Abstract
The combination of genomic and proteomic analyses is a useful tool for the study of novel Bacillus thuringiensis (Bt) strains, as these approaches allow the accurate identification of pesticidal proteins and virulence factors produced. Here, we isolated and evaluated the potential of a novel Neotropical Bt strain (TOD651) for controlling larvae of Aedes aegypti and Culex quinquefasciatus mosquitoes. Aiming for the full comprehension of the TOD651 larvicidal potential, we further evaluated the whole TOD651 genome and conducted the proteomic analysis of the TOD651 spore–crystal mixtures. Our results showed that Bt TOD651 similarly killed both A. aegypti (0.011 µg/mL) and C. quinquefasciatus (0.023 µg/mL) larvae, exhibiting similar potency to the commercial Bt strain. The genome sequence revealed that Bt TOD651 harbors cry11Aa3, cry10Aa4, cry4Aa4, cry4Ba5, cyt1Aa5, cyt1Ca1, cyt2Ba13, mpp60Aa3, and mpp60Ba3. The proteomic analysis revealed no expression of Mpp60Aa3, while all the other pesticidal proteins were expressed (Cry4Ba5 was more abundant than Cyt1Aa5). The expression of the Mppe showed the major proportions between proteases. The virulent factor neutral protease B and spore coat proteins were also expressed. The expression of relevant pesticidal proteins (e.g., Cry, Cyt, Mpp, and other pathogenic factors), whose actions can occur in a synergic relation, indicates that the biocontrol using Bt TOD651 may contribute to delaying the selection of resistant individuals.
1. Introduction
The control of insect vectors of different diseases is of great importance for public health. Mosquito species such as Aedes aegypti (Linnaeus, 1762) (Diptera: Culicidae) and Culex quinquefasciatus (Say, 1823) (Diptera: Culicidae) can transmit several diseases that affect human life. For instance, A. aegypti can transmit yellow fever virus, dengue virus, Chikungunya virus, and Zika virus, while C. quinquefasciatus is capable of transmitting arboviruses such as West Nile Virus (WNV) and the Wuchereria bancrofi nematode, responsible for lymphatic filariasis disease [1].
The use of chemical insecticides for the control of mosquitoes, which harm the environment, has been slowly substituted around the world by biological control strategies, such as Bacillus thuringiensis (Bt) [2]. Bt is a Gram-positive bacterium known for its toxicity and specificity towards insect hosts due to its ability to produce and release crystal proteins (Cry and Cyt) during the sporulation stage [3]. Bacillus thuringiensis serovar israelensis (Bti) is one of the subspecies’ most effective larvicides for mosquito control, being recommended by the World Health Organization (WHO) [4,5]. Bti produces Cry and Cyt crystals (Cry4Aa, Cry4Ba, Cry10Aa, Cry11Aa, Cyt1Aa, and Cyt2Ba) that exhibit toxicity against mosquito species from the genus Aedes, Anopheles, and Culex, and for the black fly (Simuliidae) [4,6,7,8]. These proteins synergistically interact and can decrease the incidence of resistance in insect populations [4]. Some Bti strains can also harbor Mpp60A and Mpp60B proteins, which are present in other subspecies such as jegathesan and malayensis [8].
Improvements in the control of mosquitoes using Bt have involved the constant identification and characterization of novel strains and pesticidal proteins [9,10,11,12]. In addition, next-generation sequencing (NGS) has allowed the whole-genome sequencing of novel Bt strains and their characterization. Genome information has been important in research and applications of Bt because pesticidal genes are easily detected [13,14,15]. Furthermore, other genes related to the pathogenicity of Bt can be explored through this technique, such as virulence factors and other secondary metabolites [16,17].
Despite the characterization of genes coding pesticidal proteins allowing strain classification, it is the expression of these genes that determines their spectrum of activity [18,19]. In the genome sequence, not all annotated coding regions are expressed, and the genomic approach may not suffice to fully explain the toxicity differences between Bt strains [20,21]. For instance, many pesticidal proteins are cryptic or have insignificant levels of expression [22]. Thus, proteomic analysis of the pesticidal proteins that make up the parasporal crystal is essential to understand the toxicity of novel and commercial Bt strains [19]. In this context, the combination of genomic and proteomic analyses is a powerful tool for the accurate identification of pesticidal proteins and virulence factors of Bt strains [23,24,25], and estimations of the abundance of such proteins can be achieved in purified parasporal crystals and spore–crystal mixtures [25,26].
Several studies have been conducted to investigate the genomics of mosquitocidal Bt strains [21,27,28,29]. Interestingly, the proteomic analysis for pesticidal proteins responsible for mosquitocidal activities in Bt strains remains scarce and underexploited. Therefore, we isolated a novel Bt strain (Bt TOD651) and evaluated its insecticidal activity against larvae of A. aegypti and C. quinquefasciatus. To explore and better understand its toxicity, we sequenced the whole genome of TOD651 and performed proteomic analysis of the spore–crystal mixture.
2. Materials and Methods
2.1. Origin and Culture of Bt TOD651 Strain
The Bt TOD651 strain was isolated from a soil sample, collected in the state of Tocantins, Brazil (11°43′45″ S; 49°04′07″ W), according to Monnerat et al. [30]. This strain was cultured at 28 °C for 12 h in Luria–Bertani (LB) solid medium (10 gL−1 tryptone, 5 gL−1 yeast extract, 10 gL−1 NaCl, and 20 gL−1 agar). Posteriorly, a single colony of Bt TOD651 was transferred to an LB liquid medium and incubated (28 °C at 200 rpm for 16 h) for sporulation and DNA extraction steps. The Bti AM65-52 strain was isolated from a commercial sample (VectoBac®, Sumitomo, Tokyo, Japan) and used as a reference strain.
2.2. Crystal Protein Purification and SDS-PAGE Analysis
An aliquot of LB culture (3 mL) was transferred to CCY medium (30 mL) (13 mM KH2P04, 26 mM K2HP04, 0.002% (w/v) L-glutamine, 0.1% (w/v) casein hydrolysate, 0.1% (w/v) bacto casitone, 0.04% bacto yeast extract, 0.6% (w/v) glycerol, 0.05 M ZnCl2, 0.5 M MgCl2, 0.01 M MnCI2, 0.2 M CaCl2, 0.05 M FeCl3) and incubated for sporulation (28 °C at 200 rpm for 72 h). Then, the spore–crystal mixture was collected, and the crystal proteins were purified according to a previously described method [31]. Purified crystals were suspended in a small volume of phosphate-buffered saline (136 mM NaCl, 1.4 mM KH2PO4, 2.6 mM KCl, 8 mM Na2HPO4, and 4.2 mL H2O, pH 7.4), and fractionated by electrophoresis on a 10% SDS-PAGE gel [30].
