Abstract
The primary goal of this study was to improve the rheological properties of water-based drilling mud using a combination of TiO2-coated ZnO nanoparticles and activated carbon (AC) from banana peels. The TiO2/ZnO nanocomposites were prepared using polyvinyl alcohol (PVA) as a binder under magnetic stirring and ultrasonic sonication to ensure uniform coating, followed by washing and controlled thermal treatment. NaOH-assisted chemical activation of banana peel produced activated carbon with better porosity and surface functionality than raw banana peel. The base water-based mud used in this study had different concentrations of both additives mixed in, and rheological parameters such as mud density, plastic viscosity (PV), yield point (YP), and gel strength were measured according to standard API methods. X-ray diffraction (XRD) and scanning electron microscopy (SEM) were used for structural and morphological characterization, which proved the successful coating and uniform dispersion of TiO2 on ZnO nanoparticles. The use of mixed additives resulted in a significant improvement in mud properties, such as viscosity, gel strength, and yield point, proving to be more effective in suspension capacity and overall rheological stability. The use of this hybrid bio-nanocomposite mud system is a very economical and eco-friendly way of enhancing the drilling fluid performance, thus proving to be a supporting factor in conducting drilling operations that are both safe and efficient. Additionally, this study provides a sustainable hybrid TiO2-ZnO and activated carbon additive that results in synergistic improvement of drilling-mud rheology and stability.
1. Introduction
Water-based drilling muds (WBMs) are the primary option for oil-drilling operations because they are less expensive, easier to handle, and have less environmental impact than oil-based muds. However, WBMs usually have drawbacks such as low thermal stability, substantial fluid loss, and poor rheological control under high-pressure, high-temperature (HPHT) conditions. Therefore, it is necessary to enhance WBMs’ rheological performance and filtration control of WBMs to improve the transport of cuttings, wellbore stability, and drilling efficiency [1].
Recent studies have recognized nanomaterials as an important factor in the enhancement of drilling fluid properties. For instance, ZnO and TiO2 nanoparticles, are notable for their large surface area, thermal stability, and reactivity, which can alter the microstructure and flow properties of mud systems [2]. The TiO2-coated ZnO nanocomposite not only provides the beneficial effects of both individual particles but also goes beyond their capabilities with respect to the enhancement of colloidal stability, surface charge interactions, and heat resistance.
Conversely, the use of agricultural waste in the form of activated carbon as a cost-effective and environmentally friendly drilling fluid additive is a growing area of research. Banana peel, which is a large amount of agro-waste, is mainly composed of cellulose and lignin; thus, it is very suitable for the production of activated carbon (AC), which has high porosity and adsorption capacity [3]. The development of a hybrid nanocomposite with environmentally friendly carbon is in harmony with the global movement to adopt sustainable drilling techniques.
Activated carbon from banana peel has recently gained attention as a practical and sustainable alternative to traditional coal-based activated carbon. Because banana peel is rich in lignocellulosic fibers, it develops a highly porous structure and abundant surface functional groups after activation, which enhances its adsorption capacity and interaction with drilling-fluid components [4]. In contrast, coal-based activated carbon generally exhibits a more inert, graphitic morphology with fewer active sites, often resulting in weaker rheological improvement and lower environmental compatibility. Similar advantages have been reported for other fruit-peel-derived carbons, which offer low cost, easy availability, and meaningful reductions in environmental footprint when used as drilling-fluid additives. These characteristics make banana-peel-based activated carbon a compelling, eco-efficient alternative to conventional carbon sources for enhancing water-based drilling muds.
Thus, the current research is centered around the use of TiO2-coated ZnO nanoparticles and activated charcoal from banana peel together as additives to improve the rheological and environmental performance of WBMs. This study is mainly concerned with the production of materials, their physical and chemical characterization, and testing of their flow properties. Through this, the researchers uncovered the ways in which these materials improve the quality of drilling muds.
Recent developments in drilling fluid engineering have demonstrated the ability of nanoparticles (NPs) and bio-derived additives to completely change the performance of water-based mud. Among the many carbon-based nanomaterials, the combination of carbon nanotubes with NPs has emerged as a champion, improving the rheological behavior and filtration control under extremely high-pressure, high-temperature (HPHT) conditions [5]. Concurrently, eco-friendly bio-waste derivatives, such as activated carbon derived from fruit and banana peels, not only present a natural alternative but also improve the yield point (YP), viscosity, and thermal stability, proving that green additives can compete with or even outperform traditional materials in terms of properties [6].
Metal oxide nanoparticles, especially TiO2 and ZnO, have shown high dispersion, viscosity retention, and shale inhibition even when used alone or in hybrid formulations with synergistic combinations, leading to a further reduction in settling time and an increase in gel strength (GS) [7,8]. The incorporation of these additives into polymers or nanoclays makes it possible to accurately adjust the fluid density, sagging control, and fluid-loss reduction, thus giving rise to extremely stable and versatile drilling fluids that can be used in different reservoir situations [8].
Such considerable research supports the merging of nanotechnology with green chemistry. For instance, multifunctional mud systems that perform well in all properties, that is, rheological, filtration, thermal resistance, and shale inhibition, can be created through the synergies between nanoparticles, bio-derived carbons, and conventional additives. Nanoparticle and mud interaction mechanisms, long-term stability of hybrid formulations under HPHT conditions, and environmentally friendly additive-scalable production should all be areas of focus in future research, thus leading to the next generation of eco-compatible and high-performance drilling fluids that match operational and environmental sustainability, as shown in Table 1.

Table 1.
Summary of literature reviewed for drilling mud enhancement.
Thus, this study focuses on the application of TiO2-coated ZnO and banana peel as an eco-friendly way to enhance the rheological stability of water-based drilling mud and emphasizes the ability to protect the environment.
