Abstract
Cerebral palsy generates an elevated burden on both patients and health-care systems. Cost-effective therapies such as action observation therapy (AOT), have been proposed to enhance motor performance in these patients. Objective: This systematic review with meta-analysis aimed to evaluate the effectiveness of AOT in children and adolescents with CP and describe its prescription parameters. Results: Fourteen studies involving a total of 393 patients with CP were included. Most studies presented some concerns on risk of bias. Meta-analyses compared AOT to placebo (no motor content) observation and found inconclusive results for the following: unilateral upper limb function (g = 0.565; 95% CI −0.174, 1.305), assisting hand function during bimanual activities (g = 0.200; 95% CI −0.742, 1.143), manual function daily activities (g = −0.022; 95% CI −3.134, 3.090), and hand grip strength (MD (kg) = 1.175; 95% CI −0.280, 2.630). Meta-analysis comparing AOT and physical therapy also yielded inconclusive findings for standing (g = 0.363; 95% CI −5.172, 5.898), as well as the combined dimension of walking, standing, and jumping (g = 0.798; 95% CI −8.821, 10.417) within gross motor function. Conclusions: Current evidence is imprecise and does not support definitive conclusions regarding the effectiveness of AOT over placebo observation, or over physical therapy, on functional outcomes including upper limb, hand, and lower limb functioning parameters. Current findings prevent recommending AOT for its employment in clinical practice. Further evidence is required to draw precise conclusions.
1. Introduction
Cerebral palsy (CP) has a global prevalence of 2.11 per 1000 live births, a prevalence that has shown an increasing trend from 1988 to 2019 [1]. CP is a condition that imposes high functional limitations, its severity evidenced by the fact that 61.8% of these patients have a Gross motor function classification system (GMFCS) level from II to V, and 33% have no independent gait [2]. Due to these limitations, the economic costs of this pathology are estimated to reach near one million dollars per new CP patient per patient [3].
Action observation therapy (AOT) involves observing movement as displayed on a video or performed live, which can be applied alone or in combination with the execution of the observed movement [4]. AOT has been widely investigated in neurorehabilitation [4], and recently, in treating musculoskeletal disorders and pain [5,6].
Neuroimaging studies have shown that both AO and execution activate similar brain regions, considered the equivalent of the mirror neuron system (MNS) in non-human primates. These regions include the premotor cortex, supplementary motor cortex, occipital cortex, parietal cortex, basal ganglia, and cerebellum [7,8]. Based on these findings, it is hypothesized that the functional changes observed after AOT rely on neuroplastic changes in these neural systems [9].
Studies have attempted to determine which parameters most effectively activate the MNS in humans during AO. Kemmerer et al. [10] proposed a combination of such parameters to facilitate AOT prescription. However, they may differ in pediatric populations. Recent research has shown that the neurological substrates involved in AO differ between healthy children (7–10 years) and younger adults. Specifically, the overlap between brain regions activated by both AO and action execution is more extensive in young adults. Moreover, within the pediatric population, this AO–execution overlap tends to increase with age, and is associated with better motor performance [11]. In contrast, children with neurological conditions show altered AO and imitation abilities compared to typically developed children [12]. Additionally, it remains unclear which subgroups within the CP population may benefit most from AOT.
Notably, AOT could be a promising intervention for children with CP, as healthy children are known to improve motor learning when exposed to structured AO [13].
AOT is supported by a well-documented neurophysiological mechanisms and has demonstrated effectiveness under various neurological conditions, such as Parkinson’s disease and stroke [14,15]. An influential systematic review including the traffic light system by Novak et al. [16] strongly recommended AOT for clinical implementation. However, this recommendation was primarily based on only two small clinical trials [17,18], raising concerns about the robustness for supporting the therapy.
More recently, two meta-analyses have attempted to update the evidence for AOT in CP [19,20]. Nevertheless, several methodological limitations comprise the strength of their conclusions. For example, Demeco et al. [20] appeared to combine in a single meta-analysis different types of comparisons, such as AOT versus placebo [21], and studies evaluating the additive effect of observation to repeated practice [22], blurring the interpretation of what is actually being tested.
Yang et al. [19], on the other hand, focused exclusively on the effectiveness of AOT versus placebo for upper limb function. It provided inconclusive results due to imprecise estimates, and did not explore the effectiveness in other functional outcome measures.
Current evidence remains insufficient to determine whether AOT is superior to placebo, whether adding observation enhances the effects of repeated practice, or whether AOT is more beneficial than other therapies. This information should be provided for functional measures including upper limb, hand, or lower limb functions.
This systematic review with meta-analysis aims to analyze the effectiveness of AOT in children and adolescents with CP. In addition, AOT prescription parameters will be extracted to provide a detailed description of AOT protocols for their replicability.
2. Materials and Methods
This systematic review was conducted following the guidelines of the Preferred Reporting Guidelines for Systematic Reviews and Meta-Analyses (PRISMA) [23], and it was registered in the International Prospective Register of Systematic Reviews (PROSPERO), CRD42022347350.
2.1. Eligibility Criteria
Eligibility of studies was structured following the PICOS strategy. Studies should include the following: (1) Population diagnosed with CP; (2) preschool children (2–5 years), children (6–12 years), and/or adolescents (13–18 years); (3) comparisons of interest included AOT compared to placebo (no motor content) observation, the addition of AOT to physical therapy, and AOT against conventional physical therapy; (4) analysis focused on functional outcomes, including manual tasks, functions in upper limb movement, strength, balance, lower limb performance, and gross motor function immediately after the end of treatment. Kinematic measures were obviated because the primary objective was to evaluate practical changes in task performance rather than the specific movement strategies or joint kinematics involved, which, while informative, are indirect measures of task performance. Finally, (5) randomized clinical trial (RCT) studies were eligible.
Study protocols, non-scientific articles, and articles without full text were excluded. No language restrictions were applied.
2.2. Searches and Selection Process
Two independent reviewers (JFM and BRdR) conducted the same search strategy across PubMed, EMBASE, Web of Science, EBSCO, Cochrane Central, Google Scholar, and PEDro in April 2022. Independent manual searches were also performed until October 2022. Additionally, the same search strategy was updated until July 2024, including systematic and manual searches.
Searches were conducted using free terms, descriptors, and Boolean operators in English, and additionally with Spanish terms (in Google Scholar). No language, population, study design, or temporal filters were applied. Databases employed and search equations are provided in Supplementary Materials.
In both search phases, the screening process of title–abstract and full-text evaluation followed the same procedure and applied the same eligibility criteria. However, in the first round (April 2022), screening was conducted independently and in duplicate, with discrepancies resolved by a third reviewer (SLL) through consensus. In contrast, in the updated search (July 2024), the screening process was carried out by a single reviewer only.
2.3. Data Extraction
Study information regarding authors, publication date, study design, population, inclusion and exclusion criteria, demographic data, interventions, sample size, outcome measures, measurement tools, and immediately after intervention between-group results were extracted.
