Abstract
Background/Objectives: Obesity is a major risk factor for diabetes, but the underlying mechanisms remain incompletely understood. Obesity is associated with alterations in circulating lipids. This study aimed to determine whether, and to what extent, circulating lipids mediate the diabetogenic effect of obesity. Methods: This mediation analysis included 26,627 adult participants. Parallel mediation analysis included total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides as simultaneous mediators. Low-density lipoprotein (LDL) cholesterol was excluded from the parallel model due to collinearity with total cholesterol and was assessed separately using simple mediation analysis adjusted for confounders. Results: After adjustment for tested confounders, parallel mediation analysis showed that increases in triglycerides and reductions in HDL cholesterol mediated 24.0% (indirect effect coefficient = 0.23; 95% CI: 0.20–0.26; p < 0.05) and 3.8% (indirect effect coefficient = 0.04; 95% CI: 0.01–0.06; p < 0.05) of the diabetogenic effect of obesity, respectively. An increase in total cholesterol modestly attenuated the diabetogenic effect of obesity by 2.3% (indirect effect coefficient = −0.02; 95% CI: −0.03 to −0.01; p < 0.05), a magnitude that is unlikely to be clinically meaningful. Simple mediation analysis indicated that LDL cholesterol was not a significant mediator. Conclusions: Triglycerides are the most influential circulating lipid in mediating the diabetogenic effect of obesity, accounting for 24% of the total effect. Targeting triglyceride levels might represent an underrecognized therapeutic strategy to reduce obesity-related diabetes risk.
1. Introduction
Obesity is defined as excessive fat accumulation in the body that can impair health [1]. Over the past three decades (1990–2021), global adult obesity prevalence has risen dramatically—by 105% in females (from 10.2% to 20.8%) and by 155% in males (from 5.8% to 14.8%) [2]. The World Health Organization (WHO) has classified obesity as a global epidemic [3]. According to the WHO, 890 million adults worldwide are living with diabetes, representing 16% of the global population [4]. Obesity in adults is particularly common in high-income countries, with prevalence rates of 28% in the UK [5], 29.5% in Canada [6], 32% in Australia [7], and 41.9% in the United States [8]. It is projected that by 2050, the number of adults with obesity will reach 1.95 billion [2].
Obesity has wide-ranging consequences for health and well-being [1]. Individuals with obesity often experience stigma and discrimination, which negatively impact quality of life [9,10] and increase the risk of depression [11]. Excess adipose tissue can also impair organ and tissue function; for example, it can damage joints, leading to osteoarthritis, pain, and reduced mobility [12]. Furthermore, obesity is a major risk factor for numerous diseases, including liver disease [13], chronic kidney disease, cardiovascular disease, and cancer [14,15].
Of particular concern, obesity substantially increases the risk of diabetes [16]. Currently, diabetes affects 537 million people worldwide [17], and both its prevalence and incidence continue to rise [17,18]. Diabetes can lead to severe complications, including blindness, kidney failure, heart attacks, stroke, and lower-limb amputation [19]. The World Obesity Federation and the International Diabetes Federation estimate that obesity accounts for 43% of type 2 diabetes cases [20], which itself represents approximately 90% of all diabetes diagnoses [21]. Both organizations emphasize that halting the global rise in type 2 diabetes requires prioritizing action on obesity [20].
Obesity contributes to diabetes through multiple mechanisms [16,22,23]. A key pathway involves obesity-induced insulin resistance and β-cell dysfunction [16], driven by increased oxidative stress and chronic inflammation [22,23]. Obesity is also strongly associated with dyslipidemia, characterized by elevated triglycerides [24], increased total cholesterol [25], higher low-density lipoprotein (LDL) cholesterol [26], and reduced high-density lipoprotein (HDL) cholesterol [27,28,29]. Most individuals with obesity (60–70%) exhibit dyslipidemia [30]. However, the extent to which these circulating lipids mediate the diabetogenic effect of obesity remains unclear.
To address this question, we analyzed data from 26,627 US adults who participated in the National Health and Nutrition Examination Survey (NHANES) between 1988 and 2014. Circulating lipids assessed included total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides.
2. Materials and Methods
2.1. Study Participants
This study included US civilian noninstitutionalized individuals who attended the NHANES from 1988 to 2014. These surveys were organized by the Centers for Disease Control and Prevention (CDC) [31]. The inclusion criteria of the current study were age of 20 years or older, and the availability of the following data: body mass index, fasting triglycerides, total cholesterol, HDL cholesterol, fasting plasma glucose, and blood hemoglobin A1c (HbA1c). A total of 26,724 participants met these criteria. The following individuals with unknown education status (n = 74), unknown smoking status (n = 13), or unknown physical activity status (n = 10), were excluded. Therefore, 26,627 participants were included in the final analysis (Figure 1).
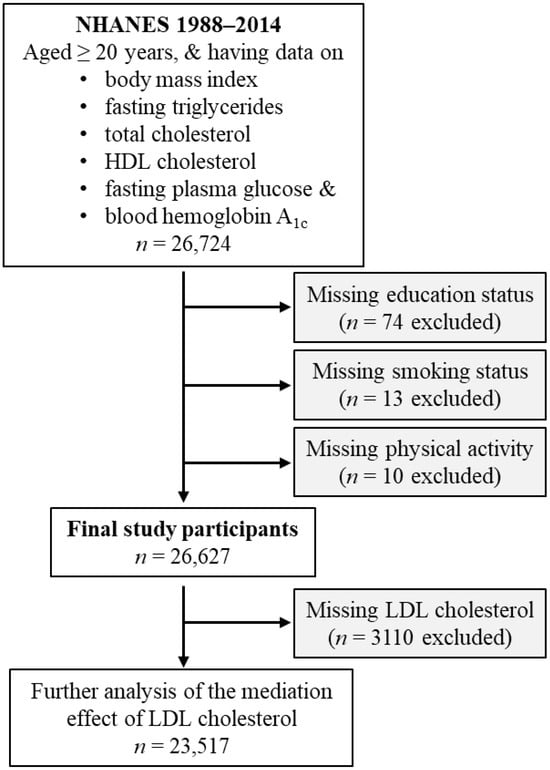
Figure 1.
Flow diagram of the study participants. HDL, high-density lipoprotein; LDL, low-density lipoprotein; NHANES, National Health and Nutrition Examination Survey.
In a further analysis investigating the effect of LDL cholesterol in mediating the diabetogenic effect of obesity, 3110 participants were excluded due to missing LDL cholesterol values. Therefore, a total of 23,517 participants were included in this further analysis (Figure 1).
2.2. Exposure Variable
The exposure variable was obesity, which was defined as a body mass index of 30 kg/m2 or above [32,33]. In addition, body mass index (continuous) was used as the exposure variable in further analysis.
2.3. Outcome Variable
Diabetes was the outcome variable. It was classified by one of the following conditions: an HbA1c value of ≥6.5%, a fasting plasma glucose value of ≥126 mg/dL, a 2-h plasma glucose value during oral glucose tolerance test of ≥200 mg/dL, the use of anti-diabetic drugs, or self-reported diabetes diagnosis [34,35].
2.4. Candidate Mediators
The mediators assessed in this study included total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides. Fasting blood samples were collected from participants who had fasted for at least 8 h after their last caloric intake [35,36,37].