2. Materials and Methods
2.1. Materials
In this study, zinc oxide (ZnO) and titanium oxide (TiO2) were purchased from BDH England Pvt. Polyvinyl alcohol (PVA) was used as the coating binder. Banana peels were collected from local waste sources, thoroughly washed, and dried. Sodium hydroxide (NaOH) and distilled water were used for the activation and washing processes, respectively. The base mud was formulated using standard drilling-grade bentonite and barite supplied by a local drilling fluid company. The materials used and their functions are shown in Table 2.

Table 2.
Chemicals and equipment used.
2.2. Synthesis of TiO2-Coated ZnO Nanoparticles
ZnO nanoparticles were synthesized using the hydrothermal method in which ZnO was dissolved in deionized water, and NaOH solution was added dropwise under magnetic stirring to achieve a homogeneous solution. The obtained ZnO precipitate was washed and dried at 80 °C.
TiO2 powder (3 g) was gradually incorporated into the ZnO/PVA mixture. The mixture was stirred continuously at 60–70 °C for 2 h. To ensure a uniform TiO2 coating on ZnO, sonication was used for 30–45 min following stirring. After coating, the particles were then filtered using filter paper and washed to remove excess PVA or TiO2. The coated ZnO was dried in an oven at 250 °C for 2 h.
2.3. Preparation of Activated Carbon from Banana Peel
Banana peels were cut into small pieces, dried at 100 °C, and carbonized at 500 °C in a muffle furnace under limited oxygen to form char [9]. The char was chemically activated by soaking in a 1 M NaOH solution for 12 h, followed by filtration and repeated washing to achieve a neutral pH. The activated carbon was then dried at 50 °C for 6 h and ground into a fine powder, as shown in Figure 1.

Figure 1.
Dried banana peel: (a) raw dried peels and (b) peels wrapped in aluminum foil for carbonization.
2.4. Preparation of Water-Based Drilling Mud
The rheological investigation in this study was conducted in accordance with API RP 13B-1 recommended practice and focuses on standard drilling-fluid parameters, including plastic viscosity (PV), yield point (YP), and gel strength, which are widely used for preliminary evaluation and comparative screening of water-based drilling fluid additives. The base mud was prepared using water-based drilling fluids to provide the basic rheological properties. These components were gradually added to water while continuously stirring with a mechanical stirrer [10]. The mixture was blended for 30 min to ensure thorough dispersion and uniformity. This base mud was used as a reference sample to compare with the modified mud with TiO2-coated ZnO and activated carbon, as shown in Table 3. All additive concentrations (0.10, 0.50, and 1.50 wt%) are expressed as wt% relative to the total mass of the drilling mud (total mud weight basis). For combined systems, TiO2-coated ZnO nanoparticles and activated carbon were added in a fixed 1:1 weight ratio (Table 4). In accordance with API laboratory practice, all mud formulations were prepared on a 350 mL laboratory barrel basis, where 350 mL of mud represents 1 bbl. Thus, additive concentrations of 0.10, 0.50, and 1.50 wt% are defined relative to the total mud weight and correspond to absolute concentrations of approximately 0.0008, 0.0039, and 0.0116 lb/bbl for single-additive systems. For the combined TiO2–ZnO/activated carbon system, a fixed 1:1 weight ratio was used, giving individual concentrations of 0.0004/0.0004, 0.0020/0.0020, and 0.0058/0.0058 lb/bbl, respectively. This approach ensures consistent comparison among base, single-additive, and hybrid mud systems.

Table 3.
Materials used for the preparation of base mud.

Table 4.
Complete formulation of base and modified water-based drilling mud samples (per 350 mL laboratory barrel).
2.5. Analytical Characterization
X-ray diffraction (XRD) was used to determine the crystalline structure and phase identification of the nanoparticles. Fourier-transform infrared spectroscopy (FTIR) was used to investigate the surface functional groups. Scanning electron microscopy (SEM) was employed to study the morphology and coating uniformity. Energy-dispersive X-ray spectroscopy (EDX) was used to confirm the elemental composition [11,12].
2.6. Rheological Measurements
A Fann viscometer was used to measure the plastic viscosity (PV) and yield point (YP) at 600 rpm and 300 rpm, respectively. Gel strength was measured at 10 s and 10 min intervals. A mud balance was used to measure the mud weight and specific gravity of the mud.
3. Results and Discussion
3.1. Analytical Structural and Morphological Analysis of TiO2 and ZnO
X-ray diffraction (XRD) was employed to explain the crystalline structure, phase purity, and crystallite size of the TiO2 and ZnO nanoparticles [13,14]. This technique is based on Bragg’s Law (nλ = 2d sinθ), where the constructive interference of X-rays diffracted by crystal planes provides information about the interplanar spacing (d) and lattice parameters. For TiO2, the diffraction peaks corresponded to the anatase (tetragonal, JCPDS No. 21-1272) and rutile (tetragonal, JCPDS No. 21-1276) phases, distinguished by their characteristic reflections at 2θ ≈ 25.3°, 37.8°, and 48.0° for anatase, and 27.4°, 36.1°, and 54.3° for rutile. In contrast, ZnO exhibits a wurtzite hexagonal structure (JCPDS No. 36-1451) with prominent diffraction peaks at 2θ ≈ 31.7°, 34.4°, and 36.2°, corresponding to the (100), (002), and (101) planes, respectively [15,16]. The absence of extraneous peaks confirmed phase purity, while the broadening of peaks indicated nanoscale crystallite dimensions, as shown in Figure 2a,b ZnO and TiO2.
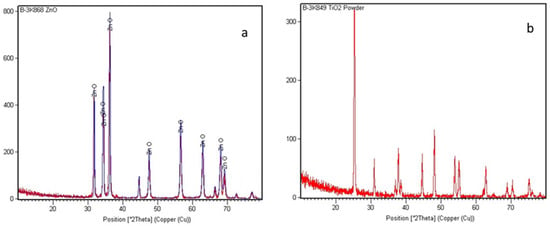
Figure 2.
