Abstract
Background: Mock circulatory loops (MCLs) are benchtop experimental platforms that reproduce key features of the human cardiovascular system, providing a safe, controlled, and reproducible environment for haemodynamic investigation. This scoping review aims to systematically map the current landscape of MCLs used for aortic simulation and identify major areas of application. Methods: A systematic search of PubMed, Scopus, and Web of Science identified original studies employing MCLs for aortic simulation. Eligible studies were categorized into predefined themes: (I) (bio)mechanical aortic characterization, (II) hemodynamics, (III) device testing, (IV) diagnostics, and (V) training. Data on MCL configurations, aortic models, and study objectives were synthesized narratively. Results: Eighty-four studies met the inclusion criteria. Twenty-five investigated aortic biomechanics, 23 hemodynamics, 22 device or product testing, 13 validated diagnostic imaging techniques, and one training application. Models included porcine (n = 22), human cadaveric (n = 7), canine (n = 1), ovine (n = 1), bovine (n = 1), and 3D-printed or molded aortic phantoms (n = 55). MCLs were employed to study parameters such as aortic stiffness, flow dynamics, dissection propagation, endoleaks, imaging accuracy, and device performance. Conclusions: This review provides a comprehensive overview of MCL applications in aortic research. MCLs represent a versatile pre-clinical platform for studying aortic pathophysiology and testing endovascular therapies under controlled conditions. Standardized reporting frameworks are now required to improve reproducibility and accelerate translation to patient-specific planning.
1. Introduction
Mock Circulatory Loops (MCLs) are experimental benchtop models designed to simulate the human cardiovascular system (CVS) under both physiological and pathological conditions (see Figure 1). MCLs can mimic hemodynamic parameters such as heart rate, cardiac output, cardiac contractility, arterial compliance, peripheral resistance, and fluid inertance in vitro or ex vivo. In contrast to in vivo studies, in vitro and ex vivo experiments are preferred when precise quantitative analysis and control of physiological parameters are required, for pre-clinical device testing, and to minimize the use of animal studies.

Figure 1.
Example of a mock circulatory loop used for aortic research.
MCLs have evolved from simple hydraulic testbeds to sophisticated hybrid platforms integrating real-time control systems and anatomically accurate components. This development enables simultaneous, accurate, and repeatable measurements of (i) hemodynamics, including flow rate and pressure, and (ii) aortic wall characteristics, such as stiffness, displacement, and deformation. Moreover, they play a key role in the design, development, and in vitro assessment of implantable aortic devices (such as stent-grafts and intra-aortic balloon pumps), surgical treatments, and diagnostic imaging techniques.
Despite these advancements, no comprehensive synthesis currently exists regarding the application of MCLs in aortic research. This scoping review aims to systematically map the current state of MCL-based aortic simulation and to identify unmet methodological and reporting gaps relevant to translational and clinical implementation.
2. Materials and Methods
2.1. Review Design
This scoping review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) and was conducted according to established methodological frameworks [1,2]. The protocol was registered and made publicly available on the Open Science Framework: 10.17605/OSF.IO/Z37FY.
2.2. Objectives
The objective of this scoping review was to systematically map and synthesize the methods, applications, and outcomes of studies employing MCLs for aortic simulation and identify gaps in knowledge.
2.3. Literature Sources and Search Strategy
Two authors (F.E.N.O. and J.F.d.K.) independently conducted the literature search and study selection. In cases of discrepancies, a third and/or fourth senior author (J.A.v.H. and S.T.) was consulted to provide consensus.
A comprehensive search of PubMed, Scopus, and Web of Science was performed on 7 July 2025 (Supplementary Table S1). The search string was designed iteratively by the review team (F.E.N.O. and J.F.d.K.) in consultation with senior authors (J.A.v.H. and S.T.) and was structured around two core categories, (i) MCLs and (ii) the aorta, with additional terms reflecting device testing, biomechanical properties, and model types. Additionally, backward and forward citation tracking was performed.
2.4. Inclusion and Exclusion Criteria
All original English-language articles presenting a simulation model of the aorta, or parts of it, integrated into an MCL were included. Review articles, commentaries, abstracts without full text, and purely computational (in silico) studies were excluded.
2.5. Study Selection
All search results were imported into Rayyan.ai for organization and screening [3]. No automation or AI-assisted tools were used during the selection process. After removal of duplicates, titles and abstracts were screened manually. Full-text articles were retrieved for all potentially relevant studies. Reference lists of included studies were reviewed for additional eligible publications. The study selection phase was concluded on 15 September 2025.
2.6. Data Acquisition and Categorization
Data extraction was performed independently by two reviewers (F.E.N.O. and N.A.H.) using a predefined extraction form in Microsoft Excel (Microsoft Corp., Redmond, WA, USA). To facilitate structured comparison across the included studies, the following themes were established. Each study was discussed by three authors (F.E.N.O.; N.A.H.; and J.F.d.K.), and subsequently classified into predefined themes: (I) aortic characteristics, referring to physiological or post-intervention (bio)mechanics of the aortic wall; (II) aortic hemodynamics, focusing on flow behavior and pressure dynamics; (III) device testing, encompassing surgical, radiological, and phantom-based evaluations of products; (IV) diagnostics, addressing image-based methods for assessment and quantification of aortic pathology; and (V) training, involving the use of MCLs for procedural simulation and skill development.
2.7. Core Components of Mock Circulatory Loops
Systemic circulation MCLs typically consist of two primary elements: a flow driver and a closed circulatory loop. The driver generates continuous or pulsatile flow. In pulsatile systems, stroke volume and heart rate can be adjusted to simulate cardiac function. Various pump types are used in MCLs. Piston and diaphragm (sac-type) pumps generate controlled pulsatile flow that mimics ventricular contractions. Roller (peristaltic) pumps are widely used in current cardiac support systems and provide easy-to-control and reliable flow through flexible tubing with intrinsic backflow protection. Centrifugal pumps deliver continuous, smooth flow for non-pulsatile simulations. Gear pumps enable precise volumetric control under high pressures. Lastly, indirect compression systems reproduce ventricular motion by compressing compliant chambers using pneumatic, hydraulic, or servo-driven actuators [4].
To mimic preload, a venous compliance chamber or reservoir can be positioned upstream of the pump to represent the venous system. This reservoir collects the working fluid at the end of the loop and recirculates it, ensuring volume stability and continuous closed-loop operation. Ideally, the reservoir can be adjusted via pressure or fluid height to regulate ventricular filling conditions, i.e., preload.
To reproduce afterload, the system can incorporate an arterial compliance chamber with or without a peripheral resistance element. The arterial compliance chamber represents the elastic properties of the arterial walls, absorbing the pulsatile output of the pump and generating a physiologic pressure-flow relationship (the Wind Kessel effect). By adjusting the arterial compliance chamber, different levels of arterial stiffness can be achieved. A peripheral resistance element simulates systemic vascular resistance and can be adjusted to mimic varying levels of vasoconstriction and vasodilation to represent specific vascular beds within the circulation.
2.8. Data Presentation
The extracted data were summarized in textual and tabular formats. Data were presented as reported in the original studies, without additional statistical transformation. Categorical variables were presented as absolute numbers (n) and percentages (%), and continuous variables were reported as mean ± standard deviation (SD). Where available, ranges were also provided. Missing or unavailable data were denoted as (–). No statistical pooling or meta-analysis was performed due to heterogeneity among studies.
3. Results
3.1. Study Selection
The initial search yielded 2063 studies across three databases. Duplicate removal resulted in 1093 studies to screen on title and abstract. Eighty-one full-text articles were retrieved, of which four could not be obtained. A total of 77 full-text articles were assessed, and 69 met the inclusion criteria. An additional 15 studies were identified through backward and forward citation, resulting in a final total of 84 included studies. Details and study characteristics are provided in Supplementary Table S2, and Figure 2 provides the detailed flow diagram of study selection.
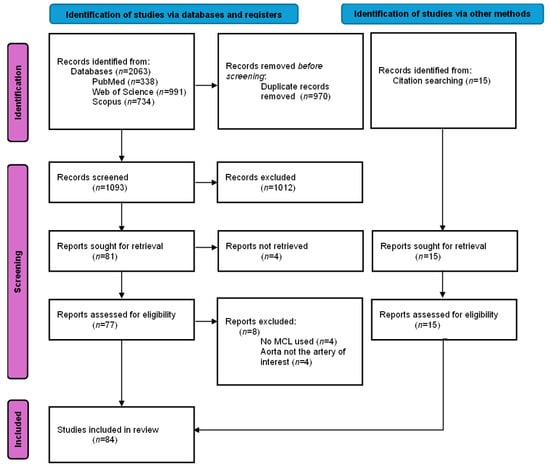
Figure 2.
