Abstract
Coagulation–flocculation, historically reliant on simple inorganic salts, has evolved into a technically sophisticated process that is central to the removal of turbidity, suspended solids, organic matter, and an expanding array of micropollutants from complex wastewaters. This review synthesizes six decades of research, charting the transition from classical aluminum and iron salts to high-performance polymeric, biosourced, and hybrid coagulants, and examines their comparative efficiency across multiple performance indicators—turbidity removal (>95%), COD/BOD reduction (up to 90%), and heavy metal abatement (>90%). Emphasis is placed on recent innovations, including magnetic composites, bio–mineral hybrids, and functionalized nanostructures, which integrate multiple mechanisms—charge neutralization, sweep flocculation, polymer bridging, and targeted adsorption—within a single formulation. Beyond performance, the review highlights persistent scientific gaps: incomplete understanding of molecular-scale interactions between coagulants and emerging contaminants such as microplastics, per- and polyfluoroalkyl substances (PFAS), and engineered nanoparticles; limited real-time analysis of flocculation kinetics and floc structural evolution; and the absence of predictive, mechanistically grounded models linking influent chemistry, coagulant properties, and operational parameters. Addressing these knowledge gaps is essential for transitioning from empirical dosing strategies to fully optimized, data-driven control. The integration of advanced coagulation into modular treatment trains, coupled with IoT-enabled sensors, zeta potential monitoring, and AI-based control algorithms, offers the potential to create “Coagulation 4.0” systems—adaptive, efficient, and embedded within circular economy frameworks. In this paradigm, treatment objectives extend beyond regulatory compliance to include resource recovery from coagulation sludge (nutrients, rare metals, construction materials) and substantial reductions in chemical and energy footprints. By uniting advances in material science, process engineering, and real-time control, coagulation–flocculation can retain its central role in water treatment while redefining its contribution to sustainability. In the systems envisioned here, every floc becomes both a vehicle for contaminant removal and a functional carrier in the broader water–energy–resource nexus.
1. Introduction
1.1. Global Context: Challenges Linked to Wastewater Pollution
Wastewater management has become one of the critical knots in the fabric of global environmental sustainability. Far from being a simple by-product of domestic, industrial, or agricultural activities, wastewater represents a dynamic interface between human development and ecological integrity. When mismanaged, it acts as a vector of instability—disrupting biogeochemical cycles, threatening public health, and undermining the hydrological resilience of ecosystems and communities [1].
Today, demographic expansion, urban densification, the growth of extractive industries, and the mounting pressure of climate change on hydrological regimes converge to create heightened exposure to multifaceted water pollution [2]. This global convergence reinforces a well-established reality: conventional sanitation infrastructures, often under-dimensioned, are no longer able to keep pace with the growing complexity of wastewater effluents [3].
At the planetary scale, wastewater now contains several thousand anthropogenic compounds, most of which escape conventional treatment. These span entire pollutant classes—reactive nutrients, trace metals, pharmaceutical residues, microplastics, per- and polyfluoroalkyl substances (PFAS), and multi-resistant biological agents [4]. Their persistence in aquatic matrices profoundly alters ecosystem functions, compromises public health security, and introduces new analytical and regulatory challenges. The limitations of current management approaches are not only technological; they are also rooted in the very architecture of water policies, which remain predominantly linear. The historical separation between the “upstream” phase (production) and the “downstream” phase (treatment) has fostered fragmented, reactive management, ill-suited to address contaminants with long environmental lifespans or diffuse impacts [5].
In an era of mounting water stress, what is needed is a systemic approach that treats wastewater as part of a closed-loop cycle. In this perspective, wastewater valorization is no longer a marginal option but a strategic imperative. The integration of advanced treatment processes, the development of reuse pathways (agricultural, industrial, urban), and the deployment of intelligent sensors for adaptive process control are emerging as essential pillars of 21st-century water engineering [6].
1.2. Limitations of Conventional Wastewater Treatment Technologies
For decades, conventional wastewater treatment technologies—such as primary sedimentation, aerobic biological processes like activated sludge, and chemical coagulation—formed the backbone of municipal and industrial sanitation systems. These approaches remain relevant for reducing overall organic loads and suspended solids [7]. However, they now face structural, technical, and environmental limitations in the face of increasingly complex effluent compositions [8,9,10].
From a technological perspective, their removal efficiency is insufficient for many emerging pollutants. Persistent organic micropollutants, pharmaceutical residues, microplastics, and trace metals often pass through untreated, as documented in numerous studies [11,12,13]. For example, in hospital wastewater treated with conventional methods, significant concentrations of active compounds such as ciprofloxacin, hormones, and disinfectants remain detectable, exerting proven ecotoxicological effects on aquatic communities [14]. Compounding the problem, these systems were designed for relatively stable pollution profiles, whereas today’s effluents fluctuate greatly in both flow rate and composition.
Energy and operational costs are another major constraint. Aerobic biological treatments require substantial oxygen injection, leading to significant energy consumption—typically estimated between 0.3 and 0.6 kWh/m3—which challenges their viability in an energy transition context [15]. Additionally, they generate large volumes of secondary sludge, whose management—dewatering, transport, incineration—remains both an environmental and economic burden, especially in densely populated urban areas.
Resilience is also a limiting factor. Studies on municipal plants have shown that variable hydraulic loads, common during extreme events such as heavy rainfall or industrial discharges, can trigger system failures: drops in treatment efficiency, nutrient release, and clogging of biological reactors [16,17,18]. This operational instability calls into question the adaptability of these technologies to contemporary urban contexts characterized by intermittent flows and diverse pollutant profiles. Furthermore, conventional systems typically operate within a linear “treat-and-discharge” paradigm, with minimal recovery of valuable resources from wastewater—such as nitrogen, phosphorus, or thermal energy. Evaluations from the European AquaFit4Use program revealed that over 60% of dissolved nutrients in municipal and industrial discharges are not recovered, primarily due to the absence of recovery modules or hybrid treatment configurations [19].
In certain industrial sectors—such as pesticide manufacturing, pharmaceuticals, and textiles—the chemical complexity of effluents exceeds the treatment capabilities of traditional systems. For instance, wastewater from a pesticide plant was found to contain chlorinated compounds that conventional biological treatments could not remove, necessitating the addition of advanced processes such as electrocoagulation or advanced oxidation to meet discharge standards [20]. In this light, while conventional technologies remain robust and well-established, they no longer meet contemporary requirements for versatility, sustainability, adaptability, and resource recovery. Their modernization—or integration with advanced treatment processes—has become a strategic priority in the development of smarter, more sustainable, and more efficient wastewater treatment systems.
1.3. The Role of Coagulants in Wastewater Treatment Chains
In modern wastewater treatment systems, coagulation–flocculation stands as a pivotal stage in both primary and tertiary treatment. Historically dominated by metal salts such as aluminum and iron, the process has evolved to meet new demands for performance, environmental safety, regenerability, and versatility in handling increasingly complex effluents. The coagulant is no longer merely a clarification aid; it has become a strategic lever for targeted treatment, cost reduction, and optimization of water circularity [21,22].
Against a backdrop of technological innovation and diversification of approaches, Figure 1 illustrates the evolution in the number of scientific publications on coagulants for wastewater treatment, compiled from Scopus and Springer databases using the keywords “coagulant” and “water” from 1960 to 2025. The trend over six decades is nearly exponential: only a few dozen articles appeared between 1960 and 1980, followed by moderate growth in the 1980s and 1990s, reflecting a growing but still limited interest. A major turning point occurred in the early 2000s with the introduction of advanced polymeric coagulants, hybrid formulations, and increasingly stringent environmental regulations. This period saw a fourfold increase in publications between 2000–2010 compared to 1990–2000. The decade 2010–2020 reached a historic peak with nearly 9000 articles, driven by concerns over sustainability, micropollutant removal, and sludge valorization. Although the volume from 2020–2025 is slightly lower, it remains high, confirming the field’s position as a priority research area. This trajectory reflects not only the scientific and technological evolution of the field but also the environmental urgency that has accelerated the search for innovative and sustainable coagulation–flocculation solutions.
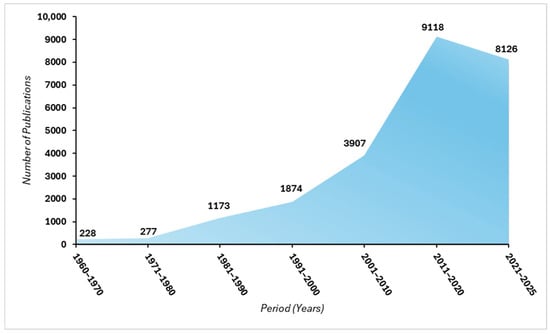
Figure 1.
Trend in the number of publications on coagulants used in wastewater treatment (1960–2025), in ten-year increments (based on a bibliographic search on Scopus, Web of Science and Google Scholar).
Recent research highlights that hybrid coagulants—combining organic and inorganic agents—outperform conventional formulations in multi-pollutant removal. Literature reviews indicate that certain hybrid coagulants (inorganic–inorganic or inorganic–biopolymer) achieve turbidity removal rates up to 98.5%, COD reduction up to 73.3%, and heavy metal removal up to 99.2% while also minimizing the need for pH adjustment—an important factor when treating complex industrial effluents [23].
In parallel, natural coagulants, often derived from plant-based materials such as starch, seeds (e.g., Moringa oleifera, Dolichos lablab), or even marine shells, are regaining interest. These agents offer higher biodegradability, lower toxicity, and comparable—or even superior—performance to chemical coagulants in removing suspended solids and phosphorus. Pilot-scale trials in Finland using starch-based coagulants achieved turbidity reductions exceeding 90%, with improved sludge quality and optimal compatibility with nutrient recovery processes [24].
From a more technological standpoint, electrocoagulation (EC) processes are emerging as innovative alternatives to traditional chemical coagulants [25,26]. EC uses metal electrodes as an in situ source of coagulants, eliminating the need for external chemical addition. These systems reduce sludge generation, show high efficiency in removing organic micropollutants, and lower the overall carbon footprint. Reviews indicate that electrocoagulation is particularly effective for heavily loaded industrial effluents, such as those from textile and petroleum industries [25,27].
The shift toward sustainable coagulants also responds to the environmental impact of sludge contaminated with aluminum or metal chlorides. A study in Current Pollution Reports demonstrated that natural coagulants provide comparable purification performance to chemical agents while reducing residual toxicity and facilitating by-product recovery [28]. Furthermore, emerging approaches involve multifunctional coagulants—such as grafted polymers or magnetic coagulants—designed to combine pollutant removal with resource recovery (e.g., phosphorus, nitrogen). These innovations align with the transition toward more circular, autonomous, and intelligent treatment systems, adapted to the pressures of urbanization, water scarcity, and industrial sustainability [29].
In sum, the role of the coagulant has expanded far beyond simple colloidal neutralization. It is now a central component of targeted, environmentally responsible, and adaptable treatment strategies—playing an active role in transforming conventional treatment chains into intelligent processing platforms.
1.4. Objective of the Review
Against the backdrop of tightening regulatory frameworks, mounting environmental pressures, and the increasing complexity of urban and industrial effluents, coagulation–flocculation processes are undergoing a significant technological renewal. The purpose of this review is to critically examine recent advances in the field, highlighting innovative, sustainable, and intelligent approaches that are reshaping the traditional paradigms of wastewater treatment.
Specifically, this review aims to:
- Identify recent progress in the development of multifunctional, hybrid, or composite coagulants capable of broadening the range of pollutants removed while minimizing environmental impact-such as magnetic or bio-functional coagulants;
- Assess emerging technological potentials, including assisted electrocoagulation, high-turbulence processes optimized through Computational Fluid Dynamics (CFD), and real-time adaptive dosing systems;
- Highlight sustainability criteria, including treatment efficiency, reduced coagulant toxicity, ease of sludge valorization, and compatibility with biological or membrane processes.
- Explore the integration of intelligent systems, notably coupling coagulation with automated technologies, IoT-enabled monitoring, and artificial intelligence to dynamically adjust treatment parameters according to load variability;
- Compare findings from applied studies on representative effluents—such as textile, agro-industrial, and petrochemical wastewater—to provide a rigorous synthesis of the optimal conditions for next-generation coagulation–flocculation technologies;
The overarching ambition of this review is to provide a structured, comparative, and forward-looking framework to guide future research and industrial practice toward treatment solutions that are more efficient, environmentally responsible, and intelligent-adapted to the water challenges of the 21st century.
2. Evolution of Coagulant Technologies for Wastewater Treatment
2.1. Traditional Mineral Coagulants (Before the 1960s)
Before the advent of polymeric and composite coagulants, wastewater treatment relied almost exclusively on simple inorganic salts such as aluminum sulfate (alum), ferric chloride (FeCl3), and ferric sulfate (Fe2(SO4)3) [30,31]. These agents primarily act through charge neutralization and sweep flocculation mechanisms, whereby unstable colloidal particles aggregate into larger, settleable flocs [30,31]. While they demonstrated proven effectiveness in reducing turbidity, chemical oxygen demand (COD), and total suspended solids (TSSs), these coagulants exhibited several critical limitations.
First, their performance is highly dependent on pH, with optimal ranges typically narrow (generally between 6 and 8). Second, they generate substantial amounts of chemical sludge, creating significant management and disposal costs. Third, the potential toxicity of residual metals (Al3+, Fe3+) in treated water raised environmental and public health concerns—notably the suspected link between aluminum salts and neurodegenerative diseases [32]. Comparative studies have shown that alum and ferric chloride, either alone or in combination with polymeric flocculants, can achieve TSS removal rates of 90–98% and COD reductions of 60–70%, depending on operational conditions [33]. However, their efficiency can be adversely affected by interactions with other constituents such as phosphates and surfactants, particularly in complex effluents from slaughterhouses or textile industries [34].
Figure 2 provides a visual synthesis of the mechanisms, performance ranges, and advantages/limitations of traditional mineral coagulants. The left panel illustrates the charge neutralization and sweep flocculation principle: small colloidal particles (blue) are aggregated by metallic salts into denser flocs (red) that settle more readily. The horizontal diagram summarizes typical performance ranges: TSS removal often exceeds 80% and can reach 90–95% under optimal conditions, while COD reduction is typically more modest, around 60–70%. These ranges are supported by recent studies. For example, using alum with 1 mg/L of polyelectrolyte, Solmaz et al. reported that COD removal increased from about 50% to 69% when the alum dose was raised to 4.05 mg/L, while turbidity and TSS removal exceeded 80% [35]. With ferric chloride, COD abatement reached approximately 70% at 1.29 mg/L and pH 5, with consistently high removal of turbidity, color, and TSS. Conversely, in studies on semi-aerobic landfill leachate, TSS removal exceeded 95%, but COD reduction was only 43%, highlighting the dependence of performance on effluent characteristics [36].
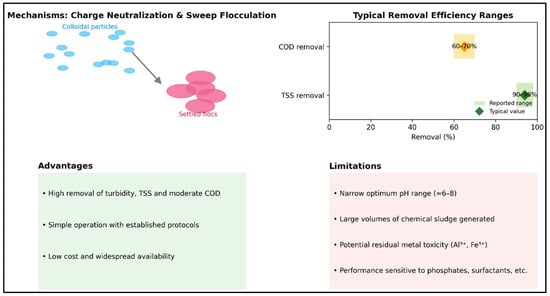
Figure 2.
Traditional mineral coagulants: mechanisms, ranges of efficacy and summary of advantages/limitations.
The lower panels of the figure (cf. Figure 2) summarize key points: these coagulants are valued for their efficiency, operational simplicity, and low cost but face major drawbacks—narrow optimal pH ranges, large volumes of sludge, risks from residual metal toxicity, and susceptibility to interference from other wastewater constituents. Thus, while traditional mineral coagulants can deliver high clarification performance, their results vary significantly with pH, dosage, and wastewater composition, as consistently shown in the literature.
In addition to sludge-related concerns, studies have found that residual aluminum in treated water can exceed regulatory thresholds when dosing is poorly controlled, necessitating careful monitoring of coagulant dosage and water quality [37]. Although these coagulants remain widely used for their simplicity and low relative cost, their gradual replacement is being driven by stricter environmental standards and the pursuit of more sustainable solutions with lower toxic residue generation.
2.2. Emergence of Synthetic Organic Coagulants and Improved Mineral Formulations (1960–1990)
From the 1960s onward, the limitations of traditional mineral coagulants led to the progressive introduction of synthetic organic coagulants and polymerized metallic salts. Two major classes defined this transition: organic polyelectrolytes (notably polyacrylamides, polyamines, and polyDADMACs) and polymerized aluminum chlorides (PACs). Together, they represented a major step forward in performance, stability, and operational safety [38].
Synthetic organic polymers were initially developed as flocculants, but their ability to form interparticle bridges and stabilize flocs allowed them to function effectively as coagulants as well. High-molecular-weight, strongly cationic polyacrylamides proved particularly efficient at removing colloidal matter and organic loads in both industrial and municipal wastewaters [39].