XRD patterns of (a) ZnO nanoparticles and (b) TiO2 nanoparticles.
Fourier Transform Infrared (FTIR) spectroscopy is helpful to provide insights into the chemical bonding, surface functional groups, and metal–oxygen interactions within TiO2 and ZnO nanoparticles [11]. The spectra typically exhibit broad absorption bands at approximately 3591 cm−1 and 1650 cm−1, corresponding to the stretching and bending vibrations of surface hydroxyl groups and adsorbed water molecules, respectively. For TiO2, characteristic Ti–O–Ti stretching vibrations were observed in the range of 400–800 cm−1, indicative of the TiO6 octahedral framework. ZnO nanoparticles display strong Zn–O stretching modes near 430–550 cm−1, signifying the formation of Zn–O bonds within the wurtzite lattice. The presence of additional peaks between 1000 and 1500 cm−1 may correspond to Ti–O–Zn linkages or surface adsorbates, confirming the successful coating of TiO2 over ZnO. Thus, FTIR analysis substantiates the formation of metal–oxygen networks and the chemical integration between the two oxide phases, as shown in Figure 3a,b ZnO TiO2 [17,18,19].
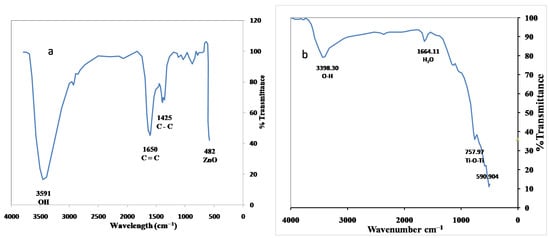
Figure 3.
(a) FTIR spectra of (a) ZnO nanoparticles and (b) TiO2 nanoparticles.
3.2. XRD and SEM Analysis of Synthesized ZnO
The XRD and SEM results presented in this section correspond exclusively to the TiO2 and ZnO nanoparticles synthesized in this study. As shown in Figure 4b, the XRD patterns of synthesized ZnO nanoparticles showed sharp peaks corresponding to the wurtzite structure. The TiO2-coated ZnO nanocomposite exhibited distinct peaks from both phases with slight broadening, confirming the successful surface coating and nanoscale particle size [20,21]. The SEM micrographs revealed that the uncoated ZnO particles exhibited a rod-like morphology, whereas the TiO2 particles were spherical [22]. The coated TiO2–ZnO nanocomposite exhibited rough surfaces with visible coating layers, indicating homogeneous TiO2 deposition over ZnO. The EDX spectra confirmed the presence of Ti, Zn, and O, verifying the coating process, as shown in Figure 4a,b SEM of coated ZnO, and XRD analysis of coated ZnO.
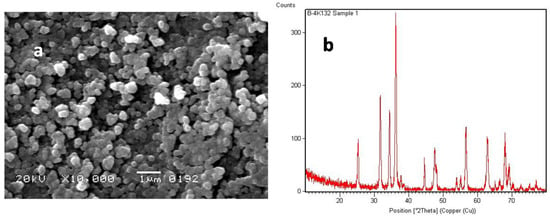
Figure 4.
(a) SEM image and (b) XRD pattern of TiO2-coated ZnO nanocomposite.
3.3. Mud Weight and Specific Gravity of Drilling Mud
The mud weight of the drilling mud prepared with synthesized ZnO was investigated in the laboratory, and it was found that with the addition of 0.10 wt% additives to the base mud, no change occurred in the mud density [22,23,24]. Subsequently, 0.50 wt% was added to the base mud, resulting in an approximately 2.02 wt% increase in mud density compared with the base mud. Finally, when 1.50 wt% additives were added to the base mud, the mud density increased by approximately 5.05 wt% compared with the base mud, as shown in Figure 5.
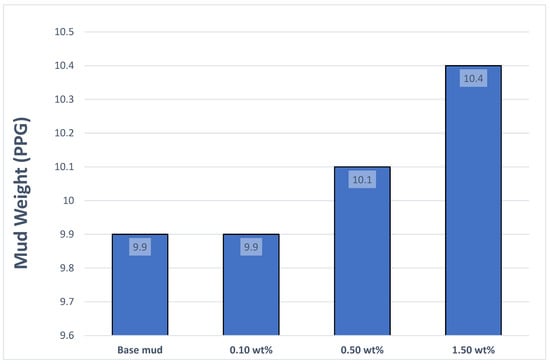
Figure 5.
Mud weight of mud samples.
Additionally, a specific investigation was performed with 0.10 wt% additives added to the base mud, which resulted in no change in the specific gravity of the mud [25,26,27]. Subsequently, 0.50 wt% additives were added to the base mud, resulting in an approximately 2.02 wt% increase in mud density compared with the base mud. Finally, when 1.50 wt% additives were added to the base mud, it resulted in an approximately 5.05 wt% increment in mud density compared with the base mud, as shown in Figure 6.
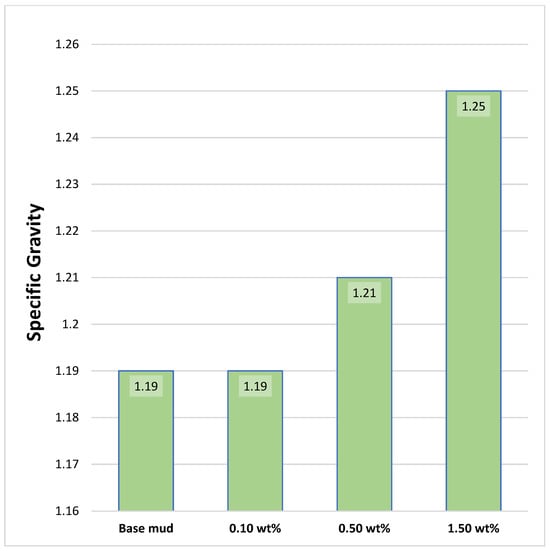
Figure 6.
