Abstract
This study aimed to use sunflower seeds (SSs) and barbatimão bark (BB) to obtain an oil enriched with phenolic compounds. For this purpose, simultaneous extractions were carried out using different proportions of SSs and BB. Subsequently, the effects of temperature and extraction time were determined. The resulting oils were evaluated for composition and physicochemical properties. BB addition decreased the mass yield by 27% to 56% but increased the total phenolic content by 5 to 13 times. The best SS/BB ratio (3:2.5) was selected for further experiments. Increasing the extraction temperature from 30 to 60 °C and the extraction time from 15 to 60 min led to a 10% increase in oil yield and enhanced the contents of phenolic acids and flavonoids by 1.1 to 10 times. Gallic, quinic, and trans-cinnamic acids were the main phenolics in enriched oils, which exhibited higher antioxidant activity via the DPPH•, FRAP, and ABTS•+ methods. Linoleic and oleic acids were identified as the major fatty acids in the tested oils. Enriched oils showed greater thermal stability than their unenriched counterpart. The application of phenolic-enriched oil at concentrations of up to 400 µg/mL did not exert cytotoxic effects on human keratinocyte HaCaT cells.
1. Introduction
In the field of complementary medicine, vegetable oils are used as an alternative and low-cost treatment for common skin diseases and related conditions [1]. Vegetable oils are rich in fat-soluble vitamins, fatty acids, steroids, phospholipids, tocopherols, and other compounds that contribute to skin health. These substances are effective in promoting skin softness and hydration by improving moisture retention and barrier function [2].
The oil obtained from sunflower seeds is widely used in the treatment and prevention of wounds, such as those caused by venous neuropathies, pressure injuries, burns, and surgical dehiscence. Sunflower oil promotes tissue repair through its anti-inflammatory and antioxidant activities, combined with its ability to stimulate cell proliferation, increase collagen synthesis, and induce neovascularization, thereby facilitating wound healing [3]. The oil mainly contains oleic and linoleic acids [4,5]. Oleic acid intensifies the penetration of other compounds, as it disrupts the skin barrier [6]. Linoleic acid, on the other hand, plays a direct role in maintaining the integrity of the water permeability barrier [7].
Phenolic compounds obtained from natural sources represent promising ingredients for the development of novel medicines and treatments. A prominent example of the applicability of phenolics is in the prevention of skin diseases as alternatives to synthetic analogs [8]. Yang et al. [9] demonstrated that gallic acid can promote wound healing by increasing cell migration. Mekhoukh et al. [10] reported that a Putoria calabrica leaf extract rich in chlorogenic acid, vitexin, and rutin showed potential as a therapeutic agent for skin protection and wound care. Furthermore, the application of a phenolic extract containing protocatechuic acid, p-hydroxybenzoic acid, caffeic acid, p-hydroxybenzaldehyde, 3-hydroxycinnamic acid, and ferulic acid accelerated the rate of wound healing in mice [11].
Enrichment with phenolic compounds is a recent strategy developed by our research group to enhance the quality of sunflower oil and expand its applicability [4,5]. In the proposed methodology, the oil and phenolics are extracted simultaneously in a single step, resulting in enhanced antioxidant capacity and oxidative stability.
Stryphnodendron adstringens, a plant commonly known as barbatimão, has aroused interest as a rich source of multiple polyphenols. It is possible to obtain bioactive extracts from barbatimão using ethyl acetate, a “green” solvent with low toxicity, low environmental impact, low cost, and high biodegradability [12,13]. Extracts of barbatimão bark are mainly composed of phenolic acids, flavonoids, and tannins [14]. These compounds are associated with anti-inflammatory [15], anticancer [16], antifungal [17], and antioxidant [18] effects. Antioxidant activity is particularly relevant for enriching vegetable oils [19,20]. Ribeiro et al. [21] reported that the antioxidant activity of barbatimão bark extract correlates with its phenolic content and can be increased by optimizing extraction conditions. Aguiar et al. [22] developed a hydrogel containing a hydroalcoholic extract of barbatimão and found that it inhibited fibroblast proliferation and exhibited pronounced healing activity.
In light of the foregoing, this study aimed to enrich sunflower oil with phenolic compounds from barbatimão bark. Specifically, the objectives were to evaluate the effect of different ratios of sunflower seeds to barbatimão bark, analyze the impact of extraction temperature and time, and characterize the resulting oils.
2. Materials and Methods
2.1. Materials
Sunflower seeds and barbatimão bark were purchased at a local market in Maria Helena, Paraná State, Brazil. Ethyl acetate (Anidrol) was used as an extraction solvent.
Total phenolic content was determined using sodium carbonate (Anidrol), Folin–Ciocalteu reagent (Dinâmica), methanol (Panreac), n-hexane (Neon), and gallic acid (Sigma–Aldrich, St. Louis, MO, USA). For analysis of antioxidant potential, the following reagents were used (all from Sigma–Aldrich): 2,2-diphenyl-1-picrylhydrazyl (DPPH•) (purity ≥ 95%), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS•+) (purity ≥ 98%), (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) (97% purity), and 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ) (purity ≥ 99%). The other reagents were potassium persulfate (Anidrol), ethanol (Anidrol, purity ≥ 99.5%), sodium acetate (Química Nova), ferric chloride (Anidrol), hydrochloric acid (Anidrol), acetic acid (Anidrol), and ferrous sulfate (Anidrol).
The following standards were used for analysis of phenolic acids and flavonoids: gallic acid, coumaric acid, ferulic acid, caffeic acid, trans-cinnamic acid, quercetin, and kaempferol (Sigma–Aldrich, >99% purity). Additionally, methanol (HPLC-grade, Merck, Rehway, NJ, USA) and formic acid (Sigma–Aldrich, 99.8% purity) were used as solvents.
For analysis of thiobarbituric acid-reactive substances (TBARS), trichloroacetic acid (TCA, Synth), butylated hydroxytoluene (BHT, Synth), 2-thiobarbituric acid (TBA, Sigma–Aldrich), and 1,1,3,3-tetraethoxypropane (TEP, Sigma–Aldrich) were used. Cytotoxicity tests were carried out using Dulbecco’s Modified Eagle Medium (DMEM; Gibco, Paisley, UK) supplemented with fetal bovine serum, streptomycin (Gibco, São Paulo, Brazil), and penicillin (Nova Biotecnologia, São Paulo, Brazil). MTT reagent (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was obtained from Sigma–Aldrich.
2.2. Preparation of Raw Materials
Sunflower seeds (1.83 ± 0.17 wt% moisture) and barbatimão bark (9.37 ± 0.40 wt% moisture) were ground separately in a blender (Cadence®, model BLD300, Bracknell, UK) and sieved through Tyler sieves (Bertel®, ASTM, Caieiras, Brazil). Seed and bark fractions with an average diameter of 0.50 and 0.15 mm, respectively, were used in the extraction tests.
2.3. Extraction
Extractions were carried out in an orbital shaker incubator (Marconi, MA 839/A, Piracicaba, SP, Brazil) under controlled temperature and agitation (100 rpm) conditions. A 250 mL Erlenmeyer flask with a glass lid was used as an extractor. Ground raw materials and the solvent were added at a ratio of 1:12 (w/v). After the extraction period, the solid material was separated by filtration, and excess solvent was removed. The mass yield was calculated as the ratio of the weight of material obtained to the sample weight (seeds + bark) used in the extraction.