2.3. Identification of Crystal Morphology
The morphological characterization of Cry protein crystals was performed by scanning electron microscopy. The spore–crystal mixture of Bt TOD651 was collected and diluted in sterile water. Then, a 100 µL aliquot of the diluted suspension was placed on metallic supports and dried for 24 h at 37 °C, covered with gold for 180 s using an Emitech apparatus (model K550; Quorum Technologies, Lewes, UK), and observed under a Zeiss scanning electron microscope (model DSM 962; Carl Zeiss AG, Oberkochen, Germany) at 10 or 20 Kv.
2.4. Larvae Rearing
The larvae of A. aegypti and C. quinquefasciatus were collected from fields without the application of insecticides, in regions of transition between urban and rural areas in the state of Tocantins, Brazil (11°40′55.7″ latitude S, 49°04′3.9″ longitude W). The insect colonies were established in the Entomology Laboratory of the Federal University of Tocantins, Gurupi Campus, according to Aguiar et al. [32]. The larvae were reared in plastic containers (40 cm × 25 cm × 8 cm) and fed a sterilized diet (an 80/20 mix of chick chow powder/yeast), and mosquitoes were provided with a 10% sucrose solution. We blood-fed the adult females five days after emergence with defibrinated sheep blood using a membrane feeder device [33].
2.5. Bioassays
Using the spore–crystal mixtures, bioassays were conducted on A. aegypti and C. quinquefasciatus third-instar larvae. The concentrations were determined according to Mclaughlin et al. [34]. Seven concentrations of the spore–crystal mixtures (0.05, 0.10, 0.15, 0.20, 0.25, 0.30, and 0.40 µg/mL) were tested, and sterile distilled water was used as a negative control. Bioassays were performed in 3 replicates with 25 larvae in 100 mL of distilled water. Treated larvae were kept at 26 ± 1 °C, 60.0 ± 5% RH, and a 12 h light–dark photoperiod. The number of dead and live larvae was counted after 24 h. The larvae that did not move when touched with a sterile stick were considered dead [35]. The spore–crystal mixture from the AM65-52 strain was used as a reference. Concentration–mortality curves were estimated by probity analysis using the PROBIT procedure in the SAS software [36].
2.6. Whole-Genome Sequencing, Assembly, and Annotation
Genomic DNA was extracted using the Wizard® Genomic DNA Purification Kit (Promega, Madison, WI, USA). Posteriorly, DNA concentration and purity were measured using the NanoDrop™ 8000 apparatus (Thermo Fisher Scientific, Waltham, MA, USA) and stored at −20 °C until further use. Sequencing was performed on Illumina Mi-Seq technologies, using a paired-end application, and reads with a mean length of 75.9 bp (Illumina, San Diego, CA, USA), generating a total of 15,425,426 reads with an average insert size of 200 bp and coverage of 426×. Sequence reads’ quality was assessed using FastQC software version 0.11.9 [37], and reads were trimmed using the Trim and Filter tool (error probability = 0.05) of Geneious version 10.2.6 [38]. The trimmed reads were used in de novo assembly with the SPAdes version 3.10.0 tool and default parameters [39], and contigs ≥ 1000 bp were discarded. The CDS of contigs were predicted using RASTtk (Domain: Bacteria; Taxonomy name: Bacillus thuringiensis; Genetic code: 11—Archaea and Bacteria). The chromosome was assembled using contigs and reference HD-789 (NCBI Accession No. CP003763) through reference-guided de novo assembly [40], using the Geneious map to reference tool to assess the virulence factors of related genes. Contigs unused in the chromosome assembly were filtered and used for predicting pesticidal protein-like genes. Related genes with virulence factors were predicted using the bacterial virulence factor database (VFDB) [41]. Putative pesticidal proteins were determined using Blastx through the Btoxin_Digger tool (scaffolds as a query) [42] and a customized database (CDS predicted as a query). The customized database was created from the Bt pesticidal protein list available at the Bt nomenclature website (http://www.lifesci.sussex.ac.uk/home/Neil_Crickmore/Bt/toxins2.html (accessed on 9 December 2022) through Geneious using the Add/Remove Database tool. CDS with homology to the Bt pesticidal proteins were filtered using parameters of an E-value of 0.001 and a word size of 6.
2.7. Phylogenetic Relationship
A phylogenetic tree was constructed using the gyrB gene (DNA gyrase subunit B), extracted from contigs sequences, and the gyrB genes of the Bacillus ssp. strains retrieved from GenBank. The alignment was performed using ClustalW, and the phylogenetic tree was created using MEGA 11 [43] from the neighbor-joining method, with 1000 replications.
2.8. LC-MS/MS Analysis
The liquid chromatography–tandem mass spectrometry (LC-MS/MS) method was used for protein detection in the spore–crystal mixture of the Bt TOD651 strain. The LC-MS/MS analysis was carried out at the Veritas/Life Sciences Department at the University of São Paulo (USP, Ribeirão Preto, SP, Brazil). Firstly, the spore–crystal sample was washed three times in 1× PBS (phosphate-buffered saline), resuspended in 750 µL of solubilization buffer (8 M urea, 0.5% octyl-glucopyranoside (OG), 0.05 M Tris-HCL, pH 8.8), and sonicated by 3 cycles (60 s, 30% amplitude, and shut off for 2 s) while maintained on ice. The quantification of solubilized protein was performed using the Bradford method (Protein Assay Dye Reagent Concentrate, Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer’s instructions. In the sample preparation for advanced mass spectrometry, 50 µg of the sample was subjected to disulfide bridge reduction (50 µg of DTT (Dithiothreitol), 60 min of incubation at 37 °C), followed by alkylation (250 µg of I.A (iodoacetamide), 60 min at room temperature in the dark). Following this, the sample was diluted five times in Tris-hydrochloride (0.05 M Tris-HCl, pH 8.8), and incubated using 2 µg of trypsin (Promega, V511A) at 37 °C overnight. The cleanup and desalting of the sample were performed using C18 resin (Supleco, Bellefonte, PA, USA). The column was calibrated using 2% acetonitrile containing 0.1% formic acid, and the elution was performed with 50% acetonitrile. The sample was then dried in a speed vac and applied to a mass spectrometer (Thermo Fisher Orbitrap Eclipse) coupled to a nanoflow Nano LC-MS/MS chromatography system (Dionex Ultimate 3000 RLSCnano System, Thermofisher, Waltham, MA, USA). Peptides were separated for 90 min in a nanoEase MZ peptide BEH C18 column (130 A, 1.7 µm, 75 µm × 250 mm, Waters, Milford, MA, USA) at 300 nL/min, with a 4–50% acetonitrile gradient. The data were obtained on MS1 in the range of M/Z 375–1500 (120,000 resolution, AGC target 1 × 106, maximum time of injection of 100 ms). The most abundant ions were submitted to MS/MS (30% collision energy, 1.2 m/z, AGC target 1 × 105, 15,000 resolution).
