Abstract
The term infertility is defined as the lack of conception within 1 year of unprotected intercourse. It affects more than 80 million individuals worldwide. It is estimated that 10-15% of couples of reproductive age are challenged by reproductive issues. Assisted reproduction techniques (ART) are responsible for more than 4% of live births. Our aim is to review the research on neurodevelopmental outcomes of newborns born after the implementation of assisted reproduction methods compared to those conceived naturally. We conducted a comprehensive search of the PubMed, Crossref and Google Scholar electronic databases for related articles up to June 2022 using the PRISMA guidelines. Our research revealed a large number of long term follow-up studies between 2 and 18 years of age, with comparable developmental outcomes. Many studies compared the effects of different infertility treatments against natural conception. The review of the literature revealed that ART is safe, as the majority of studies showed no effect on the neurodevelopmental outcomes of the offspring. In most cases when such an effect was observed, it could be attributed to confounding factors such as subfertility, multiple pregnancies and gestational age at delivery. Finally, the increase in the prevalence of neurodevelopmental disorders after ART, as described in studies with statistically significant results, is predominantly marginal, and given the low incidence of neurodevelopmental disorders in the general population, its clinical significance is debatable.
1. Introduction
The term infertility is defined as the lack of conception within 1 year of unprotected intercourse [1]. It affects more than 80 million individuals worldwide [1]. It is estimated that 10–15% of couples of reproductive age are challenged by reproductive issues [1]. These couples resort to assisted reproduction technology to achieve their reproductive goals. Assisted reproduction techniques (ART) are responsible for more than 4% of live births [2]. Concurrently, the number of oocyte donation (OD) treatment cycles performed every year in Europe and the US has reached 50,000 [3].
Assisted reproduction techniques have been associated with an increase in the prevalence of fetal morbidities such as increased blood pressure [4,5], metabolic disorders [6] and reproductive tract anomalies such as hypospadias [7]. Furthermore, it has been shown that among singleton pregnancies, adverse perinatal outcomes, including preterm delivery, placenta previa, low birth weight infants and a decreased incidence of spontaneous cephalic delivery are more common in individuals conceived with ART procedures regardless of the type of procedure used [8]. Multiple studies have suggested that the contribution of maternal factors associated with infertility to the adverse outcomes may be more important than the ART procedures themselves [8,9,10,11,12]. The results from multiple pregnancies are similar [13]. However, multiple gestations have been shown to have worse neonatal outcomes compared to singleton pregnancies [13].This has resulted in a move towards the transfer of a single embryo (SET), aiming to minimize the perinatal risks associated with children born after ART.
When evaluating the effects of ART, we must keep in mind the heterogeneity of procedures utilized to treat infertility. Assisted reproduction techniques incorporate a variety of infertility treatments aiming to achieve conception such as artificial insemination, intrauterine insemination, ovulation induction, in vitro fertilization, intracytoplasmic sperm injection, cryopreservation of gametes and embryos and oocyte donation. It is evident that due to the great number of different procedures there is an increased number of possible associations to be evaluated.
The aim of this review is to amass the research on the neurodevelopmental outcomes of newborns conceived with the use of assisted reproduction methods and compare it with newborns conceived naturally. Assisted reproduction techniques have been associated with an increase in the prevalence of fetal morbidities [4,5,6,7] and adverse perinatal outcomes. Hence, it is of the upmost importance to evaluate whether the neurodevelopmental outcomes of children born after ART are affected in comparison with children conceived naturally. In this review we will summarize all the latest data and evaluate the possible association of neurodevelopmental disorders with the different methods of assisted reproduction.
2. Materials and Methods
This review was planned, organized, and developed following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines (Figure 1) [14]. We conducted a comprehensive search of the PubMed, Crossref and Google Scholar electronic databases for articles up to June 2022, using the search terms assisted reproduction techniques (ART), neurodevelopment disorders, neurodevelopment outcomes, mental health, verbal ability, autism spectrum disorders (ASD), and cerebral palsy (CP) in conjunction with fertility treatments, assisted conception, natural conception (NC), in vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI), frozen embryo and vitrified oocytes. Titles, summaries, and abstracts of all identified studies were checked for study design, type of association and outcome. The full text of relevant articles was carefully read and analyzed. Narrative and systematic reviews were excluded from our review, while cohort studies, case control studies and clinical trials were included in our study.
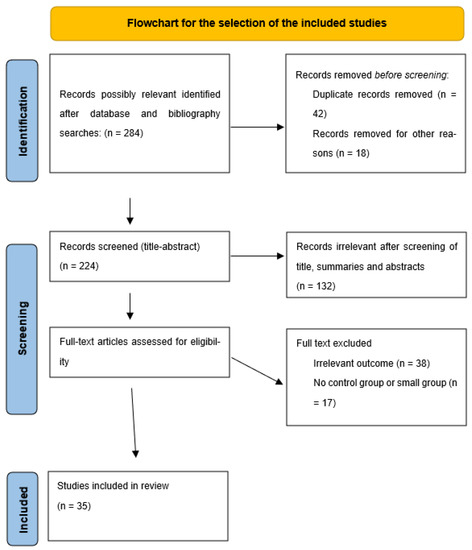
Figure 1.
