Abstract
The immobilization of enzymes in polyamide-based polymeric materials through covalent bonding is an established technique to stabilize and reuse biocatalysts in industrial processes. Traditionally, enzymes are immobilized using crosslinking agents that activate functional groups on both the support and the enzyme, creating strong bonds that securely anchor the enzyme to the surface. While effective for maintaining enzyme activity over multiple cycles, this method can reduce catalytic efficiency due to rigid binding and involves complex activation steps. Recently, in situ immobilization approaches have emerged as promising alternatives. In this method, enzymes are directly entrapped within the polymer matrix during the synthesis of the polyamide support, such as nylon, simplifying the process and offering enhanced control over enzyme distribution. For instance, studies have demonstrated that in situ immobilization can improve enzyme stability by protecting it within the polymeric network, while reducing production costs and waste. This review explores the ability of polyamide as a support material for immobilization of enzymes, analyzing key techniques, performance across applications, and future strategies to optimize polymer-enzyme interactions for industrial use.
1. Introduction
Enzymes are highly versatile, efficient, and environmentally sustainable catalysts that function under mild conditions. However, enzymes in free form present operational limitations. Immobilizing enzymes offers a promising solution, enabling enzyme recovery—a critical requirement for reuse—and providing stability against variable reaction conditions. This is crucial as enzymes are susceptible to denaturation under adverse conditions. Consequently, enzyme immobilization enhances catalytic efficiency and facilitates recycling, offering significant advantages over free enzymes under comparable reaction conditions [1,2,3].
In recent years, immobilized biocatalysts have seen increasing applications in several industries as a greener and more sustainable alternative to conventional synthetic processes. Nonetheless, several factors must be carefully considered to ensure the economic feasibility of enzymatic processes. These include the immobilization support, the structural integrity and the biochemical characteristics of the biocatalyst (enzyme), and the immobilization method, all of which significantly influence the enzyme’s activity and reusability [4,5,6,7,8].
Additionally, immobilization can optimize dispersion in the reaction medium, facilitating enzyme–substrate interactions, and allow for the efficient recovery of enzymes from the reaction medium after the reaction, enabling their reuse. This process contributes to cost efficiency by reducing the need for continuous enzyme replacement, thus increasing the economic feasibility of large-scale enzymatic processes [9,10,11,12].
The immobilization method via covalent bonding, such as acid treatment followed by functionalization with glutaraldehyde (GA), provides high enzymatic stability because of the formation of robust, direct chemical bonds between the support and the biocatalyst. This approach is suitable for requiring resistance to harsh conditions (extreme pH and temperature, and organic solvents) [6,13]. However, this process involves multiple steps and chemical reagents, such as strong acids and GA, which are often toxic [14,15,16]. Furthermore, the functionalization generates chemical waste that may require additional treatment, increasing both environmental impact and operational costs.
In contrast, the in situ polymerization of polyamides, such as nylon, offers a simpler, one-step method. In this method, the enzyme is physically entrapped within the polymeric matrix during its formation. This approach eliminates the need for toxic reagents, significantly reducing waste generation and the costs associated with effluent treatment. The encapsulated enzyme remains functional and is protected within the matrix, which can enhance its operational stability in certain applications. Despite these advantages, the main limitation of this method is the potential for restricted diffusion of substrates or products through the matrix, which may impact catalytic efficiency in high-performance systems.
This review aims to analyze the use of polyamide-based materials, particularly through in situ polymerization methods, for enzyme immobilization. By highlighting the advantages of polyamide matrices, such as their ability to physically entrap enzymes without the use of toxic reagents, the review seeks to underscore their potential as eco-friendly and cost-effective immobilization platforms. Additionally, it explores the operational stability conferred to encapsulated enzymes and addresses the challenges associated with substrate and product diffusion limitations. The goal is to provide a comprehensive understanding of the current advancements, limitations, and future perspectives of polyamide-based enzyme immobilization, with a focus on its applications in sustainable biocatalysis.
2. Immobilization Techniques
Compared to metal-based catalysts, enzymes are more cost-effective and require less energy for catalytic reactions. However, in aqueous media, enzymes are often used in a single-use manner [17,18,19,20,21,22,23,24,25]. The immobilization process involves interactions between the biocatalyst and the support material (Figure 1). For effective immobilization, a comprehensive understanding of the biocatalyst surfaces and support material is essential [6,26].
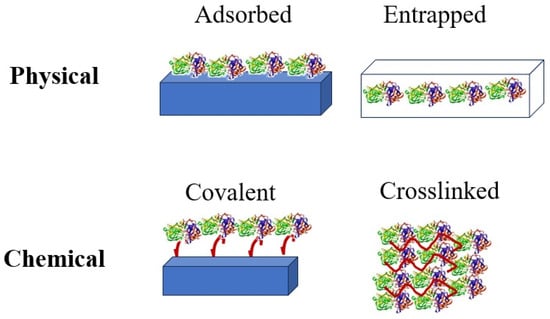
Figure 1.
Classification of enzyme immobilization methods.
Enzyme immobilization techniques commonly include adsorption onto functionalized surfaces, cross-linking with glutaraldehyde, and entrapment within chitosan beads [14,23,27,28,29,30,31]. The binding between enzymes and surfaces can occur through covalent bonding or cross-linking. Polymeric beads or glass surfaces are frequently used in industrial bioprocesses, such as packed-bed reactors [32]. Glutaraldehyde plays a key role in immobilization, particularly for cross-linking with chitosan beads, making it an important reagent in biotechnological applications [33,34].
