Abstract
Objective: The purpose of this study was to investigate the effectiveness of corticosteroid therapy for children suffering from Sydenham chorea (SC). Methods: The design of the study was observational, retrospective and conducted at the single center of the Rheumatology Unit of Policlinic Hospital of Milan, Italy, from May 1995 to May 2022. All data about the patients were collected from medical records. Results: From a total of 59 patients enrolled in the study (44 females and 15 males; median age 9.3 years, range 7.4–10.6 years), 49 were eligible for primary outcome analysis (10 patients were excluded due to incomplete data). Overall, 75% of patients received steroid therapy, while the remaining cases were treated with symptomatic drugs, including neuroleptics and antiseizure drugs. We found that the duration of chorea was significantly shorter in patients treated with corticosteroids in comparison to those receiving symptomatic treatment (median time: 31 vs. 41 days, p = 0.023). Additionally, patients with arthritis at the onset of the disease had a longer duration of chorea than those without arthritis (median time 90.5 vs. 39 days, p = 0.02). We also found that chorea recurred in 12% of the patients and seemed to be linked to a younger age at onset (p = 0.01). Conclusions: The study suggests that corticosteroid therapy can lead to a faster resolution of SC when compared to neuroleptics and antiseizure drugs treatment.
1. Introduction
Chorea is a movement disorder characterized by a continuous flow of unpredictable involuntary movements [1,2]. Sydenham chorea (SC), a major manifestation of rheumatic fever (RF), is the most common form of acute chorea in children [3], usually developing 4 to 8 weeks after an episode of group A Beta-Hemolytic Streptococcal (GABHS) pharyngitis [4]. Treatment options for SC are still largely empirical [5]. The presence of antibodies reactive with neuronal tissue in the serum of patients with SC indicates a humoral mediated autoimmune condition [6,7]. Immunomodulatory therapies to shorten the duration of the illness and to prevent aggravations include corticosteroids, intravenous immunoglobulins (IVIG) and plasma exchange [8], with steroids having the strongest literature data [9].
Here we report the clinical experience with corticosteroid treatment in pediatric patients with SC referred to our tertiary referral center for RF over nearly three decades.
2. Materials and Methods
The design of the study was observational, retrospective and conducted at the single center of the Rheumatology Unit of Policlinic Hospital of Milan, Italy, from May 1995 to May 2022.
Clinical data of patients suffering from SC at disease onset and during a follow-up of at least 12 months were collected from medical records and registered in a case report form. All patients’ names and personal data were anonymized.
The diagnosis of SC was established according to the revised Jones criteria [10]. For all patients, other causes of chorea were ruled out by appropriate investigations, including thyroid disorders, systemic lupus erythematosus, vasculitis and Wilson’s disease.
We required a positive throat culture or increased anti-streptolysin-O (ASO) titer to confirm a previous streptococcal infection, although their absence did not exclude the clinical diagnosis of SC. Indeed, 20–25% of acute chorea cases show no clinical or laboratory evidence of previous rheumatic disease [11].
The following patient data were collected: demographic data (age, sex, ethnic group), type of chorea (generalized or hemichorea), family history of RF, past medical history (including previous pharyngotonsillitis), presence of neuropsychiatric manifestations(emotional lability, irritability, difficulty in concentrating, mood disorders), presence of other major criteria of RF (carditis, arthritis, erythema marginatum, subcutaneous nodules), laboratory exams (C reactive protein, erythrocyte sedimentation rate, ASO titer, throat culture for group A streptococcus), echocardiography and brain MRI. Regarding the treatment, patients with SC were divided into two groups, the first one receiving steroid therapy and the second one receiving symptomatic treatment with neuroleptics or antiseizure drugs. The latency from onset of symptoms to diagnosis and start of treatment and the time needed for improvement and clinical remission were recorded, as well as the rate of recurrence.