Total cholesterol was measured enzymatically using a series of coupled reactions: cholesteryl esters were hydrolyzed to cholesterol by cholesterol esterase; cholesterol was then oxidized by cholesterol oxidase, producing hydrogen peroxide; and hydrogen peroxide was converted into a red dye by peroxidase in the presence of 4-aminophenazone and phenol. The color intensity, directly proportional to cholesterol concentration, was determined photometrically at 500 nm [38].
HDL cholesterol was measured directly without removing apoB-containing lipoproteins [39]. A blocking reagent rendered LDL, very low-density lipoprotein (VLDL), and chylomicrons non-reactive with the enzymatic cholesterol reagent under assay conditions, effectively excluding them from detection. HDL cholesterol esters were converted to cholesterol by polyethylene glycol (PEG)-modified cholesterol esterase, then oxidized by cholesterol oxidase to Δ4-cholestenone and hydrogen peroxide. In the presence of peroxidase, hydrogen peroxide reacted with 4-amino-antipyrine and N-(2-hydroxy-3-sulfopropyl)-3,5-dimethoxyaniline (HSDA) to form a purple-blue dye. The color intensity, proportional to HDL cholesterol concentration, was measured photometrically.
Triglycerides were measured enzymatically according to the published method [40]. LDL cholesterol was not directly measured [41]; instead, it was calculated using the Friedewald formula [42].
2.5. Confounding Variables
The study included a broad range of confounders, including age (continuous), sex, ethnicity, education status, income status, survey periods, physical activity, alcohol consumption status, smoking status, hypertension, and family history of diabetes [43,44,45].
2.6. Statistical Analyses
Baseline characteristics of participants were summarized as follows: categorical variables were presented as numbers (percentages), non-normally distributed continuous variables as medians (interquartile ranges), and normally distributed continuous variables as means (standard deviations) [46]. Differences in categorical variables were assessed using Pearson’s chi-square test [47], while differences in continuous variables were evaluated using Student’s t-test for normally distributed variables and the Mann–Whitney U test for non-normally distributed variables [48]. Correlations among the lipids were analysed using bivariate Pearson correlation analysis.
The association of obesity with diabetes was examined using binary logistic regression [49]. Mediation analysis was performed using the PROCESS Version 4.3 Macro for SPSS [50,51] with Model number 4. The 95% confidence interval (CI) for the indirect effect was estimated via bootstrapping using 5000 samples without centering for the construction of products [52,53]. First, a simple mediation analysis was conducted (Figure 2A), where candidate mediators (total cholesterol, HDL cholesterol, and triglycerides) were analyzed individually to estimate their separate mediation effects on the obesity–diabetes association. Subsequently, a parallel mediation analysis was performed (Figure 2B), in which all three mediators were included simultaneously in the same model.
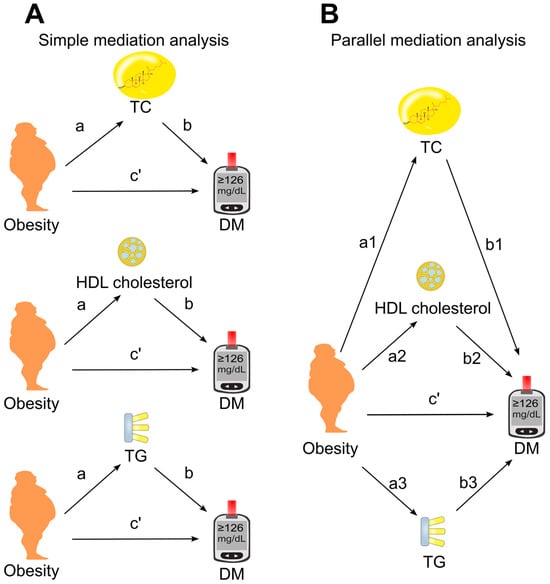
Figure 2.
Models of mediation. (A), Simple mediation model. Total cholesterol, HDL cholesterol, or triglycerides was added as single mediator between obesity and diabetes. (B), Parallel mediation model. Total cholesterol, HDL cholesterol, and triglycerides were added simultaneously to the same model. a, association coefficient of obesity with mediator; b, association coefficient of mediator with diabetes; c′, direct effect which measures the association coefficient of obesity with diabetes in the presence of mediator(s). DM, diabetes; HDL, high-density lipoprotein; TC, total cholesterol; TG, triglycerides.
Further analyses assessed the mediation effect of LDL cholesterol. Participants lacking LDL cholesterol data (n = 3310) were excluded, leaving 23,517 participants for this analysis (Figure 1). Because LDL cholesterol was highly correlated with total cholesterol (Pearson correlation coefficient = 0.917, Table 1), it was excluded from the parallel mediation model to avoid collinearity. Instead, its mediation effect was examined using simple mediation analysis, adjusting for all tested confounders as well as HDL cholesterol and triglycerides.

Table 1.
Bivariate Pearson correlation coefficient among the circulating lipids.
Association coefficients were derived from mediation analysis (Figure 2). Coefficient a represented the association of obesity with the tested mediator, while coefficient b reflected the association of the mediator with diabetes. The direct effect (c′) was the association of obesity with diabetes after accounting for the mediator(s). The indirect effect, also referred to as the mediation effect, was calculated as a × b [34].
A mediation effect was considered statistically significant (p < 0.05) if the 95% CI did not include zero [54]. The following formula was used to calculate proportion mediated: Proportion mediated = a × b/(a × b + c′). This metric indicates the extent to which the tested mediator explains the effect of obesity on diabetes [34,55].
Additional analyses were performed by replacing obesity with body mass index (continuous) in the mediation models. Sensitivity analyses were conducted by excluding participants who were using anti-diabetic or lipid-lowering medications or by omitting individuals who participated in surveys prior to 1999. NHANES sampling weights were not applied in any of the analyses.
Triglycerides, body mass index, total cholesterol, and HDL cholesterol were natural log-transformed to improve data distribution prior to inclusion in regression and mediation models [56]. Using obesity as a categorical variable enhances interpretability, making the findings more accessible to researchers, clinicians, and the general public. Therefore, the primary analysis in this study was conducted using obesity as a categorical variable. Two-tailed p-values < 0.05 were regarded as statistical significance. SPSS (version 27.0, IBM SPSS Statistics for Windows, Armonk, NY, USA, IBM Corporation) was used to perform all the statistical analyses.
3. Results
3.1. General Characteristics
This study included 26,627 adults, with a mean age of 48 years. Among these participants, 3958 individuals (14.9%) had diabetes, and 8425 individuals (31.6%) were obese. Compared to non-obese individuals, those with obesity had a higher likelihood of diabetes and hypertension, higher levels of triglycerides, higher levels of total cholesterol, higher levels of LDL cholesterol, lower levels of HDL cholesterol, and less physical activity (Table 2).

Table 2.
Characteristics of the 26,627 participants, stratified according to obesity 1.
3.2. Association of Obesity with Diabetes Diagnosis
Obesity was associated with a 2.44-fold higher odds ratio for diabetes (Model 4, Table 3) after adjustment for risk factors except total cholesterol, HDL cholesterol, and triglycerides (i.e., three tested mediators). After further adjustment for these tested mediators, obesity remained associated with a higher risk of diabetes (Model 8, Table 3), suggesting that any mediation by total cholesterol, HDL cholesterol, or triglycerides is partial rather than complete [57].

