Hospital and Patient Characteristics Associated with Neonatal Blood Stream Infection in Inpatient Care: Insights from the 2019 HCUP KID Database
Abstract
1. Introduction
- Are specific hospital characteristics (region, teaching status, rurality, ownership, size) associated with NQI 03?
- Are specific patient characteristics (age, gender, payor, race, previous major operation, service line of provider associated with hospital visits) associated with NQI 03?
2. Materials and Methods
2.1. Variables
- Hospital characteristics: hospital bed size (coded as 1: Small, 2: Medium, or 3: Large); hospital location (1: Rural, 2: Urban nonteaching, or 3: Urban teaching); hospital region (1: Northeast, 2: Midwest, 3: South, or 4: West); and hospital ownership (1: Government, non-federal (Public), 2: Private, not-for-profit (voluntary), or 3: Private, investor-owned (proprietary)).
- Patient characteristics included gender (0: Male, 1: Female); race (1: White, 2: Black, 3: Hispanic, 4: Asian/Pacific Islander, 5: Native American, 6: Other); service line (1: Maternal and Neonatal, 2: Mental health/Substance use, 3: Injury, 4: Surgical, 5: Medical); payment type (1: Medicare, 2: Medicaid, 3: Private Insurance, 4: Self-pay, 5: No charge, 6: Other); and operations on record (0: No major operating room procedure on record, or 1: Major operating room procedure on record).
- (1)
- Maternal and neonatal discharges are identified through specific Major Diagnostic Categories (MDCs), namely MDC 14 (Pregnancy, Childbirth, and Puerperium) and MDC 15 (Newborn and Other Neonates—Perinatal Period) [18].
- (2)
- Mental health/substance use discharges are characterized by the presence of diagnoses falling under MDC 19 (Mental Diseases and Disorders) and MDC 20 (Alcohol/Drug Use or Induced Mental Disorders) [18].
- (3)
- Injury discharges are identified using a predefined screen, involving Clinical Classification Software Refined (CCSR) categories for the principal ICD-10-CM diagnosis, specifically ranging from INJ001 to INJ027 and INJ032 [18].
- (4)
- Surgical discharges are determined by the presence of a surgical Diagnosis-Related Group (DRG), consistent with the definition employed prior to the data year 2019. The DRG assignment process initially places the discharge into an MDC based on the principal diagnosis, followed by the assessment of the qualifying procedure codes for operating room procedures within each MDC. If such procedures are involved, the discharge is categorized into a surgical DRG within the corresponding MDC category [18].
- (5)
- Discharges that do not meet the criteria for the Maternal/Neonatal, Mental health/substance abuse, Injury, or Surgical categories are classified as Medical discharges. In cases where the DRG does not provide sufficient information for classification as medical or surgical, discharges are typically assumed to be Medical. It is noteworthy that discharges with a principal diagnosis of injury or poisoning by intentional self-harm are categorized under the Medical service line rather than the Mental health/Substance use or Injury service lines [18].
2.2. Analysis
3. Results
4. Discussion
4.1. Public Health Implications and Recommendations
4.2. Strengths and Limitations
4.3. Recommendations for Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHRQ | Agency for Healthcare Research and Quality |
| HCUP | Healthcare Cost and Utilization Project |
| AE | Adverse Events |
| PAE | Pediatric Adverse Events |
| CLABSI | Central Line-associated Blood Stream Infection |