A review of the literature highlights the diversity of immobilization techniques and the wide range of supports available for enzyme immobilization. The choice of the most suitable technique and support depends on the specific requirements of each process and application [6,35,36,37,38,39,40,41,42,43,44,45,46,47]. Moreover, to achieve optimal performance in enzymatic reactions, the selection of an appropriate supporting matrix and immobilization method is critical. Supporting materials can be categorized into three main types: inorganic materials, biopolymers, and synthetic organic polymers. Commonly used immobilization materials include ceramics, mesoporous silica, ion-exchange resins, polyurethane, magnetic nanoparticles, polyvinyl alcohol, carboxymethyl cellulose, gelatin, chitosan, and alginate [48,49,50,51,52,53].
In situ enzyme immobilization within polymeric matrices co-occurs with the polymerization of monomers, making the process simpler and more efficient by combining it into a single step. In this method, the enzyme is physically trapped within the forming polymeric matrix, without additional chemical reactions to create bonds between the biocatalyst and the support. Studies by Nyari et al. [54], Antunes et al. [55], and Ficanha et al. [50] demonstrate that in situ immobilization in polyurethane and sol–gel matrices allows the free diffusion of substrates and products, maintaining the enzyme’s catalytic activity while avoiding potential inactivation caused by covalent bonding. This approach is particularly advantageous due to its simplicity, preservation of enzymatic activity, and potential applicability in continuous bioprocess systems, where enzyme reuse is critical for the economic and operational viability of the process.
In situ immobilization occurs during the formation of the matrix. Despite the simplicity of this single-step method, it has limitations due to the conditions required for matrix formation, which must be moderate—such as an aqueous medium, controlled pH, and low temperature—to prevent enzyme denaturation. These constraints may restrict the types of matrices utilized [56].
3. Polyamide and Polyamide Enzyme Immobilization
Polyamide, commonly known as nylon, is a class of polymers characterized by a structure comprising polyethylene segments (CH2)n interspersed with peptide units (NH−CO) (Figure 2). The most prominent types of nylon include nylon-6, nylon-6,6, nylon-6,10, nylon-6,12, nylon-11, nylon-12, and nylon MXD6.
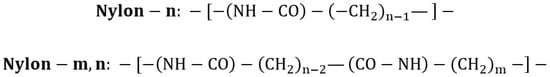
Figure 2.
Nylon structural unit.
Nylon is classified based on the number of carbon atoms between the amide groups along the polymer chain. Specifically, nylon-6, nylon-11, and nylon-12 refer to polyamides formed from a single monomer, whose structure contains the number of carbon atoms indicated by their designation.
Nylon-6,6 and nylon-6,10 macromolecules are synthesized from two base materials, with the first number in their nomenclature representing the number of carbon (C) atoms in the diamine used as a monomer, and the second indicating the number of C atoms in the corresponding diacid or dichloride. Nylon synthesis can be achieved through three main polymerization routes: (i) anionic polymerization, which employs bismide as an initiator and magnesium bromide–caprolactam as a catalyst; (ii) hydrolytic polymerization, involving the monomer ε-caprolactam in the presence of water and acetic acid; and (iii) interfacial polymerization, wherein two monomers dissolved in immiscible phases react at the interface of liquid–liquid or gas–liquid systems.
Nylon-6 is a polymer synthesized through the hydrolytic polymerization of the monomer ε-caprolactam, an organic compound containing six carbon atoms. In contrast, nylon-6,10 and nylon-6,6 are produced via condensation polymerization between dicarboxylic acids (or their dichlorides) and diamines. Nylon-6,10 is synthesized through interfacial condensation polymerization, occurring at the interface between an aqueous phase containing 1,6-hexane diamine and water, and an organic phase containing sebacoyl chloride and hexane [57]. Similarly, nylon-6,6 is obtained through interfacial polymerization between 1,6-hexane diamine and adipoyl chloride.
Polyamide nylon-6 stands out as a material of significant interest for use as a polymeric support in catalyst immobilization. This interest arises from its broad commercial availability, non-toxicity, and simple for modification and/or functionalization. The chemical structure of nylon-6 mimics the macromolecules of natural polypeptide proteins, making it a safe material that can be hydrolyzed into amino acid-like fragments. Nylon-6 and nylon-6,6 exhibit similar properties. However, nylon-6 is generally about 30% less expensive than nylon-6,6, making it an attractive option for various industrial and biotechnological applications [58].
According to Ben-Othman and Rinken [59], nylon is considered a great support for processes in food industries due to its inertness, robustness, and availability. However, the covalent attachment of enzymes to nylon often requires acid treatment, as the polyamide structure is chemically inactive [60].
Nylon modification is typically performed using acid treatment followed by GA functionalization. An alternative approach involves enzymatic treatment, wherein amino (–NH2) and carboxyl (–COOH) groups are generated on the polyamide fiber through the cleavage of amide bonds (–CONH–). Enzymatic hydrolysis with protease introduces reactive functional groups, without compromising the fiber’s strength. These groups can interact with enzymes through specific coupling reagents like GA, forming a Schiff base [61]. Table 1 provides an overview of studies employing covalent immobilization techniques on nylon-based materials.

Table 1.
Examples of immobilization of enzymes on nylon supports: methods, conditions, and applications.
One of the significant challenges in enzyme immobilization within polyamide matrices is the restricted diffusion of substrates and products. This limitation arises due to the dense and semi-crystalline structure of polyamide polymers, which can hinder the free movement of molecules within the matrix. The degree of restriction depends on some factors, such as the polymer’s porosity, size of the substrate or product molecules, and the distribution of enzymes within the matrix. When substrates are unable to efficiently access the active sites of immobilized enzymes, or when products are trapped within the matrix, the overall catalytic efficiency decreases, particularly in high-performance or continuous reaction systems.