Specific gravity of mud samples.
3.4. Rheological Characterization
Rheological investigations were performed to analyze the viscoelasticity of the drilling mud. The incorporation of TiO2–ZnO nanocomposite and activated carbon markedly improved the rheological properties of the water-based drilling mud [28,29,30].
3.4.1. Plastic Viscosity (PV)
The addition of TiO2-coated ZnO nanoparticles increased the plastic viscosity of the drilling fluid compared with that of the control sample [31,32,33]. This increase was due to the enhanced interaction between the nanoparticles and bentonite particles, which improved the flow resistance of the mud. According to the recommended API standards, the magnitude of plastic viscosity should be between 8.0 and 35 cP. In Figure 7, the red line shows the lower limit of the API of PV.
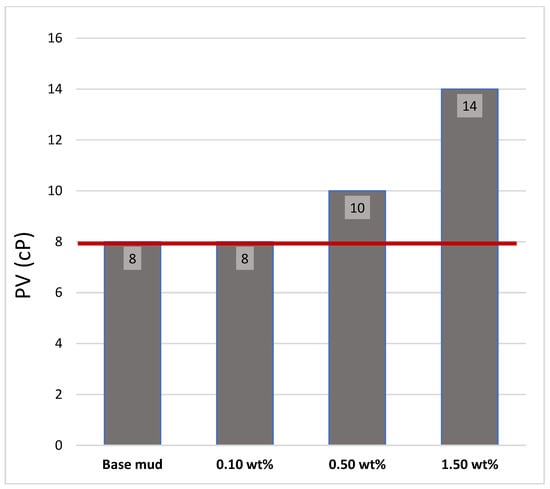
Figure 7.
PV of mud samples.
The experimental findings indicate that, at 0.10 wt% additives to the base mud, there was no change observed in the PV of the mud. With the addition of 0.50 wt% additives to the base mud, approximately 25 wt% of the PV in the mud increased. Finally, 1.50 wt% additives were added to the base mud; approximately 75 wt% PV of the mud was increased compared with that of the base mud.
Yield Point
Samples containing activated carbon from banana peels demonstrated a significant increase in YP, indicating an improved ability to suspend drill cuttings when circulation ceases [34,35,36]. The combination of both additives resulted in the highest yield point, demonstrating a synergistic effect, as shown in Figure 8. According to the API recommended standard, the yield point should be higher than 5 lb/100 ft2. The red line shows the lower limit of the API of YP.
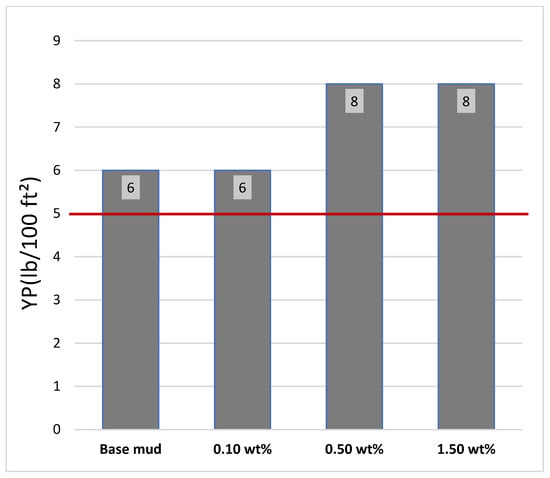
Figure 8.
Yield point (YP) of the drilling mud samples at various additive concentrations.
When 0.10 wt% additives were added to the base mud, no change occurred in the YP of the mud. When 0.50 wt% additives were added to the base mud, the YP of the mud increased by approximately 33.3 wt% compared with that of the base mud. When 1.50 wt% additives were added to the base mud, the YP of the mud increased by approximately 33.3 wt% compared with that of the base mud.
Gel Strength
Gel strength measurements showed that mud samples with combined additives developed stronger gels at both low (10 s) and high (10 min) shear times, as shown in Figure 9. A Fann Viscometer was used to obtain readings at θ600 and θ300. This ensures better suspension of solids during the drilling. According to the API recommended standard, the gel strength (10 min) should be between 2 and 35 lb/100 ft2. The red line shows the API lower limit of the gel strength.
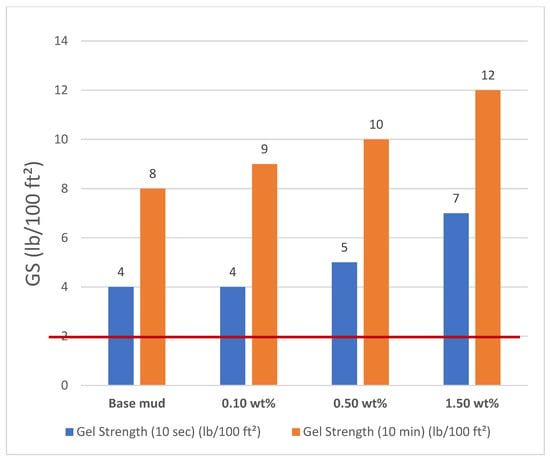
Figure 9.
Gel strength of mud samples.