PRISMA-ScR flow diagram of the study selection process for a scoping review on mock circulatory loops used in aortic research. Note: n = number of articles included.
3.2. Study Characteristics and Publication Trends
Most included articles originated from Europe and North America, with the largest contributions from the USA (n = 20; 23.8%) [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24], followed by Italy (n = 9; 10.7%) [25,26,27,28,29,30,31,32,33], The Netherlands (n = 9; 10.7%) [34,35,36,37,38,39,40,41,42], Austria (n = 6; 7.1%) [43,44,45,46,47,48], Canada (n = 6; 7.1%) [49,50,51,52,53,54], France (n = 6; 7.1%) [55,56,57,58,59,60], and Germany (n = 6; 7.1%) [61,62,63,64,65,66].
The first aortic MCL simulation study was published in 1998 in The Journal of Vascular Surgery by Schurink et al. [41]. Studies published between 1998 and 2025 were included with various themes, summarized in Table 1. Notably, 36 of 84 (42.9%) studies were published in the last 5 years. Mann–Kendall test confirmed a statistically significant upward publication trend from 2016 (τ = 0.488, p = 0.003), as shown in Figure 3.

Table 1.
Categorization of studies included in a scoping review on mock circulatory loops for aortic research. Note: n = number of articles included.
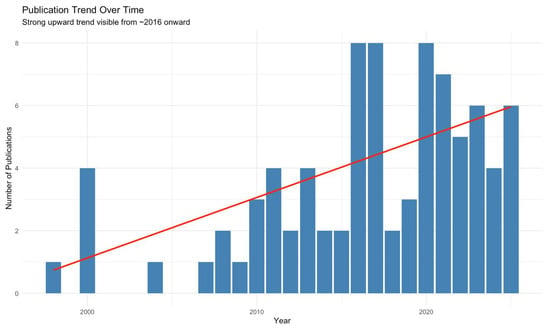
Figure 3.
The Mann–Kendall test showing the publication trend over time of studies included in a scoping review on mock circulatory loops used in aortic research. Red line shows the parametric linear increase in number of studies published per year using MCLs.
Of the 84 included studies, 48 (57.1%) were published in clinically oriented journals, while 30 (35.7%) were published in engineering journals. Only three studies (3.6%) each were published in broad-scope or physics journals.
3.3. Characteristics of Mock Circulatory Loops
The main characteristics of the mock circulatory loops used in the included studies are summarized in Table 2.

Table 2.
Characteristics of Mock Circulatory Loops (MCLs) used in studies included in a scoping review on MCLs for aortic research.
3.3.1. Flow Patterns and Pump Types
Most of the studies employed pulsatile flow (n = 75; 90.5%); six studies [6,15,27,29,39,56] used a continuous flow; two studies [8,52] used a pulsatile and (quasi)static flow. One study did not specify the flow pattern [45]. Several types of pumps were employed to generate flow within the MCLs: piston pumps (n = 43; 51.2%) were most common, followed by diaphragm or sac-type pumps (n = 8; 9.5%), gear pumps (n = 6; 7.1%), indirect compression systems (n = 5; 6.0%), centrifugal pumps (n = 4; 4.8%), and roller (peristaltic) pumps (n = 3; 3.6%). One study used a continuous-flow pump with a magnetic valve to create pulsatile flow. In 14 studies, the pump type was not reported.
3.3.2. Type of Aortic Models
Three studies employed hybrid experimental set-ups combining biological and synthetic; two combined phantom and porcine aortas [6,67], the other combined phantom and bovine aortas [18].
Twenty-nine (34.5%) used exclusively biological tissue, including seven with human cadaveric aortas [24,43,47,50,52,55,57], one with canine [16], and one with ovine and porcine [46]. The remaining 20 biological studies used porcine aortas [8,11,15,22,26,27,28,29,30,31,32,33,34,35,36,42,44,48,65,68].
In contrast, 52 studies employed synthetic aortic models, 20 of which were patient-specific phantoms fabricated via 3D printing or mold-based casting [5,12,14,17,21,23,25,37,45,56,59,60,62,63,66,69,70,71,72,73].
3.3.3. Type and Temperature of Working Fluid
In 33 studies (39.3%), glycerol or glycerin mixed with water or saline was used as the perfusion fluid. Fourteen studies (16.7%) employed saline, sometimes phosphate-buffered, aerated with 5% CO2/95% O2, or supplemented with contrast agents. Twenty-two studies (26.2%) utilized water as the perfusate. Four studies applied a commercially available blood-mimicking fluid [9,11,22,71]. Schurink et al. [41] used a starch solution with plasma-like viscosity, while Qing et al. [68] employed Gelofusine. Only Desai et al. [67] used anticoagulated blood for the perfusion of porcine aortas, and for synthetic aortas, they prepared a complex medium consisting of 8% low-molecular-weight Dextran (77 kDa) dissolved in M199 minimum essential medium, supplemented with 20% fetal bovine serum (FBS), 7.5% sodium bicarbonate, 200 mmol l-glutamine, and the antibiotics penicillin (10,000 U/mL) and streptomycin (10 mg/mL), adjusted to pH 7.20 ± 0.01. Eight studies did not report the working fluid [14,44,45,54,58,60,74,75].
A total of 37 studies reported the temperature of the working fluid, which was 37 °C in 27 studies [7,9,18,24,26,27,28,29,30,31,34,40,42,43,45,46,47,49,50,51,52,65,67,68,71,72,73].
3.3.4. Thoracic and Abdominal Environment
Thirteen articles reported a type of thoracic and/or abdominal environment simulation. Four studies mounted the aortic phantoms on a 3D-printed spine to replicate the mechanical support provided by the surrounding anatomy. Three of these used gelatine solutions [35,36,42], while one applied water [60] to mimic abdominal pressure. One study embedded a vascular model in a radiopaque anthropomorphic body phantom with a skeleton [66]. Other studies immersed the aortas in saline [67,76], glycerin [43], a glycerol-water solution [47], or a tissue-mimicking gel [6,8]. One study positioned an aortic endoleak model within a customized abdominal phantom that included tissue-mimicking material and representations of the spine, kidneys, liver, and spleen to create a realistic imaging background during fluoroscopy and CT [40]. Another study embedded the aortic phantom in a gel block to provide a static tissue reference [17].
3.3.5. Study Themes
3.3.5.1. Aortic (Bio)mechanical Characteristics
A total of 25 studies investigated the (bio)mechanical characteristics of the aorta, including 12 non-interventional and 13 post-interventional models.
Non-Interventional Characterization
Several studies investigated the intrinsic mechanical behavior of the native or untreated aorta, mainly viscoelastic and mechanical properties. Amabili et al. [49] and Franchini et al. [52] quantified the viscoelastic dynamics of the human descending thoracic aorta, reporting dynamic stiffness ratios and energy loss factors. Mascarenhas et al. [34] combined 2D ultrasound elastography with inflation testing to estimate the mechanical properties of porcine aortas.
Ene et al. [77] evaluated the mechanical impact of intraluminal thrombus in abdominal aortic aneurysm (AAA) phantoms by testing silicone models with varying intraluminal thrombus stiffness and thickness, showing that the presence and thickness of intraluminal thrombus reduced compliance and shifted maximal wall strain proximally. Wen et al. [73] combined phase-contrast magnetic resonance imaging (MRI) and computational fluid dynamics to determine flow characteristics and wall dynamics, finding that regions of high wall shear stress and pressure align with dissection-prone sites, whereas low wall shear stress and high oscillatory shear index are linked to wall thickening. Cameron et al. [53] assessed the effect of aortic compliance on pump performance, confirming that compliant mock aortas generated more physiologic waveforms, steadier flow, and reduced cardiac workload compared to rigid models.