To address these diffusion limitations, several strategies can be employed, such as (i) Increasing Matrix Porosity: Modifying the polymer synthesis process to create porous polyamide matrices can improve molecular diffusion; (ii) Surface Functionalization: Enhancing the surface properties of the matrix by functionalizing it with hydrophilic groups can promote substrate transport, especially for polar molecules; (iii) Co-immobilization Techniques: Combining polyamide supports with other materials, such as mesoporous silica or hydrogels, can create hybrid systems that balance structural stability with improved diffusivity; and (iv) Optimizing Reaction Conditions: Adjusting parameters like temperature, pH, and substrate concentration can improve diffusion by reducing viscosity and enhancing the mobility of molecules within the reaction medium. Future research should focus on tailoring polyamide matrix properties to meet the specific requirements of diverse enzymatic processes. By overcoming diffusion challenges, these materials can achieve their full potential as supports for sustainable and efficient biocatalytic systems [67,68,69].
Alatawi et al. [58] chemically modified nylon-6 fibers to functionalize them through a graft copolymerization reaction. The material was employed as a support for immobilizing the horseradish peroxidase (HRP) enzyme. The hydrazide-containing fibers were activated with GA, enabling the coupling of HRP protein macromolecules. The optimal pH of the immobilized HRP enzyme shifted to a higher value compared to the free enzyme. The residual activity of the immobilized enzyme was higher at elevated temperatures in comparison with the enzyme in free form, indicating enhanced thermal stability after immobilization. Furthermore, kinetic studies revealed a reduced enzyme-substrate affinity post-immobilization. Nevertheless, the immobilized HRP retained more than 89% of its original activity and went up to 10 reuse cycles, representing a promising outcome for potential applications in environmental and biotechnological fields.
A commercial extracellular invertase from Saccharomyces cerevisiae, immobilized via covalent bonding onto novel nylon-6 microspheres using GA, was applied in a fixed-bed tubular reactor to evaluate its performance in the continuous hydrolysis of sucrose [70]. Oliveira et al. [71] immobilized a commercial pectinolytic enzyme through non-covalent adsorption in nylon-6 microparticles. The support was evaluated with and without magnetic properties. Compared to the free enzyme, the immobilized systems exhibited enhanced activity, greater substrate affinity, faster catalytic performance, and improved resistance to inhibition. Additionally, the immobilized enzyme demonstrated reusability, maintaining effectiveness for up to three cycles in the complete clarification of rosé must.
Laccase obtained from Trametes hirsuta was immobilized on nylon-6,6 and utilized for continuous textile effluent decolorization, demonstrating its applicability in a membrane reactor [72]. Similarly, Jankowska et al. [73] developed electrospun-modified nylon-6 fibers for laccase immobilization. These immobilized enzymes effectively decolorized selected dyes, achieving 63% efficiency for the azo dye Reactive Black 5 and 77% for the anthraquinone dye Reactive Blue 4. Remarkably, the catalyst showed over 60% and 70% of its activity after 10 consecutive catalytic cycles for the adsorbed and covalently bound forms, respectively. These findings highlight the potential scalability and effectiveness of these systems for removing phenolic pollutants from wastewater.
Maryšková et al. [74] immobilized laccase from Trametes versicolor onto a polyamide/polyethyleneimine matrix, which demonstrated high efficiency in degrading a mixture of endocrine-disrupting chemicals present in real wastewater. This immobilization system facilitated the effective removal of these pollutants and showed potential for practical application in wastewater treatment processes.
The immobilization of pectinases on nylon was also reported by Ben-Othman and Rinken [59], who utilized nylon 6,6 in the form of pellets and fibers as support for immobilizing the pectinase Pectinex® Ultra Tropical. The nylon was activated using dimethyl sulfate (DMS) and treated with a GA solution before incubation with the enzyme solution. The immobilization efficiency per gram of support was higher for the fibers compared to the pellets. However, in both forms, fibers and pellets retained 40% of their activity over five consecutive cycles.
In a study from another research group, Laccase from Trametes versicolor was immobilized either by adsorption onto pre-fabricated polyamide 4 (PA4) microparticles or through in situ physical entrapment during synthesis. The newly synthesized PA4-laccase complexes were successfully applied in dye decolorization, demonstrating the potential for effluent remediation. All complexes achieved near-complete decolorization (~100%) of positively charged dyes within 15 min. In a second decolorization cycle, efficiency decreased by only 5–10%, highlighting the reusability potential of the PA4-laccase conjugates [75].
4. Potential Applications and Future Outcomes
Enzyme immobilization, particularly using polyamide-based materials, presents vast potential for industrial and biotechnological applications. The versatility of immobilized enzymes, combined with the inherent stability and operational resilience imparted by polyamide matrices, positions this technology as a sustainable alternative in processes requiring robust catalytic performance. From pharmaceutical synthesis to food production and environmental remediation, immobilized enzymes offer improved efficiency, reduced waste, and enhanced cost-effectiveness through enzyme recovery and reuse [76].
The simplicity of in situ polymerization for polyamides, such as nylon, further enhances the appeal of these materials for enzyme immobilization. By eliminating toxic reagents and reducing process complexity, this method aligns with the principles of green chemistry, providing an eco-friendly solution for industries transitioning towards sustainable practices. Additionally, the protective environment afforded by polyamide matrices ensures enzyme functionality under diverse operational conditions, enabling applications in scenarios with extreme pH, temperature, or solvent presence.
Looking ahead, advancements in material design and immobilization techniques could address existing challenges, such as substrate diffusion limitations within polyamide matrices. Optimizing polymer structures to improve permeability while maintaining enzyme stability could unlock broader applications, particularly in high-performance catalytic systems. Future research may also explore novel polyamide composites and hybrid materials that enhance enzyme compatibility and activity.