In parallel, polymerized aluminum coagulants such as polyaluminum chloride (PAC) were introduced to overcome the weaknesses of simple salts. PAC contains polymeric species such as Al13, which has a high charge density and enables rapid, efficient colloid neutralization, even at low dosages and across a broad pH range [40]. Optimized dosing and pH adjustments have been shown to achieve 90–100% removal of turbidity and organic dyes from textile effluents [41]. Improvements in PAC formulations—including enrichment in Al13 and the addition of specific anions such as sulfates or silicates—led to variants like polyaluminum silicate chloride (PASiC), offering better coagulation efficiency, reduced sludge generation, and improved stability [42,43,44].
Figure 3 summarizes the major advances observed between 1960 and 1990, when two new families emerged beyond simple metallic salts: high-molecular-weight organic polyelectrolytes and polymerized mineral coagulants such as PAC and its variants. The left-hand vignette illustrates two distinct mechanisms: interparticle bridging by polymer chains, typical of polyacrylamides, polyamines, and polyDADMACs; and the rapid particle aggregation induced by high-charge-density Al13 clusters in PAC and composite coagulants. The horizontal diagram outlines typical ranges reported in the literature: advanced coagulants generally achieve >90% suspended solids removal, up to 95% turbidity and color reduction, and COD decreases between 68% and 90%, depending on effluent type and optimal dosing. For example, in chemically enhanced primary treatment (CEPT) using PAC, suspended solid removal reached 95% and COD 68% at pH 7, while another study reported 97.34% TSS and 75.76% COD removal at pH 7.5 [45]. At 10 mg/L PAC, removals of ~90% TSS and 72% COD were achieved, and coupling PAC with a biological filter increased COD abatement to 96%. Cationic polyacrylamides were capable of removing nearly all turbidity in a simulated water sample (≈99.5%), although their efficiency dropped to ~50% for complex petrochemical effluents [46]. Coagulation assisted by ferrate and PAC has yielded COD reductions of 83–93% with near-complete turbidity removal [47]. Conversely, hybrid polyamide/PAC systems applied with dissolved air flotation achieved ~90% turbidity removal but only 15% COD reduction [30], underscoring how performance varies with effluent composition and coagulant formulation.
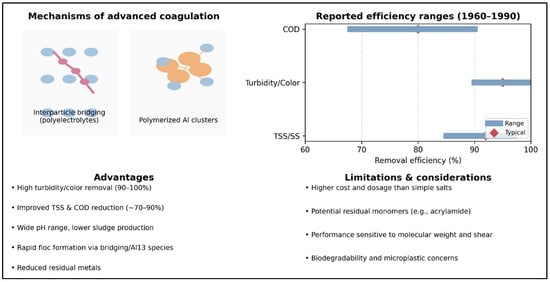
Figure 3.
Advanced Coagulants (1960–1990): Mechanisms, Efficiency Ranges, and Practical Considerations.
The lower panel of Figure 3 emphasizes key findings: these advanced coagulants deliver decisive advantages—very high turbidity and color removal, greater reductions in COD and suspended solids, effectiveness over a wider pH range, faster formation of denser flocs, and reduced levels of residual metals. However, limitations remain: higher costs and dosage requirements, potential residual monomers (e.g., acrylamide), sensitivity to molecular weight and shear, and ongoing concerns regarding biodegradability and microplastic generation. Collectively, these developments show that the 1960–1990 period marked a turning point toward more efficient and adaptable coagulation processes, setting the stage for the sustainable innovations that emerged in the following decades.
In industrial applications, these advanced coagulants proved effective in reducing COD, turbidity, and color loads in various effluents, notably in the paper [48] and textile industries. Their advantages also include greater solubility, faster reaction rates, and reduced formation of toxic by-products such as free metal ions [49]. As such, this period represents a clear shift toward more controlled, versatile, and efficient coagulation—laying the foundation for the sustainable and intelligent treatment approaches developed after the 2000s.
2.3. Revival of Natural and Bio-Based Coagulants (1990–2010)
From the 1990s onward, growing environmental concerns, the rising costs of managing chemical sludge, and the potential toxicity of conventional coagulants spurred renewed interest in natural coagulants, particularly those derived from plant and marine sources. This revival aligned with a broader shift toward sustainability and the valorization of local, renewable resources [50,51,52]. Among the most extensively studied natural coagulants are chitosan—derived from chitin in crustacean exoskeletons—and plant-based extracts such as Moringa oleifera, condensed tannins, and modified starches. These biopolymers function through mechanisms of charge neutralization, molecular bridging, and surface adsorption.
Figure 4 illustrates the principle of interparticle aggregation: biopolymers such as chitosan and cationic proteins from Moringa oleifera neutralize colloidal charges and form molecular bridges between particles, leading to their agglomeration. The panel on “Typical Removal Efficiencies” highlights ranges reported in the literature: natural coagulants generally achieve 80–95% removal of turbidity and suspended solids, and 65–90% removal of COD/BOD.
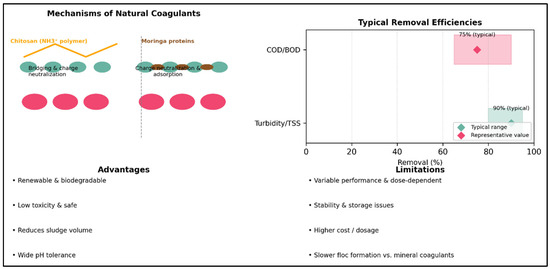
Figure 4.
Natural Bio-Sourced Coagulants (1990–2010)—Mechanisms, Efficiencies, and Summary of Advantages and Limitations.
Chitosan quickly emerged as an effective alternative to synthetic coagulants for treating diverse effluents, including municipal, aquaculture, and agro-industrial wastewaters. It consistently delivers high removal rates for turbidity, phosphorus, and COD while generating smaller volumes of biodegradable sludge [53]. For instance, one study reported that chitosan achieved 84% turbidity reduction at an optimized pH of 6 [54]. Moringa oleifera, rich in cationic proteins, operates primarily through charge neutralization and has demonstrated COD/BOD reductions of up to 90% in certain industrial effluents [54]. Although its performance is sometimes lower than that of chitosan, its low cost and minimal toxicity make it an attractive option in rural regions and developing countries [55]. Beyond these, tannins (plant-derived polyphenols) and modified starches have also shown strong performance in removing phosphorus, suspended solids, and organic matter, while enabling sludge reuse in agricultural applications [56]. Some tannins have even been reported to outperform starches in reducing pollutant loads at low concentrations [53].
Despite their promise, bio-based coagulants are not without limitations. Their efficiency varies with raw material source; they can be unstable in aqueous solutions, and their performance is highly dosage-dependent. Current research is therefore focusing on stabilizing these biopolymers through mild chemical modifications (e.g., grafting, cross-linking) and on combining them with mineral or synthetic coagulants in hybrid formulations [57]. In summary, the period from 1990 to 2010 established a new generation of coagulants firmly embedded within the framework of the circular bioeconomy—agents that combine effectiveness with public health safety and environmental compatibility.
2.4. Recent Innovative and Hybrid Technologies (2010–2025)
Since the early 2010s, coagulation engineering has undergone profound transformation, driven by the emergence of hybrid and composite coagulants designed to meet the increasing complexity of industrial and municipal wastewaters [29,58,59]. Unlike traditional single-component coagulants, these new materials are based on intentional synergies between inorganic agents, natural polymers, and functional nanomaterials. The goal is to simultaneously enhance treatment efficiency, pollutant selectivity, and environmental sustainability [23,60,61].
One of the most widely studied hybrids is the PAC–chitosan composite, which combines the strong charge neutralization of polyaluminum chloride with the biodegradable flocculation properties of chitosan. Studies have demonstrated that such composites not only reduce PAC dosage requirements by up to 30% but also improve performance in turbidity removal (>95%), COD reduction (>85%), and phosphate removal (>70%) [62]. Similarly, a FeCl3-modified starch system—comparable to cationic/amphoteric starches tested by Chen et al.—achieved >98% turbidity reduction and showed strong potential for heavy metal removal at neutral pH [63].
Beyond biopolymers, nanostructured materials are increasingly incorporated into coagulant formulations. Metal oxide nanoparticles (e.g., Fe3O4, ZnO), clay–polymer nanocomposites, and functionalized silica-based structures play active roles in capturing organic and inorganic micropollutants. Zeng et al. reported that a magnetic activated carbon composite (AC@MNPs) combined with PACl achieved 74.02% UV254 reduction and 76.53% TOC removal, compared to only 33.4% and 36.2%, respectively, for PACl alone [64]. Another promising approach involves functionalized bio-based coagulants derived from renewable resources or agro-industrial waste. Activated biochar, produced from sewage sludge or nutshells, has proven to be an excellent support for complexing chitosan or clays, providing both rapid flocculation and extensive adsorption. For example, Mian and Liu demonstrated that a TiO2/Fe3O4/biochar composite derived from sludge achieved a lead adsorption capacity of 216 mg/g, with magnetic recovery in under five minutes [65].
In the context of dye and textile effluents, composites such as PAC–alginate, chitosan–montmorillonite, and nanocellulose–tannin have achieved >95% color removal, >90% COD reduction, and complete sedimentation in under 15 min [66,67]. Parallel advances include integrating these composites into coupled treatment systems—such as coagulation–flocculation followed by ultrafiltration or advanced oxidation (AOP). For instance, Alibeigi-Beni et al. showed that a PAC–iron nanoparticle hybrid system combined with membranes achieved 97% turbidity and 91% COD removal, with significantly reduced membrane fouling [68].
A particularly dynamic area is the development of intelligent multifunctional coagulants, designed with self-adjusting pH responsiveness or endowed with magnetic, thermosensitive, or even bioactive properties. Future perspectives include 3D-printable coagulants, nanoencapsulation of reagents, and the integration of embedded chemical sensors for real-time adaptive dosing. The drive toward next-generation low-carbon materials is equally critical, supported by approaches that combine mechanical performance, durability, and drastic reductions in greenhouse gas emissions. Many of these materials are designed to promote circular economy practices by leveraging renewable resources, minimizing waste, and enhancing recycling [69]. Recent advances include bio-inspired construction materials produced at low temperatures using natural binders, which provide cement-comparable strength while reducing carbon footprints by an order of magnitude [70,71]. Similarly, biomass-derived functional carbons are being developed for high-performance applications in energy storage, batteries, and supercapacitors, bridging treatment efficiency with long-term sustainability [72].
In summary, the evolution of coagulants from the 1960s through 2025 reveals a clear trajectory toward materials that are more reactive, selective, recyclable, and compatible with increasingly stringent discharge standards. This progression is driven by advances in materials science, nanotechnology, and green chemistry. A chronological timeline (cf. Figure 5) can visually illustrate this trajectory—from traditional mineral salts to next-generation multi-scale composites with low-carbon footprints.
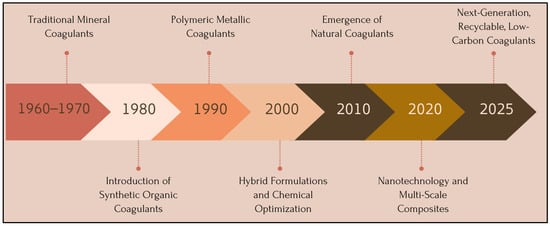
Figure 5.
Timeline of Technological Advancements in Coagulants—From Traditional Mineral Salts to Next-Generation Low-Carbon Materials (1960–2025).
3. Coagulation/Flocculation Mechanisms and Performance Criteria
3.1. Fundamentals of Coagulation/Flocculation Mechanisms
Wastewater treatment through coagulation and flocculation relies on destabilizing colloidal particles, which are naturally present in suspension. These submicron particles typically carry a negative surface charge (with zeta potentials often below −25 mV), preventing spontaneous aggregation due to strong electrostatic repulsion [73]. The addition of a coagulant interrupts this colloidal stability and promotes the aggregation of particles into larger, settleable flocs. This process involves four fundamental physico-chemical mechanisms, often interconnected and highly dependent on operating conditions. These mechanisms are schematically illustrated in Figure 6
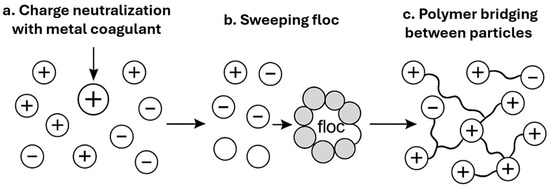
Figure 6.
Schematic representation of destabilization mechanisms: (a) charge neutralization by a metal salt, (b) sweep flocculation, (c) polymer bridging between particles.
The first and most critical mechanism is charge neutralization. When a trivalent metal coagulant such as Al3+ or Fe3+ is added, it compresses the electrical double layer surrounding the colloidal particles and partially or completely neutralizes their negative charge. Once the charge is neutralized, attractive Van der Waals forces dominate, allowing aggregation to occur. This mechanism is particularly effective at low coagulant doses and under slightly acidic conditions. Duan and Gregory demonstrated that aluminum-based coagulants achieve optimal performance at pH values between 5.5 and 7 [74].
At higher dosages, a second mechanism—known as sweep flocculation or enmeshment—becomes dominant. Here, amorphous metal hydroxide precipitates (Al(OH)3, Fe(OH)3) form in situ and physically entrap colloidal particles within their settling matrix. This mechanism tends to prevail when coagulants are added in excess under slightly alkaline conditions. According to Ghernaout and Ghernaout, this “sweeping” type of flocculation ensures greater efficiency on highly turbid waters, while offering flexibility in the face of variations in organic load [75].
In parallel, when polymeric coagulants are applied—whether synthetic (e.g., polyacrylamide, PAM) or natural (e.g., chitosan)—interparticle bridging can occur. In this mechanism, long macromolecular chains partially adsorb onto several particles at once, creating physical bridges that bind them together. This produces large, voluminous flocs that are easier to separate. Zhang et al. reported that the addition of an anionic polymer in combination with ferric coagulants increased average floc size by more than 50%, resulting in a 96% turbidity reduction [76].
Finally, specific adsorption and affinity-based interactions can also play an important role. This mechanism is characteristic of some biopolymers (e.g., chitosan, tannins, alginate) or functionalized materials (e.g., biochar, modified clays), which contain active groups capable of selectively binding metal ions, hydrophobic organics, or micropollutants. For instance, Ramlee et al. demonstrated that carboxyl groups in cactus powder interacted with metallic ions in industrial effluents, achieving removal efficiencies above 80% for cadmium and chromium [77].
It is essential to emphasize that the efficiency of these mechanisms depends heavily on the physico-chemical environment, especially pH, which influences both colloid charge and the speciation of metallic coagulants. Ersoy et al. showed that turbidity removal is maximized for aluminum-based coagulants when pH is maintained between 6 and 8 [78]. Other parameters, such as alkalinity, temperature, the intensity of rapid mixing (which ensures proper coagulant dispersion), and the duration of slow mixing (crucial for floc growth), are equally important. Finally, determining the optimal coagulant dose is critical. Underdosing leaves residual colloids in suspension, while overdosing can lead to charge reversal and restabilization of the suspension. For this reason, experimental optimization—most often through laboratory jar tests—remains indispensable for each specific wastewater matrix.
These three fundamental mechanisms—charge neutralization, polymer bridging, and sweep flocculation—serve as the physicochemical basis for most coagulant formulations and are further discussed in application-specific contexts in the following sections.
3.2. Comparative Performance of Different Categories of Coagulants
The performance of coagulants used in wastewater treatment varies widely depending on their chemical nature, structural properties, and dominant mechanisms of action [28,79,80]. It is therefore essential to comparatively evaluate the main classes of coagulants—mineral, synthetic polymers, natural, and hybrid—in order to guide technological choices.
Mineral coagulants such as aluminum sulfate and ferric chloride remain the most widely applied due to their efficiency in removing turbidity and suspended solids. Their action is mainly based on charge neutralization and the formation of insoluble metal hydroxides, which sweep colloidal particles during sedimentation. For example, ferric sulfate has been shown to remove up to 98% of turbidity and 97% of color in textile wastewater [81], while composite aluminum–zinc–iron coagulants (PAZF) achieved 99.4% turbidity removal and 77% COD reduction [82]. However, these coagulants produce significant volumes of chemical sludge, and their efficiency is highly dependent on pH, with narrow optimal ranges near neutrality [83,84,85].
Synthetic organic polymers, such as polyacrylamide and its cationic or anionic copolymers, offer greater application flexibility. Their dominant mechanism is interparticle bridging, which produces large, dense flocs. Several studies have shown that PAM-based polymers can reduce turbidity by more than 90% at very low dosages (<10 mg/L) while maintaining efficiency over a broad pH range [86,87,88]. Nevertheless, concerns remain regarding potential toxicity from residual acrylamide monomers, poor biodegradability, and higher operational costs compared to mineral salts.