| KID | Kids’ Inpatient Databases |
| NBSI | Neonatal Blood Stream Infection |
| NQI 03 | Neonatal Quality Indicator |
| PDI | Pediatric Quality Indicator |
| PCLASS_ORPROC | Procedure Classes Refined for ICD-10-PCS |
| WHO | World Health Organization |
| MDCs | Major Diagnostic Categories |
| DRG | Diagnosis-Related Group |
| AHA | Annual Survey of Hospitals |
Appendix A
| 10 Variables: | AGE SEX RACE I10_SERVICELINE PAY1 PCLASS_ORPROC HOSP_BEDSIZE HOSP_LOCTEACH HOSP_REGION1 H_CONTRL | |||||||||
| Simple Statistics | ||||||||||
| Variable | N | Mean | Std Dev | Sum | Minimum | Maximum | Label | |||
| AGE | 4,571,036 | 5.76424 | 7.81784 | 26,348,548 | 0 | 20.00000 | Age in years at admission | |||
| SEX | 4,569,130 | 1.47427 | 0.49934 | 6,736,116 | 1.00000 | 2.00000 | Indicator of sex | |||
| RACE | 4,250,309 | 2.02445 | 1.39698 | 8,604,530 | 1.00000 | 6.00000 | Race (uniform) | |||
| I10_SERVICELINE | 4,571,036 | 2.30483 | 1.74136 | 10,535,477 | 1.00000 | 5.00000 | ICD-10-CM/PCS Hospital Service Line | |||
| PAY1 | 4,562,501 | 2.63280 | 0.85708 | 12,012,167 | 1.00000 | 6.00000 | Primary expected payer (uniform) | |||
| PCLASS_ORPROC | 4,571,036 | 1.88391 | 0.32033 | 8,611,429 | 1.00000 | 2.00000 | Indicates operating room (major diagnostic or therapeutic) procedure on the record | |||
| HOSP_BEDSIZE | 4,571,036 | 2.28911 | 0.80497 | 10,463,605 | 1.00000 | 3.00000 | Bed size of hospital | |||
| HOSP_LOCTEACH | 4,571,036 | 2.52114 | 0.75153 | 11,524,225 | 1.00000 | 3.00000 | Location/teaching status of hospital | |||
| HOSP_REGION1 | 4,571,036 | 2.67167 | 0.99289 | 12,212,279 | 1.00000 | 4.00000 | Region of hospital | |||
| H_CONTRL | 4,571,036 | 1.99197 | 0.50772 | 9,105,379 | 1.00000 | 3.00000 | Control/ownership of hospital | |||
| Pearson Correlation Coefficients Prob > |r| under H0: Rho = 0 Number of Observations | ||||||||||
| AGE | SEX | RACE | I10_SERVICELINE | PAY1 | PCLASS_ORPROC | HOSP_BEDSIZE | HOSP_LOCTEACH | HOSP_REGION1 | H_CONTRL | |
| AGE Age in years at admission | 1.00000 4,571,036 | −0.20238 <0.0001 4,569,130 | −0.04180 <0.0001 4,250,309 | 0.30891 <0.0001 4,571,036 | −0.01622 <0.0001 4,562,501 | −0.33560 <0.0001 4,571,036 | −0.00704 <0.0001 4,571,036 | −0.03152 <0.0001 4,571,036 | −0.01026 <0.0001 4,571036 | 0.00198 <0.0001 4,571,036 |
| SEX Indicator of sex | −0.20238 <0.0001 4,569,130 | 1.00000 4,569,130 | −0.00227 <0.0001 4,248,966 | 0.07046 <0.0001 4,569,130 | 0.03537 <0.0001 4,560,603 | 0.06901 <0.0001 4,569,130 | 0.00827 <0.0001 4,569,130 | 0.03813 <0.0001 4,569,130 | 0.00893 <0.0001 4,569130 | 0.00039 0.4026 4,569,130 |
| RACE Race (uniform) | −0.04180 <0.0001 4,250,309 | −0.00227 <0.0001 4,248,966 | 1.00000 4,250,309 | 0.00282 <0.0001 4,250,309 | −0.05614 <0.0001 4,243,736 | 0.00858 <0.0001 4,250,309 | 0.05178 <0.0001 4,250,309 | 0.11552 <0.0001 4250,309 | 0.09380 <0.0001 4,250,309 | 0.01458 <0.0001 4,250,309 |
| I10_SERVICELINE ICD-10-CM/PCS Hospital Service Line | 0.30891 <0.0001 4,571,036 | 0.07046 <0.0001 4,569,130 | 0.00282 <0.0001 4,250,309 | 1.00000 4,571,036 | −0.00239 <0.0001 4,562,501 | −0.10171 <0.0001 4,571,036 | 0.05416 <0.0001 4,571,036 | 0.12103 <0.0001 4,571,036 | −0.00247 <0.0001 4,571,036 | −0.03633 <0.0001 4,571,036 |
| PAY1 Primary expected payer (uniform) | −0.01622 <0.0001 4,562,501 | 0.03537 <0.0001 4,560,603 | −0.05614 <0.0001 4,243,736 | −0.00239 <0.0001 4,562,501 | 1.00000 4,562,501 | −0.01452 <0.0001 4,562,501 | −0.01467 <0.0001 4,562,501 | 0.01545 <0.0001 4,562,501 | 0.00524 <0.0001 4,562,501 | 0.00819 <0.0001 4,562,501 |