2.9. Proteomic Data Analysis
The proteomic data were processed using the PatternLabV [44]. Firstly, the customized database was created using translated CDS of the TOD651 genome through the Generate Search DB option, including a contaminant library (MS contaminant sequences, e.g., trypsin, keratins, and albumin). Then, proteomic data were analyzed against the customized database using the following parameters: The modifications selected in the search were carbamidomethyl (C), deamination (NQ), and oxidation (M). Enzyme trypsin (fully specific), two maximum missed cleavages, an initial precursor mass tolerance of 35 ppm, MS and MS/MS tolerance errors of 10 ppm, and acceptable FDR (false discovery rate) estimates of 3% at spectral, 2% at peptide, and 1% at protein levels were added as advanced parameters.
The functional annotation of the identified proteins was performed with the UniProtKB/Swiss-Prot database, and the summary graphic of functional classification was created using GO terms through the WEGO 2.0 tool (Web Gene Ontology Annotation Plot) [45].
2.10. Data Availability
The clean reads of the Bt TOD651 strain have been deposited at the Sequence Read Archive (SRA) under the accession number PRJNA907848.
3. Results
3.1. Protein Profile, Crystal Morphology, and Mosquitocidal Activity
The protein profile of Bt TOD651-purified crystals in an SDS-PAGE showed the main proteins with molecular weights of approximately 130, 70, and 27 kDa in size (Figure 1a). The ultra-structural analysis of the spore–crystal mixture indicated the presence of spherical crystals (Figure 1b).
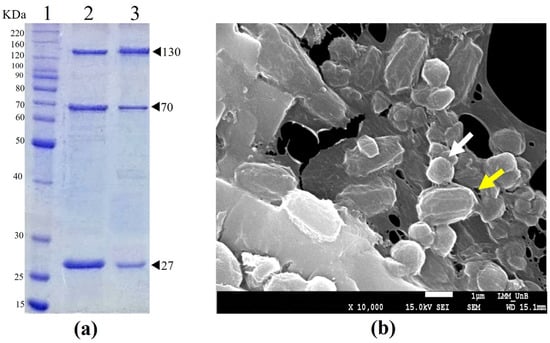
Figure 1.
SDS-PAGE analysis and scanning electron microscopic image of Bt TOD651-purified crystals and the spore–crystal mixture. (a) Protein profile of Bt TOD651-purified crystals: Lane 1—molecular mass marker; Lane 2—AM65-52-purified crystals; Lane 3—TOD651-purified crystals. (b) Scanning electron micrograph of spore–crystal mixture produced by Bt TOD651. Arrows indicate spore (yellow) and crystal (white).
The Bt TOD651 spore–crystal mixture showed mosquitocidal activity towards A. aegypti and C. quinquefasciatus, with 50% lethal concentration (LC50) values of 0.011 and 0.023 µg/mL, respectively (Table 1). High toxicity was observed, but the lethal concentration did not differ between the reference strain and Bt TOD651 for both species.

Table 1.
Lethal concentration estimations of Bt TOD651 to larvae of Aedes aegypti and Culex quinquefasciatus.
3.2. General Genomic Features
Most genes were classified into different functional classes using the RASTtk tool’s analysis. The amino acids and derivatives metabolism class (579 genes), carbohydrate metabolism (320 genes), cofactors, vitamins, prosthetic groups, pigment metabolism subsystems (215 genes), protein metabolism (155 genes), cell wall and capsule (163), nucleosides and nucleotides (159 genes), and protein metabolism were the most common (Figure 2). One hundred and eight (108) genes were grouped in the subsystem class “virulence, disease, and defense.” To assess the virulence factors, the chromosome was assembled at the draft level. This sequence consisted of a chromosome with ~5.4 Mb bp containing 35.9% GC, 5130 CDS, 1 rRNA, and 71 tRNA genes (Table 2). The sequences not used in the chromosome, and presumably belonging to plasmid sequences, were used for the cry/cyt gene screening.
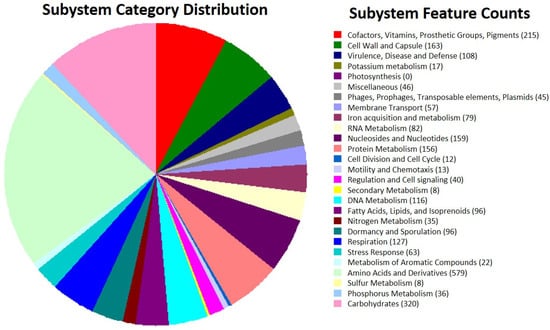
Figure 2.
Subsystem category distributions in the genome of Bt TOD651-based functional classification.

Table 2.
Draft chromosome features of the Bt TOD651 strain.
3.3. Phylogenetic Analysis
Phylogenetic analysis using the gyrB gene showed that the Bt TOD651 strain formed a group closely related to three Bti strains (BGSC 4Q1, BGSC 4Q7rifR, and AM65-52), Bt MYBT18246, Bt ATCC 10792, and Bt serovar thuringiensis IS5056 (Figure 3).
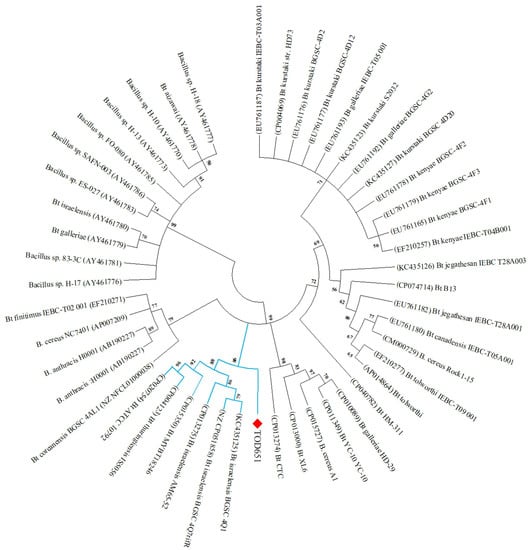
Figure 3.
Phylogenetic analysis of Bt TOD651 with other Bacillus spp. based on gyrB sequences. The phylogenetic tree was constructed using the neighbor-joining method with 1000 bootstrap replications. Bootstrap values < 50% were disregarded for branches.
3.4. Genes Associated with Bt TOD651 Pathogenicity
Different virulence-associated genes were detected in the genome sequence (Table S1, Figure 4). Among them, enzymes (inhA1 and sph), immune evasion (bpsC and polysaccharide capsule genes), iron acquisition genes (dhbA-C, dhbE, dhbF, hal, ilsA, and asbA-F), regulation genes (pagR-XO2, papR, plcR, and cheA), and toxins (hlyI-III, hblA, and nheA-C) were identified (Supplementary Table S1, Figure 4a).
Figure 4.
A graphical representation of the virulence factors and pesticidal protein-like genes found in the draft genome of Bt TOD651. (a) Distribution of virulence factor genes in the draft chromosome. (b) Position of pesticidal protein-like genes in contigs unused in chromosome assembly.
