Abstract
There are various organic compounds that can be utilized in the organic Rankine cycle as working fluids. The selection of a suitable working fluid is complicated due to the large number of options and factors affecting the choice, such as thermodynamic properties, environmental impact, cost, etc. This study evaluates seven different pure organic compounds and twenty-one of their binary zeotropic mixtures as potential working fluids for the organic Rankine cycle powered by a heat source at 200 °C. The pure organic fluids show higher exergy efficiency, higher specific net power output, and lower heat exchange area requirements compared to the binary mixtures. Among the pure fluids, RE347mcc performs the best in terms of exergy efficiency, followed by neopentane, isopentane, and pentane. Cyclopentane exhibits the highest power production capacity per unit mass flow rate of the working fluid. Two mixtures, pentane/Novec 649 and cyclopentane/Novec 649, showed significantly higher exergy efficiency than their individual components, but at significantly lower specific power production capacity. The study presents an interesting trade-off between exergy efficiency and heat exchange area, indicating that a small increase in exergy efficiency can lead to a large decrease in the required heat exchange area. The outcomes of this study can help in selecting suitable working fluids for ORC operation with a heat source at 200 °C.
1. Introduction
The demand for energy is rising, and thermal energy of lesser grade has gained importance in meeting this demand. Conventional cycles like the steam Rankine cycle exhibit limited efficiency when operating at low temperatures. As a result, alternative thermodynamic cycles have emerged to capture and transform this energy into mechanical and electrical power. One of them is organic Rankine cycle (ORC). The ORC is particularly noteworthy for its reliability, wide applicability, uncomplicated structure, intuitive working principle, and minimal maintenance needs [1], making it the most promising choice. The ORC functions similarly to the steam Rankine cycle, but employs organic working fluids with lower boiling points than water. This characteristic allows it to extract energy from low temperatures effectively.
The versatility of organic Rankine cycles (ORCs) is due to their ability to utilize a diverse range of organic fluids; this lets them effectively harness various heat sources. Nonetheless, this advantageous feature comes with added complexity in optimizing these systems. The choice of the working fluid and the cycle layout is of utmost importance because using an inappropriate working fluid will lead to an underwhelming cycle performance [2]. Furthermore, the choice of the working fluid for an ORC is affected by factors such as the cost and market availability of organic fluids. The expense of the working fluid can account for up to 10% of the overall plant cost [3]. Hydrocarbons, in general, tend to be more cost effective than other options, and are readily available.
Research work has been published in recent years proposing employing binary mixtures as the working fluids in the organic Rankine cycle (ORC) in place of pure fluids. For pure fluids, temperature remains constant during the phase change process essentially occurring in Evaporator and Condenser. This leads to large temperature differences between hot and cold streams during the heat exchange process, and therefore irreversibilities. The approach of employing mixed working fluids mainly targets the minimization of irreversibility in the Evaporator and Condenser by utilizing fluids with non-isothermal phase change behavior; this approach facilitates improved temperature profile alignment between the working fluid and the heat source as well as the heat sink [4,5]. In a recent study conducted by Shahrooz et al. [6], the performance of binary mixtures in organic Rankine cycles (ORCs) was investigated. The findings indicate that the glide temperature has the potential to reduce exergy losses in both the Evaporator and Condenser. However, it should be noted that employing a glide temperature does not always guarantee improved performance of the cycle in comparison to using a pure working fluid.
Several studies have been conducted in recent decades on the exploitation of the potential of binary mixture organic fluids as working fluid in organic Rankine cycles [7]. Some researchers aimed to utilize waste heat [8,9], while some investigated it for renewable sources, like solar and geothermal sources [10,11]. Compared to cycles utilizing high-grade heat sources, organic Rankine cycles (ORCs) often exhibit lower efficiency due to the utilization of low-grade heat [12]. Binary mixtures in ORCs could potentially aid in the energy conversion process of low-grade heat by reducing irreversibilities and ultimately improving the exergy performance of the cycle [13,14,15,16,17,18,19].
In addition to the potential enhancements offered by binary mixtures in ORCs, the utilization of binary mixtures often necessitates the use of specialized heat exchangers capable of accommodating the changing composition during the phase change process. Blondel et al. recently experimentally investigated and developed correlations to predict the heat transfer coefficient of Hydrofluoroether pure fluids and zeotropic mixtures in plate-type heat exchangers for ORCs [20]. The paper examined the condensation behavior of a zeotropic mixture consisting of R134a and R245fa in a small, brazed-plate heat exchanger (BPHE) [21]. In a recent publication by Kapustenko et al. regarding plate-type heat exchangers, it was proposed that increasing the corrugation angles results in enhanced heat transfer intensity during vapor condensation. However, this improvement came at the expense of an increased friction factor [22].
The performance of ORCs, either operating with pure organic fluids or a binary mixtures, greatly depends on the fluid’s thermophysical properties. Therefore, most of the studies conducted and found in the literature are devoted to the selection of organic fluids for the cycle. For example, Herath et al. assessed the performance of simple ORC using seven working fluids: R-134a, R-245fa, benzene, methanol, ethanol, acetone, and propane (R-290) [23]. Their work indicates that ORC systems utilizing benzene and methanol as working fluids exhibit higher efficiency than other fluids studied for Evaporator temperatures in the range of 100 to 200 °C. González et al. studied basic ORCs with eight working fluids, including hydrocarbons and fourth-generation refrigerants [24]. Their work provides a set of tools and recommendations specifically designed to aid in the optimization process of these systems, including the selection of appropriate working fluid for a given operating condition.