Future research could focus on developing polyamide composites specifically designed for immobilization, incorporating surface modifications or functional additives to enhance substrate diffusion and enzyme stability. This approach would improve the applicability of polyamides in diverse catalytic environments. Leveraging nanotechnology, such as embedding nanoparticles within polyamide matrices, could enhance surface area, catalytic efficiency, and substrate accessibility. This innovation may also enable multifunctional composites capable of combining enzyme immobilization with additional properties, such as magnetic recovery.
For specific applications, research can investigate immobilized pectinases on polyamide supports for their use in clarifying wines and fruit juices. This application could benefit from enhanced enzyme stability and reusability, reducing costs and waste in the beverage industry. Immobilized lipases could be explored for the enzymatic synthesis of esters, which are valuable in flavor, fragrance, and biodiesel industries. The use of polyamide supports may improve operational stability in non-aqueous systems, ensuring consistent production efficiency.
As industries increasingly prioritize sustainability and economic efficiency, enzyme immobilization using polyamide matrices is poised to play a pivotal role in reshaping biotechnological processes. Its continued development promises innovative solutions that balance environmental responsibility with industrial demands, driving progress in fields ranging from renewable energy production to precision medicine.
5. Conclusions
Enzyme immobilization represents a transformative approach for enhancing the efficiency, stability, and reusability of biocatalysts in diverse industrial and biotechnological applications. Among the various immobilization techniques, the use of polyamide-based materials, particularly through in situ polymerization, stands out for its simplicity, eco-friendliness, and cost-effectiveness. Polyamide matrices not only provide a protective environment for enzymes, enhancing their operational stability but also eliminate the need for toxic reagents, aligning with the principles of green chemistry and sustainable development.
In situ enzyme immobilization represents a promising advancement in biocatalyst technology by integrating enzyme fixation directly into the material processing phase. This innovative approach simplifies the immobilization process and improves enzyme activity, stability, and uniform distribution on the support. By reducing production complexity, time, and cost, in situ immobilization paves the way for more efficient and sustainable applications in biotechnology, environmental science, and the chemical industry. Nylon as a support material highlights its potential to drive the development of robust enzyme–polymer systems tailored to meet evolving industrial needs, paving the way for wider adoption and new applications in the future.
Despite the promising advantages, challenges such as substrate and product diffusion limitations within polyamide matrices remain critical areas for further exploration. Advances in material science, including the development of tailored polyamide composites and hybrid supports, hold potential for overcoming these limitations and optimizing catalytic performance in high-demand processes.
This review highlights the significant progress achieved in the field of enzyme immobilization and underscores the immense potential of polyamide-based systems to address industrial challenges. Future research focusing on material innovation and process optimization is essential to unlock the full capabilities of this technology, ensuring its widespread adoption in sectors ranging from food production to pharmaceuticals and environmental management. As industries continue to seek sustainable and efficient solutions, polyamide-based enzyme immobilization can be the future of biocatalysis.
Author Contributions
Conceptualization, C.E.D.O. and B.M.S.P.; methodology, C.E.D.O. and B.M.S.P.; validation, C.E.D.O. and B.M.S.P.; investigation, C.E.D.O. and B.M.S.P.; resources, L.D.V.; data curation, L.D.V., R.M.D. and M.V.T.; writing—original draft preparation, C.E.D.O. and B.M.S.P.; writing—review and editing, C.E.D.O., B.M.S.P., L.D.V., R.M.D. and M.V.T.; supervision, R.M.D. and M.V.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) (project number 151580/2024-3).
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors thanks CNPq, URI and UFSM for the support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- El-Shishtawy, R.M.; Ahmed, N.S.E.; Almulaiky, Y.Q. Immobilization of Catalase on Chitosan/Zno and Chitosan/Zno/Fe2o3 Nanocomposites: A Comparative Study. Catalysts 2021, 11, 820. [Google Scholar] [CrossRef]
