Abstract
Sinapic acid (SA) is a poorly water-soluble substance which could result in poor bioavailability. The aim of this study was to determine the “hydroxypropyl β-cyclodextrin (HPβCD)” solubilization of SA in the presence of the auxiliary substance hydroxypropyl methylcellulose (HPMC) and to evaluate the ternary inclusion complex prepared by microwave technology. Phase-solubility profiles showed that HPβCD exhibited the greatest solubilizing effect on SA in the presence of HPMC. The enhanced rate of SA dissolution was exhibited by a ternary complex. Outcomes of analyses such as “DSC, FTIR, NMR, and SEM” confirmed the embedding of SA into the cavity of the HPβCD and the formation of a ternary inclusion complex. The outcomes of antioxidant activity (ABTS and nitric oxide scavenging activity) demonstrated that SA ternary inclusion complex (TIC) presented strong antioxidant activity, which might be a result of the enhanced solubility of SA in the TIC prepared by microwave technology. Hence, SA-TIC formulation could be a better dosage form which may protect the body from free radical damage and oxidative stress. Microwave technology greatly boosted the interaction of SA with HPβCD and HPMC, and such findings are expected to contribute to raising the solubility of SA, thereby improving the bioavailability of SA.
1. Introduction
Drugs that are poorly water-soluble have been extensively complexed with cyclodextrins (CDs) in order to increase their solubility in water and their dissolution rate [1]. CDs’ hydrophilic properties have made them popular, because they increase drug solubility, enhance physicochemical properties, and improve chemical stability [2]. It has been observed that CDs form stable complexes with guest molecules with appropriate sizes, because they have hydrophobic central cavities [3]. In recent years, chemically modified CDs, such as “hydroxypropyl β-cyclodextrin (HPβCD)”, have gained importance due to their small cavities and hydrophilicity [4,5]. It was reported that the formation of ternary complexes can improve the solubility of drugs in aqueous solutions when some water-soluble polymer(s) is added [6,7]. As a result of the combination of CD and polymer, the process of drug solubilization was enhanced by a synergistic effect [8]. The use of water-soluble polymers aids in the stabilization of complex aggregates and pharmaceutical particle systems. In addition, they can decrease the CD mobility and increase the solubility of complexes by altering the hydration properties of CD molecules [9]. In a previous study, “hydroxypropyl methylcellulose (HPMC)” was reported to increase daidzein, vinpocetine, and piroxicam complexation with CD [10,11,12,13]. HPMC is a polymer that is soluble in water. The consequences of adding poorly soluble drugs, for instance, SA, to HPβCD/HPMC solubilization have not yet been investigated. Further, it has been also reported that CDs can improve the antioxidant properties of various agents by enhancing their release rate from the inclusion complex [14,15].
Research has shown that flavonoids can provide powerful antioxidant protection against oxidative stress and free radicals in the body [16]. Hydroxycinnamic acid is a phenolic acid that contains bioactive carboxylic acids; examples include caffeic acid, ferulic acid, and sinapic acid (SA) [17,18,19]. SA is abundant in variety of plant sources, such as berry fruits, cereals, citrus, spices, oilseed crops, and vegetables [20]. SA has a molecular weight of 224.21 g/mol, and it is a yellow-brown crystalline powder [18]. SA is considered to be poorly soluble in water, as it exhibits a poor dissolution profile, which leads to poor bioavailability. Researchers have demonstrated that SA has powerful “antioxidant, anti-diabetic, anxiolytic, antibacterial, neuroprotection, anti-inflammatory, hepatoprotection, anti-cancer, renal protection, cardioprotection properties” [17,21]. SA and its derivatives are useful in the pharmaceutical, cosmetic, and food industries due to their antioxidative properties [17,18]. Further, SA may be able to attenuate certain toxic effects that are caused by chemicals [17].
Several reports are available that demonstrate the role of SA in the management of diabetes [22,23,24,25,26]. A key characteristic of diabetes mellitus is hyperglycemia, which causes tissue damage, mediated by oxidative stress [27]. It was reported that “insulin-stimulated glucose uptake into peripheral tissues” is augmented by SA administration. Additionally, SA increases glucose uptake in primary target organs, which likely explains its insulin sensitization properties [16].
This study examined the influence of the ternary inclusion complex of HPβCD/HPMC on SA’s aqueous solubility and dissolution rate, as well as its antioxidant activity. Microwave irradiation (MI) was used to prepare the ternary inclusion complex of SA with HPβCD + HPMC. A phase solubility study was conducted to establish the stability constant of the ternary mixture. Further, the prepared TPM and SA-TIC were compared with SA alone in terms of their dissolution characteristics. In addition, the solid-state properties of pure SA, HPβCD, HPMC, and SA-TIC were characterized by “Differential scanning calorimetry (DSC), Fourier transform infrared spectroscopy (FTIR), nuclear magnetic resonance (NMR), and Scanning electron microscopy (SEM)”. Furthermore, “ABTS (2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)” radical cation and nitric oxide scavenging activity were used to determine the antioxidant activity of prepared SA-TIC and compared with pure SA.
2. Materials and Methods
2.1. Materials
SA was obtained from “Carbosynth Limited, Berkshire, UK”. HPMC and HPβCD were procured from “Sigma-Aldrich, St. Louis, MO, USA”. Ethanol and methanol were purchased from “Scharlab S. L., Sentmenat, Spain and Fisher Scientific UK, Limited, Loughborough, UK”, respectively. Other materials of analytical reagent grade were used.