In addition, several studies focused specifically on aortic stiffness. Mandigers et al. [31] investigated the association between aortic arch geometry and aortic stiffness, demonstrating that increased arch angulation is associated with higher pulse wave velocity (PWV), blood pressures, and amplifies the stiffening effect of stent-grafts. Jarman et al. [46] investigated the effect of different loading conditions on aortic stiffness indices and concluded that aortic elasticity is highly dependent on blood pressure. Complementary imaging-based approaches were reported by Boese et al. [61], Gaddum et al. [74], and Moravia et al. [59]. Boese et al. [61] evaluated the feasibility and accuracy of measuring PWV using phase-contrast MRI techniques. Gaddum et al. [74] validated a real-time MRI sequence to quantify beat-to-beat variations in PWV during respiratory maneuvers. Moravia et al. [59] applied particle image velocimetry (PIV) to assess PWV and corresponding stiffness using the lnD–U method, a one-point technique that relates instantaneous changes in flow velocity to logarithmic variations in aortic diameter, enabling localized stiffness assessment without dual-point pressure measurements. Zimmermann et al. [5] further examined the impact of vessel wall stiffness on 4D-flow MRI hemodynamic quantification.
Post-Interventional Characterization
Several studies investigated the biomechanical effects of endovascular interventions in human and porcine aortas. Agrafiotis et al. [43] investigated the impact of thoracic endovascular aortic repair (TEVAR) in human thoracic aortas, showing that stent-graft implantation significantly reduced distensibility and increased stiffness. Nauta et al. [27,29] assessed longitudinal [29] and radial [27] strain after TEVAR in porcine aortas, showing significant reductions in both longitudinal and radial strain. Similarly, in porcine models, de Beaufort et al. [26,28] and Bianchi et al. [33] demonstrated increased PWV following stent-graft deployment for TEVAR, with effect depending on the extent of stent graft coverage, while Mandigers et al. [30] confirmed the stiffening effects when comparing two generations of Valiant grafts. Schellinger et al. [65] determined that endograft placement in the abdominal aorta induced stiffness gradients and altered circumferential strain.
Yusefi et al. [47] bridged the comparison with open arch replacement (OAR) by evaluating both OAR and TEVAR in human aortas, showing that TEVAR led to higher systolic pressure and input impedance, indicating increased left ventricular afterload and altered wave dynamics. De Kort et al. [32] extended this to open surgical repair, showing increased PWV after Dacron graft replacement in a porcine descending aorta.
Other in vitro experiments focused on device design. Morris et al. [72] compared four commercial abdominal stent-grafts with a multilayer flow modulator, showing that most grafts reduced compliance and increased stiffness, while the multilayer device preserved compliance best. Moreover, Legerer et al. [78] tested an external spring casing around Dacron grafts, aiming to restore aortic compliance after open surgical repair, which reduced PWV and pulse pressure.
Lastly, Timaran et al. [10] showed that increasing type II endoleak volume raised aneurysm wall pressure, especially at the site of maximum diameter.
3.3.5.2. Hemodynamics
A total of 23 studies investigated the aortic hemodynamics within MCLs, focusing on aortic dissection dynamics, flow behavior, pressure distribution, and treatment-related hemodynamic changes. Ten studies did an intervention, while thirteen studies did not perform an intervention; two of those were in healthy aortas and eleven in pathologic aortas.
Several of these studies explored the propagation mechanisms of type B aortic dissections (TBAD), demonstrating how primary entry tear size [9], configuration [79], location [44,57], pulse pressure [11], and re-entry tears [22] influence false lumen pressurization and dissection flap dynamics. Birjniuk et al. [23] used 4-dimensional phase-contrast MRI and flexible aortic models to reveal significant flow reversal, vortices, and flap motion within the false lumen. Chung et al. [19,20] demonstrated that true-lumen collapse in TBAD strongly depends on the difference in ratios of inflow capacity to outflow capacity in the true and false lumina, both anatomic and physiologic factors can affect true-lumen collapse [19], and that covering the primary entry tear with a stent-graft is the most effective way to relieve true-lumen collapse [20]. Morris et al. [71] developed a patient-specific in vitro model of TBAD to assess the hemodynamics within the true and false lumina.
Nair et al. [12] combined an MCL with fluid–structure interaction simulations to evaluate how exercise affects pressure gradients across aortic coarctations, showing that pressure drop increases nonlinearly with cardiac output.
Five studies investigated the effects of certain interventions on aortic hemodynamics. Ong et al. [80] assessed a novel stent graft design with slit perforations in thoracic aortic aneurysm repair that can positively alter the hemodynamics at the aortic arch while maintaining blood flow to supra-aortic branches. Morris et al. [70] investigated the effect of variations in stent-graft types, demonstrating that commercial stent-grafts reduce aortic compliance, increase PWV, cause pressure wave reflections, and disturb flow at the bifurcation, while a tapered geometry improved flow uniformity. Pasta et al. [25] investigated how excessive bird-beak configurations in thoracic endografts influence the risk of infolding using a patient-specific pulsatile flow phantom. Williamson et al. [81] developed a silicone aortic phantom with a surrogate Frozen Elephant Trunk to study Type 1B endoleaks using PIV. Qing et al. [68] demonstrated that after TEVAR for TBAD in porcine aortas, the false lumen can remain pressurized despite successful closure of the primary entry tear. Moreover, nearly 80% of the pressure remained in the thrombosed false lumen. Lastly, Mirgolbabee et al. [37] used contrast-enhanced ultrasound PIV in a patient-specific phantom to study limb thrombosis after endovascular aortic repair.
Schurink et al. [41] and Blackwood et al. [21] also investigated endoleaks and their relation to aortic hemodynamics. Schurink et al. [41] found in an in vitro aneurysm model that every endoleak caused pressure greater than the systemic diastolic pressure within the aneurysmal sac, and all endoleaks could be visualized with a delayed computed tomography angiography (CTA) protocol. Blackwood et al. [21] demonstrated that endotension can result from a non-visualized type Ia endoleak with no flow, while also confirming the findings of Schurink et al.
From a methodological perspective, three studies applied PIV. Yazdi et al. [82] investigated the effect of compliance on aortic arch hemodynamics experiencing pulsatility, finding that compliant aortic phantoms exhibit larger and more irregular recirculation zones than rigid ones, especially under deceleration, in regions earlier identified as high-risk areas for atherosclerosis. Büsen et al. [62] studied the effects of aortic stiffness on hemodynamics in a compliant silicone aortic phantom, demonstrating that reduced compliance increased pressure change rates and mean flow velocity while diminishing vortex formation. Steinlauf et al. [83] examined the effects of the treatment approach on unsteady hemodynamics and blood perfusion to the upper vessels in models of an aortic arch aneurysm, and of the three common repair approaches: open-chest surgical repair, chimney, and hybrid approach.
Lastly, Chen et al. [69] developed a patient-specific MCL to simulate normal and dissected aortic flow in silicone aortic phantoms, which reproduced physiological pressures and flow distributions, revealing higher true lumen velocities and pressure differences in aortic dissection models.
3.3.5.3. Device Testing
Twenty-two studies were conducted to evaluate devices or products, including 11 surgical devices, 6 radiological devices, and 5 phantoms.
Surgical Device Testing
Several studies focused on surgical devices for aortic interventions. Stent-graft fixation and oversizing were both investigated by Kratzberg et al. [18] and Canaud et al. [55] Kratzberg et al. examined the role of barbs and oversizing of abdominal grafts in bovine aortas and synthetic phantoms [18], finding that excessive oversizing compromised fixation and increased risk of migration. Canaud et al. investigated the impact of aortic arch angulation on thoracic stent-graft apposition in human cadaveric aortas [55], showing that radial force and open stent segments were more important for secure fixation than hooks alone.
Mechanical properties of graft materials were assessed by Amabili et al. [49] and Ferrari et al. [51], who characterized the viscoelastic and dynamic behavior of Dacron grafts. Hauck et al. [45] and Zimpfer et al. [48] assessed the feasibility of novel aortic endografts in phantom and porcine ascending aortas, respectively.
Three studies focused on improving the safety and performance of resuscitative endovascular balloon occlusion of the aorta. Maleckis et al. [24] found that safe balloon inflation parameters in cadaveric human aortas depend on anatomical, physiological, and demographic characteristics, and that pressure-guided rather than volume-guided inflation may reduce aortic rupture risk. Madurska et al. [15] compared inflation characteristics and rupture risk of compliant versus semi-compliant balloons in porcine aortas. Lastly, McCarthy et al. [58] introduced an intelligent automated balloon pressure management system, showing that the technique is feasible in porcine aortas.
Jansen et al. [39] preclinically evaluated Fiber Optic RealShape (FORS) technology, which enables real-time 3-dimensional visualization of endovascular devices without fluoroscopy, demonstrating the safety and feasibility in phantom models.
Finally, a novel injectable hydrogel to improve endoleak embolization after EVAR was evaluated by Zehtabi et al. [7], showing successful occlusion in an aortic phantom model.