Natural coagulants, derived from plant or animal sources such as chitosan, Moringa proteins, or modified biochars, have emerged as more sustainable alternatives. Their advantages include biodegradability, renewability, and non-toxicity. For instance, chitosan has been shown to remove up to 90% of turbidity and 80% of bacterial loads in wastewater [89,90]. However, their performance can be limited in heavily loaded effluents or those containing dissolved pollutants. Standardization and long-term stability also remain challenges for large-scale deployment [91,92,93].
Hybrid and composite coagulants combine inorganic agents (e.g., PAC, FeCl3) with natural or synthetic polymers, leveraging synergies between charge neutralization and polymer bridging. These systems often demonstrate superior performance compared to their individual components. For example, PAFC–CPAM hybrids have achieved 98.5% turbidity reduction, 99.2% heavy metal removal, and 73.3% COD reduction [87,94,95,96]. Some advanced materials—such as PAZF or nanostructured composites—also enable magnetic recovery or selective adsorption, making them multifunctional. However, questions remain regarding their cost-effectiveness and scalability for full industrial implementation.
A comparative summary (cf. Table 1) synthesizes these findings across key criteria, including coagulant type, typical efficiency (%), optimal dosage, effective pH range, advantages, limitations, and application domains. Such a framework is critical to visualizing trade-offs between cost, performance, and sustainability, and to inform rational technological choices in wastewater treatment.

Table 1.
Comparison of coagulant families by performance.
3.3. Criteria for Selecting a Coagulant (Efficiency, Cost, Sustainability, Toxicity, Etc.)
The optimal choice of a coagulant for wastewater treatment requires more than just assessing its removal efficiency. While technical performance, such as turbidity or pollutant removal, remains essential, decision-makers must now balance this with economic feasibility, environmental sustainability, operational constraints, and regulatory compliance. Recent research emphasizes that efficient coagulants must also minimize sludge production, reduce environmental toxicity, and be compatible with existing treatment infrastructure [52]. Furthermore, durability of performance and resilience to variations in wastewater composition are crucial for long-term sustainability [115]. Decision frameworks like the Analytic Hierarchy Process (AHP) have been used to weigh trade-offs between cost, sludge production, sensitivity to pH, and health impacts of coagulants [116]. Additionally, natural and hybrid coagulants are gaining attention as eco-friendly and safer alternatives to conventional chemical options, aligning with green chemistry principles and global sustainability goals [117].
The first determinant is treatment efficiency against the specific pollutants present. For highly turbid waters dominated by mineral particulates, conventional metallic salts such as aluminum sulfate (Al2(SO4)3) or ferric chloride remain highly effective. A year-long monitoring campaign showed significant reductions in turbidity and associated physico-chemical parameters after alum treatment [101]. However, when treating waters rich in color or dissolved organic matter (DOC, COD), cationic polymers or mixed formulations (e.g., PAC combined with a polymer) generally outperform simple mineral coagulants. Their superior interaction with negatively charged humic substances allows for greater organic matter removal [118,119].
Cost considerations are equally decisive. Traditional coagulants such as alum and ferric chloride remain inexpensive (≈0.02–0.05 €/m3 treated) and are globally available, making them attractive for large-scale industrial plants [115]. In contrast, synthetic polymers, purified bio-based coagulants, or advanced hybrid formulations (e.g., magnetic or grafted coagulants) can be five to ten times more expensive per unit dose. Nonetheless, their higher efficiency often allows for reduced dosing requirements, partially offsetting the upfront costs [117].
From a sustainability perspective, bio-based coagulants (e.g., Moringa seeds, chitosan, starch derivatives) offer significant advantages: biodegradability, lower greenhouse gas emissions during production, reduced sludge volumes, and potential reuse of sludge in agriculture [24,120]. However, their large-scale deployment is constrained by challenges such as variability in raw material quality, limited shelf-life, and difficulties in standardization and mass production.
Toxicity concerns are central, particularly in drinking water applications. Aluminum salts have been scrutinized for their potential neurotoxic effects, though evidence remains debated. Synthetic cationic polymers may contain residual monomers such as acrylamide, a compound with recognized carcinogenic potential, necessitating strict monitoring. By contrast, natural coagulants are generally regarded as non-toxic—some being derived from edible plants—making them intrinsically safer [121].
Finally, regulatory frameworks strongly influence coagulant selection. For example, the maximum permissible concentration of residual aluminum in drinking water (typically 0.2 mg/L, as recommended by WHO) has prompted the adoption of PAC or natural alternatives in certain contexts. At the same time, crude natural coagulants may themselves carry risks, such as trace heavy metals or microbial contamination, requiring purification and quality control prior to use [118,119].
Although numerous studies report impressive pollutant removal efficiencies for both conventional and emerging coagulants, these data are often obtained under non-comparable experimental conditions. Variations in influent type, pH, initial turbidity, mixing regime, and coagulant dosage make direct comparisons difficult and may lead to overestimating performance differences. To address this issue, a comparative summary of representative studies was compiled (cf. Table 2) to contextualize reported removal efficiencies under normalized operational parameters. The analysis reveals that, while traditional mineral coagulants (e.g., alum, FeCl3) maintain consistent turbidity reductions above 90%, their performance rapidly declines at neutral or high pH values or in the presence of organic colloids. In contrast, hybrid and biosourced coagulants achieve comparable or superior removal rates at lower dosages, though their performance is strongly matrix-dependent and varies with extraction method and molecular weight. These discrepancies highlight the urgent need for standardized benchmarking protocols and meta-analytical frameworks that normalize results per unit dosage or per charge equivalent, ensuring fair evaluation across coagulant classes and environmental contexts.

Table 2.
Comparative performance of selected coagulant types under normalized conditions.
In summary, the choice of a coagulant cannot be universalized. It requires a holistic evaluation that weighs removal efficiency, economic viability, environmental sustainability, toxicity risks, and compliance with evolving regulations. Such a systemic approach is critical to enabling the transition toward smarter, more sustainable treatment strategies—an agenda explored in the following sections.
4. Scientific Gaps, Technological Limits and Current Challenges
4.1. Scientific Gaps in the Understanding of Coagulation/Flocculation Mechanisms
Despite the widespread application of coagulation–flocculation in wastewater treatment, significant blind spots remain in our understanding of the underlying physico-chemical mechanisms. A critical examination of recent literature reveals several major gaps, spanning both fundamental science and practical implementation.
Recent studies have identified a significant knowledge gap in the treatment of emerging pollutants—most notably microplastics, metallic nanoparticles, and per- and polyfluoroalkyl substances (PFASs)—using coagulation processes. Traditional coagulants, while effective for conventional contaminants, often fail to adequately remove these newer classes of pollutants due to the absence of robust predictive models that account for critical physicochemical parameters such as particle morphology, size distribution, and surface properties. As highlighted by Khan et al., the lack of such models hinders the development and optimization of coagulant formulations specifically adapted to the diverse and complex characteristics of these emerging contaminants [133].
For instance, the coagulation–flocculation–sedimentation (CFS) process has been shown to be largely ineffective in removing micro- and nanoplastics, with removal efficiencies often below 2%, particularly for smaller particle sizes [134]. Nevertheless, the presence of biofilms on plastic surfaces has been found to enhance aggregation and sedimentation, suggesting that biological interactions can influence removal outcomes.
In response to these challenges, several studies have explored the use of advanced and alternative coagulants. Poly-aluminum chloride (PACl), for example, has demonstrated improved performance in the simultaneous removal of dissolved organic matter, microplastics, and silver nanoparticles, especially when used at optimized dosages [135]. Additionally, electrocoagulation has emerged as a highly effective method, achieving over 95% removal efficiency of nanoplastics under controlled conditions, with the added benefit of enabling physical separation of contaminants via a foam-like floc layer [136].
In complex industrial effluents, multiple pollutants (organic, mineral, particulate) coexist and may interact during coagulation, but these interactions are little studied. For example, recent research is examining the simultaneous removal of composite pollutants—such as microplastics in the presence of organic pollutants (dyes, pharmaceuticals)—to understand how the presence of one contaminant can influence the capture of the other in flocs [137]. Khan et al. recommend further study of chemical interactions between microplastics and flocs, as the forces involved (surface bonds, floc density, buoyancy vs. Brownian forces) strongly determine the sedimentation efficiency of these unconventional particles [137].
Regarding PFAS, removal efficiency has been shown to depend strongly on molecular structure. Longer-chain compounds are more effectively removed via coagulation, particularly when using specialized agents such as Perfluor Ad® or composite coagulants containing activated carbon or cationic polymers. However, shorter-chain PFAS remain recalcitrant under standard treatment conditions [138]. In parallel, there is growing interest in natural and bio-based coagulants such as chitosan and sodium alginate, which have shown potential as sustainable alternatives when combined with conventional agents, particularly in enhancing floc formation and reducing chemical dosage requirements [139].
A second major area of uncertainty in the field of coagulation-based water treatment pertains to the use of natural coagulants. While their potential for sustainable and low-cost water treatment has been widely recognized, the underlying molecular mechanisms that drive their coagulating activity remain poorly characterized. Plant-derived polysaccharides, such as those extracted from Moringa oleifera seeds or Opuntia ficus-indica cladodes, have demonstrated significant removal efficiencies for turbidity, chemical oxygen demand (COD), and even some heavy metals. However, the chemical basis for these effects—particularly the role of active functional groups like carboxyl, hydroxyl, and amino moieties—has not been systematically investigated in most studies.
As noted by Ref. [81] the majority of publications focus on performance metrics (e.g., percentage removal of turbidity or COD) without exploring the molecular-level interactions between coagulant molecules and target pollutants. This lack of mechanistic insight significantly limits the ability to predict performance across different water matrices or to rationally optimize coagulant formulations [29]. These authors highlight the need for further investigations to isolate and understand the main active agents in natural extracts and their modes of action, technological limitations and application constraints [29]. Similarly, for recently developed biological or magnetic coagulants, the mechanisms of action are not fully elucidated, which limits optimization of their efficacy. Moreover, advanced analytical techniques capable of elucidating structure-function relationships—such as Fourier Transform Infrared Spectroscopy (FTIR), Nuclear Magnetic Resonance (NMR), and even X-ray diffraction (XRD)—are underutilized in the characterization of natural coagulants. When employed, FTIR often identifies broad functional group regions, but rarely is this data correlated with coagulation performance or pollutant-binding capacity. NMR, which could provide precise structural insights, is almost entirely absent from the literature on natural coagulants.
Furthermore, variability in extraction methods, plant maturity, storage conditions, and even geographic origin can lead to significant heterogeneity in coagulant composition—yet these factors are rarely standardized or reported in detail. As a result, reproducibility and scalability of natural coagulants remain major barriers to their widespread implementation in municipal or industrial water treatment systems.
A third limitation involves the structure and dynamics of flocs. Zaki et al. emphasize the scarcity of studies combining electron microscopy, zeta potential measurements, and numerical modeling to capture floc architecture in terms of porosity, density, and fractal dimension [140]. This knowledge gap is particularly problematic for integrated processes, such as coagulation coupled with membrane filtration, where floc morphology directly affects fouling and separation efficiency.
A fourth challenge lies in the kinetics of floc formation and breakage. As Mohamed Noor and Ngadi point out, few studies propose universal kinetic models describing floc growth, sedimentation velocity, and pollutant concentration profiles over time [141]. Most experimental work remains focused on end-point measurements, neglecting the dynamic evolution of the process.
In the absence of robust predictive models, determining the optimum dose of coagulant/flocculant is still largely based on empirical trials (JAR tests). Indeed, the chemistry of coagulation is complex and depends on numerous parameters (pH, turbidity, conductivity, nature of pollutants, etc.), making it difficult to predict the dose and effectiveness of treatment theoretically [142]. For example, Fang et al. note that the complexity of physico-chemical interactions in a coagulation basin makes it difficult to accurately control the dose using conventional methods, motivating the development of intelligent algorithms to overcome this shortcoming.
Finally, there is a lack of studies that bridge experimental and multi-scale modeling approaches. Al-Jadabi et al. highlight the importance of integrating advanced physico-chemical analysis, CFD modulizations, and real-time monitoring tools (e.g., turbidity, zeta potential sensors) to develop adaptive dosing strategies for heterogeneous effluents [143].
Taken together, these gaps point toward four priority research directions:
- Advanced molecular characterization of natural and hybrid coagulants;
- Multi-scale analysis of floc structures using microscopy, spectroscopy, and modeling;
- Development of robust kinetic models, experimentally validated across different effluent types;
- Integration of experiments, online sensing, and simulation to enable real-time process optimization.
4.2. Technological Limitations and Application Constraints
While the scientific gaps outlined earlier underscore the need to strengthen fundamental knowledge of coagulation–flocculation mechanisms, it is equally crucial to examine the technological and operational barriers that currently limit the efficiency and widespread adoption of these processes in both municipal and industrial contexts. These challenges stem not only from the intrinsic performance of coagulants but also from their practical integration into complex treatment chains. Addressing these obstacles is indispensable to ensuring both the effectiveness and long-term sustainability of real-scale systems.
A first major issue concerns the management of by-products, particularly the sludge generated during coagulation–flocculation. Although these processes are well established for reducing turbidity, organic matter, and heavy metals, the large sludge volumes produced remain a serious environmental and economic burden. For instance, Turna and Yıldız reported that, in the treatment of vegetable oil industry effluents, optimal doses of aluminum salts (up to 800 mg/L) achieved COD removals above 90% yet simultaneously produced substantial amounts of non-recoverable sludge, generating significant logistical and secondary treatment costs [144]. Similarly, Teh et al. identified excessive sludge generation as one of the main limitations of coagulation, highlighting the lack of scalable, sustainable valorization strategies [145]. The problem becomes more complex when the sludge contains residual metals or refractory organics, which compromise its agricultural or energy recovery potential.
A second key limitation lies in the adaptability of coagulation to complex effluents. Numerous studies show that textile, hospital, or agro-industrial wastewaters contain heterogeneous pollutant matrices for which coagulation alone is insufficient. Silva et al. demonstrated that, while pre-coagulation significantly improved turbidity and suspended solids removal in textile effluents, hydrosoluble dyes and persistent organics required additional downstream processes in a combined coagulation/flocculation–direct contact membrane distillation (CF-DCMD) system [146]. Marques et al. similarly reported that coupling coagulation with hydrodynamic cavitation and ozonation enabled COD removals above 90% while reducing sludge production compared to coagulation alone [147]. However, as Tzoupous & Zouboulis noted, such hybrid approaches involve higher capital costs, greater technical expertise, and increased energy demands—factors that restrict their deployment in resource-limited settings, especially in the Global South [148].
Economic constraints represent a transversal challenge. According to Mohamed Noor and Ngadi, coagulants account for 20–35% of total operating costs in wastewater treatment plants, a proportion particularly burdensome in lower-income countries where most coagulants are imported [141]. This financial pressure often leads operators to underdose coagulants or to use lower-quality products, ultimately compromising treatment efficiency. Although local production of bio-based coagulants is sometimes proposed as a cost-effective alternative, their extraction and purification remain energy- and resource-intensive. Owodunni et al., for instance, showed that isolating active compounds from Moringa oleifera or Opuntia ficus-indica requires significant energy and processing inputs, reducing competitiveness compared to conventional mineral coagulants [149]. Moreover, some natural formulations exhibit poor shelf stability, requiring additives or stabilization processes to extend their usability.
Beyond cost and technical factors, environmental and health concerns further complicate adoption. Matesun et al., demonstrated that certain synthetic polymers, while effective at low doses, can release non-biodegradable fragments prone to accumulation and chronic toxicity in aquatic environments [12]. Even natural coagulants are not exempt from scrutiny: Mohamed Noor and Ngadi reported contradictory results regarding phytotoxicity and cytotoxicity, emphasizing the need for long-term toxicological assessments [141]. Such uncertainties not only hinder regulatory approval but also slow the market acceptance of bio-sourced alternatives.
On the other hand, operational challenges remain critical, particularly regarding process control. Parameters such as pH and optimal coagulant dose strongly influence removal efficiency and floc morphology. Conventional inorganic coagulants are often sensitive to pH and dosage. The effectiveness of the process can drop outside an optimal pH range, requiring the pH of the effluent to be adjusted (by adding acid or base) before coagulation. For example, the Fenton process (coagulation/oxidation with Fe2+/H2O2) only works at a pH of ~3, which is a major limitation for its large-scale application [22]. Similarly, overdosing with coagulant can generate a positive residual charge and restabilize particles or lead to chemical waste, while underdosing leaves high residual turbidity. Continuous dose control remains difficult in practice, and plants often operate at a fixed or overcompensated dose to ensure safety, at the expense of economic optimization.
In cosmetic wastewater treatment, Zhang et al. showed that polyaluminum chloride (PAC) performance depended heavily on maintaining a narrow pH window (6–7.5), which is difficult to achieve under real conditions without advanced automation. Oktaviani & Adachi further highlighted that even minor deviations in mixing intensity or flocculation time can destabilize microflocs, impair sedimentation, and compromise overall process efficiency [150]. These findings underline the need for precise control systems, which remain a challenge in small-scale or batch-operated treatment facilities.