2.2. Experimental Analysis of Phase Solubility
A phase solubility analysis was performed to determine the stability constant and complexation efficiency of the ternary mixture containing SA/HPβCD/HPMC. In this study, an excess quantity of SA was added into aqueous solutions containing HPβCD (2–10 mM), with and without HPMC (5%) [28]. At 25 °C, the flasks containing samples were repeatedly shaken for 72 h. Supernatants of the samples were carefully pipetted out and filtered across the membrane filter (0.45 µm), then the filtrate was spectrophotometrically analyzed at 322 nm [29,30]. In Equation (1), the stability constant (Kc) is determined by the slope of the phase solubility plot [31,32,33,34]. Equation (2) was used to assess each sample’s complexation efficiency (Ce) [32,35]. S0 is the solubility of SA in water at equilibrium [36,37,38].
2.3. Preparation of Physical Mixture and Inclusion Complex
The SA-ternary physical mixture (SA-TPM, 1:1) was prepared by proper mixing of each component of SA-TPM [SA: HPβCD: HPMC (5%)] with a mortar and pestle. The prepared TPM mixture was stored in a desiccator for dissolution testing. In this study, SA-TIC was prepared by adding the precise amount of SA to ethanolic water (7:3 v/v) along with HPMC. Following the addition of the HPMC to this mixture, the mixture was then placed in a microwave oven which was programmed to emit 600 W of microwave radiation “(Samsung ME0113M1; Malaysia)” [2,39,40]. Samples that had been irradiated were collected and cooled. Following the drying process, the dried mass was ground using a mortar and pestle and then passed through a #80 mesh sieve. A well-sealed desiccator was used to store the prepared dried ternary complex.
2.4. Dissolution Study
The test was carried out using the “USP dissolution apparatus II paddle system” for in vitro dissolution studies. For the assessment of dissolution profiles for SA, TPM, and TIC samples (equivalent to 100 mg of SA) were placed in a dissolution test vessel. The dissolution media, “phosphate buffered with pH 6.8 (900 mL), was kept at 37 °C ± 0.5 °C” and the paddle rotation speed was fixed at 50 rpm. The sample (5 mL) was withdrawn from the dissolution test vessels at every time point up to 60 min, and at the same time refilled with an equivalent volume of dissolution media. The filtered pipetted samples (4 mL) were then assessed for drug concentration using a UV spectrophotometer at 322 nm [29,30].
2.5. Differential Scanning Calorimetry
“DSC, Perkin Elmer, Pyris 6 System, Shelton, CT, USA” was used to assess the thermal analytical determinations of pure SA, HPβCD alone, HPMC alone, and TIC. The test sample of 5 mg was placed in an aluminum pan that had been crimped, and an empty aluminum pan was considered as a standard. During the testing process, a steady rate of 10 °C/min was applied to the DSC system during its operation at a temperature range of 50 °C to 300 °C [41].
2.6. Fourier Transform Infrared Spectroscopy
Further, the FTIR spectra of the pure SA, HPβCD alone, HPMC alone, and TIC sample were analyzed by “Alpha FTIR spectroscopy (Bruker, Karlsruhe, Germany)”. All samples were determined across a spectral range between 400 and 4000 cm−1 [42].
2.7. Nuclear Magnetic Resonance Spectroscopy
The test specimens of the neat SA, HPβCD, and HPMC, as well as the SA-TIC, were solubilized in acetone-d6. Measurements of 1H NMR were conducted by “Bruker NMR spectroscopy”. The values for the spectrum and chemical shift (δ) were reported as ppm [43,44].
2.8. Scanning Electron Microscopy
A SEM microscope (“EVO LS10, Carl Zeiss, Oberkochen, Germany”) was used for the analysis of surface features of pure SA, pure HPβCD, and HPMC, as well as the prepared complex SA-TIC. A SEM scan of the all samples was performed, and they were photographed under magnification and electronically captured [38,45].
2.9. ABTS Radical Cation and Nitric Oxide Scavenging Activity
The SA-TIC radical scavenging activity against the radical cation of ABTS was calculated [46]. Separately, a potassium persulfate solution (2.45 mmol/L) and an ABTS solution (7 mmol/L) were prepared in water. Both solutions were mixed 1:1 and stored at room temperature in the dark for 6 h. This period was marked by the production of the ABTS radical. An aliquot (0.1 mL) of SA-TIC was mixed with 2.9 mL of diluted ABTS radical cation solution. An absorbance measurement at 734 nm was taken after incubating the reaction for 20 min at 30 °C. The potency of the test sample to quench the ABTS free radical was assessed in accordance with Equation (3).
Nitric oxide radical inhibition was estimated using Griess Illosvory reaction [47,48]. The nitric oxide scavenging activity of SA-TIC was estimated by using Equation (3) [49].
2.10. Statistical Analysis
“The findings of phase solubility and dissolution study were assessed statistically via one-way ANOVA followed by Dunnett test and Tukey test, whereas the unpaired t-test was performed for evaluating the significance of antioxidant efficacy. Analyses were conducted by GraphPad InStat® 3.06 (GraphPad Software, Inc., San Diego, CA, USA) and *, $ p < 0.05 was considered significant”.
3. Results and Discussion
3.1. Phase Solubility
Analyses of phase solubility were performed on SA’s solubility in HPβCD with HPMC. It can be seen that SA solubility was augmented in increments in the concentration of HPβCD (Figure 1).