A total of 10 CDS predicted in the Bt TOD651 genome were highly homologous to pesticidal proteins (Figure 4b, Table 3). Four cry genes (cry11Aa3, cry10Aa4, cry4Aa4, and cry4Ba5), three cyt (cyt1Aa5, cyt1Ca1, and cyt2Ba13), two mpp (mpp60Aa3 and mpp60Ba3), and one spp (spp1Aa1) genes were identified (Figure 4b, Table 3).

Table 3.
Identification of genes coding pesticidal protein-like genes in the Bt TOD651.
3.5. Proteomics of Spore–Crystal Mixture
The detected protein sequences were functionally classified into 10 GO terms related to cellular components, 8 GO terms related to molecular functions, and 14 terms related to biological processes (Figure 5). In the cellular component groups, most proteins were mainly related to the cell part and the cell, and the molecular function classification was represented by proteins with catalytic and binding activities. Moreover, in the biological process category, the major portion of proteins belonged to metabolic and cellular processes (Figure 5).
Figure 5.
Functional annotation and classification of proteins identified in the spore–crystal mixture of TOD651.
A comparison of genomic and proteomic data was performed to identify the predicted CDS that were expressed. A total of 43 CDS regions annotated in the genome were detected in the proteomic analysis with at least two peptides (Table 4). With respect to pesticidal proteins, only mpp60Aa3 did not show a unique peptide, and therefore its expression was not confirmed. Thus, the expression of Cry11Aa3, Cry10Aa4, Cry4Aa4, Cry4Ba5, Cyt1Aa5, Cyt1Ca1, Cyt2Ba13, and Mpp60Ba3 was confirmed. Cry4Ba5 was the most abundant peptide discovered (60), followed by Cry4Aa4 (47), Cry11Aa3 (46), Mpp60Aa3 (29), and Cry10Aa4 (29) (Table 4, Supplementary Table S2). Among the cytolytic proteins, Cyt1Aa was the most abundant, showing 13 unique peptides, while Cyt2Ba13 and Cyt1Ca1 showed 7 and 5 unique peptides, respectively (Table 4, Supplementary Table S2). Besides pesticidal proteins, other proteins were also identified. Metallophosphoesterase (Mppe) was the most abundant protease and showed 25 unique peptides (Table 4). Three unique peptides were from the virulence factor, extracellular neutral protease B. Proteins involved in sporulation (spore coat proteins and exosporium protein), protein biosynthesis (chaperone protein, heat-shock protein, translation elongation factor Tu, ribosomal proteins), and other functions (e.g., aminopeptidase, enolase, DNA-binding protein, RNA-binding proteins, and superoxide dismutase) were also identified (Table 4).

Table 4.
Pesticidal and other proteins identified in the spore–crystal mixture of the Bt TOD651 strain.
4. Discussion
Here, we demonstrated that a novel Neotropical Bt strain (TOD651), isolated from Brazilian soil samples, exhibited significant larvicidal activities against larvae of A. aegypti and C. quinquefasciatus. Such larvicidal activities were similarly potent to those recorded in Bt strains that are already commercialized. In addition to the characterization of Bt TOD651 potential to be integrated into biorational programs of mosquito control, we further explored its genome and proteome, and discovered that the pathogenicity may be derived from the expression of pesticidal proteins (e.g., Cry11Aa3, Cry10Aa4, Cry4Aa4, Cry4Ba5, Cyt1Aa5, Cyt2Ba13, and Mpp60Ba3), virulence factors (e.g., Mppe and NprB), and spore coat proteins (e.g., CoatB, CoatE, CoatG, and CotY/CotZ family proteins).
The protein profiles and ultrastructure of the parasporal crystals of the Bt TOD651 strain were observed to be similar to those of other anti-dipteran Bt strains, including the reference strain [46,47]. The SDS-PAGE 130 kDa protein band represents the Cry4 protein, the 70 kDa band indicates the presence of Cry11/Cry10, and the 27 kDa band suggests the presence of the Cyt protein [4,8]. Further, the spherical crystals observed by scanning electron microscopy showed the usual the crystal morphology found in this type of Bt strain [48].
The phylogenetic analysis based on the gyrB gene showed that Bt TOD651 was closely related to Bti strains. In addition, a nematocidal strain, Bt MYBT18246 [49], a type strain, Bt ATCC 10792 (i.e., the nomenclatural type of the species Bt), and a strain toxic against lepidopteran insects, Bt thuringiensis IS5056 [50], were also closely related to Bt TOD651.
The Bt TOD651 strain’s whole-genome analysis revealed the following genes: cry11Aa3, cry10Aa4, cry4Aa4, cry4Ba5, cyt1Aa5, cyt1Ca1, cyt2Ba13, mpp60Aa3, and mpp60Ba3. Other genomic studies revealed a similar gene content in Bt strains with high mosquitocidal activity. For example, Bt AR23 has been described to harbor cry10Aa4, cry11Aa3, cry4Ba5, cry4Aa4, cyt2Ba, cyt1Aa, and cyt1Ca [28], while Bt LLP29 harbors cry4Aa4, cry10Aa4, cry11Aa4, cyt1Aa6, cyt2Ba1, and cry22Aa [29]. The Bt TOD651 genome also harbors different virulence factor genes, such as hemolysins, enterotoxins, proteases, and phospholipases. These virulence factor genes, conserved in the Bacillus cereus group, are responsible for the colonization and adaptation of Bt in insect hosts [51,52,53].
The proteomic analysis revealed the expression of Cry11Aa3, Cry10Aa4, Cry4Aa4, Cry4Ba5, Cyt1Aa5, Cyt2Ba13, and Mpp60Ba3 proteins. Thus, these proteins were responsible for the toxic activity of Bt TOD651 against A. aegypti and C. quinquefasciatus. Stein et al. [54] detected the transcripts of cyt1Ca but did not find Cyt1Ca protein. Here, Cyt1Ca1 protein was expressed. However, it may not be related to the toxicity of Bt TOD651, since neither mosquitocidal nor larvicidal activity function has been reported for Cyt1Ca [55].
Bt TOD651 showed the lower CL50 for A. aegypti. When tested individually, mosquitocidal Cry proteins have different toxicity levels between Aedes and Culex genera. The Cry4Ba protein is highly toxic against A. aegypti, while the Cry4Aa is highly toxic for Culex mosquitoes [56,57]. Cry11Aa has been linked to high toxicity against both the Aedes and Culex genera [56], whereas Cry10Aa is toxic to A. aegypti insects [8]. Mpp60 proteins show moderate insecticidal activity against C. quinquefasciatus larvae [58]. Cyt1Aa and Cyt2Ba exhibit toxicity towards both A. aegypti and C. quinquefasciatus [59,60]. However, these proteins act synergistically, which is mainly attributed to the Cyt1Aa toxin that can increase the activity of Cry4Aa, Cry4Ba, Cry11Aa, or Cry10Aa [61].