- Kuepethkaew, S.; Zhang, Y.; Kishimura, H.; Kumagai, Y.; Simpson, B.K.; Benjakul, S.; Damodaran, S.; Klomklao, S. Enzymological Characteristics of Pepsinogens and Pepsins Purified from Lizardfish (Saurida micropectoralis) Stomach. Food Chem. 2022, 366, 130532. [Google Scholar] [CrossRef] [PubMed]
- Romero-Fernández, M.; Moreno-Perez, S.; Martins de Oliveira, S.; Santamaría, R.I.; Guisan, J.M.; Rocha-Martin, J. Preparation of a Robust Immobilized Biocatalyst of β-1,4-Endoxylanase by Surface Coating with Polymers for Production of Xylooligosaccharides from Different Xylan Sources. New Biotechnol. 2018, 44, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Cipolatti, E.P.; Valério, A.; Henriques, R.O.; Cerqueira Pinto, M.C.; Lorente, G.F.; Manoel, E.A.; Guisán, J.M.; Ninow, J.L.; de Oliveira, D.; Pessela, B.C. Production of New Nanobiocatalysts via Immobilization of Lipase B from C. Antarctica on Polyurethane Nanosupports for Application on Food and Pharmaceutical Industries. Int. J. Biol. Macromol. 2020, 165, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Patra, T.; Gupta, M.K. Evaluation of Sodium Alginate for Encapsulation-Vitrification of Testicular Leydig Cells. Int. J. Biol. Macromol. 2020, 153, 128–137. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Virgen-Ortíz, J.J.; dos Santos, J.C.S.; Berenguer-Murcia, Á.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Immobilization of Lipases on Hydrophobic Supports: Immobilization Mechanism, Advantages, Problems, and Solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef]
- Mehdi, W.A.; Mehde, A.A.; Özacar, M.; Özacar, Z. Characterization and Immobilization of Protease and Lipase on Chitin-Starch Material as a Novel Matrix. Int. J. Biol. Macromol. 2018, 117, 947–958. [Google Scholar] [CrossRef]
- Katsimpouras, C.; Stephanopoulos, G. Enzymes in Biotechnology: Critical Platform Technologies for Bioprocess Development. Curr. Opin. Biotechnol. 2021, 69, 91–102. [Google Scholar] [CrossRef]
- Rehm, F.B.H.; Chen, S.; Rehm, B.H.A. Enzyme Engineering for in Situ Immobilization. Molecules 2016, 21, 1370. [Google Scholar] [CrossRef]
- Zhang, C.; Dong, X.; Guo, Z.; Sun, Y. Remarkably Enhanced Activity and Substrate Affinity of Lipase Covalently Bonded on Zwitterionic Polymer-Grafted Silica Nanoparticles. J. Colloid Interface Sci. 2018, 519, 145–153. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Sun, Y. Lipase Immobilized to a Short Alkyl Chain-Containing Zwitterionic Polymer Grafted on Silica Nanoparticles: Moderate Activation and Significant Increase of Thermal Stability. Biochem. Eng. J. 2019, 146, 124–131. [Google Scholar] [CrossRef]
- Facin, B.R.; Valério, A.; de Oliveira, D.; Oliveira, J.V. Developing an Immobilized Low-Cost Biocatalyst for FAME Synthesis. Biocatal. Agric. Biotechnol. 2020, 29, 101752. [Google Scholar] [CrossRef]
- Wu, P.; Li, G.; He, Y.; Luo, D.; Li, L.; Guo, J.; Ding, P.; Yang, F. High-Efficient and Sustainable Biodegradation of Microcystin-LR Using Sphingopyxis sp. YF1 Immobilized Fe3O4@chitosan. Colloids Surfaces B Biointerfaces 2020, 185, 110633. [Google Scholar] [CrossRef]
- Zaitsev, S.Y.; Savina, A.A.; Zaitsev, I.S. Biochemical Aspects of Lipase Immobilization at Polysaccharides for Biotechnology. Adv. Colloid Interface Sci. 2019, 272, 102016. [Google Scholar] [CrossRef]
- Kamel Ariffin, M.F.; Idris, A. Fe2O3/Chitosan Coated Superparamagnetic Nanoparticles Supporting Lipase Enzyme from Candida antarctica for Microwave Assisted Biodiesel Production. Renew. Energy 2022, 185, 1362–1375. [Google Scholar] [CrossRef]
- Han, J.; Wang, L.; Wang, Y.; Dong, J.; Tang, X.; Ni, L.; Wang, L. Preparation and Characterization of Fe3O4-NH2@4-Arm-PEG-NH2, a Novel Magnetic Four-Arm Polymer-Nanoparticle Composite for Cellulase Immobilization. Biochem. Eng. J. 2018, 130, 90–98. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Zhang, Z.F.; Kaewlaoyoong, A.; Huang, J.T.; Cheruiyot, N.K.; Lin, C.; Huang, H.C. Improved Bioremediation of PCDD/Fs Contaminated Soil by Mycelium-Free Liquids Induced by Agro-Industrial Residues. Bioresour. Technol. Rep. 2023, 22, 101435. [Google Scholar] [CrossRef]
- Zhou, L.; Xie, X.; Wu, T.; Chen, M.; Yao, Q.; Zhu, H.; Zou, W. Compound Enzymatic Hydrolysis of Feather Waste to Improve the Nutritional Value. Biomass Convers. Biorefin. 2020, 12, 287–298. [Google Scholar] [CrossRef]
- Ramos, K.R.M.; Valdehuesa, K.N.G.; Nisola, G.M.; Lee, W.K.; Chung, W.J. Identification and Characterization of a Thermostable Endolytic β-Agarase Aga2 from a Newly Isolated Marine Agarolytic Bacteria Cellulophaga omnivescoria W5C. New Biotechnol. 2018, 40, 261–267. [Google Scholar] [CrossRef]