Flowchart of the selection of the included studies.
3. Results
Given the variability of Assisted Reproduction Technologies used in current practice, we elected to present the results comparing the neurodevelopmental outcomes of ART vs. natural conception in general and then analyze specific aspects of ART such as intracytoplasmic sperm injection (ICSI), which has been the focus of a lot of research in the past few years. Furthermore, we compared the incidence of autism in offspring born via natural conception versus ART. All the studies analyzed below are also available in Table 1.

Table 1.
Overview of studies on the neurodevelopmental outcomes of infants conceived via assisted reproduction techniques versus naturally conceived children.
3.1. ART versus Natural Conception
There are a lot of studies that have evaluated the neurodevelopmental outcomes of offspring conceived by ART vs. children conceived naturally. A very important prospective cohort study conducted by the Integrated Research Network in Perinatology of Quebec and Eastern Ontario in Canada compared cognitive, motor, and language neurodevelopmental outcomes between ART and natural conception groups at 24 months of age [17]. This study recruited 2366 pregnant women, of whom 278 conceived with ART between 2010 to 2012. This study, also known as the 3D-Study (Découvrir, Développer, Devenir), revealed no difference in cognitive scores, composite motor scores and language scores during the neurodevelopmental assessment at the age of 2 years old. Furthermore, no difference was observed when independent ART techniques were compared nor when comparing in vivo (ovarian stimulation or intrauterine insemination) or in vitro (in vitro fertilization, intracytoplasmic sperm injection, or in vitro maturation) techniques [17].
Another very important population-based, prospective cohort study was conducted using the Swedish national health archive [39]. This study included children born between 1982 and 2007 and followed for a clinical diagnosis of autistic disorder or mental retardation until the end of 2009 [39]. Out of more than 2.5 million infants born, 30.959 (1.2%) were conceived by ART, and they were followed up for a mean of ten years. There was no statistically significant association for either outcome after restricting analysis to singleton pregnancies [39].
Two studies from Denmark, with data from 1995 to 2003 and 2003 to 2008, respectively, compared IQ scores and selective or sustained attention scores between children conceived via ART and children born after natural conception [18,25]. These studies included 588,967 children (33,139 ART) and 1782 children (205 infertility group & 1577 spontaneously) respectively [18,25]. No statistically different outcomes were documented after correcting for confounding factors such as birthweight, maternal age and parity.
Τwo smaller cohort studies evaluated the effects of ovarian stimulation on the neurodevelopmental outcomes of offspring. Τhe Groningen ART cohort study which included a total of 254 children (66 ovarian stimulation/IVF, 87 natural conception-subfertile and 101 reference group) and the Netherland prospective cohort study which included a total of 665 singletons (68 after controlled ovarian stimulation-IVF/ICSI, 57 natural cycle-IVF/ICSI, 90 naturally conceived singletons of subfertile couples and 450 control group) both concluded that the neurological outcome is not influenced by ovarian hyperstimulation [2,36].
The New York prospective cohort study included 5841 children (1830 ART & 2074 twins) through 36 months of age [46]. It analyzed five developmental domains (fine motor, gross motor, communication, personal-social functioning, and problem-solving ability), as measured by the parental completion of the Ages and Stages Questionnaires at 4, 8, 12, 18, 24, 30, and 36 months of age [46]. The study indicated that infertility treatment was not associated with an increased risk of the children failing in any developmental domain after excluding multiple deliveries [46].
Finally, a case control study from an Iranian Assisted Reproduction Center, which included 400 children conceived via ART and 420 controls conceived naturally, compared the neurodevelopmental status at 9 months old [28]. This study revealed no difference in the neurodevelopmental status at nine months between naturally conceived children and children born with ART.
It is apparent that in the absence of confounding factors, the literature overwhelmingly shows that the neurodevelopmental outcomes of children born after infertility treatment are on par with the neurodevelopmental outcomes of naturally conceived offspring.
3.2. Long-Term Follow Up
For the most part, studies that compare the neurodevelopmental outcomes of children born with ART vs. naturally conceived offspring focus in the first three years of life. However, given that several mental disorders are diagnosed later in life, it is important to assess the neurodevelopmental outcome of children during the first two decades of their life [20,47].
Zhu et al, utilized data from three population-based birth cohorts (the Aalborg-Odense Birth Cohort, the Aarhus Birth Cohort, and the Danish National Birth Cohort) from Denmark to compare the incidence of behavioral problems in children born to fertile and infertile couples [20]. The children studied were between the ages of 7 and 21 years. No statistically significant difference was detected regarding behavioral problems regardless of the presence of infertility and the infertility treatment used [20].