It can be stated that using 0.10 wt% additives to base mud, no change occurred in the GS (10 s) of mud [37,38,39]. When adding 0.50 wt% additives were added to the base mud, approximately 25 wt% GS (10 s) of mud was increased compared with the base. Finally, when 1.50 wt% additives were added to the base mud, approximately 75 wt% GS (10 s) of mud was increased compared with the base mud. Similar experimental findings were obtained after 10 min. When 0.10 wt % additives were added to the base mud, approximately 12.5 wt% GS (10 min) of the mud was increased compared with the base mud. However, when 0.50 wt% additives were added to the base mud, approximately 25 wt% GS (10 min) of mud was increased compared with the base mud. When 1.50 wt% additives are added to base mud, approximately 50 wt% GS (10 min) of mud is increased compared with base mud.
It can be stated that TiO2 coating improves the dispersion and stability of ZnO nanoparticles in drilling mud [38,40,41]. Combining the coated nanoparticles with activated carbon enhanced rheological properties. Combining these additives yields better overall performance, making it a promising approach for environmentally friendly drilling mud enhancement [42,43,44]. These findings align with recent studies where metal oxide nanoparticles and agricultural waste-based activated carbon improved drilling mud properties. The sustainable use of banana peel activated carbon not only adds value to waste but also contributes to reducing the environmental footprint of drilling operations.
The rheological measurements presented in this study were performed under ambient conditions; nevertheless, a full high-temperature, high-pressure (HPHT) testing protocol (150 °C, 30 MPa, 16 h aging) has been clearly defined to test the formulated mud systems. The HPHT measurements could not be conducted in this phase due to the specifications of the equipment. Yet, the protocol is presented here to record the overall intended methodology, which will be enacted in the next phase of the research.
4. Mechanism of Enhancement
The following mechanism is proposed based on experimental observations of the synthesized nanoparticles (SEM analysis) combined with established literature, rather than direct in situ measurements within the drilling mud. The proposed improvement in rheology arises from the synergistic effects of TiO2-coated ZnO nanoparticles, which provide enhanced surface charge stability and interaction with bentonite platelets, reducing particle agglomeration and improving dispersion. (i) Activated carbon contributes through the adsorption of free water and the formation of a microporous structure, increasing the effective viscosity. (ii) The combined system proposed a strong particle–fluid–solid network formation, which enhances the gelation, suspension, and stability of the mud, as shown in Figure 10.
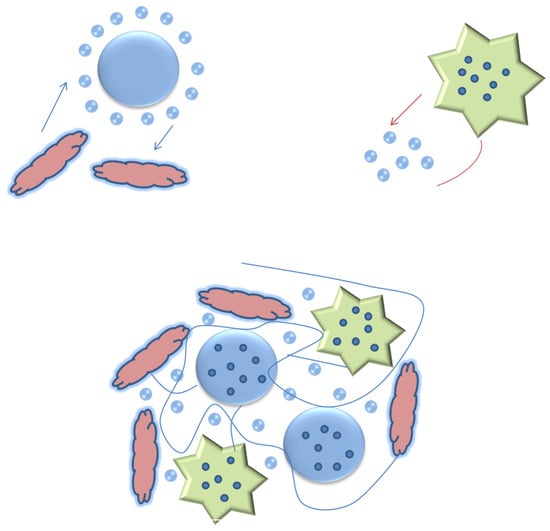
Figure 10.
Schematic illustration of the proposed mechanism for rheological enhancement of water-based drilling mud using TiO2-coated ZnO nanoparticles and activated carbon.
5. Environmental and Economic Implications
The application of banana peel waste as a carbon precursor represents a circular and sustainable approach for transforming agricultural biomass into a high-value nanomaterial. This strategy not only mitigates waste disposal challenges but also reduces dependence on petroleum-derived polymers that are traditionally used in drilling fluid formulations [45]. The PVA-assisted synthesis of the TiO2–ZnO nanocomposite ensured uniform coating, enhanced material dispersion, and minimal chemical by-products, aligning with the principles of green chemistry.
From an environmental perspective, both TiO2 and ZnO are nontoxic, chemically stable, and exhibit excellent recyclability, minimizing the ecological footprint during drilling and post-use disposal [46]. The incorporation of bio-derived activated carbon further enhances the adsorption and rheological performance without introducing hazardous residues into the ecosystem.
Economically, one can state that the process capitalizes on abundant, low-cost agricultural waste and readily available metal oxide precursors, making large-scale synthesis financially viable. The reusability and durability of the nanocomposite reduce the frequency of mud replacement and additive consumption, translating into lower operational costs. Therefore, this sustainable formulation provides the dual benefit of enhanced drilling efficiency with reduced environmental impact, making it a promising candidate for next-generation eco-efficient drilling fluid systems.
6. Conclusions
This research investigated the application of TiO2-coated ZnO nanoparticles and banana peel as activated carbon to improve the rheological properties of water-based drilling muds. The key experimental findings are written below:
- 1.
- Successful synthesis and characterization.
- PVA helped in the formation of the uniform TiO2 coating on ZnO that was investigated by XRD and SEM analysis in Figure 4a,b.
- 2.
- Significant rheological improvements.
- Plastic viscosity was increased by ~25% at 0.50 wt% and ~75% at 1.50 wt%.
- Yield point was increased by ~33% at 0.50 wt% and 1.50 wt% additive levels.
- Gel strength improved by 25–75% (10 s) and 12.5–50% (10 min), depending on dosage, indicating stronger structural integrity during static time.
- 3.
- Synergistic effect on TiO2-coated ZnO nanoparticles.
- The mixture of additives performed well, and the effects of each separate component showed better dispersion and stronger particle networks.
- 4.
- Environmental and economic considerations.
- Agricultural waste conversion into activated carbon is an eco-friendly and economical option compared with the regular ones.
- The composite configuration is completely in line with green nanoparticles, and it also lessens the negative impact on the environment while performing at a good level.
- 5.
- Oilfield application.
- The improved viscosity, yield point, and gel strength make this additive system suitable for operational drilling conditions requiring stable and efficient WBM performance.
- The formulation remains compatible with standard mud systems and does not adversely affect mud density.