| PCLASS_ORPROC Indicates operating room (major diagnostic or therapeutic) procedure on the record | −0.33560 <0.0001 4,571,036 | 0.06901 <0.0001 4,569,130 | 0.00858 <0.0001 4,250,309 | −0.10171 <0.0001 4,571,036 | −0.01452 <0.0001 4,562,501 | 1.00000 4,571,036 | −0.00590 <0.0001 4,571,036 | −0.02304 <0.0001 4,571,036 | −0.01690 <0.0001 4,571,036 | −0.01271 <0.0001 4,571,036 |
| HOSP_BEDSIZE Bedsize of hospital | −0.00704 <0.0001 4,571,036 | 0.00827 <0.0001 4,569,130 | 0.05178 <0.0001 4,250,309 | 0.05416 <0.0001 4,571,036 | −0.01467 <0.0001 4,562,501 | −0.00590 <0.0001 4,571,036 | 1.00000 4,571,036 | 0.05958 <0.0001 4,571,036 | 0.06144 <0.0001 4,571,036 | −0.07654 <0.0001 4,571,036 |
| HOSP_LOCTEACH Location/teaching status of hospital | −0.03152 <0.0001 4571,036 | 0.03813 <0.0001 4,569,130 | 0.11552 <0.0001 4,250,309 | 0.12103 <0.0001 4,571,036 | 0.01545 <0.0001 4,562,501 | −0.02304 <0.0001 4,571,036 | 0.05958 <0.0001 4,571,036 | 1.00000 4,571,036 | −0.04933 <0.0001 4,571,036 | 0.05362 <0.0001 4,571,036 |
| HOSP_REGION1 Region of hospital | −0.01026 <0.0001 4,571,036 | 0.00893 <0.0001 4,569,130 | 0.09380 <0.0001 4,250,309 | −0.00247 <0.0001 4,571,036 | 0.00524 <0.0001 4,562,501 | −0.01690 <0.0001 4,571,036 | 0.06144 <0.0001 4,571,036 | −0.04933 <0.0001 4,571,036 | 1.00000 4,571,036 | 0.03591 <0.0001 4,571,036 |
| H_CONTRL Control/ownership of hospital | 0.00198 <0.0001 4,571,036 | 0.00039 0.4026 4,569,130 | 0.01458 <0.0001 4,250,309 | −0.03633 <0.0001 4,571,036 | 0.00819 <0.0001 4,562,501 | −0.01271 <0.0001 4,571,036 | −0.07654 <0.0001 4,571,036 | 0.05362 <0.0001 4,571,036 | 0.03591 <0.0001 4,571,036 | 1.00000 4,571,036 |
Appendix B
Appendix B.1. Further Variable Description for Selected Variables
- The hospital’s bed size category (H_BEDSZ) is nested within location and teaching status (H_LOCTCH) [18].
| Location and Teaching Status | Bed Size | ||
| Small | Medium | Large | |
| Rural | 1–49 | 50–99 | 100+ |
| Urban, nonteaching | 1–99 | 100–199 | 200+ |
| Urban, teaching | 1–299 | 300–499 | 500+ |
- B.
- The data element PCLASS_ORPROC indicates whether a major operating room procedure was reported on the discharge record [18].
- Minor Diagnostic: Non-operating-room procedures that are diagnostic (e.g., BW2800Z CT scan of head using high osmolar contrast);
- Minor Therapeutic—Non-operating-room procedures that are therapeutic (e.g., 079030Z drainage of head lymph, percutaneous approach);
- Major Diagnostic—All procedures considered valid operating room procedures by the Diagnosis-Related Group (DRG) grouper and that are performed for diagnostic reasons (e.g., 00B00ZX excision of brain, open approach, diagnostic);
- Major Therapeutic—All procedures considered valid operating room procedures by the Diagnosis-Related Group (DRG) grouper and that are performed for therapeutic reasons (e.g., 021008W bypass coronary artery, one artery from aorta with zooplastic tissue, open approach).
| Variable | Description | Value | Value Description |
| PCLASS_ORPROC | Major operating room ICD-10-PCS procedure indicator | 0 | No major operating room procedure reported on discharge record |
| 1 | Major operating room procedure reported on discharge record |
- C.
- All discharges were categorized into five hospitalization types (i.e., service lines) in the following hierarchical order: Maternal/Neonatal, Mental health/Substance abuse, Injury, Surgical, and Medical. The criteria for identifying the hospitalization types varies across data years.