Only functional or performance measures were extracted. The information regarding AOT prescription parameters was extracted regarding AOT media display, dose, and dose adaptation. This information was collected from the articles’ text and figures, Supplementary Materials (videos, etc.), and previously published and referenced AOT trial protocols.
The AOT media display analysis included the patient’s perspective, the actor, the body parts in action, and the visible body parts. To explore the therapeutic dose, we extracted the number of activities, type of actions, session distribution, durations, and frequency. The dose adaptation was determined according to the patient’s functional level, progression, and incorporation of ludic activities.
2.4. Risk of Bias Assessment
Risk of bias was analyzed with the Cochrane Risk of Bias 2.0 (RoB) tool [24]. This tool assesses 5 domains of bias: the randomization process, deviations from intended interventions, missing outcome data, outcome measurement, and selection of reported outcomes. The risk of bias for each of the 5 domains and overall were classified as low risk of bias, some concerns, or high risk of bias [24].
Two independent reviewers (JFM and CDCL) assessed blindly the included studies. Interrater item agreement was analyzed using the Kappa coefficient. An almost perfect level of agreement was established when κ was 0.81–1.00; substantial when 0.61–0.80; moderate when 0.41–0.6; fair when 0.21–0.4; slight when 0.00–0.20; and poor when <0.00 [25]. Disagreements between reviewers were resolved by consensus including a third reviewer (SLL).
2.5. Meta-Analysis and Qualitative Synthesis
A meta-analysis was performed in the following conditions: the presence of 2 or more studies including the same comparisons and outcome measures; and availability of the number of participants, outcome point measures, and variability measures.
Although a random allocation process was performed in the studies, baseline imbalances could appear when small sample sizes were included. Therefore, reported mean and SD of post–pre changes and sample size were extracted for the meta-analysis. When this information was not available, the mean change and SD difference was calculated as follows:
A meta-analysis was executed following the random-effects model, with the number of subjects, mean, and SD difference for each outcome. Numeric data extractions from studies were taken from tables and/or text. Data were also extracted manually from graphics if not shown in tables or text. Additionally, if the data were presented in medians and quartiles, conversions to mean and SD were performed following the equations nº14 and 15 proposed by Wan et al. [26]. Standard errors of mean were also transformed to SD according to Section 6.3 of the Cochrane Handbook for Systematic Reviews of Interventions [27].
Meta-analyses were conducted employing random effects, with the Maximum Restricted Likelihood method, following a t-distribution. Outcomes were reported employing the Hedges’ g with a 95% CI [28], considering its result as “very small” if <0.20; “small” if 0.20–0.49; “medium” if 0.5–0.79; and “large” ≥ 0.8 [29].
Heterogeneity was explored with the inconsistency index (I2) and Cochran’s Q statistic test. Inconsistency was considered small if I2 > 25%, medium if I2 > 50%, and large when I2 > 75%. Both statistical tests present a problem of power with a small number of studies; thus, heterogeneity was considered if both of the following cases were fulfilled: I2 > 75%; Q-test was significant (p < 0.1). Publication and selection bias were qualitatively assessed by employing funnel plots with 95% CI limits, while also exploring the presence of possible outliers. Finally, a leave-one-out analysis was carried out for meta-analysis of 3 or more studies to explore possible changes in the overall effect (determined through the precision of 95% CI) with the extraction of individual studies.
Statistical analyses were conducted with R Software version 4.4.1 [30]. The package “metafor” version 4.6–0 was employed for Hedges’ g calculations and for conducting the meta-analyses [31].
Finally, this information was synthesized employing the Grading of Recommendations Assessment, Development, and Evaluation, which classifies overall certainty of evidence based on 5 domains: study design, risk of bias, imprecision, indirectness, inconsistency, and publication bias [32]. Each domain is classified with “not serious”, “serious”, or “very serious” limitations. Overall certainty of evidence is classified into 4 levels: “high certainty”, “moderate certainty”, “low certainty”, and “very low certainty”. Overall certainty of evidence is initially classified into “high certainty”; however, based on the number of classifications of “serious” or “very serious”, that certainty level is downgraded once or twice, respectively, based on their amount across domains.
3. Results
3.1. Selection Process
Original searches provided a total of 11 studies included in the review [17,18,21,22,33,34,35,36,37,38,39]. Additionally, other 3 studies were included in the review through actualized searches [40,41,42], accounting for a total of 14 studies. See Figure 1.
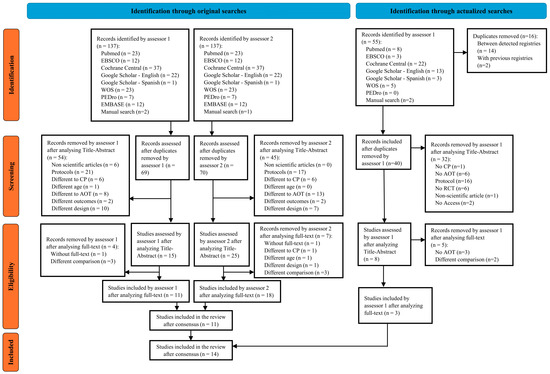
Figure 1.
Prisma flow chart of selection process.
3.2. Study Features
Among the 14 RCTs included in the review, 13 presented a parallel group design [17,18,21,22,33,34,35,36,38,39,40,41,42] and 1 presented a cross-over design [37].
A total of 393 patients with CP were enrolled in the included studies. Seven studies included only patients with unilateral CP (UCP), accounting for 268 patients [18,22,38,39,40,41,42], two studies included a total of 48 patients only with bilateral CP (BCP) [34,36], and five studies enrolled patients with either UCP or BCP, for a total of 77 patients [17,21,33,35,37].
The age range of participants varied across studies, from 2 years [36] to up to 18 years [42]. The proportion of female participants ranged between 23% [33] and 57% [35]. All studies reported including exclusively spastic CP-type patients [17,18,21,33,34,35,36,37,39,40,41,42], except for 2 studies that did not report the type of CP [22,38].
Regarding hand function, several studies included participants based on the Manual Ability Classification System [43], selecting participants with ≤2 [41], 2–3 [40], ≤3 [38,39], and ≤4 [21,37]. In studies using the House Functional Classification System [44], inclusion criteria included scores of ≥ 2 [42] and 4–8 [18,38].
Most studies included patients with spastic CP [17,18,21,33,34,35,36,37,39,40,41,42], and applied different thresholds using the Modified Ashworth Scale [45], including participants with ≤1+ [34], ≤2 [33,35], 1–2 [33], 1+–2 [40], and ≤3 [39].
Gross motor function was assessed with the GMFCS [46], with inclusion levels within 1–2 [39], 1–3 [34,35,36], 1–4 [37], and 2–3 [40].