Overall, the hybrid TiO2–ZnO/activated-carbon additive system showed clear improvements in API-standard rheological parameters (PV, YP, and gel strength) and environmental compatibility of the water-based drilling mud, suggesting its potential as a sustainable additive for drilling-fluid applications.
Limitations
- The current research was applied to the rheological characterization at ambient temperature; due to temporary limitations in the HPHT (high-pressure, high-temperature) aging-cell availability, the HPHT rheological evaluations were not carried out. To overcome this, targeted HPHT tests (150 °C, 30 MPa; 16 h aging) on the base, single-additive, and hybrid formulations have been planned to find out the variations in PV, YP, and gel strength and to set the temperature–pressure resistance limits of the materials. These experiments will be done as part of specific follow-up research.
- This research did not investigate long-term stability for 7-day and 14-day or 100-cycle simulated circulation experiments. The long-term stability tests used to measure the time-dependent variations of PV, YP, and gel strength, as well as the possible additive degradation mechanisms, will be carried out in the next phase of the study to analyze the long-term stability evaluation of the formulated drilling fluids.
- The limitation of this study is the comparison of banana-peel-derived activated carbon with other activated carbons, including coal-based products, due to laboratory limitations. However, in literature, the use of fruit-peel-derived activated carbons typically possesses higher porosity, greater surface functionalization, and enhanced adsorption behavior relative to coal-based activated carbon.
Author Contributions
Conceptualization, C.L.; Methodology, W.Z.; Validation, T.W. and Z.A.L.; Formal analysis, T.W., Z.A.L. and W.Z.; Resources, C.L. and Z.A.L.; Data curation, Z.A.L.; Writing—original draft, C.L., T.W., Z.A.L. and W.Z.; Writing—review & editing, Z.A.L.; Supervision, W.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (grant number: 52474036, grant number: 52174022, grant number: 52074088), Heilongjiang Provincial Natural Science Foundation Project (grant number: LH2024E008), Heilongjiang Province Postdoctoral Research Initiation Project (grant number: LBH-Q21086), ‘Open bidding for selecting the best candidates’ Heilongjiang Province Science and Technology Research Project (grant number: DQYT-2022-JS-758), Basic Research Expenses of Heilongjiang provincial Colleges and Universities: Northeast Petroleum University Control Science and Engineering Team Special Project (grant number: 2022TSTD-04), 2024 Leading Scientific and Technological Innovation Talent Team: Shale Oil Geology and Engineering Integrated Fracturing and Prevention Technology Innovation Team (grant number: CYCX24015).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
Author Chunping Liu was employed by the company Daqing Oilfield Company, Limited. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Lysakova, E.I.; Skorobogatova, A.D.; Neverov, A.L.; Minakov, A.V. Investigation of the effect of spherical nanoparticle additives on the properties of drilling fluids modified by carbon nanotubes. Nano-Struct. Nano-Objects 2025, 41, 101442. [Google Scholar] [CrossRef]
- Qu, Z.; Song, G.; Olortegui-Revoredo, J.; Kwon, P.; Chung, H. Dispersant-Induced Enhancement of Rheological Properties in Metal–Photopolymer Mixtures for 3D Printing. J. Manuf. Mater. Process. 2025, 9, 244. [Google Scholar] [CrossRef]
- Xu, K.; Yang, J.; He, H.; Wei, J.; Zhu, Y. Influences of Additives on the Rheological Properties of Cement Composites: A Review of Material Impacts. Materials 2025, 18, 1753. [Google Scholar] [CrossRef]
- Asad, M.S.; Jaafar, M.T.; Rashid, F.L.; Togun, H.; Rasheed, M.K.; Al-Obaidi, M.A.; Al-Amir, Q.R.; Mohammed, H.I.; Sarris, I.E. Sustainable Drilling Fluids: A Review of Nano-Additives for Improved Performance and Reduced Environmental Impact. Processes 2024, 12, 2180. [Google Scholar] [CrossRef]
- Mohamed, A.; Al-Afnan, S.; Elkatatny, S.; Hussein, I. Prevention of Barite Sag in Water-Based Drilling Fluids by A Urea-Based Additive for Drilling Deep Formations. Sustainability 2020, 12, 2719. [Google Scholar] [CrossRef]
- Wakeel, S.; Aslam, A.; Zhang, J. Advances in Polymer Nanocomposites for Drilling Fluids: A Review. Materials 2025, 18, 4809. [Google Scholar] [CrossRef]
- AlBajalan, A.R.; Rasol, A.A.A.; Norddin, M.N.A.M. Graphene and bio-graphene nanosheets in water-based mud (WBM): A pathway to sustainable and high-performance drilling muds. Emergent Mater. 2025. [Google Scholar] [CrossRef]