Another large population-based cohort study from the United Kingdom investigated the possible influence of infertility treatment to the cognitive development of offspring at the ages of 3 and 5 [42]. It included 18.818 children and concluded that neither subfertility nor ART adversely affected children’s cognitive development at ages 3 and 5 [42].
Similarly, other smaller studies observed no statistically significant differences in the neurological outcomes after long-term follow up of offspring conceived via ART vs. children conceived naturally. Takeshige et al. compared neurological outcomes of offspring conceived from vitrified oocytes after ICSI with the national average data from Japan at regular intervals between three months and six years of age [22]. No statistically significant differences were observed regarding the neurological outcomes [22]. Punamaki et al. prospectively followed up 255 singleton children born after ART (164 IVF/76 ICSI) and compared their cognitive development and mental health at the age of 7–8 years old with 278 naturally conceived children without detecting any statistically significant differences [9]. Wagenar et al. compared 139 adolescents born after IVF with 143 control adolescents regarding attention, information processing and visual-motor function, and did not detect any statistically significant differences [43]. A recent study from Farhi et al. compared different neurodevelopmental measures of children conceived by ART (n = 358) compared to spontaneously conceived offspring (n = 401), concluding that there is no statistically significant difference between the two groups [22]. Finally, Bay et al. conducted a prospective register-based cohort study in Denmark and included all the children born in Denmark between 1995 and 2003, with follow-up in 2012 when they were aged between 8 and 17 years old [19]. The numbers of the children studied were 33,139 conceived after fertility treatment and 555,828 after spontaneous conception. Conversely to the previously mentioned studies, the study from Denmark revealed a statistically significant increase in mental disorders in offspring born after ovulation induction/intrauterine insemination (iui) compared to naturally conceived offspring [19]. However, the same was not true about in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) in the same population [19]. No specific type of hormone drug treatment was related to the higher risk of mental disorders [19].
3.3. ICSI vs. Natural Conception
The term intracytoplasmic sperm injection (ICSI) is used to describe the assisted reproductive method that involves choosing a single sperm cell and injecting it manually into the ovum [48].
There are a lot of studies evaluating the neurodevelopmental outcomes of children born after ICSI in comparison either with natural conception or other assisted reproduction techniques. The majority of both cohort [16,26,31,38,39,42,44] and case control studies [40,41] are in accordance with the notion that children conceived via ICSI are not at higher risk of mental disorders.
One of the largest population-based cohort studies was conducted by the Taipei Medical University between 2004 and 2016 [44]. It included 23,885 children, (23,148 born after natural conception and 737 after ICSI) and concluded that there is no difference in the risk of developing neurodevelopmental disorders among the two groups [44]. On par with the previous study, a prospective cohort study conducted by Agarwal et al. compared the neurodevelopmental outcome at 2 years of age between 76 offspring conceived via ICSI and 261 matched controls, and concluded that neurodevelopmental and functional outcomes were similar in both groups [16].
A study conducted by Ponjaert-Kristoffersen et al. matched three hundred singleton children conceived via ICSI with spontaneously conceived controls from Belgium, Sweden, and the USA [38]. They compared their psychological well-being and cognitive development at the age of 5 and concluded that there was no statistically significant difference between the two groups [38]. A cohort study conducted in Australia included 89 children born after ICSI, 84 born after IVF and 80 conceived naturally [31]. The results of this study suggest that children conceived using ICSI do not have an increased risk of delayed mental development at 5 years of age [31].
Ludwig et al. conducted a prospective controlled single-blinded study to assess the neurodevelopmental health of children born after ICSI [33]. The study included 276 children born after ICSI and 273 naturally conceived singletons at 5.5 years old [33]. The results showed no difference regarding neurologic examination, motor skills, emotional/behavioral development, and intelligence [33]. Similarly, Sutcliffe et al. compared the outcomes of 208 children conceived via ICSI with 221 naturally conceived children at 17 months old. As with previously mentioned studies, no significant difference was detected regarding the neurodevelopmental ability of children conceived after ICSI vs. children conceived naturally [41]. A smaller study from the same lead author of a cohort of Australian children yielded the same results [40].
A small number of studies have associated ICSI with poorer neurodevelopmental outcomes of the offspring. However, most of these studies have included a very small number of patients and have neglected to account for confounding factors.