Cognitive state was evaluated through different criteria, selecting participants with IQ ≥ 70 [42], IQ > 70 [17,21,37], MMSE ≥ 24 [41], or described as “within normal limits” [18].
Six studies explored the efficacy of AOT comparing it against placebo (no motor content) observation [17,18,21,37,38,39]. Two studies explored the effect of adding AOT to protocol of task execution [22,35]. Finally, five studies explored its effectiveness against other active therapies, such as conventional physical therapy [33,34,36,40], or bimanual arm training [41].
A great part of studies explored manual performance measures of the hand, including unimanual dexterity [33,38,41,42], manual function during daily activities [18,22,33,38], hand sensorimotor function [41], spontaneous use of assisting hand [18,21,22,37,38,42], bimanual dexterity [38], and hand grip strength [38,39]. Studies also explored outcomes involving the functioning of specific and general domains of the unilateral upper limb [17,18,21,22,37,38,39,40,42]. Additionally, gross motor function was explored during sitting [34,36], crawling and kneeling [34,36], standing [34,35,36], and walking [34,35,36], running and jumping, along with the combined result of domains [34,36]. Some studies explored results on balance [34,35], function in timed-up-and-go task, sit-to-stand tasks, walking performance, and stair climbing performance [35].
Additional information is presented in Table 1.

Table 1.
Summary data from included studies.
3.3. AOT Prescription Parameters
AOT protocols were displayed on video in 13 studies [17,18,21,33,34,35,36,37,38,39,40,41,42] and live in 1 study [22].
In terms of point of view, 6 studies displayed only a first-person perspective [18,22,37,38,39,42], 2 studies offered a third-person perspective employing several perspectives [34,40], 2 studies employed multiple perspectives but did not specify them [17,21], and 4 additional studies did not specify the point of view [33,35,36,41].
Regarding the body parts performing the actions, 1 study displayed only unimanual tasks [38], 1 study showed only bimanual tasks [22], 9 studies included both unimanual and bimanual tasks [17,18,21,33,37,39,40,41,42], and 3 studies only displayed both lower limbs in action [34,35,36].
Out of the studies that employed manual tasks, 6 studies reported only mirroring to match patient’s more-affected side in unimanual tasks [17,18,21,37,38,42], while the information was not stated in 4 studies [33,39,40,41]. For studies including bimanual tasks, only 1 study reported the role of each hand during asymmetrical bimanual tasks [22], while the other studies did not report this information [17,18,21,33,37,39,40,41,42].
The mean number of activities performed in the AOT protocols was 16.3, ranging from 6 [35] to 60 [39]. Session durations ranged from 15 to 60 min, with a weekly frequency ranging from 3 to 6 sessions per week, except for Simon-Martinez et al. [38], with 1 or 2 daily sessions for 5 consecutive days.
All AOTs of the upper limbs employed goal-directed and ludic activities [17,18,21,22,33,37,38,39,40,41,42], whereas AOT protocols of the lower limbs were neither ludic nor goal-directed [34,35,36].
AOT protocols were adapted to patient’s functional level in 5 studies [18,22,38,39,42], and only 9 studies reported procedures for progressing AOT prescription through the intervention [1,18,34,35,36,37,38,39,42].
Additional information is shown in Table 2.

Table 2.
Action observation therapy prescription parameters from included studies.
3.4. Risk of Bias Evaluation
The overall risk of bias assessment revealed 2 studies with a low risk of bias [18,36], 9 with some concerns [17,21,22,34,35,37,38,39,42], and 3 with a high risk of bias [33,40,41]. Studies presented a higher prevalence of low risk of bias across randomization, missing data analysis, and outcome measurement procedures. Contrarily, a high number of studies presented concerns in the reported result domain. A substantial level of agreement for the RoB assessment tool (κ = 0.732) was observed. Results are presented in Figure 2 and Figure 3.
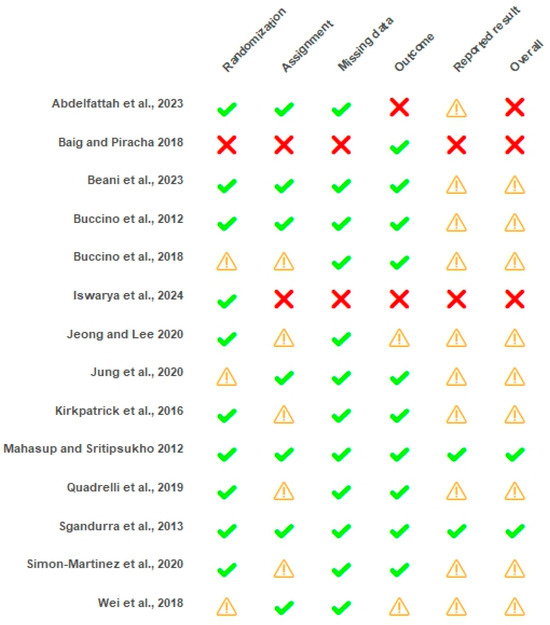
Figure 2.
Risk of bias of individual studies with ROB 2.0. ✔: low risk of bias; ⚠: some concerns; ☒: high risk of bias [17,18,21,22,33,34,35,36,37,38,39,40,41,42].
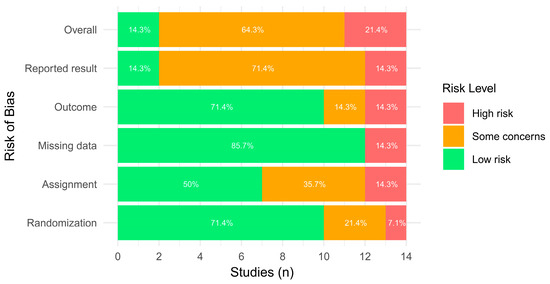
Figure 3.
Risk of bias across domains with ROB 2.0.
3.5. Meta-Analysis and Qualitative Synthesis Results
The eligibility process for study selection in meta-analysis is provided in Table 3. Finally, four meta-analyses were conducted for AOT compared to placebo observation (in addition to other therapies), and two meta-analyses explored the comparative effectiveness of an AOT protocol against physical therapy. Funnel plots are provided in the Supplementary Materials.

Table 3.
Data availability, extraction, and processing for meta-analyses between healthy older and younger adults.
3.5.1. AOT Versus Placebo—Unilateral Upper Limb Function (More-Affected Limb)
Four studies with 5 comparison groups were included in the meta-analysis [17,18,21,39]. These studies presented low risk of bias [18], and some concerns [17,21,39]. The meta-analysis provided a non-significant effect (g = 0.565; 95% CI −0.174, 1.305), with 95% CI indicating imprecise results where the effect could range from “very small” in favor of placebo observation to a “large” in favor of AOT. Heterogeneity was not relevant (Q = 6.510, p = 0.164; I2 = 39.39%), see Figure 4. Publication bias was present, due to visual asymmetry in the funnel plot and the presence of Wei et al. [39] groups D vs. B as possible outliers. Leave-one-out analysis did not change the precision or conclusions of the estimate.