- Amini, Z.; Ilham, Z.; Ong, H.C.; Mazaheri, H.; Chen, W.H. State of the Art and Prospective of Lipase-Catalyzed Transesterification Reaction for Biodiesel Production. Energy Convers. Manag. 2017, 141, 339–353. [Google Scholar] [CrossRef]
- Costa-Silva, T.A.; Carvalho, A.K.F.; Souza, C.R.F.; De Castro, H.F.; Said, S.; Oliveira, W.P. Enzymatic Transesterification of Coconut Oil Using Chitosan-Immobilized Lipase Produced by Fluidized-Bed System. Energy Fuels 2017, 31, 12209–12216. [Google Scholar] [CrossRef]
- Binhayeeding, N.; Klomklao, S.; Prasertsan, P.; Sangkharak, K. Improvement of Biodiesel Production Using Waste Cooking Oil and Applying Single and Mixed Immobilised Lipases on Polyhydroxyalkanoate. Renew. Energy 2020, 162, 1819–1827. [Google Scholar] [CrossRef]
- Badgujar, V.C.; Badgujar, K.C.; Yeole, P.M.; Bhanage, B.M. Investigation of Effect of Ultrasound on Immobilized C. Rugosa Lipase: Synthesis of Biomass Based Furfuryl Derivative and Green Metrics Evaluation Study. Enzym. Microb. Technol. 2021, 144, 109738. [Google Scholar] [CrossRef] [PubMed]
- Sarno, M.; Iuliano, M.; Polichetti, M.; Ciambelli, P. High Activity and Selectivity Immobilized Lipase on Fe3O4 Nanoparticles for Banana Flavour Synthesis. Process Biochem. 2017, 56, 98–108. [Google Scholar] [CrossRef]
- Ficanha, A.M.M.; Oro, C.E.D.; Franceschi, E.; Dallago, R.M.; Mignoni, M.L. Evaluation of Different Ionic Liquids as Additives in the Immobilization of Lipase CAL B by Sol-Gel Technique. Appl. Biochem. Biotechnol. 2021, 193, 2162–2181. [Google Scholar] [CrossRef] [PubMed]
- Quayson, E.; Amoah, J.; Hama, S.; Kondo, A.; Ogino, C. Immobilized Lipases for Biodiesel Production: Current and Future Greening Opportunities. Renew. Sustain. Energy Rev. 2020, 134, 110355. [Google Scholar] [CrossRef]
- Wang, R.; Ni, N.; Zhang, Q.; Duan, X.; He, Q.; Chi, Y. long Adsorption and Separation of Flavonoid Aglycones and Flavonol Aglycones by Using Zr(IV) Immobilized Collagen Fiber Adsorbent as Column Packing Material. Sep. Purif. Technol. 2022, 289, 120684. [Google Scholar] [CrossRef]
- Konovalova, V.; Guzikevich, K.; Burban, A.; Kujawski, W.; Jarzynka, K.; Kujawa, J. Enhanced Starch Hydrolysis Using α-Amylase Immobilized on Cellulose Ultrafiltration Affinity Membrane. Carbohydr. Polym. 2016, 152, 710–717. [Google Scholar] [CrossRef]
- Cortés-Ríos, J.; Valdivia-Olivares, R.Y.; Álvarez-Figueroa, M.J.; Rodriguez-Fernandez, M.; González-Aramundiz, J.V. Optimization of Physicochemical Properties of Novel Multiple Nanoemulsion for Complex Food Matrices through Iterative Mathematical Modelling. J. Food Eng. 2020, 276, 109883. [Google Scholar] [CrossRef]
- Urrutia, P.; Bernal, C.; Wilson, L.; Illanes, A. Use of Chitosan Heterofunctionality for Enzyme Immobilization: β-Galactosidase Immobilization for Galacto-Oligosaccharide Synthesis. Int. J. Biol. Macromol. 2018, 116, 182–193. [Google Scholar] [CrossRef]
- Chiou, S.H.; Wu, W.T. Immobilization of Candida rugosa Lipase on Chitosan with Activation of the Hydroxyl Groups. Biomaterials 2004, 25, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Işık, M. High Stability of Immobilized Acetylcholinesterase on Chitosan Beads. ChemistrySelect 2020, 5, 4623–4627. [Google Scholar] [CrossRef]
- Mendes, A.A.; De Castro, H.F.; De S. Rodrigues, D.; Adriano, W.S.; Tardioli, P.W.; Mammarella, E.J.; De C. Giordano, R.; De L. C. Giordano, R. Multipoint Covalent Immobilization of Lipase on Chitosan Hybrid Hydrogels: Influence of the Polyelectrolyte Complex Type and Chemical Modification on the Catalytic Properties of the Biocatalysts. J. Ind. Microbiol. Biotechnol. 2011, 38, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Adriano, W.S.; Mendonça, D.B.; Rodrigues, D.S.; Mammarella, E.J.; Giordano, R.L.C. Improving the Properties of Chitosan as Support for the Covalent Multipoint Immobilization of Chymotrypsin. Biomacromolecules 2008, 9, 2170–2179. [Google Scholar] [CrossRef] [PubMed]
- Filho, D.G.; Silva, A.G.; Guidini, C.Z. Lipases: Sources, Immobilization Methods, and Industrial Applications. Appl. Microbiol. Biotechnol. 2019, 103, 7399–7423. [Google Scholar] [CrossRef]
- Moazeni, F.; Chen, Y.C.; Zhang, G. Enzymatic Transesterification for Biodiesel Production from Used Cooking Oil, a Review. J. Clean. Prod. 2019, 216, 117–128. [Google Scholar] [CrossRef]
- Silva, J.d.C.; de França, P.R.L.; Converti, A.; Porto, T.S. Kinetic and Thermodynamic Characterization of a Novel Aspergillus Aculeatus URM4953 Polygalacturonase. Comparison of Free and Calcium Alginate-Immobilized Enzyme. Process Biochem. 2018, 74, 61–70. [Google Scholar] [CrossRef]
- Quilles, J.C.J.; Brito, R.R.; Borges, J.P.; Aragon, C.C.; Fernandez-Lorente, G.; Bocchini-Martins, D.A.; Gomes, E.; da Silva, R.; Boscolo, M.; Guisan, J.M. Modulation of the Activity and Selectivity of the Immobilized Lipases by Surfactants and Solvents. Biochem. Eng. J. 2015, 93, 274–280. [Google Scholar] [CrossRef]