The literature reveals that numerous organic compounds can potentially be utilized to operate organic Rankine cycle systems. The organic compounds most commonly used in the ORCs reported in the literature include hydrocarbons (HCs), hydrofluorocarbons (HFCs), hydrofluoroolefins (HFOs), and chlorofluorocarbons (CFCs). The availability of a large variety of the organic compounds as potential candidates for ORC make selection difficult [2,25]. Many factors can affect the selection of an organic compound as working fluid, for example, its availability, its cost, its performance in the cycle, its thermophysical properties, and, especially, its critical properties and normal boiling point [26]. HCs are relatively less expensive and are readily available in the market. They are generally environmentally friendly (i.e., they possess low GWP and zero ODP). One major concern with HCs is their high flammability, leading to increased security concerns. HFCs have zero ODP, but relatively higher GWP. HFOs have zero ODP with low GWP, making them more environmentally friendly than HFCs. CFCs are less viable candidates due to their higher GWP and higher ODP values.
The work found in the literature on ORCs utilizing zeotropic mixtures mainly utilizes simple ORCs operating in cold regions. The condensing temperature in most of the papers available in the literature typically ranges from 10 °C to 25 °C. The ORC performance is highly dependent on the normal boiling temperature of the organic fluid used, and therefore on the Condenser temperature. In the current study, we aim to investigate the performance of an ORC system for use in warm regions, such as Saudi Arabia and the gulf countries, where the ambient temperatures are typically high throughout the year. This study was conducted using seven pure organic fluids and their possible binary mixtures. Because the selection of the working fluid depends on multiple factors, in the first step, the exergy efficiency of the cycle was assessed to compare the potential of each working fluid in the energy conversion process. In the next step, the specific net power output (SNPO) of the cycle with each working fluid was determined. SNPO is one of the important factors in the selection process, as lower SNPO values often lead to higher overall cost when operating the cycle with a targeted net power output. Finally, the heat exchanger conductance was estimated for each case to assess the overall heat exchanger size needed for the plant.
2. System Configuration and Working Parameters
2.1. Cycle Layout
Figure 1 presents the layout of the organic Rankine cycle investigated in the current study. A cycle with internal heat recovery is used to recover the heat from the working fluid, leaving the Expander. Hot air is employed as the heating source to heat the working fluid, whereas water is used in the Condenser as the cooling medium. The Expander generates shaft power by expanding the high-temperature and high-pressure working fluid between state points 1 and 2, leading to a decrease in pressure and temperature. Before the heat is released in the Condenser, the heat recovery unit recovers a significant portion of the heat from the stream exiting the Expander, enabling its reuse. In the heat recovery unit (HRU), the hot stream (between state points 2 and 3) transfers heat to the cold stream (between state points 5 and 6). The cold stream leaving the HRU (state point 6) enters the Evaporator, where it absorbs heat from the hot air.
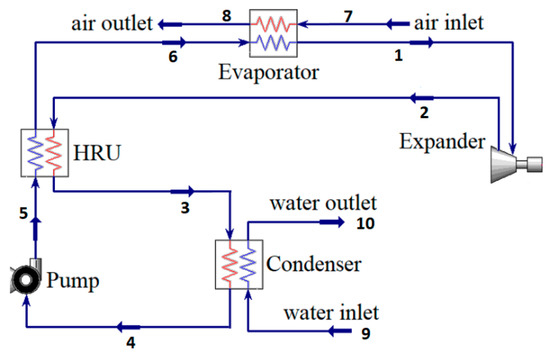
Figure 1.
Organic Rankine cycle with internal heat regeneration.
2.2. Simulation Environment and Fixed Parameters
The cycle shown in Figure 1 is simulated in Aspen HYSYS V11 (licensed–2019). The Peng–Robinson equation of state is used to calculate thermophysical properties of the working fluid. The heat source in the cycle is air, with a mass flow rate of 1 kg/s at 200 °C and 101 kPa. The minimum pinch temperature in the Evaporator is 10 °C; therefore, the maximum temperature of the working fluid at the Expander inlet can reach up to 190 °C, depending on the type of the working fluid and the cycle operating pressures. This study focuses on the performance of the cycle when applied in warm regions; therefore, the Condenser temperature is fixed at 40 °C. The cycle operates at a steady state and the pressure drops in the heat exchangers and pipelines are ignored. The minimum pressure in the cycle is 101 kPa in order to prevent negative pressure in the system.
The complete list of operating parameters and their values in the design process of the cycle are shown in Table 1.

Table 1.
Fixed parameters and their values considered in the design.
2.3. Organic Fluids and Their Zeotropic Mixtures
Using zeotropic mixtures of organic fluids may help to decrease irreversibilities in Evaporators and Condensers. During the phase change in a zeotropic mixture, the temperature difference between the refrigerant and the heat source or sink gradually decreases. This gradual decrease in temperature difference can play a crucial role in minimizing entropy generation and reducing irreversibilities within the system. This helps to improve the heat transfer between fluids. Moreover, the use of zeotropic mixtures often results in lower pressure drops during evaporation and condensation compared to pure refrigerants. Lower pressure drops represent lower entropy generation, leading to a decrease in irreversibilities. The overall impact could lead to higher cycle efficiency and lower energy consumption for zeotropic refrigerant mixture cycles.