First, extractions were conducted to evaluate the effect of raw material composition by testing different seed/bark ratios while keeping the temperature fixed at 60 °C and the extraction time at 60 min. After the optimal composition was selected, the effects of temperature (30, 45, and 60 °C) and extraction time (15, 30, 45, and 60 min) were evaluated.
2.4. Analytical Methods
2.4.1. Total Phenolics, Antioxidant Potential, and Phenolic Profile
For analysis of total phenolic content, antioxidant potential, and phenolic profile, a hydromethanolic extract was prepared as described elsewhere [23] and subjected to filtration through a PVDF hydrophobic membrane (0.45 µm pore size, 25 mm diameter). Subsequently, total phenolic content was determined according to Singleton et al. [24] via the colorimetric method using the Folin–Ciocalteu reagent. The absorbance was determined at 760 nm (Shimadzu, UV 1900, Tokyo, Japan). The total phenolic content was quantified against a standard curve of gallic acid (R2 ≥ 0.99).
The antioxidant potential of extracts was determined according to the DPPH• [25], ferric reducing antioxidant power (FRAP) [26], and ABTS•+ [27] assays. The absorbance was determined at 517, 595, and 734 nm (Shimadzu, UV 1900, Tokyo, Japan), respectively. Quantification was performed based on calibration curves prepared using Trolox (R2 ≥ 0.99).
Hydromethanolic extracts were analyzed by high-performance liquid chromatography (HPLC, Prominence 20A, Shimadzu, Kyoto, Japan). The chromatograph was coupled to a UV detector (SPD-20A, operated at 280 and 320 nm) and a C-18 column (Shim-pack CLC-ODS (H)™, 25 cm × 4.6 mm × 5 mm, Shimadzu) maintained at 25 °C (CTO-20 column oven). The mobile phases were injected at a flow rate of 0.8 mL/min (quaternary pump, LC-20AT). Ultrapure water acidified with 0.05% formic acid (A) and methanol acidified with 0.1% formic acid (B) were used in gradient elution mode, as follows: 0.01 to 5 min, 20% B; 5 to 25 min, 50% B; and 25 to 30 min, 80% B. Solutions of phenolic acids and flavonoids (1 to 10 mg/mL) were prepared to construct the analytical curves (R2 ≥ 0.99).
2.4.2. Fatty Acid Profile
Fatty acids were identified via gas chromatography–mass spectrometry (GC-MS) (Shimadzu, GC-MS-QP2010 SE, Tokyo, Japan). For each analysis, samples were previously derivatized as described by Stevanato et al. [28]. Subsequently, the samples were injected into the GC-MS system equipped with a Zebron™ ZB-Wax capillary column (Phenomenex, 30 m × 0.25 mm × 0.25 µm, Torrance, CA, USA). Chromatographic conditions, as well as quantification methods, were previously described by Mello et al. [29].
2.4.3. Thermogravimetric Analysis
A simultaneous thermal analyzer (STA 6000, PerkinElmer Inc., Waltham, MA, USA) was used to evaluate the thermal stability of the experimental oils. In each analysis, the oil sample (~10 mg) was heated from 30 to 750 °C at a rate of 10 °C/min under a synthetic air atmosphere (50 mL/min). Data were processed using Pyris™ software. Thermogravimetric (TG) and derivative thermogravimetric (DTG) curves were evaluated using Origin 8.6 software (OriginLab, Northampton, MA, USA).
2.4.4. TBARS Assay
The TBARS assay was performed according to Sridhar and Charles [30]. Briefly, 0.3 mL of oil and 3 mL of TBARS reagent were added to a capped test tube. The mixture was homogenized and heated (95 °C) in a water bath (MA093/1) for 20 min until a pink color was observed. Samples were cooled in an ice bath, and then 5 mL of chloroform was added. Samples were then homogenized and centrifuged at 3000 rpm for 10 min. The supernatant was collected, and the absorbance was read in a spectrophotometer at 532 nm. The blank was prepared using 3 mL of TBARS reagent and 5 mL of chloroform. Quantification was achieved against a standard curve of TEP (0.5–22.5 µg/mL). TBARS values are expressed as mg of malondialdehyde (MDA) per kg of dry sample.
2.4.5. Cytotoxicity Analysis
Cell viability was determined using human keratinocyte HaCaT cells in the presence of oils. Initially, the cells were cultured in DMEM supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin at 37 °C in a humidified atmosphere with 5% CO2. Upon reaching ~80% confluence, the cells were seeded in 96-well plates (1 × 104 cells per well) and treated with different concentrations of oils (50, 100, 200, and 400 µg/mL) for 24 and 48 h. Ethanol (70%) was used as a negative control. Cytotoxicity was determined according to Malich et al. [31], using the MTT assay, with absorbance reading at 570 nm on a plate reader (Agilent, Santa Clara, CA, USA). Cell viability was calculated using Equation (1).
2.5. Statistical Analysis
Extractions and assays were conducted in duplicate. Data were subjected to a one-way analysis of variance (ANOVA), followed by a Tukey’s test. The level of significance was set at 5% (Microsoft Excel® 2010). Pearson correlation analysis was conducted using PAST software (version 4.03).
3. Results and Discussion
3.1. Effect of Raw Material Ratio
Table 1 presents the effect of sunflower seed/barbatimão bark ratio on mass yield and the total phenolic content for extractions conducted at 60 °C for 60 min. Barbatimão bark addition caused a 27% to 56% decrease in mass yield. However, treatments containing barbatimão bark at ratios up to 3:2.5 (seeds/bark) resulted in an increase in the total phenolic content, reaching a value about 13 times higher than that obtained using sunflower seeds only. These findings are explained by differences in composition between the raw materials.

Table 1.
Effect of raw material ratio on mass yield and total phenolic content.
The total phenolic content of barbatimão bark (43,289.1 ± 2397.5 mg GAE/100 g, Table 1) was within the range reported in previous studies, such as 3700–40,000 [21], 22,200 [32], and 608,700 mg GAE/100 g [33]. Sunflower seeds had a lower total phenolic content (13.8 ± 0.7 mg GAE/100 g), similar to the values obtained by Kumar et al. [34] for extractions using supercritical carbon dioxide and hexane (49 and 56 mg GAE/100 g, respectively). In the study by Kumar et al. [34], the mass yield varied from 41% to 44%, which was similar to the mass yield obtained in the current study for sunflower seeds without barbatimão bark addition (40%).
The highest total phenolic content was obtained using sunflower seeds/barbatimão bark ratios of 3:2.5 and 3:3. As the 3:2.5 ratio resulted in a higher mass yield, it was chosen for subsequent assays.
3.2. Effects of Extraction Temperature and Time
Table 2 presents the effects of extraction temperature and time on the mass yield and phenolic composition of enriched oils, unenriched oil obtained from sunflower seeds only, and barbatimão bark extract. There was good agreement between total phenolic contents determined using the Folin–Ciocalteu method and the results of phenolic acids and flavonoids determined via HPLC. However, the Folin–Ciocalteu method, a colorimetric assay, is influenced by numerous interferents, such as proteins, inorganic ions, and other non-phenolic reducing compounds, that may lead to overestimation of phenolic content [24]. Therefore, given the specificity of HPLC analysis, it was deemed more appropriate to use the sum of the detected compounds as the total phenolic content from this stage of the study onward.

Table 2.