- Kharazmi, S.; Taheri-Kafrani, A.; Soozanipour, A.; Nasrollahzadeh, M.; Varma, R.S. Xylanase Immobilization onto Trichlorotriazine-Functionalized Polyethylene Glycol Grafted Magnetic Nanoparticles: A Thermostable and Robust Nanobiocatalyst for Fruit Juice Clarification. Int. J. Biol. Macromol. 2020, 163, 402–413. [Google Scholar] [CrossRef]
- Reichardt, C.; Utgenannt, S.; Stahmann, K.P.; Klepel, O.; Barig, S. Highly Stable Adsorptive and Covalent Immobilization of Thermomyces Lanuginosus Lipase on Tailor-Made Porous Carbon Material. Biochem. Eng. J. 2018, 138, 63–73. [Google Scholar] [CrossRef]
- de Melo, R.R.; de Lima, E.A.; Persinoti, G.F.; Vieira, P.S.; de Sousa, A.S.; Zanphorlin, L.M.; de Giuseppe, P.O.; Ruller, R.; Murakami, M.T. Identification of a Cold-Adapted and Metal-Stimulated β-1,4-Glucanase with Potential Use in the Extraction of Bioactive Compounds from Plants. Int. J. Biol. Macromol. 2021, 166, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Feng, Y.; Wang, G.; Wang, Z.; Bilal, M.; Lv, H.; Jia, S.; Cui, J. Production and Use of Immobilized Lipases in/on Nanomaterials: A Review from the Waste to Biodiesel Production. Int. J. Biol. Macromol. 2020, 152, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Ureta, M.M.; Martins, G.N.; Figueira, O.; Pires, P.F.; Castilho, P.C.; Gomez-Zavaglia, A. Recent Advances in β-Galactosidase and Fructosyltransferase Immobilization Technology. Crit. Rev. Food Sci. Nutr. 2020, 61, 2659–2690. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Goomber, S.; Kaur, J. Engineering Lipases for Temperature Adaptation: Structure Function Correlation. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 140261. [Google Scholar] [CrossRef]
- Karishma, S.; Saravanan, A.; Deivayanai, V.C.; Ajithkumar, U.; Yaashikaa, P.R.; Vickram, A.S. Emerging Strategies for Enhancing Microbial Degradation of Petroleum Hydrocarbons: Prospects and Challenges. Bioresour. Technol. Rep. 2024, 26, 101866. [Google Scholar] [CrossRef]
- Cipolatti, E.P.; Valério, A.; Henriques, R.O.; Moritz, D.E.; Ninow, J.L.; Freire, D.M.G.; Manoel, E.A.; Fernandez-Lafuente, R.; De Oliveira, D. Nanomaterials for Biocatalyst Immobilization-State of the Art and Future Trends. RSC Adv. 2016, 6, 104675–104692. [Google Scholar] [CrossRef]
- Liu, S.; Bilal, M.; Rizwan, K.; Gul, I.; Rasheed, T.; Iqbal, H.M.N. Smart Chemistry of Enzyme Immobilization Using Various Support Matrices—A Review. Int. J. Biol. Macromol. 2021, 190, 396–408. [Google Scholar] [CrossRef]
- Guo, R.; Zheng, X.; Wang, Y.; Yang, Y.; Ma, Y.; Zou, D.; Liu, Y. Optimization of Cellulase Immobilization with Sodium Alginate-Polyethylene for Enhancement of Enzymatic Hydrolysis of Microcrystalline Cellulose Using Response Surface Methodology. Appl. Biochem. Biotechnol. 2021, 193, 2043–2060. [Google Scholar] [CrossRef]
- Ficanha, A.M.M.; Antunes, A.; Oro, C.E.D.; Franceschi, E.; Dallago, R.M.; Mignoni, M.L. Immobilization of Lipase CALB in Organically Modified Silica. Biointerface Res. Appl. Chem. 2021, 11, 7814–7825. [Google Scholar] [CrossRef]
- Ficanha, A.M.M.; Antunes, A.; Oro, C.E.D.; Valduga, A.T.; Matuella Moreira, C.; Dallago, R.M.; Mignoni, M. Study of Drying Conditions of the Aerogel Obtained by the Sol-Gel Technique for Immobilization in Situ of Lipase Candida antarctica B. Ind. Biotechnol. 2019, 15, 350–356. [Google Scholar] [CrossRef]
- Bustamante-Vargas, C.E.; De Oliveira, D.; Nyari, N.L.D.; Valduga, E.; Soares, M.B.A.; Backes, G.T.; Dallago, R.M. In Situ Immobilization of Commercial Pectinase in Rigid Polyurethane Foam and Application in the Hydrolysis of Pectic Oligosaccharides. J. Mol. Catal. B Enzym. 2015, 122, 35–43. [Google Scholar] [CrossRef]
- Gaio, I.; Oro, C.E.D.; Graboski, A.M.; Bustamante-Vargas, C.E.; Tres, M.V.; Junges, A.; Dallago, R.M.; Valduga, E.; Furigo, A., Jr. Liquefied Petroleum Gas as Solvent Medium for the Treatment of Immobilized Pectinases. Biocatal. Agric. Biotechnol. 2017, 11, 108–115. [Google Scholar] [CrossRef]
- Babaki, M.; Yousefi, M.; Habibi, Z.; Brask, J.; Mohammadi, M. Preparation of Highly Reusable Biocatalysts by Immobilization of Lipases on Epoxy-Functionalized Silica for Production of Biodiesel from Canola Oil. Biochem. Eng. J. 2015, 101, 23–31. [Google Scholar] [CrossRef]
- Nyari, N.L.D.; Fernandes, I.A.; Bustamante-Vargas, C.E.; Steffens, C.; De Oliveira, D.; Zeni, J.; Rigo, E.; Dallago, R.M. In Situ Immobilization of Candida antarctica B Lipase in Polyurethane Foam Support. J. Mol. Catal. B Enzym. 2016, 124, 52–61. [Google Scholar] [CrossRef]
- Antunes, A.; Oro, C.E.D.; Denti, A.F.; da Silva, L.M.; Ficanha, A.M.M.; Mulinari, J.; Venquiaruto, L.D.; Zeni, J.; Mignoni, M.L.; Dallago, R.M. In Situ CALB Immobilization in Xerogel and Sonogel Employing TMOS as Silica Precursor and Polyethylene Glycol as Additive. Processes 2024, 12, 2411. [Google Scholar] [CrossRef]