Appendix B.2. Beginning in Data Year 2019
- MDC 14 Pregnancy, Childbirth, and Puerperium
- MDC 15 Newborn and Other Neonates (Perinatal Period)
- MDC 19 Mental Diseases and Disorders
- MDC 20 Alcohol/Drug Use or Induced Mental Disorders
- Clinical Classification Software-Refined categories for the principal ICD-10-CM diagnosis:
- ○
- INJ001–INJ027
- ○
- INJ032
Appendix B.3. Dependent Variable Definition
| PDI Number | PDI Name | PDI Description | PDI Numerator |
|---|---|---|---|
| NQI 03 | Neonatal Blood Stream Infection Rate (https://qualityindicators.ahrq.gov/Downloads/Modules/PDI/V2019/TechSpecs/NQI_03_Neonatal_Blood_Stream_Infection_Rate.pdf accessed on 29 October 2022) | Discharges with healthcare-associated blood stream infection per 1000 discharges for newborns and outborns with birth weight of 500 g or more but less than 1500 g; with gestational age between 24 and 30 weeks; or with birth weight of 1500 g or more, and death, an operating room procedure, mechanical ventilation, or transferring from another hospital within two days of birth. Excludes discharges with a length of stay less than 3 days and discharges with a principal diagnosis of sepsis, bacteremia, or newborn bacteremia. | Discharges among cases meeting the inclusion and exclusion rules for the denominator, with either: • any secondary ICD-10-CM diagnosis codes for newborn sepsis (BSI5DX) or • any secondary ICD-10-CM diagnosis codes for newborn septicemia or bacteremia codes requiring a separate organism code (BSI2DX) and any secondary ICD-10-CM diagnosis codes for staphylococcal or Gram-negative bacterial infection (BSI3DX) |
Appendix C. Descriptive Number of Observations of Each Tested Independent Variable in the HCUP KID Database
| Hospital Characteristics | ||||
| Variable Name | Level | Number of Observations | Percent % | Missing % |
| Hospital bed size | Small | 470,770 | 15.2 | 0.0 |
| Medium | 742,057 | 24.0 | ||
| Large | 1,876,456 | 60.7 | ||
| Hospital location | Rural | 189,298 | 6.1 | 0.0 |
| Urban nonteaching | 356,963 | 11.6 | ||
| Urban teaching | 2,543,022 | 82.3 | ||
| Hospital region | Northeast | 529,073 | 17.1 | 0.0 |
| Midwest | 696,645 | 22.6 | ||
| South | 1,183,705 | 38.3 | ||
| West | 679,860 | 22.0 | ||
| Hospital ownership | Public | 365,784 | 11.8 | 0.0 |
| Private, not-for-profit | 2,382,758 | 77.1 | ||
| Private, investor-owned | 340,741 | 11.0 | ||
| Patient Characteristics | ||||
| Gender | Female | 1,587,394 | 51.4 | 0.0 |
| Male | 1,500,745 | 48.6 | ||
| Race | White | 1,407,652 | 45.6 | 7.2 |
| Black | 513,619 | 16.6 | ||
| Hispanic | 607,329 | 19.7 | ||
| Asian/Pacific Islander | 123,698 | 4.0 | ||
| Native American | 26,306 | .9 | ||
| Other | 188,042 | 6.1 | ||
| Service line | Maternal and Neonatal | 1,716,825 | 55.6 | 0.0 |
| Mental health/Substance use | 209,939 | 6.8 | ||
| Injury | 97,434 | 3.2 | ||
| Surgical | 219,576 | 7.1 | ||
| Medical | 845,509 | 27.4 | ||
| Payment source | Medicare | 10,554 | 0.3 | 0.1 |
| Medicaid | 1,567,452 | 50.7 | ||
| Private Insurance | 1,270,547 | 41.1 | ||
| Self-pay | 131,918 | 4.3 | ||
| No charge | 3843 | 0.1 | ||
| Other | 100,660 | 3.3 | ||
| Operation on record | No operation on record | 2,718,390 | 88.0 | 0.0 |
| Major operation on record | 370,893 | 12.0 | ||
References
- Agency for Healthcare Research and Quality: A Profile. Content Last Reviewed July 2022. Agency for Healthcare Research and Quality, Rockville, MD. Available online: https://www.ahrq.gov/cpi/about/profile/index.html (accessed on 29 October 2022).
- Garrouste-Orgeas, M.; Philippart, F.; Bruel, C.; Max, A.; Lau, N.; Misset, B. Overview of medical errors and adverse events. Ann. Intensive Care 2012, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Agency for Healthcare Research and Quality. Quality Indicators Software Instructions and Data Dictionary SAS QI® v2020. Rockville. Retrieved from Chrome Extension. July 2020. Available online: https://qualityindicators.ahrq.gov/Downloads/Software/SAS/v2020/Software_Inst_SASQI_v2020_July_2020.pdf (accessed on 29 October 2022).
- Falcone, M.L.; Van Stee, S.K.; Tokac, U.; Fish, A.F. Adverse Event Reporting Priorities: An Integrative Review. J. Patient Saf. 2022, 18, e727–e740. [Google Scholar] [CrossRef] [PubMed]
- Jarry, J. Medical Error Is Not the Third Leading Cause of Death. Office for Science and Society. 27 August 2021. Available online: https://www.mcgill.ca/oss/article/critical-thinking-health/medical-error-not-third-leading-cause-death (accessed on 27 December 2022).