Table 3.
Obesity-associated odds ratio for diabetes in 26,627 individuals.
3.3. Role of Circulating Lipids in Mediating the Effect of Obesity on Diabetes
The mediation coefficients of total cholesterol, HDL cholesterol, and triglycerides for the effect of obesity on diabetes are displayed in Figure 3, Figure 4 and Figure 5. When total cholesterol, HDL cholesterol, and triglycerides was added separately into the analysis model, they all mediated the association of obesity with diabetes (Figure 3A), with triglycerides as the most dominant mediator, accounting for 18% of the diabetogenic effect of obesity (Figure 3A).
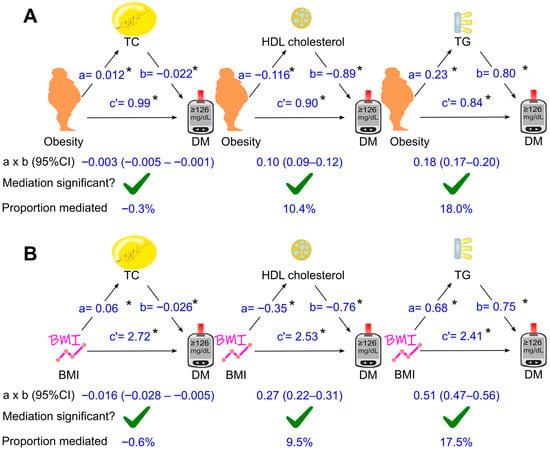
Figure 3.
Simple mediation analysis. Total cholesterol, HDL cholesterol, or triglycerides was added separately into the model using obesity (A) or BMI (B) as the exposure variable. a, association coefficient of obesity with mediator or that of BMI with mediator; b, association coefficient of mediator with diabetes; c′, association coefficient of obesity with diabetes or that of BMI with diabetes in the presence of mediator. BMI, body mass index; CI, confidence interval; DM, diabetes; HDL, high-density lipoprotein; TC, total cholesterol; TG, triglycerides. Green ticks indicate statistical significance. *, p <0.05.
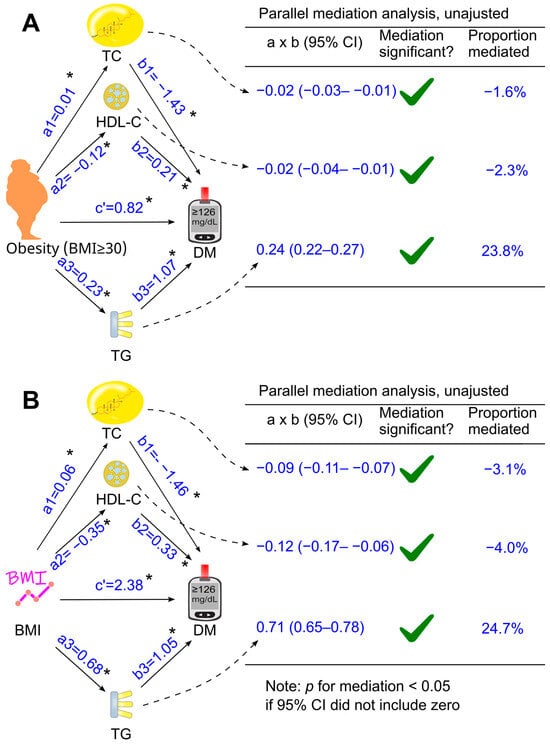
Figure 4.
Parallel mediation analysis without adjustment. Total cholesterol, HDL cholesterol, and triglycerides were added simultaneously into the same model using obesity (A) or BMI (B) as the exposure variable. These analyses were not adjusted for confounders. a, association coefficient of obesity with the tested mediator or that of BMI with the tested mediator; b, association coefficient of the tested mediator with diabetes; c′, association coefficient of obesity with diabetes or that of BMI with diabetes. BMI, body mass index; CI, confidence interval; DM, diabetes; HDL-C, high-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides. Green ticks indicate statistical significance. *, p <0.05.
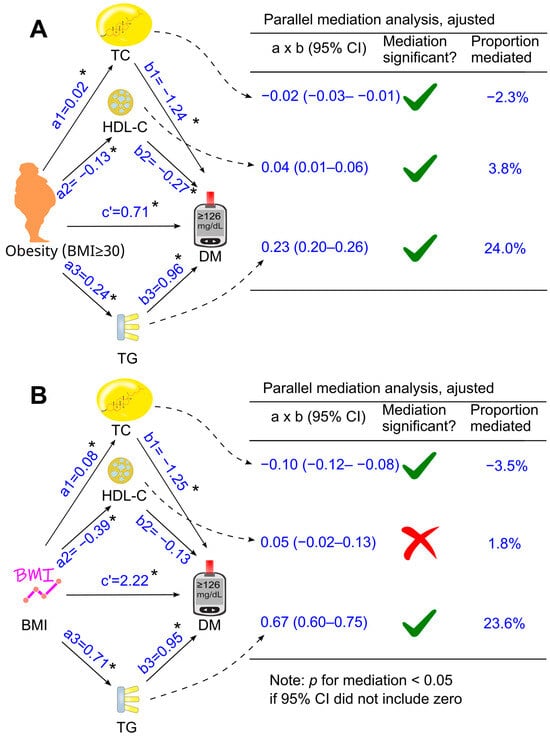
Figure 5.
Adjusted parallel mediation analysis. Total cholesterol, HDL cholesterol, and triglycerides were placed simultaneously into the same model using obesity (A) or BMI (B) as the exposure variable. This analysis was adjusted for confounding factors, including age, sex, ethnicity, poverty-income ratio, education status, survey period, lifestyle confounding factors (physical activity, alcohol consumption status, and smoking status), clinical confounding factors (hypertension and family history of diabetes). Abbreviations: a, association coefficient of obesity with mediator, or that of BMI with mediator; b, association coefficient of mediator with diabetes; c′, association coefficient of obesity with diabetes or that of BMI with diabetes in the presence of mediators. BMI, body mass index; CI, confidence interval; DM, diabetes; HDL-C, high-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides. Green ticks indicate statistical significance, while red crosses indicate non-significance. *, p <0.05.
When total cholesterol, HDL cholesterol, and triglycerides were included together as mediators in a parallel mediation model, all three parameters continued to mediate the relationship between obesity and diabetes, both without adjustment (Figure 4A) and after adjusting for confounders (Figure 5A). Increases in triglycerides and reductions in HDL cholesterol mediated 24.0% and 3.8% of the total effect after adjustment, respectively, whereas an increase in total cholesterol modestly attenuated the diabetogenic effect of obesity by 2.3%, a magnitude unlikely to be clinically meaningful (Figure 5A).
Similar results were observed after excluding participants taking anti-diabetic or lipid-lowering medications (Supplementary Figure S1). Triglycerides remained the most influential circulating lipid, mediating 21% of the diabetogenic effect of obesity. A reduction in HDL cholesterol explained 8% of the effect, whereas the small effect of total cholesterol was no longer statistically significant (Supplementary Figure S1).