In the current study, a total of seven pure organic compounds are considered, allowing us to develop twenty-one possible zeotropic mixtures. These include five HCs, one HFO, and one engineered fluid called Novec 649, which has zero ozone depletion potential (ODP) and very low global warming potential (GWP~1). Table 2 shows the selected organic fluids with their basic thermophysical properties obtained from Aspen HYSYS.

Table 2.
Organic fluids and their basic thermophysical properties.
3. Mathematical Model
3.1. Exergy Analysis
The cycle exergy efficiency ( represents how effectively the available potential of a resource is utilized during the energy conversion process. Mathematically, it can be represented as:
where and represent the net exergy loss and exergy input to the cycle, respectively. The net exergy loss is the summation of exergy losses occurring in each component of the cycle discussed below.
The exergy ( of the working fluid in any given state or condition can be calculated as follows:
where and represent the specific enthalpy and the specific entropy of the working fluid, whereas refers to the ambient temperature. Once the exergy has been calculated for each state point in the cycle, the exergy loss can be found by calculating the exergy difference between inlets and outlets of any component of the cycle. Component-wise exergy loss in the cycle can be represented as:
In the above set of equations, and represent the power produced and consumed by the Expander and the Pump, respectively. Moreover, the net exergy input to the cycle is estimated by calculating the exergy difference of the hot air entering and leaving the Evaporator, i.e.,
3.2. Heat Exchanger Size Estimation
The cycle shown in Figure 1 needs three primary heat exchangers: an Evaporator, a Condenser, and an HRU. The specific design of each of these heat exchangers is not covered in the current study. However, their size is roughly approximated by estimating the heat exchange area needed in the cycle. This is done by calculating the convective heat transfer coefficient (UA value) of the heat exchanger. For a given heat exchanger, this can be represented as:
where is the amount of heat transfer between the hot and the cold streams, and represents the log-mean temperature difference between the hot and the cold streams. The total heat transfer coefficient needed by the cycle can be written as:
4. Simulation Results and Discussion
4.1. Binary Mixtures
Considering the use of a binary mixture as a working fluid for operating ORCs has certain advantages. Unlike pure working fluids, the phase change processes of binary mixtures at a given pressure are not isothermal. This characteristic of binary mixtures can be exploited to achieve better temperature profile matching in the Evaporator and Condenser during phase change, which ultimately results in a reduction in the associated irreversibilities [27,28]. For the selected organic fluids, the temperature glide (the difference between bubble point and dew point temperatures) is calculated utilizing the Peng–Robinson equation of state in Aspen HYSYS. In Figure 2, the temperature glide of binary mixtures of pentane is presented with other remaining organic fluids selected in the study. It can be seen that all these mixtures are zeotropic in nature, and the temperature glide increases to its maximum value with increasing mixture ratio, and then drops to zero. It can also be observed that increasing the pressure does not always decrease the temperature glide. For some mixtures (e.g., for pentane/RE347mcc) it increases, while for others it decreases and then increases (e.g., for pentane/cyclopentane). This indicates that for some compositions (binary mixtures), the glide is higher in the Evaporator than in the Condenser. Higher glide values help to improve the irreversibilities in the heat exchange process by reducing the temperature difference between the hot and cold streams [6]. Moreover, for some zeotropic mixtures, the temperature glide is very small. Take, for instance, combinations like pentane/isopentane and pentane/RE347mcc. These binary mixtures exhibit an incredibly minimal temperature glide of less than half a degree Celsius. This remarkable characteristic suggests that the phase transition of these mixtures closely resembles an isothermal process, akin to that of a pure substance. This means that some mixtures may not facilitate temperature matching during the heat exchange process in the Evaporator and the Condenser. On the other hand, some zeotropic mixtures, such as pentane/neopentane and pentane/Novec 649, can be seen to have a higher temperature glide.
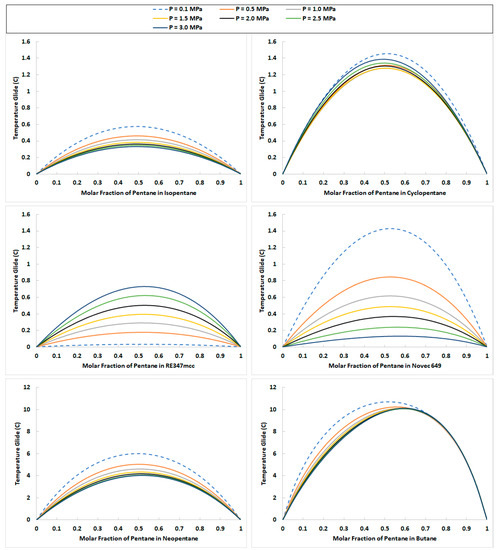
Figure 2.
Temperature glide of the zeotropic mixtures of pentane with isopentane, cyclopentane, neopentane, butane, RE347mcc, and Novec 649 at various mixture pressures from 0.1 MPa to 3.0 MPa.