Effects of extraction temperature and time on mass yield and phenolic composition.
An increase in temperature from 30 to 60 °C led to a 10% increase in mass yield. This increment also favored the extraction of phenolic acids (gallic, trans-cinnamic, caffeic, and quinic acids) and flavonoids (kaempferol and quercetin), whose contents increased from 1.1 to 6.1 times. Accordingly, the total content of phenolic acids and flavonoids was enhanced 3-fold at the highest extraction temperature.
This result may be explained by the fact that heat enhances the permeability of cell walls, thereby increasing the solubility and diffusion of compounds. Additionally, the viscosity of solvents is reduced, further promoting extraction [35]. Qian et al. [36] observed an increase in caffeic acid extraction from sorghum straw via oscillation-assisted mild hydrothermal pretreatment, with an increase in temperature from 50 to 60 °C. Temperatures of 20 to 50 °C are typically applied in conventional extraction techniques, given that temperatures greater than 70 °C can cause the degradation of phenolic compounds [37,38].
For extractions conducted at 60 °C, an increase in extraction time from 15 to 60 min resulted in a 10% increase in mass yield. Moreover, the contents of phenolic acids (gallic, trans-cinnamic, caffeic, and quinic acids) and quercetin increased gradually with extraction time. A 60 min extraction period also promoted kaempferol extraction, with no differences in kaempferol levels between 15 and 45 min of extraction. Long extraction (60 min) afforded a 10-fold increase in the levels of the identified phenolic compounds compared with short extraction (15 min). Novais et al. [38] observed that an increase in the perchlorination extraction time led to an increase in the total phenolic content of barbatimão bark, particularly from 5 to 45 min. Qian et al. [36] found an increase in the extraction of caffeic acid from sorghum straw at 60 °C with a longer extraction time.
In view of the above, the most promising extraction conditions for obtaining phenolics-enriched oils are 60 °C and 60 min. As expected, under these conditions, barbatimão bark extract had higher contents of phenolic acids and flavonoids than sunflower seed oil. Sunflower oil contained 2 to 12 times lower levels of trans-cinnamic acid, caffeic acid, kaempferol, and quercetin than enriched oils, in addition to lacking gallic and quinic acids.
Gallic, caffeic, and quinic acids were the major phenolic acids in barbatimão bark extract. Ribeiro et al. [21] reported several phenolic acids in barbatimão bark, with gallic, caffeic, and protocatechuic acids being the predominant ones. Ribeiro et al. [21] also detected quinic acid in barbatimão bark extract. These compounds have significant antioxidant and anti-inflammatory properties, can promote cell proliferation and collagen synthesis, and may contribute to combating oxidative stress [39,40].
Similar to the results of this study, Pellenz et al. [41] identified quercetin and kaempferol in barbatimão bark extracts using a mixture of ethanol and water (70% v/v) as solvent. The flavonoids showed good potential as antioxidants, in addition to having antibacterial and anti-inflammatory properties, promoting growth factor production and collagen synthesis [42,43].