Similar results were observed after excluding individuals who participated in surveys prior to 1999 (Supplementary Figure S2). Triglycerides remained the most influential circulating lipid, mediating 24.5% of the diabetogenic effect of obesity. In contrast, the reduction in HDL cholesterol did not significantly contribute to this mediation effect, and the attenuating influence of total cholesterol remained minimal (Supplementary Figure S2).
3.4. Role of Circulating Lipids in Mediating the Effect of Body Mass Index on Diabetes
Further analyses were conducted when obesity was replaced with a continuous variable, i.e., body mass index (Figure 3B, Figure 4B and Figure 5B). After adjustment for all the tested confounders, the parallel mediation analysis showed that triglycerides remained the most dominant mediator among the three tested mediators, mediated 23.6% of the association between body mass index and diabetes, and total cholesterol slightly attenuated the association by 3.5% (Figure 5B). However, HDL cholesterol did not mediate the effect of body mass index on diabetes (Figure 5B, p > 0.05)
3.5. Further Analyses of the Role of LDL Cholesterol in Mediating the Effect of Obesity (Or Body Mass Index) on Diabetes
Further analyses were conducted in a sub-cohort of 23,517 participants after 3110 participants were excluded due to missing LDL cholesterol. After adjustment for all the tested confounders, an increase in LDL cholesterol slightly attenuated the diabetogenic effect of obesity by 1%, which was not significant (p > 0.05, Figure 6). However, when obesity was replaced by the body mass index, an increase in LDL cholesterol attenuated the diabetogenic effect of higher BMI by 3% (p < 0.05, Figure 6). Similar findings were observed after excluding participants who were taking anti-diabetic or lipid-lowering medications (Supplementary Figure S3) and after omitting individuals who participated in surveys prior to 1999 (Supplementary Figure S4). Notably, LDL cholesterol did not substantially contribute to the diabetogenic effect of obesity, with proportions mediated of −0.4% and −1.1%, respectively (Supplementary Figures S3 and S4).
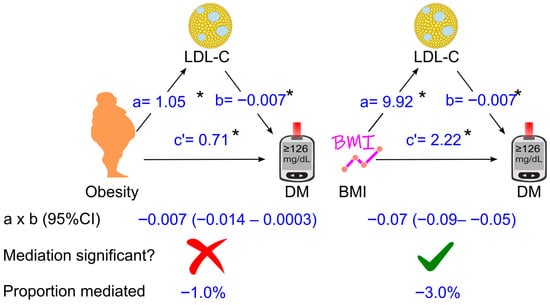
Figure 6.
Association coefficients of LDL cholesterol for mediating the effect of obesity or BMI on diabetes in 23,517 participants. The analysis was adjusted for age, sex, ethnicity, poverty-income ratio, education status, survey period, lifestyle confounding factors (physical activity, alcohol consumption status, and smoking status), clinical confounding factors (hypertension and family history of diabetes), high-density lipoprotein (HDL) cholesterol, and triglycerides. Abbreviations: a, association coefficient of obesity with LDL cholesterol, or that of BMI with LDL cholesterol; b, association coefficient of LDL cholesterol with diabetes; c′, association coefficient of obesity with diabetes or that of BMI with diabetes in the presence of mediators and confounders. BMI, body mass index; CI, confidence interval; DM, diabetes. Green ticks indicate statistical significance, while red crosses indicate non-significance. *, p <0.05.
4. Discussion
Utilizing a large sample of US adults (n = 26,627), this study found that the increase in triglycerides and the decrease in HDL cholesterol might mediate 24.0% (p < 0.05) and 3.8% (p < 0.05) of the diabetogenic effect of obesity, respectively, after adjusting for tested confounders. In contrast, an increase in total cholesterol modestly attenuated the diabetogenic effect of obesity by 2.3% (p < 0.05), a magnitude that is unlikely to be clinically meaningful. In addition, LDL cholesterol was not a significant factor mediating the diabetogenic effect of obesity. These findings indicate that triglycerides are the most influential circulating lipid mediating the diabetogenic effect of obesity.
Our analysis indicated that an increase in total cholesterol attenuated the diabetogenic effect of obesity by 2.3%. Although statistically significant, this effect is unlikely to be clinically meaningful. LDL cholesterol did not influence the diabetogenic effect of obesity. However, when BMI was used instead of obesity, an increase in LDL cholesterol moderately attenuated the diabetogenic effect of higher BMI by 3.0%. Despite statistical significance, this association also appears to have negligible clinical relevance.
Overall, these results suggest that although obesity is associated with increased total and LDL cholesterol, these lipids do not play a major role in mediating its diabetogenic effect. They may even provide slight protection against obesity-related diabetes risk. The mechanisms underlying these observations remain unclear. Nevertheless, our findings align with existing literature showing that lowering cholesterol and LDL cholesterol with statins slightly increases type 2 diabetes risk by approximately 10–12% [58,59,60,61,62]. Statins reversibly and competitively inhibit HMG-CoA reductase, the rate-limiting enzyme in cholesterol biosynthesis [63], thereby reducing cholesterol production and circulating total cholesterol. Additionally, statin-induced reductions in intracellular cholesterol upregulate LDL receptors (LDLR) in the liver and peripheral tissues, enhancing LDL clearance and lowering circulating LDL cholesterol [62,64].
This study also found that a reduction in HDL cholesterol mediated 3.8% of the diabetogenic effect of obesity. Obesity was associated with decreased HDL cholesterol, consistent with previous reports [27,28,29]. This reduction contributed to increased diabetes risk, supporting evidence that HDL cholesterol protects against type 2 diabetes [65,66] through mechanisms such as stimulating pancreatic insulin synthesis and secretion [67] and enhancing skeletal muscle glucose uptake [68]. Similarly, treatment with cholesteryl ester transfer protein (CETP) inhibitors to raise HDL cholesterol improved glycemic control in patients with type 2 diabetes [69].
The reduction in HDL cholesterol appeared to play only a minor role (3.8%) in mediating the diabetogenic effect of obesity, and this effect disappeared when BMI replaced obesity in the analysis. Notably, the use of anti-diabetic and lipid-lowering medications may influence this relationship. In our sensitivity analysis excluding individuals on these drugs, a decrease in HDL cholesterol mediated 8% of the diabetogenic effect of obesity and 5.5% of that associated with higher BMI.
Triglycerides, unlike total cholesterol, LDL cholesterol, and HDL cholesterol, played a substantially greater role in mediating the diabetogenic effect of obesity, accounting for 24% of the total effect. We observed that obesity was associated with elevated triglyceride levels, consistent with previous reports [24,26,70]. Furthermore, this increase in triglycerides was linked to a higher risk of diabetes, in agreement with existing literature [36,71,72]. Mechanistically, elevated circulating triglycerides promote intracellular triglyceride accumulation and reduce the capacity of cells to store excess glucose as triglycerides, thereby inducing insulin resistance [73]. In addition, high triglyceride levels contribute to β-cell dysfunction [74,75] and apoptosis [76], as well as increased hepatic gluconeogenesis [73,77,78]. Collectively, these mechanisms underscore the role of triglycerides in diabetes development.