Finally, we evaluated the compliance of secondary prophylaxis for GABHS, consisting of intramuscular penicillin G benzathine every 3 weeks for at least 5 years in patients without carditis and 10 years in those with carditis.
Statistical Analysis
Categorical variables are described as number and percentage, while continuous variables as median and interquartile range (IQR).
To assess the correlation between treatment and outcome parameters (time of improvement, time of remission and recurrence), Pearson chi-square test or Pearson correlation coefficient or the non-parametric Mann–Whitney test were used, when appropriate. p-values lower than 0.05 were considered significant.
The analyses were carried out leveraging SciPy, the open source scientific computing library for the Python programming language [12].
3. Results
A total of fifty-nine SC patients (44 females, 15 males), aged between 2 and 18 years (mean age 9.3 years) were enrolled for the study. Demographic characteristics and clinical features of the 59 patients with SC enrolled are illustrated below in Table 1.

Table 1.
Demographic characteristics and clinical features of the 59 patients with SC enrolled in the study, including 39 patients treated with prednisone and 10 patients treated with symptomatic drugs.
The median time from onset of symptoms to the diagnosis and start of treatment was 9 days (IQR = 3–15 days). Chorea’s improvement began after a median of 1 day after starting treatment (IQR 1–3,75 days), and clinical remission was achieved in 40 days (IQR 29–61 days).
The relationship between type of therapy (steroid or symptomatic drugs) and symptoms’ duration was established in only 49 of the 59 patients enrolled, since the date of remission was not available in six patients and SC was diagnosed only after spontaneous remission in four patients. Among the 49 patients considered, 39 patients (79.5%) received steroid therapy as first line therapy or second choice when symptomatic therapy failed (neuroleptics, diazepam, valproate, respectively in nine, two, and one patients), while the remaining ten patients were treated only with symptomatic drugs (neuroleptics, valproate, diazepam respectively in eight, one, and one patients). Steroid therapy consisted of prednisone at the initial dose of 2 mg/Kg/day for 1–2 weeks, followed by tapering and discontinuation within 1–2 months. The duration of treatment with symptomatic drugs was variable, depending on the severity of chorea. Corticosteroids treatment was associated with transitory weight gain, while therapy with neuroleptics was complicated by unilateral foot dystonia in one patient, that disappeared after 6 months.
Demographic characteristics and clinical features of the 39 patients treated with prednisone and of the ten patients treated with symptomatic drugs are showed below in Table 1.
The relationship between type of treatment and primary outcomes are detailed in Table 2 and represented in Figure 1.

Table 2.
The time for improvement, for remission (primary outcome) and the recurrence rate in the patients treated with steroid therapy among other drugs and only with symptomatic therapy.
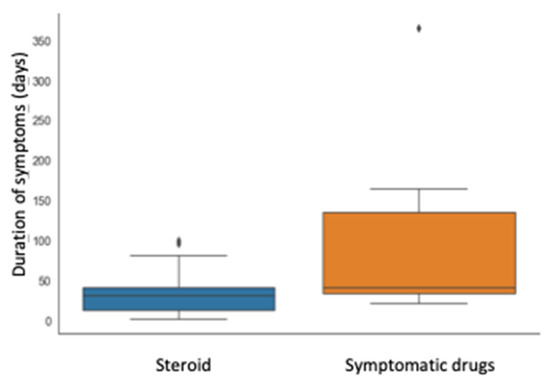
Figure 1.
Correlation between therapy (prednisone or not) and symptom duration (days).
Although the interval for first improvement was not statistically different in the two groups, the time of remission was significantly shorter in patients treated with prednisone compared with those treated with symptomatic drugs (median time 31 vs. 41 days, p = 0.023). Furthermore, the group that received steroid presented an IQR of time for remission remarkably shorter (28 vs. 102 days), as shown in Figure 1.
Finally, the presence of arthritis at SC onset was associated with a longer duration of symptoms (median time 90.5 versus 39 days, p = 0.02), as shown in Figure 2.