Radiological Device Testing
Six studies focused on radiological devices and image-guidance technologies. Nagel et al. [40] optimized the radiopacity of a novel injectable polymer for type II endoleak treatment, aiming to identify the minimum tantalum concentration sufficient for safe injection into the aneurysmal sac. Two studies by Riga et al. [84,85] tested remotely steerable robotic catheters in arch cannulation [84] and fenestrated stent-grafting [85], showing shorter cannulation times, fewer movements, and reduced radiation exposure compared with conventional techniques. Sidhu et al. [86] evaluated an electromagnetic 3-dimensional navigation system for arterial cannulation, demonstrating feasibility and a significant reduction in fluoroscopy use during arterial cannulation tasks. Rengier et al. [64] assessed the effect of nitinol stent grafts on flow measurements by 3-dimensional velocity-encoded cine MRI, revealing a systematic positive bias but overall feasibility of in-stent flow quantification. Lan et al. [17] validated a reduced unified continuum formulation for fluid–structure interaction against in vitro 4-dimensional flow MRI in a patient-specific compliant phantom.
Phantom Validation for Radiology and Training
Five studies focused on physiologically realistic aortic phantoms for device evaluation, imaging, and surgical purposes. Mirgolbabee et al. [38] introduced a framework to generate a 50-patient cohort-averaged AAA phantom, showing a good match with its reference model by fabricating a 3D-printed thin-walled phantom and demonstrating the feasibility of flow-field quantification within the phantom. Desai et al. [67] manufactured a physiologically relevant nanocomposite aortic model designed for long-term fatigue analysis of stent-grafts, outperforming conventional latex models. Urbina et al. [87] developed an MRI-compatible phantom to simulate normal and coarctation conditions, with flow and pressure measurements aligning closely with healthy volunteers and patient data. Perrot et al. [60] developed a patient-specific abdominal aortic aneurysm phantom to reproduce realistic biomechanical behavior. Through combining ultrasound and digital image stereo-correlation, they validated the setup as a reliable platform to study vessel wall motion and potential complications of endovascular aortic repair in a controlled environment. Finally, Mohl et al. [63] created a perfused, patient-specific TBAD phantom with a flexible dissection flap, enabling the simulation of TEVAR procedures in a real endovascular operating environment.
3.3.5.4. Diagnostics—Image Validation
Thirteen studies had the aim of testing diagnostics or image validation. Li et al. [16] aimed to improve the clinical utility of Pulse Wave Imaging using conventional ultrasound (US) by evaluating spatial or temporal resolution in ex vivo canine aortas. Building upon these efforts in ultrasound-based assessment, de Hoop et al. demonstrated that multi-perspective acquisitions with dual transducers improved motion tracking and strain estimation in porcine aortas [36] and later expanded this concept to bistatic 3-dimensional acquisitions, further enhancing motion tracking accuracy and spatial resolution for vascular elastography [35]. Van Disseldorp et al. [42] validated a 4-dimensional US-based non-invasive aortic stiffness quantification in porcine aortas, which was highly reproducible and showed moderate agreement with biaxial tensile testing of excised aortic tissue. Wang et al. [54] validated an ultrafast principal strain estimator combining tissue-Doppler Imaging and optical flow tracking to evaluate aortic stiffness, demonstrating that the method can detect and evaluate aortic aneurysm stiffness.
MRI–based methods were also validated in MCLs. Roberts et al. [75] proved the feasibility of real-time cardiovascular MRI to measure PWV during exercise stress testing by validating it against standard phase-contrast MRI in phantoms, while Xu et al. [8] and Zhang et al. [6] validated magnetic resonance elastography to assess global and regional aortic stiffness, respectively, in phantoms and porcine aortas. Potthast et al. [88] validated intra-arterial magnetic resonance aortography with high-speed parallel acquisition in phantoms, reducing contrast dose and scan time without loss of contrast-to-noise ratio.
Other groups focused on imaging accuracy for interventions. Moresco et al. [13] compared CO2 angiography with iodinated contrast and intravascular ultrasound in a flow phantom, demonstrating that CO2 angiography consistently yields significantly larger vessel measurements. Sieren et al. [66] tested fusion imaging registration in a pulsatile anthropomorphic aortic phantom and showed that 3D–3D methods were significantly more accurate than 2D–3D methods, with computational tomography and magnetic resonance angiography performing equivalently.
Lastly, Saida et al. [76] and Cosset et al. [56] focused on identifying and classifying endoleaks. Saida et al. [76] aimed to optimize an unenhanced MRI technique using motion sensitized driven equilibrium combined with balanced turbo field echo sequences to detect and classify endoleaks in an aortic phantom, while Cosset et al. [56] investigated the feasibility of identifying and characterizing the three most common within a thoracic aorta aneurysm model using bicolour K-edge imaging with a spectral photon-counting CT system in combination with a biphasic contrast agent injection.
3.3.5.5. Training
Only one study used an MCL specifically for training purposes. Meess et al. [14] simulated fenestrated EVAR in a patient-specific 3D-printed AAA phantom, allowing the clinical team to optimize their procedural strategy.
4. Discussion
This scoping review provides the first comprehensive overview of MCL applications in aortic research, identifying 84 eligible studies published between 1998 and 2025. These studies encompassed five major research themes: (I) (bio)mechanical characterization of the aorta, (II) hemodynamics, (III) device and product testing, (IV) diagnostics, and (V) training. A broad range of biological and synthetic aortic models were employed, including porcine, canine, ovine, bovine, and human cadaveric specimens, as well as various 3D-printed or molded synthetic phantoms, demonstrating the versatility of MCL methodology. Collectively, these models have been used to research clinically relevant questions such as aortic stiffness after stent-grafting, flow disruption in dissection, false lumen pressurization, endoleak mechanisms, device validation, and radiological performance and imaging accuracy. This shows that MCLs are not limited to engineering proof-of-concept studies but are increasingly used to explore mechanisms directly impacting endovascular outcomes and patient safety.
MCLs occupy a translational niche between computational simulations and in vivo experimentation. Unlike purely numerical models, they reproduce physiological pulsatility, tissue compliance, and boundary conditions, while avoiding the ethical and logistical challenges of animal or human studies. As such, they are highly suitable for pre-clinical evaluation of stents, other devices, and novel imaging or navigation techniques such as FORS guidance [39,45,89]. Despite these advantages, MCLs inherently isolate components of the cardiovascular system, and therefore cannot fully reproduce the global interdependence of the aorta, where local biomechanical changes propagate system-wide effects.
In addition to their mechanistic and device-focused applications, MCLs also support procedural planning and decision-making in complex aortic anatomies. Recent technological advances, including the integration of 3D-printed, patient-specific aortic phantoms with multimodal imaging platforms (e.g., 4D-flow MRI, ultrasound elastography, FORS) and increasingly sophisticated image-guided workflows, position MCLs at the forefront of precision aortic research. Patient-specific phantoms enable rehearsal of fenestrated EVAR, TEVAR, and Frozen Elephant Trunk–related steps under physiologic flow [25,63]. MCL platforms further facilitate pre-procedural simulation and trainee skill development, exemplified by the patient-specific fenestrated EVAR training model developed by Meess et al. [14] Together, these applications illustrate the translational potential of MCLs for optimizing strategy, improving operator preparedness through pre-operative simulation, and supporting complex endovascular interventions.
Limitations
Importantly, this review also reveals a fragmented methodological landscape, with substantial variability in MCL designs, configurations, and reporting quality across studies. Essential methodological details, such as pump characteristics, compliance and resistance settings, Wind Kessel simulation, and calibration procedures, were frequently missing or inconsistently described, limiting reproducibility and cross-study comparison.
Therefore, a dedicated reporting framework is urgently needed, tailored to cardiovascular MCL research. The authors’ group is currently developing a dedicated MCL quality assessment scale to address this gap and promote reproducibility and comparability among future investigations.
Moreover, all included studies share an inherent limitation: The aorta functions as an integrated organ, so any local modification in mechanical or hemodynamic properties affects the system as a whole. This fundamental constraint contributes to discrepancies between benchtop findings and clinical-physiological observations. Future progress, therefore, requires a multidisciplinary approach that combines physiology, biomechanics, and computational modeling to bridge these gaps and advance the physiological and pathophysiological understanding of the aorta.