- Makary, M.A.; Daniel, M. Medical error-the third leading cause of death in the US. BMJ Br. Med. J. 2016, 353, i2139. [Google Scholar] [CrossRef] [PubMed]
- Agarwal-Harding, K.J.; von Allmen, D.; Deans, K.J. Adverse events in pediatric surgery: A review of the literature and analysis of incident reports from a surgical safety registry. In Pediatric Surgery International; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Sharek, P.J.; Horbar, J.D.; Mason, W.; Bisarya, H.; Thurm, C.W.; Suresh, G.; Classen, D. Adverse events in the neonatal intensive care unit: Development, testing and findings of a NICU-focused trigger tool to identify harm in North American NICUs. Pediatrics 2010, 126, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Davenport, M.C.; Domínguez, P.A.; Ferreira, J.P.; Kannemann, A.L.; Paganini, A.; Torres, F.A. Measuring adverse events in pediatric inpatients with the Global Trigger Tool. Arch. Argent. Pediatr. 2017, 115, 357–363, (In English and Spanish). [Google Scholar] [CrossRef] [PubMed]
- Stockwell, D.C.; Landrigan, C.P.; Toomey, S.L.; Loren, S.S.; Jang, J.; Quinn, J.A.; Ashrafzadeh, S.; Wang, M.J.; Wu, M.; Sharek, P.J.; et al. Adverse events in hospitalized pediatric patients. Pediatrics 2018, 142, e20173360. [Google Scholar] [CrossRef] [PubMed]
- Stoll, B.J.; Puopolo, K.M.; Hansen, N.I.; Sánchez, P.J.; Bell, E.F.; Carlo, W.A.; Cotten, C.M.; D’Angio, C.T.; Kazzi, S.N.J.; Poindexter, B.B.; et al. Early-Onset Neonatal Sepsis 2015 to 2017, the Rise of Escherichia coli, and the Need for Novel Prevention Strategies. JAMA Pediatr. 2020, 174, e200593, Erratum in JAMA Pediatr. 2021, 175, 212. [Google Scholar] [CrossRef] [PubMed]
- Schrag, S.J.; Farley, M.M.; Petit, S.; Reingold, A.; Weston, E.J.; Pondo, T.; Jain, J.H.; Lynfield, R. Epidemiology of Invasive Early-Onset Neonatal Sepsis, 2005 to 2014. Pediatrics 2016, 138, e20162013. [Google Scholar] [CrossRef] [PubMed]
- Flannery, D.D.; Chiotos, K.; Gerber, J.S.; Puopolo, K.M. Neonatal multidrug-resistant gram-negative infection: Epidemiology, mechanisms of resistance, and management. Pediatr. Res. 2022, 91, 380–391. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vachirapuranon, S.; Vijarnsorn, C.; Kanjanauthai, S.; Tocharoenchok, T.; Durongpisitkul, K.; Chanthong, P.; Phachiyanukul, A. Major infections following pediatric cardiac surgery pre-and post-CLABSI bundle implementation. PeerJ 2022, 10, e14279. [Google Scholar] [CrossRef] [PubMed]
- HCUP Databases. Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality, Rockville, MD. February 2022. Available online: www.hcup-us.ahrq.gov/kidoverview.jsp (accessed on 29 October 2022).
- Lyren, A.; Haines, E.; Fanta, M.; Gutzeit, M.; Staubach, K.; Chundi, P.; Ward, V.; Srinivasan, L.; Mackey, M.; Vonderhaar, M.; et al. Racial and ethnic disparities in common inpatient safety outcomes in a children’s hospital cohort. BMJ Qual. Saf. 2024, 33, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Heller, K.O.; Souter, K.J. Disclosure of Adverse Events and Medical Errors: A Framework for Anesthesiologists. Anesthesiology Clinics. Available online: https://www.anesthesiology.theclinics.com/article/S1932-2275(23)00121-0/fulltext (accessed on 6 January 2024).
- Healthcare Cost and Utilization Project (HCUP). KID Description of Data Elements. (n.d.). Available online: https://hcup-us.ahrq.gov/db/nation/kid/kiddde.jsp (accessed on 29 October 2022).
- Feyissa, D.; Kebede, B.; Zewudie, A.; Mamo, Y. Medication Error and Its Contributing Factors Among Pediatric Patients Diagnosed with Infectious Diseases Admitted to Jimma University Medical Center, Southwest Ethiopia: Prospective Observational Study. Integr. Pharm. Res. Pract. 2020, 9, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Parikh, K.; Hall, M.; Tieder, J.S.; Dixon, G.; Ward, M.C.; Hinds, P.S.; Goyal, M.K.; Rangel, S.J.; Flores, G.; Kaiser, S.V. Disparities in Racial, Ethnic, and Payer Groups for Pediatric Safety Events in US Hospitals. Pediatrics 2024, 153, e2023063714. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.B.; Dynan, L.; Fairbrother, G.; Chabi, G.; Simpson, L. Medicaid, hospital financial stress, and the incidence of adverse medical events for children. Health Serv. Res. 2012, 47, 1621–1641. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guth, M.; Garfield, R.; Rudowitz, R. The Effects of Medicaid Expansion under the ACA: Studies from January 2014 to January 2020. KFF The Independent Source for Health Policy Research, Polling, and News. 17 February 2023. Available online: https://www.kff.org/affordable-care-act/report/the-effects-of-medicaid-expansion-under-the-aca-updated-findings-from-a-literature-review/ (accessed on 5 May 2024).