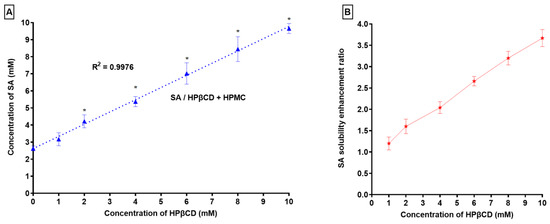
Figure 1.
(A) Phase solubility profile of SA in presence of HPβCD and HPMC (5%) and (B) SA solubility enhancement ratio in presence of HPβCD and HPMC (5%), * p < 0.05 as compared to S0.
The solubility of SA was found to be 3.67 times higher in water in the presence of HPβCD/HPMC. The Kc and Ce values were found to be 961.90 M−1 and 2536.56, respectively. Antecedent reports have revealed that a Kc value between 50 and 5000 M−1 was found to be most suitable for improving the stability and solubility of poorly water-soluble drugs [28,50,51,52,53,54]. In our case, the Kc value was found to be 961.90 M−1; an assessment concluded that the preparation of a SA/HPβCD/HPMC complex was stable [55]. Furthermore, HPMC increased the stability constant of the mixture, which led to enhanced SA solubilization. The outcomes advocating for a strong interaction between SA and HPβCD/HPMC have been accomplished, and this is evident from the higher Kc value.
3.2. Dissolution Study
In Figure 2, the dissolution profiles for SA, SA-TPM, and SA-TIC are illustrated. It was noted that the lowest rate of dissolution of the pure SA (27.63 ± 2.44%) was observed at 60 min compared to the rest of the formulations, such as SA-TPM and SA-TIC.
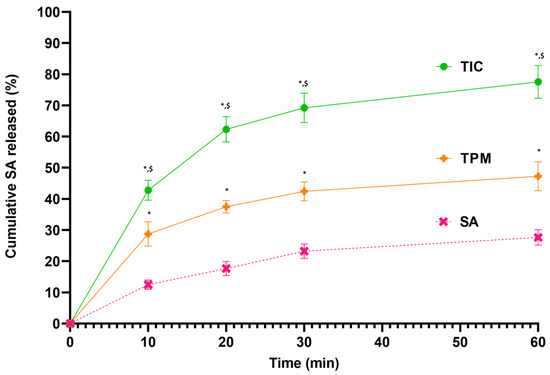
Figure 2.
Dissolution profile of SA, TPM, and TIC, * p < 0.05 as compared to SA, $ p < 0.05 as compared to TPM.
It has been speculated that this may be a result of SA’s poor solubility. Both the TPM and TIC formulations greatly enhanced the rate of dissolution of SA. During the dissolution study, it was found that approximately 47.27 ± 4.65% of the SA was released from TPM at 60 min. SA-TIC further improved the release of SA by a considerable amount compared with the pure SA and TPM. A maximum release of 77.56 ± 5.25% of SA was observed at 60 min from prepared complex SA-TIC (Figure 2). Recently, Ahad et al. developed a binary inclusion complex of SA with HPβCD using the solvent evaporation process. Authors stated that 2.59 times better solubility of SA in water was measured in the existence of HPβCD, and 46.27% drug release was reported for the prepared inclusion complex [56]. Another study evaluated SA-loaded “SNEDDS (self-nanoemulsifying drug delivery systems)” formulation. Comparing SNEDDS with SA suspension, the dissolution of SA was substantially improved. A considerable difference in efficacy was observed between SA suspension and SA in SNEDDS in in vivo anti-inflammatory studies in animal models [57].
In further steps of this study, samples of SA, HPβCD, HPMC, and SA-TIC were evaluated for solid state characterization.
3.3. Differential Scanning Calorimetry
DSC analysis is an important tool to determine the formation of an inclusion complex. In the DSC thermogram of the inclusion complex, the absence of an endothermic peak of the drug signifies that the guest molecule has melted, and this indicates that the complex has formed.
According to Figure 3, a notable absorption peak for SA can be found at 193.07 °C. In another study, Torrisi et al. concluded that the absorption peak is caused by the melting of SA [58]. The sample of HPβCD has a wide endothermic response, ranging from 50 to 100 °C, that can be linked to the water loss from the structure of the HPβCD [59]. It is interesting to note that as opposed to pure SA, the DSC curve of the TIC does not show any endothermic peak of SA at 193 °C. In this way, the disappearance of the endothermic drug peak could be interpreted as an indicator of SA/HPβCD/HPMC ternary complex formation.
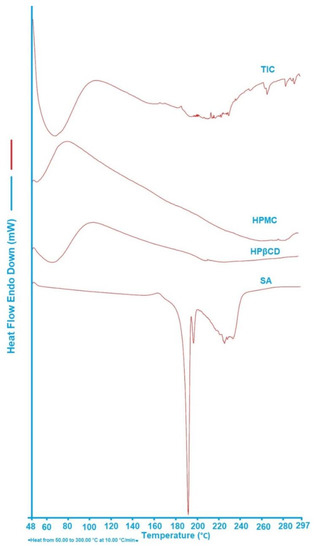
Figure 3.
DSC thermograph of SA, HPβCD, HPMC, and TIC.