Table 3 describes the antioxidant potential of samples (as listed in Table 2). The phenolic compounds in barbatimão bark are associated with antioxidant action, as reported by Sabino et al. [18]. According to the authors, these phenolics are capable of quenching DPPH and ABTS radicals by donating electrons and hydrogen atoms to remove the odd electron that confers radical reactivity. Reducing power is associated with action against oxidized intermediates of lipid peroxidation, thereby preventing oxidative stress. Although in vitro methods do not yet exhibit a clear correlation with in vivo effects, for instance, through topical application, the results of this study can motivate future studies to investigate the physiological effects of the enriched oils.

Table 3.
Effect of temperature and time on the antioxidant potential (µMol Trolox equivalents/g) of enriched oils.
It is known that antioxidant activity is closely associated with anti-inflammatory effects, as the reduction in oxidative stress attenuates the production of pro-inflammatory mediators, thereby protecting tissues from cellular damage [44]. The literature provides evidence of improved tissue repair and highlights the hemostatic, antioxidant, and anti-inflammatory properties of phenolics in barbatimão bark, supporting their potential topical applicability due to their cutaneous bioactivity. This was demonstrated by Alves et al. [45], who evaluated the incorporation of barbatimão bark extract into hydrophilic matrices for wound healing applied to rat skin. The authors observed a marked reparative effect, associated with the stimulation of keratinocyte proliferation (re-epithelialization), which led to accelerated healing and a reduced inflammatory response, directly linked to the presence of phenolic compounds.
The addition of barbatimão bark to sunflower seed extraction increased the antioxidant potential of the resulting oils, varying according to extraction conditions. FRAP and DPPH• activities doubled when increasing the extraction temperature from 30 to 60 °C, and ABTS•+ activity increased 8-fold. Under an extraction temperature of 60 °C, the resulting oils showed increasing antioxidant potential with longer extraction periods. For instance, oils extracted for the maximum time (60 min) had four-times-higher antioxidant potential by the FRAP method and two-times-higher activities via the DPPH• and ABTS•+ methods than oils obtained over 15 min of reaction.
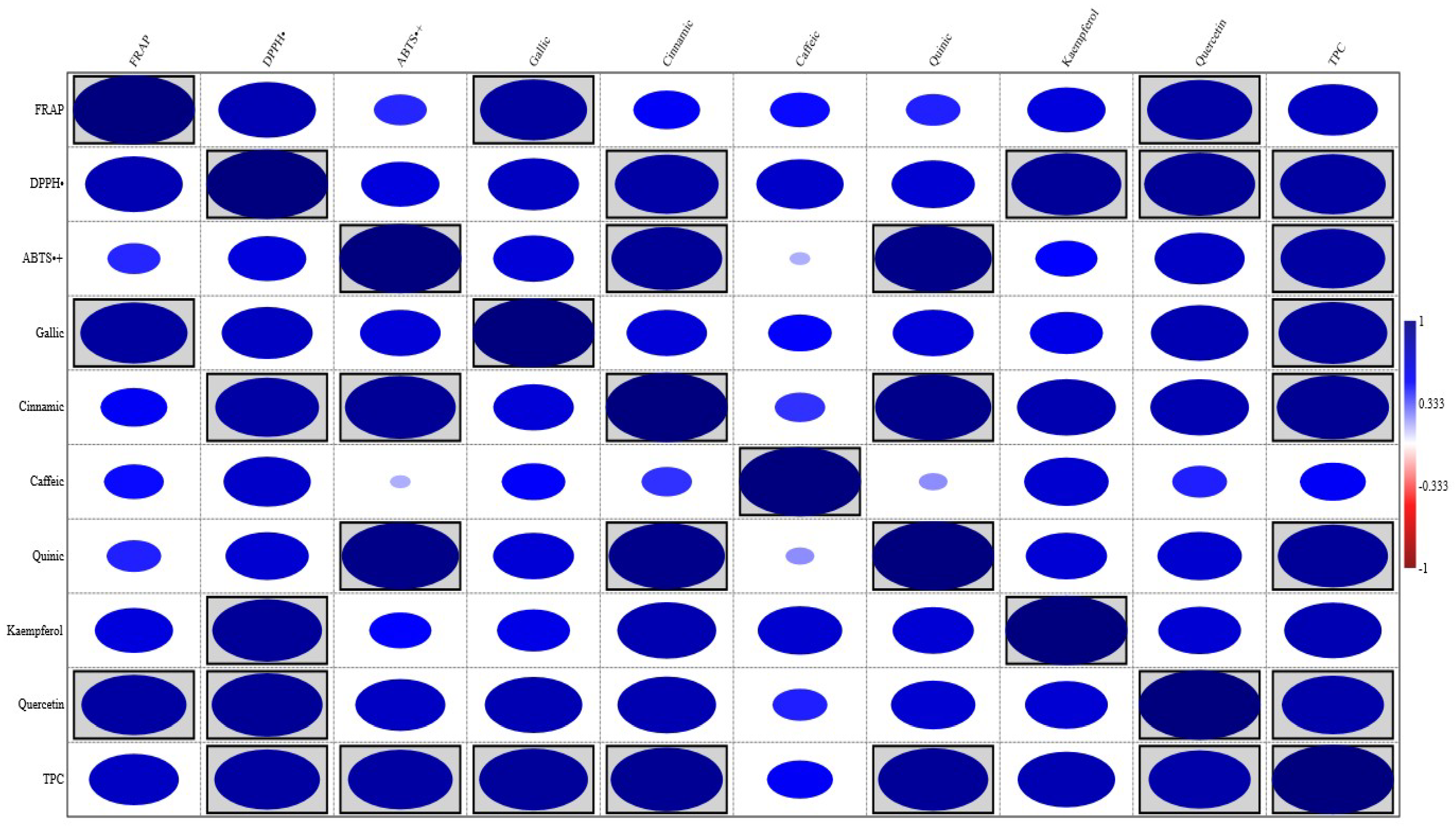
A Pearson’s correlation matrix was generated to assess the relationship of antioxidant potential with oil chemical composition, as shown in Figure 1. FRAP values were highly correlated with gallic acid (r = 0.876) and quercetin (r = 0.859) contents, whereas DPPH• activity correlated with trans-cinnamic acid (r = 0.848), kaempferol (r = 0.898), and quercetin (r = 0.908) levels. Finally, ABTS•+ activity was correlated with trans-cinnamic (r = 0.907) and quinic (r = 0.960) acids. The differences in correlations between antioxidant potentials and chemical composition can be attributed to the different mechanisms of action of the analytical techniques. FRAP and ABTS•+ methods are based on electron donation, whereas DPPH• is based on the transfer of hydrogen atoms [46]. Pearson’s correlation coefficient suggested that total phenolic content is a good predictor of antioxidant potential, as determined by DPPH• (r = 0.863) and ABTS•+ (r = 0.856) methods. All correlations were significant (p < 0.05). The findings demonstrated that phenolic acids and flavonoids were responsible for the antioxidant activity of experimental oils.
Figure 1.