4.2. Cycle Operating Pressures
The cycle shown in Figure 1 was studied using the pure organic fluids shown in Table 2 and their possible zeotropic mixtures. For any two organic fluids, the investigation is performed for mixture ratios from 0.1 to 0.9 with an increment of 0.1. For any working fluid, the performance of the cycle depends on the operating conditions, such as the pressures and the temperatures in the Evaporator and the Condenser. The parameter to be optimized in this work is the Evaporator or Expander inlet pressure, with the objective of maximizing exergy efficiency. Table 1 lists parameters the assumed or fixed in the current work. The Condenser temperature is fixed at 40 °C and its pressures is either the saturation pressure of the working fluid or 101 kPa, in case the saturation pressure drops below ambient pressure to avoid vacuum. The working fluid state at the Expander inlet is set to be a saturated vapor, because superheating at the Expander inlet increases exergy losses [6,29]. Therefore, the Expander inlet pressure is optimized to maximize the overall exergy efficiency of the cycle. Hence, for each binary mixture of any composition, the Expander inlet pressure that maximizes the exergy efficiency is obtained. Figure 3 represents the plots of the exergy efficiencies at various Expander inlet pressures for 50/50 ratio binary mixtures of pentane with isopentane, cyclopentane, neopentane, butane, RE347mcc, and Novec 649. In general, the exergy efficiencies increase with increasing Expander inlet pressure. However, for some binary mixtures, the exergy efficiency of the cycle increases to a maximum, and then declines. This is a similar behavior to that observed for all of the binary mixtures of any composition considered in this study. It should be noted that the upper limit of Expander operating pressure was set to 90% of the critical pressure of the working fluid.
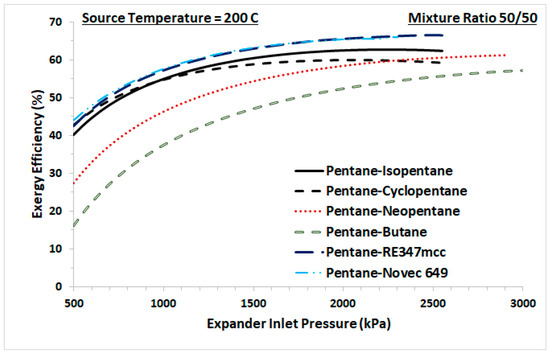
Figure 3.
Exergy efficiency of the cycle versus Expander inlet pressure for 50/50 zeotropic mixtures of pentane with isopentane, cyclopentane, neopentane, butane, RE347mcc, and Novec 649.
4.3. Exergy Performance of the Cycle
The exergy efficiency of the cycle is assessed for all working fluids listed in Table 2. For each working fluid, whether pure or zeotropic mixture, the optimal operating pressure of the Expander is determined using the method described in the previous section. Figure 4 illustrates the exergy efficiencies of the cycle with various working fluids. Figure 4a demonstrates the impact of increasing the mole fraction of additives (isopentane, cyclopentane, neopentane, butane, RE347mcc, and Novec 649) in pentane. It is observed that a binary mixture with any composition of pentane and other hydrocarbons (isopentane, cyclopentane, neopentane, butane) does not enhance the exergy efficiency of the cycle; instead, it decreases significantly for some mixtures, such as pentane–butane and pentane–cyclopentane. Conversely, the binary mixtures of pentane with RE347mcc and Novec 649 produce some improvement in the cycle’s exergy performance. This indicates that blending any two pure working fluids does not always result in performance enhancement. Remember, the exergy performance of the cycle relies on the thermophysical properties of the working fluid and the associated irreversibilities in various components.
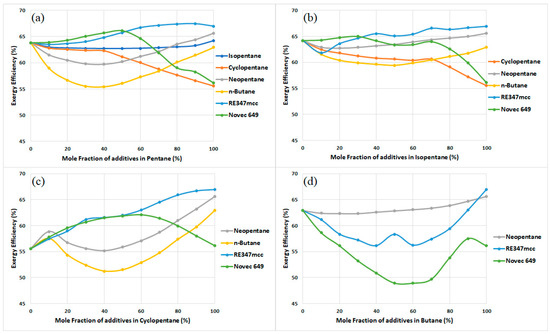
Figure 4.
Exergy efficiency of the cycle for zeotropic mixtures with (a) pentane, (b) isopentane, (c) cyclopentane, and (d) butane.
Just like in ref. [30], we undertook an investigation to understand the factors influencing the significant fluctuations in exergy efficiency. The main objective was to pinpoint the specific components accountable for these variations. To achieve this, we computed the individual exergy losses for each component and visually represented them as a bar chart in Figure 5 for pentane/isopentane, butane/Novec 649, and cyclopentane/Novec 649 binary mixtures. Upon initial observation of this figure, we notice a consistent irreversibility loss of approximately 13% in the Expander. Furthermore, the exergy loss in the pump is negligible, and can be safely disregarded. Thus, the substantial exergy loss occurs predominantly during the heat exchange process. When analyzing the data for cyclopentane/Novec 649 from Figure 4c, we found that the exergy efficiency exhibits an increasing trend, reaching a peak before declining. This behavior is attributed to the net irreversibilities in the heat exchangers (namely, the Condenser, the Evaporator, and the HRU). These irreversibilities decrease when the Novec 649 mole fraction in the mixture is in the range from 0% to almost 60%, thereby leading to the observed increase in exergy efficiency. However, beyond this point, the net irreversibilities start to increase, causing the exergy efficiency to decrease. The same rationale applies to all other cases, as well.