The significance of triglycerides in mediating the diabetogenic effect of obesity is further supported by evidence from bariatric surgery. Weight loss induced by bariatric surgery improves glycemic control and often leads to diabetes remission [79,80,81]. Notably, these improvements occur without significant changes in total cholesterol [79,81] or LDL cholesterol [80,81], reinforcing our observation that these lipids play only a minor role in mediating obesity’s diabetogenic effect. Although bariatric surgery is frequently associated with increased HDL cholesterol [79,80], HDL cholesterol does not appear to be a major contributor to the anti-diabetic effect. For example, Genua et al. reported that blood glucose levels declined within three months post-surgery, while HDL cholesterol decreased during this period; although HDL cholesterol subsequently increased and remained elevated for up to five years, changes in body mass index were not associated with changes in HDL cholesterol [81].
In contrast, bariatric surgery consistently reduces circulating triglyceride levels [79,80,82,83]. This reduction parallels a decrease in intracellular triglyceride deposition in the liver and skeletal muscle [84]. These findings suggest that triglycerides may play a central role in mediating the anti-diabetic effects of weight loss, consistent with our observation that triglycerides accounted for 24% of the total effect of obesity.
Evidence from dietary energy restriction-induced weight loss further supports our findings. Lim et al. [85] reported that dietary energy restriction led to weight loss and reduced blood glucose levels within one week of intervention. However, at this time point, no changes in HDL cholesterol or LDL cholesterol were observed, suggesting that these lipids may not play a major role in mediating the antidiabetic effect of dietary energy restriction-induced weight loss—consistent with our results. In contrast, Lim et al. [85] found that the antidiabetic effect of dietary energy restriction-induced weight loss was accompanied by a reduction in circulating triglycerides. Moreover, a decline in intracellular triglyceride content in the pancreas was associated with restored insulin secretion, while a similar decline in the liver corresponded with reduced hepatic glucose production and lower fasting plasma glucose [85,86]. These findings suggest that lowering circulating triglycerides may contribute significantly to the antidiabetic effect of dietary energy restriction-induced weight loss.
Taken together, our results and evidence from weight-loss studies [79,80,81,82,83,84,85,86], support the notion that circulating triglycerides—rather than total cholesterol, HDL cholesterol, or LDL cholesterol—play a key role in mediating the diabetogenic effect of obesity. Therefore, targeting circulating triglycerides may represent a promising therapeutic strategy to reduce obesity-induced diabetes risk. Research indicates that lowering triglycerides with fenofibrate improves insulin sensitivity and reduces plasma glucose in mice [87]. Furthermore, fenofibrate has been shown to protect against T2DM in mice [88] and to slow the progression of albuminuria [89] and retinopathy [90] in patients with T2DM. In addition, bezafibrate has been reported to reduce the incidence of T2DM in humans [91].
Strengths of this study include its large sample size and adjustment for multiple confounding factors. However, the findings were based on US participants and may not be generalizable to other populations. In addition, although this study adjusted for a broad range of confounding factors, the presence of unmeasured confounders cannot be ruled out and may have influenced the results. Moreover, because diabetes can influence lipid levels and BMI, the possibility of reciprocal mediation cannot be excluded. A longitudinal study is warranted to confirm these findings.
5. Conclusions
This study demonstrates that among circulating lipids, triglycerides play the central mediating role in the diabetogenic effect of obesity. Consequently, targeting circulating triglycerides might be an underrecognized therapeutic approach for managing obesity-related diabetes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines14010011/s1, Supplementary Figure S1: Sensitivity analysis of the parallel analysis after excluding participants who were taking anti-diabetic or lipid-lowering drugs; Supplementary Figure S2: Sensitivity analysis of the parallel analysis after excluding participants who participated in surveys prior to 1999; Supplementary Figure S3: Sensitivity analysis of association coefficients of LDL cholesterol for mediating the effect of obesity or BMI on diabetes after excluding those who were taking anti-diabetic or lipid-lowering drugs; Supplementary Figure S4: Sensitivity analysis of association coefficients of LDL cholesterol for mediating the effect of obesity or BMI on diabetes after excluding those who participated in surveys prior to 1999.
Author Contributions
Conceptualization, Y.W.; formal analysis, Y.W.; data curation, Y.W. and Y.F.; writing—original draft preparation, Y.W., Y.F., F.J.C., G.R.D. and C.G.S.; writing—review and editing, Y.W., Y.F., F.J.C., G.R.D. and C.G.S.; visualization, Y.W.; funding acquisition, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
Y.W., G.R.D., and C.G.S. were supported by grants from the National Health and Medical Research Council of Australia (Y.W.: 1062671; G.R.D.: 2020452; G.G.S.: 2003156, 203760).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the NHANES Institutional Review Board (https://www.cdc.gov/nchs/nhanes/about/erb.html; accessed on 1 July 2025). Approval Code: NHANES Protocol #98–12, #2005–06, and #2011–17.
Informed Consent Statement
All participants provided written informed consent. The participants’ records were anonymized before being accessed by the author.
Data Availability Statement
All data included in this study are publicly available on the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm; accessed on 1 July 2025).
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BMI | Body mass index |
| CDC | Centers for Disease Control and Prevention |
| CETP | Cholesteryl ester transfer protein |
| CI | Confidence interval |
| DM | Diabetes |
| HbA1c | Hemoglobin A1c |
| HDL | High-density lipoprotein |
| HDL-C | High-density lipoprotein cholesterol |
| HSDA | N-(2-hydroxy-3-sulfopropyl)-3,5-dimethoxyaniline |
| IQR | Interquartile range |
| LDL | Low-density lipoprotein |
| LDL-C | Low-density lipoprotein cholesterol |
| LDLR | LDL receptor |
| n | Number |
| NA | Not applicable |
| NCHS | National Center for Health Statistics |
| NHANES | National Health and Nutrition Examination Survey |
| OR | Odds ratio |
| PEG | Polyethylene glycol |
| SD | Standard deviation |
| TC | Total cholesterol |
| TG | Triglyceride |
| VLDL | Very low-density lipoprotein |
| WHO | World Health Organization |
References
- Lingvay, I.; Cohen, R.V.; Roux, C.W.L.; Sumithran, P. Obesity in adults. Lancet 2024, 404, 972–987. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Gakidou, E.; Lo, J.; Abate, Y.H.; Abbafati, C.; Abbas, N.; Abbasian, M.; Abd ElHafeez, S.; Abdel-Rahman, W.M.; Abd-Elsalam, S.; et al. Global, regional, and national prevalence of adult overweight and obesity, 1990–2021, with forecasts to 2050: A forecasting study for the Global Burden of Disease Study 2021. Lancet 2025, 405, 813–838. [Google Scholar] [CrossRef]
- World Health Organization. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ. Tech. Rep. Ser. 2000, 894, 1–253. [Google Scholar]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 14 October 2025).
- House of Commons Library. Obesity Statistics. Available online: https://commonslibrary.parliament.uk/research-briefings/sn03336/ (accessed on 15 October 2025).
- Public Health Agency of Canada. Obesity Statistics in Canada: Report. Available online: https://www.canada.ca/en/public-health/services/publications/healthy-living/obesity-statistics-canada.html#a6 (accessed on 15 October 2025).
