam, M.T.; Ansar Rasul Suleria, H.; Martorell, M.; Daştan, S.D.; et al. Ethnomedicinal use, phytochemistry, and pharmacology of Xylocarpus granatum J. Koenig. Evid. Based Complementary Altern. Med. 2021, 2021, 8922196. [Google Scholar] [CrossRef]
- Algra, A.M.; Rothwell, P.M. Effects of regular aspirin on long-term cancer incidence and metastasis: A systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012, 13, 518–527. [Google Scholar] [CrossRef]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer chemoprevention by carotenoids. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef]
- Narayanan, B.A. Chemopreventive agents alters global gene expression pattern: Predicting their mode of action and targets. Curr. Cancer Drug Targets 2006, 6, 711–727. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, L.; Cheung, P.C. Physicochemical properties and antitumor activities of water-soluble native and sulfated hyperbranched mushroom polysaccharides. Carbohydr. Res. 2006, 341, 2261–2269. [Google Scholar] [CrossRef]
- Brown, E.M.; Gill, C.I.; McDougall, G.J.; Stewart, D. Mechanisms underlying the anti-proliferative effects of berry components in in vitro models of colon cancer. Curr. Pharm. Biotechnol. 2012, 13, 200–209. [Google Scholar] [CrossRef]
- Kendall, C.W.; Esfahani, A.; Truan, J.; Srichaikul, K.; Jenkins, D. Health benefits of nuts in prevention and management of diabetes. Asia Pac. J. Clin. Nutr. 2010, 19, 110–116. [Google Scholar]
- Halliwell, B. Antioxidants and human disease: A general introduction. Nutr. Rev. 1997, 55, S44–S49. [Google Scholar] [CrossRef]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef]
- Heiss, E.; Herhaus, C.; Klimo, K.; Bartsch, H.; Gerhäuser, C. Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J. Biol. Chem. 2001, 276, 32008–32015. [Google Scholar] [CrossRef]
- Cochran, F.R.; Selph, J.; Sherman, P. Insights into the role of nitric oxide in inflammatory arthritis. Med. Res. Rev. 1996, 16, 547–563. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure-function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- Yi, Y.; Zhang, M.-W.; Liao, S.-T.; Zhang, R.-F.; Deng, Y.-Y.; Wei, Z.-C.; Tang, X.-J.; Zhang, Y. Structural features and immunomodulatory activities of polysaccharides of longan pulp. Carbohydr. Polym. 2012, 87, 636–643. [Google Scholar] [CrossRef]
- Yi, Y.; Liao, S.T.; Zhang, M.W.; Shi, J.; Zhang, R.F.; Deng, Y.Y.; Wei, Z.C. Physicochemical characteristics and immunomodulatory activities of three polysaccharide-protein complexes of longan pulp. Molecules 2011, 16, 6148–6164. [Google Scholar] [CrossRef]
- Rong, Y.; Yang, R.; Yang, Y.; Wen, Y.; Liu, S.; Li, C.; Hu, Z.; Cheng, X.; Li, W. Structural characterization of an active polysaccharide of longan and evaluation of immunological activity. Carbohydr. Polym. 2019, 213, 247–256. [Google Scholar] [CrossRef]
- Jiang, J.; Meng, F.Y.; He, Z.; Ning, Y.L.; Li, X.H.; Song, H.; Wang, J.; Zhou, R. Sulfated modification of longan polysaccharide and its immunomodulatory and antitumor activity in vitro. Int. J. Biol. Macromol. 2014, 67, 323–329. [Google Scholar] [CrossRef]
- Li, X.; Jiao, L.L.; Zhang, X.; Tian, W.M.; Chen, S.; Zhang, L.P. Anti-tumor and immunomodulating activities of proteoglycans from mycelium of Phellinus nigricans and culture medium. Int. Immunopharmacol. 2008, 8, 909–915. [Google Scholar] [CrossRef]
- Sakurai, M.H.; Matsumoto, T.; Kiyohara, H.; Yamada, H. B-cell proliferation activity of pectic polysaccharide from a medicinal herb, the roots of Bupleurum falcatum L. and its structural requirement. Immunology 1999, 97, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef]
- Pineiro, M.; Asp, N.G.; Reid, G.; Macfarlane, S.; Morelli, L.; Brunser, O.; Tuohy, K. FAO Technical meeting on prebiotics. J. Clin. Gastroenterol. 2008, 42, S156–S159. [Google Scholar] [CrossRef] [PubMed]
- Gómez, B.; Gullón, B.; Yáñez, R.; Schols, H.; Alonso, J.L. Prebiotic potential of pectins and pectic oligosaccharides derived from lemon peel wastes and sugar beet pulp: A comparative evaluation. J. Funct. Foods 2016, 20, 108–121. [Google Scholar] [CrossRef]
- Scott, K.P.; Martin, J.C.; Duncan, S.H.; Flint, H.J. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol. Ecol. 2014, 87, 30–40. [Google Scholar] [CrossRef]