- Kannan, S.; Bruch, J.D.; Song, Z. Changes in Hospital Adverse Events and Patient Outcomes Associated with Private Equity Acquisition. JAMA 2023, 330, 2365–2375. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.; Hsia, Y.; Basmaci, R.; Bielicki, J.; Heath, P.T.; Versporten, A.; Goossens, H.; Sharland, M. Global Divergence from World Health Organization Treatment Guidelines for Neonatal and Pediatric Sepsis. Pediatr. Infect. Dis. J. 2019, 38, 1104–1106. [Google Scholar] [CrossRef] [PubMed]
| NBSIs | Frequency | Percent |
|---|---|---|
| NBSI abscent | 1,833,049 | 78.61 |
| NBSI present | 498,829 | 21.39 |
| Patient Characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Estimate | SE | Wald Chi-Square | p-Value | OR | Wald 95% Confidence Limits for OR | ||
| Gender | Female | −0.0909 | 0.00231 | 1544.9792 | 0.5876 | 0.998 | 0.992 | 1.005 |
| Male § | -- | -- | -- | -- | -- | -- | -- | |
| Race | Black | 0.3737 | 0.00474 | 6210.2915 | <0.0001 | 1.453 | 1.440 | 1.467 |
| Hispanic | 0.3005 | 0.00456 | 4335.7171 | <0.0001 | 1.351 | 1.339 | 1.363 | |
| Asian/Pacific Islander | 0.3514 | 0.00731 | 2309.3090 | <0.0001 | 1.421 | 1.401 | 1.442 | |
| Native American | −0.6356 | 0.0211 | 905.0704 | <0.0001 | 0.530 | 0.508 | 0.552 | |
| Others | 0.2732 | 0.00656 | 1735.7776 | <0.0001 | 1.314 | 1.297 | 1.331 | |
| White § | -- | -- | -- | -- | -- | -- | -- | |
| Service line | Maternal and Neonatal | −0.5369 | 0.0154 | 1212.1621 | <0.0001 | 0.585 | 0.567 | 0.603 |
| Mental health/Substance use | 0.2149 | 0.2036 | 1.1139 | 0.2912 | 1.240 | 0.832 | 1.848 | |
| Injury | 0.2358 | 0.0915 | 6.6453 | 0.0099 | 1.266 | 1.058 | 1.515 | |
| Surgical | 0.5785 | 0.0286 | 409.9134 | <0.0001 | 1.783 | 1.686 | 1.886 | |
| Medical § | -- | -- | -- | -- | -- | -- | -- | |
| Payment Source | Medicare | −0.4039 | 0.0385 | 109.8998 | <0.0001 | 0.668 | 0.619 | 0.720 |
| Medicaid | 0.2659 | 0.00837 | 1009.4189 | <0.0001 | 1.305 | 1.283 | 1.326 | |
| Private insurance | 0.4226 | 0.00837 | 2548.9116 | <0.0001 | 1.526 | 1.501 | 1.551 | |
| No charge | −0.5472 | 0.0518 | 111.5880 | <0.0001 | 0.579 | 0.523 | 0.640 | |
| Other | 0.4556 | 0.0126 | 1304.3793 | <0.0001 | 1.577 | 1.539 | 1.617 | |
| Self-pay § | -- | -- | -- | -- | -- | -- | -- | |
| Operation on record | Major operating room procedure on record | 0.6831 | 0.0125 | 3003.8294 | <0.0001 | 1.980 | 1.932 | 2.029 |
| Hospital Characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Estimate | SE | Wald Chi-Square | p-Value | OR | Wald 95% CL for OR | ||