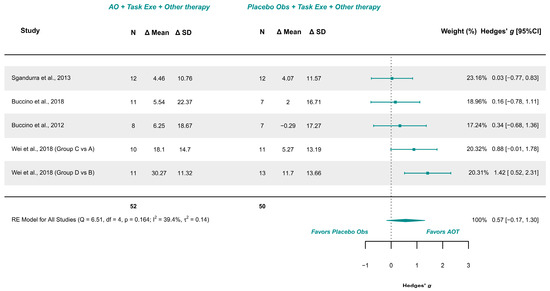
Figure 4.
Forest plot of unilateral upper limb function [17,18,21,39].
GRADE synthesis provided a “very low” certainty, mainly due to imprecision of the effect, and publication bias concerns. The present findings can be potentially changed with further studies. See Table 4.

Table 4.
Grading of Recommendations Assessment, Development and Evaluation (GRADE) certainty of evidence of meta-analyzed results.
3.5.2. AOT Versus Placebo—Assisting Hand Function During Bimanual Activities
Three studies explored this outcome and were meta-analyzed [18,21,38]. Risk of bias included low risk [18] and some concerns [21,38]. The meta-analysis provided a non-significant effect (g = 0.200; 95% CI −0.742, 1.143), with 95% CI indicating imprecise results where the effect could range from “medium” in favor of placebo observation to a “large” in favor of AOT. Heterogeneity was not relevant (Q = 0.571, p = 0.752; I2 = 0%), see Figure 5. Publication bias was present, due to visual asymmetry in the funnel plot with no presence of outliers. GRADE synthesis provided a “very low” certainty, mainly due to imprecision of the effect, and publication bias concerns. The present findings can be potentially changed with further studies. See Table 4.
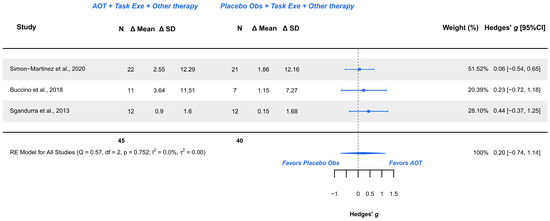
Figure 5.
Forest plot AHA scale [18,21,38].
3.5.3. AOT Versus Placebo—Manual Function During Daily Activities
Two studies explored this outcome measure and were meta-analyzed [18,38]. They presented low risk [18] and some concerns [38]. The meta-analysis provided a non-significant effect (g = −0.022; 95% CI −3.134, 3.090), with 95% CI indicating imprecise results where the effect could range from “large” in favor of placebo observation to a “large” in favor of AOT. Heterogeneity was not relevant (Q = 0.595, p = 0.440; I2 = 0%), see Figure 6. Publication bias was absent, due to symmetry in the funnel plot with no presence of outliers.
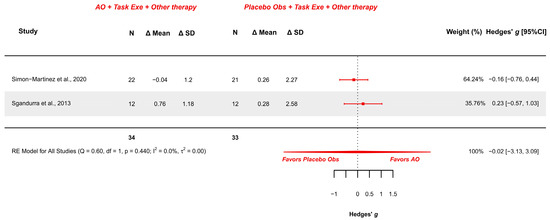
Figure 6.
Forest plot of AbilHand scale [18,38].
GRADE synthesis provided a “low” certainty mainly due to imprecision of the effect. The present findings can be potentially changed with further studies. See Table 4.
3.5.4. AOT Versus Placebo—Hand Grip Strength (More-Affected Limb)
Two studies explored this variable and were meta-analyzed [38,39]. Both studies presented some concerns. The meta-analysis provided a non-significant effect (MD (kg) = 1.175; 95% CI −0.280, 2.630), with 95% CI indicating imprecise results where the effect could range from a trivial difference (−0.28 kg) to a relevant difference (2.63 kg) in favor of AOT. Heterogeneity was not relevant (Q = 2.195, p = 0.334; I2 = 21.18), see Figure 7. Publication bias was present, due to visual asymmetry in the funnel plot with no presence of relevant outliers.
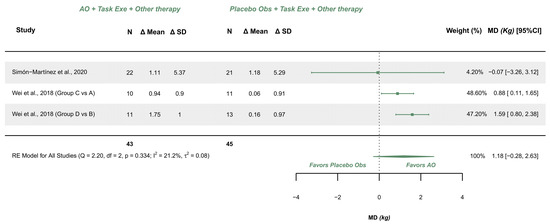
Figure 7.
Forest plot of grip strength [38,39].
GRADE synthesis provided a “very low” certainty, mainly due to imprecision of the effect, and publication bias concerns. The present findings can be potentially changed with further studies. See Table 4.
3.5.5. AOT Versus Physical Therapy—Gross Motor Function in Standing Dimension
Two studies explored this outcome and were meta-analyzed [34,36]. They presented low risk [36] and some concerns [34]. The meta-analysis provided a non-significant effect (g = 0.363; 95% CI −5.172, 5.898), with 95% CI indicating imprecise results where the effect could range from “large” in favor of physical therapy to a “large” in favor of AOT (AO combined with execution). Heterogeneity was not relevant (I2 = 51.41%; Q = 2.058, p = 0.151), see Figure 8. Publication bias was absent, due to visual symmetry in the funnel plot with no presence of outliers.
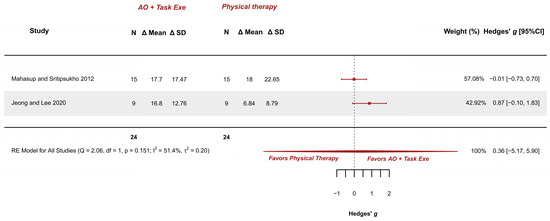
Figure 8.
Forest plot of gross motor function measurement—standing dimension [34,36].
GRADE synthesis provided a “low” certainty mainly due to the great imprecision of the effect. The present findings can be potentially changed with further studies. See Table 4.
3.5.6. AOT Versus Physical Therapy—Gross Motor Function in Walking, Standing, and Jumping Dimensions
Two studies explored this variable and were meta-analyzed [34,36]. They presented low risk [36] and some concerns [34]. The meta-analysis provided a non-significant effect (g = 0.798; 95% CI −8.821, 10.417), with 95% CI indicating imprecise results where the effect could range from “large” in favor of physical therapy to a “large” in favor of AOT (AO combined with execution). Heterogeneity was relevant (I2 = 81.43%; Q = 5.386, p = 0.020), see Figure 9. Publication bias was present, due to asymmetry in the funnel plot being both studies possible outliers.
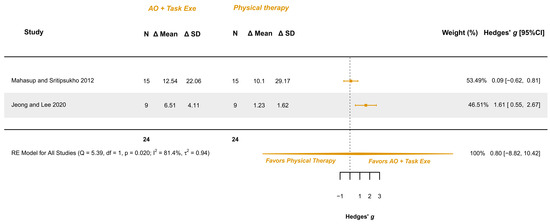
Figure 9.