- Channei, D.; Jannoey, P.; Thammaacheep, P.; Khanitchaidecha, W.; Nakaruk, A. From Waste to Value: Banana-Peel-Derived Adsorbents for Efficient Removal of Polar Compounds from Used Palm Oil. Appl. Sci. 2025, 15, 2205. [Google Scholar] [CrossRef]
- Tadesse, M.G.; Kasaw, E.; Lübben, J.F. Valorization of Banana Peel Using Carbonization: Potential Use in the Sustainable Manufacturing of Flexible Supercapacitors. Micromachines 2023, 14, 330. [Google Scholar] [CrossRef]
- Alkalbani, A.K.; Chala, G.T.; Alkalbani, A.M. Experimental investigation of the rheological properties of water base mud with silica nanoparticles for deep well application. Ain Shams Eng. J. 2023, 14, 102147. [Google Scholar] [CrossRef]
- Pasieczna-Patkowska, S.; Cichy, M.; Flieger, J. Application of Fourier Transform Infrared (FTIR) Spectroscopy in Characterization of Green Synthesized Nanoparticles. Molecules 2025, 30, 684. [Google Scholar] [CrossRef]
- Surdu, V.-A.; Győrgy, R. X-ray Diffraction Data Analysis by Machine Learning Methods A Review. Appl. Sci. 2023, 13, 9992. [Google Scholar] [CrossRef]
- Caraveo-Castro, C.d.R.; Rodríguez-Guerra, Y.; Fuentes-Montero, L.; González-Jacquez, A.I.; Fuentes-Cobas, L.E.; Montero-Cabrera, M.E. Procedures for X-Ray Diffraction Phase Analysis: The Case of Fine Sediments from Peña Blanca, Chihuahua, Mexico. Crystals 2025, 15, 169. [Google Scholar] [CrossRef]
- Sukarman; Kristiawan, B.; Khoirudin; Abdulah, A.; Enoki, K.; Wijayanta, A.T. Characterization of TiO2 nanoparticles for nanomaterial applications: Crystallite size, microstrain and phase analysis using multiple techniques. Nano-Struct. Nano-Objects 2024, 38, 101168. [Google Scholar] [CrossRef]
- Lashari, Z.A.; Lalji, S.M.; Parveen, K.; Qureshi, M.F.; Nasreen, S.; Al-Khayri, J.M.; Al-Dossary, O.; Alsubaie, B.; Shehata, W.F.; Almaghasla, M.I. Optimization of nanofluid stability using response surface methodology: A study on PAM/sodium alginate-ZnO systems. Chem. Pap. 2025, 79, 8131–8143. [Google Scholar] [CrossRef]
- Lashari, Z.A.; Zhao, W.; Ali-zada, A.; El-Bahy, S.M.; Lalji, S.M. Stability and settling dynamics of Al2O3/TiO2 nanofluids: An ImageJ-based quantification with response surface methodology. Chem. Pap. 2025. [Google Scholar] [CrossRef]
- Lashari, Z.A.; Qureshi, M.F.; Bhutto, D.K.; Parveen, K.; Lalji, S.M.; Khan, M.A.; Li, M.; Gurbanova, L.; Al-Onozi, W.A. Full factorial analysis with ANOVA and physiochemical investigation of ZnO/Al2O3 with pure bore at low salinity to improve oil recovery. Chem. Pap. 2025, 79, 6279–6294. [Google Scholar] [CrossRef]
- Lashari, Z.A.; Lalji, S.M.; Yasin, Q.; Bentalib, A.; Bin Jumah, A. Performance of nanoparticle MgO/TiO2 nanofluids with Pure bore: Insight into statistical and analytical approach. Chem. Pap. 2025, 79, 1523–1538. [Google Scholar] [CrossRef]
- Orudzhev, F.; Gadzhiev, M.; Abdulkerimov, M.; Muslimov, A.; Krasnova, V.; Il’ichev, M.; Kulikov, Y.; Chistolinov, A.; Volchkov, I.; Tyuftyaev, A.; et al. Plasma-Assisted Synthesis of TiO2/ZnO Heterocomposite Microparticles: Phase Composition, Surface Chemistry, and Photocatalytic Performance. Molecules 2025, 30, 3371. [Google Scholar] [CrossRef]
- Marny, M.; Sowa, M.; Kazek-Kęsik, A.; Rokosz, K.; Raaen, S.; Chapon, P.; Viter, R.; Pshenychnyi, R.; Simka, W.; Michalska, J. Shaping the Structure and Properties of TiO2-ZnO Oxide Coatings Produced by Plasma Electrolytic Oxidation on Titanium Substrate. Materials 2023, 16, 7400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, C. TiO2 Coated ZnO Nanorods by Mist Chemical Vapor Deposition for Application as Photoanodes for Dye-Sensitized Solar Cells. Nanomaterials 2019, 9, 1339. [Google Scholar] [CrossRef]
- Gao, L.; Nefzaoui, E.; Marty, F.; Erfan, M.; Bastide, S.; Leprince-Wang, Y.; Bourouina, T. TiO2-Coated ZnO Nanowire Arrays: A Photocatalyst with Enhanced Chemical Corrosion Resistance. Catalysts 2021, 11, 1289. [Google Scholar] [CrossRef]
- Ahmed, A.; Pervaiz, E.; Ahmed, I.; Noor, T. Remarkable improvement in drilling fluid properties with graphitic-carbon nitride for enhanced wellbore stability. Heliyon 2025, 11, e41237. [Google Scholar] [CrossRef]
- Medved, I.; Gaurina-Međimurec, N.; Novak Mavar, K.; Mijić, P. Waste Mandarin Peel as an Eco-Friendly Water-Based Drilling Fluid Additive. Energies 2022, 15, 2591. [Google Scholar] [CrossRef]
- Leusheva, E.; Brovkina, N.; Morenov, V. Investigation of Non-Linear Rheological Characteristics of Barite-Free Drilling Fluids. Fluids 2021, 6, 327. [Google Scholar] [CrossRef]
- Ali, J.A.; Gailani, R.; Abdullah, A.D.; Jaf, P.T.; Simo, S.M.; Abdalqadir, M.; Faris, V.M. Performance evaluation of the nano-biodegradable drilling fluid using the greenly synthesized zinc nanorods and gundelia seed waste. Environ. Sci. Pollut. Res. 2024, 31, 51381–51400. [Google Scholar] [CrossRef]