3.4. Fourier Transform Infrared Spectroscopy
Character absorption peaks of SA and HPβCD were founded at 621.53, 1109.20, 1258.81, 1658.46 cm−1, 1021.94, and 3344.17 cm−1, respectively (Figure 4), while the stretching vibration at 1049.05 cm−1 was the main noticeable peak for HPMC.
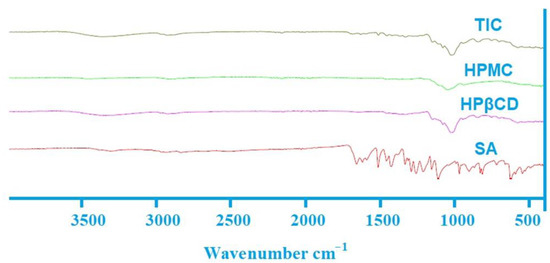
Figure 4.
FTIR spectra of SA, HPβCD, HPMC and TIC.
In this study, it was found that the inclusion complex spectrum differed significantly when compared with the FTIR spectra of pure SA. In addition, substantial decreases in intensity and expanded peak shapes were observed in the FTIR spectra of SA-TIC. This could be due to an interaction that had transpired between SA, HPβCD, and HPMC, resulting in a decrease in peak intensity and displacement. As shown in Figure 4, the characteristic peaks of SA at 621.53 to 1658.46 cm−1 were nearly absent in the inclusion complex spectrum. All such changes in the spectra effectively demonstrated the inclusion of SA into the hydrophobic interior of HPβCD.
3.5. Nuclear Magnetic Resonance Spectroscopy
The proton NMR spectrum of SA in acetone-d6 showed a distinct singlet at 7.62 ppm, which was attributed to the Ar-CH = CH group. The singlet OH and OCH3 peak for the SA was also observed at 𝛿 6.41 and 3.91 ppm, respectively [60,61], while the structure of HPβCD exhibited triplet and duplet peaks at 𝛿 values of 2.07 and 2.09 ppm, respectively (Figure 5). One singlet peak was observed at 𝛿 value 2.06 ppm, and two distinct peaks were also seen at 𝛿 2.86 and 2.90 ppm in the NMR spectra of HPβCD. However, in the NMR spectra of HPMC, distinct peaks were seen at 𝛿 2.06, 2.86, and 2.89 ppm (Figure 5).
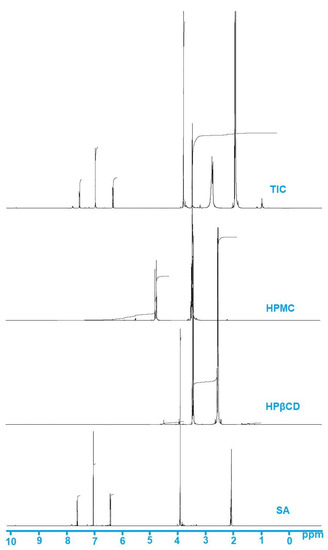
Figure 5.
NMR spectra of SA, HPβCD, HPMC, and TIC.
NMR spectra of the prepared complex SA-TIC portrayed changes in the 𝛿 values, which were observed to be different from the NMR spectra of pure SA, HPβCD, and HPMC. The 1H NMR values of SA-TIC demonstrated that peaks of HPβCD and HPMC showed considerable shifting. Some additional peaks were also detected in SA-TIC samples that could be attributed to the HPβCD and HPMC, demonstrating the development of TIC. The NMR spectroscopic findings depicted evidence of the interaction between the pure SA and HPβCD and HPMC, which contributes to the development of inclusion complexes.
3.6. Scanning Electron Microscopy
As shown in Figure 6, TIC, HPβCD, and HPMC were characterized using SEM. The surface of SA gives the impression of being fairly smooth and compact. HPMC appears to be elongated, thin, crumpled, and slightly distorted. The HPβCD sample has a rough surface and a thick appearance.

Figure 6.
SEM graph of SA, HPβCD, HPMC, and TIC.
In micrographs of TIC, however, SA crystals and HPβCD exhibit different characteristic patterns. No evidence of SA’s compact solid appearance could be found in the TIC complex. When the sample of TIC was observed, it appeared as if HPMC was layered on the surface of SA/HPβCD. The formation of the inclusion complex is strongly supported by the changes in both the crystal structure and morphology observed in TIC sample.
3.7. ABTS Radical Cation and Nitric Oxide Scavenging Activity
Antioxidant activity is more readily recognized using the ABTS assay. This might be due to its more rapidly reaction kinetics and more potent reaction time to antioxidants [62,63]. Nithya and Subramanian found that SA exhibits 86.5% and 88.6% ABTS radical scavenging and superoxide scavenging activity at 50 μM concentration, and this clearly demonstrates its antioxidant potential. The findings of our investigation are consistent with those of the preceding literature, providing evidence that SA strongly scavenges free radicals [64].
SA’s ABTS radical scavenging activity was strongly influenced by the complexation with HPβCD and HPMC. It was demonstrated that the SA-TIC is able to scavenge the ABTS radical cation to a higher extent than pure SA (Figure 7).
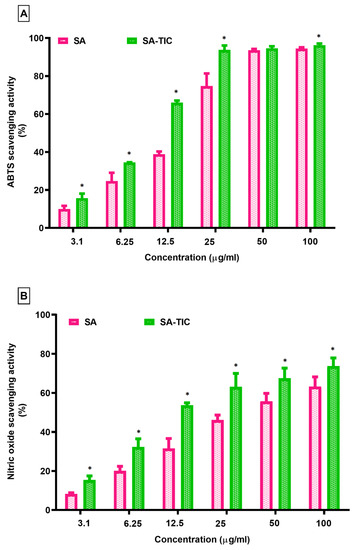
Figure 7.