Forest plot of gross motor function measurement—walking: walking, standing, and jumping dimensions [34,36].
GRADE synthesis provided a “low” certainty mainly due to the great imprecision of the effect. The present findings can be potentially changed with further studies. See Table 4.
4. Discussion
The present systematic review with meta-analysis aimed to analyze the effectiveness of AOT on various functional outcomes in children and adolescents with CP. AOT consists of observing various body-related movements, and it has demonstrated significant motor function improvement in adult patients with stroke [52,53]. The findings have been supported by a degree of functional reorganization of the motor system, as observed from significant modifications in functional magnetic resonance imaging activation during an object manipulation task [54,55]. Although the physiological mechanism behind AOT in children with CP is not fully understood, it is believed to be related to a neural plasticity process derived from activation of the MNS [56]. This therapeutic approach, combining the observation and execution of movement, might activate and promote the connections between these mirror neurons, accelerating the maturation of the corticospinal tract, adaptively shaping the spinal motor circuits, and potentially leading to improvements in motor function in children with CP [37,57,58]. Following these principles, all the included studies combined AOT protocols with observing and executing the observed tasks. Based on the summary data from the included studies, the majority of AOT protocols presented favorable results, as previously reported in other systematic reviews [59,60,61].
Nevertheless, none of the included meta-analyses yielded conclusive results. The wide 95% CI indicated a lack of precision, preventing firm conclusions about the effect on this population. This was evident for AOT over placebo observation in UL and hand functions. For instance, the meta-analysis on unilateral upper limb function, including interventions ranging from 3 [17,18,21] to 12 weeks [39], with the longer duration showing a greater tendency towards positive effects over placebo [39].
The meta-analyses of gross motor function presented even fewer subjects (24–45 patients per group), resulting in limited statistical power to detect significant differences. Another factor leading to low statistical power is to the comparison of two potentially effective interventions, such as AOT vs. physical therapy, leading to wide 95% CI, further preventing drawing clear conclusions.
Previous research has examined the effectiveness of AOT in individuals with CP, with findings generally consistent with those reported in the current literature. Although recent reviews have endorsed AOT for these patients, issuing a green-light recommendation [16], the present results together with earlier meta-analyses [20,62] suggest caution. Given the inconclusive evidence regarding its effectiveness, such recommendations may be premature for clinical practice. It should be noted that although AOT for upper limb and manual outcomes produced low to moderate effect sizes, as well as a possibly relevant effect size for hand grip strength, readers should consider that this evidence is not sufficient to determine the real effect of the therapy, as observed through the imprecision in 95% CI, preventing drawing conclusions of its effectiveness and its possible implementation in clinical practice as a sole therapy.
Other interventions based on movement representation, such as mirror therapy or visual feedback therapies, have been investigated in children with CP. In particular, the network meta-analysis by Yang et al. [19] compared several therapies, including AOT and mirror therapy. Their findings were consistent with those of our present review, where AOT did not show any significant effect compared to placebo in improving upper limb function. In contrast, mirror therapy demonstrated a significant and beneficial effect, not only over placebo but also superior to AOT.
Several factors might explain the lack of effectiveness of AOT observed across the meta-analysis. These factors are likely related to both patient demographics and therapy characteristics.
Firstly, a key demographic variable is age. According to Morales et al. [11], a young adult (~18 years) would be expected to show a greater overlap of AO–execution brain regions than an older child (~10 years), and even more compared to a younger child (~7 years) [11]. If these developmental patterns were fully applicable to patients with CP, which current evidence suggests they are not [12], then adolescents with CP might benefit more from AOT in terms of motor learning than younger children.
Secondly, a factor that bridges both patient and therapy characteristics is the level of engagement and motivation during AOT. Elements such as the patient’s commitment to therapy, their connection with the therapist, and the emotional and motivational components of therapy were believed to influence AOT effectiveness [63].
Finally, the minimum effective dose of AOT is another critical factor that requires further investigation. For instance, the total amount of sessions or the duration of therapy might determine whether AOT produces benefits. This trend is reflected in the meta-analysis of AOT over placebo on the unilateral upper limb function, where the study of Wei et al. [39] with 12 weeks of therapy and a total of 60 sessions showed favorable effects, whereas the studies of Sgandurra et al. [18] and Buccino et al. [17,21] with only 3 week (15 sessions) did not show favorable outcomes.
These factors warrant further exploration to determine the optimal conditions for recommending AOT in clinical practice.
4.1. Srengths and Limitations
The present systematic review with meta-analysis presents several strengths compared to the previous reviews [20]. One notable advantage the larger number of studies had included AOT, which increases the generalizability of the findings. Additionally, this review offers a clearer inclusion criteria for the meta-analysis, providing more precise comparisons of AOT against specific interventions, such as placebo and conventional physical therapy.
In contrast, previous meta-analyses [20] encountered methodological issues that hindered clear interpretation of the results. These included, for example, the duplication of control group data from studies such Molinaro et al. [64] and Buccino et al. [21], as well as the inclusion of heterogeneous comparisons within a meta-analysis, such as AOT vs. placebo [21], and AOT as an adjunct to physical practice [22], which blurred the interpretation of the intervention’s true effect.
Although the updated searches in the current review could have been further improved by ensuing a paired and blinded screening and data extraction process, which, in this case, was only performed by a single researcher, the data curation was rigorous and the selection of studies for the meta-analysis was conducted carefully (see Table 3). Furthermore, the methodological approach was robust, employing the Maximum Restricted Likelihood Method, which provides more accurate estimates of heterogeneity than other methods [65], random effect meta-analysis, and determining significance testing, as well as 95% CI based on t-distribution rather than z-distribution, thereby enhancing the validity of the results.
4.2. Clinical Implications
The findings of this review indicate that there is insufficient evidence to support the clinical use of AOT in children and adolescents with CP. Current studies do not provide consistent or robust results to conclude that AOT is more effective than placebo or conventional physical therapy in improving functional outcomes.
As such, AOT should not be currently recommended for clinical implementation in this population with the present evidence. Clinical guidelines and therapeutic decision-making should be cautious and rely on interventions with stronger empirical support.
5. Conclusions
Current findings are imprecise and prevent drawing clear conclusions about the real effect of AOT over placebo observation, and over physical therapy, on functional outcomes including upper limb, hand, and lower limb functioning parameters. Current findings prevent recommending the employment of AOT in clinical practice for treating children and adolescents with CP.
Further evidence is required to determine the real effectiveness of AOT in children and adolescents with CP.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/children12070810/s1; Table S1: Search engines, databases, search equations and results. Figure S1: Funnel plot of unilateral upper limb function meta-analysis. Figure S2: Funnel plot of assisting hand ability during bimanual activities meta-analysis. Figure S3: Funnel plot of manual function during daily activities meta analysis. Figure S4: Funnel plot of hand grip strength meta analysis. Figure S5: Funnel plot gross motor function standing dimension meta analysis. Figure S6: Funnel plot gross motor function walking, standing and jumping dimensions, dimension meta analysis.