Finally, it should be noted that the coagulation–flocculation process itself remains relatively energy-efficient. In fact, energy requirements are mainly limited to the mechanical agitation required for the rapid mixing (coagulation) and slow mixing (flocculation) phases, without the need for heat sources or complex equipment. However, if we broaden our thinking to include the entire treatment chain, indirect energy consumption items appear: pumping the water to be treated, activating the mixing systems, and downstream operations related to sludge management, such as dewatering, transport, or final disposal, can represent a significant portion of the system’s overall energy footprint.
Furthermore, the role of coagulation–flocculation is particularly important in hybrid configurations where it is integrated upstream of so-called “advanced” treatments, such as ozonation, UV irradiation, or membrane filtration. In these contexts, its main objective is not only to clarify the water but above all to reduce the organic or colloidal load, so as to limit energy consumption in subsequent, more resource-intensive stages. Lucas et al. illustrate this point by showing that pre-coagulation of heavily loaded industrial effluents significantly reduces the energy required for residual carbon oxidation during advanced treatment by oxidation processes [22]. Thus, far from being a simple pretreatment step, coagulation–flocculation plays a strategic role in the overall energy optimization of modern treatment processes. In this regard, Vadasarukkai and Gagnon demonstrated that the rapid mixing intensity applied in treatment plants is often higher than necessary and that a strategic reduction in this energy would make it possible to maintain equivalent water quality while reducing energy requirements [151].
Recent literature reviews agree that only an integrated approach (combining chemical, biological, and/or advanced methods) and multi-criteria optimization (performance, cost, environment) will enable these challenges to be met [12,22].
Taken together, the current limitations of coagulation–flocculation can be grouped into four main categories: (i) sludge management and valorization, (ii) adaptability to complex effluents via hybrid processes, (iii) economic optimization of coagulant sourcing and use, and (iv) rigorous control of environmental and health impacts. Addressing these interconnected challenges requires a systemic shift in treatment practices, driven by technological innovation, diversification of coagulant sources, and intelligent integration into multi-barrier treatment chains—a transition explored in the following section.
The table below (cf. Table 3) summarizes the main scientific gaps, technological limitations, and current challenges concerning the coagulation–flocculation process in industrial water treatment, as identified in recent literature.

Table 3.
Summary of Scientific Gaps, Technological Limitations, and Current Challenges in Coagulation–Flocculation for Industrial Wastewater Treatment.
4.3. Current Challenges and Open Questions
Building on the previously identified scientific gaps and technological constraints, it becomes clear that the future of coagulation–flocculation will depend on how effectively the research community addresses a series of unresolved questions. These challenges are not confined to laboratory optimization; they touch upon the broader imperatives of environmental sustainability, economic feasibility, operational resilience, and regulatory legitimacy.
One of the most pressing issues is the design of coagulants that reconcile high efficiency with environmental safety and affordability. Traditional metallic salts are effective but carry a heavy ecological burden, from their energy-intensive production to the accumulation of residual metals in sludge. Research is therefore shifting toward multifunctional bio-based and hybrid coagulants, which aim to combine the biodegradability and low toxicity of natural polymers with the robustness of inorganic agents [141]. Studies such as Zaharia et al. confirm that hybrids derived from natural extracts can achieve high color and COD removal, but the scalability and reproducibility of these formulations remain uncertain [152]. Beyond performance, a crucial open question is how to ensure consistent quality and shelf stability of bio-derived coagulants, especially when sourced from variable agricultural or marine biomass.
Another challenge relates to the operational optimization and digitalization of coagulation–flocculation. Laboratory-scale work has already demonstrated the potential of statistical tools like RSM to fine-tune parameters, achieving removal efficiencies above 90% [153]. However, the real breakthrough will come from the integration of smart sensors, real-time monitoring, and machine learning algorithms to dynamically adjust coagulant dosing under fluctuating water qualities. This perspective aligns with the broader paradigm of Industry 4.0 for water treatment, where coagulation is no longer a static process but part of a self-adaptive treatment train. Yet, the cost of advanced instrumentation and the lack of skilled personnel to manage such systems present barriers that cannot be overlooked.
Equally important is the integration of coagulation with complementary advanced technologies. Alone, coagulation cannot fully address persistent organic pollutants, pharmaceuticals, or PFAS. Recent successes with cavitation, ozonation, or direct membrane distillation underscore the potential of hybrid approaches to tackle these contaminants more comprehensively [147,154]. Beyond pollutant removal, such integration also promises reduced sludge volumes and enhanced energy efficiency when processes are designed synergistically. Nevertheless, hybrid systems must be carefully engineered to avoid simply shifting the burden—for example, from sludge management to higher energy demands. This raises an open question: can next-generation hybrid systems achieve true resource recovery (e.g., recovery of nutrients, reuse of treated water, valorization of sludge) rather than just pollution removal?
A further dimension concerns the holistic assessment of coagulation–flocculation systems. While bench-scale studies frequently highlight impressive removal efficiencies, few consider the full life cycle costs and impacts, from raw material sourcing to end-of-life sludge disposal. Zaki et al. argue that, without robust life cycle assessments (LCA) and techno-economic evaluations, many “green” coagulants risk being unsustainable at scale [155]. A more comprehensive framework must include not only greenhouse gas emissions and energy use but also social and economic indicators such as affordability for low-income communities and resilience against supply chain disruptions.
Beyond environmental performance, the large-scale viability of novel coagulants is ultimately determined by their techno-economic balance between synthesis complexity, operational efficiency, and cost competitiveness. While laboratory-scale studies frequently report remarkable removal efficiencies, the translation of these results to industrial practice remains hindered by the high cost of reagents, process energy, and limited regeneration capacity. Recent techno-economic evaluations have demonstrated that optimized electrocoagulation systems can achieve treatment costs as low as 1.5 USD m−3 when operated under energy-efficient regimes, offering a competitive alternative to conventional alum- or ferric-based treatments [156]. Similar assessments on hybrid processes coupling coagulation with thermochemical valorization pathways—such as pyrolysis or hydrothermal carbonization—suggest that the integrated recovery of coagulant metals and energy carriers could reduce the overall life-cycle cost by 20–30% while closing material loops [157]. Nevertheless, the synthesis and stabilization of advanced nanostructured or functionalized coagulants often rely on costly precursors, and their economic feasibility depends on developing scalable, solvent-free, and modular production methods. Standardized cost models and techno-economic metrics are therefore required to harmonize future assessments and to identify thresholds beyond which hybrid or biosourced coagulants can become not only environmentally but also economically sustainable.
Finally, the regulatory landscape remains a decisive factor shaping adoption. Many natural and hybrid coagulants still lack approval for use in potable water treatment, partly due to uncertainties around toxicological safety, microbiological risks, and by-product formation. Aragaw and Bogale highlight the urgent need for harmonized international standards that could accelerate market access for bio-based coagulants while maintaining public health safeguards [31]. Beyond compliance, there is also the question of public perception and acceptance: how willing are communities to embrace coagulants derived from agricultural waste, marine biomass, or nanomaterials? Addressing this socio-regulatory dimension is as crucial as solving technical inefficiencies.
In essence, the coagulation–flocculation field now stands at a crossroads. Progress will depend on its ability to move beyond narrow efficiency metrics toward a multidimensional approach that integrates green chemistry, digital process control, hybrid system design, life cycle sustainability, and adaptive regulation. Only by tackling these open questions in concert can the next generation of coagulants deliver truly sustainable solutions for complex wastewater treatment.
5. Recommendations and Future Perspectives
In light of the challenges identified around mechanistic understanding, coagulant design, and sustainable industrial deployment, a strategic synthesis is warranted. The following distinguishes near-term, operational recommendations from medium- to long-term, disruptive perspectives.
5.1. Immediate and Practical Recommendations
5.1.1. Research Directions
A priority is the fine-scale exploration of interactions between coagulants and pollutants, especially at the molecular level. Recent molecular modelling work has, for example, examined five plant-derived coagulant proteins (Moringa oleifera, Arachis hypogaea, Bertholletia excelsa, Brassica napus, Helianthus annuus) and highlighted the decisive role of specific amino-acid residues in coagulation. Hydrogen bonding and π–π interactions dominate aggregation mechanisms, and proteins from Brassica napus and Helianthus annuus can outperform the well-known Moringa oleifera protein depending on treatment conditions [158,159]. These results confirm the need to characterize, at molecular resolution, adsorption and charge-neutralization phenomena.
Building on this, advanced structural analytics are essential to elucidate functional interactions at the surface of natural coagulants. Techniques such as Fourier-transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and Raman spectroscopy provide insight into the structure and active groups of biopolymer coagulants. For example, combining FTIR, SEM and dynamic light scattering (DLS) enabled detailed characterization of a seed gum extracted from Cassia fistula. At pilot scale (30 L reactor), this natural coagulant achieved pollutant removals up to ~93.8% at an optimized dose of 1.17 mg L−1 [160]. Such studies show that in-depth physicochemical characterization (morphology, molecular weight, functional sites) helps target dominant action mechanisms (H-bonding, bridging, etc.) and improve the performance of bio-based coagulants.
To enable reliable comparison of alternative versus conventional mineral coagulants, standardized experimental protocols are critical. Methodologies combining zeta-potential measurements with statistical design of experiments have proven effective for optimizing coagulation conditions. A case study showed that, by finely tuning the dose of a cationic polymer (polydadmac), one can reach near-complete removal of turbidity and suspended solids at precisely defined dosages—i.e., once optimal charge neutralization is achieved, floc formation maximizes clarification [161]. Extending these methodological efforts—e.g., through inter-laboratory standardized tests—will close fundamental gaps and make results more comparable and transferable.
Beyond classical views, some authors propose broadening coagulation mechanistic concepts by introducing co-adsorption [162]. This integrates coagulation, adsorption, and agglomeration effects to reflect the multifactor complexity of heterogeneous systems. A specific co-adsorption indicator has been suggested to quantify and predict coagulant efficiency against given pollutant classes. This holistic (yet emergent) approach deserves further study, as it could sharpen predictive models and support dose setting under complex conditions.
Development of Innovative Coagulants
Innovation in coagulants is a major lever for more effective, sustainable, and environmentally safe water treatment. A near-term strategic avenue is controlled chemical modification of natural biopolymers such as chitosan, tannins, or plant proteins. Such modifications have already produced higher-performance hybrid materials: for instance, carboxymethyl grafting onto cellulosic matrices stabilizes Ag and CuO nanoparticles while imparting long-lasting antimicrobial action [163]. Likewise, functionalizing chitosan—a polysaccharide from crustacean waste—with inorganic nanoparticles markedly improves its thermal stability and charge-dispersion capacity. Hybrid membranes combining chitosan with modified nano-silica display enhanced thermal stability and optimized charge dispersion, particularly around ~3 wt% nano-silica [164]. Leveraging such organo-mineral hybrid coagulants is promising because it exploits synergies among adsorption, ionic complexation, and floc formation to target a broad pollutant spectrum.
Nanotechnology also opens new avenues for “smart” coagulation systems. Embedding magnetic nanoparticles within microspheres functionalized with coagulant proteins (e.g., from Moringa oleifera) optimizes capture of colloids and enables floc recovery by applying an external magnetic field [165]. Researchers have engineered superparamagnetic microspheres grafted with Moringa proteins that act as coagulants and can be extracted post-use with a magnet. This recoverability and reusability, along with low reagent demand, are major assets for reducing OPEX and environmental impacts.
Finally, stimuli-responsive composite materials open the way to adaptive coagulants. By combining natural biopolymers with inorganic fillers that respond to external stimuli (pH, temperature, magnetic field), one can devise “smart” coagulants that adjust conformation and reactivity to the medium. For example, pH-responsive particles may reversibly shift solubility or charge. These multifunctional systems—still at research stage—could offer unprecedented control over coagulation by modulating efficiency in real time as influent composition varies.
Integration into Industrial Treatment Trains
In industry, overall performance hinges on coherently orchestrating multiple technologies. It is therefore judicious to embed coagulation as a key step within larger treatment trains rather than treating it in isolation. Many studies show that coupling coagulation–flocculation with membrane processes (UF/MF/NF) significantly reduces fouling, extends equipment lifetime, and improves product water quality [166]. Dosing a coagulant upstream of UF limits pore blocking and cake resistance by precipitating colloids, easing cleaning and maintaining flux. This benefit is especially marked for industrial effluents rich in persistent organics or colloids (e.g., food & beverage, fine chemicals).
Similarly, combining coagulation with advanced oxidation processes (AOPs) is promising for refractory pollutants. Sequences such as coagulation + ozonation or coagulation + UV/H2O2 achieve higher removals of difficult compounds (e.g., dyes, pesticides) than either step alone, by (i) removing a large fraction of COD and solids first and (ii) oxidizing the remaining dissolved organics more efficiently—often with lower overall sludge generation due to synergy.
To enable broader adoption of such hybrid systems, comprehensive technical guides should be made available to operators. These should specify optimal operating windows for each combined technology (coagulant dose, contact times, filtration/oxidation settings), outline automation strategies, define compatible control systems (SCADA, PLCs), and list real-time KPIs (turbidity, UV254, etc.). Near-term dissemination of such operational know-how can remove adoption barriers for alternative coagulants and hybrid trains across sectors facing increasingly strict discharge limits.
5.2. Long-Term Perspectives
5.2.1. Toward Intelligent Coagulation
Progress toward intelligent treatment systems is among the most promising axes for simultaneously boosting effectiveness and sustainability. With online sensors and AI algorithms, automated, adaptive, and predictive coagulant dosing is now feasible. In practice, systems continuously measure influent quality (turbidity, organics, pH, etc.) and adjust dose in real time, anticipating variations rather than reacting late. Full-scale trials in Norway illustrate this potential: implementing multi-parameter predictive control stabilized plant performance despite influent variability while reducing coagulant consumption by up to ~30%—a dual economic and environmental gain via lower reagent use and less sludge production [167].
In parallel, cloud/IoT architectures are transforming infrastructure. Connected supervisory platforms embed predictive models within industrial control (e.g., SCADA). A recent platform leverages deep-learning models (deep belief networks) tied to an IoT sensor network to optimize WWTP operation continuously [168]. IoT–SCADA integration enables proactive control: early detection of drifts (organic surges, overflow risk) triggers automatic adjustments (dose increases, flow regime shifts) before effluent quality degrades—and reduces downtime through predictive maintenance.
A notable advance is the emergence of soft sensors (virtual sensors). These model-based or ML estimators instantly infer quality parameters (e.g., clarifier effluent turbidity) before sedimentation completes. One such soft sensor predicted settled-water turbidity from inlet conditions and unit hydrodynamics [169]. Validated on site, it achieved ~86% accuracy and, crucially, provided an early estimate—allowing dose adjustments without waiting for hours-long settling. This responsiveness stabilizes operations and reduces prolonged under- or over-dosing.
Further research explores hybridizing AI with mechanistic models to combine domain knowledge with data. For example, PHREEQC (aqueous equilibrium simulator) can be coupled with optimization algorithms to simulate metal precipitation or coagulation as a function of physicochemical conditions (temperature, redox, etc.). A recent study used PHREEQC to model chemically assisted coagulation with online sensors, better explaining removal mechanisms and enabling real-time control [170]. Predictions of floc formation (e.g., Fe(OH)3) versus pH and temperature agreed with experiments. Such hybrid approaches (physics-based + ML) can safely anticipate system behavior and refine operating strategies beyond black-box AI.
A recent review underscores that these intelligent systems—real-time control, algorithmic optimization, and hybrid trains—are central to future chemical water treatment [168]. As technologies mature, autonomous, self-learning, high-efficiency coagulation–flocculation is likely to become standard, minimizing resource use while maximizing water-quality outcomes.
5.2.2. Disruptive Innovation: Electrocoagulation and Biocoagulation
Among disruptive pathways, replacing conventional chemicals with I generation or biological agents is gaining traction. Electrocoagulation (EC), in particular, produces coagulant species directly in the reactor via controlled anodic dissolution—no external reagents required. Optimized EC (durable electrodes, green electricity) has strong sustainability potential: it limits manufactured chemicals and can reduce hydroxide-sludge volumes; dosage can be controlled precisely via current; and EC can synergize with other electrochemical effects (anodic oxidation of organics, H2 flotation). Conceptually, EC mirrors chemical coagulation (forming Al or Fe hydroxides) but in short-loop mode, with the sacrificial anode as the coagulant source [171].
Biocoagulation, in parallel, uses natural flocculants—extracellular biopolymers, plant extracts, even whole microorganisms. These biodegradable agents are often low-toxicity, renewable (from agro-waste), and yield sludges with valorization potential. Used alone, they may lack robustness on very strong or toxic effluents—hence the appeal of hybrid EC + biocoagulant systems. For example, EC assisted by a plant-derived biocoagulant achieved ~99% COD/DBO removal on brewery wastewater under optimized conditions (pH ≈ 7, 0.5 A, 40 min) with modest energy demand (~0.54 kWh m−3) [172]. Similarly, treating dairy whey with EC plus an Opuntia ficus-indica biopolymer removed ~98.9% turbidity and ~83.8% COD at optimum settings [173]. These synergistic setups combine the strengths of metal-based coagulation and gentle biopolymer flocculation while mitigating drawbacks (e.g., biopolymer can reduce electrode passivation; EC compensates lower standalone biocoagulant yields).