- Huang, F.; Liu, H.; Zhang, R.; Dong, L.; Liu, L.; Ma, Y.; Jia, X.; Wang, G.; Zhang, M. Physicochemical properties and prebiotic activities of polysaccharides from longan pulp based on different extraction techniques. Carbohydr. Polym. 2019, 206, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Thitiratsakul, B.; Anprung, P. Prebiotic activity score and bioactive compounds in longan (Dimocarpus longan Lour.): Influence of pectinase in enzyme-assisted extraction. J. Food Sci. Technol. 2014, 51, 1947–1955. [Google Scholar] [CrossRef]
- Rahman, M.A.; Rahman, M.H.; Biswas, P.; Hossain, M.S.; Islam, R.; Hannan, M.A.; Uddin, M.J.; Rhim, H. Potential therapeutic role of phytochemicals to mitigate mitochondrial dysfunctions in Alzheimer’s disease. Antioxidants 2020, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, G.G.; Locatelli, J.; Freitas, P.C.; Silva, G.L. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz. J. Microbiol. 2000, 31, 247–256. [Google Scholar] [CrossRef]
- Janssen, A.M.; Scheffer, J.J.; Baerheim Svendsen, A. Antimicrobial activity of essential oils: A 1976–1986 literature review. Aspects of the test methods. Planta Med. 1987, 53, 395–398. [Google Scholar] [CrossRef]
- Saxena, G.; McCutcheon, A.R.; Farmer, S.; Towers, G.H.; Hancock, R.E. Antimicrobial constituents of Rhus glabra. J. Ethnopharmacol. 1994, 42, 95–99. [Google Scholar] [CrossRef]
- Connolly, J.E., Jr.; McAdams, H.P.; Erasmus, J.J.; Rosado-de-Christenson, M.L. Opportunistic fungal pneumonia. J. Thorac. Imaging 1999, 14, 51–62. [Google Scholar] [CrossRef]
- Rindum, J.L.; Stenderup, A.; Holmstrup, P. Identification of Candida albicans types related to healthy and pathological oral mucosa. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 1994, 23, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Sorrell, T.C.; Chen, S.C. Pulmonary Cryptococcosis. Semin. Respir. Crit. Care Med. 2015, 36, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Spies, F.S.; de Oliveira, M.B.; Krug, M.S.; Severo, C.B.; Severo, L.C.; Vainstein, M.H. Cryptococcosis in patients living with hepatitis C and B viruses. Mycopathologia 2015, 179, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Trk receptors: Roles in neuronal signal transduction. Annu. Rev. Biochem. 2003, 72, 609–642. [Google Scholar] [CrossRef]
- Kaplan, D.R.; Miller, F.D. Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 2000, 10, 381–391. [Google Scholar] [CrossRef]
- Figurov, A.; Pozzo-Miller, L.D.; Olafsson, P.; Wang, T.; Lu, B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature 1996, 381, 706–709. [Google Scholar] [CrossRef]
- Korte, M.; Carroll, P.; Wolf, E.; Brem, G.; Thoenen, H.; Bonhoeffer, T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc. Natl. Acad. Sci. USA 1995, 92, 8856–8860. [Google Scholar] [CrossRef]
- Gage, F.H. Mammalian neural stem cells. Science 2000, 287, 1433–1438. [Google Scholar] [CrossRef]
- Taupin, P.; Gage, F.H. Adult neurogenesis and neural stem cells of the central nervous system in mammals. J. Neurosci. Res. 2002, 69, 745–749. [Google Scholar] [CrossRef]
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms and functional implications of adult neurogenesis. Cell 2008, 132, 645–660. [Google Scholar] [CrossRef]
- Goldberg, J.L.; Barres, B.A. The relationship between neuronal survival and regeneration. Annu. Rev. Neurosci. 2000, 23, 579–612. [Google Scholar] [CrossRef]
- McAllister, A.K.; Katz, L.C.; Lo, D.C. Neurotrophin regulation of cortical dendritic growth requires activity. Neuron 1996, 17, 1057–1064. [Google Scholar] [CrossRef]
- Gutierrez-Merino, C.; Lopez-Sanchez, C.; Lagoa, R.; Samhan-Arias, A.K.; Bueno, C.; Garcia-Martinez, V. Neuroprotective actions of flavonoids. Curr. Med. Chem. 2011, 18, 1195–1212. [Google Scholar] [CrossRef] [PubMed]
- Leonardo, C.C.; Doré, S. Dietary flavonoids are neuroprotective through Nrf2-coordinated induction of endogenous cytoprotective proteins. Nutr. Neurosci. 2011, 14, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Campos-Esparza Mdel, R.; Torres-Ramos, M.A. Neuroprotection by natural polyphenols: Molecular mechanisms. Cent. Nerv. Syst. Agents Med. Chem. 2010, 10, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Rahman, M.D.H.; Hossain, M.S.; Biswas, P.; Islam, R.; Uddin, M.J.; Rahman, M.H.; Rhim, H. Molecular insights into the multifunctional role of natural compounds: Autophagy modulation and cancer prevention. Biomedicines 2020, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- López-Alarcón, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef]