Figure 2.
Correlation between arthritis (present or not) and symptom duration (days).
More details about the demographic characteristics and the clinical features of the patients with and without arthritis are shown in Table 3.

Table 3.
Demographic characteristics and clinical features of patients with and without arthritis.
Finally, we looked for a possible correlation between any demographic and clinical features and the occurrence of one or more recurrences of the SC. We found that the incidence of a recurrence was higher in the patients with a younger age at onset (median age at onset of patients with recurrence is 6.06 years versus 9.37 years, p-value 0.011), as shown in the Figure 3.

Figure 3.
Correlation between the recurrence of SC and the median age of patients at the onset of the disease.
4. Discussion
The main result of our study was that corticosteroids induce faster remission of chorea in comparison to neuroleptics and antiseizure drugs. This result seems to agree with other studies reporting that corticosteroid therapy induces a rapid response if compared with standard therapy for chorea [13], placebo [6] or natural history of SC [14]. Different treatments of chorea have been suggested in patients with SC, including symptomatic drugs (antipsychotic, antiseizure drugs) and immunomodulatory therapy (steroids, IVIG, and plasma exchange) [15]. Both neuroleptics and antiseizure drugs are “off-label” therapies for SC, with no demonstrated efficacy established by randomized control studies [5]. Furthermore, SC may represent a risk factor for drug induced parkinsonism, and there is a need for caution when treating patients with SC with neuroleptics [16]. The pathophysiology of SC raises the possibility of immunomodulatory therapies being effective [2]. According to Barash et al. [14], we found that a short course of corticosteroids is associated with marked improvement of the involuntary movements, without side effects besides transitory weight gain.
We wondered if the presence of carditis could have influenced the decision of starting steroid treatment in SC, but, contrary to our expectations, we found that eight patients with SC and carditis had never received steroids. All these patients were admitted before the 2006, i.e., before the first randomized double-blind study demonstrating the superiority of steroids over placebo to shorten the symptoms of SC [6].
Our study also suggested that corticosteroid treatment was not the only factor influencing the time of remission of chorea. Indeed, patients with arthritis at disease onset had a longer duration of chorea compared to patients without arthritis. In our opinion, this finding could be explained by the higher phase activity of the inflammatory disease in SC patients with arthritis compared to those without arthritis.
Finally, our study showed that recurrence of chorea seems to be related only to a younger age at onset. Although SC is usually a monophasic disease [17], 13% to 42% of the cases recur, usually in the first few years [4,17,18,19,20]. Suggested risk factors associated with SC recurrence include irregular usage of antibiotic prophylaxis, failure to achieve remission within 6 months and prolongation of symptoms for more than 1 year [21]. Although some authors reported that penicillin G prophylaxis reduces recurrence compared with no prophylaxis [21,22,23], suggesting that most recurrences are due to re-exposure to streptococcal infection [19], a few reports mentioned that prophylactic treatment does not have any effect on recurrences [24,25]. Several patients with delayed recurrences of chorea that were not accompanied by active RF have been reported, suggesting that other unidentified triggers have induced an increase in the immunological reaction within the central nervous system [17]. However, the efficacy of immunomodulatory therapies in preventing recurrent episodes of SC remains controversial. Although Barash et al. suggested that commencement of corticosteroid treatment at an early stage could explain the lack of recurrence of SC [14], Paz et al. reported that recurrence rates were the same in children treated with steroids compared with placebo controls in a randomized, double-blind, parallel study [6]. Favaretto et al. reported that steroid therapy does not increase the risk of relapses but rather, on the contrary, could be effective in their prevention [13]. Recognizing that some authors suggest that recurrent episodes of SC may not be immune mediated, future studies of immunomodulatory therapies should attempt to correlate outcomes with measurements of proposed biomarkers [15].