Many such technologies are still at lab/pilot stage, yet their potential is well documented and results are encouraging. Over time, they could credibly partially replace classical chemical coagulants while meeting sustainability and environmental-safety requirements.
5.2.3. Toward a Circular Economy of Coagulants
From a resource-responsible standpoint, valorizing wastes and by-products as coagulants is a promising circular strategy. Instead of relying on virgin metal salts (aluminum/iron, energy-intensive to produce), residual materials can be upcycled into alternative coagulants.
An emblematic case is chitosan, a polysaccharide extracted from shrimp/crab shells (fishery waste) [174]. This abundant, biodegradable biopolymer carries cationic amines in acidic solution, conferring excellent flocculation toward negatively charged colloids. It has been widely studied as an alternative coagulant, alone or in blends, with well-established efficacy for fine particles and heavy metals—and, crucially, it originates from waste.
Likewise, mineral wastes can be valorized. Coal fly ash contains alumina and lime and has been tested as a base for alternative coagulants. Composite coagulants derived from fly ash have, in some cases, rivaled commercial reagents in clarifying wastewater by forming dense, stable flocs and capturing impurities efficiently [175]. Agricultural residues (corn stalks, fruit peels, coconut fibers), once transformed (biochar, ash, or extracted polysaccharides), are also explored for charge neutralization/adsorption. Beyond coagulant action, such biomasses can play adsorbent roles for dissolved molecules.
Another circular dimension concerns sludge valorization. Traditionally treated as waste (landfill/incineration), coagulation sludges—rich in metal hydroxides and fixed organics—can be resources. Initiatives show they can be reused, e.g., incorporated into clay bodies to make bricks (organics act as internal fuel; metal oxides add pozzolanic properties) [176], applied as soil amendments after stabilization (subject to metal-safety checks), or processed as secondary ores to recover valuable metals [177,178]. Acid/base leaching can retrieve aluminum from alum sludges (recycling it into coagulant or other products), and even rare earths in specific cases.
Ambitious pilots have integrated these strategies into fully circular local schemes: coagulants produced from local wastes, used in treatment, with resulting sludges recycled into building materials or agriculture—closing loops, cutting waste-management costs, and creating local value (e.g., bricks, fertilizers) while securing coagulant supply.
5.2.4. Interdisciplinary Approach and Multi-Stakeholder Partnerships
Accelerating innovation in coagulation–flocculation requires crossing disciplinary boundaries. Synergies among chemistry (synthesizing/derivatizing coagulants), process engineering (embedding them into industrial trains, reactor design), biotechnology (biopolymer production, microbial flocculants), materials science (composites, functional nanostructures), and computing/AI (real-time control, smart sensing, predictive modeling) are essential to deliver robust, high-performance solutions. Research programs should thus adopt genuinely interdisciplinary approaches.
This scientific cross-fertilization must pair with stronger institutional cooperation. Durable partnerships among universities, applied research centers, engineering firms, water-treatment companies, and local authorities are needed. Exchange platforms (clusters/consortia) let each actor contribute: labs develop/test concepts; engineers/industry assess techno-economic feasibility at scale; municipalities provide pilot sites and benefit from innovation. Living labs for water/environment or dedicated competitiveness clusters facilitate co-construction of transferable technologies by involving end-users from the outset.
For example, projects on sludge reuse or biocoagulant development moved faster to demonstrators thanks to joint involvement of academia, plant operators, and startups (real-time analytics, green reagents). Field trials—e.g., deploying plant-based coagulants in a municipal plant or converting sludges into materials—under rigorous scientific monitoring have shown tangible benefits (reduced mineral-coagulant dose without loss of performance; prototype bricks with X% sludge substitution). Such multi-actor partnerships are pivotal for scaling and adoption, sharing risk, accelerating collective learning, and ensuring innovations meet field constraints. Public funders can catalyze this through collaborative calls and mission-driven programs. For clarity, Table 4 provides a concise synthesis of our recommendations (near-term) and outlook (mid/long-term) across the strategic axes: mechanistic understanding, innovative coagulants, process integration, intelligent control, disruptive alternatives and circular economy.

Table 4.
Summary of recommendations and outlook for the future of coagulation–flocculation.
5.3. Digital Transformation, Circular Economy, and SDG Synergies in Coagulant Development
The convergence of digital technologies, sustainable resource management, and global development goals has paved the way for transformative innovations in the design and application of coagulants. The concept of “Coagulation 4.0”—inspired by Industry 4.0 principles—aims to integrate smart automation, real-time monitoring, and predictive analytics to optimize coagulation processes while promoting environmental sustainability and socio-economic benefits.
5.3.1. Digital Transformation and Smart Coagulation Systems
The ongoing digital transformation under the framework of Industry 4.0 is profoundly reshaping water and wastewater treatment systems. Through the integration of cyber-physical systems, artificial intelligence (AI), the Internet of Things (IoT), big data analytics, and digital twins, treatment processes are becoming increasingly intelligent, adaptive, and resource-efficient. Within this paradigm, smart coagulation systems have emerged as a pivotal innovation, offering real-time monitoring and optimization of coagulant dosing to achieve higher treatment efficiency while minimizing chemical and energy consumption [179].
These systems rely on data-driven feedback loops and predictive control models that dynamically adjust coagulant dosing according to water quality parameters such as turbidity, pH, and organic load. Such adaptive automation significantly reduces chemical wastage and the generation of secondary sludge, contributing to a lower environmental footprint and improved process resilience [180]. Moreover, digital twin technologies—virtual replicas of physical treatment systems—enable process simulation and performance prediction under varying operating conditions, providing a powerful tool for preventive maintenance and optimization [181].
The integration of Industry 4.0 technologies into water treatment aligns directly with the Sustainable Development Goals (SDGs), particularly SDG 6 (Clean Water and Sanitation) and SDG 12 (Responsible Consumption and Production). Beyond efficiency gains, these digital systems facilitate traceability, compliance with environmental regulations, and green procurement strategies, thus embedding sustainability within the operational and governance dimensions of the water sector [21].
Ultimately, smart coagulation systems embody the convergence of environmental intelligence and technological innovation. They represent a transition from reactive, empirically controlled processes to proactive, self-learning systems capable of reducing costs, enhancing treatment reliability, and supporting circular resource flows. This evolution mirrors a broader industrial transformation where digitalization and sustainability are no longer parallel objectives but deeply interconnected imperatives for resilient water management [182].
5.3.2. Circular and Green Economy Synergies, SDGs, and Indigenous Biomaterial Coagulants
The transition toward a circular and green economy has become an essential pillar of sustainable water and wastewater treatment, particularly in the development of environmentally benign coagulants and flocculants. The circular economy (CE) promotes a restorative and regenerative industrial model, seeking to close material loops and minimize waste generation, while the green economy emphasizes low-carbon, resource-efficient, and socially inclusive growth. Both frameworks contribute directly to the realization of the Sustainable Development Goals (SDGs)—notably SDG 6 (Clean Water and Sanitation), SDG 9 (Industry, Innovation, and Infrastructure), and SDG 12 (Responsible Consumption and Production) [183].
In the context of coagulant development, circular economy strategies have enabled the valorization of water treatment residues such as drinking water treatment sludge (DWTS) and metal-rich industrial by-products into reusable coagulants. Such approaches not only reduce the dependency on virgin raw materials like aluminum or iron salts but also mitigate the environmental burdens associated with sludge disposal [184].
Concurrently, the rediscovery of traditional and indigenous biomaterials for water treatment has gained global attention. Coagulants and flocculants derived from plant-based resources (such as Moringa oleifera, Opuntia ficus-indica, and Cactus mucilage), animal by-products (notably chitosan from crustacean shells), and fungal polysaccharides (known as mycoflocculants) demonstrate high performance in turbidity removal, heavy metal adsorption, and microbial reduction. These bio-based materials, supported by indigenous knowledge systems, offer low-cost, biodegradable, and locally available alternatives to conventional synthetic coagulants, particularly in rural and resource-constrained regions of Africa, Asia, and Latin America [185].
A striking example of this evolution is the continuous grafting process used to produce LENFLOC™, a bio-based coagulant derived from lentil waste. This innovative process is supported by real-time IoT sensors that analyze the feedstock’s biochemical composition and automatically adjust production parameters to ensure consistent polymerization performance. In jar tests, LENFLOC™ achieved 99% turbidity removal using significantly lower dosages than raw lentil extracts, while also producing denser and faster-settling flocs [21]. This case exemplifies how digital transformation and bio-based innovation converge to yield sustainable, high-efficiency coagulants that minimize waste and enhance water quality.
At the regional and global levels, integrating digital monitoring tools and life cycle assessment (LCA) frameworks enables better quality assurance and sustainability evaluation of these bio-coagulants. Moreover, Industry 4.0-enabled traceability systems—through blockchain and IoT—can ensure transparency in sourcing, production, and environmental performance tracking. This integration bridges traditional ecological knowledge with technological innovation, fostering a hybrid knowledge system that enhances social inclusivity, environmental protection, and economic viability within the water sector [181].
In sum, the synergy between digital transformation, circular economy, and indigenous biomaterial innovation represents a promising pathway toward achieving the SDGs. It redefines the coagulant value chain into a digitally enabled, bio-inspired, and circular system, capable of balancing technological progress with ecological integrity and cultural heritage.
6. Conclusions
Coagulation–flocculation is often labeled a “mature” technology, yet its long history belies a dynamic evolution rather than stagnation. In response to increasingly complex wastewater matrices and stringent regulations, the field has advanced from reliance on simple mineral salts to sophisticated hybrid coagulants and multifunctional flocculation platforms. Modern coagulants, including biosourced polymers and nanostructured hybrids, now integrate multiple removal mechanisms—charge neutralization, polymer bridging, adsorption, and even catalytic degradation—within single formulations. These innovations have dramatically improved performance, achieving contaminant removals once out of reach for conventional reagents. In cutting-edge designs, the floc itself becomes a functional entity (e.g., embedding magnetic nanoparticles or reactive sites) that can be recovered and repurposed, effectively transforming each floc into a vehicle not just for contaminant removal but also for resource capture and reuse. Taken together, these developments demonstrate a clear shift toward selective, adaptive, and resource-conscious coagulation strategies that align with circular water–economy principles established in the broader water–energy nexus.
Despite this progress, critical knowledge gaps and technical limitations persist. The mechanistic interactions between advanced coagulants and certain emerging pollutants remain poorly understood. For example, the exact PFAS removal mechanisms in coagulation are not fully elucidated—longer-chain PFAS can be partially captured by specialized coagulants, but shorter-chain variants often escape, indicating an incomplete molecular-level understanding of coagulant–pollutant binding. Similarly, the nucleation, growth, and breakage dynamics of flocs are seldom tracked in real time, leaving only a fragmented picture of how floc microstructure influences overall separation efficiency. This lack of real-time floc characterization hinders the development of robust, predictive models. Indeed, coagulant dosing in industry still relies heavily on empirical jar tests and rules of thumb, rather than on AI-driven or mechanistically grounded models that would link influent chemistry, coagulant properties, and operational parameters. Such empirical approaches make it difficult to optimize treatment for fluctuating water conditions or novel contaminants. Addressing these scientific blind spots is essential to moving beyond trial-and-error and fully unlocking the potential of next-generation coagulation systems.
Looking ahead, a number of actionable pathways can guide coagulation–flocculation toward smarter and more resilient performance. First, the process should be embedded into modular, integrated treatment trains alongside membranes, advanced oxidation, biological reactors, and electrochemical units. Such integration means each unit process can compensate for others’ limitations, amplifying overall contaminant removal and enabling synergistic benefits (for instance, coagulation pretreatment reducing fouling in downstream membranes). Second, the adoption of real-time sensing and AI-based control is imperative for what might be termed “Coagulation 4.0”. By continuously monitoring key indicators (e.g., turbidity, zeta potential, natural organic matter fractions, or specific micropollutant fingerprints) and feeding this data into machine-learning algorithms, future systems can dynamically adjust coagulant dose, mixing intensity, and pH to respond to changing water quality within seconds. Early studies already show that multi-parameter feedback control can stabilize effluent quality under variable influent while minimizing chemical use, foreshadowing the benefits of full-scale AI-optimized coagulation. Under this paradigm, decisions occur nearly instantaneously, chemical sludge production is minimized, and flocs can be tailored for easier valorization (e.g., as a source of nutrients or materials) in downstream processes. Finally, advancing coagulation–flocculation will require more than just technology—it calls for a cross-disciplinary collaboration that brings together materials scientists to design novel coagulant materials, chemists and environmental engineers to unravel reaction mechanisms, data scientists to develop predictive control algorithms, and policymakers to craft supportive regulatory frameworks. Such an alliance can ensure that research breakthroughs translate into field applications and that operational practices keep pace with scientific insights.
The stakes are high. If coagulation–flocculation fails to evolve, it risks becoming obsolete in the face of emerging contaminants and evolving regulatory standards. Conversely, by embracing the above innovations, coagulation–flocculation can secure its role as a cornerstone of integrated water management, meeting ever-tightening discharge limits while also contributing to broader sustainability goals. A successfully transformed coagulation process would not only produce clear water but also recover valuable resources, move toward carbon-neutral operation, and seamlessly integrate into circular economy frameworks for water and waste. Ultimately, the next generation of coagulation systems should be judged not only by how effectively they clarify water but by how intelligently they convert waste into resources and adapt to changing conditions. By clearly delineating recent advancements, persisting challenges, and future directions, this conclusion underscores a path forward: one that leverages innovation and interdisciplinary effort to ensure coagulation–flocculation remains both an indispensable water treatment technology and a driver of sustainable resource management.