- Sicard, C. In Situ Enzyme Immobilization by Covalent Organic Frameworks. Angew. Chem. Int. Ed. 2023, 62, e202213405. [Google Scholar] [CrossRef]
- Pahujani, S.; Kanwar, S.S.; Chauhan, G.; Gupta, R. Glutaraldehyde Activation of Polymer Nylon-6 for Lipase Immobilization: Enzyme Characteristics and Stability. Bioresour. Technol. 2008, 99, 2566–2570. [Google Scholar] [CrossRef]
- Alatawi, F.S.; Elsayed, N.H.; Monier, M. Immobilization of Horseradish Peroxidase on Modified Nylon-6 Fibers. ChemistrySelect 2020, 5, 6841–6850. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Rinken, T. Immobilization of Pectinolytic Enzymes on Nylon 6/6 Carriers. Appl. Sci. 2021, 11, 4591. [Google Scholar] [CrossRef]
- Rather, A.H.; Khan, R.S.; Wani, T.U.; Beigh, M.A.; Sheikh, F.A. Overview on Immobilization of Enzymes on Synthetic Polymeric Nanofibers Fabricated by Electrospinning. Biotechnol. Bioeng. 2022, 119, 9–33. [Google Scholar] [CrossRef]
- Song, J.E.; Kim, H.R.; Lee, S.H. Effect of Enzymatic Hydrolysis on Developing Support of Polyamide Woven Fabric for Enzyme Immobilization. Text. Res. J. 2019, 89, 1345–1360. [Google Scholar] [CrossRef]
- Zaidi, A.; Gainer, J.L.; Carta, G.; Mrani, A.; Kadiri, T.; Belarbi, Y.; Mir, A. Esterification of Fatty Acids Using Nylon-Immobilized Lipase in n-Hexane: Kinetic Parameters and Chain-Length Effects. J. Biotechnol. 2002, 93, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Bruno, L.M.; Saavedra Pinto, G.A.; Ferreira De Castro, H.; Jose’luiz De Lima-Filho, J.; Henrique De Magalhães, E.; Melo, M. Variables That Affect Immobilization of Mucor Miehei Lipase on Nylon Membrane. World J. Microbiol. Biotechnol. 2004, 20, 371–375. [Google Scholar] [CrossRef]
- Shukla, S.; Saxena, S.; Thakur, J.; Gupta, R. Immobilization of Polygalacturonase from Aspergilus Niger onto Glutaraldehyde Activated Nylon-6 and Its Application in Apple Juice Clarification. Acta Aliment. 2010, 39, 277–292. [Google Scholar] [CrossRef]
- Zeng, H.Y.; Liu, X.Y.; He, P.; Peng, D.H.; Fan, B.; Xia, K. Lipase Adsorption on Woven Nylon-6 Membrane: Optimization, Kinetic and Thermodynamic Analyses. Biocatal. Biotransform. 2014, 32, 188–197. [Google Scholar] [CrossRef]
- Halin, N.I.A.; Al-Khatib, M.F.R.; Salleh, H.M.; Nasef, M.M. Immobilization of Candida rugosa Lipase on Aminated Polyvinyl Benzyl Chloride-Grafted Nylon-6 Microfibers. Bull. Chem. React. Eng. Catal. 2019, 14, 369–379. [Google Scholar] [CrossRef]
- Laird, M.; Yokoyama, J.; Carcel, C.; Unno, M.; Bartlett, J.R.; Wong Chi Man, M. Sol–Gel Processing of Polyhedral Oligomeric Silsesquioxanes: Nanohybrid Materials Incorporating T8 and T10 Cages. J. Sol-Gel Sci. Technol. 2020, 95, 760–770. [Google Scholar] [CrossRef]
- Coelho, A.L.S.; Orlandelli, R.C. Immobilized Microbial Lipases in the Food Industry: A Systematic Literature Review. Crit. Rev. Food Sci. Nutr. 2020, 61, 1689–1703. [Google Scholar] [CrossRef]
- Ambrogio, M.W.; Thomas, C.R.; Zhao, Y.L.; Zink, J.I.; Stoddart, J.F. Mechanized Silica Nanoparticles: A New Frontier in Theranostic Nanomedicine. Acc. Chem. Res. 2011, 44, 903–913. [Google Scholar] [CrossRef]
- Amaya-Delgado, L.; Hidalgo-Lara, M.E.; Montes-Horcasitas, M.C. Hydrolysis of Sucrose by Invertase Immobilized on Nylon-6 Microbeads. Food Chem. 2006, 99, 299–304. [Google Scholar] [CrossRef]
- Oliveira, S.C.; Dencheva, N.V.; Denchev, Z.Z. Immobilization of Enological Pectinase on Magnetic Sensitive Polyamide Microparticles for Wine Clarification. Foods 2024, 13, 420. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Silva, C.J.; Zille, A.; Guebitz, G.M.; Cavaco-Paulo, A. Laccase Immobilization on Enzymatically Functionalized Polyamide 6,6 Fibres. Enzyme Microb. Technol. 2007, 41, 867–875. [Google Scholar] [CrossRef]
- Jankowska, K.; Grzywaczyk, A.; Piasecki, A.; Kijeńska-Gawrońska, E.; Nguyen, L.N.; Zdarta, J.; Nghiem, L.D.; Pinelo, M.; Jesionowski, T. Electrospun Biosystems Made of Nylon 6 and Laccase and Its Application in Dyes Removal. Environ. Technol. Innov. 2021, 21, 101332. [Google Scholar] [CrossRef]
- Maryšková, M.; Schaabová, M.; Tománková, H.; Novotnỳ, V.; Rysová, M. Wastewater Treatment by Novel Polyamide/Polyethylenimine Nanofibers with Immobilized Laccase. Water 2020, 12, 588. [Google Scholar] [CrossRef]
- Dencheva, N.; Braz, J.; Scheibel, D.; Malfois, M.; Denchev, Z.; Gitsov, I. Polymer-Assisted Biocatalysis: Polyamide 4 Microparticles as Promising Carriers of Enzymatic Function. Catalysts 2020, 10, 767. [Google Scholar] [CrossRef]
- Maghraby, Y.R.; El-Shabasy, R.M.; Ibrahim, A.H.; Azzazy, H.M.E.S. Enzyme Immobilization Technologies and Industrial Applications. ACS Omega 2023, 8, 5184–5196. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).