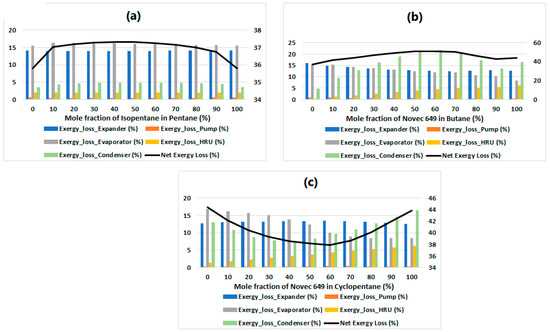
Figure 5.
Total and component-wise exergy losses for (a) pentane/isopentane, (b) butane/Novec 649, and (c) cyclopentane/Novec 649 binary mixtures.
The highest exergy efficiency of the cycle for each zeotropic mixture (a total of 21) is calculated and presented in Table 3. The table’s upper triangle displays the exergy efficiency values as percentages, while the lower triangle provides the composition of the best working fluid for each case. It can be observed that RE347mcc is the best candidate in terms of exergy performance for the cycle among the pure working fluids, with an exergy efficiency of approximately 67%. However, the binary mixture of RE347mcc and pentane (0.9/0.1 mixing ratio) has a marginally higher exergy efficiency value (i.e., 67.4%) than pure RE347mcc. Choosing the best candidate (working fluid) for the actual installation of the power plant is a complex process. Several factors must be considered, including the cost of the organic fluid and its availability. In general, hydrocarbons (pentane, isopentane, cyclopentane, neopentane, butane) are significantly more affordable and widely available in the market compared to other organic fluids.

Table 3.
Exergy performance of the cycle with pure and zeotropic mixtures. The upper triangle shows the values of the exergy efficiencies in percentages, while the lower triangle reports the composition of the working fluid maximizing the exergy efficiency.
4.4. Power Generation Capacity of the Cycle
The power generation capacity of the cycle is one of the key factors in decision making. This capacity is determined by calculating the amount of power generated by the cycle per unit mass flow rate of the working fluid. Generally, the higher the specific net power generation capacity, the smaller the power plant and equipment needed to generate the desired electrical power from the plant. The specific net power output (SNPO) from the plant depends mainly on the thermophysical properties of the working fluid. To assess the power generation capabilities of each working fluid in the cycle, SNPO is calculated as follows:
The SNPO values of the cycle are graphically presented in Figure 6. When considering pure organic fluids, hydrocarbons can be observed to have higher SNPO than RE347mcc and Novec 649. Cyclopentane has the highest SNPO (≈102 kJ/kg), followed by pentane (≈86 kJ/kg) and isopentane (≈79 kJ/kg). Neopentane and butane have nearly the same value of SNPO (≈60 kJ/kg). Besides being the best performer with respect to exergy assessment, RE347mcc has a significantly low SNPO value (≈28 kJ/kg), while the lowest is that of Novec 649 (≈20 kJ/kg).
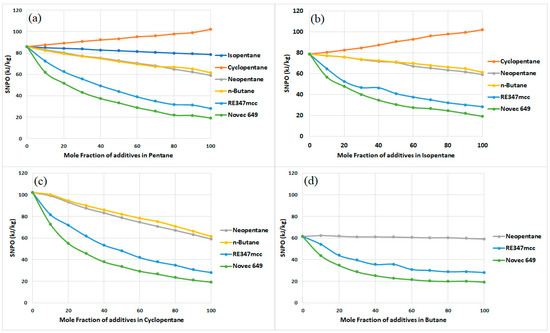
Figure 6.
Specific net power output of the cycle for zeotropic mixtures with (a) pentane, (b) isopentane, (c) cyclopentane, and (d) butane.
Table 4 presents the numerical values of SNPO for the cycle operating with the working fluid corresponding to the maximum exergy efficiency, as per Table 3. It can be observed that no binary mixtures of organic fluids were found to have a higher value of SNPO than their pure constituents. Hence, pure constituents can be prioritized as working fluids over their slightly lower exergy efficiency, considering their superior power generation capabilities. For instance, when examining the isopentane/Novec 649 binary mixture, the highest exergy efficiency achieved is 65%, which results from a blend consisting of 70% isopentane and 30% Novec (see Table 3). With this specific mixture ratio, the Specific Net Power Output (SNPO) of the cycle is 40.2 kJ/kg. Conversely, if a pure isopentane is employed instead of the binary mixture, the exergy efficiency of the cycle decreases slightly to 64.2%. Nevertheless, this trade-off is compensated by the increased SNPO of 78.7 kJ/kg, which is nearly double the value obtained with the binary mixture.

Table 4.
Upper triangle: SNPO of the cycle in kJ/kg with working fluid maximizing the exergy efficiency as listed in Table 3. Lower triangle: the composition of the working fluid maximizing the exergy efficiency.
Therefore, to estimate the gain in SNPO with respect to the anticipated decline in exergy efficiency, we calculate the differences between the exergy efficiencies of the working fluids listed in Table 3 and the exergy efficiencies of the pure constituents with the highest SNPO values. The calculated values are shown in Table 5. The upper triangle of this table presents the decline in exergy efficiency, whereas the lower triangle presents the gain in SNPO.