Cry10Aa has been reported as the minor protein component of Bti crystals [6]. In the proteomic analysis of Bti 4Q2-72, Cry10Aa presented a low abundance of peptides [62]. Cyt2Aa is also considered a minority pesticidal protein produced by Bti [63]. In the proteomic analysis of the commercial Bti AM65-52, Cry10Aa and Cyt2Ba were not expressed [19]. In line with the Cyt1Aa protein, the Cyt2Ba protein is also highly synergistic with the Cry proteins, and hence their combinations prevent the emergence of resistance in the target insects [4]. In addition, Cry10Aa seems to contribute to the overall toxicity of Bti [4]. Cry10Aa4 was one of the most abundant proteins in the spore–crystal mixture of Bt TOD651, and the peptide number of Cyt2Ba13 was sufficient to not consider it as a trace-level protein.
Cyt1Aa has been reported as the major component of Bti AM65-52 crystals [19], while Bti 4Q2-72 expressed higher levels of Cry11Aa [62]. In contrast, Bt TOD651 expressed mainly Cry4Ba5. Cyt1Aa plays an important role in the synergism of Bti strains and may also contribute to delaying the evolution of resistance to Cry proteins in low proportions [64].
With respect to Mpp proteins, only Mpp60Ba3 expression was detected in this study. Even though the mpp60Aa3 and mpp60Ba3 genes are part of the same operon, neither of them depends on the other to be expressed [58]. SDS-PAGE gel analysis revealed no detectable protein band for Mpp60Ba3. Sauka et al. [65] discovered an mpp homolog in a B. toyonensis strain’s genome but no protein bands by SDS-PAGE analysis, implying that these proteins are secreted and present in remnant fractions. The expression of Mpp60Aa and Mpp60Ba proteins has been detected in Bti AM65-52 [19] and Bt jegathesan [58] in low abundance. In contrast, Mpp60Ba3 represented a high proportion in Bt TOD651, suggesting that it plays an important role in the toxicity of this bacteria.
Metalloproteinases have been described as virulence factors involved in Bt pathogenesis, increasing the toxicity of pesticidal proteins [52]. The immune inhibitor A (InhA) has been identified as the main metalloproteinase associated with pesticidal proteins in spore–crystal mixtures of Bt strains [25,66]. Interestingly, in this study, the high protein homology to Metallophosphoesterase (Mppe) was the most abundant protease in Bt TOD651. A gene of the Mppe family has been identified in the B. cereus genome [67]. Mppe should play an important role in the stress resistance of bacteria to adapt to the environment/host [67]. Neutral protease B (NprB) (also named NprA and Npr99), also present in the spore–crystal mixture of Bt TOD651, has been associated with the virulence of Bacillus anthracis, degrading host tissues and increasing tissue permeability to the pathogen [68].
Other proteins that may contribute to Bt TOD651 toxicity were detected in the spore–crystal mixture, such as spore coat proteins. It has been demonstrated that spores in the spore–crystal mixture play a major role in Bt toxicity, not only due to septicemia from spore germination and outgrowth, but also due to a synergy between the spore coat protein and the crystal protein [69]. Heat-shock proteins and the elongation factor Tu were also detected, which are necessary for the formation of crystals in Bt strains [70,71].
The genomic and proteomic analysis of the Bt TOD651 and other Bt strains can provide insights into the genetic makeup and protein expression of the bacteria. This information can help identify the genes responsible for the bacterium’s insecticidal properties, which can be used for developing new, more effective insecticides. Additionally, genomic and proteomic analyses can provide information on the evolutionary history and diversity of different Bt strains, which can inform their classification to aid in the development of new strains with desired traits.
The Bt TOD651 strain can be used as an alternative for A. aegypti and C. quinquefasciatus control, and the combined genomic and proteomic analyses revealed the proteins directly related to their toxicity. In addition, Bt TOD651 exhibits a variety of protein content that can potentially delay the evolution of resistance. Furthermore, we detected other proteins that can also contribute to Bt TOD651’s pathogenicity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr11051455/s1, Table S1: Virulence factors identified in the chromosome draft sequence of Bt TOD651 from the VFDB database. Table S2: Peptides identified for pesticidal proteins classified as unique (true) or non-unique (false).
Author Contributions
Conceptualization, all authors; methodology, G.B.A. and M.L.D.; writing—original draft preparation, G.B.A., M.L.D. and R.W.d.S.A.; writing—review and editing, B.M.R., G.R.d.S. and E.E.d.O.; supervision, R.W.d.S.A., G.R.d.S., E.E.d.O. and B.M.R.; project administration, R.W.d.S.A.; funding acquisition, R.W.d.S.A. and B.M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Council of Scientific and Technological Development (CNPq—process numbers: 313455/2019-8, 427304/2018-0, 308576/2018-7), the Tocantins State Foundation for Research Aid (FAPT-SESAU/TO-DECIT/SCTIE/MS_CNPQ/No. 01/2017), and the Federal University of Tocantins (PROPESQ)—EDITAL No. 29/2020 PROPESQ, and PPGBIOTEC/UFT/GURUPI—Chamada pública para auxílio de tradução e/ou publicação de artigos cientificos—EDITAL No. 011/2020.