Author Contributions
Conceptualization, R.L.T. and S.L.-L.; methodology, R.L.T. and J.F.-M.; software, J.F.-M.; validation, J.F.-M., R.L.T., S.L.-L., C.D.C.-L., and B.R.d.R.-R.; formal analysis, R.L.T. and J.F.-M.; investigation, J.F.-M. and S.L.-L.; resources, R.L.T. and S.L.-L.; data curation, J.F.-M., C.D.C.-L., B.R.d.R.-R., and A.L.-M.; writing—original draft preparation, J.F.-M. and M.O.-A.; writing—review and editing, J.F.-M., R.L.T., A.L.-M., and S.L.-L.; visualization, S.L.-L.; supervision, S.L.-L.; project administration, S.L.-L.; funding acquisition, S.L.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundación IberCaja grant “Impulso Solidario” 2021.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
This study was conducted with the financial support of “imPULSO Solidario” award 2021 from the Ibercaja Foundation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yang, S.; Xia, J.; Gao, J.; Wang, L. Increasing Prevalence of Cerebral Palsy among Children and Adolescents in China 1988–2020: A Systematic Review and Meta-Analysis. J. Rehabil. Med. 2021, 53, jrm00195. [Google Scholar] [CrossRef] [PubMed]
- Kirby, R.S.; Wingate, M.S.; Braun, K.V.N.; Doernberg, N.S.; Arneson, C.L.; Benedict, R.E.; Mulvihill, B.; Durkin, M.S.; Fitzgerald, R.T.; Maenner, M.J.; et al. Prevalence and Functioning of Children with Cerebral Palsy in Four Areas of the United States in 2006: A Report from the Autism and Developmental Disabilities Monitoring Network. Res. Dev. Disabil. 2011, 32, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Tonmukayakul, U.; Shih, S.T.F.; Bourke-Taylor, H.; Imms, C.; Reddihough, D.; Cox, L.; Carter, R. Systematic Review of the Economic Impact of Cerebral Palsy. Res. Dev. Disabil. 2018, 80, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.; Fullen, B.; Rio, E.; Segurado, R.; Stokes, D.; O’Sullivan, C. Effect of Action Observation Therapy in the Rehabilitation of Neurologic and Musculoskeletal Conditions: A Systematic Review. Arch. Rehabil. Res. Clin. Transl. 2021, 3, 100106. [Google Scholar] [CrossRef]
- Cuenca-Martínez, F.; Reina-Varona, Á.; Castillo-García, J.; Touche, R.L.; Angulo-Díaz-Parreño, S.; Suso-Martí, L. Pain Relief by Movement Representation Strategies: An Umbrella and Mapping Review with Meta-Meta-Analysis of Motor Imagery, Action Observation and Mirror Therapy. Eur. J. Pain. 2021, 26, 284–309. [Google Scholar] [CrossRef]
- Herranz-Gómez, A.; Gaudiosi, C.; Angulo-Díaz-Parreño, S.; Suso-Martí, L.; Touche, R.L.; Cuenca-Martínez, F. Effectiveness of Motor Imagery and Action Observation on Functional Variables: An Umbrella and Mapping Review with Meta-Meta-Analysis. Neurosci. Biobehav. Rev. 2020, 118, 828–845. [Google Scholar] [CrossRef]
- Errante, A.; Fogassi, L. Activation of Cerebellum and Basal Ganglia during the Observation and Execution of Manipulative Actions. Sci. Rep. 2020, 10, 12008. [Google Scholar] [CrossRef]
- Hardwick, R.M.; Caspers, S.; Eickhoff, S.B.; Swinnen, S.P. Neural Correlates of Action: Comparing Meta-Analyses of Imagery, Observation, and Execution. Neurosci. Biobehav. Rev. 2018, 94, 31–44. [Google Scholar] [CrossRef]
- Agosta, F.; Gatti, R.; Sarasso, E.; Volonté, M.A.; Canu, E.; Meani, A.; Sarro, L.; Copetti, M.; Cattrysse, E.; Kerckhofs, E.; et al. Brain Plasticity in Parkinson’s Disease with Freezing of Gait Induced by Action Observation Training. J. Neurol. 2017, 264, 88–101. [Google Scholar] [CrossRef]
- Kemmerer, D. What Modulates the Mirror Neuron System during Action Observation?: Multiple Factors Involving the Action, the Actor, the Observer, the Relationship between Actor and Observer, and the Context. Prog. Neurobiol. 2021, 205, 102128. [Google Scholar] [CrossRef]
- Morales, S.; Bowman, L.C.; Velnoskey, K.R.; Fox, N.A.; Redcay, E. An fMRI Study of Action Observation and Action Execution in Childhood. Dev. Cogn. Neurosci. 2019, 37, 100655. [Google Scholar] [CrossRef] [PubMed]
- Bieber, E.; Smits-Engelsman, B.C.M.; Sgandurra, G.; Martini, G.; Guzzetta, A.; Cioni, G.; Feys, H.; Klingels, K. Insights on Action Observation and Imitation Abilities in Children with Developmental Coordination Disorder and Typically Developing Children. Res. Dev. Disabil. 2023, 139, 104556. [Google Scholar] [CrossRef] [PubMed]
- Foti, F.; Martone, D.; Orrù, S.; Montuori, S.; Imperlini, E.; Buono, P.; Petrosini, L.; Mandolesi, L. Are Young Children Able to Learn Exploratory Strategies by Observation? Psychol. Res. 2018, 82, 1212–1223. [Google Scholar] [CrossRef] [PubMed]
- Borges, L.R.; Fernandes, A.B.; Melo, L.P.; Guerra, R.O.; Campos, T.F. Action Observation for Upper Limb Rehabilitation after Stroke. Cochrane Database Syst. Rev. 2018, 10, CD011887. [Google Scholar] [CrossRef]
- Temporiti, F.; Adamo, P.; Cavalli, E.; Gatti, R. Efficacy and Characteristics of the Stimuli of Action Observation Therapy in Subjects with Parkinson’s Disease: A Systematic Review. Front. Neurol. 2020, 11, 808. [Google Scholar] [CrossRef]
- Novak, I.; Morgan, C.; Fahey, M.; Finch-Edmondson, M.; Galea, C.; Hines, A.; Langdon, K.; Namara, M.M.; Paton, M.C.; Popat, H.; et al. State of the Evidence Traffic Lights 2019: Systematic Review of Interventions for Preventing and Treating Children with Cerebral Palsy. Curr. Neurol. Neurosci. Rep. 2020, 20, 3. [Google Scholar] [CrossRef]
- Buccino, G.; Arisi, D.; Gough, P.; Aprile, D.; Ferri, C.; Serotti, L.; Tiberti, A.; Fazzi, E. Improving Upper Limb Motor Functions through Action Observation Treatment: A Pilot Study in Children with Cerebral Palsy. Dev. Med. Child. Neurol. 2012, 54, 822–828. [Google Scholar] [CrossRef]