Table 5.
Comparison of the exergy efficiency decline in percentage point and SNPO gain. The upper triangle of the table showcases the decline in exergy efficiency, while the lower triangle displays the corresponding gains in SNPO. “pp” represents percentage points.
Among the binary mixtures with other organic fluids, the SNPO gain is significantly greater for binary mixtures obtained through the addition of RE347mcc or Novec 649 or cyclopentane. Moreover, the compromise in exergy efficiency is not very high for most of the mixtures. For instance, when utilizing RE347mcc/butane, the exergy efficiency undergoes a minimal decrease of only 1.2 percentage points, while the SNPO shows a substantial gain of approximately 31 kJ/kg. Similarly, in the case of Novec 649/isopentane, the exergy efficiency decreases by a mere 0.8 percentage points, but results in a remarkable increase in SNPO of 38.4 kJ/kg. Conversely, in some cases, the enhancement of Specific Net Power Output (SNPO) is accompanied by a significant decrease in exergy efficiency. For instance, in the case of a cyclopentane/neopentane, if pure cyclopentane is employed instead of neopentane, the SNPO increases by 43.1 kJ/kg. However, this gain is achieved at the expense of a substantial decline in exergy efficiency, which decreasese by 10 percentage points. This demonstrates the potential trade-offs and impacts on system performance resulting from the choice of working fluid.
4.5. Heat Exchanger Size Assessment
The net size of the heat exchange area needed for the cycle was calculated according to the procedure described in Section 3.2. It should be noted that the cycle operates with different working fluids, but the heat input to the cycle remains constant. A hot air stream at 200 °C and 1 kg/s (please refer to Table 1 for the operating parameters of the cycle) was used as a heat source. As a result, the mass flow rate of the working fluid varied with each change in the organic fluid used in the cycle, leading to a varying power output in each case. In order to be able to assess and obtain a fair comparison of the heat exchange areas needed in the cycle for each working fluid, the heat transfer coefficient of the cycle (), normalized by the net power output of the cycle, was calculated, and the results are plotted in Figure 7. Considering pure organic fluids, we observed that n-butane requires the highest value of UA per unit net power output, followed by neopentane, RE347mcc, Novec 649, isopentane, pentane, and cyclopentane. The greater the value of , the larger the heat exchange area needed per unit power output produced by the cycle.
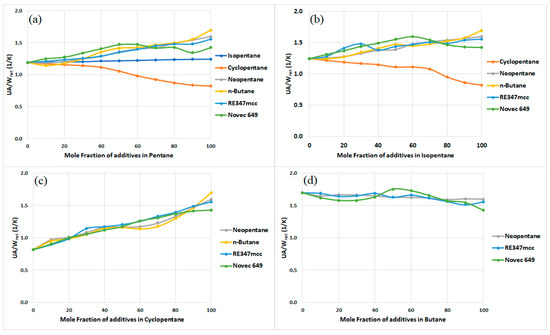
Figure 7.
Total conductance of the heat exchanger () per unit net power output of the cycle required to operate the cycle using zeotropic mixtures with (a) pentane, (b) isopentane, (c) cyclopentane, and (d) butane.
Table 6 displays the numerical values of for the cycle utilizing a working fluid that aligns with the maximum exergy efficiency indicated in Table 3. It can be observed that none of the binary mixtures of organic fluids exhibit a lower value than their pure constituents. Therefore, accepting a slightly lower exergy efficiency and choosing a pure constituent as the working fluid could be considered, which could lead to a considerable reduction in plant size and overall cost.

Table 6.
Upper triangle: of the cycle with working fluid maximizing the exergy efficiency as listed in Table 3. Lower triangle: the composition of the working fluid maximizing the exergy efficiency.
To gauge the decrease in the heat exchange area () due to the expected decline in the exergy efficiency, we compute the differences between the exergy efficiencies of the working fluids outlined in Table 3 and the exergy efficiencies of the pure constituent that exhibits the lowest values of . This calculation allows us to estimate the net reduction in . Table 7 displays the computed values. The upper triangle in the table illustrates the decrease in exergy efficiency, while the lower triangle demonstrates the corresponding percentage reduction in plant size. The findings of this study are intriguing, revealing that even a slight compromise in exergy efficiency can lead to a significant decrease in net heat exchange area. For instance, in the case of using 70% isopentane and 30% Novec 649, switching to pure isopentane results in a reduction in the heat exchange area of nearly 14%, accompanied by a decrease of only 0.8 percentage points in terms of exergy efficiency. This demonstrates the potential trade-offs and impacts on system performance based on the choice of working fluids.

Table 7.
Comparison of the exergy efficiency decline and the reduction in heat exchange area needed. The upper triangle of the table showcases the decline in exergy efficiency, while the lower triangle displays the corresponding decline in . “pp” represents percentage point.
5. Conclusions
The possibility of harnessing energy from low-grade heat sources with a temperature of 200 °C was assessed using a regenerative organic Rankine cycle. This study essentially focused on the selection of organic fluid, either in pure form or as a zeotropic mixture, based on exergy efficiency of the cycle. The assessment of the cycle’s performance based on other parameters, like specific net power output and heat exchanger size, provided a broader spectrum to be considered when selecting an appropriate working fluid. The study was conducted using seven pure organic fluids (pentane, isopentane, cyclopentane, neopentane, butane, RE347mcc, and Novec 649) and their binary mixtures (21 in total). The key outcomes of the study are highlighted and summarized below.