Author Contributions
F.E.O.: Investigation, Resources, Conceptualization, Methodology, Writing—Original Draft Preparation. A.L. and E.A.M.S.: Supervision, Writing—Review & Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were generated or analyzed in this study; therefore, data sharing is not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Padilla-Rivera, A.; Morgan-Sagastume, J.M.; Güereca-Hernández, L.P. Sustainability Assessment of Wastewater Systems: An Environmental and Economic Approach. J. Environ. Prot. 2019, 10, 241–259. [Google Scholar] [CrossRef]
- Javan, K.; Altaee, A.; BaniHashemi, S.; Darestani, M.; Zhou, J.; Pignatta, G. A review of interconnected challenges in the water–energy–food nexus: Urban pollution perspective towards sustainable development. Sci. Total Environ. 2024, 912, 169319. [Google Scholar] [CrossRef] [PubMed]
- Lema, M.W. Wastewater crisis in East African cities: Challenges and emerging opportunities. Discov. Environ. 2025, 3, 18. [Google Scholar] [CrossRef]
- Ruprecht, J.E.; Birrer, S.C.; Dafforn, K.A.; Mitrovic, S.M.; Crane, S.L.; Johnston, E.L.; Wemheuer, F.; Navarro, A.; Harrison, A.J.; Turner, I.L.; et al. Wastewater effluents cause microbial community shifts and change trophic status. Water Res. 2021, 200, 117206. [Google Scholar] [CrossRef]
- Parween, M.; Ramanathan, A.L. Wastewater Management to Environmental Materials Management. In Handbook of Environmental Materials Management; Springer: Cham, Switzerland, 2018; pp. 1–24. [Google Scholar] [CrossRef]
- Drechsel, P.; Qadir, M.; Galibourg, D. The WHO Guidelines for Safe Wastewater Use in Agriculture: A Review of Implementation Challenges and Possible Solutions in the Global South. Water 2022, 14, 864. [Google Scholar] [CrossRef]
- El Ouadrhiri, F.; Elyemni, M.; Lahkimi, A.; Lhassani, A.; Chaouch, M.; Taleb, M. Mesoporous carbon from optimized date stone hydrochar by catalytic hydrothermal carbonization using response surface methodology: Application to dyes adsorption. Int. J. Chem. Eng. 2021, 2021, 5555406. [Google Scholar] [CrossRef]
- Yeoh, J.X.; Siti Nurul, S.N.A.; Syukri, F.; Koyama, M.; Nourouzi Mobarekeh, M. Comparison between Conventional Treatment Processes and Advanced Oxidation Processes in Treating Slaughterhouse Wastewater: A Review. Water 2022, 14, 3778. [Google Scholar] [CrossRef]
- Kato, S.; Kansha, Y. Comprehensive review of industrial wastewater treatment techniques. Environ. Sci. Pollut. Res. 2024, 31, 51064–51097. [Google Scholar] [CrossRef]
- Shamshad, J.; Ur Rehman, R. Innovative approaches to sustainable wastewater treatment: A comprehensive exploration of conventional and emerging technologies. Environ. Sci. Adv. 2025, 4, 189–222. [Google Scholar] [CrossRef]
- Tarigan, M.; Raji, S.; Al-Fatesh, H.; Czermak, P.; Ebrahimi, M. The Occurrence of Micropollutants in the Aquatic Environment and Technologies for Their Removal. Processes 2025, 13, 843. [Google Scholar] [CrossRef]
- Matesun, J.; Petrik, L.; Musvoto, E.; Ayinde, W.; Ikumi, D. Limitations of wastewater treatment plants in removing trace anthropogenic biomarkers and future directions: A review. Ecotoxicol. Environ. Saf. 2024, 281, 116610. [Google Scholar] [CrossRef]
- El Ouadrhiri, F.; Althomali, R.H.; Adachi, A.; Abdu Musad Saleh, E.; Husain, K.; Lhassani, A.; Hassan, I.; Mostafa Moharam, M.; Kassem, A.F.; Chaouch, M.; et al. Nitrogen and phosphorus co-doped carbocatalyst for efficient organic pollutant removal through persulfate-based advanced oxidation processes. J. Saudi Chem. Soc. 2023, 27, 101648. [Google Scholar] [CrossRef]
- Khan, N.A.; Ahmed, S.; Vambol, S.; Vambol, V.; Farooqi, I.H. Field hospital wastewater treatment scenario. Ecol. Quest. 2019, 30, 57–69. [Google Scholar] [CrossRef]
- Hosomi, M. New challenges on wastewater treatment. Clean Technol. Environ. Policy 2016, 18, 627–628. [Google Scholar] [CrossRef]
- Pariente, M.I.; Segura, Y.; Molina, R.; Martínez, F. Wastewater treatment as a process and a resource. In Wastewater Treatment Residues as Resources for Biorefinery Products and Biofuels; Elsevier: Amsterdam, The Netherlands, 2019; pp. 19–45. [Google Scholar] [CrossRef]
- Ketheesan, B.; Stuckey, D.C. Effects of hydraulic/organic shock/transient loads in anaerobic wastewater treatment: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2693–2727. [Google Scholar] [CrossRef]
- Jianlong, W.; Hanchang, S.; Yi, Q. Wastewater treatment in a hybrid biological reactor (HBR): Effect of organic loading rates. Process Biochem. 2000, 36, 297–303. [Google Scholar] [CrossRef]
- De Gisi, S.; Notarnicola, M. Industrial Wastewater Treatment. In Encyclopedia of Sustainable Technologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 23–42. ISBN 9780128046777. [Google Scholar]
- Bhagawan, D.; Chandan, V.; Srilatha, K.; Shankaraiah, G.; Rani, M.Y.; Himabindu, V. Industrial wastewater treatment using electrochemical process. In IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: New York, NY, USA, 2018; Volume 191. [Google Scholar]
- Usman, I.M.T.; Ho, Y.C.; Lam, M.K.; Show, P.L.; Sujarwo, W. Continuous-Flow Grafting of LENFLOCTM Coagulant for Water Treatment toward Circular Economy. Water 2023, 15, 2484. [Google Scholar] [CrossRef]
- Lucas, M.S.; Teixeira, A.R.; Jorge, N.; Peres, J.A. Industrial Wastewater Treatment by Coagulation–Flocculation and Advanced Oxidation Processes: A Review. Water 2025, 17, 1934. [Google Scholar] [CrossRef]
- Abujazar, M.S.S.; Karaağaç, S.U.; Abu Amr, S.S.; Alazaiza, M.Y.D.; Bashir, M.J. Recent advancement in the application of hybrid coagulants in coagulation-flocculation of wastewater: A review. J. Clean. Prod. 2022, 345, 131133. [Google Scholar] [CrossRef]
- Righetto, I.; Al-Juboori, R.A.; Kaljunen, J.U.; Mikola, A. Wastewater treatment with starch-based coagulants for nutrient recovery purposes: Testing on lab and pilot scales. J. Environ. Manag. 2021, 284, 112021. [Google Scholar] [CrossRef]
- Mao, Y.; Zhao, Y.; Cotterill, S. Examining Current and Future Applications of Electrocoagulation in Wastewater Treatment. Water 2023, 15, 1455. [Google Scholar] [CrossRef]
- Etafo, N.O.; Adekanmi, D.G.; Awobifa, O.S.; Torres, J.R.P.; Herrera, L.A.I.; Awobifa, O.A. Clean and green: The multifaceted solution of the electrocoagulation technology in emerging contaminants in wastewater. Discov. Civ. Eng. 2025, 2, 103. [Google Scholar] [CrossRef]
- Jo, S.; Kadam, R.; Jang, H.; Seo, D.; Park, J. Recent Advances in Wastewater Electrocoagulation Technologies: Beyond Chemical Coagulation. Energies 2024, 17, 5863. [Google Scholar] [CrossRef]
- Bahrodin, M.B.; Zaidi, N.S.; Hussein, N.; Sillanpää, M.; Prasetyo, D.D.; Syafiuddin, A. Recent Advances on Coagulation-Based Treatment of Wastewater: Transition from Chemical to Natural Coagulant. Curr. Pollut. Rep. 2021, 7, 379–391. [Google Scholar] [CrossRef]
- Badawi, A.K.; Salama, R.S.; Mostafa, M.M.M. Natural-based coagulants/flocculants as sustainable market-valued products for industrial wastewater treatment: A review of recent developments. RSC Adv. 2023, 13, 19335–19355. [Google Scholar] [CrossRef]
- Miranda, R.; Latour, I.; Blanco, A. Understanding the Efficiency of Aluminum Coagulants Used in Dissolved Air Flotation (DAF). Front. Chem. 2020, 8, 27. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Bogale, F.M. Role of coagulation/flocculation as a pretreatment option to reduce colloidal/bio-colloidal fouling in tertiary filtration of textile wastewater: A review and future outlooks. Front. Environ. Sci. 2023, 11, 1142227. [Google Scholar] [CrossRef]
- Jagaba, A.; Abubakar, S.; Lawal, I.; Latiff, A.; Umaru, I. Wastewater Treatment Using Alum, the Combinations of Alum-Ferric Chloride, Alum-Chitosan, Alum-Zeolite and Alum- Moringa oleifera as Adsorbent and Coagulant. Int. J. Eng. 2018, 2, 67. [Google Scholar] [CrossRef]
- Abu Bakar, A.F.; Halim, A.A. Treatment of automotive wastewater by coagulation-flocculation using poly-aluminum chloride (PAC), ferric chloride (FeCl3) and aluminum sulfate (alum). AIP Conf. Proc. 2013, 1571, 524–529. [Google Scholar] [CrossRef]
- Amuda, O.S.; Alade, A. Coagulation/flocculation process in the treatment of abattoir wastewater. Desalination 2006, 196, 22–31. [Google Scholar] [CrossRef]
- Solmaz, A.; Bölükbaşi, Ö.S.; Sari, Z.A. Green industry work: Production of FeCl3 from iron and steel industry waste (mill scale) and its use in wastewater treatment. Environ. Sci. Pollut. Res. 2024, 31, 19795–19814. [Google Scholar] [CrossRef]
- Hamidi, A.A.; Alias, S.; Assari, F.; Adlan, M.N. The use of alum, ferric chloride and ferrous sulphate as coagulants in removing suspended solids, colour and COD from semi-aerobic landfill leachate at controlled pH. Waste Manag. Res. 2007, 25, 556–565. [Google Scholar] [CrossRef]
- Demissie, E.; Baylie, H.; Tesfa, G.; Asefa, E. Comparison on The Effectiveness of Hydrated Aluminum Sulfate and Ferric Chloride as A Coagulant in Brewery Wastewater Treatment. The Case of Walia Brewery, Heineken Ethiopia. Glob. Sci. J. 2019, 7, 325–336. [Google Scholar]
- Kawamura, S. Integrated Design of Water Treatment Facilities; Choice Review: Marrickville, Australia, 1991; Volume 29, pp. 29–2124. [Google Scholar] [CrossRef]
- Cainglet, A.; Tesfamariam, A.; Heiderscheidt, E. Organic polyelectrolytes as the sole precipitation agent in municipal wastewater treatment. J. Environ. Manag. 2020, 271, 111002. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.Y.; Chu, Y.B.; Yue, Q.Y.; Wang, B.J.; Wang, S.G. Characterization and coagulation of a polyaluminum chloride (PAC) coagulant with high Al13 content. J. Environ. Manag. 2005, 76, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Lan, S.H.; Wang, C.L.; Sun, J.H.; Zhang, H. Study on treatment of dyeing wastewater by Fe0-H2O2 system. Adv. Mater. Res. 2014, 1048, 507–510. [Google Scholar] [CrossRef]
- Gao, B.Y.; Yue, Q.Y.; Wang, B.J.; Chu, Y.B. Poly-aluminum-silicate-chloride (PASiC)—A new type of composite inorganic polymer coagulant. Colloids Surfaces A Physicochem. Eng. Asp. 2003, 229, 121–127. [Google Scholar] [CrossRef]
- Gao, B.Y.; Hahn, H.H.; Hoffmann, E. Evaluation of aluminum-silicate polymer composite as a coagulant for water treatment. Water Res. 2002, 36, 3573–3581. [Google Scholar] [CrossRef]
- Liao, L.; Zhang, P. Preparation and Characterization of Polyaluminum Titanium Silicate and its Performance in the Treatment of Low-Turbidity Water. Processes 2018, 6, 125. [Google Scholar] [CrossRef]
- Amoohadi, V.; Pasalari, H.; Esrafili, A.; Gholami, M.; Farzadkia, M. A comparative study on polyaluminum chloride (PACl) and Moringa oleifera (MO) chemically enhanced primary treatment (CEPT) in enhanced biogas production: Anaerobic digestion performance and the Gompertz model. RSC Adv. 2023, 13, 17121–17129. [Google Scholar] [CrossRef]
- Wei, Z.; Long, W.; Li, S.; Zhao, Y.; Yu, S.; Zhou, F. Preparation of Cationic Polyacrylamide Suspension and Its Application in Oilfield Wastewater Treatment. Polymers 2024, 16, 151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, X.; Chen, M.; Ding, N.; Liu, H. Response Surface Optimization on Ferrate-Assisted Coagulation Pretreatment of SDBS-Containing Strengthened Organic Wastewater. Int. J. Environ. Res. Public Health 2023, 20, 5008. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.K.; Kumar, S.; Sharma, C. Removal of chloro-organics and color from pulp and paper mill wastewater by polyaluminium chloride as coagulant. Desalin. Water Treat. 2015, 53, 697–708. [Google Scholar] [CrossRef]
- Wang, J.; Yue, W.; Wang, Z.; Bai, Y.; Song, J. Removal effect of trihalomethanes (THMs) and halogenated acetic acids (HAAs) precursors in reclaimed water by polyaluminum chloride (PACl) coagulation. Water Sci. Technol. 2023, 87, 672–684. [Google Scholar] [CrossRef]
- Juliana, J.; Jesintha, K.L.; Mariaamalraj, S.; Padmanaban, V.C.; Neelakandan, A.R.; Rajanikant, G.K. Biopolymers as a Sustainable Approach for Wastewater Treatment. In Sustainable Technologies for Water and Wastewater Treatment; CRC Press: Boca Raton, FL, USA, 2021; pp. 259–274. [Google Scholar] [CrossRef]
- Manohara, H.M.; Nayak, S.S.; Franklin, G.; Nataraj, S.K.; Mondal, D. Progress in marine derived renewable functional materials and biochar for sustainable water purification. Green Chem. 2021, 23, 8305–8331. [Google Scholar] [CrossRef]
- Karnena, M.K.; Saritha, V. Natural Coagulants for the Treatment of Water and Wastewater: A Futuristic Option for Sustainable Water Clarification. Recent Innov. Chem. Eng. (Formerly Recent Patents Chem. Eng.) 2020, 14, 120–147. [Google Scholar] [CrossRef]
- Turunen, J.; Karppinen, A.; Ihme, R. Effectiveness of biopolymer coagulants in agricultural wastewater treatment at two contrasting levels of pollution. SN Appl. Sci. 2019, 1, 210. [Google Scholar] [CrossRef]
- Tong, C.Y.; Che Yusuf, F.H.B.; Derek, C.J.C. Optimization of Moringa oleifera seed extract and chitosan as natural coagulant in treatment of fish farm wastewater. Desalin. Water Treat. 2022, 256, 99–113. [Google Scholar] [CrossRef]
- Vigneshwaran, S.; Karthikeyan, P.; Sirajudheen, P.; Meenakshi, S. Optimization of sustainable chitosan/Moringa. oleifera as coagulant aid for the treatment of synthetic turbid water—A systemic study. Environ. Chem. Ecotoxicol. 2020, 2, 132–140. [Google Scholar] [CrossRef]
- Ahmed, H.M.; El-Khateeb, M.A.; Mohamed, N.Y.; Sobhy, N.A.; Fawzy, M.E. Evaluation of different natural waste materials as bio-coagulants for domestic wastewater treatment. Desalin. Water Treat. 2024, 317, 100034. [Google Scholar] [CrossRef]
- Lau, S.Y.; Tien, P.T.K.; Choy, S.Y.; Jeevanandam, J.; Show, P.L.; Lam, M.K.; Tan, Y.H.; Lim, S. Unleashing the power of plant-based modified starch as a game-changing natural coagulant. Process Biochem. 2024, 147, 213–227. [Google Scholar] [CrossRef]
- Mishra, B.; Kallem, P.; Yadavalli, R.; Mandal, S.K.; Reddy, C.N.; Sumithra, B.; Lakshmayya, N.S.V.; Bana, F. Industrial wastewater treatment using extracellular polymer substances/bioflocculants: A review. Appl. Water Sci. 2025, 15, 58. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Abdullah, S.R.S.; Imron, M.F.; Said, N.S.M.; Ismail, N.; Hasan, H.A.; Othman, A.R.; Purwanti, I.F. Challenges and Opportunities of Biocoagulant/Bioflocculant Application for Drinking Water and Wastewater Treatment and Its Potential for Sludge Recovery. Int. J. Environ. Res. Public Health 2020, 17, 9312. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Chen, J. Developing Magnetic Material for Remediation of Aquatic Nitrogen Pollution in Water Facilities. J. Met. Mater. Res. 2021, 4, 22–25. [Google Scholar] [CrossRef]
- Coello-Cabezas, J.; Verdezoto Carvajal, M.; Mejía Cabezas, N.; Sánchez-Moreno, H.; Basantes Basantes, E.; Estrella Semblantes, M.; Gavilanez Alvarez, I.; Ormaza Hugo, R. Organic coagulant combined with magnetite nanoparticles for the treatment of mercury-contaminated waters. Case Stud. Chem. Environ. Eng. 2024, 9, 100579. [Google Scholar] [CrossRef]
- Karyab, H.; Ghasemi, M.; Ghotbinia, F.; Nazeri, N. Efficiency of chitosan nanoparticle with polyaluminum chloride in dye removal from aqueous solutions: Optimization through response surface methodology (RSM) and central composite design (CCD). Int. J. Biol. Macromol. 2023, 249, 125977. [Google Scholar] [CrossRef]