The study by Sandin et al. included a very large number of patients (2.5 million, 30.959 of whom born after ART) and followed them for a considerable amount of time (10 years) [39]. However, initial results showing an increase in the prevalence of mental retardation and autistic disorder in children born after ART were swiftly dismissed when restricting the analysis to singleton pregnancies [39]. As for the effects of ICSI on the development of offspring, after adjusting for singleton pregnancies, a statistically significantly association of ICSI with mental retardation was observed [39]. However, there are certain limitations one needs to consider in order to properly interpret these results. Firstly, the prevalence of mental retardation is very low, and the risk associated with the procedure is very small. Secondly, important confounding factors such as parental socioeconomic status were not evaluated during the analysis. Hence, the results of this study must be interpreted carefully.
Knoester et al. conducted a study of singletons born between June and December 1996 at Leiden University Medical Center aged between 5 and 8 years old [29]. The researchers compared the IQ scores of 252 infants (83 born after ICSI, 83 born after IVF and 86 spontaneously conceived), and found that cognitive development was lower among singletons born after ICSI compared to the other two categories [29]. Although the results seem alarming, one has to consider the limited sample size, possible selection bias, unavailable parental IQ scores, and lack of clinical significance of the mean difference in IQ, which was between 3 and 7 points [29].
3.4. Autism Spectrum Disorders and ART
An aspect of the neurodevelopmental outcome of children which has come under a lot of attention in recent years is autism. Autism spectrum disorder (ASD) is a neurological and developmental disorder that affects the way people interact, communicate with others, learn, and behave [49]. It is important to investigate if offspring after ART are at an increased risk of autism spectrum disorders compared to those conceived naturally.
There are a few case-control studies that claim no association between autism spectrum disorders and ART. One study was conducted in Finland and studied 4164 cases with autism and 16,582 matched controls born in 1991–2005 [30]. Another study was conducted in Iran, and it included 100 cases with ASD and 200 controls between 2 and 10 years of age [27].
Larger studies, which reached the same conclusions, were conducted in Massachusetts, Taiwan, and California. The Massachusetts study included 10,147 children born after ART, 8072 offspring born from subfertile couples without the use of assisted reproduction and 441,898 children born from fertile couples and concluded that compared to children born to fertile women, children born to ART, ICSI, or IVF, or subfertile women are not at increased risk of receiving an ASD diagnosis [21]. The Taiwan Birth Cohort Study, using a national birth cohort dataset, reached the same conclusion regarding the lack of association between ART and ASD [34]. An observational cohort study from California studied all 5,926,251 live births from 1997 to 2007 and revealed no association between ART and ASD, while adjusting for possible confounding factors [23]. Another large population-based prospective cohort study using the Swedish national health registers from 1982 to 2007 showed no association between ART and ASD overall, while other possible associations between ASD and specific techniques such as ICSI were not statistically significant when the analysis was restricted to singleton pregnancies [39]. Finally, a prospective cohort study based on data from the Danish National Health Register which included all children born alive between 1995 and 2003 showed a comparable risk for ASD between children conceived naturally and children born after IVF or ICSI [19]. A marginal but statistically significant association was noted between induced ovulation and intrauterine insemination and ASD, which was not the case with any other study [19].
After reviewing all available research on the possible association between ART and ASD, the overwhelming majority of data support the absence of association. Hence, development of ASD should not be a concern for couples resorting to ART, as no association has been documented for the majority of ART techniques. However, since a possible mild association was noted in one of the published studies, further evaluation is warranted to determine the validity of the association between ovulation induction/iui and ASD.
4. Discussion
This review summarizes the existing literature on the neurodevelopmental outcomes in offspring born after ART compared to those conceived naturally. Despite the initial perception that there is an association between ART and neurodevelopmental disorders, the elimination of confounding factors results in a lack of such an association in the majority of studies.
When discussing neurodevelopmental outcomes, it is of great importance to analyze long-term outcomes. Several studies have looked into long-term neurodevelopmental outcomes of children born after ART. Long-term outcomes include comparisons of neurodevelopmental outcomes not only during early childhood but for the first two decades of their life [9,20,22,29,42,43,45,46]. None of the studies showed a greater risk of mental disorders for children born from ART, with the exception of a follow-up study from Denmark that showed a low but statistically significant risk of mental disorders after ovulation induction [19].
ICSI, being based on the non-natural selection of sperm, came under a lot of research regarding offspring outcomes. Recent data suggest that children born after ICSI do not have an increased risk of developing mental disorders [16,26,32,38,39,40,41,42,44]. However, two studies [38,44] observed a higher incidence of mental and psychological disorders in male offspring born after ICSI, and one study suggested a possible relationship between ICSI and mental retardation [39].