- For the heat source temperature considered in this study (i.e., 200 °C), pure organic fluids performed better than binary mixtures in terms of exergy efficiency, SNPO, and heat exchange area needed for the cycle to operate.
- Among pure organic fluids, RE347mcc achieved the highest exergy efficiency of nearly 67%, followed by neopentane (≈65.6%) isopentane (≈64.2%), pentane (≈63.8%), butane (≈62.9%), Novec 649 (≈56.2%), and cyclopentane (≈55.6%).
- Cyclopentane, despite being the worst performer in exergy assessment, was found to have the highest capacity to generate power per unit mass flow rate (i.e., SNPO), at nearly 102 kJ/kg, followed by pentane (≈86 kJ/kg), isopentane (≈78.7 kJ/kg), butane (≈61.5 kJ/kg), neopentane (≈59.1 kJ/kg), RE347mcc (≈28.2 kJ/kg), and Novec 649 (≈19.4 kJ/kg).
- Binary mixtures of 50% pentane/50% Novec 649 and 40% cyclopentane/60% Novec 649 were found to have substantially higher exergy performance than their pure constituents. However, their SNPO values were significantly lower. For instance, pentane/Novec 649 achieved a maximum exergy efficiency of 66.1%, which is only 2.3 percentage points higher than what was obtained with pure pentane; however, its SNPO was nearly 61% lower than that of pure pentane.
- The analysis of component-wise irreversibility losses revealed that the primary contributors to exergy losses were the heat exchange processes.
- The study revealed an intriguing relationship between exergy efficiency and net heat exchange area, illustrating that a slight compromise in exergy efficiency can lead to a substantial decline in the required heat exchange area.
Author Contributions
Conceptualization, M.E.S. and E.A.; Methodology, M.E.S.; Software, M.E.S. and U.S.; Validation, M.E.S.; Formal analysis, M.E.S., E.A. and U.S.; Investigation, M.E.S.; Writing—original draft, M.E.S.; Writing—review & editing, E.A. and U.S.; Funding acquisition, M.E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was funded by Institutional Fund Projects under grant no. (IFPIP: 577-135-1443). The authors gratefully acknowledge technical and financial support provided by the Ministry of Education and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bao, J.; Zhao, L. A review of working fluid and expander selections for organic Rankine cycle. Renew. Sustain. Energy Rev. 2013, 24, 325–342. [Google Scholar] [CrossRef]
- Sanchez, C.J.N.; Gosselin, L.; da Silva, A.K. Designed binary mixtures for subcritical organic Rankine cycles based on multiobjective optimization. Energy Convers. Manag. 2018, 156, 585–596. [Google Scholar] [CrossRef]
- Astolfi, M.; Martelli, E.; Pierobon, L. Thermodynamic and Technoeconomic Optimization of Organic Rankine Cycle Systems. In Organic Rankine Cycle (ORC) Power Systems: Technologies and Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 173–249. [Google Scholar] [CrossRef]
- Abadi, G.B.; Kim, K.C. Investigation of organic Rankine cycles with zeotropic mixtures as a working fluid: Advantages and issues. Renew. Sustain. Energy Rev. 2017, 73, 1000–1013. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, R.; Deng, S.; Zhao, L.; Mao, S.S. Is zeotropic working fluid a promising option for organic Rankine cycle: A quantitative evaluation based on literature data. Renew. Sustain. Energy Rev. 2021, 148, 111267. [Google Scholar] [CrossRef]
- Shahrooz, M.; Lundqvist, P.; Nekså, P. Performance of binary zeotropic mixtures in organic Rankine cycles (ORCs). Energy Convers. Manag. 2022, 266, 115783. [Google Scholar] [CrossRef]
- Siddiqui, M.E.; Almatrafi, E.; Bamasag, A.; Saeed, U. Adoption of CO2-based binary mixture to operate transcritical Rankine cycle in warm regions. Renew. Energy 2022, 199, 1372–1380. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, H.; Wang, E.; Song, S.; Bei, C.; Chang, Y.; Wang, H.; Yao, B. Study on Mixed Working Fluids with Different Compositions in Organic Rankine Cycle (ORC) Systems for Vehicle Diesel Engines. Entropy 2014, 16, 4769–4787. [Google Scholar] [CrossRef]
- Cignitti, S.; Andreasen, J.G.; Haglind, F.; Woodley, J.M.; Abildskov, J. Integrated working fluid-thermodynamic cycle design of organic Rankine cycle power systems for waste heat recovery. Appl. Energy 2017, 203, 442–453. [Google Scholar] [CrossRef]
- Ganjehsarabi, H. Mixed refrigerant as working fluid in Organic Rankine Cycle for hydrogen production driven by geothermal energy. Int. J. Hydrogen Energy 2019, 44, 18703–18711. [Google Scholar] [CrossRef]