Although we did not use standardized neuropsychiatric interviews, we noticed that a faster resolution of chorea in our patients treated with prednisone seemed associated with a better social reintegration at home and school. This personal observation could be consistent with the interesting finding reported by Moreira et al. that social phobia was more frequent in SC patients compared to the general population [26].
Our study has several limitations, including the retrospective nature of the study, the imbalance between patients treated with prednisone and those treated with symptomatic drugs (39 versus 10 respectively), and the lack of use of standardized chorea rating scales.
In conclusion, our study suggests that early treatment with corticosteroids in SC is associated with rapid improvement of the involuntary movement and could be considered as first-line therapy in all patients with SC unless contraindicated. We currently try to avoid symptomatic treatment with neuroleptics owing to the risk of drug induced parkinsonism, dystonia or both [1,27]. Larger, possibly comparative studies, using standardized assessment scales, are necessary if therapeutic decisions for SC are to be based on meaningful information [14,15].
Author Contributions
Conceptualization, A.M.C. and A.P.; methodology, A.M.C. and A.P.; software, G.R.; validation, A.M.C., G.F. and A.P.; formal analysis, G.R.; investigation, G.R.; resources, A.P.; data curation, G.R. and A.P.; writing—original draft preparation, A.M.C.; writing—review and editing, A.M.C.; visualization, A.P. and G.F.; supervision, A.M.C. and A.P.; project administration, A.M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Ricerca Corrente’ (IRCCS RC-2023 Grant no. 01) grant from the Italian Ministry of Health.
Informed Consent Statement
Patient’s written consent forms was not required owing to the retrospective nature of the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to thank Carducci Francesco for his invaluable contribution to the development of the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cardoso, F. Autoimmune choreas. J. Neurol. Neurosurg. Psychiatry 2017, 88, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, S.; Mink, J.W. Treatment of Chorea in Childhood. Pediatr. Neurol. 2020, 102, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Eduardo, C.; Silva, A.P.; Mota, C.C.C. Chorea in fifty consecutive patients with rheumatic fever. Mov. Disord. 1997, 12, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Nardocci, N.; Fernandez-Alvarez, E. Movement Disorders in Children: A Clinical Update with Video Recordings. In Movement Disorders in Children: A Clinical Update with Video Recordings; John Libbey Eurotext: Montrouge, France, 2007; pp. 113–124. [Google Scholar]
- Vasconcelos, L.P.B.; Vasconcelos, M.C.; Nunes, M.D.C.P.; Teixeira, A.L. Sydenham’s chorea: An update on pathophysiology, clinical features and management. Expert Opin. Orphan Drugs 2019, 7, 501–511. [Google Scholar] [CrossRef]
- Paz, J.A.; Silva, C.A.A.; Marques-Dias, M.J. Randomized Double-Blind Study With Prednisone in Sydenham’s Chorea. Pediatr. Neurol. 2006, 34, 264–269. [Google Scholar] [CrossRef]
- Swedo, S.E. Sydenham’s Chorea: A Model for Childhood Autoimmune Neuropsychiatric Disorders. JAMA 1994, 272, 1788–1791. [Google Scholar] [CrossRef]
- Garvey, M.A.; Snider, L.A.; Leitman, S.F.; Werden, R.; Swedo, S.E. Treatment of Sydenham’s chorea with intravenous immunoglobulin, plasma exchange, or prednisone. J. Child Neurol. 2005, 20, 424–429. [Google Scholar] [CrossRef]
- Walker, K.G.; Wilmshurst, J.M. An update on the treatment of Sydenham’s chorea: The evidence for established and evolving interventions. Ther. Adv. Neurol. Disord. 2010, 3, 301–309. [Google Scholar] [CrossRef]
- Guidelines for the Diagnosis of Rheumatic Fever: Jones Criteria, 1992 Update|JAMA|JAMA Network [Internet]. Available online: https://jamanetwork.com/journals/jama/article-abstract/400639 (accessed on 11 November 2022).