5. Conclusions
This scoping review maps current applications of mock circulation loops (MCLs) in aortic research, highlighting the value for studying (bio)mechanics, hemodynamics, devices, diagnostics, and training under controlled conditions. Despite growing use, methodological heterogeneity persists. Standard reporting is essential to enhance reproducibility and support translation from benchtop experiments to clinical practice. Progress in this field will depend on multidisciplinary approaches that couple MCL experimentation with physiological, biomechanical, and computational models to capture the inherently integrated behavior of the aorta.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines14010028/s1. Table S1: Search String for PUBMED, Web of Science, and Scopus, used for a scoping review on mock circulatory loops in aortic research. Table S2: Study characteristics of all included studies included in a scoping review on mock circulatory loops in aortic research.
Author Contributions
F.E.N.O.: conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, writing—original draft, visualization; N.A.H.: conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, writing—original draft, visualization; J.F.d.K.: conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, visualization, writing—review and editing, project administration; E.-L.M.: methodology, validation, formal analysis, investigation, resources, data curation, visualization, writing—review and editing; M.D.: writing—review and editing; M.S.: writing—review and editing; A.C.: writing—review and editing; M.C.: writing—review and editing; C.E.V.B.H.: conceptualization, writing—review and editing; F.A.: writing—review and editing; J.L.d.B.: conceptualization, resources, writing—review and editing, supervision; J.A.v.H.: conceptualization, resources, writing—review and editing, supervision; S.T.: conceptualization, resources, writing—review and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially funded by the Italian Ministry of Health, current research IRCCS (RC2025).
Data Availability Statements
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
J.A. van Herwaarden is or has been a proctor or consultant for Gore Medical, Terumo Aortic, and Cook Medical, and a speaker for Artivion Inc. C.E.V.B. Hazenberg is or has been a proctor or consultant for Gore Medical, Terumo Aortic, and Cook Medical. S. Trimarchi is a consultant and speaker for Medtronic Inc., W.L. Gore, and Terumo Aortic. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The companies above had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| 2D | Two-Dimensional |
| 3D | Three-Dimensional |
| 4D | Four-Dimensional |
| AAA | Abdominal Aortic Aneurysm |
| CO2 | Carbon Dioxide |
| CTA | Computed Tomography Angiography |
| CVS | Cardiovascular System |
| EVAR | Endovascular Aneurysm Repair |
| FBS | Fetal Bovine Serum |
| FORS | Fiber Optic RealShape |
| kDa | Kilodalton |
| MRI | Magnetic Resonance Imaging |
| MCL | Mock Circulatory Loop |
| n | Number (sample size) |
| O2 | Oxygen |
| OAR | Open Arch Replacement |
| PIV | Particle Image Velocimetry |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-Analyses—Extension for Scoping Reviews |
| PWV | Pulse Wave Velocity |
| SD | Standard Deviation |
| TBAD | Type B Aortic Dissection |
| TEVAR | Thoracic Endovascular Aortic Repair |
| U/mL | Units per Milliliter |
| US | Ultrasound |
| USA | United States of America |
References
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Tewari, V.; Chen, J.; Sawchuk, A.P.; Yu, H. A Comprehensive Review of Mock Circulation Loop Systems for Experimental Hemodynamics of Cardiovascular Diseases. Fluids 2025, 10, 166. [Google Scholar] [CrossRef]
- Zimmermann, J.; Loecher, M.; Kolawole, F.O.; Bäumler, K.; Gifford, K.; Dual, S.A.; Levenston, M.; Marsden, A.L.; Ennis, D.B. On the impact of vessel wall stiffness on quantitative flow dynamics in a synthetic model of the thoracic aorta. Sci. Rep. 2021, 11, 6703. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, J.; Yin, M.; Glaser, K.J.; Xu, L.; Ehman, R.L. Quantification of regional aortic stiffness using MR elastography: A phantom and ex-vivo porcine aorta study. Magn. Reson. Imaging 2016, 34, 91–96. [Google Scholar] [CrossRef]
- Zehtabi, F.; Gangrade, A.; Tseng, K.; Haghniaz, R.; Abbasgholizadeh, R.; Montazerian, H.; Khorsandi, D.; Bahari, J.; Ahari, A.; Mohaghegh, N.; et al. Injectable Shear-Thinning Hydrogels with Sclerosing and Matrix Metalloproteinase Modulatory Properties for the Treatment of Vascular Malformations. Adv. Funct. Mater. 2023, 33, 2305880. [Google Scholar] [CrossRef]
- Xu, L.; Chen, J.; Glaser, K.J.; Yin, M.; Ms, P.J.R.; Ehman, R.L. MR elastography of the human abdominal aorta: A preliminary study. J. Magn. Reson. Imaging 2013, 38, 1549–1553. [Google Scholar] [CrossRef]
- Tsai, T.T.; Schlicht, M.S.; Khanafer, K.; Bull, J.L.; Valassis, D.T.; Williams, D.M.; Berguer, R.; Eagle, K.A. Tear size and location impacts false lumen pressure in an ex vivo model of chronic type B aortic dissection. J. Vasc. Surg. 2008, 47, 844–851. [Google Scholar] [CrossRef]
- Timaran, C.H.; Ohki, T.; Veith, F.J.; Lipsitz, E.C.; Gargiulo, N.J.; Rhee, S.J.; Malas, M.B.; Suggs, W.D.; Pacanowski, J.P. Influence of type II endoleak volume on aneurysm wall pressure and distribution in an experimental model. J. Vasc. Surg. 2005, 41, 657–663. [Google Scholar] [CrossRef]
- Peelukhana, S.V.; Wang, Y.; Berwick, Z.; Kratzberg, J.; Krieger, J.; Roeder, B.; Clough, R.E.; Hsiao, A.; Chambers, S.; Kassab, G.S. Role of Pulse Pressure and Geometry of Primary Entry Tear in Acute Type B Dissection Propagation. Ann. Biomed. Eng. 2017, 45, 592–603, Erratum in Ann. Biomed. Eng. 2017, 45, 604. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.J.; Perra, E.; McElhinney, D.B.; Marsden, A.L.; Ennis, D.B.; Dual, S.A. Experiments and Simulations to Assess Exercise-Induced Pressure Drop Across Aortic Coarctations. J. Biomech. Eng. 2025, 147, 071007. [Google Scholar] [CrossRef] [PubMed]
- Moresco, K.P.; Patel, N.; Johnson, M.S.; Trobridge, D.; Bergan, K.A.; Lalka, S.G. Accuracy of CO2 Angiography in Vessel Diameter Assessment: A Comparative Study of CO2 versus Iodinated Contrast Material in an Aortoiliac Flow Model. J. Vasc. Interv. Radiol. 2000, 11, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Meess, K.M.; Izzo, R.L.; Dryjski, M.L.; Curl, R.E.; Harris, L.M.; Springer, M.; Siddiqui, A.H.; Rudin, S.; Ionita, C.N. 3D printed abdominal aortic aneurysm phantom for image guided surgical planning with a patient specific fenestrated endovascular graft system. In Proceedings of the SPIE Medical Imaging 2017, Orlando, FL. USA, 11–16 February 2017; p. 101380P. [Google Scholar]
- Madurska, M.J.; Franklin, C.; Richmond, M.; Adnan, S.M.; Stansby, G.P.; White, J.M.; Morrison, J.J. Improving the safety of resuscitative endovascular balloon occlusion of the aorta—Compliant versus semi-compliant balloon systems. Vascular 2020, 28, 612–618. [Google Scholar] [CrossRef]