| Hospital bed size | Small | −0.8163 | 0.00485 | 28,347.8775 | <0.0001 | 0.442 | 0.438 | 0.446 |
| Medium | −0.2816 | 0.00389 | 5232.2092 | <0.0001 | 0.755 | 0.749 | 0.760 | |
| Large § | -- | -- | -- | -- | -- | -- | -- | |
| Hospital location | Rural | −2.6062 | 0.0106 | 60,150.4947 | <0.0001 | 0.074 | 0.072 | 0.075 |
| Urban nonteaching | −0.7646 | 0.00466 | 26946.8518 | <0.0001 | 0.466 | 0.461 | 0.470 | |
| Urban teaching § | -- | -- | -- | -- | -- | -- | -- | |
| Hospital region | Northeast | −0.0741 | 0.00555 | 178.5115 | <0.0001 | 0.929 | 0.919 | 0.939 |
| Midwest | −0.2399 | 0.00509 | 2217.5831 | <0.0001 | 0.787 | 0.779 | 0.795 | |
| South | 0.1026 | 0.00435 | 556.8232 | <0.0001 | 1.108 | 1.099 | 1.118 | |
| West § | -- | -- | -- | -- | -- | -- | -- | |
| Hospital ownership | Public | −0.3219 | 0.00690 | 2177.1313 | <0.0001 | 0.725 | 0.715 | 0.735 |
| Private, not-for-profit | 0.0247 | 0.00499 | 24.5644 | <0.0001 | 1.025 | 1.015 | 1.035 | |
| Private, investor-owned § | -- | -- | -- | -- | -- | -- | -- | |
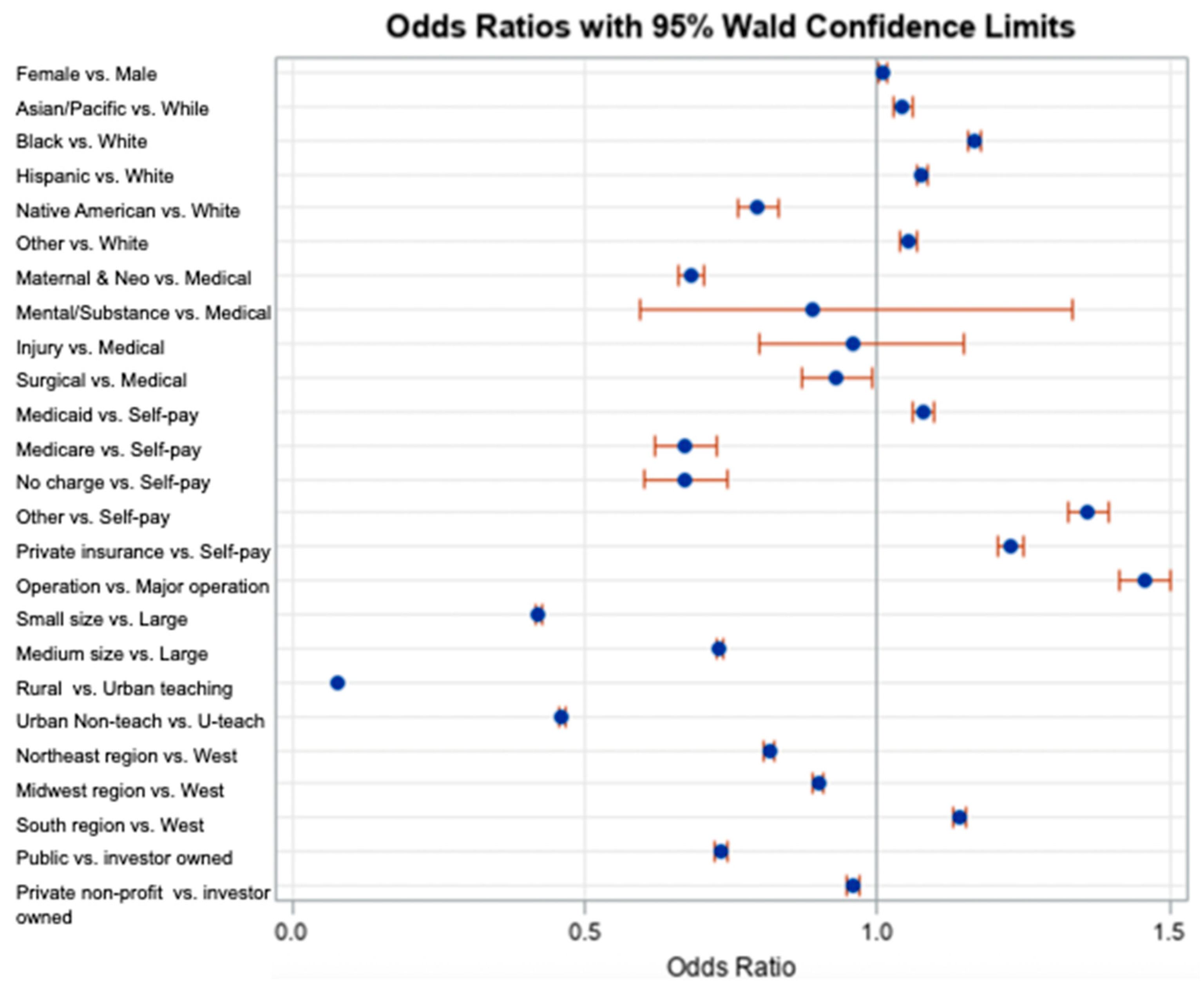
| Variable | Estimate | SE | Wald Chi-Square Test | p-Value | AOR | Wald 95% CL for AOR | ||
|---|---|---|---|---|---|---|---|---|
| Intercept | −0.4337 | 0.0194 | 501.5069 | <0.0001 | 0.648 | - | - | |
| Sex | Female | 0.00847 | 0.00351 | 5.8222 | 0.0158 | 1.009 | 1.002 | 1.015 |
| Male § | -- | -- | -- | -- | -- | -- | -- | |
| Race | Black | 0.1535 | 0.00517 | 881.5084 | <0.0001 | 1.166 | 1.154 | 1.178 |
| Hispanic | 0.0739 | 0.00503 | 215.7485 | <0.0001 | 1.077 | 1.066 | 1.087 | |
| Asian/Pacific Islander | 0.0423 | 0.00759 | 31.0443 | <0.0001 | 1.043 | 0.962 | 0.990 | |
| Native American | −0.2294 | 0.0225 | 103.8504 | <0.0001 | 0.795 | 0.761 | 0.831 | |
| Others | 0.0508 | 0.00683 | 55.2842 | <0.0001 | 1.052 | 1.038 | 1.066 | |
| White § | -- | -- | -- | -- | -- | -- | -- | |
| Service line | Maternal and Neonatal | −0.3831 | 0.0161 | 567.0396 | <0.0001 | 0.682 | 0.661 | 0.704 |
| Mental health/Substance use | −0.1174 | 0.2069 | 0.3217 | 0.5706 | 0.889 | 0.593 | 1.334 | |
| Injury | −0.0435 | 0.0928 | 0.2201 | 0.6389 | 0.957 | 0.798 | 1.148 | |
| Surgical | −0.0737 | 0.0329 | 5.0169 | 0.0251 | 0.929 | 0.871 | 0.991 | |
| Medical § | -- | -- | -- | -- | -- | -- | -- | |
| Payment Source | Medicare | −0.3971 | 0.0402 | 97.5495 | <0.0001 | 0.672 | 0.621 | 0.727 |
| Medicaid | 0.0759 | 0.00881 | 74.1780 | <0.0001 | 1.079 | 1.060 | 1.098 | |
| Private Insurance | 0.2057 | 0.00884 | 542.0145 | <0.0001 | 1.228 | 1.207 | 1.250 | |
| No charge | −0.4010 | 0.0538 | 55.6353 | <0.0001 | 0.670 | 0.603 | 0.744 | |