Data Availability Statement
All data and code generated appear in the submitted article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Ramos-Nino, M.E.; Fitzpatrick, D.M.; Eckstrom, K.M.; Tighe, S.; Hattaway, L.M.; Hsueh, A.N.; Cheetham, S. Metagenomic analysis of Aedes aegypti and Culex quinquefasciatus mosquitoes from Grenada, West Indies. PLoS ONE 2020, 15, e0231047. [Google Scholar] [CrossRef] [PubMed]
- Boukedi, H.; Hman, M.; Khedher, S.B.; Tounsi, S.; Abdelkefi-Mesrati, L. Promising active bioinsecticides produced by Bacillus thuringiensis strain BLB427. J. Adv. Res. Rev. 2020, 8, 026–035. [Google Scholar]
- AL-FAR, I.M. Bacillus thuringiensis and its pesticidal crystal proteins. Int. J. Fauna Biol. 2020, 3, 157–162. [Google Scholar]
- Ben-Dov, E. Bacillus thuringiensis subsp. israelensis and its dipteran-specific toxins. Toxins 2014, 6, 1222–1243. [Google Scholar]
- Lutinski, J.A.; Kuczmainski, A.G.; de Quadros, S.; Busato, M.A.; Weirich, C.M.M.; Malgueiro, A.; Garcia, F.R.M. Bacillus thuringiensis var. israelensis como alternativa para o controle populacional de Aedes aegypti (Linnaeus, 1762) (Diptera: Culicidae). Ciência Nat. 2017, 39, 211–220. [Google Scholar]
- Berry, C.; O’Neil, S.; Ben-Dov, E.; Jones, A.F.; Murphy, L.; Quail, M.A.; Holden, M.T.G.; Harris, D.; Zaritsky, A.; Parkhill, J. Complete sequence and organization of pBtoxis, the toxin-coding plasmid of Bacillus thuringiensis subsp. Israelensis. Appl. Environ. Microbiol. 2002, 68, 5082–5095. [Google Scholar] [CrossRef]
- Gray, E.W.; Fusco, R. Microbial control of black flies (Diptera: Simuliidae) with Acillus thuringiensis subsp. israelensis. In Microbial Control of Insect and Mite Pests; Lacey, L.A., Ed.; Academic Press: Yakima, WA, USA, 2017; pp. 367–377. [Google Scholar]
- Valtierra-de-Luis, D.; Villanueva, M.; Berry, C.; Caballero, P. Potential for Bacillus thuringiensis and Other Bacterial Toxins as Biological Control Agents to Combat Dipteran Pests of Medical and Agronomic Importance. Toxins 2020, 12, 773. [Google Scholar] [CrossRef]
- Viana, J.L.; Soares-da-Silva, J.; Vieira-Neta, M.R.A.; Tadei, W.P.; Oliveira, C.D.; Abdalla, F.C.; Peixoto, C.A.; Pinheiro, V.C.S. Isolates of Bacillus thuringiensis from Maranhão biomes with potential insecticidal action against Aedes aegypti larvae (Diptera, Culicidae). Braz. J. Biol. 2020, 81, 114–124. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, Z.; Zhang, J.; Wan, Y.; Jin, W.; Li, Y.; Fang, X. Bacillus thuringiensis novel toxin Epp is toxic to mosquitoes and Prodenia litura larvae. Braz. J. Microbiol. 2020, 51, 437–445. [Google Scholar] [CrossRef]
- Wu, J.; Wei, L.; He, J.; Fu, K.; Li, X.; Jia, L.; Wang, R.; Zhang, W. Characterization of a novel Bacillus thuringiensis toxin active against Aedes aegypti larvae. Acta Trop. 2021, 223, 106088. [Google Scholar] [CrossRef]
- Roy, M.; Chatterjee, S.; Dangar, T.K. Characterization and mosquitocidal potency of a Bacillus thuringiensis strain of rice field soil of Burdwan, West Bengal, India. Microb. Pathog. 2021, 158, 105093. [Google Scholar] [CrossRef] [PubMed]
- Day, M.; Ibrahim, M.; Dyer, D.; Bulla, L. Genome Sequence of Bacillus thuringiensis subsp. kurstaki strain HD-1. Genome Announc. 2014, 2, e00613-14. [Google Scholar] [PubMed]
- Jeong, H.; Choi, S.-K.; Park, S.-H. Genome Sequences of Bacillus thuringiensis serovar kurstaki strain BP865 and B. thuringiensis, serovar aizawai strain HD-133. Genome Announc. 2017, 5, e01544-16. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Geng, C.; Li, M.; Wang, Y.; Liu, H.; Zheng, J.; Sun, M. Whole-genome analysis of Bacillus thuringiensis revealing partial genes as a source of novel cry toxins. Appl. Environ. Microbiol. 2018, 84, e00277-18. [Google Scholar] [CrossRef] [PubMed]
- Lechuga, A.; Lood, C.; Salas, M.; van Noort, V.; Lavigne, R.; Redrejo-Rodríguez, M. Completed genomic sequence of Bacillus thuringiensis HER1410 reveals a Cry-containing chromosome, two megaplasmids, and an integrative plasmidial prophage. G3 Genes Genom. Genet. 2020, 10, 2927–2939. [Google Scholar]
- Susič, N.; Janežič, S.; Rupnik, M.; Stare, B.G. Whole genome sequencing and comparative genomics of two nematicidal Bacillus strains reveals a wide range of possible virulence factors. G3 Genes Genom. Genet. 2020, 10, 881–890. [Google Scholar] [CrossRef]
- Wu, D.; He, J.; Gong, Y.; Chen, D.; Zhu, X.; Qiu, N.; Sun, M.; Li, M.; Yu, Z. Proteomic analysis reveals the strategies of Bacillus thuringiensis YBT-1520 for survival under long-term heat stress. Proteomics 2011, 11, 2580–2591. [Google Scholar] [CrossRef]
- Caballero, J.; Jiménez-Moreno, N.; Orera, I.; Williams, T.; Fernández, A.B.; Villanueva, M.; Ferré, J.; Caballero, P.; Ancín-Azpilicueta, C. Unraveling the composition of insecticidal crystal proteins in Bacillus thuringiensis: A proteomics approach. Appl. Environ. Microbiol. 2020, 86, e00476-20. [Google Scholar] [CrossRef] [PubMed]
- Rang, J.; He, H.; Wang, T.; Ding, X.; Zuo, M.; Quan, M.; Sun, Y.; Yu, Z.; Hu, S.; Xia, L. Comparative analysis of genomics and proteomics in Bacillus thuringiensis 4.0718. PLoS ONE 2015, 10, e0119065. [Google Scholar] [CrossRef]
- Alves, G.B.; Melo, F.L.; Oliveira, E.E.; Haddi, K.; Costa, L.; Dias, M.L.; Campos, F.S.; Pereira, E.J.G.; Côrrea, R.F.T.; Ascêncio, S.D.; et al. Comparative genomic analysis and mosquito larvicidal activity of four Bacillus thuringiensis subsp. israelensis strains. Sci. Rep. 2020, 10, 5518. [Google Scholar] [CrossRef]
- Dankocsik, C.; Donovan, W.P.; Jany, C.S. Activation of a cryptic crystal protein gene of Bacillus thuringiensis subspecies kurstaki by gene fusion and determination of the crystal protein insecticidal specificity. Mol. Microbiol. 1990, 4, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Quan, M.; Xie, J.; Liu, X.; Li, Y.; Rang, J.; Zhang, T.; Zhou, F.; Xia, F.; Hu, S.; Sun, Y.; et al. Comparative analysis of genomics and proteomics in the new isolated Bacillus thuringiensis X022 revealed the metabolic regulation mechanism of carbon flux following Cu2+ treatment. Front. Microbiol. 2016, 7, 792. [Google Scholar] [CrossRef] [PubMed]