- Li, R.X.; Qaqish, W.; Konofagou, E.E. Performance assessment of pulse wave imaging using conventional ultrasound in canine aortas ex vivo and normal human arteries in vivo. Artery Res. 2015, 11, 19–28. [Google Scholar] [CrossRef]
- Lan, I.S.; Liu, J.; Yang, W.; Zimmermann, J.; Ennis, D.B.; Marsden, A.L. Validation of the Reduced Unified Continuum Formulation Against In Vitro 4D-Flow MRI. Ann. Biomed. Eng. 2023, 51, 377–393. [Google Scholar] [CrossRef]
- Kratzberg, J.A.; Golzarian, J.; Raghavan, M.L. Role of graft oversizing in the fixation strength of barbed endovascular grafts. J. Vasc. Surg. 2009, 49, 1543–1553. [Google Scholar] [CrossRef]
- Chung, J.W.; Elkins, C.; Sakai, T.; Kato, N.; Vestring, T.; Semba, C.P.; Slonim, S.M.; Dake, M.D. True-Lumen Collapse in Aortic Dissection Part I. Evaluation of Causative Factors in Phantoms with Pulsatile Flow. Radiology 2000, 214, 87–98. [Google Scholar] [CrossRef]
- Chung, J.W.; Elkins, C.; Sakai, T.; Kato, N.; Vestring, T.; Semba, C.P.; Slonim, S.M.; Dake, M.D. True-Lumen Collapse in Aortic Dissection Part II. Evaluation of Treatment Methods in Phantoms with Pulsatile Flow. Radiology 2000, 214, 99–106. [Google Scholar] [CrossRef]
- Blackwood, S.; Mix, D.; Chandra, A.; Dietzek, A.M. A model to demonstrate that endotension is a nonvisualized type I endoleak. J. Vasc. Surg. 2016, 64, 779–787. [Google Scholar] [CrossRef]
- Canchi, S.; Guo, X.; Phillips, M.; Berwick, Z.; Kratzberg, J.; Krieger, J.; Roeder, B.; Haulon, S.; Chambers, S.; Kassab, G.S. Role of Re-entry Tears on the Dynamics of Type B Dissection Flap. Ann. Biomed. Eng. 2018, 46, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Birjiniuk, J.; Timmins, L.H.; Young, M.; Leshnower, B.G.; Oshinski, J.N.; Ku, D.N.; Veeraswamy, R.K. Pulsatile Flow Leads to Intimal Flap Motion and Flow Reversal in an In Vitro Model of Type B Aortic Dissection. Cardiovasc. Eng. Technol. 2017, 8, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Maleckis, K.; Keiser, C.; Jadidi, M.; Anttila, E.; Desyatova, A.; MacTaggart, J.; Kamenskiy, A. Safe balloon inflation parameters for resuscitative endovascular balloon occlusion of the aorta. J. Trauma Acute Care Surgery 2021, 91, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Pasta, S.; Scardulla, F.; Rinaudo, A.; Raffa, G.M.; D’Ancona, G.; Pilato, M.; Scardulla, C. An in vitro phantom study on the role of the bird-beak configuration in endograft infolding in the aortic arch. J. Endovasc. Ther. 2016, 23, 172–181. [Google Scholar] [CrossRef]
- de Beaufort, H.W.; Conti, M.; Kamman, A.V.; Nauta, F.J.; Lanzarone, E.; Moll, F.L.; van Herwaarden, J.A.; Auricchio, F.; Trimarchi, S. Stent-Graft Deployment Increases Aortic Stiffness in an Ex Vivo Porcine Model. Ann. Vasc. Surg. 2017, 43, 302–308. [Google Scholar] [CrossRef]
- Nauta, F.J.H.; de Beaufort, H.W.L.; Conti, M.; Marconi, S.; Kamman, A.V.; Ferrara, A.; van Herwaarden, J.A.; Moll, F.L.; Auricchio, F.; Trimarchi, S. Impact of thoracic endovascular aortic repair on radial strain in an ex vivo porcine model. Eur. J. Cardio-Thorac. Surg. 2017, 51, 783–789, Erratum in Eur. J. Cardiothorac. Surg. 2018, 53, 1299. [Google Scholar]
- de Beaufort, H.W.L.; Coda, M.; Conti, M.; van Bakel, T.M.J.; Nauta, F.J.H.; Lanzarone, E.; Moll, F.L.; van Herwaarden, J.A.; Auricchio, F.; Trimarchi, S. Changes in aortic pulse wave velocity of four thoracic aortic stent grafts in an ex vivo porcine model. PLoS ONE 2017, 12, e0186080. [Google Scholar] [CrossRef]
- Nauta, F.J.; Conti, M.; Marconi, S.; Kamman, A.V.; Alaimo, G.; Morganti, S.; Ferrara, A.; van Herwaarden, J.A.; Moll, F.L.; Auricchio, F.; et al. An experimental investigation of the impact of thoracic endovascular aortic repair on longitudinal strain. Eur. J. Cardio Thoracic Surg. 2016, 50, 955–961. [Google Scholar] [CrossRef]
- Mandigers, T.J.; Conti, M.; Allievi, S.; Dedola, F.; Bissacco, D.; Bianchi, D.; Marconi, S.; Domanin, M.; Van Herwaarden, J.A.; Auricchio, F.; et al. Comparison of Two Generations of Thoracic Aortic Stent Grafts and Their Impact on Aortic Stiffness in an Ex Vivo Porcine Model. EJVES Vasc. Forum 2023, 59, 8–14. [Google Scholar] [CrossRef]
- Mandigers, T.J.; Pascaner, A.F.; Conti, M.; Schembri, M.; Jelic, S.; Favilli, A.; Bissacco, D.; Domanin, M.; van Herwaarden, J.A.; Auricchio, F.; et al. Type III aortic arch angulation increases aortic stiffness: Analysis from an ex vivo porcine model. JTCVS Open 2024, 17, 37–46. [Google Scholar] [CrossRef]
- de Kort, J.F.; Mandigers, T.J.; Pascaner, A.F.; Conti, M.; Schembri, M.; Jelic, S.; Caimi, A.; Bissacco, D.; Domanin, M.; Heijmen, R.H.; et al. Impact of Open Surgical Descending Repair on Aortic Stiffness in an Ex Vivo Porcine Model. Ann. Vasc. Surg. 2025, 112, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, D.; Conti, M.; Bissacco, D.; Domanin, M.; Trimarchi, S.; Auricchio, F. Impact of thoracic endovascular aortic repair on aortic biomechanics: Integration of in silico and ex vivo analysis using porcine model. Int. J. Numer. Methods Biomed. Eng. 2023, 39, e3594. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, E.J.; Peters, M.F.; Nijs, J.; Rutten, M.C.; van de Vosse, F.N.; Lopata, R.G. Assessment of mechanical properties of porcine aortas under physiological loading conditions using vascular elastography. J. Mech. Behav. Biomed. Mater. 2016, 59, 185–196. [Google Scholar] [CrossRef] [PubMed]
- de Hoop, H.; Maas, E.; Muller, J.-W.; Schwab, H.-M.; Lopata, R. 3-D motion tracking and vascular strain imaging using bistatic dual aperture ultrasound acquisitions. Phys. Med. Biol. 2025, 70, 045013. [Google Scholar] [CrossRef]
- de Hoop, H.; Petterson, N.J.; van de Vosse, F.N.; van Sambeek, M.R.H.M.; Schwab, H.M.; Lopata, R.G.P. Multiperspective Ultrasound Strain Imaging of the Abdominal Aorta. IEEE Trans. Med. Imaging. 2020, 39, 3714–3724, Erratum in IEEE Trans. Med. Imaging. 2021, 40, 3968. [Google Scholar] [CrossRef]
- Mirgolbabaee, H.; van de Velde, L.; Geelkerken, R.H.; Versluis, M.; Jebbink, E.G.; Reijnen, M.M.P.J. Ultrasound Particle Image Velocimetry to Investigate Potential Hemodynamic Causes of Limb Thrombosis After Endovascular Aneurysm Repair with the Anaconda Device. J. Endovasc. Ther. 2023, 32, 2223–2235. [Google Scholar] [CrossRef]
- Mirgolbabaee, H.; Nagel, J.R.; Plomp, J.; Ghanbarzadeh-Dagheyan, A.; Simmering, J.A.; Versluis, M.; Reijnen, M.M.P.J.; Groot Jebbink, E. Vascular Flow Phantom of A Cohort-Based Averaged Abdominal Aortic Aneurysm: Design, Fabrication and Characterization. Ann. Biomed. Eng. 2025, 53, 1439–1452. [Google Scholar] [CrossRef]
- Jansen, M.; Khandige, A.; Kobeiter, H.; Vonken, E.J.; Hazenberg, C.; van Herwaarden, J. Three Dimensional Visualisation of Endovascular Guidewires and Catheters Based on Laser Light instead of Fluoroscopy with Fiber Optic RealShape Technology: Preclinical Results. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 135–143. [Google Scholar] [CrossRef]