- Ryan, A.S. Exercise in aging: Its important role in mortality, obesity and insulin resistance. Aging Health 2010, 6, 551–563. [Google Scholar] [CrossRef]
- Rahman, A.; Hamdani, S.U.; Awan, N.R.; Bryant, R.A.; Dawson, K.S.; Khan, M.F.; Azeemi, M.M.-U.-H.; Akhtar, P.; Nazir, H.; Chiumento, A. Effect of a multicomponent behavioral intervention in adults impaired by psychological distress in a conflict-affected area of Pakistan: A randomized clinical trial. JAMA 2016, 316, 2609–2617. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; Jiménez, M.; Cabanes, J.; García-Carmona, F.; Escribano, J. Tyrosinase inhibitory activity of cucumber compounds: Enzymes responsible for browning in cucumber. J. Agric. Food Chem. 2003, 51, 7764–7769. [Google Scholar] [CrossRef] [PubMed]
- McEvily, A.J.; Iyengar, R.; Otwell, W.S. Inhibition of enzymatic browning in foods and beverages. Crit. Rev. Food Sci. Nutr. 1992, 32, 253–273. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ferrer, A.; Rodríguez-López, J.N.; García-Cánovas, F.; García-Carmona, F. Tyrosinase: A comprehensive review of its mechanism. Biochim. Biophys. Acta 1995, 1247, 1–11. [Google Scholar] [CrossRef]
- Tuan, H.M.; Lee, C.Y.; Tang, H.C. Phenolic compounds in food and their effects on health. v. 1. Analysis, occurrence, and chemistry—v. 2. Antioxidants and cancer prevention. ACS Symp. Ser. 1992, 507. [Google Scholar]
- Prasad, K.N.; Yang, B.; Shi, J.; Yu, C.; Zhao, M.; Xue, S.; Jiang, Y. Enhanced antioxidant and antityrosinase activities of longan fruit pericarp by ultra-high-pressure-assisted extraction. J. Pharm. Biomed. Anal. 2010, 51, 471–477. [Google Scholar] [CrossRef]
- Song, K.K.; Huang, H.; Han, P.; Zhang, C.L.; Shi, Y.; Chen, Q.X. Inhibitory effects of cis- and trans-isomers of 3,5-dihydroxystilbene on the activity of mushroom tyrosinase. Biochem. Biophys. Res. Commun. 2006, 342, 1147–1151. [Google Scholar] [CrossRef]
- Yang, B.; Zhao, M.; Jiang, Y. Optimization of tyrosinase inhibition activity of ultrasonic-extracted polysaccharides from longan fruit pericarp. Food Chem. 2008, 110, 294–300. [Google Scholar] [CrossRef]
- Rout, S.; Banerjee, R. Free radical scavenging, anti-glycation and tyrosinase inhibition properties of a polysaccharide fraction isolated from the rind from Punica granatum. Bioresour. Technol. 2007, 98, 3159–3163. [Google Scholar] [CrossRef]
- Chooi, Y.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Lee, E.M.; Lee, D.Y.; Lee, J.H.; Oh, S. Longan fruit increase bone mineral density in zebrafish and ovariectomized rat by suppressing RANKL-induced osteoclast differentiation. Phytomed. Int. J. Phytother. Phytopharm. 2019, 59, 152910. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.H.; Son, Y.; Goh, S.H.; Oh, S. Longan (Dimocarpus longan Lour.) fruit extract stimulates osteoblast differentiation via Erk1/2-dependent RUNX2 activation. J. Microbiol. Biotechnol. 2016, 26, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Jiang, Y.; Yang, B.; Yu, C.; Tsao, R.; Zhang, H.; Chen, F. Structural characteristics and antioxidant activities of oligosaccharides from longan fruit pericarp. J. Agric. Food Chem. 2009, 57, 9293–9298. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhao, M.; Jiang, Y. Anti-glycated activity of polysaccharides of longan (Dimocarpus longan Lour.) fruit pericarp treated by ultrasonic wave. Food Chem. 2009, 114, 629–633. [Google Scholar] [CrossRef]
- Zheng, G.; Xu, L.; Wu, P.; Xie, H.; Jiang, Y.; Chen, F.; Wei, X. Polyphenols from longan seeds and their radical-scavenging activity. Food Chem. 2009, 116, 433–436. [Google Scholar] [CrossRef]
- Chung, Y.C.; Lin, C.C.; Chou, C.C.; Hsu, C.P. The effect of Longan seed polyphenols on colorectal carcinoma cells. Eur. J. Clin. Investig. 2010, 40, 713–721. [Google Scholar] [CrossRef]
- Aziz, H.A.; Rahim, N.A.; Ramli, S.F.; Alazaiza, M.Y.; Omar, F.M.; Hung, Y.-T. Potential use of Dimocarpus longan seeds as a flocculant in landfill leachate treatment. Water 2018, 10, 1672. [Google Scholar] [CrossRef]
- Ho, S.C.; Hwang, L.S.; Shen, Y.J.; Lin, C.C. Suppressive effect of a proanthocyanidin-rich extract from longan (Dimocarpus longan Lour.) flowers on nitric oxide production in LPS-stimulated macrophage cells. J. Agric. Food Chem. 2007, 55, 10664–10670. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).