(A) ABTS and (B) nitric oxide scavenging activity of SA and TIC, * p < 0.05, as compared to SA.
The antioxidant capacity of pure SA was noted to be 94.47% at 100 µg/mL concentration, and it has been found to be significantly higher for SA TIC (96.31%). There was also a substantial difference in nitric oxide scavenging activity between the pure SA and the SA-TIC sample. The sample of SA-TIC demonstrated 73.80% scavenging activity at 100 μg/mL concentration, in contrast to pure SA, which showed 63.28%. The outcomes of ABTS radical cation and nitric oxide scavenging activity demonstrated that the SA-TIC presented strong antioxidant activity. This might be a result of the enhanced solubility of SA in TIC.
Based on the outcomes of present study, it can be concluded that CDs can be used as excipients in SA-loaded formulations, since they form inclusion complexes with SA, showing improvements in physicochemical properties compared to free SA. Further, this study showed improvement in the aqueous solubility of SA without the addition of the organic solvents, surfactants, or lipids. This further increases the dissolution rate, and could contribute to ameliorating the oral bioavailability of SA. In addition to this, SA-TIC exhibited better antioxidant activity compared to SA alone. Hence, SA-TIC formulation could be a better dosage form to protect the body from free radical damage and oxidative stress.
4. Conclusions
It was demonstrated in the present study that the microwave irradiation method was successful in preparing SA ternary inclusion complex with HPβCD/HPMC. Outcomes of phase solubility exhibited that HPβCD/HPMC caused solubilization of SA. Compared to water, SA’s solubility was 3.67 times higher in the presence of HPβCD/HPMC. The formulation of SA-TIC greatly enhanced the rate of dissolution of SA. A release of 77.56% of SA was observed at 60 min from the prepared complex SA-TIC. Further analysis viz. DSC, FTIR, NMR, and SEM confirmed the embedding of SA into the cavity of the HPβCD and the formation of the ternary inclusion complex. Moreover, the SA/HPβCD/HPMC ternary inclusion complex substantially augmented the antioxidant activity of SA. The inclusion complex of SA with HPβCD/HPMC depicted a high dissolution rate and, consequently, it is an excellent candidate for developing pharmaceutical formulations of SA. It is anticipated that the formation of inclusion complexes with CDs may improve the properties of SA formulations, ameliorating the antioxidant effectiveness for future applications.
Author Contributions
“Conceptualization, A.A. and F.I.A.-J.; formal analysis, A.A.; funding acquisition, Y.A.B.J.; writing—original draft, A.A.; writing—review and editing, F.I.A.-J., A.M.A.-M., Y.A.B.J. and M.R. All authors have read and agreed to the published version of the manuscript.”
Funding
“This research is funded by the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia- project number IFKSURG-2-235.”
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data generated from the experiments have been presented in the results.
Acknowledgments
“The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project no. (IFKSURG-2-235).”
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aleem, O.; Kuchekar, B.; Pore, Y.; Late, S. Effect of beta-cyclodextrin and hydroxypropyl beta-cyclodextrin complexation on physicochemical properties and antimicrobial activity of cefdinir. J. Pharm. Biomed. Anal. 2008, 47, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Tan, F.; Jing, Z.; Liu, Z. Preparation and study the 1:2 inclusion complex of carvedilol with beta-cyclodextrin. J. Pharm. Biomed. Anal. 2004, 34, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhu, S. Preparation and characterization of inclusion complexes of prazosin hydrochloride with beta-cyclodextrin and hydroxypropyl-beta-cyclodextrin. J. Pharm. Biomed. Anal. 2006, 40, 122–127. [Google Scholar] [CrossRef]
- Chakraborty, K.K.; Naik, S.R. Therapeutic and hemolytic evaluation of in-situ liposomal preparation containing amphotericin - beta complexed with different chemically modified beta-cyclodextrins. J. Pharm. Pharm. Sci. 2003, 6, 231–237. [Google Scholar] [PubMed]
- Zhu, G.; Xiao, Z. Fabrication and characterization of ethyl acetate-hydroxypropyl-beta-cyclodextrin inclusion complex. J. Food Sci. 2021, 86, 3589–3597. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Cyclodextrins as functional excipients: Methods to enhance complexation efficiency. J. Pharm. Sci. 2012, 101, 3019–3032. [Google Scholar] [CrossRef]