- Nagel, J.R.; Groot Jebbink, E.; Smorenburg, S.P.M.; Hoksbergen, A.W.J.; Lely, R.J.; Versluis, M.; Reijnen, M.M.P.J. Optimizing the Radiopacity of an Injectable Polymer on Fluoroscopy used for Treatment of Type II Endoleak After Endovascular Aneurysm Repair. Cardiovasc. Eng. Technol. 2025, 16, 377–385. [Google Scholar] [CrossRef]
- Schurink, G.W.H.; Aarts, N.J.M.; Wilde, J.; van Baalen, J.M.; Chuter, T.A.M.; Schultze Kool, L.J.; van Bockel, J.H. Endoleakage after stent-graft treatment of abdominal aneurysm: Implications on pressure and imaging-an in vitro study. J. Vasc. Surg. 1998, 28, 232–241. [Google Scholar] [CrossRef]
- van Disseldorp, E.M.J.; van den Hoven, M.H.M.H.; van de Vosse, F.N.; van Sambeek, M.R.H.M.; Lopata, R.G.P. Reproducibility assessment of ultrasound-based aortic stiffness quantification and verification using Bi-axial tensile testing. J. Mech. Behav. Biomed. Mater. 2020, 103, 103571. [Google Scholar] [CrossRef] [PubMed]
- Agrafiotis, E.; Mayer, C.; Grabenwöger, M.; Zimpfer, D.; Regitnig, P.; Mächler, H.; Holzapfel, G.A. Global and local stiffening of ex vivo-perfused stented human thoracic aortas: A mock circulation study. Acta Biomater. 2023, 161, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Dziodzio, T.; Juraszek, A.; Reineke, D.; Jenni, H.; Zermatten, E.; Zimpfer, D.; Stoiber, M.; Scheikl, V.; Schima, H.; Grimm, M.; et al. Experimental acute type B aortic dissection: Different sites of primary entry tears cause different ways of propagation. Ann. Thorac. Surg. 2011, 91, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Hauck, S.R.; Kupferthaler, A.; Stelzmüller, M.; Eilenberg, W.; Ehrlich, M.; Neumayer, C.; Wolf, F.; Loewe, C.; Funovics, M.A. Endovascular Stent-Graft Repair of the Ascending Aorta: Assessment of a Specific Novel Stent-Graft Design in Phantom, Cadaveric, and Clinical Application. Cardiovasc. Intervent. Radiol. 2021, 44, 1448–1455. [Google Scholar] [CrossRef]
- Jarman, J.; Navarro, D.; Ticar, J.; Tosto, G.; Dittrich, M.; Santer, D.; Radloff, J.; Mora, B.; Moore, M.; Czerny, M.; et al. Evaluation of different indices to measure elastic properties of the aorta in a pulsatile mock circulation loop. Eur. J. Cardio Thorac. Surg. 2025, 67, ezaf073. [Google Scholar] [CrossRef]
- Yusefi, M.; Agrafiotis, E.; Regitnig, P.; Laufer, G.; Sommer, G.; Holzapfel, G.A.; Mächler, H. TEVAR versus open aortic arch replacement in ex vivo perfused human thoracic aortas. Acta Biomater. 2025, 192, 140–150. [Google Scholar] [CrossRef]
- Zimpfer, D.; Schima, H.; Czerny, M.; Kasimir, M.-T.; Sandner, S.; Seebacher, G.; Losert, U.; Simon, P.; Grimm, M.; Wolner, E.; et al. Experimental Stent-Graft Treatment of Ascending Aortic Dissection. Ann. Thorac. Surg. 2008, 85, 470–473. [Google Scholar] [CrossRef]
- Amabili, M.; Balasubramanian, P.; Ferrari, G.; Franchini, G.; Giovanniello, F.; Tubaldi, E. Identification of viscoelastic properties of Dacron aortic grafts subjected to physiological pulsatile flow. J. Mech. Behav. Biomed. Mater. 2020, 110, 103804. [Google Scholar] [CrossRef]
- Amabili, M.; Balasubramanian, P.; Bozzo, I.; Breslavsky, I.D.; Ferrari, G.; Franchini, G.; Giovanniello, F.; Pogue, C. Nonlinear Dynamics of Human Aortas for Material Characterization. Phys. Rev. X 2020, 10, 011015. [Google Scholar] [CrossRef]
- Ferrari, G.; Balasubramanian, P.; Tubaldi, E.; Giovanniello, F.; Amabili, M. Experiments on dynamic behaviour of a Dacron aortic graft in a mock circulatory loop. J. Biomech. 2019, 86, 132–140. [Google Scholar] [CrossRef]
- Franchini, G.; Giovanniello, F.; Amabili, M. Viscoelasticity of human descending thoracic aorta in a mock circulatory loop. J. Mech. Behav. Biomed. Mater. 2022, 130, 105205. [Google Scholar] [CrossRef] [PubMed]
- Cameron, K.; El Hassan, M.; Sabbagh, R.; Freed, D.H.; Nobes, D.S. Experimental investigation into the effect of compliance of a mock aorta on cardiac performance. PLoS ONE 2020, 15, e0239604. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chayer, B.; Destrempes, F.; Toumoux, F.; Cloutier, G. Stiffness Evaluation of Aortic Aneurysms Using an Ultrafast Principal Strain Estimator: In Vitro Validation. In Proceedings of the IEEE International Ultrasonics Symposium (IUS), Kobe, Japan, 22–25 October 2018. [Google Scholar]
- Canaud, L.; Alric, P.; Laurent, M.; Baum, T.-P.; Branchereau, P.; Marty-Ané, C.H.; Berthet, J.P. Proximal fixation of thoracic stent-grafts as a function of oversizing and increasing aortic arch angulation in human cadaveric aortas. J. Endovasc. Ther. 2008, 15, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Cosset, B.; Sigovan, M.; Boccalini, S.; Farhat, F.; Douek, P.; Boussel, L.; Si-Mohamed, S.A. Bicolor K-edge spectral photon-counting CT imaging for the diagnosis of thoracic endoleaks: A dynamic phantom study. Diagn. Interv. Imaging 2023, 104, 235–242. [Google Scholar] [CrossRef]
- Faure, E.M.; Canaud, L.; Cathala, P.; Serres, I.; Marty-Ané, C.; Alric, P. Human ex-vivo model of Stanford type B aortic dissection. J. Vasc. Surg. 2014, 60, 767–775. [Google Scholar] [CrossRef]
- McCarthy, C.; Kanterman, I.; Trauzettel, F.; Jaeger, H.A.; Goetz, A.-A.; Colvard, B.; Swanstrom, L.; Cantillon-Murphy, P. Automated Balloon Control in Resuscitative Endovascular Balloon Occlusion of the Aorta. IEEE Trans. Biomed. Eng. 2019, 66, 1723–1729. [Google Scholar] [CrossRef]
- Moravia, A.; Simoëns, S.; El Hajem, M.; Bou-Saïd, B.; Menut, M.; Kulisa, P.; Lermusiaux, P.; Della-Schiava, N. Particle Image Velocimetry to Evaluate Pulse Wave Velocity in Aorta Phantom with the lnD–U Method. Cardiovasc. Eng. Technol. 2023, 14, 141–151. [Google Scholar] [CrossRef]
- Perrot, V.; Meier, S.; Bel-Brunon, A.; Berre, H.W.-L.; Bou-Saïd, B.; Chaudet, P.; Detti, V.; Vray, D.; Liebgott, H. Biofidelic Abdominal Aorta Phantom: Cross-Over Preliminary Study Using UltraSound and Digital Image Stereo-Correlation. IRBM 2017, 38, 238–244. [Google Scholar] [CrossRef]
- Boese, J.M.; Bock, M.; O Schoenberg, S.; Schad, L.R. Estimation of aortic compliance using magnetic resonance pulse wave velocity measurement. Phys. Med. Biol. 2000, 45, 1703–1713. [Google Scholar] [CrossRef]
- Büsen, M.; Arenz, C.; Neidlin, M.; Liao, S.; Schmitz-Rode, T.; Steinseifer, U.; Sonntag, S.J. Development of an In Vitro PIV Setup for Preliminary Investigation of the Effects of Aortic Compliance on Flow Patterns and Hemodynamics. Cardiovasc. Eng. Technol. 2017, 8, 368–377. [Google Scholar] [CrossRef]