- Australian Institute of Health and Welfare. Overweight and Obesity. Available online: https://www.aihw.gov.au/reports/overweight-obesity/overweight-and-obesity/contents/summary (accessed on 14 October 2025).
- Stierman, B.; Afful, J.; Carroll, M.D.; Chen, T.-C.; Davy, O.; Fink, S.; Fryar, C.D.; Gu, Q.; Hales, C.M.; Hughes, J.P. National Health and Nutrition Examination Survey 2017-March 2020 prepandemic data files-development of files and prevalence estimates for selected health outcomes. Natl. Health Stat. Rep. 2021, 158, 1–21. [Google Scholar] [CrossRef]
- Buckell, J.; Mei, X.W.; Clarke, P.; Aveyard, P.; Jebb, S.A. Weight loss interventions on health-related quality of life in those with moderate to severe obesity: Findings from an individual patient data meta-analysis of randomized trials. Obes. Rev. 2021, 22, e13317. [Google Scholar] [CrossRef]
- Ul-Haq, Z.; Mackay, D.F.; Fenwick, E.; Pell, J.P. Meta-analysis of the association between body mass index and health-related quality of life among adults, assessed by the SF-36. Obesity 2013, 21, E322–E327. [Google Scholar] [CrossRef] [PubMed]
- Luppino, F.S.; de Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.; Zitman, F.G. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry 2010, 67, 220–229. [Google Scholar] [CrossRef]
- Nedunchezhiyan, U.; Varughese, I.; Sun, A.R.; Wu, X.; Crawford, R.; Prasadam, I. Obesity, Inflammation, and Immune System in Osteoarthritis. Front. Immunol. 2022, 13, 907750. [Google Scholar] [CrossRef]
- Pipitone, R.M.; Ciccioli, C.; Infantino, G.; La Mantia, C.; Parisi, S.; Tulone, A.; Pennisi, G.; Grimaudo, S.; Petta, S. MAFLD: A multisystem disease. Ther. Adv. Endocrinol. Metab. 2023, 14, 20420188221145549. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Obesity and Cancer. Available online: https://www.cdc.gov/cancer/risk-factors/obesity.html#:~:text=Overweight%20and%20obesity%20can%20cause,longer%20a%20person%20is%20overweight (accessed on 15 October 2025).
- Onstad, M.A.; Schmandt, R.E.; Lu, K.H. Addressing the Role of Obesity in Endometrial Cancer Risk, Prevention, and Treatment. J. Clin. Oncol. 2016, 34, 4225–4230. [Google Scholar] [CrossRef]
- Klein, S.; Gastaldelli, A.; Yki-Järvinen, H.; Scherer, P.E. Why does obesity cause diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef]
- Hossain, M.J.; Al-Mamun, M.; Islam, M.R. Diabetes mellitus, the fastest growing global public health concern: Early detection should be focused. Health Sci. Rep. 2024, 7, e2004. [Google Scholar] [CrossRef]
- Klonoff, D.C. The increasing incidence of diabetes in the 21st century. J. Diabetes Sci. Technol. 2009, 3, 1169–1173. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 18 October 2025).
- World Obesity Federation. Obesity and Type 2 Diabetes: A Joint Approach to Halt the Rise. Available online: https://www.worldobesity.org/news/idf-and-wof-release-new-policy-brief-to-address-obesity-and-type-2-diabetes (accessed on 15 October 2025).
- International Diabetes Federation. Diabetes Facts and Figures. Available online: https://idf.org/about-diabetes/diabetes-facts-figures/ (accessed on 1 October 2025).
- Wondmkun, Y.T. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab. Syndr. Obes. 2020, 13, 3611–3616. [Google Scholar] [CrossRef]
- Ruze, R.; Liu, T.; Zou, X.; Song, J.; Chen, Y.; Xu, R.; Yin, X.; Xu, Q. Obesity and type 2 diabetes mellitus: Connections in epidemiology, pathogenesis, and treatments. Front. Endocrinol. 2023, 14, 1161521. [Google Scholar] [CrossRef] [PubMed]
- Shamai, L.; Lurix, E.; Shen, M.; Novaro, G.M.; Szomstein, S.; Rosenthal, R.; Hernandez, A.V.; Asher, C.R. Association of body mass index and lipid profiles: Evaluation of a broad spectrum of body mass index patients including the morbidly obese. Obes. Surg. 2011, 21, 42–47. [Google Scholar] [CrossRef]
- Denke, M.A.; Sempos, C.T.; Grundy, S.M. Excess body weight. An under-recognized contributor to dyslipidemia in white American women. Arch. Intern. Med. 1994, 154, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Klop, B.; Elte, J.W.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef]
- Feingold, K.R. Obesity and Dyslipidemia; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK305895/ (accessed on 1 August 2025).
- Bays, H.E.; Toth, P.P.; Kris-Etherton, P.M.; Abate, N.; Aronne, L.J.; Brown, W.V.; Gonzalez-Campoy, J.M.; Jones, S.R.; Kumar, R.; La Forge, R.; et al. Obesity, adiposity, and dyslipidemia: A consensus statement from the National Lipid Association. J. Clin. Lipidol. 2013, 7, 304–383. [Google Scholar] [CrossRef]
- Oda, E. LDL cholesterol was more strongly associated with percent body fat than body mass index and waist circumference in a health screening population. Obes. Res. Clin. Pract. 2018, 12, 195–203. [Google Scholar] [CrossRef]
- Bays, H.E.; Chapman, R.H.; Grandy, S. The relationship of body mass index to diabetes mellitus, hypertension and dyslipidaemia: Comparison of data from two national surveys. Int. J. Clin. Pract. 2007, 61, 737–747. [Google Scholar] [CrossRef]
- Zipf, G.; Chiappa, M.; Porter, K.S.; Ostchega, Y.; Lewis, B.G.; Dostal, J. National Health and Nutrition Examination Survey: Plan and Operations, 1999–2010. National Center for Health Statistics; Vital Health Statistics 1; U.S. Department of Health and Human Services: Washington, DC, USA, 2013; 56, pp. 1–37.
- Garvey, W.T.; Mechanick, J.I.; Brett, E.M.; Garber, A.J.; Hurley, D.L.; Jastreboff, A.M.; Nadolsky, K.; Pessah-Pollack, R.; Plodkowski, R. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients with Obesity. Endocr. Pract. 2016, 22, 842–884. [Google Scholar] [CrossRef]
- Chauvin, S. Role of Granulosa Cell Dysfunction in Women Infertility Associated with Polycystic Ovary Syndrome and Obesity. Biomolecules 2025, 15, 923. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, Y.; Vrablik, M. Homeostasis Model Assessment for Insulin Resistance Mediates the Positive Association of Triglycerides with Diabetes. Diagnostics 2024, 14, 733. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef]
- Wang, Y. Higher fasting triglyceride predicts higher risks of diabetes mortality in US adults. Lipids Health Dis. 2021, 20, 181. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef] [PubMed]
- Lipid Laboratory Johns Hopkins. Total Cholesterol. Laboratory Procedure Manual. NHANES 2005–2006. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/public/2005/labmethods/tchol_d_met_h717.pdf (accessed on 1 October 2025).