- Sgandurra, G.; Ferrari, A.; Cossu, G.; Guzzetta, A.; Fogassi, L.; Cioni, G. Randomized Trial of Observation and Execution of Upper Extremity Actions versus Action Alone in Children with Unilateral Cerebral Palsy. Neurorehabilit. Neural Repair 2013, 27, 808–815. [Google Scholar] [CrossRef]
- Yang, F.-A.; Lee, T.-H.; Huang, S.-W.; Liou, T.-H.; Escorpizo, R.; Chen, H.-C. Upper Limb Manual Training for Children with Cerebral Palsy: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Clin. Rehabil. 2023, 37, 516–533. [Google Scholar] [CrossRef]
- Demeco, A.; Molinaro, A.; Ambroggi, M.; Frizziero, A.; Fazzi, E.; Costantino, C.; Buccino, G. Cognitive Approaches in the Rehabilitation of Upper Limbs Function in Children with Cerebral Palsy: A Systematic Review and Meta-Analysis. Eur. J. Phys. Rehabil. Med. 2024, 60, 445–457. [Google Scholar] [CrossRef]
- Buccino, G.; Molinaro, A.; Ambrosi, C.; Arisi, D.; Mascaro, L.; Pinardi, C.; Rossi, A.; Gasparotti, R.; Fazzi, E.; Galli, J. Action Observation Treatment Improves Upper Limb Motor Functions in Children with Cerebral Palsy: A Combined Clinical and Brain Imaging Study. Neural Plast. 2018, 2018, 4843985. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, E.; Pearse, J.; James, P.; Basu, A. Effect of Parent-Delivered Action Observation Therapy on Upper Limb Function in Unilateral Cerebral Palsy: A Randomized Controlled Trial. Dev. Med. Child. Neurol. 2016, 58, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ (Clin. Res. Ed.) 2021, 372, 71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ (Clin. Res. Ed.) 2019, 366, l4898. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Li, T.; Deeks, J.J. Chapter 6: Choosing effect measures and computing estimates of effect. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.5; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane Training: London, UK, 2022. [Google Scholar]
- Hedges, L.V. Estimation of Effect Size from a Series of Independent Experiments. Psychol. Bull. 1982, 92, 490–499. [Google Scholar] [CrossRef]
- Cohen, J. The Statistical Power of Abnormal-Social Psychological Research: A Review. J. Abnorm. Soc. Psychol. 1962, 65, 145–153. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Viechtbauer, W. Conducting Meta-Analyses in R with the Metafor Package. J. Stat. Soft. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Baig, M.O.; Piracha, S. Effect of Action Observation Therapy In Spastic Kinds Of Cerebral Palsy. J. Riphah Coll. Rehabil. Sci. 2018, 6, 84–89. [Google Scholar]
- Jeong, Y.A.; Lee, B.H. Effect of Action Observation Training on Spasticity, Gross Motor Function, and Balance in Children with Diplegia Cerebral Palsy. Children 2020, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Chung, E.J.; Chun, H.L.; Lee, B.H. Effects of Whole-Body Vibration Combined with Action Observation on Gross Motor Function, Balance, and Gait in Children with Spastic Cerebral Palsy: A Preliminary Study. J. Exerc. Rehabil. 2020, 16, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Mahasup, P.; Sritipsukho, P. Effects of Mirror Neurons Stimulation on Motor Skill Rehabilitation in Children with Cerebral Palsy: A Clinical Trial. J. Med. Assoc. Thail. 2012, 95 (Suppl. 1), S166–S172. [Google Scholar]
- Quadrelli, E.; Anzani, A.; Ferri, M.; Bolognini, N.; Maravita, A.; Zambonin, F.; Turati, C. Electrophysiological Correlates of Action Observation Treatment in Children with Cerebral Palsy: A Pilot Study. Dev. Neurobiol. 2019, 79, 934–948. [Google Scholar] [CrossRef]
- Simon-Martinez, C.; Mailleux, L.; Hoskens, J.; Ortibus, E.; Jaspers, E.; Wenderoth, N. Randomized Controlled Trial Combining Constraint-Induced Movement Therapy and Action-Observation Training in Unilateral Cerebral Palsy: Clinical Effects and Influencing Factors of Treatment Response. Ther. Adv. Neurol. Disord. 2020, 13, 1–19. [Google Scholar] [CrossRef]
- Wei, Y.-M.; Jiang, Z.-M.; Tang, J.-H.; Du, J.-Y.; Li, X.-M.; Wang, Y.-N.; Li, M.-Q. Effect of Action Observation Therapy on Upper Limb Function in Children with Spastic Hemi-Plegic Cerebral Palsy. Chin. J. Rehabil. Theory Pract. 2018, 24, 432–436. [Google Scholar] [CrossRef]
- Abdelfattah, H.E.; ElHadidy, E.I.; Al-Nemr, A.F. Effect of Action Observation Physical Training on Quality of Upper Limb and Functional Independence in Children with Hemiplegia. Egypt. J. Hosp. Med. 2023, 93, 6908–6913. [Google Scholar] [CrossRef]
- Iswarya, S.; Jagatheesan, A.; Senthil Kumar, N.; Shruthi, J. Effect of Action Observation Training and Bimanual Arm Training on Hand Function for Children with Hemiparetic Cerebral Palsy. IJPOT 2024, 18, 254–260. [Google Scholar] [CrossRef]
- Beani, E.; Menici, V.; Sicola, E.; Ferrari, A.; Feys, H.; Klingels, K.; Mailleux, L.; Boyd, R.; Cioni, G.; Sgandurra, G. Effectiveness of the Home-Based Training Program Tele-UPCAT (Tele-Monitored UPper Limb Children Action Observation Training) in Unilateral Cerebral Palsy: A Randomized Controlled Trial. Eur. J. Phys. Rehabil. Med. 2023, 59, 554–563. [Google Scholar] [CrossRef]
- Eliasson, A.-C.; Krumlinde-Sundholm, L.; Rösblad, B.; Beckung, E.; Arner, M.; Ohrvall, A.-M.; Rosenbaum, P. The Manual Ability Classification System (MACS) for Children with Cerebral Palsy: Scale Development and Evidence of Validity and Reliability. Dev. Med. Child. Neurol. 2006, 48, 549–554. [Google Scholar] [CrossRef] [PubMed]
- House, J.H.; Gwathmey, F.W.; Fidler, M.O. A Dynamic Approach to the Thumb-in Palm Deformity in Cerebral Palsy. J. Bone Jt. Surg. Am. 1981, 63, 216–225. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Smith, M.B. Interrater Reliability of a Modified Ashworth Scale of Muscle Spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef]