- Hirlekar, R.S.; Sonawane, S.N.; Kadam, V.J. Studies on the effect of water-soluble polymers on drug-cyclodextrin complex solubility. AAPS PharmSciTech 2009, 10, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Frikdriksdóttir, H.; Sigurkdardóttir, A.M.; Ueda, H. The effect of watersoluble polymers on drug-cyclodextrin complexation. Int. J. Pharm. 1994, 110, 169–177. [Google Scholar] [CrossRef]
- Loftsson, T.; Jarho, P.; Masson, M.; Jarvinen, T. Cyclodextrins in drug delivery. Expert Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef]
- Borghetti, G.S.; Pinto, A.P.; Lula, I.S.; Sinisterra, R.D.; Teixeira, H.F.; Bassani, V.L. Daidzein/cyclodextrin/hydrophilic polymer ternary systems. Drug Dev. Ind. Pharm. 2011, 37, 886–893. [Google Scholar] [CrossRef]
- Ribeiro, L.; Loftsson, T.; Ferreira, D.; Veiga, F. Investigation and physicochemical characterization of vinpocetine-sulfobutyl ether beta-cyclodextrin binary and ternary complexes. Chem. Pharm. Bull. 2003, 51, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Ammar, H.O.; Salama, H.A.; Ghorab, M.; Mahmoud, A.A. Implication of inclusion complexation of glimepiride in cyclodextrin-polymer systems on its dissolution, stability and therapeutic efficacy. Int. J. Pharm. 2006, 320, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Jug, M.; Becirevic-Lacan, M.; Cetina-Cizmek, B.; Horvat, M. Hydroxypropyl methylcellulose microspheres with piroxicam and piroxicam-hydroxypropyl-beta-cyclodextrin inclusion complex. Pharmazie 2004, 59, 686–691. [Google Scholar] [PubMed]
- Shiozawa, R.; Inoue, Y.; Murata, I.; Kanamoto, I. Effect of antioxidant activity of caffeic acid with cyclodextrins using ground mixture method. Asian J. Pharm. Sci. 2018, 13, 24–33. [Google Scholar] [CrossRef]
- Li, S.; Yuan, L.; Chen, Y.; Zhou, W.; Wang, X. Studies on the Inclusion Complexes of Daidzein with beta-Cyclodextrin and Derivatives. Molecules 2017, 22, 2183. [Google Scholar] [CrossRef]
- Nithya, R.; Subramanian, S. Sinapic acid, a naturally occurring carboxylic acid derivative ameliorates hyperglycemia in high fat diet-low dose stz induced experimental diabetic rats. Int. J. Sci. Eng. Tech. Res. 2015, 4, 5746–5750. [Google Scholar]
- Chen, C. Sinapic Acid and Its Derivatives as Medicine in Oxidative Stress-Induced Diseases and Aging. Oxid. Med. Cell Longev. 2016, 2016, 3571614. [Google Scholar] [CrossRef]
- Niciforovic, N.; Abramovic, H. Sinapic Acid and Its Derivatives: Natural Sources and Bioactivity. Compr Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef]
- Bunzel, M.; Ralph, J.; Kim, H.; Lu, F.; Ralph, S.A.; Marita, J.M.; Hatfield, R.D.; Steinhart, H. Sinapate dehydrodimers and sinapate-ferulate heterodimers in cereal dietary fiber. J. Agric. Food Chem. 2003, 51, 1427–1434. [Google Scholar] [CrossRef]
- Engels, C.; Schieber, A.; Gänzle, M.G. Sinapic acid derivatives in defatted Oriental mustard (Brassica juncea L.) seed meal extracts using UHPLC-DAD-ESI-MSn and identification of compounds with antibacterial activity. Eur. Food Res. Technol. 2012, 234, 535–542. [Google Scholar] [CrossRef]
- Pandi, A.; Kalappan, V.M. Pharmacological and therapeutic applications of sinapic acid-an updated review. Mol. Biol. Rep. 2021, 48, 3733–3745. [Google Scholar] [CrossRef] [PubMed]
- Altindag, F.; Ragbetli, M.C.; Ozdek, U.; Koyun, N.; Ismael Alhalboosi, J.K.; Elasan, S. Combined treatment of sinapic acid and ellagic acid attenuates hyperglycemia in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2021, 156, 112443. [Google Scholar] [CrossRef] [PubMed]
- Cherng, Y.G.; Tsai, C.C.; Chung, H.H.; Lai, Y.W.; Kuo, S.C.; Cheng, J.T. Antihyperglycemic action of sinapic acid in diabetic rats. J. Agric. Food Chem. 2013, 61, 12053–12059. [Google Scholar] [CrossRef] [PubMed]
- Alaofi, A.L. Sinapic Acid Ameliorates the Progression of Streptozotocin (STZ)-Induced Diabetic Nephropathy in Rats via NRF2/HO-1 Mediated Pathways. Front. Pharmacol. 2020, 11, 1119. [Google Scholar] [CrossRef] [PubMed]
- Zych, M.; Wojnar, W.; Borymski, S.; Szalabska, K.; Bramora, P.; Kaczmarczyk-Sedlak, I. Effect of Rosmarinic Acid and Sinapic Acid on Oxidative Stress Parameters in the Cardiac Tissue and Serum of Type 2 Diabetic Female Rats. Antioxidants 2019, 8, 579. [Google Scholar] [CrossRef] [PubMed]