- Gomis-Cebolla, J.; Scaramal, R.A.P.; Ferré, J. A genomic and proteomic approach to identify and quantify the expressed Bacillus thuringiensis proteins in the supernatant and parasporal crystal. Toxins 2018, 10, 193. [Google Scholar] [CrossRef] [PubMed]
- Khorramnejad, A.; Gomis-Cebolla, J.; Talaei-Hassanlouei, R.; Bel, Y.; Escriche, B. Genomics and proteomics analyses revealed novel candidate pesticidal proteins in a lepidopteran-toxic Bacillus thuringiensis strain. Toxins 2020, 12, 673. [Google Scholar] [CrossRef]
- Baragamaarachchi, R.Y.; Samarasekera, J.K.R.R.; Weerasena, O.V.D.S.J.; Lamour, K.; Jurat-Fuentes, J.L. Identification of a native Bacillus thuringiensis strain from Sri Lanka active against Dipel-resistant Plutella xylostella. PeerJ 2019, 7, e7535. [Google Scholar] [CrossRef]
- Doggett, N.A.; Stubben, C.J.; Chertkov, O.; Bruce, D.C.; Detter, J.C.; Johnson, S.L.; Han, C.S. Complete genome sequence of Bacillus thuringiensis serovar israelensis strain HD-789. Genome Announc. 2013, 1, e01023-13. [Google Scholar] [CrossRef]
- Fayad, N.; Patiño-Navarrete, R.; Kambris, Z.; Antoun, M.; Osta, M.; Chopineau, J.; Mahillon, J.; El Chamy, L.; Sanchis, V.; Awad, M.K. Characterization and whole genome sequencing of AR23, a highly toxic Bacillus thuringiensis strain isolated from Lebanese soil. Curr. Microbiol. 2019, 76, 1503–1511. [Google Scholar] [CrossRef]
- Ma, W.; Chen, H.; Jiang, X.; Wang, J.; Gelbič, I.; Guan, X.; Zhang, L. Whole genome sequence analysis of the mosquitocidal Bacillus thuringiensis LLP29. Arch. Microbiol. 2020, 202, 1693–1700. [Google Scholar] [CrossRef]
- Monnerat, R.G.; Batista, A.C.; de Medeiros, P.T.; Martins, E.S.; Melatti, V.M.; Praça, L.B.; Dumas, V.F.; Morinaga, C.; Demo, C.; Gomes, A.C.M.; et al. Screening of Brazilian Bacillus thuringiensis isolates active against Spodoptera frugiperda, Plutella xylostella and Anticarsia gemmatalis. Biol. Control 2007, 41, 291–295. [Google Scholar] [CrossRef]
- Mounsef, J.R.; Salameh, D.; Kallassy, A.M.; Chamy, L.; Brandam, C.; Lteif, R. A simple method for the separation of Bacillus thuringiensis spores and crystals. J. Microbiol. Methods 2014, 107, 147–149. [Google Scholar] [CrossRef]
- Aguiar, R.W.S.; dos Santos, S.F.; Morgado, F.S.; Ascencio, S.D.; Lopes, M.M.; Viana, K.F.; Didonet, J.; Ribeiro, B.M. Insecticidal and repellent activity of Siparuna guianensis Aubl. (Negramina) against Aedes aegypti and Culex quinquefasciatus. PLoS ONE 2015, 10, e0116765. [Google Scholar]
- Valbon, W.; Andreazza, F.; Oliveira, E.E.; Liu, F.; Feng, B.; Hall, M.; Klimavicz, J.; Coats, J.R.; Dong, K. Bioallethrin activates specific olfactory sensory neurons and elicits spatial repellency in Aedes aegypti. Pest Manag. Sci. 2022, 78, 438–445. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, R.E.; Dulmage, H.T.; Alls, R.; Couch, T.L.; Dame, D.A.; Hall, I.M.; Rose, R.I.; Versoi, P.L. US standard bioassay for the potency assessment of Bacillus thuringiensis serotype H-14 against mosquito larvae. Bull. ESA 1984, 30, 26–29. [Google Scholar]
- Dulmage, H.T.; Yousten, A.A.; Singer, S.L.L.A.; Lacey, L.A. Guidelines for Production of Bacillus thuringiensis H-14 and Bacillus sphaericus; No. TDR/BCV/90.1; Unpublished; World Health Organization: Geneva, Switzerland, 1990. [Google Scholar]
- SAS Institute. SAS/STAT User’s Guide; SAS Institute: Cary, NC, USA, 2008. [Google Scholar]
- Andrews, S. FastQC: A Quality-Control Tool for High-Throughput Sequence. 2015. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 26 October 2021).
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Lischer, H.E.; Shimizu, K.K. Reference-guided de novo assembly approach improves genome reconstruction for related species. BMC Bioinform. 2017, 18, 474. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, D.; Jin, Q.; Chen, L.; Yang, J. VFDB 2019: A comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res. 2019, 47, D687–D692. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, J.; Bo, D.; Yu, Y.; Ye, W.; Peng, D.; Sun, M. BtToxin_Digger: A comprehensive and high-throughput pipeline for mining toxin protein genes from Bacillus thuringiensis. Bioinformatics 2021, 38, 250–251. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Santos, M.D.; Lima, D.B.; Fischer, J.S.; Clasen, M.A.; Kurt, L.U.; Camillo-Andrade, A.C.; Monteiro, L.C.; de Aquino, P.F.; Neves-Ferreira, A.G.C.; Valente, R.H.; et al. Simple, efficient and thorough shotgun proteomic analysis with PatternLab V. Nat. Protoc. 2022, 17, 1553–1578. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, Y.; Cui, H.; Liu, J.; Wu, Y.; Cheng, Y.; Xu, H.; Huang, X.; Li, S.; Zhou, A.; et al. WEGO 2.0: A web tool for analyzing and plotting GO annotations, 2018 update. Nucleic Acids Res. 2018, 46, W71–W75. [Google Scholar] [CrossRef] [PubMed]
- Elleuch, J.; Jaoua, S.; Darriet, F.; Chandre, F.; Tounsi, S.; Zghal, R.Z. Cry4Ba and Cyt1Aa proteins from Bacillus thuringiensis israelensis: Interactions and toxicity mechanism against Aedes aegypti. Toxicon 2015, 104, 83–90. [Google Scholar] [CrossRef] [PubMed]
- El-Kersh, T.A.; Ahmed, A.M.; Al-Sheikh, Y.A.; Tripet, F.; Ibrahim, M.S.; Metwalli, A.A. Isolation and characterization of native Bacillus thuringiensis strains from Saudi Arabia with enhanced larvicidal toxicity against the mosquito vector Anopheles gambiae (sl). Parasites Vectors 2016, 9, 647. [Google Scholar] [CrossRef] [PubMed]
- Nair, K.; Al-Thani, R.; Al-Thani, D.; Al-Yafei, F.; Ahmed, T.; Jaoua, S. Diversity of Bacillus thuringiensis strains from Qatar as shown by crystal morphology, δ-endotoxins and cry gene content. Front. Microbiol. 2018, 9, 708. [Google Scholar] [CrossRef]