Correlation matrix describing the relationship between antioxidant activity and phenolic acid, flavonoid, and total phenolic contents (TPCs). The size of the ellipse is proportional to the Pearson’s correlation coefficient. Ellipses highlighted with rectangles indicate significant correlations (p < 0.05).
3.3. Oil Characterization
Table 4 shows the fatty acid profile of enriched oil and sunflower seed oil. Both oils had a similar fatty acid profile, differing only in the percentages of palmitic and linoleic acids. Santos et al. [47] indicated palmitic acid as the major fatty acid in barbatimão bark, which explains the increase in its content by ~13% in enriched oil.

Table 4.
Fatty acid profile of enriched oil and sunflower seed oil.
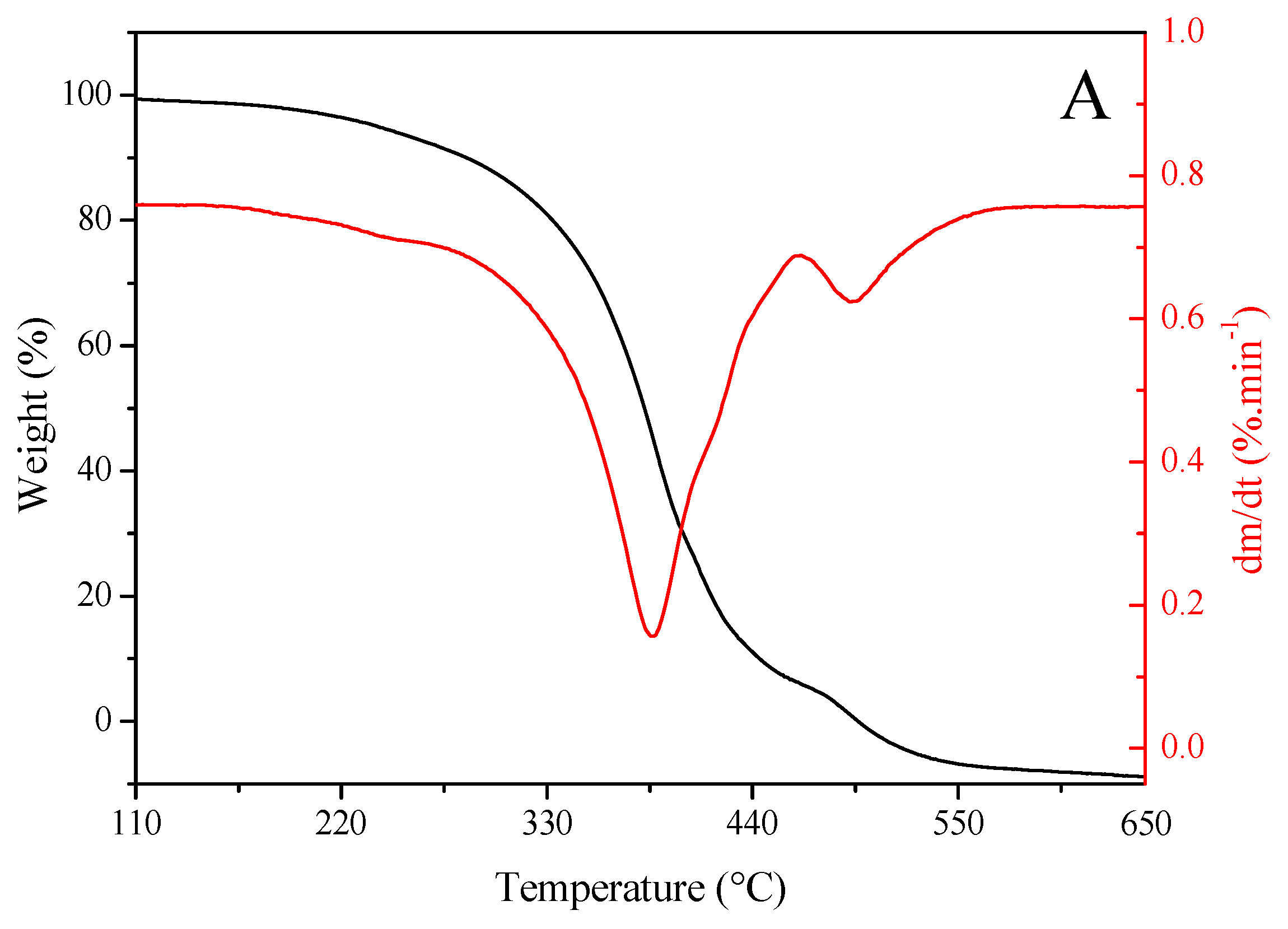
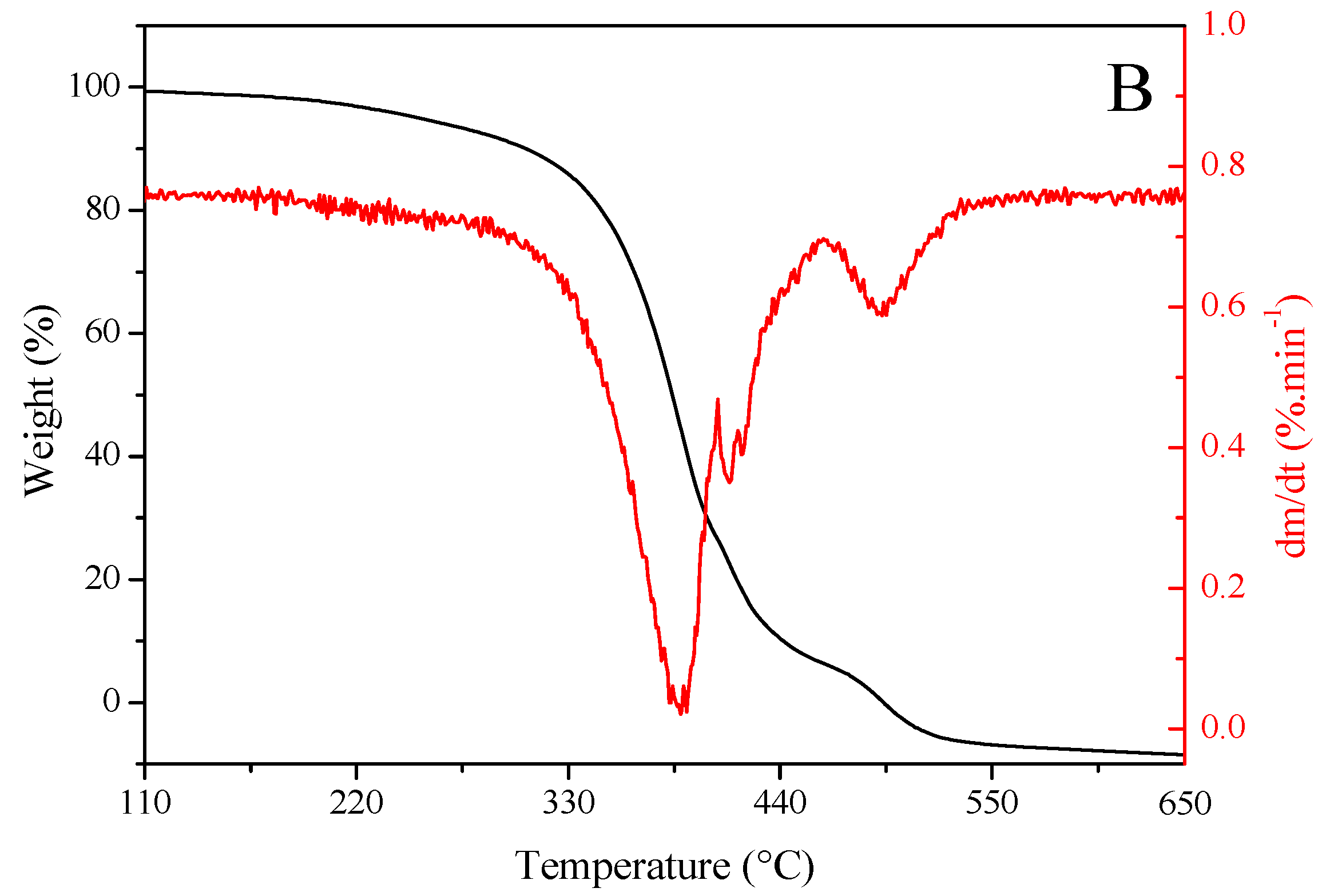
Figure 2 shows the results of TG and DTG analyses of sunflower seed oil (Figure 2A) and enriched oil (Figure 2B). Oils exhibited a similar trend in thermal degradation up to ~220 °C. However, enriched oil had higher thermal stability than sunflower seed oil from this temperature onward. Getachew et al. [48] argued that thermal stability is related to low mass loss during thermal degradation. This property indicates that the oil can be heated to high temperatures or for longer periods without undergoing oxidative degradation. Such an effect may be explained by the higher levels of saturated fatty acids in enriched oil (15.70%) than in sunflower seed oil (13.32%), as well as the presence of major natural antioxidants in the former (Table 2 and Table 3). Antioxidants positively influence oxidative stability [4]. In similar studies, enriched oil exhibited a higher stability than sunflower seed oil in the treatment of skin wounds [49] and in relation to the synthetic antioxidant butylated hydroxyanisole [50].
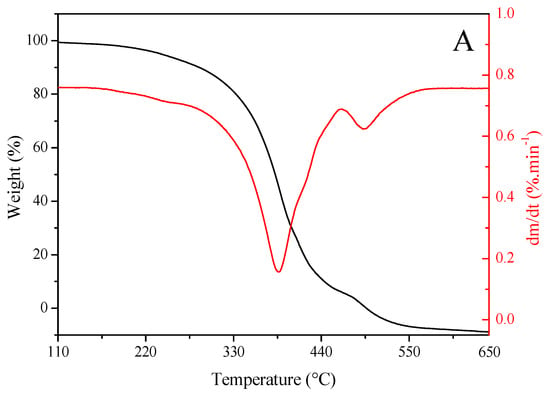
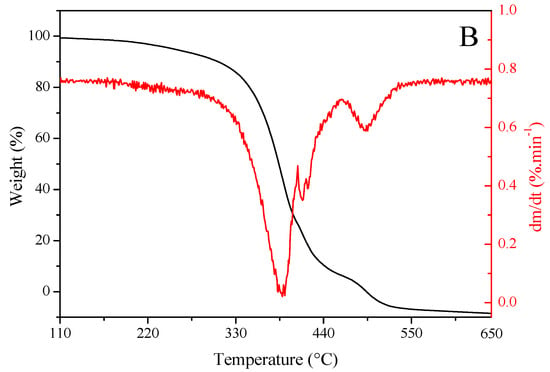
Figure 2.
Thermogravimetric (red) and derivative thermogravimetric (black) curves of (A) sunflower seed oil and (B) enriched oil.
The degradation curve obtained using the DTG analysis was found at ~380–400 °C, suggesting that the oils are suitable for high-temperature applications [51]. Furthermore, the burning temperature of samples was determined from TG–DTG curves, which was found to be 650 °C for both oils. These data indicate that, at 600 °C, the oils were completely degraded, and oxidation was completed. This stage was achieved via the decomposition of monounsaturated fatty acids, represented predominantly by oleic acid. The rupture of double bonds gives rise to saturated fatty acids, which sequentially undergo degradation, resulting in residual carbonaceous substances [52].
The TBARS assay revealed MDA contents of 983.02 ± 10.19 and 850.14 ± 5.54 mg/100 g in sunflower seed oil and essential oil, respectively. These values corroborate those obtained by TG and DTG analyses.
3.4. Cytotoxicity Analysis
The enriched oil was not found to be toxic toward HaCaT cells at the evaluated concentrations (Table 5). Overall, cell viability remained >90% after 24 h and >97% after 48 h, regardless of extract concentration. These findings indicate that, in addition to a lack of cytotoxic effects [53], the enriched oil promoted cell growth at certain concentrations. The possible increase in cell number, observed mainly after 48 h, might be related to the presence of phenolic compounds from barbatimão bark, as reported in Table 2.

Table 5.