- Raish, M.; Ahmad, A.; Bin Jardan, Y.A.; Shahid, M.; Alkharfy, K.M.; Ahad, A.; Ansari, M.A.; Abdelrahman, I.A.; Al-Jenoobi, F.I. Sinapic acid ameliorates cardiac dysfunction and cardiomyopathy by modulating NF-kappaB and Nrf2/HO-1 signaling pathways in streptozocin induced diabetic rats. Biomed. Pharmacother. 2022, 145, 112412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, J.; Chen, X.Q.; Zan, K.; Wen, X.D.; Chen, H.; Wang, Q.; Lai, M.X. Antidiabetic and antioxidant effects of extracts from Potentilla discolor Bunge on diabetic rats induced by high fat diet and streptozotocin. J. Ethnopharmacol. 2010, 132, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Loh, G.O.; Tan, Y.T.; Peh, K.K. Effect of HPMC concentration on beta-cyclodextrin solubilization of norfloxacin. Carbohydr. Polym. 2014, 101, 505–510. [Google Scholar] [CrossRef]
- Khattab, R.; Eskin, M.; Aliani, M.; Thiyam, U. Determination of Sinapic Acid Derivatives in Canola Extracts Using High-Performance Liquid Chromatography. J. Am. Oil Chem. Soc. 2010, 87, 147–155. [Google Scholar] [CrossRef]
- Cai, R.; Arntfield, S.D. A rapid high-performance liquid chromatographic method for the determination of sinapine and sinapic acid in canola seed and meal. J. Am. Oil Chem. Soc. 2001, 78, 903–910. [Google Scholar] [CrossRef]
- Ajit Shankarrao, K.; Dhairysheel Mahadeo, G.; Pankaj Balavantrao, K. Formulation and In-vitro Evaluation of Orally Disintegrating Tablets of Olanzapine-2-Hydroxypropyl-beta-Cyclodextrin Inclusion Complex. Iran. J. Pharm. Res. 2010, 9, 335–347. [Google Scholar] [PubMed]
- Maeda, H.; Tanaka, R.; Nakayama, H. Inclusion complexes of trihexyphenidyl with natural and modified cyclodextrins. SpringerPlus 2015, 4, 218. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Roy, S.; Kumar, A.; Mahmood, S.; Khodapanah, N.; Thomas, S.; Agatemor, C.; Ghosal, K. Physicochemical Characterization, Molecular Docking, and In Vitro Dissolution of Glimepiride-Captisol Inclusion Complexes. ACS Omega 2020, 5, 19968–19977. [Google Scholar] [CrossRef] [PubMed]
- Talegaonkar, S.; Khan, A.Z.; Khar, R.K.; Ahmad, F.J.; Khan, Z.I. Development and characterization of paracetamol complexes with hydroxypropyl-ß-cyclodextrin. Iran. J. Pharm. Res. 2007, 6, 95–99. [Google Scholar]
- Brewster, M.E.; Vandecruys, R.; Peeters, J.; Neeskens, P.; Verreck, G.; Loftsson, T. Comparative interaction of 2-hydroxypropyl-beta-cyclodextrin and sulfobutylether-beta-cyclodextrin with itraconazole: Phase-solubility behavior and stabilization of supersaturated drug solutions. Eur. J. Pharm. Sci. 2008, 34, 94–103. [Google Scholar] [CrossRef]
- Jansook, P.; Loftsson, T. CDs as solubilizers: Effects of excipients and competing drugs. Int. J. Pharm. 2009, 379, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Hreinsdóttir, D.; Másson, M. The complexation efficiency. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 545–552. [Google Scholar] [CrossRef]
- Da Silva Mourao, L.C.; Ribeiro Batista, D.R.M.; Honorato, S.B.; Ayala, A.P.; de Alencar Morais, W.; Barbosa, E.G.; Raffin, F.N.; de Lima e Moura, T.F.A. Effect of hydroxypropyl methylcellulose on beta cyclodextrin complexation of praziquantel in solution and in solid state. J. Incl. Phenom. Macrocycl. Chem. 2016, 86, 151–160. [Google Scholar] [CrossRef]
- Moneghini, M.; Zingone, G.; Zordi, N. Influence of microwave technology on the physical-chemical properties of solid dispersion with nimesulide. Powder Tech. 2009, 195, 259–263. [Google Scholar] [CrossRef]
- Moneghini, M.; Bellich, B.; Baxa, P.; Princivalle, F. Microwave generated solid dispersions containing Ibuprofen. Int. J. Pharm. 2008, 361, 125–130. [Google Scholar] [CrossRef]
- Burga-Sanchez, J.; Ferreira, L.E.N.; Volpato, M.C.; Cabeca, L.F.; Braga, M.; Fraceto, L.F.; de Paula, E.; Groppo, F.C. Physicochemical characterization and cytotoxicity of articaine-2-hydroxypropyl-beta-cyclodextrin inclusion complex. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Sbarcea, L.; Tanase, I.M.; Ledeti, A.; Circioban, D.; Vlase, G.; Barvinschi, P.; Miclau, M.; Varut, R.M.; Suciu, O.; Ledeti, I. Risperidone/Randomly Methylated beta-Cyclodextrin Inclusion Complex-Compatibility Study with Pharmaceutical Excipients. Molecules 2021, 26, 1690. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Stalin, T. Study of inclusion complex between 2,6-dinitrobenzoic acid and beta-cyclodextrin by 1H NMR, 2D 1H NMR (ROESY), FT-IR, XRD, SEM and photophysical methods. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 130, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Lima, B.D.S.; Campos, C.A.; da Silva Santos, A.C.R.; Santos, V.C.N.; Trindade, G.; Shanmugam, S.; Pereira, E.W.M.; Marreto, R.N.; Duarte, M.C.; Almeida, J.; et al. Development of morin/hydroxypropyl-beta-cyclodextrin inclusion complex: Enhancement of bioavailability, antihyperalgesic and anti-inflammatory effects. Food Chem. Toxicol. 2019, 126, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Al-Qubaisi, M.S.; Rasedee, A.; Flaifel, M.H.; Eid, E.E.M.; Hussein-Al-Ali, S.; Alhassan, F.H.; Salih, A.M.; Hussein, M.Z.; Zainal, Z.; Sani, D.; et al. Characterization of thymoquinone/hydroxypropyl-beta-cyclodextrin inclusion complex: Application to anti-allergy properties. Eur. J. Pharm. Sci. 2019, 133, 167–182. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Afsar, T.; Razak, S.; Shabbir, M.; Khan, M.R. Antioxidant activity of polyphenolic compounds isolated from ethyl-acetate fraction of Acacia hydaspica R. Parker. Chem. Cent. J. 2018, 12, 5. [Google Scholar] [CrossRef]