- Hollensteiner, J.; Poehlein, A.; Spröer, C.; Bunk, B.; Sheppard, A.E.; Rosentstiel, P.; Schulenburg, H.; Liesegang, H. Complete genome sequence of the nematicidal Bacillus thuringiensis MYBT18246. Stand. Genom. Sci. 2017, 12, 48. [Google Scholar] [CrossRef]
- Swiecicka, I.; Bideshi, D.K.; Federici, B.A. Novel isolate of Bacillus thuringiensis subsp. thuringiensis that produces a Quasicuboidal crystal of Cry1Ab21 toxic to larvae of Trichoplusia ni. Appl. Environ. Microbiol. 2008, 74, 923–930. [Google Scholar]
- Vilas-Bôas, G.T.; Alvarez, R.C.; dos Santos, C.A.; Vilas-Boas, L.A. Fatores de virulência de Bacillus thuringiensis: O que existe além das proteínas Cry. EntomoBrasilis 2012, 5, 1–10. [Google Scholar] [CrossRef]
- Guillemet, E.; Cadot, C.; Tran, S.L.; Guinebretiere, M.H.; Lereclus, D.; Ramarao, N. The InhA metalloproteases of Bacillus cereus contribute concomitantly to virulence. J. Bacteriol. 2010, 192, 286–294. [Google Scholar] [CrossRef]
- Pohare, M.B.; Wagh, S.G.; Udayasuriyan, V. Bacillus thuringiensis as potential biocontrol agent for sustainable agriculture. In Current Trends in Microbial Biotechnology for Sustainable Agriculture, 1st ed.; Yadav, A.N., Singh, J., Singh, C., Yadav, N., Eds.; Springer: Singapore, 2020; pp. 439–468. [Google Scholar]
- Stein, C.; Jones, G.W.; Chalmers, T.; Berry, C. Transcriptional analysis of the toxin-coding plasmid pBtoxis from Bacillus thuringiensis subsp. israelensis. Appl. Environ. Microbiol. 2006, 72, 1771–1776. [Google Scholar] [CrossRef]
- Cohen, S.; Albeck, S.; Ben-Dov, E.; Cahan, R.; Firer, M.; Zaritsky, A.; Dym, O. Cyt1Aa toxin: Crystal structure reveals implications for its membrane-perforating function. J. Mol. Biol. 2011, 413, 804–814. [Google Scholar] [CrossRef]
- Otieno-Ayayo, Z.N.; Zaritsky, A.; Wirth, M.C.; Manasherob, R.; Khasdan, V.; Cahan, R.; Ben-Dov, E. Variations in the mosquito larvicidal activities of toxins from Bacillus thuringiensis ssp. israelensis. Environ. Microbiol. 2008, 10, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.A.F.; Alzate, O.; Mohammad, M.; McNall, R.J.; Adang, M.J.; Dean, D.H. Introduction of Culex toxicity into Bacillus thuringiensis Cry4Ba by protein engineering. Appl. Environ. Microbiol. 2003, 69, 5343–5353. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Q.; Xia, L.; Ding, X.; Hu, Q.; Federici, B.A.; Park, H.W. Identification and characterization of three previously undescribed crystal proteins from Bacillus thuringiensis subsp. jegathesan. Appl. Environ. Microbiol. 2013, 79, 3364–3370. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Pérez, V.; Guerchicoff, A.; Rubinstein, C.; Delecluse, A. Characterization of Cyt2Bc toxin from Bacillus thuringiensis subsp. medellin. Appl. Environ. Microbiol. 2002, 68, 1228–1231. [Google Scholar] [CrossRef]
- Torres-Quintero, M.C.; Gómez, I.; Pacheco, S.; Sánchez, J.; Flores, H.; Osuna, J.; Mendoza, G.; Soberón, M.; Bravo, A. Engineering Bacillus thuringiensis Cyt1Aa toxin specificity from dipteran to lepidopteran toxicity. Sci. Rep. 2018, 8, 4989. [Google Scholar] [CrossRef]
- Cantón, P.E.; Reyes, E.Z.; Ruiz De Escudero, I.; Bravo, A.; Soberon, M. Binding of Bacillus thuringiensis subsp. israelensis Cry4Ba to Cyt1Aa has an important role in synergism. Peptides 2011, 32, 595–600. [Google Scholar] [PubMed]
- Fu, Z.; Sun, Y.; Xia, L.; Ding, X.; Mo, X.; Li, X.; Huang, K.; Zhang, Y. Assessment of protoxin composition of Bacillus thuringiensis strains by use of polyacrylamide gel block and mass spectrometry. Appl. Environ. Microbiol. 2008, 79, 875–880. [Google Scholar] [CrossRef]
- Valtierra-de-Luis, D.; Villanueva, M.; Lai, L.; Williams, T.; Caballero, P. Potential of Cry10Aa and Cyt2Ba, two minority δ-endotoxins produced by Bacillus thuringiensis ser. israelensis, for the control of Aedes aegypti larvae. Toxins 2020, 12, 355. [Google Scholar]
- Wirth, M.C.; Park, H.W.; Walton, W.E.; Federici, B.A. Cyt1A of Bacillus thuringiensis delays evolution of resistance to Cry11A in the mosquito Culex quinquefasciatus. Appl. Environ. Microbiol. 2005, 71, 185–189. [Google Scholar] [CrossRef]
- Sauka, D.H.; Peralta, C.; Pérez, M.P.; Onco, M.I.; Fiodor, A.; Caballero, J.; Caballero, P.; Berry, C.; Del Valle, E.E.; Palma, L. Bacillus toyonensis biovar thuringiensis: A novel entomopathogen with insecticidal activity against lepidopteran and coleopteran pests. Biol. Control 2022, 167, 104838. [Google Scholar] [CrossRef]
- Banik, A.; Chattopadhyay, A.; Ganguly, S.; Mukhopadhyay, S.K. Characterization of a tea pest specific Bacillus thuringiensis and identification of its toxin by MALDI-TOF mass spectrometry. Ind. Crops Prod. 2019, 137, 549–556. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Xie, T.; Huang, Q.; Xiong, X.; Liu, Q.; Wang, G. The YmdB protein regulates biofilm formation dependent on the repressor SinR in Bacillus cereus 0–9. World J. Microbiol. Biotechnol. 2020, 36, 165. [Google Scholar] [CrossRef]
- Chung, M.C.; Popova, T.G.; Millis, B.A.; Mukherjee, D.V.; Zhou, W.; Liotta, L.A.; Petricoin, E.F.; Chandhoke, V.; Bailey, C.; Popov, S.G. Secreted neutral metalloproteases of Bacillus anthracis as candidate pathogenic factors. J. Biol. Chem. 2006, 281, 31408–31418. [Google Scholar] [CrossRef]
- Johnson, D.E.; Oppert, B.; McGaughey, W.H. Spore coat protein synergizes Bacillus thuringiensis crystal toxicity for the Indianmeal moth (Plodia interpunctella). Curr. Microbiol. 1998, 36, 278–282. [Google Scholar] [CrossRef]
- Ding, X.; Huang, J.; Xia, L.; Li, X.; Yuan, C.; Dan, S. A proteomic analysis approach to study insecticidal crystal proteins from different strains of Bacillus thuringiensis. Biocontrol Sci. Technol. 2009, 19, 289–299. [Google Scholar] [CrossRef]
- Xie, J.; Peng, J.; Yi, Z.; Zhao, X.; Li, S.; Zhang, T.; Quan, M.; Yang, S.; Lu, J.; Zhou, P.; et al. Role of hsp20 in the Production of Spores and Insecticidal Crystal Proteins in Bacillus thuringiensis. Front. Microbiol. 2019, 10, 2059. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).