| Other | 0.3081 | 0.0133 | 538.8821 | <0.0001 | 1.361 | 1.326 | 1.397 | |
| Self-pay § | -- | -- | -- | -- | -- | -- | -- | |
| Operation on record | Major operating room procedure on record | 0.3762 | 0.0153 | 605.3762 | <0.0001 | 1.457 | 1.414 | 1.501 |
| Hospital bed size | Small | −0.8640 | 0.00502 | 29,669.8567 | <0.0001 | 0.421 | 0.417 | 0.426 |
| Medium | −0.3144 | 0.00408 | 5930.7825 | <0.0001 | 0.730 | 0.724 | 0.736 | |
| Large § | -- | -- | -- | -- | -- | -- | -- | |
| Hospital location | Rural | −2.5734 | 0.0108 | 57,294.8291 | <0.0001 | 0.076 | 0.075 | 0.078 |
| Urban nonteaching | −0.7742 | 0.00477 | 26,318.7635 | <0.0001 | 0.461 | 0.457 | 0.465 | |
| Urban teaching § | -- | -- | -- | -- | -- | -- | -- | |
| Hospital region | Northeast | −0.2032 | 0.00591 | 1181.1937 | <0.0001 | 0.816 | 0.807 | 0.826 |
| Midwest | −0.1065 | 0.00558 | 364.6463 | <0.0001 | 0.899 | 0.889 | 0.909 | |
| South | 0.1321 | 0.00474 | 777.7836 | <0.0001 | 1.141 | 1.131 | 1.152 | |
| West § | -- | -- | -- | -- | -- | -- | -- | |
| Hospital ownership | Public | −0.3099 | 0.00735 | 1780.3272 | <0.0001 | 0.733 | 0.723 | 0.744 |
| Private, not-for-profit | −0.0433 | 0.00536 | 65.1875 | <0.0001 | 0.958 | 0.948 | 0.968 | |
| Private, investor-owned § | -- | -- | -- | -- | -- | -- | -- | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samawi, M.; Shah, G.H.; Kimsey, L.; Waterfield, K.C.; Hendrix, S. Hospital and Patient Characteristics Associated with Neonatal Blood Stream Infection in Inpatient Care: Insights from the 2019 HCUP KID Database. Children 2024, 11, 923. https://doi.org/10.3390/children11080923
Samawi M, Shah GH, Kimsey L, Waterfield KC, Hendrix S. Hospital and Patient Characteristics Associated with Neonatal Blood Stream Infection in Inpatient Care: Insights from the 2019 HCUP KID Database. Children. 2024; 11(8):923. https://doi.org/10.3390/children11080923
Chicago/Turabian StyleSamawi, Michael, Gulzar H. Shah, Linda Kimsey, Kristie C. Waterfield, and Susan Hendrix. 2024. "Hospital and Patient Characteristics Associated with Neonatal Blood Stream Infection in Inpatient Care: Insights from the 2019 HCUP KID Database" Children 11, no. 8: 923. https://doi.org/10.3390/children11080923
APA StyleSamawi, M., Shah, G. H., Kimsey, L., Waterfield, K. C., & Hendrix, S. (2024). Hospital and Patient Characteristics Associated with Neonatal Blood Stream Infection in Inpatient Care: Insights from the 2019 HCUP KID Database. Children, 11(8), 923. https://doi.org/10.3390/children11080923