The majority of the studies that reported a possible association between ART and neurodevelopmental disorders in offspring did not take into account multiple gestation, maternal age, prematurity, birthweight, socioeconomic and health lifestyle differences. One of the most important contributors to poor neurodevelopmental outcomes is multiple gestation [23,25,35,45]. In many cases, limiting the analysis to singleton pregnancies diminishes any previously occurring statistically significant differences [45]. Furthermore, apart from multiple gestations, subfertility factors such as maternal age and paternal infertility have been associated with poorer neurodevelopmental outcomes in offspring [39]. Both Schendelaar et al. and Goldsmith et al. showed that poorer neurodevelopmental outcomes are associated with infertility rather than the assisted reproduction procedures [2,24]. Other factors highly associated with an increased prevalence of mental disorders include preterm delivery and low birth weight, [15,18,37,45]. Another weakness of the studies that show poorer neurodevelopmental outcomes after ART is the fact that the type of procedure linked to poorer neurodevelopmental outcomes varies between studies (ICSI, ovulation induction, intrauterine insemination etc.). Finally, the increase in the prevalence of neurodevelopmental disorders after ART, as described in studies with statistically significant results, is predominantly marginal, and given the low incidence of neurodevelopmental disorders in the general population, its clinical significance is debatable.
On the other hand, one should not overlook the facts that some of the studies that have observed statistically significantly worse neurodevelopmental outcomes in children conceived after various ART procedures include a large number of patients [19,39]. These studies, despite their weaknesses, have been conducted with the proper methodology and have taken into account a number of confounding factors. It should be noted that adjusting the analysis for multiple confounding factors may be necessary, but it results in lowering the power of the study and hence results are less likely to be statistically significant.
This review summarizes the existing literature on the potential relationship between assisted reproduction techniques and the risk of increased incidence of neurodevelopmental disorders in the offspring compared to pregnancies conceived naturally. Our study has some limitations. Firstly, great heterogeneity was observed among most of the analyzed studies. This is not surprising, as the included studies investigated diverse populations, from numerous geographic regions, different age groups, fertility treatments, ART procedures and delivery year (1984–2020). Consequently, the antenatal care varied depending on the country and year of delivery. However, it is important that the majority of the studies adjusted the statistical analysis for the most common confounding factors such as multiple gestations, birthweight, gestational age and maternal age. However, other confounding factors such as the socioeconomic status of the parents, mental health and educational level, which may affect the incidence of neurodevelopmental outcomes in the offspring, were not always considered. Finally, the term neurodevelopmental disorder is very diverse, including abnormalities ranging from mild disorders to very serious, debilitating conditions. Hence, comparison between different studies is even more challenging.
5. Conclusions
The greater part of the literature shows no association between ART procedures and poorer neurodevelopmental outcomes in offspring. However, there is a small number of studies that have demonstrated possible associations between various ART procedures and different neurodevelopmental disorders. The interpretation of these studies must be made carefully, as accounting for confounding factors may negate the proposed association. In any case, even when an association is proposed between ART and a worse neurodevelopmental outcome, the clinical impact is expected to be very small. Hence, ART procedures should be considered safe regarding the incidence of neurodevelopmental disorders in offspring.
Author Contributions