- Liang, Z.; Liang, Y.; Luo, X.; Chen, J.; Yang, Z.; Wang, C.; Chen, Y. Synthesis and simultaneous optimization of multi-heat source multi-pressure evaporation organic Rankine cycle with mixed working fluid. Energy Convers. Manag. 2022, 251, 114930. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, L.; Mao, S.S.; Deng, S. Towards novel low temperature thermodynamic cycle: A critical review originated from organic Rankine cycle. Appl. Energy 2020, 270, 115186. [Google Scholar] [CrossRef]
- Lecompte, S.; Huisseune, H.; Van Den Broek, M.; Vanslambrouck, B.; De Paepe, M. Review of organic Rankine cycle (ORC) architectures for waste heat recovery. Renew. Sustain. Energy Rev. 2015, 47, 448–461. [Google Scholar] [CrossRef]
- Miao, Z.; Zhang, K.; Wang, M.; Xu, J. Thermodynamic selection criteria of zeotropic mixtures for subcritical organic Rankine cycle. Energy 2019, 167, 484–497. [Google Scholar] [CrossRef]
- Collings, P.; Yu, Z.; Wang, E. A dynamic organic Rankine cycle using a zeotropic mixture as the working fluid with composition tuning to match changing ambient conditions. Appl. Energy 2016, 171, 581–591. [Google Scholar] [CrossRef]
- Li, J.; Ge, Z.; Duan, Y.; Yang, Z. Effects of heat source temperature and mixture composition on the combined superiority of dual-pressure evaporation organic Rankine cycle and zeotropic mixtures. Energy 2019, 174, 436–449. [Google Scholar] [CrossRef]
- Deethayat, T.; Asanakham, A.; Kiatsiriroat, T. Performance analysis of low temperature organic Rankine cycle with zeotropic refrigerant by Figure of Merit (FOM). Energy 2016, 96, 96–102. [Google Scholar] [CrossRef]
- Lecompte, S.; Ameel, B.; Ziviani, D.; Van den Broek, M.; De Paepe, M. Exergy analysis of zeotropic mixtures as working fluids in Organic Rankine Cycles. Energy Convers. Manag. 2014, 85, 727–739. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, C.-L.; Zhang, Z.-Y. Stepped pressure cycle—A new approach to Lorenz cycle. Int. J. Refrig. 2017, 74, 283–294. [Google Scholar] [CrossRef]
- Blondel, Q.; Tauveron, N.; Lhermet, G.; Caney, N. Zeotropic mixtures study in plate heat exchangers and ORC systems. Appl. Therm. Eng. 2023, 219, 119418. [Google Scholar] [CrossRef]
- Zhang, J.; Elmegaard, B.; Haglind, F. Condensation heat transfer and pressure drop characteristics of zeotropic mixtures of R134a/R245fa in plate heat exchangers. Int. J. Heat Mass Transf. 2021, 164, 120577. [Google Scholar] [CrossRef]
- Kapustenko, P.; Klemeš, J.J.; Arsenyeva, O.; Tovazhnyanskyy, L. PHE (Plate Heat Exchanger) for Condensing Duties: Recent Advances and Future Prospects. Energies 2023, 16, 524. [Google Scholar] [CrossRef]
- Herath, H.; Wijewardane, M.; Ranasinghe, R.; Jayasekera, J. Working fluid selection of Organic Rankine Cycles. Energy Rep. 2020, 6, 680–686. [Google Scholar] [CrossRef]
- González, J.; Garrido, J.M.; Quinteros-Lama, H. Analysis of the Maximum Efficiency and the Maximum Net Power as Objective Functions for Organic Rankine Cycles Optimization. Entropy 2023, 25, 882. [Google Scholar] [CrossRef] [PubMed]
- Bell, I.H.; Lemmon, E.W. Overview of Thermophysical Properties of Fluids and Their Application to Organic Rankine Cycle Systems. 2017. Available online: http://www.sciencedirect.com:5070/book/9780081005101/organic-rankine-cycle-orc-power-systems (accessed on 21 May 2023).
- Siddiqui, M.E.; Almatrafi, E.; Saeed, U.; Taimoor, A.A. Selection of Organic Fluid Based on Exergetic Performance of Subcritical Organic Rankine Cycle (ORC) for Warm Regions. Energies 2023, 16, 5149. [Google Scholar] [CrossRef]
- Bell, I.; Lemmon, E. Organic fluids for Organic Rankine Cycle systems: Classification and calculation of thermodynamic and transport properties. In Organic Rankine Cycle (ORC) Power Systems: Technologies and Applications; Woodhead Publishing: Sawston, UK, 2017; pp. 91–119. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, L.; Wang, X. A comparative study of pure and zeotropic mixtures in low-temperature solar Rankine cycle. Appl. Energy 2010, 87, 3366–3373. [Google Scholar] [CrossRef]
- Shahrooz, M.; Lundqvist, P.; Nekså, P. Natural refrigerants for low temperature power cycles. In Proceedings of the 13th IIR Gustav Lorentzen Conference on Natural Refrigerants (GL2018), Valencia, Spain, 18–20 June 2018; Volume 2018, pp. 1373–1380. [Google Scholar] [CrossRef]
- Arefdehgani, S.; Khosroshahi, A.R. The Effect of Ambient Temperature to Tabriz Power Plant Efficiency. In Exergy for a Better Environment and Improved Sustainability 1; Springer: Cham, Switzerland, 2018; pp. 727–736. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).