- Mohl, L.; Karl, R.; Hagedorn, M.N.; Runz, A.; Skornitzke, S.; Toelle, M.; Bergt, C.S.; Hatzl, J.; Uhl, C.; Böckler, D.; et al. Simulation of thoracic endovascular aortic repair in a perfused patient-specific model of type B aortic dissection. Int. J. Comput. Assist. Radiol. Surg. 2025, 20, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Rengier, F.; Delles, M.; Unterhinninghofen, R.; Ley, S.; Partovi, S.; Dillmann, R.; Kauczor, H.U.; von Tengg-Kobligk, H. Impact of an Aortic Nitinol Stent Graft on Flow Measurements by Time-resolved Three-dimensional Velocity-encoded MRI. Acad. Radiol. 2012, 19, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Schellinger, I.N.; Naumann, J.; Hoffmann, A.; Barnard, S.-J.; Düsing, S.; Wagenhäuser, M.U.; Haunschild, J.; Scheinert, D.; Hasenfuß, G.; Etz, C.D.; et al. Abdominal Aortic Endograft Implantation Immediately Induces Vascular Stiffness Gradients That May Promote Adverse Aortic Neck Dilatation: Results of A Porcine Ex Vivo Study. J. Endovasc. Ther. 2025, 32, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Sieren, M.M.; Schareck, C.; Kaschwich, M.; Horn, M.; Matysiak, F.; Stahlberg, E.; Wegner, F.; Oechtering, T.H.; Barkhausen, J.; Goltz, J. Accuracy of registration techniques and vascular imaging modalities in fusion imaging for aortic endovascular interventions: A phantom study. CVIR Endovasc. 2021, 4, 51. [Google Scholar] [CrossRef]
- Desai, M.; Ahmed, M.; Darbyshire, A.; You, Z.; Hamilton, G.; Seifalian, A.M. An aortic model for the physiological assessment of endovascular stent-grafts. Ann. Vasc. Surg. 2011, 25, 530–537. [Google Scholar] [CrossRef]
- Qing, K.-X.; Chan, Y.-C.; Ting, A.; Cheng, S. Persistent Intraluminal Pressure after Endovascular Stent Grafting for Type B Aortic Dissection. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 656–663. [Google Scholar] [CrossRef]
- Chen, D.; Liang, S.; Li, Z.; Mei, Y.; Dong, H.; Ma, Y.; Zhao, J.; Xu, S.; Zheng, J.; Xiong, J. A Mock Circulation Loop for In Vitro Hemodynamic Evaluation of Aorta: Application in Aortic Dissection. J. Endovasc. Ther. 2022, 29, 132–142. [Google Scholar] [CrossRef]
- Morris, L.; Stefanov, F.; McGloughlin, T. Stent graft performance in the treatment of abdominal aortic aneurysms: The influence of compliance and geometry. J. Biomech. 2013, 46, 383–395. [Google Scholar] [CrossRef]
- Morris, L.; Tierney, P.; Hynes, N.; Sultan, S. An in vitro Assessment of the Haemodynamic Features Occurring Within the True and False Lumens Separated by a Dissection Flap for a Patient-Specific Type B Aortic Dissection. Front. Cardiovasc. Med. 2022, 9, 797829. [Google Scholar] [CrossRef]
- Morris, L.; Stefanov, F.; Hynes, N.; Diethrich, E.B.; Sultan, S. An Experimental Evaluation of Device/Arterial Wall Compliance Mismatch for Four Stent-Graft Devices and a Multi-layer Flow Modulator Device for the Treatment of Abdominal Aortic Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 44–55. [Google Scholar] [CrossRef]
- Wen, C.-Y.; Yang, A.-S.; Tseng, L.-Y.; Chai, J.-W. Investigation of pulsatile flowfield in healthy thoracic aorta models. Ann. Biomed. Eng. 2010, 38, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Gaddum, N.R.; Schaeffter, T.; Bührer, M.; Rutten, M.; Smith, L.; Chowienczyk, P.J.; Beerbaum, P.B. Beat-to-beat variation in pulse wave velocity during breathing maneuvers. Magn. Reson. Med. 2014, 72, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.A.; Cowan, B.R.; Liu, Y.; Lin, A.C.W.; Nielsen, P.M.F.; Taberner, A.J.; Stewart, R.A.; Lam, H.I.; Young, A.A. Real-time aortic pulse wave velocity measurement during exercise stress testing. J. Cardiovasc. Magn. Reson. 2015, 17, 86. [Google Scholar] [CrossRef] [PubMed]
- Saida, T.; Mori, K.; Yabe, H.; Shindo, M.; Nasu, K.; Shiigai, M.; Takahashi, H.; Minami, M. Noninvasive visualization of endoleaks after endovascular aortic aneurysm repair through unenhanced MRI with motion-sensitized driven equilibrium preparation: Phantom experiments. J. Magn. Reson. Imaging 2013, 38, 714–721. [Google Scholar] [CrossRef]
- Ene, F.; Gachon, C.; Delassus, P.; Carroll, R.; Stefanov, F.; O’Flynn, P.; Morris, L. In vitro evaluation of the effects of intraluminal thrombus on abdominal aortic aneurysm wall dynamics. Med. Eng. Phys. 2011, 33, 957–966. [Google Scholar] [CrossRef]
- Legerer, C.; Stevens, M.; Vazquez, G.; Müller, T.; Ferrington, L. An experimental evaluation of a concept to improve conventional aortic prostheses. J. Biomech. 2020, 112, 110010. [Google Scholar] [CrossRef]
- Rudenick, P.A.; Bijnens, B.H.; García-Dorado, D.; Evangelista, A. An in vitro phantom study on the influence of tear size and configuration on the hemodynamics of the lumina in chronic type B aortic dissections. J. Vasc. Surg. 2013, 57, 464–474.e5. [Google Scholar] [CrossRef]
- Ong, C.W.; Xiong, F.; Kabinejadian, F.; Kumar, G.P.; Cui, F.; Chen, G.; Ho, P.; Leo, H. Hemodynamic analysis of a novel stent graft design with slit perforations in thoracic aortic aneurysm. J. Biomech. 2019, 85, 210–217. [Google Scholar] [CrossRef]
- Williamson, P.N.; Docherty, P.D.; Yazdi, S.G.; Khanafer, A.; Kabaliuk, N.; Jermy, M. PIV Analysis of Haemodynamics Distal to the Frozen Elephant Trunk Stent Surrogate. Cardiovasc. Eng. Technol. 2021, 12, 373–386. [Google Scholar] [CrossRef]
- Yazdi, S.G.; Docherty, P.D.; Williamson, P.N.; Jermy, M.; Kabaliuk, N.; Khanafer, A.; Geoghegan, P.H. In vitro pulsatile flow study in compliant and rigid ascending aorta phantoms by stereo particle image velocimetry. Med. Eng. Phys. 2021, 96, 81–90. [Google Scholar] [CrossRef]
- Steinlauf, S.; Shenberger, S.H.; Halak, M.; Liberzon, A.; Avrahami, I. Aortic arch aneurysm repair—Unsteady hemodynamics and perfusion at different heart rates. J. Biomech. 2021, 121, 110351. [Google Scholar] [CrossRef]
- Riga, C.V.; Bicknell, C.D.; Hamady, M.S.; Cheshire, N.J.W. Evaluation of robotic endovascular catheters for arch vessel cannulation. J. Vasc. Surg. 2011, 54, 799–809. [Google Scholar] [CrossRef]
- Riga, C.V.; Cheshire, N.J.W.; Hamady, M.S.; Bicknell, C.D. The role of robotic endovascular catheters in fenestrated stent grafting. J. Vasc. Surg. 2010, 51, 810–820. [Google Scholar] [CrossRef]
- Sidhu, R.; Weir-McCall, J.; Cochennec, F.; Riga, C.; DiMarco, A.; Bicknell, C.D. Evaluation of an electromagnetic 3D navigation system to facilitate endovascular tasks: A feasibility study. Eur. J. Vasc. Endovasc. Surg. 2012, 43, 22–29. [Google Scholar] [CrossRef]
- Urbina, J.; Sotelo, J.A.; Springmüller, D.; Montalba, C.; Letelier, K.; Tejos, C.; Irarrázaval, P.; Andia, M.E.; Razavi, R.; Valverde, I.; et al. Realistic aortic phantom to study hemodynamics using MRI and cardiac catheterization in normal and aortic coarctation conditions. J. Magn. Reson. Imaging 2016, 44, 683–697. [Google Scholar] [CrossRef]
- Potthast, S.; Bongartz, G.M.; Huegli, R.; Schulte, A.-C.; Schwarz, J.G.; Aschwanden, M.; Bilecen, D. Intraarterial contrast-enhanced MR aortography with and without parallel acquisition technique in patients with peripheral arterial occlusive disease. Am. J. Roentgenol. 2007, 188, 823–829. [Google Scholar] [CrossRef]
- Bonanni, M.; Russo, G.; De Siati, M.; Tomao, F.; Massaro, G.; Benedetto, D.; Longoni, M.; Matteucci, A.; Maffi, V.; Mariano, E.G.; et al. Holographic mixed reality for planning transcatheter aortic valve replacement. Int. J. Cardiol. 2024, 412, 132330. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.