Conceptualization, A.R., G.D.; methodology, P.A., A.P.; validation, P.A., M.T., M.S.; formal analysis, P.P., A.P.; investigation, P.P., A.K., T.N.; resources, P.P., T.N.; data curation, A.K., Z.F.; writing—original draft preparation, P.P., A.P., Z.F.; writing—review and editing, P.A., M.T., P.A.; visualization, G.D., A.R.; supervision, G.D., A.R.; project administration, G.D., A.R.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Luke, B. Pregnancy and birth outcomes in couples with infertility with and without assisted reproductive technology: With an emphasis on US population-based studies. Am. J. Obstet. Gynecol. 2017, 217, 270–281. [Google Scholar] [CrossRef]
- Schendelaar, P.; Middelburg, K.J.; Bos, A.F.; Heineman, M.J.; Jongbloed-Pereboom, M.; Hadders-Algra, M. The Groningen ART cohort study: The effects of ovarian hyperstimulation and the IVF laboratory procedures on neurological condition at 2 years. Hum. Reprod. 2011, 26, 703–712. [Google Scholar] [CrossRef][Green Version]
- Storgaard, M.; Loft, A.; Bergh, C.; Wennerholm, U.B.; Söderström-Anttila, V.; Romundstad, L.B.; Aittomaki, K.; Oldereid, N.; Forman, J.; Pinborg, A. Obstetric and neonatal complications in pregnancies conceived after oocyte donation: A systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 561–572. [Google Scholar] [CrossRef]
- Scherrer, U.; Rimoldi, S.F.; Rexhaj, E.; Stuber, T.; Duplain, H.; Garcin, S.; de Marchi, S.F.; Nicod, P.; Germond, M.; Allemann, Y.; et al. Systemic and Pulmonary Vascular Dysfunction in Children Conceived by Assisted Reproductive Technologies. Circulation 2012, 125, 1890–1896. [Google Scholar] [CrossRef]
- Meister, T.A.; Rimoldi, S.F.; Soria, R.; von Arx, R.; Messerli, F.H.; Sartori, C.; Scherrer, U.; Rexhaj, E. Association of Assisted Reproductive Technologies with Arterial Hypertension During Adolescence. J. Am. Coll. Cardiol. 2018, 72, 1267–1274. [Google Scholar] [CrossRef]
- Guo, X.Y.; Liu, X.M.; Jin, L.; Wang, T.T.; Ullah, K.; Sheng, J.Z.; Huang, H.-F. Cardiovascular and metabolic profiles of offspring conceived by assisted reproductive technologies: A systematic review and meta-analysis. Fertil. Steril. 2017, 107, 622–631.e5. [Google Scholar] [CrossRef]
- Eisenberg, E. Long-term outcomes in children born after assisted conception. Semin. Reprod. Med. 2012, 30, 123–130. [Google Scholar] [CrossRef]
- Hayashi, M.; Nakai, A.; Satoh, S.; Matsuda, Y. Adverse obstetric and perinatal outcomes of singleton pregnancies may be related to maternal factors associated with infertility rather than the type of assisted reproductive technology procedure used. Fertil. Steril. 2012, 98, 922–928. [Google Scholar] [CrossRef]
- Punamäki, R.L.; Tiitinen, A.; Lindblom, J.; Unkila-Kallio, L.; Flykt, M.; Vänskä, M.; Poikkeus, P.; Tulppala, M. Mental health and developmental outcomes for children born after ART: A comparative prospective study on child gender and treatment type. Hum. Reprod. 2016, 31, 100–107. [Google Scholar] [CrossRef]
- Sazonova, A.; Kllen, K.; Thurin-Kjellberg, A.; Wennerholm, U.B.; Bergh, C. Factors affecting obstetric outcome of singletons born after IVF. Hum. Reprod. 2011, 26, 2878–2886. [Google Scholar] [CrossRef][Green Version]
- Pinborg, A.; Loft, A.; Schmidt, L.; Andersen, A.N. Morbidity in a Danish national cohort of 472 IVF/ICSI twins, 1132 non-IVF/ICSI twins and 634 IVF/ICSI singletons: Health-related and social implications for the children and their families. Hum. Reprod. 2003, 18, 1234–1243. [Google Scholar] [CrossRef]
- Place, I.; Englert, Y. A prospective longitudinal study of the physical, psychomotor, and intellectual development of singleton children up to 5 years who were conceived by intracytoplasmic sperm injection compared with children conceived spontaneously and by in vitro fertilization. Fertil. Steril. 2003, 80, 1388–1397. [Google Scholar]
- Qin, J.; Wang, H.; Sheng, X.; Liang, D.; Tan, H.; Xia, J. Pregnancy-related complications and adverse pregnancy outcomes in multiple pregnancies resulting from assisted reproductive technology: A meta-analysis of cohort studies. Fertil. Steril. 2015, 103, 1492–1508.e7. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Abdel-Latif, M.E.; Bajuk, B.; Ward, M.; Oei, J.L.; Badawi, N. Neurodevelopmental outcomes of extremely premature infants conceived after assisted conception: A population based cohort study. Arch. Dis. Child.-Fetal Neonatal Ed. 2013, 98, F205–F211. [Google Scholar] [CrossRef]