- Chen, Y.; Nan, J.; Guo, M.; Zhang, Y.; Wang, J.; Wang, Q.; Fang, R. Novel cationic and amphoteric starch-modified coagulants for efficient treatment of relatively high turbidity and large organic matter source waters: Performance, predictive modeling and mechanism analysis. Sep. Purif. Technol. 2025, 359, 130845. [Google Scholar] [CrossRef]
- Zeng, H.; Sun, X.; Zhang, J.; Li, D. Enhanced ballasted magnetic coagulation process based on one-step pyrolysis synthesis of sludge-derived AC@MNPs for advanced purification of secondary effluent from wastewater treatment plants: Specific removal of low molecular weight organic matter. Sep. Purif. Technol. 2025, 359, 130457. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G. Sewage sludge-derived TiO2/Fe/Fe3C-biochar composite as an efficient heterogeneous catalyst for degradation of methylene blue. Chemosphere 2019, 215, 101–114. [Google Scholar] [CrossRef]
- Raja, A.G.; Selva Arasu, K.A.; Rajaram, R. Synthesis, Characterization and Application of Chitosan-TPP-ZnO Nanocomposite for Efficient Treatment of Effluent Containing Sulphur Dye. Mater. Today Proc. 2022, 68, 483–490. [Google Scholar] [CrossRef]
- El Foulani, A.A.; Ounas, O.; Tahiri, M.; Chafi, M. A Comparative Study of the Performance of Polyaluminum Chloride-Sodium Alginate and Polyaluminum Chloride-Chitosan Composite Coagulants in Dam Water Treatment. J. Polym. Environ. 2023, 31, 4909–4918. [Google Scholar] [CrossRef]
- Alibeigi-Beni, S.; Habibi Zare, M.; Pourafshari Chenar, M.; Sadeghi, M.; Shirazian, S. Design and optimization of a hybrid process based on hollow-fiber membrane/coagulation for wastewater treatment. Environ. Sci. Pollut. Res. 2021, 28, 8235–8245. [Google Scholar] [CrossRef] [PubMed]
- Das, O.; Restás, Á.; Shanmugam, V.; Sas, G.; Försth, M.; Xu, Q.; Jiang, L.; Hedenqvist, M.S.; Ramakrishna, S. Demystifying Low-Carbon Materials. Mater. Circ. Econ. 2021, 3, 26. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Z.; Wan, X.; Wang, Z.; Zhang, Y.; Meng, J.; Jiang, L.; Wang, S. Colonial sandcastle-inspired low-carbon building materials. Matter 2023, 6, 3864–3876. [Google Scholar] [CrossRef]
- Chen, Z.; Lai, J.; Kang, Y.; Wei, Y.; Xu, X.; Wang, S. A Tough and Sustainable Bioinspired Low-Carbon Building Material Reinforced by Glass Fibers. Small 2025, 21, e2500740. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, Z. The rational design of biomass-derived carbon materials towards next-generation energy storage: A review. Renew. Sustain. Energy Rev. 2020, 134, 110308. [Google Scholar] [CrossRef]
- Dawood, A.T.; Abdul-Bary, H.I.; Alazzawi, K.S.A.; Salman, I.H.; Kafeel, A. Removal of Colloidal Suspension through Coagulation–Flocculation Process In Water Purification—A Review. J. Biotechnol. Res. Cent. 2024, 18, 38–64. [Google Scholar] [CrossRef]
- Duan, J.; Gregory, J. Coagulation by hydrolysing metal salts. Adv. Colloid Interface Sci. 2003, 100, 475–502. [Google Scholar] [CrossRef]
- Ghernaout, D.; Ghernaout, B. Sweep flocculation as a second form of charge neutralisation—A review. Desalin. Water Treat. 2012, 44, 15–28. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, H.; Li, Q.; Cheng, C.; Shen, H.; Zhang, Z.; Zhang, Z.; Wang, H. Removal of refractory organics in wastewater by coagulation/flocculation with green chlorine-free coagulants. Sci. Total Environ. 2021, 787, 147654. [Google Scholar] [CrossRef]
- Ramlee, A.A.; Som, A.M.; Puasa, S.W.; Hamid, H.A.A. Coagulation–flocculation mechanism and characterisation of Hylocereus undatus foliage as a natural coagulant in industrial wastewater treatment. Chem. Pap. 2023, 77, 6083–6093. [Google Scholar] [CrossRef]
- Ersoy, B.; Tosun, I.; Günay, A.; Dikmen, S. Turbidity Removal from Wastewaters of Natural Stone Processing by Coagulation/Flocculation Methods. Clean-Soil Air Water 2009, 37, 225–232. [Google Scholar] [CrossRef]
- Mailler, R.; Mèche, P.; Sauvignet, P.; Azimi, S.; Rocher, V. Normalization of wastewater coagulation-flocculation trials and implications in terms of variability in treatment performance and comparison of commercial coagulants. Environ. Technol. 2021, 42, 4015–4026. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Liu, S.; Tan, Y.; Liu, Y.; Yuan, T.; Shen, Z.; Cheng, Z. Application of QSAR for investigation on coagulation mechanisms of textile wastewater. Ecotoxicol. Environ. Saf. 2022, 244, 114035. [Google Scholar] [CrossRef] [PubMed]
- Badawi, A.K.; Zaher, K. Hybrid treatment system for real textile wastewater remediation based on coagulation/flocculation, adsorption and filtration processes: Performance and economic evaluation. J. Water Process Eng. 2021, 40, 101963. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, J.; Wang, Y.; Lu, N. Application performance of a new coagulant in wastewater reuse. Water Sci. Technol. 2016, 73, 2101–2107. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Zouboulis, A.I. Application of Composite Pre-Polymerized Coagulants for the Treatment of High-Strength Industrial Wastewaters. Water 2020, 12, 1258. [Google Scholar] [CrossRef]
- Maknakorn, W.; Jutaporn, P.; Khongnakorn, W. Coagulation and adsorption as pretreatments of thin-film composite-forward osmosis (TFC-FO) for ink printing wastewater treatment. Water Sci. Technol. 2019, 79, 877–887. [Google Scholar] [CrossRef]
- Jiang, J.Q. The role of coagulation in water treatment. Curr. Opin. Chem. Eng. 2015, 8, 36–44. [Google Scholar] [CrossRef]
- Xiong, J.; Ma, R.; Tian, Y.; Yang, L.; He, Y.; Qian, J.; Wu, W.; Gao, Y.; Luo, J.; Xu, D.; et al. Improvement of Coagulation and Membrane Filtration Performance in Hybrid Coagulation-UF-RO System for Chemical Mechanical Polishing Wastewater Treatment: Effect of Coagulants. Water Air Soil Pollut. 2024, 235, 505. [Google Scholar] [CrossRef]
- Sun, C.; Qiu, J.; Zhang, Z.; Marhaba, T.F.; Zhang, Y. Coagulation behavior and floc characteristics of a novel composite poly-ferric aluminum chloride-polydimethyl diallylammonium chloride coagulant with different OH/(Fe3+ + Al3+) molar ratios. Water Sci. Technol. 2016, 74, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Fu, M.; Li, F.; Wu, N.; Yang, J.; Jia, H.; Wang, B.; Cheng, R. Preparation of a New Inorganic-Organic Composite Dual-Coagulant and Application of Oily Wastewater Treatment. Adv. Mater. Res. 2011, 233–235, 523–527. [Google Scholar] [CrossRef]
- Precious Sibiya, N.; Rathilal, S.; Kweinor Tetteh, E. Coagulation Treatment of Wastewater: Kinetics and Natural Coagulant Evaluation. Molecules 2021, 26, 698. [Google Scholar] [CrossRef] [PubMed]
- Tsoutsa, E.K.; Tolkou, A.K.; Kyzas, G.Z.; Katsoyiannis, I.A. New Trends in Composite Coagulants for Water and Wastewater Treatment. Macromol 2024, 4, 509–532. [Google Scholar] [CrossRef]
- Zouboulis, A.I.; Sarasidis, V.; Moussas, P.A. Removal of Copper From Synthetic Wastewaters by the Hybrid Coagulation–Microfiltration Process. Sep. Sci. Technol. 2010, 45, 1658–1666. [Google Scholar] [CrossRef]
- Qian, S. Study on Treatment of Simulated Wastewater by Composite Aluminum-zinc-iron(PAZF) Coagulant. Liaoning Chem. Ind. 2013, 9, 1026–1028. [Google Scholar]
- Lee, K.E.; Hanafiah, M.M.; Halim, A.A.; Mahmud, M.H. Primary Treatment of Dye Wastewater Using Aloe Vera-aided Aluminium and Magnesium Hybrid Coagulants. Procedia Environ. Sci. 2015, 30, 56–61. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, H.; Yang, X. Preparation and performance of a novel starch-based inorganic/organic composite coagulant for textile wastewater treatment. Sep. Purif. Technol. 2019, 210, 93–99. [Google Scholar] [CrossRef]
- Sakthivel, E.; Manoj Suburam, R. Performance evaluation of composite coagulant in treating textile wastewater. Mater. Today Proc. 2022, 62, 1708–1711. [Google Scholar] [CrossRef]
- Jin, X.; Liu, Y.; Wang, Y.; Zhang, S.; Zhang, W.; Jin, P.; Xu, L.; Shi, X.; Wang, X.C.; Lv, S. Towards a comparison between the hybrid ozonation-coagulation (HOC) process using Al- and Fe-based coagulants: Performance and mechanism. Chemosphere 2020, 253, 126625. [Google Scholar] [CrossRef]
- Chen, H.; Li, C.; Zhang, Y.; Fang, W.; Zou, L.; Chi, R. Investigation of Solution Microstructure in Ferric Sulfate Coagulation-Assisted Precipitation of Fluoride Ions. Molecules 2025, 30, 1362. [Google Scholar] [CrossRef] [PubMed]
- Iman, L.; Afshin, E.; Mehdi, H. Comparison study of turbidity removal using synthetized poly-aluminum chloride-sulfate and poly-aluminum chloride in aqueous solutions. Int. J. Environ. Health Eng. 2014, 3, 32. [Google Scholar] [CrossRef]
- Narges, S.; Ghorban, A.; Hassan, K.; Mohammad, K. Prediction of the optimal dosage of coagulants in water treatment plants through developing models based on artificial neural network fuzzy inference system (ANFIS). J. Environ. Health Sci. Eng. 2021, 19, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Dhrubo, A.A.K.; Jannat, M.; Hossain, M.S. Enhancing the performance of coagulants for wastewater treatment by varying and optimizing the experimental parameters. J. Water Process Eng. 2023, 55, 104144. [Google Scholar] [CrossRef]
- Tahraoui, H.; Toumi, S.; Boudoukhani, M.; Touzout, N.; Sid, A.N.E.H.; Amrane, A.; Belhadj, A.E.; Hadjadj, M.; Laichi, Y.; Aboumustapha, M.; et al. Evaluating the Effectiveness of Coagulation–Flocculation Treatment Using Aluminum Sulfate on a Polluted Surface Water Source: A Year-Long Study. Water 2024, 16, 400. [Google Scholar] [CrossRef]
- Yuan, M.; Bustamante, H.; Mahmoudi, N.; Gradzielski, M. Colloidal Chemistry in Water Treatment: The Effect of Ca2+ on the Interaction between Humic Acid and Poly(diallyldimethylammonium chloride) (PDADMAC). Langmuir 2024, 40, 4108–4121. [Google Scholar] [CrossRef]
- Gotlib, I.Y.; Korchak, P.A.; Safonova, E.A.; Volkova, A.V.; Victorov, A.I. Poly(diallyldimethylammonium chloride) in water and aqueous salt solution: Explicit solvent molecular dynamics and experiment. J. Mol. Liq. 2025, 422, 126946. [Google Scholar] [CrossRef]
- Levakov, I.; Maor, I.; Barak, C.; Kirshenbaum, Y.; Rytwo, G. Colorimetric Quantification for Residual Poly-DADMAC in Water Treatment. Water 2023, 15, 3352. [Google Scholar] [CrossRef]
- Chen, J.; Lin, J.; Li, W.; Wang, Y.; Huang, H. Coagulative removal of polyethylene microplastics using polyaluminum chloride in conjunction with laminarin. Chem. Eng. Res. Des. 2024, 212, 230–239. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, X.; Qu, C.; Zhang, R. Efficient treatment of polyacrylamide wastewater by cascade heterogeneous Fenton and magnetic flocculation process. Geoenergy Sci. Eng. 2024, 242, 213287. [Google Scholar] [CrossRef]
- Koul, B.; Bhat, N.; Abubakar, M.; Mishra, M.; Arukha, A.P.; Yadav, D. Application of Natural Coagulants in Water Treatment: A Sustainable Alternative to Chemicals. Water 2022, 14, 3751. [Google Scholar] [CrossRef]
- Otálora, M.C.; Wilches-Torres, A.; Lara, C.R.; Díaz-Gómez, J.; Gómez Castaño, J.A.; Cifuentes, G.R. Assessment of Prickly Pear Fruit Peel Mucilage in Form of Gel as a Green Coagulant for the Tertiary Treatment of Domestic Wastewater. Gels 2023, 9, 723. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.K.; Abdelwahab, O.; El-Ashtoukhy, E.S.Z.; Abdel-Aziz, M.H. Comparative analysis of new natural coagulant extracts for turbidity removal in water systems. Water Sci. Technol. 2025, 91, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Dandesa, B.; Akuma, D.A.; Alemayehu, E. Water purification improvement using Moringa oleifera seed extract pastes for coagulation follow scoria filtration. Heliyon 2023, 9, e17420. [Google Scholar] [CrossRef]
- Prasetyo, S.; Santos, C.A.; Sugih, A.K.; Kristianto, H. Utilization of chitosan as a natural coagulant for polyethylene microplastic removal. Sustain. Chem. Environ. 2025, 9, 100225. [Google Scholar] [CrossRef]
- Nasir, M.; Javaid, F.; Masood, M.T.; Arshad, M.; Yasir, M.; Sedlarik, V.; Qadir, M.A.; Qiblawey, H.; Zhang, W.; Deen, K.M.; et al. Regenerable chitosan-embedded magnetic iron oxide beads for nitrate removal from industrial wastewater. Environ. Sci. Adv. 2024, 3, 572–584. [Google Scholar] [CrossRef]
- Shadan, B.; Jafari, A.; Gharibshahi, R. Enhanced drilling waste-water treatment through magnetic nano-composite coagulant application: A central composite design study. Heliyon 2024, 10, e40450. [Google Scholar] [CrossRef]
- Singh, S.; Sivaram, N.; Nath, B.; Khan, N.A.; Singh, J.; Ramamurthy, P.C. Metal organic frameworks for wastewater treatment, renewable energy and circular economy contributions. npj Clean Water 2024, 7, 124. [Google Scholar] [CrossRef]
- Dash, S.; Raj, A.; Vara, S. Experimental screening and selection criteria of natural coagulants towards wastewater treatment. J. Appl. Nat. Sci. 2024, 16, 1096–1105. [Google Scholar] [CrossRef]
- Asadollahfardi, G.; Zangooei, H.; Motamedi, V.; Davoodi, M. Selection of coagulant using jar test and analytic hierarchy process: A case study of Mazandaran textile wastewater. Adv. Environ. Res. 2018, 7, 1–11. [Google Scholar]
- Bhamidipati, S.H.; Vadlamudi, D.P.; Moka, S. Polymers as Coagulants for Wastewater Treatment. In Advanced Materials and Technologies for Wastewater Treatment; CRC Press: Boca Raton, FL, USA, 2021; pp. 85–114. [Google Scholar] [CrossRef]
- Ozgur, C.; Gurhan, A.B.; Bekaroglu, S.S.K. A multicriteria decision-making model for the selection of conventional/hybrid coagulants in water treatment. Integr. Environ. Assess. Manag. 2025, 21, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Kweinor Tetteh, E.; Rathilal, S. Application of Organic Coagulants in Water and Wastewater Treatment. In Organic Polymers; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Diver, D.; Nhapi, I.; Ruziwa, W.R. The potential and constraints of replacing conventional chemical coagulants with natural plant extracts in water and wastewater treatment. Environ. Adv. 2023, 13, 100421. [Google Scholar] [CrossRef]
- Tzfati, E.; Sein, M.; Rubinov, A.; Raveh, A.; Bick, A. Pretreatment of wastewater: Optimal coagulant selection using Partial Order Scaling Analysis (POSA). J. Hazard. Mater. 2011, 190, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Castro-Jiménez, C.C.; Saldarriaga-Molina, J.C.; García, E.F. Physical-chemical characterisation of an alum-based water treatment sludge in different raw water turbidity scenarios. Heliyon 2024, 10, e37579. [Google Scholar] [CrossRef]
- El Hafidi, E.M.; Mortadi, A.; Chahid, E.G.; Laasri, S. Optimization of Domestic Wastewater Treatment Using Ferric Chloride Coagulant: Physicochemical Analysis and Impedance Spectroscopy Studies. Water. Air. Soil Pollut. 2024, 235, 68. [Google Scholar] [CrossRef]
- Coleman, C.K.; Oza, H.H.; Bailey, E.S.; Sobsey, A.; Kyzas, G.Z.; Coleman, C.K.; Oza, H.H.; Bailey, E.S.; Sobsey, M.D. A Review of Chitosan as a Coagulant of Health-Related Microorganisms in Water and Wastewater. Environments 2024, 11, 211. [Google Scholar] [CrossRef]
- Long, F.; Liu, H. Enhancing resource recovery from acid whey through chitosan-based pretreatment and machine learning optimization. Bioresour. Technol. 2025, 418, 131932. [Google Scholar] [CrossRef]