- Tahir, I.; Khan, M.R.; Shah, N.A.; Aftab, M. Evaluation of phytochemicals, antioxidant activity and amelioration of pulmonary fibrosis with Phyllanthus emblica leaves. BMC Complement. Altern. Med. 2016, 16, 406. [Google Scholar] [CrossRef]
- Ansari, M.A.; Raish, M.; Ahmad, A.; Ahmad, S.F.; Mudassar, S.; Mohsin, K.; Shakeel, F.; Korashy, H.M.; Bakheet, S.A. Sinapic acid mitigates gentamicin-induced nephrotoxicity and associated oxidative/nitrosative stress, apoptosis, and inflammation in rats. Life Sci. 2016, 165, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Patel, M. Preparation and evaluation of inclusion complex of the lipid lowering drug lovastatin with ?-Cyclodextrin. Dhaka Univ. J. Pharm. Sci. 2007, 6, 25–36. [Google Scholar] [CrossRef]
- Sapkal, N.P.; Kilor, V.A.; Shewale, B.D.; Bhusari, K.P.; Daud, A.S. Study of the Complexation Behaviour of Fexofenadine with beta-Cyclodextrin. Indian J. Pharm. Sci. 2010, 72, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Batt, D.K.; Garala, K.C. Preparation and evaluation of inclusion complexes of diacerein with ?-cyclodextrin and hydroxypropyl ?-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2013, 77, 471–481. [Google Scholar] [CrossRef]
- Pu, H.; Sun, Q.; Tang, P.; Zhao, L.; Li, Q.; Liu, Y.; Li, H. Characterization and antioxidant activity of the complexes of tertiary butylhydroquinone with beta-cyclodextrin and its derivatives. Food Chem. 2018, 260, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Hreinsdottir, D.; Masson, M. Evaluation of cyclodextrin solubilization of drugs. Int. J. Pharm. 2005, 302, 18–28. [Google Scholar] [CrossRef]
- Heydari, A.; Iranmanesh, M.; Doostan, F.; Hassan, S. Preparation of inclusion complex between nifedipine and ethylenediamine-?-Cyclodextrin as nanocarrier agent. Pharm. Chem. J. 2015, 49, 605–612. [Google Scholar] [CrossRef]
- Ahad, A.; Bin Jardan, Y.A.; Raish, M.; Al-Mohizea, A.M.; Al-Jenoobi, F.I. Hydroxypropyl-?-cyclodextrin for delivery of sinapic acid via inclusion complex prepared by solvent evaporation method. Processes 2022, 10, 2046. [Google Scholar] [CrossRef]
- Shakeel, F.; Raish, M.; Anwer, M.K.; Al-Shdefat, R. Self-nanoemulsifying drug delivery system of sinapic acid: In vitro and in vivo evaluation. J. Mol. Liq. 2016, 224, 351–358. [Google Scholar] [CrossRef]
- Torrisi, C.; Morgante, A.; Malfa, G.; Acquaviva, R.; Castelli, F.; Pignatello, R.; Sarpietro, M.G. Sinapic acid release at the cell level by incorporation into nanoparticles: Experimental evidence using biomembrane models. Micro 2021, 1, 120–128. [Google Scholar] [CrossRef]
- Linares, M.S.; Longhi, M.R. Effects of hydroxypropyl-beta-cyclodextrin on the chemical stability of a naphthoquinone in aqueous solutions. Pharmazie 2003, 58, 32–37. [Google Scholar]
- Padmashali, B.; Chidananda, B.N.; Govindappa, B.; Basavaraj, S.M.; Chandrashekharappa, S.; Venugopala, K.N. Synthesis and characterization of novel 1,6-dihydropyrimidine derivatives for their pharmacological properties. J. Appl. Pharm. Sci. 2019, 9, 133–140. [Google Scholar]
- Rubino, M.I.; Arntfield, S.D.; Charlton, J.L. Evaluation of alkaline conversion of sinapic acid to thomasidioic acid. J. Agric. Food Chem. 1996, 44, 1399–1402. [Google Scholar] [CrossRef]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef] [PubMed]
- Balaci, T.; Velescu, B.; Karampelas, O.; Musuc, A.M.; Nitulescu, G.M.; Ozon, E.A.; Nitulescu, G.; Gîrd, C.E.; Fita, C.; Lupuliasa, D. Physico-chemical and pharmaco-technical characterization of inclusion complexes formed by rutoside with ?-cyclodextrin and hydroxypropyl-?-cyclodextrin used to develop solid dosage forms. Processes 2021, 9, 26. [Google Scholar] [CrossRef]
- Nithya, R.; Subramanian, S. Antioxidant properties of sinapic acid: In vitro and in vivo approach. Asian J. Pharm. Clin. Res. 2017, 10, 255–262. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).