- Dike, H.N.; Chibueze, L.N.; Ipinsokan, S.; Adewumi, C.N.; Olabode, O.; Olaniyan, D.D.; Pius, I.E.; Oke, M.A. An Evaluation of the Rheological and Filtration Properties of Cow Bone Powder and Calcium Carbonate as Fluid-Loss Additives in Drilling Operations. Processes 2025, 13, 2205. [Google Scholar] [CrossRef]
- Davoodi, S.; Al-Shargabi, M.; Wood, D.A.; Rukavishnikov, V.S.; Minaev, K.M. Synthetic polymers: A review of applications in drilling fluids. Pet. Sci. 2024, 21, 475–518. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, W.; Li, J.; Sun, H.; Li, X.; Wei, Q. A decoupling model for fatigue life assessment of double wellhead system with subsea suction anchor. Ocean Eng. 2024, 311, 118896. [Google Scholar] [CrossRef]
- Sui, Y.; Guo, T.; Cao, G. Characteristics of sodium p-styrenesulfonate modified polyacrylamide at high temperature under dual scale boundary. Phys. Fluids 2025, 37, 073115. [Google Scholar] [CrossRef]
- Ge, L.; Liu, Z.; Liu, S.; Xiao, X.; Yuan, Y.; Yin, Z. Electromagnetic Tomography for Multiphase Flow in the Downhole Annulus. IEEE Trans. Instrum. Meas. 2025, 74, 1–13. [Google Scholar] [CrossRef]
- Hossain, M.E.; Al-Majed, A. Fundamentals of Sustainable Drilling Engineering; Wiley: Hoboken, NJ, USA, 2021. [Google Scholar]
- Wang, J.; Pan, W.; Cao, Y.; Zhang, Y.; Ba, X.; Guo, L.; Han, Y. Mitigation Effects and Prediction Formulae of Stratum Disturbance by Different Slurry Pressures and Filter Cake Parameters in Slurry Shield Tunneling. Rock Mech. Rock Eng. 2025. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; He, H.; Peng, Z.; Pu, J.; Zheng, L.; Gu, X. A Thermo-Stable Polymeric Surfactant for Enhanced Heavy Oil Recovery via Hot Water Chemical Flooding. Langmuir 2025, 41, 29180–29195. [Google Scholar] [CrossRef] [PubMed]
- Maiti, M.; Bhaumik, A.K.; Mandal, A. Performance of water-based drilling fluids for deepwater and hydrate reservoirs: Designing and modelling studies. Pet. Sci. 2021, 18, 1709–1728. [Google Scholar] [CrossRef]
- Ahmed, A.; Alsaihati, A.; Elkatatny, S. An Overview of the Common Water-Based Formulations Used for Drilling Onshore Gas Wells in the Middle East. Arab. J. Sci. Eng. 2021, 46, 6867–6877. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, D.; Gao, H.; Hu, Y.; Duan, L. Real-Time Measurement of Drilling Fluid Rheological Properties: A Review. Sensors 2021, 21, 3592. [Google Scholar] [CrossRef] [PubMed]
- Lashari, Z.A.; Aamir, M.; Kumar, B.; Aziz, H.; Soomro, N.A.; Lalji, S.M.; Tahir, F. Stability analysis of nanofluid with aluminum oxide and polyacrylamide for enhanced oil recovery: Insight into experimental investigation. Multiscale Multidiscip. Model. Exp. Des. 2024, 8, 63. [Google Scholar] [CrossRef]
- Lashari, Z.A.; Aamir, M.; Lalji, S.M.; Murtaza, M.; Yamin, Y.; Haider, M.; Bilal, M.; Al-Onozi, W.A.; Al-Mohaimeed, A.M.; Iqbal, R.; et al. Stability and Efficiency of Magnesium Oxide-Sodium Alginate Combination in Low Salinity EOR Processes: An Integrated Approach of CCD. Chem. Pap. 2025, 79, 4363–4377. [Google Scholar] [CrossRef]
- Lalji, S.M.; Ali, S.I.; Lashari, Z.A.; Panjwani, S.K.; Burney, M. Performance evaluation of Si/Fe3O4 nanoparticles in water-based mud in presence of different Mg2+, K+, Na+ salts: Experimental and stability visualization study. Chem. Pap. 2024, 78, 8379–8396. [Google Scholar] [CrossRef]
- Lashari, Z.A.; Lalji, S.M.; Kumar, D.; Bilal, A. Physiochemical analysis of titanium dioxide and polyacrylamide nanofluid at low salinity. Chem. Pap. 2024, 78, 3629–3637. [Google Scholar] [CrossRef]
- Lashari, Z.A.; Haq, B.; Al-Shehri, D.; Zaman, E.; Al-Ahmed, A.; Lashari, N. Recent Development of Physical Hydrogen Storage: Insights into Global Outlook and Future Applications. Chem. Asian J. 2024, 19, e202300926. [Google Scholar] [CrossRef]
- Chaudhry, A.U.; Muneer, R.; Lashari, Z.A.; Hashmet, M.R.; Osei-Bonsu, K.; Abdala, A.; Rabbani, H.S. Recent advancements in novel nanoparticles as foam stabilizer: Prospects in EOR and CO2 sequestration. J. Mol. Liq. 2024, 407, 125209. [Google Scholar] [CrossRef]
- Soomro, N.A. Laboratory evaluation of an innovative polyfraction nanoemulsion for enhanced oil recovery in carbonate reservoirs. Unconv. Resour. 2025, 8, 100212. [Google Scholar] [CrossRef]
- Soomro, N.A.; Ansari, U.; Shams, B.; Memon, M.K.; Bhutto, D.K.; Rui, Z.; Pan, Y. Optimizing Gas Well Deliquification: Experimental Analysis of Surfactant-Based Strategies for Liquid Unloading in Gas Wells for Enhanced Recovery. Unconv. Resour. 2025, 7, 100200. [Google Scholar] [CrossRef]
- Soomro, N.A.; Ansari, U.; Shams, B.; Memon, M.K.; Bhutto, D.K.; Rui, Z.; Pan, Y. Experimental Assessment of the Stability and Impact of Water-Based Fracturing Fluid with and without Triethanolamine (TEA). Fuel Commun. 2025, 23, 100137. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.