Cytotoxic effects of enriched oil on HaCaT cells.
The phenolic compounds present in enriched oil may have promoted in vitro cell viability, likely due to their ability to modulate responses to oxidative stress and support cell survival and proliferation. According to Chanaj-Kaczmarek et al. [54], mechanistically, the antioxidant activity of phenolic compounds is considered multidirectional, and such mechanisms can contribute to the maintenance of cellular homeostasis, making it possible to promote cell survival and create favorable conditions for cell proliferation.
Lim et al. [55] investigated the effect of dried green tea leaf extracts on intestinal Caco-2 cells subjected to H2O2-induced oxidative stress. The results demonstrated that cell viability significantly increased when probiotics were coated with phenolic compounds. These results indicate that phenolic compounds not only prevent potential cytotoxic effects but also promote cell viability.
Research on the effects of oil enriched with barbatimão bark on cell viability is still incipient. Nevertheless, Gomes et al. [56] assessed the cytotoxicity of barbatimão bark extract against MRC-5 fibroblasts and reported no cytotoxic effect at the tested concentration (40 µg/mL). Cecílio et al. [57] determined the maximum non-toxic concentration of ethanolic extract of barbatimão leaves; cytotoxicity was observed at 500 and 5000 µg/mL for MA-104 cell monolayers, indicating that cytotoxicity may vary according to plant part, extract concentration, and cell type. However, at the concentrations tested here, enriched oil was not considered toxic.
4. Conclusions
This study successfully obtained sunflower oil enriched with phenolic compounds from barbatimão bark under the proposed extraction conditions. The oil with the highest phenolic content (182 mg GAE/100 g oil) was produced at 60 °C for 60 min, using a sunflower seed/barbatimão bark ratio of 3:2.5. Although the addition of barbatimão bark during sunflower seed extraction caused a reduction in mass yield (from 40.20% to 21.49%), it increased the levels of phenolic acids (gallic, trans-cinnamic, caffeic, coumaric, and ferulic), flavonoids (quercetin and kaempferol), and antioxidant activity. The enriched oil exhibited greater thermal stability and was non-toxic, as confirmed by cytotoxicity assays using HaCaT cells. These results demonstrate the effectiveness of the proposed green extraction method for obtaining a bioactive oil potentially suitable for topical applications. Furthermore, the method is easily scalable, as it involves simple and environmentally friendly operational steps.
Author Contributions
Conceptualization, J.d.O.C., B.C.B.B. and C.d.S.; methodology, J.d.O.C., B.C.B.B. and C.d.S.; software, C.d.S. and B.C.B.B.; validation, J.d.O.C., N.S., D.T.R. and C.d.S.; formal analysis, investigation, and data curation, J.d.O.C., I.A.d.C.F., D.T.R., N.S., V.A.d.S.G., B.C.B.B. and C.d.S.; writing—original draft, J.d.O.C., B.C.B.B. and C.d.S.; writing—review and editing, C.d.S.; visualization, C.d.S.; supervision, B.C.B.B. and C.d.S.; project administration, C.d.S.; funding acquisition, C.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001 (88887.658985/2021-00).
Data Availability Statement
The datasets supporting the conclusions of this article are included within the manuscript.
Acknowledgments
The authors would like to thank the State University of Maringá (UEM).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sousa, J.d.P.d.S.; Feitosa, R.S.; Lira, B.S.d.M.M.; Medeiros, M.d.G.F.d.; Carvalho, A.L.M. Óleos vegetais como promotores de permeação cutânea em formulações tópicas e transdérmicas de anti-inflamatórios: Uma revisão integrativa. Res. Soc. Dev. 2021, 10, e541101220308. [Google Scholar] [CrossRef]
- Sanches, S.C.d.C.; Silva-Júnior, J.O.C.; Ribeiro-Costa, R.M. O uso dos óleos vegetais na prevenção do envelhecimento da pele. Res. Soc. Dev. 2021, 10, e44010111941. [Google Scholar] [CrossRef]
- Torres, S.B.; de Queiroz, A.L.F.G.; dos Santos, A.N.A.; Alves, G.Q.; da Silva, I.A.; Brito, J.K.C.; Sultanun, R.F.d.S.; Monteiro, A.C.S. Óleo de girassol (Helianthus annus L.) como cicatrizante de feridas em idosos diabéticos. Braz. J. Health Rev. 2021, 4, 4692–4703. [Google Scholar] [CrossRef]
- da Rosa, A.C.S.; Costa, A.J.N.; Santos Júnior, O.O.; da Silva, C. Ultrasound-Assisted Extraction of Sunflower Seed Oil Enriched with Active Compounds from Jambolan Leaf. J. Braz. Chem. Soc. 2025, 36, 1–10. [Google Scholar] [CrossRef]
- Segantini, K.C.d.O.; Santos Junior, O.d.O.S.; Garcia, V.A.D.S.; Raspe, D.T.; da Silva, C. Sunflower Seed Oil Enriched with Compounds from the Turmeric Rhizome: Extraction, Characterization and Cell Viability. Separations 2025, 12, 121. [Google Scholar] [CrossRef]
- Correa, M.C.M.; Mao, G.; Saad, P.; Flach, C.R.; Mendelsohn, R.; Walters, R.M. Molecular interactions of plant oil components with stratum corneum lipids correlate with clinical measures of skin barrier function. Exp. Dermatol. 2014, 23, 39–44. [Google Scholar] [CrossRef]
- Elias, P.M.; Brown, B.E.; Ziboh, V.A. The Permeability Barrier in Essential Fatty Acid Deficiency: Evidence for a Direct Role for Linoleic Acid in Barrier Function. J. Investig. Dermatol. 1980, 74, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Cižmárová, B.; Hubková, B.; Tomečková, V.; Birková, A. Flavonoids as Promising Natural Compounds in the Prevention and Treatment of Selected Skin Diseases. Int. J. Mol. Sci. 2023, 24, 6324. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.J.; Moh, S.H.; Son, D.H.; You, S.; Kinyua, A.W.; Ko, C.M.; Song, M.; Yeo, J.; Choi, Y.-H.; Kim, K.W. Gallic Acid Promotes Wound Healing in Normal and Hyperglucidic Conditions. Molecules 2016, 21, 899. [Google Scholar] [CrossRef]