- Lipid Laboratory Johns Hopkins. HDL-Cholesterol. Laboratory Procedure Manual. NHANES 2005–2006. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/public/2005/labmethods/hdl_d_met_cholesterol_hdl_h717.pdf (accessed on 1 October 2025).
- Lipid Laboratory Johns Hopkins. Triglycerides. Laboratory Procedure Manual. NHANES 2005–2006. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/public/2005/labmethods/trigly_d_met_triglyceride_h717.pdf (accessed on 1 October 2025).
- NHANES. Cholesterol—LDL & Triglycerides. Available online: https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2009/DataFiles/TRIGLY_F.htm (accessed on 1 March 2025).
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fang, Y.; Witting, P.K.; Charchar, F.J.; Sobey, C.G.; Drummond, G.R.; Golledge, J. Dietary fatty acids and mortality risk from heart disease in US adults: An analysis based on NHANES. Sci. Rep. 2023, 13, 1614. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, Y.; Magliano, D.J.; Charchar, F.J.; Sobey, C.G.; Drummond, G.R.; Golledge, J. Fasting triglycerides are positively associated with cardiovascular mortality risk in people with diabetes. Cardiovasc. Res. 2023, 119, 826–834. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, Y.; Aberson, C.L.; Charchar, F.J.; Ceriello, A. Postprandial Plasma Glucose between 4 and 7.9 h May Be a Potential Diagnostic Marker for Diabetes. Biomedicines 2024, 12, 1313. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Qian, T.; Sun, H.; Xu, Q.; Hou, X.; Hu, W.; Zhang, G.; Drummond, G.R.; Sobey, C.G.; et al. Reduced renal function may explain the higher prevalence of hyperuricemia in older people. Sci. Rep. 2021, 11, 1302. [Google Scholar] [CrossRef]
- Wang, Y. Stage 1 hypertension and risk of cardiovascular disease mortality in United States adults with or without diabetes. J. Hypertens. 2022, 40, 794–803. [Google Scholar] [CrossRef]
- Qian, T.; Sun, H.; Xu, Q.; Hou, X.; Hu, W.; Zhang, G.; Drummond, G.R.; Sobey, C.G.; Charchar, F.J.; Golledge, J.; et al. Hyperuricemia is independently associated with hypertension in men under 60 years in a general Chinese population. J. Hum. Hypertens. 2021, 35, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Wen, S.; Wang, Y.; Qian, Z.; Tan, Y.; Li, H.; Hou, Y.; Hu, H.; Golledge, J.; Yang, G. The association between serum uric acid and blood pressure in different age groups in a healthy Chinese cohort. Medicine 2017, 96, e8953. [Google Scholar] [CrossRef]
- Preacher, K.J.; Hayes, A.F. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav. Res. Methods 2008, 40, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.F. The PROCESS Macro for SPSS, SAS, and R. Available online: https://processmacro.org/index.html (accessed on 21 July 2025).
- DiCiccio, T.J.; Efron, B. Bootstrap confidence intervals. Stat. Sci. 1996, 11, 189–228. [Google Scholar] [CrossRef]
- Li, L.; Zhong, H.Y.; Xiao, T.; Xiao, R.H.; Yang, J.; Li, Y.L.; Yao, Q.; Chen, X.J. Association between self-disclosure and benefit finding of Chinese cancer patients caregivers: The mediation effect of coping styles. Support. Care Cancer 2023, 31, 684. [Google Scholar] [CrossRef]
- Soares, M.J.; Calton, E.K.; Pathak, K.; Zhao, Y. Hypothesized pathways for the association of vitamin D status and insulin sensitivity with resting energy expenditure: A cross sectional mediation analysis in Australian adults of European ancestry. Eur. J. Clin. Nutr. 2022, 76, 1457–1463. [Google Scholar] [CrossRef]
- Ananth, C. Proportion mediated in a causal mediation analysis: How useful is this measure? J. Obstet. Gynaecol. 2019, 126, 983. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Definition, prevalence, and risk factors of low sex hormone-binding globulin in US adults. J. Clin. Endocrinol. Metab. 2021, 106, e3946–e3956. [Google Scholar] [CrossRef] [PubMed]
- Gunzler, D.; Chen, T.; Wu, P.; Zhang, H. Introduction to mediation analysis with structural equation modeling. Shanghai Arch. Psychiatry 2013, 25, 390–394. [Google Scholar] [CrossRef]
- Betteridge, D.J.; Carmena, R. The diabetogenic action of statins—Mechanisms and clinical implications. Nat. Rev. Endocrinol. 2016, 12, 99–110. [Google Scholar] [CrossRef]
- Carter, A.A.; Gomes, T.; Camacho, X.; Juurlink, D.N.; Shah, B.R.; Mamdani, M.M. Risk of incident diabetes among patients treated with statins: Population based study. BMJ 2013, 346, f2610. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cai, R.; Yuan, Y.; Varghese, Z.; Moorhead, J.; Ruan, X.Z. Association between reductions in low-density lipoprotein cholesterol with statin therapy and the risk of new-onset diabetes: A meta-analysis. Sci. Rep. 2017, 7, 39982. [Google Scholar] [CrossRef] [PubMed]
- Zaharan, N.L.; Williams, D.; Bennett, K. Statins and risk of treated incident diabetes in a primary care population. Br. J. Clin. Pharmacol. 2013, 75, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Jebari, S.; Larrea-Sebal, A.; Uribe, K.B.; Siddiqi, H.; Ostolaza, H.; Benito-Vicente, A.; Martín, C. Statin Treatment-Induced Development of Type 2 Diabetes: From Clinical Evidence to Mechanistic Insights. Int. J. Mol. Sci. 2020, 21, 4725. [Google Scholar] [CrossRef] [PubMed]
- Endo, A. A gift from nature: The birth of the statins. Nat. Med. 2008, 14, 1050–1052. [Google Scholar] [CrossRef]
- Endo, A. A historical perspective on the discovery of statins. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Femlak, M.; Gluba-Brzózka, A.; Ciałkowska-Rysz, A.; Rysz, J. The role and function of HDL in patients with diabetes mellitus and the related cardiovascular risk. Lipids Health Dis. 2017, 16, 207. [Google Scholar] [CrossRef]
- Barter, P.J. High Density Lipoprotein: A Therapeutic Target in Type 2 Diabetes. Endocrinol. Metab. 2013, 28, 169–177. [Google Scholar] [CrossRef]
- Fryirs, M.A.; Barter, P.J.; Appavoo, M.; Tuch, B.E.; Tabet, F.; Heather, A.K.; Rye, K.A. Effects of high-density lipoproteins on pancreatic beta-cell insulin secretion. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1642–1648. [Google Scholar] [CrossRef] [PubMed]