- Agarwal, P.; Loh, S.K.E.; Lim, S.B.; Sriram, B.; Daniel, M.L.; Yeo, S.H.; Heng, D. Two-year neurodevelopmental outcome in children conceived by intracytoplasmic sperm injection: Prospective cohort study. BJOG Int. J. Obstet. Gynaecol. 2005, 112, 1376–1383. [Google Scholar] [CrossRef]
- Balayla, J.; Sheehy, O.; Fraser, W.D.; Séguin, J.R.; Trasler, J.; Monnier, P.; MacLeod, A.; Simard, M.-N.; Muckle, G.; Bérard, A. Neurodevelopmental Outcomes After Assisted Reproductive Technologies. Obstet. Gynecol. 2017, 129, 265–272. [Google Scholar] [CrossRef]
- Bay, B.; Mortensen, E.L.; Kesmodel, U.S. Fertility treatment and child intelligence, attention, and executive functions in 5-year-old singletons: A cohort study. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 1642–1651. [Google Scholar] [CrossRef]
- Bay, B.; Mortensen, E.L.; Hvidtjørn, D.; Kesmodel, U.S. Fertility treatment and risk of childhood and adolescent mental disorders: Register based cohort study. BMJ 2013, 347, f3978. [Google Scholar] [CrossRef]
- Carson, C.; Kelly, Y.; Kurinczuk, J.J.; Sacker, A.; Redshaw, M.; Quigley, M.A. Effect of pregnancy planning and fertility treatment on cognitive outcomes in children at ages 3 and 5: Longitudinal cohort study. BMJ 2011, 343, d4473. [Google Scholar] [CrossRef]
- Diop, H.; Cabral, H.; Gopal, D.; Cui, X.; Stern, J.E.; Kotelchuck, M. Early Autism Spectrum Disorders in Children Born to Fertile, Subfertile, and ART-Treated Women. Matern. Child Health J. 2019, 23, 1489–1499. [Google Scholar] [CrossRef]
- Farhi, A.; Glasser, S.; Gabis, L.V.; Hirsh-Yechezkel, G.; Frank, S.; Brinton, L.; Scoccia, B.; Ron-El, R.; Orvieto, R.; Lerner-Geva, L. How Are They Doing? Neurodevelopmental Outcomes at School Age of Children Born Following Assisted Reproductive Treatments. J. Child Neurol. 2021, 36, 262–271. [Google Scholar] [CrossRef]
- Fountain, C.; Zhang, Y.; Kissin, D.M.; Schieve, L.A.; Jamieson, D.J.; Rice, C.; Bearman, P. Association between assisted reproductive technology conception and autism in California, 1997–2007. Am. J. Public Health 2015, 105, 963–971. [Google Scholar] [CrossRef]
- Goldsmith, S.; Mcintyre, S.; Badawi, N.; Hansen, M. Cerebral palsy after assisted reproductive technology: A cohort study. Dev. Med. Child Neurol. 2018, 60, 73–80. [Google Scholar] [CrossRef]
- Hvidtjørn, D.; Grove, J.; Schendel, D.; Sværke, C.; Schieve, L.A.; Uldall, P.; Ernst, E.; Jacobsson, B.; Thorsen, P. Multiplicity and early gestational age contribute to an increased risk of cerebral palsy from assisted conception: A population-based cohort study. Hum. Reprod. 2010, 25, 2115–2123. [Google Scholar] [CrossRef]
- Husen, S.C.; Koning, I.V.; Go, A.T.J.I.; Groenenberg, I.A.L.; Willemsen, S.P.; Rousian, M.; Steegers-Theunissen, R.P.M. IVF with or without ICSI and the impact on human embryonic brain development: The Rotterdam Periconceptional Cohort. Hum. Reprod. 2021, 36, 596–604. [Google Scholar] [CrossRef]
- Jenabi, E.; Seyedi, M.; Hamzehei, R.; Bashirian, S.; Rezaei, M.; Razjouyan, K.; Khazaei, S. Association between assisted reproductive technology and autism spectrum disorders in Iran: A case-control study. Clin. Exp. Pediatr. 2020, 63, 368–372. [Google Scholar] [CrossRef]
- Kermani, R.M.K.; Nedaeifard, L.; Nateghi, M.; Fazeli, A. Neurodevelopmental Status of Infants Conceived Assisted Reproductive Techniques in Royan Institute. J. Fam. Reprod. Health 2011, 5, 79–83. [Google Scholar]
- Knoester, M.; Helmerhorst, F.M.; Vandenbroucke, J.P.; van der Westerlaken, L.A.J.; Walther, F.J.; Veen, S. Cognitive development of singletons born after intracytoplasmic sperm injection compared with in vitro fertilization and natural conception. Fertil. Steril. 2008, 90, 289–296. [Google Scholar] [CrossRef]
- Lehti, V.; Brown, A.S.; Gissler, M.; Rihko, M.; Suominen, A.; Sourander, A. Autism spectrum disorders in IVF children: A national case-control study in Finland. Hum. Reprod. 2013, 28, 812–818. [Google Scholar] [CrossRef]
- Leslie, G.I.; Gibson, F.L.; McMahon, C.; Cohen, J.; Saunders, D.M.; Tennant, C. Children conceived using ICSI do not have an increased risk of delayed mental development at 5 years of age. Hum. Reprod. 2003, 18, 2067–2072. [Google Scholar] [CrossRef]
- Leunens, L.; Celestin-Westreich, S.; Bonduelle, M.; Liebaers, I.; Ponjaert-Kristoffersen, I. Follow-up of cognitive and motor development of 10-year-old singleton children born after ICSI compared with spontaneously conceived children. Hum. Reprod. 2008, 23, 105–111. [Google Scholar] [CrossRef]