- Boivin, S.; Okuda, T.; Fujioka, T. Effects of Moringa oleifera-based pre-coagulation and hydraulic flushing on the reduction of submerged nanofiltration membrane fouling. Desalin. Water Treat. 2025, 323, 101249. [Google Scholar] [CrossRef]
- Shah, A.; Manning, G.; Zakharova, J.; Arjunan, A.; Batool, M.; Hawkins, A.J. Particle size effect of Moringa oleifera Lam. seeds on the turbidity removal and antibacterial activity for drinking water treatment. Environ. Chem. Ecotoxicol. 2024, 6, 370–379. [Google Scholar] [CrossRef]
- Yi, J.; Chen, Z.; Xu, D.; Wu, D.; Howard, A. Preparation of a coagulant of polysilicate aluminum ferric from foundry dust and its coagulation performance in treatment of swine wastewater. J. Clean. Prod. 2024, 434, 140400. [Google Scholar] [CrossRef]
- Kong, Y.; Zhou, Y.; Zhang, P.; Nie, Y.; Ma, J. Coagulation performance and mechanism of different novel covalently bonded organic silicon-aluminum/iron composite coagulant for As(V) removal from water: The role of hydrolysate species and the effect of coexisting microplastics. J. Hazard. Mater. 2024, 480, 135819. [Google Scholar] [CrossRef]
- Zeng, H.; Liu, C.; Xu, H.; Hao, R.; Zhang, J.; Li, D. Preparation of Fe3O4@C with water treatment residuals and its potential in the magnetic coagulation process. J. Clean. Prod. 2022, 362, 132327. [Google Scholar] [CrossRef]
- Du, S.; Liu, C.; Cheng, P.; Liang, W. Chitosan-Based Grafted Cationic Magnetic Material to Remove Emulsified Oil from Wastewater: Performance and Mechanism. Processes 2024, 12, 797. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Du, S.; Cheng, P.; Liang, W. Magnetic coagulation and flocculation of kaolin suspension using Fe3O4 with plant polyphenol self-assembled flocculants. Int. J. Biol. Macromol. 2023, 253, 126578. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Ashraf, S.; Alhuzaymi, T.M.; Ghani, L.; Um, W. Low level radioactive waste treatment by coagulation flocculation technique: A review. J. Radioanal. Nucl. Chem. 2024, 333, 6079–6091. [Google Scholar] [CrossRef]
- Zhang, Y.; Diehl, A.; Lewandowski, A.; Gopalakrishnan, K.; Baker, T. Removal efficiency of micro- and nanoplastics (180 nm–125 μm) during drinking water treatment. Sci. Total Environ. 2020, 720, 137383. [Google Scholar] [CrossRef]
- Keawchouy, S.; Na-Phatthalung, W.; Keaonaborn, D.; Jaichuedee, J.; Musikavong, C.; Sinyoung, S. Enhanced coagulation process for removing dissolved organic matter, microplastics, and silver nanoparticles. J. Environ. Sci. Health-Part A Toxic/Hazardous Subst. Environ. Eng. 2022, 57, 1084–1098. [Google Scholar] [CrossRef]
- Pawak, V.S.; Loganathan, V.A.; Sabapathy, M. Efficient removal of nanoplastics from synthetic wastewater using electrocoagulation. arXiv 2023, arXiv:2302.08451. [Google Scholar] [CrossRef]
- Khan, M.T.; Ahmad, M.; Hossain, M.F.; Nawab, A.; Ahmad, I.; Ahmad, K.; Panyametheekul, S. Microplastic removal by coagulation: A review of optimizing the reaction conditions and mechanisms. Water Emerg. Contam. Nanoplast. 2023, 2, 22. [Google Scholar] [CrossRef]
- Hubert, M.; Meyn, T.; Hansen, M.C.; Hale, S.E.; Arp, H.P.H. Per- and polyfluoroalkyl substance (PFAS) removal from soil washing water by coagulation and flocculation. Water Res. 2024, 249, 120888. [Google Scholar] [CrossRef]
- Facchino, M.; Pietrelli, L.; Menegoni, P.; Capocelli, M.; Limiti, E.; Trombetta, M.; Basoli, F.; De Falco, M. Greener Microplastics Removal: Progressive Replacement of Iron-Based Coagulants with Sodium Alginate and Chitosan to Enhance Sustainability. Chempluschem 2025, 90, e202400736. [Google Scholar] [CrossRef]
- Zaki, N.; Hadoudi, N.; Charki, A.; Bensitel, N.; El Ouarghi, H.; Amhamdi, H.; Ahari, M. Advancements in the chemical treatment of potable water and industrial wastewater using the coagulation–flocculation process. Sep. Sci. Technol. 2023, 58, 2619–2630. [Google Scholar] [CrossRef]
- Mohamed Noor, M.H.; Ngadi, N. Ecotoxicological risk assessment on coagulation-flocculation in water/wastewater treatment: A systematic review. Environ. Sci. Pollut. Res. 2024, 31, 52631–52657. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zhai, Z.; Zang, J.; Zhu, Y. An Intelligent Dosing Algorithm Model for Wastewater Treatment Plant. J. Phys. Conf. Ser. 2022, 2224, 012027. [Google Scholar] [CrossRef]
- Al-Jadabi, N.; Laaouan, M.; El Hajjaji, S.; Mabrouki, J.; Benbouzid, M.; Dhiba, D. The Dual Performance of Moringa oleifera Seeds as Eco-Friendly Natural Coagulant and as an Antimicrobial for Wastewater Treatment: A Review. Sustainability 2023, 15, 4280. [Google Scholar] [CrossRef]
- Turna, T.; Yıldız, Y. Treatment of Vegetable Oil Industry Wastewaters with Coagulation-flocculation Methods. DÜMF Mühendislik Derg. 2024, 15, 533–540. [Google Scholar] [CrossRef]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent Advancement of Coagulation-Flocculation and Its Application in Wastewater Treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- de Sousa Silva, R.; Rengel, H.D.; Voigt, F.D.; Machado, R.A.F.; de Fátima Peralta Muniz Moreira, R.; Marangoni, C. Treatment of real textile wastewater by coagulation/flocculation integrated with direct contact membrane distillation. Sep. Sci. Technol. 2023, 58, 2394–2410. [Google Scholar] [CrossRef]
- Marques, D.G.; de Melo Franco Domingos, J.; Nolasco, M.A.; Campos, V. Textile effluent treatment using coagulation-flocculation and a hydrodynamic cavitation reactor associated with ozonation. Chem. Eng. Sci. 2025, 304, 121094. [Google Scholar] [CrossRef]
- Tzoupanos, N.D.; Zouboulis, A.I. Coagulation-Flocculation Processes in Water/Wastewater Treatment: The Application of New Generation of Chemical Reagents. In Proceedings of the 6th IASME/WSEAS international conference on heat transfer, thermal engineering and environment (HTE’08), Rhodes, Greece, 20–22 August 2008; pp. 309–317. [Google Scholar]
- Owodunni, A.A.; Ismail, S.; Kurniawan, S.B.; Ahmad, A.; Imron, M.F.; Abdullah, S.R.S. A review on revolutionary technique for phosphate removal in wastewater using green coagulant. J. Water Process Eng. 2023, 52, 103573. [Google Scholar] [CrossRef]
- Oktaviani; Adachi, Y. Effect of mixing intensity on flocculation kinetics of polystyrene latex particles with high-charge density polyelectrolyte at various ionic strengths. Colloid Polym. Sci. 2018, 296, 1945–1951. [Google Scholar] [CrossRef]
- Vadasarukkai, Y.S.; Gagnon, G.A. Influence of the Mixing Energy Consumption Affecting Coagulation and Floc Aggregation. Environ. Sci. Technol. 2017, 51, 3480–3489. [Google Scholar] [CrossRef] [PubMed]
- Zaharia, C.; Musteret, C.P.; Afrasinei, M.A. The Use of Coagulation–Flocculation for Industrial Colored Wastewater Treatment—(I) The Application of Hybrid Materials. Appl. Sci. 2024, 14, 2184. [Google Scholar] [CrossRef]
- Haddaji, C.; Khattabi Rifi, S.; Digua, K.; Madinzi, A.; Chatoui, M.; Driouich, A.; Ettaloui, Z.; Agustiono Kurniawan, T.; Anouzla, A.; Souabi, S. Optimization of the coagulation-flocculation process using response surface methodology for wastewater pretreatment generated by vegetable oil refineries: A path towards environmental sustainability. Environ. Nanotechnol. Monit. Manag. 2024, 22, 100973. [Google Scholar] [CrossRef]
- Khalidi-Idrissi, A.; Hartal, O.; Madinzi, A.; El-Abbadi, K.; Souabi, S. Natural flotation and coagulation–flocculation: A dual approach to refinery wastewater treatment. Euro-Mediterr. J. Environ. Integr. 2025, 10, 1425–1443. [Google Scholar] [CrossRef]
- Zaki, N.; Charki, A.; Hadoudi, N.; Fraiha, O.; El Ouarghi, H.; Salhi, A.; Amhamdi, H.; Ahari, M. Coagulation-flocculation parameters for simultaneous removal of nitrates, nitrites, phosphates, and ammonium from wastewater: A mini review. E3S Web Conf. 2024, 527, 03015. [Google Scholar] [CrossRef]
- Hellal, M.S.; Doma, H.S.; Abou-Taleb, E.M. Techno-economic evaluation of electrocoagulation for cattle slaughterhouse wastewater treatment using aluminum electrodes in batch and continuous experiment. Sustain. Environ. Res. 2023, 33, 2. [Google Scholar] [CrossRef]
- Msemwa, G.G.; Nasr, M.; Abdelhaleem, A.; Fujii, M.; Ibrahim, M.G. Coagulation-Flocculation/Pyrolysis Integrated System for Dye-Laden Wastewater Treatment: A Techno-Economic and Sustainable Approach. Water. Air. Soil Pollut. 2025, 236, 57. [Google Scholar] [CrossRef]
- Adeleke, V.T.; Madlala, N.E.; Adeniyi, A.A.; Lokhat, D. Molecular Interactions Associated with Coagulation of Organic Pollutants by 2S Albumin of Plant Proteins: A Computational Approach. Molecules 2022, 27, 1685. [Google Scholar] [CrossRef]
- Leon Zerpa, F.; Chirinza, N.P.; Muguirrima, P.V.; Mendieta Pino, C.A.; Ramos Martin, A. Moringa oleifera Seed Extract As a Coagulant in the Treatment Of Drinking Water. DYNA 2025, 100, 5. [Google Scholar] [CrossRef]
- Dao, M.-T.; Nguyen, V.-C.-N.; Tran, T.-N.; Nguyen, X.-D.; Vo, D.-T.; Nguyen, V.-K.; Hoang, L.-T.-T.-T. Pilot-Scale Study of Real Domestic Textile Wastewater Treatment Using Cassia fistula Seed-Derived Coagulant. J. Chem. 2021, 2021, 7608856. [Google Scholar] [CrossRef]
- López-Maldonado, E.A.; Oropeza-Guzmán, M.T.; Ochoa-Terán, A. Improving the efficiency of a coagulation-flocculation wastewater treatment of the semiconductor industry through zeta potential measurements. J. Chem. 2014, 2014, 969720. [Google Scholar] [CrossRef]
- Alekseev, E.; Shambina, S. Coagulation of waste water from the point of view of physic-chemical interactions. E3S Web Conf. 2021, 263, 04011. [Google Scholar] [CrossRef]
- Macieja, S.; Środa, B.; Zielińska, B.; Roy, S.; Bartkowiak, A.; Łopusiewicz, Ł. Bioactive Carboxymethyl Cellulose (CMC)-Based Films Modified with Melanin and Silver Nanoparticles (AgNPs)—The Effect of the Degree of CMC Substitution on the In Situ Synthesis of AgNPs and Films’ Functional Properties. Int. J. Mol. Sci. 2022, 23, 15560. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Khastgir, D. Hybrid composite membranes of chitosan/sulfonated polyaniline/silica as polymer electrolyte membrane for fuel cells. Carbohydr. Polym. 2018, 179, 152–163. [Google Scholar] [CrossRef]
- Saladino, G.M.; Hamawandi, B.; Vogt, C.; Rajarao, G.K.; Toprak, M.S. Click chemical assembly and validation of bio-functionalized superparamagnetic hybrid microspheres. Appl. Nanosci. 2020, 10, 1861–1869. [Google Scholar] [CrossRef]
- Malkoske, T.A.; Bérubé, P.R.; Andrews, R.C. Coagulation/flocculation prior to low pressure membranes in drinking water treatment: A review. Environ. Sci. Water Res. Technol. 2020, 6, 2993–3023. [Google Scholar] [CrossRef]
- Manamperuma, L.; Wei, L.; Ratnaweera, H. Multi-parameter based coagulant dosing control. Water Sci. Technol. 2017, 75, 2157–2162. [Google Scholar] [CrossRef]
- Dwarakanath, B.; Kalpana Devi, P.; Ranjith Kumar, A.; Metwally, A.S.M.; Ashraf, G.A.; Thamineni, B.L. Smart IoT-based water treatment with a Supervisory Control and Data Acquisition (SCADA) system process. Water Reuse 2023, 13, 411–431. [Google Scholar] [CrossRef]
- Liu, W.; Ratnaweera, H. Feed-forward-based software sensor for outlet turbidity of coagulation process considering plug flow condition. Int. J. Environ. Sci. Technol. 2017, 14, 1689–1696. [Google Scholar] [CrossRef]
- Sarp Akarsu, M.; Tokgöz Güneş, S. The Role of PHREEQC Model and Sensor Analysis in Chemical Coagulation Processes Supported by Online Sensors. J. Anatol. Environ. Anim. Sci. 2024, 9, 45–52. [Google Scholar] [CrossRef]
- Patil, B.; Patil, S.; Ravande, K. Use of electrocoagulation for treatment of wastewater. In Materials Research Proceedings; Association of American Publishers: Washington, DC, USA, 2025; Volume 48, pp. 764–773. [Google Scholar]
- Garomsa, F.S.; Berhanu, Y.M.; Desta, W.M.; Bidira, F. Indigenous bio-coagulant assisted electrocoagulation process for the removal of contaminants from brewery wastewater: Performance evaluation and response surface methodology optimization. Heliyon 2024, 10, e40394. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, H.G.J.; Elguera, N.Y.; Ancco, M.; Castro, A.E.L.F.; Meza, M.E.B.; Almeida, V.C. Combined coagulation-electrocoagulation process using biocoagulant from the Opuntia ficus-indica for treatment of cheese whey wastewater. Environ. Monit. Assess. 2023, 195, 491. [Google Scholar] [CrossRef] [PubMed]
- Issahaku, I.; Tetteh, I.K.; Tetteh, A.Y. Chitosan and chitosan derivatives: Recent advancements in production and applications in environmental remediation. Environ. Adv. 2023, 11, 100351. [Google Scholar] [CrossRef]
- Luo, F.; Wu, Z.; Wang, M.; Shu, X.; Shu, X.; Jia, P.; Li, Q. High-Performance Flocculants for Purification: Solving the Problem of Waste Incineration Bottom Ash and Unpurified Water. ACS Omega 2020, 5, 13259–13267. [Google Scholar] [CrossRef]
- Sabahaudin, M.F.H.; Kadir, A.A.; Detho, A.; Hassan, M.I.H.; Hashar, N.N.H.; Hissham, N.F.N. Utilization of Sludge from Water Treatment Plant as Fired Clay Brick. Arch. Metall. Mater. 2025, 70, 253–258. [Google Scholar] [CrossRef]
- Vargas-Velez, C.D.; Sánchez Ortiz, I.A.; Masumoto, T. Recovery of coagulants via acid treatment in potabilization sludges and their reuse in raw and urban wastewaters. Water Sci. Technol. 2025, 91, 1010–1021. [Google Scholar] [CrossRef]
- Mora-León, A.G.; Castro-Jiménez, C.C.; Saldarriaga-Molina, J.C.; García A, E.F.; Correa-Ochoa, M.A. Aluminium recovered coagulant from water treatment sludge as an alternative for improving the primary treatment of domestic wastewater. J. Clean. Prod. 2022, 346, 131229. [Google Scholar] [CrossRef]
- Matheri, A.N.; Mohamed, B.; Ntuli, F.; Nabadda, E.; Ngila, J.C. Sustainable circularity and intelligent data-driven operations and control of the wastewater treatment plant. Phys. Chem. Earth 2022, 126, 103152. [Google Scholar] [CrossRef]
- Md Nuruzzaman Abir, A.Z. Industry 4.0 Technologies for a Data-Driven, Secure, Green and Circular Manufacturing Economy. In Proceedings of the 2024 IEEE Green Technologies Conference, Springdale, AR, USA, 3–5 April 2024; pp. 26–31. [Google Scholar] [CrossRef]
- Spaltini, M.; Poletti, A.; Acerbi, F.; Taisch, M. A quantitative framework for Industry 4.0 enabled Circular Economy. In Procedia CIRP; Elsevier B.V.: Amsterdam, The Netherlands, 2021; Volume 98, pp. 115–120. [Google Scholar]
- Nobre, G.C.; Tavares, E. The role of Industry 4.0 technologies in the transition to a circular economy: A practice perspective. Sustain. Sci. Pract. Policy 2023, 19, 2289260. [Google Scholar] [CrossRef]
- Singh, R.; Joshi, A.; Dissanayake, H.; Iddagoda, A.; Khan, S.; Félix, M.J.; Santos, G. Integrating Industry 4.0, Circular Economy, and Green HRM: A Framework for Sustainable Transformation. Sustainability 2025, 17, 3082. [Google Scholar] [CrossRef]
- Castro-Jiménez, C.C.; Saldarriaga-Molina, J.C.; García, E.F.; Correa-Ochoa, M.A. Primary Treatment of Domestic Wastewater with the Use of Unmodified and Chemically Modified Drinking Water Treatment Sludge. Sustainability 2022, 14, 9827. [Google Scholar] [CrossRef]
- De, T.; Verma, P.; Gangaraju, P.K.; Nibhanupudi Siva Bhaskar, A.; Mahlawat, S.; Kumar, V.; Singh, S. Exploring the synergistic effects of circular economy, Industry 4.0 technology, and green human resource management practices on sustainable performance: Empirical evidence from Indian companies. Bus. Strateg. Dev. 2024, 7, e70002. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).