- Mekhoukh, N.; Chougui, N.; Vilas-Boas, A.A.; Pintado, M.; Bendif, H.; Zancato, M.; Bellik, Y.; Sid, N.; Peron, G. Development and characterization of natural phenolics-rich extracts and formulations based on Putoria calabrica leaf for wound healing applications. J. Pharm. Pharmacol. 2025, 77, 970–982. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zeng, R.; Hu, L.; Maffucci, K.G.; Ren, X.; Qu, Y. In vivo wound healing and in vitro antioxidant activities of Bletilla striata phenolic extracts. Biomed. Pharmacother. 2017, 93, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Janicka, P.; Płotka-Wasylka, J.; Jatkowska, N.; Chabowska, A.; Fares, M.Y.; Andruch, V.; Kaykhaii, M.; Gębicki, J. Trends in the new generation of green solvents in extraction processes. Curr. Opin. Green Sustain. Chem. 2022, 37, 100670. [Google Scholar] [CrossRef]
- Piotrowski, W.; Kubica, R. Integration of the process for production of ethyl acetate by an enhanced extraction process. Processes 2021, 9, 1425. [Google Scholar] [CrossRef]
- Ribeiro, M.M.d.S.; dos Santos, L.C.; de Novais, N.S.; Viganó, J.; Veggi, P.C. An evaluative review on Stryphnodendron adstringens extract composition: Current and future perspectives on extraction and application. Ind. Crops Prod. 2022, 187, 115325. [Google Scholar] [CrossRef]
- Henriques, B.O.; Corrêa, O.; Azevedo, E.P.C.; Pádua, R.M.; Oliveira, V.L.S.; de Oliveira, T.H.C.; Boff, D.; Dias, A.C.F.; de Souza, D.G.; Amaral, F.A.; et al. In vitro TNF-α inhibitory activity of brazilian plants and anti-inflammatory effect of Stryphnodendron adstringens in an acute arthritis model. Evid.-Based Complement. Altern. Med. 2016, 2016, 9872598. [Google Scholar] [CrossRef]
- Baldivia, D.D.S.; Leite, D.F.; Castro, D.T.H.D.; Campos, J.F.; Santos, U.P.D.; Paredes-Gamero, E.J.; Carollo, C.A.; Silva, D.B.; Souza, K.P.; Santos, E.L. Evaluation of in vitro antioxidant and anticancer properties of the aqueous extract from the stem bark of Stryphnodendron adstringens. Int. J. Mol. Sci. 2018, 19, 2432. [Google Scholar] [CrossRef]
- Ishida, K.; Mello, J.C.P.; Cortez, D.A.G.; Dias Filho, B.P.; Ueda-Nakamura, T.; Nakamura, C.V. Influence of tannins from Stryphnodendron adstringens on growth and virulence factors of Candida albicans. J. Antimicrob. Chemother. 2006, 58, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Sabino, A.P.L.; Eustáquio, L.M.S.; Miranda, A.C.F.; Biojone, C.; Mariosa, T.N.; Gouvêa, C.M.C.P. Stryphnodendron adstringens (“Barbatimão”) leaf fraction: Chemical characterization, antioxidant activity, and cytotoxicity towards human breast cancer cell lines. Appl. Biochem. Biotechnol. 2018, 184, 1375–1389. [Google Scholar] [CrossRef]
- Trevisan, D.A.C.; Silva, P.V.; Farias, A.B.P.; Campanerut-Sá, P.A.Z.; Ribeiro, T.D.V.R.; Faria, D.R.; Mendonça, P.S.B.; Mello, J.C.P.; Seixas, F.A.V.; Mikcha, J.M.G. Antibacterial activity of Barbatimão (Stryphnodendron adstringens) against Staphylococcus aureus: In vitro and in silico studies. Lett. Appl. Microbiol. 2020, 71, 259–271. [Google Scholar] [CrossRef]
- Braga, M.N.S.; Batista, D.M.; Souza, D.B.; Lima, E.S.; Pantoja, T.M.A.; Xavier, R.A.T.; Lima, R.A. Estudo Etnobotânico de Plantas Medicinais da Família Fabaceae Na Comunidade Cristolândia, Humaitá-AM. Rev. Biodivers 2022, 21, 14–26. [Google Scholar]
- Ribeiro, M.M.d.S.; Viganó, J.; de Novais, N.S.; Mesquita, L.M.d.S.; Kamikawachi, R.C.; Vilegas, W.; Lopes, P.S.; da Silva, C.S.; Rostagno, M.A.; Veggi, P.C. The effect of ultrasound on improving the extraction of tannins from the Stryphnodendron adstringens bark. Sustain. Chem. Pharm. 2023, 33, 101044. [Google Scholar] [CrossRef]
- Aguiar, P.d.S.d.; Correa, Á.P.; Antunes, F.T.T.; Ferraz, A.F.d.B.; Vencato, S.B.; Amado, G.J.V.; Wiiland, E.; Corrêa, D.S.; Grivicich, I.; de Souza, A.H. Benefits of Stryphnodendron adstringens when associated with hydrogel on wound healing in diabetic rats. Clin. Phytoscience 2021, 7, 22. [Google Scholar] [CrossRef]
- Haiyan, Z.; Bedgood, D.R., Jr.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Endogenous biophenol, fatty acid and volatile profiles of selected oils. Food Chem. 2007, 100, 1544–1551. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Gu, C.; Howell, K.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS Characterisation of Phenolic Acids and Flavonoids in Polyphenol-Rich Fruits and Vegetables and Their Potential Antioxidant Activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, N.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F.; Serafini, M. Total Antioxidant Capacity of Plant Foods, Beverages and Oils Consumed in Italy Assessed by Three Different In Vitro Assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef]
- Stevanato, N.; de Mello, B.T.F.; Saldaña, M.D.A.; Cardozo-Filho, L.; da Silva, C. Production of ethyl esters from forage radish seed: An integrated sequential route using pressurized ethanol and ethyl acetate. Fuel 2023, 332, 126075. [Google Scholar] [CrossRef]
- de Mello, B.T.F.; Stevanato, N.; Filho, L.C.; da Silva, C. Pressurized liquid extraction of radish seed oil using ethanol as solvent: Effect of pretreatment on seeds and process variables. J. Supercrit. Fluids 2021, 176, 105307. [Google Scholar] [CrossRef]
- Sridhar, K.; Charles, A.L. Grape skin extracts as a sustainable source of antioxidants in an oil-in-water emulsion: An alternate natural approach to synthetic antioxidants using principal component analysis. Int. J. Food Sci. Technol. 2021, 56, 1937–1945. [Google Scholar] [CrossRef]
- Malich, G.; Markovic, B.; Winder, C. The sensitivity and specificity of the MTS tetrazolium assay for detecting the in vitro cytotoxicity of 20 chemicals using human cell lines. Toxicology 1997, 124, 179–192. [Google Scholar] [CrossRef]
- de Queiroz, J.E.; dos Santos, D.M.; Verde, G.M.V.; de Paula, J.R.; de Aquino, G.L.B. Microwave irradiation to the rapid extraction of Stryphnodendron adstringens (Barbatimão) compounds by statistical planning. Nat. Prod. Res. 2019, 35, 354–358. [Google Scholar] [CrossRef]
- Cruz, J.E.R.; Costa, J.L.G.; Teixeira, T.A.; Freitas, G.R.O.; Gomes, M.S.; Morais, E.R. Phenolic compounds, antioxidant and antibacterial activity of extract from leaves and bark of Stryphnodendron adstringens (Mart.) Coville. Rev. Ciênc. Agron. 2022, 53, e20217903. [Google Scholar] [CrossRef]
- Kumar, S.; Rai, A.; Prasad, K. Enhancing sustainability and quality: A comparative study of sunflower seed oil extraction methods and physico-chemical characterization. Sustain. Chem. One World 2025, 6, 100060. [Google Scholar] [CrossRef]