- Drew, B.G.; Duffy, S.J.; Formosa, M.F.; Natoli, A.K.; Henstridge, D.C.; Penfold, S.A.; Thomas, W.G.; Mukhamedova, N.; de Courten, B.; Forbes, J.M.; et al. High-density lipoprotein modulates glucose metabolism in patients with type 2 diabetes mellitus. Circulation 2009, 119, 2103–2111. [Google Scholar] [CrossRef]
- Barter, P.J.; Rye, K.A.; Tardif, J.C.; Waters, D.D.; Boekholdt, S.M.; Breazna, A.; Kastelein, J.J. Effect of torcetrapib on glucose, insulin, and hemoglobin A1c in subjects in the Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events (ILLUMINATE) trial. Circulation 2011, 124, 555–562. [Google Scholar] [CrossRef]
- Hollister, L.E.; Overall, J.E.; Snow, H.L. Relationship of Obesity to Serum Triglyceride, Cholesterol, and Uric Acid, and to Plasma-Glucose Levels1. Am. J. Clin. Nutr. 1967, 20, 777–782. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, Y.; Zhang, X.; Wu, N.-Q. Non-Fasting Plasma Triglycerides Are Positively Associated with Diabetes Mortality in a Representative US Adult Population. Targets 2024, 2, 93–103. [Google Scholar] [CrossRef]
- Wilson, P.W.; Meigs, J.B.; Sullivan, L.; Fox, C.S.; Nathan, D.M.; D’Agostino, R.B., Sr. Prediction of incident diabetes mellitus in middle-aged adults: The Framingham Offspring Study. Arch. Intern. Med. 2007, 167, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Triglycerides, Glucose Metabolism, and Type 2 Diabetes. Int. J. Mol. Sci. 2025, 26, 9910. [Google Scholar] [CrossRef] [PubMed]
- Terauchi, Y.; Takamoto, I.; Kubota, N.; Matsui, J.; Suzuki, R.; Komeda, K.; Hara, A.; Toyoda, Y.; Miwa, I.; Aizawa, S.; et al. Glucokinase and IRS-2 are required for compensatory β cell hyperplasia in response to high-fat diet–induced insulin resistance. J. Clin. Investig. 2007, 117, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; Chen, X.; Rosner, J.; Barton, M. Effects of a 48-h Fat Infusion on Insulin Secretion and Glucose Utilization. Diabetes 1995, 44, 1239–1242. [Google Scholar] [CrossRef]
- Cunha, D.A.; Hekerman, P.; Ladrière, L.; Bazarra-Castro, A.; Ortis, F.; Wakeham, M.C.; Moore, F.; Rasschaert, J.; Cardozo, A.K.; Bellomo, E.; et al. Initiation and execution of lipotoxic ER stress in pancreatic β-cells. J. Cell Sci. 2008, 121, 2308–2318. [Google Scholar] [CrossRef]
- Weinman, E.O.; Strisower, E.H.; Chaikoff, I.L. Conversion of Fatty Acids to Carbohydrate: Application of Isotopes to this Problem and Role of the Krebs Cycle as a Synthetic Pathway. Physiol. Rev. 1957, 37, 252–272. [Google Scholar] [CrossRef]
- Borrebaek, B.; Bremer, J.; Davis, E.J.; Thienen, W.D.V.-v.; Singh, B. The effect of glucagon on the carbon flux from palmitate into glucose, lactate and ketone bodies, studied with isolated hepatocytes. Int. J. Biochem. 1984, 16, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.B.; O’Brien, P.E.; Playfair, J.; Chapman, L.; Schachter, L.M.; Skinner, S.; Proietto, J.; Bailey, M.; Anderson, M. Adjustable Gastric Banding and Conventional Therapy for Type 2 DiabetesA Randomized Controlled Trial. JAMA 2008, 299, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, J.P.; Courcoulas, A.P.; Cummings, D.E.; Goldfine, A.B.; Kashyap, S.R.; Simonson, D.C.; Arterburn, D.E.; Gourash, W.F.; Vernon, A.H.; Jakicic, J.M.; et al. Diabetes Remission in the Alliance of Randomized Trials of Medicine Versus Metabolic Surgery in Type 2 Diabetes (ARMMS-T2D). Diabetes Care 2022, 45, 1574–1583. [Google Scholar] [CrossRef]
- Genua, I.; Ramos, A.; Caimari, F.; Balagué, C.; Sánchez-Quesada, J.L.; Pérez, A.; Miñambres, I. Effects of Bariatric Surgery on HDL Cholesterol. Obes. Surg. 2020, 30, 1793–1798. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, L.; Lindroos, A.-K.; Peltonen, M.; Torgerson, J.; Bouchard, C.; Carlsson, B.; Dahlgren, S.; Larsson, B.; Narbro, K.; Sjöström Carl, D.; et al. Lifestyle, Diabetes, and Cardiovascular Risk Factors 10 Years after Bariatric Surgery. N. Engl. J. Med. 2004, 351, 2683–2693. [Google Scholar] [CrossRef]
- Heffron, S.P.; Lin, B.-X.; Parikh, M.; Scolaro, B.; Adelman, S.J.; Collins, H.L.; Berger, J.S.; Fisher, E.A. Changes in High-Density Lipoprotein Cholesterol Efflux Capacity After Bariatric Surgery Are Procedure Dependent. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 245–254. [Google Scholar] [CrossRef]
- Kawano, Y.; Ohta, M.; Hirashita, T.; Masuda, T.; Inomata, M.; Kitano, S. Effects of Sleeve Gastrectomy on Lipid Metabolism in an Obese Diabetic Rat Model. Obes. Surg. 2013, 23, 1947–1956. [Google Scholar] [CrossRef]
- Lim, E.L.; Hollingsworth, K.G.; Aribisala, B.S.; Chen, M.J.; Mathers, J.C.; Taylor, R. Reversal of type 2 diabetes: Normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011, 54, 2506–2514. [Google Scholar] [CrossRef]
- Taylor, R. Type 2 Diabetes: Etiology and reversibility. Diabetes Care 2013, 36, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jeon, S.; Lee, M.; Yoon, M. Fenofibrate alleviates insulin resistance by reducing tissue inflammation in obese ovariectomized mice. Nutr. Diabetes 2023, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Araki, M.; Nakagawa, Y.; Oishi, A.; Han, S.I.; Kumagai, K.; Ohno, H.; Mizunoe, Y.; Iwasaki, H.; Sekiya, M.; Matsuzaka, T.; et al. The Peroxisome Proliferator-Activated Receptor α (PPARα) Agonist Pemafibrate Protects against Diet-Induced Obesity in Mice. Int. J. Mol. Sci. 2018, 19, 2148. [Google Scholar] [CrossRef]
- Ginsberg, H.N.; Elam, M.B.; Lovato, L.C.; Crouse, J.R., 3rd; Leiter, L.A.; Linz, P.; Friedewald, W.T.; Buse, J.B.; Gerstein, H.C.; Probstfield, J.; et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N. Engl. J. Med. 2010, 362, 1563–1574. [Google Scholar] [CrossRef]
- Keech, A.C.; Mitchell, P.; Summanen, P.A.; O’Day, J.; Davis, T.M.; Moffitt, M.S.; Taskinen, M.R.; Simes, R.J.; Tse, D.; Williamson, E.; et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): A randomised controlled trial. Lancet 2007, 370, 1687–1697. [Google Scholar] [CrossRef]
- Tenenbaum, A.; Motro, M.; Fisman, E.Z.; Schwammenthal, E.; Adler, Y.; Goldenberg, I.; Leor, J.; Boyko, V.; Mandelzweig, L.; Behar, S. Peroxisome Proliferator–Activated Receptor Ligand Bezafibrate for Prevention of Type 2 Diabetes Mellitus in Patients with Coronary Artery Disease. Circulation 2004, 109, 2197–2202. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.