- Bodkin, A.W.; Robinson, C.; Perales, F.P. Reliability and Validity of the Gross Motor Function Classification System for Cerebral Palsy. Pediatr. Phys. Ther. 2003, 15, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Simon-Martinez, C.; Mailleux, L.; Ortibus, E.; Fehrenbach, A.; Sgandurra, G.; Cioni, G.; Desloovere, K.; Wenderoth, N.; Demaerel, P.; Sunaert, S.; et al. Combining Constraint-Induced Movement Therapy and Action-Observation Training in Children with Unilateral Cerebral Palsy: A Randomized Controlled Trial. BMC Pediatr. 2018, 18, 250. [Google Scholar] [CrossRef]
- Simon-Martinez, C.; Mailleux, L.; Jaspers, E.; Ortibus, E.; Desloovere, K.; Klingels, K.; Feys, H. Effects of Combining Constraint-Induced Movement Therapy and Action-Observation Training on Upper Limb Kinematics in Children with Unilateral Cerebral Palsy: A Randomized Controlled Trial. Sci. Rep. 2020, 10, 10421. [Google Scholar] [CrossRef]
- Kim, D.H. Comparison of Short- and Long-Time Action Observation Training (AOT) on Upper Limb Function in Children with Cerebral Palsy. Physiother. Pract. Res. 2020, 41, 53–58. [Google Scholar] [CrossRef]
- Sgandurra, G.; Cecchi, F.; Beani, E.; Mannari, I.; Maselli, M.; Falotico, F.P.; Inguaggiato, E.; Perazza, S.; Sicola, E.; Feys, H.; et al. Tele-UPCAT: Study Protocol of a Randomised Controlled Trial of a Home-Based Tele-Monitored UPper Limb Children Action Observation Training for Participants with Unilateral Cerebral Palsy. BMJ Open 2018, 8, e017819. [Google Scholar] [CrossRef]
- Sgandurra, G.; Ferrari, A.; Cossu, G.; Guzzetta, A.; Biagi, L.; Tosetti, M.; Fogassi, L.; Cioni, G. Upper Limb Children Action-Observation Training (UP-CAT): A Randomised Controlled Trial in Hemiplegic Cerebral Palsy. BMC Neurol. 2011, 11, 80. [Google Scholar] [CrossRef]
- Ertelt, D.; Small, S.; Solodkin, A.; Dettmers, C.; McNamara, A.; Binkofski, F.; Buccino, G. Action Observation Has a Positive Impact on Rehabilitation of Motor Deficits after Stroke. Neuroimage 2007, 36, T164–T173. [Google Scholar] [CrossRef]
- Franceschini, M.; Ceravolo, M.G.; Agosti, M.; Cavallini, P.; Bonassi, S.; Dall’Armi, V. Clinical Relevance of Action Observation in Upper-Limb Stroke Rehabilitation: A Possible Role in Recovery of Functional Dexterity. A Randomized Clinical Trial. Neurorehabil. Neural Repair 2012, 26, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Dinomais, M.; Chinier, E.; Lignon, G.; Richard, I.; Minassian, A.T.; Tich, S.N.T. The Effect of Video-Guidance on Passive Movement in Patients with Cerebral Palsy: fMRI Study. Res. Dev. Disabil. 2013, 34, 3487–3496. [Google Scholar] [CrossRef] [PubMed]
- Errante, A.; Cesare, G.; Pinardi, C.; Fasano, F.; Sghedoni, S.; Costi, S. Mirror Neuron System Activation in Children with Unilateral Cerebral Palsy During Observation of Actions Performed by a Pathological Model. Neurorehabil. Neural Repair 2019, 33, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.P. Early Intervention after Perinatal Stroke: Opportunities and Challenges. Dev. Med. Child. Neurol. 2014, 56, 516–521. [Google Scholar] [CrossRef]
- Bazzini, M.C.; Nuara, A.; Scalona, E.; Marco, D.; Rizzolatti, G.; Avanzini, P. The Proactive Synergy Between Action Observation and Execution in the Acquisition of New Motor Skills. Front. Hum. Neurosci. 2022, 16, 793849. [Google Scholar] [CrossRef]
- Riddell, M.; Kuo, H.C.; Zewdie, E.; Kirton, A. Mirror Movements in Children with Unilateral Cerebral Palsy Due to Perinatal Stroke: Clinical Correlates of Plasticity Reorganization. Dev. Med. Child. Neurol. 2019, 61, 943–949. [Google Scholar] [CrossRef]
- Bae, S.-Y.; Jung, N.-H. A Systematic Review of Action Observation Therapy Intervention Program for Children with Cerebral Palsy. J. Korean Soc. Occup. Ther. 2020, 28, 85–98. [Google Scholar] [CrossRef]
- Sakzewski, L.; Ziviani, J.; Boyd, R.N. Efficacy of Upper Limb Therapies for Unilateral Cerebral Palsy: A Meta-Analysis. Pediatrics 2014, 133, e175–e204. [Google Scholar] [CrossRef]
- Oliva-Sierra, M.; Ríos-León, M.; Abuín-Porras, V.; Martín-Casas, P. Effectiveness of Mirror Therapy and Action Observation Therapy in Infantile Cerebral Palsy: A Systematic Review. An. Sist. Sanit. Navar. 2022, 45, e1003. [Google Scholar] [CrossRef]
- Abdelhaleem, N.; Taher, S.; Mahmoud, M.; Hendawy, A.; Hamed, M.; Mortada, H.; Magdy, A.; El-Din, M.R.E.; Zoukiem, I.; Elshennawy, S. Effect of Action Observation Therapy on Motor Function in Children with Cerebral Palsy: A Systematic Review of Randomized Controlled Trials with Meta-Analysis. Clin. Rehabil. 2021, 35, 51–63. [Google Scholar] [CrossRef]
- King, G.; Chiarello, L.A.; Ideishi, R.; D’Arrigo, R.; Smart, E.; Ziviani, J.; Pinto, M. The Nature, Value, and Experience of Engagement in Pediatric Rehabilitation: Perspectives of Youth, Caregivers, and Service Providers. Dev. Neurorehabil. 2020, 23, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, A.; Micheletti, S.; Pagani, F.; Garofalo, G.; Galli, J.; Rossi, A.; Fazzi, E.; Buccino, G. Action Observation Treatment in a Tele-Rehabilitation Setting: A Pilot Study in Children with Cerebral Palsy. Disabil. Rehabil. 2022, 44, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Langan, D.; Higgins, J.P.T.; Jackson, D.; Bowden, J.; Veroniki, A.A.; Kontopantelis, E.; Viechtbauer, W.; Simmonds, M. A Comparison of Heterogeneity Variance Estimators in Simulated Random-Effects Meta-Analyses. Res. Synth. Methods 2019, 10, 83–98. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).