- Ludwig, A.; Katalinic, A.; Thyen, U.; Sutcliffe, A.G.; Diedrich, K.; Ludwig, M. Neuromotor development and mental health at 5.5 years of age of singletons born at term after intracytoplasmatic sperm injection ICSI: Results of a prospective controlled single-blinded study in Germany. Fertil. Steril. 2009, 91, 125–132. [Google Scholar] [CrossRef]
- Lung, F.W.; Chiang, T.L.; Lin, S.J.; Lee, M.C.; Shu, B.C. Assisted reproductive technology has no association with autism spectrum disorders: The Taiwan Birth Cohort Study. Autism 2018, 22, 377–384. [Google Scholar] [CrossRef]
- Maheshwari, A.; Raja, E.A.; Bhattacharya, S. Obstetric and perinatal outcomes after either fresh or thawed frozen embryo transfer: An analysis of 112,432 singleton pregnancies recorded in the Human Fertilisation and Embryology Authority anonymized dataset. Fertil. Steril. 2016, 106, 1703–1708. [Google Scholar] [CrossRef]
- Middelburg, K.J.; Heineman, M.J.; Bos, A.F.; Pereboom, M.; Fidler, V.; Hadders-Algra, M. The Groningen ART cohort study: Ovarian hyperstimulation and the in vitro procedure do not affect neurological outcome in infancy. Hum. Reprod. 2009, 24, 3119–3126. [Google Scholar] [CrossRef]
- Middelburg, K.J.; Haadsma, M.L.; Heineman, M.J.; Bos, A.F.; Hadders-Algra, M. Ovarian hyperstimulation and the in vitro fertilization procedure do not influence early neuromotor development; a history of subfertility does. Fertil. Steril. 2010, 93, 544–553. [Google Scholar] [CrossRef]
- Ponjaert-Kristoffersen, I.; Tjus, T.; Nekkebroeck, J.; Squires, J.; Verté, D.; Heimann, M.; Bonduelle, M.-L.; Palermo, G.; Wennerholm, U.-B. Psychological follow-up study of 5-year-old ICSI children. Hum. Reprod. 2004, 19, 2791–2797. [Google Scholar] [CrossRef]
- Sandin, S.; Nygren, K.G.; Iliadou, A.; Hultman, C.M.; Reichenberg, A. Autism and mental retardation among offspring born after in vitro fertilization. JAMA 2013, 310, 75–84. [Google Scholar] [CrossRef]
- Sutcliffe, A.G.; Saunders, K.; McLachlan, R.; Taylor, B.; Edwards, P.; Grudzinskas, G.; Leiberman, B.; Thornton, S. A retrospective case-control study of developmental and other outcomes in a cohort of Australian children conceived by intracytoplasmic sperm injection compared with a similar group in the United Kingdom. Fertil. Steril. 2003, 79, 512–516. [Google Scholar] [CrossRef]
- Sutcliffe, A.G.; Taylor, B.; Saunders, K.; Thornton, S.; Lieberman, B.A.; Grudzinskas, J.G. Outcome in the second year of life after in-vitro fertilisation by intracytoplasmic sperm injection: A UK case-control study. Lancet 2001, 357, 2080–2084. [Google Scholar] [CrossRef]
- Takeshige, Y.; Takahashi, M.; Hashimoto, T.; Kyono, K. Six-year follow-up of children born from vitrified oocytes. Reprod. BioMed. Online 2021, 42, 564–571. [Google Scholar] [CrossRef]
- Wagenaar, K.; van Weissenbruch, M.M.; Knol, D.L.; Cohen-Kettenis, P.T.; Delemarre-Van De Waal, H.A.; Huisman, J. Information processing, attention and visual-motor function of adolescents born after in vitro fertilization compared with spontaneous conception. Hum. Reprod. 2009, 24, 913–921. [Google Scholar] [CrossRef]
- Wang, C.W.; Chang, T.H.; Chuang, N.C.; Au, H.K.; Chen, C.H.; Tseng, S.H. Association between intracytoplasmic sperm injection and neurodevelopmental outcomes among offspring. PLoS ONE 2021, 16, e0257268. [Google Scholar] [CrossRef]
- Yeung, E.H.; Sundaram, R.; Bell, E.M.; Druschel, C.; Kus, C.; Ghassabian, A.; Bello, S.; Xie, Y.; Louis, G.B. Examining Infertility Treatment and Early Childhood Development in the Upstate KIDS Study. JAMA Pediatr. 2016, 170, 251–258. [Google Scholar] [CrossRef]
- Zhu, J.L.; Obel, C.; Basso, O.; Henriksen, T.B.; Bech, B.H.; Hvidtjãrn, D.; Olsen, J. Infertility, infertility treatment and behavioural problems in the offspring. Paediatr. Perinat. Epidemiol. 2011, 25, 466–477. [Google Scholar] [CrossRef]
- Barfield, W.D. Public Health Implications of Very Preterm Birth. Clin. Perinatol. 2018, 45, 565–577. [Google Scholar] [CrossRef]
- Chen, H.; Feng, G.; Zhang, B.; Zhou, H.; Wang, C.; Shu, J.; Gan, X.; Lin, R.; Huang, D.; Huang, Y. A new insight into male fertility preservation for patients with completely immotile spermatozoa. Reprod. Biol. Endocrinol. 2017, 15, 74. [Google Scholar] [CrossRef]
- NIMH. Autism Spectrum Disorder. Available online: https://www.nimh.nih.gov/health/topics/autism-spectrum-disorders-asd (accessed on 3 August 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).