- Barsottini, O.G.; Ferraz, H.B.; Seviliano, M.M.; Barbieri, A. Brain SPECT imaging in Sydenham’s chorea. Braz. J. Med. Biol. Res. 2002, 35, 431–436. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Favaretto, E.; Gortani, G.; Simonini, G.; Pastore, S.; Di Mascio, A.; Cimaz, R.; Taddio, A. Preliminary data on prednisone effectiveness in children with Sydenham chorea. Eur. J. Pediatr. 2020, 179, 993–997. [Google Scholar] [CrossRef]
- Barash, J.; Margalith, D.; Matitiau, A. Corticosteroid treatment in patients with Sydenham’s chorea. Pediatr. Neurol. 2005, 32, 205–207. [Google Scholar] [CrossRef]
- Dean, S.L.; Singer, H.S. Treatment of Sydenham’s Chorea: A Review of the Current Evidence. Tremor Other Hyperkinetic Mov. 2017, 7, 456. [Google Scholar] [CrossRef]
- Teixeira, A.L. Sydenham’s chorea may be a risk factor for drug induced parkinsonism. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1350–1351. [Google Scholar] [CrossRef]
- Korn-Lubetzki, I.; Brand, A.; Steiner, I. Recurrence of Sydenham chorea: Implications for pathogenesis. Arch. Neurol. 2004, 61, 1261–1264. [Google Scholar] [CrossRef]
- Demiroren, K.; Yavuz, H.; Cam, L.; Oran, B.; Karaaslan, S.; Demiroren, S. Sydenham’s Chorea: A Clinical Follow-Up of 65 Patients. J. Child Neurol. 2007, 22, 550–554. [Google Scholar] [CrossRef]
- Tumas, V.; Caldas, C.T.; Santos, A.C.; Nobre, A.; Fernandes, R.M.F. Sydenham’s chorea: Clinical observations from a Brazilian movement disorder clinic. Park. Relat. Disord. 2007, 13, 276–283. [Google Scholar] [CrossRef]
- Kılıç, A.; Ünüvar, E.; Tatlı, B.; Gökçe, M.; Ömeroğlu, R.E.; Oğuz, F.; Sıdal, M. Neurologic and Cardiac Findings in Children With Sydenham Chorea. Pediatr. Neurol. 2007, 36, 159–164. [Google Scholar] [CrossRef]
- Gurkas, E.; Karalok, Z.S.; Taskin, B.D.; Aydogmus, U.; Guven, A.; Degerliyurt, A.; Bektas, O.; Yilmaz, C. Predictors of recurrence in Sydenham’s chorea: Clinical observation from a single center. Brain Dev. 2016, 38, 827–834. [Google Scholar] [CrossRef]
- al-Eissa, A. Sydenham’s chorea: A new look at an old disease. Br. J. Clin. Pract. 1993, 47, 14–16. [Google Scholar]
- Gebremariam, A. Sydenham’s chorea: Risk factors and the role of prophylactic benzathine penicillin G in preventing recurrence. Ann. Trop. Paediatr. 1999, 19, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Berrios, X.; Quesney, F.; Morales, A.; Blazquez, J.; Bisno, A.L. Are all recurrences of “pure” Sydenham chorea true recurrences of acute rheumatic fever? J. Pediatr. 1985, 107, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Manyemba, J.; Mayosi, B.M. Penicillin for secondary prevention of rheumatic fever. Cochrane Database Syst. Rev. 2002, CD002227. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.; Kummer, A.; Harsányi, E.; Cardoso, F. Psychiatric disorders in persistent and remitted Sydenham’s chorea. Park. Relat. Disord. 2014, 20, 233–236. [Google Scholar] [CrossRef]
- Cappellari, A.M.; Lanfranchi, C.; Bruschi, G.; Petaccia, A. Sydenham’s chorea: A diagnosis not to miss. Rev. Neurol. 2022, 178, 855–856. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).