- Sibhatu, H.K.; Jabasingh, S.A.; Yimam, A.; Ahmed, S. Ferulic acid production from brewery spent grains, an agro-industrial waste. LWT 2021, 135, 110009. [Google Scholar] [CrossRef]
- Qian, S.; Lu, M.; Zhou, X.; Sun, S.; Han, Z.; Song, H. Improvement in caffeic acid and ferulic acid extraction by oscillation-assisted mild hydrothermal pretreatment from sorghum straws. Bioresour. Technol. 2024, 396, 130442. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- de Novais, N.S.; Ribeiro, M.M.d.S.; Viganó, J.; Coelho, D.B.; Falcão, L.d.S.; de Moraes, M.A.; Veggi, P.C. High- and low-pressure fixed bed extraction behaviors to obtain phenolic compounds from barbatimão (Stryphnodendron adstringens) bark. Sustain. Chem. Pharm. 2023, 36, 101314. [Google Scholar] [CrossRef]
- Romana-Souza, B.; dos Santos, J.S.; Monte-Alto-Costa, A. Caffeic acid phenethyl ester promotes wound healing of mice pressure ulcers affecting NF-κB, NOS2 and NRF2 expression. Life Sci. 2018, 207, 158–165. [Google Scholar] [CrossRef]
- Singh, M.P.; Gupta, A.; Sisodia, S.S. Wound healing activity of Terminalia bellerica Roxb and gallic acid in experimentally induced diabetic animals. J. Complement. Integr. Med. 2019, 17, 20190133. [Google Scholar] [CrossRef]
- Pellenz, N.L.; Barbisan, F.; Azzolin, V.F.; Duarte, T.; Bolignon, A.; Mastella, M.H.; Teixeira, C.F.; Ribeiro, E.E.; da Cruz, I.B.M.; Duarte, M.M.M.F. Analysis of in vitro cyto-and genotoxicity of barbatimão extract on human keratinocytes and fibroblasts. BioMed Res. Int. 2018, 2018, 1942451. [Google Scholar] [CrossRef] [PubMed]
- Özay, Y.; Güzel, S.; Yumrutaş, Ö.; Pehlivanoğlu, B.; Erdoğdu, I.H.; Yildirim, Z.; Türk, B.A.; Darcan, S. Wound healing effect of kaempferol in diabetic and nondiabetic rats. J. Surg. Res. 2019, 233, 284–296. [Google Scholar] [CrossRef]
- Subramanian, S.; Duraipandian, C.; Alsayari, A.; Ramachawolran, G.; Wong, L.S.; Sekar, M.; Gan, S.H.; Subramaniyan, V.; Seethalakshmi, S.; Jeyabalan, S.; et al. Wound healing properties of a new formulated flavonoid-rich fraction from Dodonaea viscosa Jacq. leaves extract. Front. Pharmacol. 2023, 14, 1096905. [Google Scholar] [CrossRef]
- Souza-Moreira, T.M.; Queiroz-Fernandes, G.M.; Pietro, R.C.L.R. Stryphnodendron Species Known as “Barbatimão”: A Comprehensive Report. Molecules 2018, 23, 910. [Google Scholar] [CrossRef]
- Alves, M.C.M.A.; Nascimento, M.F.; de Almeida, B.M.; Alves, M.M.A.; Lima-Verde, I.B.; Costa, D.S.; Araújo, D.C.M.; de Paula, M.N.; de Mello, J.C.P.; Cano, A.; et al. Hydrophilic Scaffolds Containing Extracts of Stryphnodendron adstringens and Abarema cochliacarpa for Wound Healing: In Vivo Proofs of Concept. Pharmaceutics 2022, 14, 2150. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Jing, L.; Zhao, K.; Su, C.; Zhang, B.; Zhang, Q.; Han, L.; Yu, X.; Li, W. The phenolic compounds profile, quantitative analysis and antioxidant activity of four naked barley grains with different color. Food Chem. 2021, 335, 127655. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.R.D.; Barbosa, C.R.; Souza, C.G.; Fernandes, T.; Chagas, R.A.; Navarro, S.R.; Fernandes, A.R.M.; Vargas Junior, F.M. Barbatimão bark as an alternative to sodium lasalocid in finishing diets for lambs: Carcass characteristics and meat quality. Meat Sci. 2025, 225, 109831. [Google Scholar] [CrossRef]
- Getachew, A.T.; Saravana, P.S.; Cho, Y.J.; Woo, H.C.; Chun, B.S. Concurrent extraction of oil from roasted coffee (Coffea arabica) and fucoxanthin from brown seaweed (Saccharina japonica) using supercritical carbon dioxide. J. CO2 Util. 2018, 25, 137–146. [Google Scholar] [CrossRef]
- Rocha, K.F.; Melo, E.S.d.P.; Cardozo, C.M.L.; Guimarães, R.d.C.A.; Freitas, K.d.C.; Coelho, M.L.; Ramos, C.A.D.N.; de Oliveira, L.C.S.; Cavalheiro, L.F.; Nascimento, V.A.D. Data on fatty acid profile, optical properties and oxidative stability of sunflower oils used in the treatment of skin wounds. Data Brief. 2023, 47, 109009. [Google Scholar] [CrossRef]
- Lu, L.; Luo, K.; Luan, Y.; Zhao, M.; Wang, R.; Zhao, X.; Wu, S. Effect of caffeic acid esters on antioxidant activity and oxidative stability of sunflower oil: Molecular simulation and experiments. Food Res. Int. 2022, 160, 117060. [Google Scholar] [CrossRef]
- Roy, V.C.; Getachew, A.T.; Cho, Y.-J.; Park, J.-S.; Chun, B.-S. Recovery and bio-potentialities of astaxanthin-rich oil from shrimp (Penaeus monodon) waste and mackerel (Scomberomous niphonius) skin using concurrent supercritical CO2 extraction. J. Supercrit. Fluids 2020, 159, 104773. [Google Scholar] [CrossRef]
- Machate, D.J.; Melo, E.S.P.; de Oliveira, L.C.S.; Bogo, D.; Michels, F.S.; Pott, A.; Cavalheiro, L.F.; Guimarães, R.d.C.A.; Freitas, K.d.C.; Hiane, P.A.; et al. Oxidative stability and elemental analysis of sunflower (Helianthus annuus) edible oil produced in Brazil using a domestic extraction machine. Front. Nutr. 2022, 9, 977813. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Chanaj-Kaczmarek, J.; Wysocki, M.; Karachitos, A.; Wojcinska, M.; Bartosz, G.; Matławska, I.; Kmita, H. Effects of plant extract antioxidative phenolic compounds on energetic status and viability of Saccharomyces cerevisiae cells undergoing oxidative stress. J. Funct. Foods 2015, 16, 364–377. [Google Scholar] [CrossRef]
- Lim, J.; Na, G.; Kang, J. A green nanocoating approach to Lactobacillus plantarum using tea residue-derived phenolic compounds and cellulose nanocrystals. Food Hydrocoll. 2025, 167, 111469. [Google Scholar] [CrossRef]
- Gomes, P.W.P.; Pamplona, T.C.D.L.; Navegantes-Lima, K.C.; Quadros, L.B.G.; Oliveira, A.L.B.; Santos, S.M.; Silva, C.Y.Y.; Silva, M.J.C.; Souza, J.N.S.; Quirós-Guerrero, L.M.; et al. Chemical composition and antibacterial action of Stryphnodendron pulcherrimum bark extract, “barbatimão” species: Evaluation of its use as a topical agent. Arab. J. Chem. 2021, 14, 103183. [Google Scholar] [CrossRef]
- Cecílio, A.B.; de Faria, D.B.; Oliveira, P.d.C.; Caldas, S.; de Oliveira, D.A.; Sobral, M.E.G.; Duarte, M.G.R.; Moreira, C.P.d.S.; Silva, C.G.; de Almeida, V.L. Screening of Brazilian medicinal plants for antiviral activity against rotavirus. J. Ethnopharmacol. 2012, 141, 975–981. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).