Abstract
An estimated 2.6 billion individuals are currently living with overweight or obesity, and this number is projected to exceed 4 billion by 2035. Consequently, unless this increasing trajectory is effectively addressed, the trend is expected to continue in the coming years. The gut microbiome has emerged as a central regulator of host metabolism and energy homeostasis, making its detailed characterization crucial for the advancement of innovative therapeutic strategies and for elucidating mechanisms underlying metabolic health and disease. This review examines human obesity through the lens of the gut microbiome, providing a comprehensive overview of its role by addressing gut microbiome alterations, microbiome-driven mechanisms, dietary influences, prebiotic effects, microbiome-based therapeutics, and other approaches in the treatment of obesity and related metabolic disorders. The composition of the gut microbiome is altered in obesity and characterized by reduced microbial diversity and inconsistent shifts in dominant bacterial phyla, which collectively contribute to metabolic dysregulation. The gut microbiome influences obesity through multiple mechanisms. These include regulating energy balance and insulin sensitivity via short-chain fatty acids, inducing chronic inflammation, modulating metabolic and appetite genes, altering bile acid signaling, and promoting fat storage by inhibiting fasting-induced adipose factor. Dietary patterns exert a profound influence on gut microbiome composition and function, with plant-based diets conferring protective effects against obesity and its comorbidities. Microbiome-based therapeutics, including probiotics, synbiotics, and fecal microbiota transplantation, have demonstrated potential in modulating key metabolic and inflammatory pathways associated with obesity. As the scientific understanding of the human gut microbiome continues to advance, the integration of microbiome-based therapies into standard clinical practice is poised to become increasingly feasible and therapeutically transformative, particularly for obesity, a complex condition that demands innovative and customized interventions.
1. Introduction
Obesity results from the chronic accumulation of adipose tissue, primarily driven by a sustained imbalance between caloric intake and energy expenditure [1]. At present, an estimated 2.6 billion individuals, equivalent to 40% of the global population, are living with overweight or obesity. It is projected that this number could exceed 4 billion by 2035, approaching half of the world’s population [2,3]. The persistent rise in obesity rates reflects the convergence of numerous interrelated factors, including but not limited to high-fat diets, sedentary lifestyles, neurohormonal dysregulation, and genetic and epigenetic susceptibility, as well as environmental and behavioral influences. Unless this increasing trajectory is effectively addressed, the trend is expected to continue in the coming years [4].
Individuals with obesity are predisposed to a multitude of obesity-related pathologies, including metabolic dysfunction-associated steatotic liver disease (formerly non-alcoholic fatty liver disease), metabolic syndrome (e.g., dyslipidemia, insulin resistance, hypertension, and elevated fasting glucose), type 2 diabetes mellitus (T2D), hepatic disorders, cardiovascular diseases, and certain cancers [5,6,7]. Moreover, obesity has been associated with diminished life satisfaction and the emergence of adverse mental health conditions, such as anxiety and depression [8]. Consequently, obesity constitutes a major contributor to the increased morbidity and mortality rates observed in contemporary populations [9]. This can be explained by evidence indicating that excessive adipose tissue accumulation in obesity activates cells of the innate immune system, which in turn promotes a state of chronic low-grade inflammation [10].
Traditionally linked to caloric intake and physical inactivity, obesity is now increasingly recognized as being influenced by the gut microbiome (GM), a diverse and dynamic microbial community residing in the gastrointestinal tract [10,11]. Emerging evidence indicates that the GM affects host metabolism through bioactive metabolites, which may regulate pivotal processes such as insulin sensitivity, lipogenesis, neurohormonal signaling, and systemic inflammation [5,12]. However, although research links the GM to obesity, an integrative synthesis connecting microbial mechanisms, dietary interventions, and GM-targeted therapies remains lacking. In this context, the prevailing hypothesis suggests that an imbalance in GM composition, known as dysbiosis, contributes to the activation of numerous signals via multiple pathways, resulting in obesity and its related complications. Indeed, dysbiosis plays a crucial role by influencing hunger regulation, satiety, and nutrient absorption [13]. It also promotes oxidative stress, inflammatory responses, increased adiposity, and metabolic dysfunction, including disturbances in glucose metabolism and adipocyte distribution. Despite these insights, the development of effective screening, diagnostic, and therapeutic approaches for obesity and its comorbidities remains limited, largely due to incomplete understanding of its underlying pathophysiology [5].
Classical approaches to obesity management have included pharmacotherapy, bariatric surgery, and lifestyle interventions [14]. Although significant progress has been made in pharmacological treatments in recent years [15,16], most anti-obesity drugs remain contraindicated for use during adolescence and pregnancy [17]. In turn, bariatric surgery carries considerable risks and potential complications [18]. Evidence-based lifestyle approaches typically encompass a combination of dietary modification, increased physical activity, and behavioral change therapies, which synergistically support sustainable weight management and contribute to improved metabolic outcomes [19,20,21]. Despite lifestyle-based interventions increasing the likelihood of achieving clinically significant weight loss in individuals with obesity, long-term weight maintenance remains a major obstacle, as weight regain over time is common, even among individuals who adhere to treatment protocols [22]. In light of these challenges, emerging therapeutic approaches, such as probiotic and prebiotic supplementation, brown adipose tissue transplantation, fecal microbiota transplantation (FMT), glucagon-like peptide-1 receptor agonists, poly-agonist pharmacotherapies, ultrasound stimulation of the vagus nerve, and precision nutrition strategies, have shown promise for clinical application [15,23,24,25,26,27,28].
Excessive adiposity significantly impairs quality of life and is associated with numerous comorbidities, collectively heightening the risk of preventable mortality. The GM has emerged as a central regulator of host metabolism and energy homeostasis, making its detailed characterization crucial for the advancement of innovative therapeutic strategies and for elucidating mechanisms underlying metabolic health and disease. Understanding these microbiome-mediated mechanisms is therefore essential for developing targeted interventions to manage obesity. In fact, these insights may also reveal that modulation of the GM can offer broader human health outcomes beyond metabolism. Based on these considerations, it is hypothesized that GM dysbiosis contributes causally to obesity by modulating energy balance, inflammation, and metabolic signaling, highlighting a knowledge gap in current obesity research. This review offers a novel and integrative perspective by connecting current evidence on GM functions with potential GM-focused strategies for obesity therapy. More specifically, it examines human obesity through the lens of the GM, providing a comprehensive overview of its role by addressing GM alterations, microbiome-driven mechanisms, dietary influences, prebiotic effects, microbiome-based therapeutics, and other approaches in the treatment of obesity and related metabolic disorders.
2. Gut Microbiome Composition in Individuals with Obesity
The composition and function of the human GM play a pivotal role in the development and management of obesity. Several studies have demonstrated that GM composition differs not only between obese and lean subjects but also across geographic regions, with marked variations observed between countries [29]. A review of the literature highlights consistent alterations in the GM of individuals with obesity compared to eutrophic subjects, notably a decrease in microbial richness and diversity [30,31,32]. These compositional changes have been linked to increased adiposity, dyslipidemia, heightened low-grade inflammation, and impaired glucose metabolism, underscoring the relevance of GM alterations in obesity pathophysiology [33].
In terms of GM composition, individuals with obesity often exhibit an increased Bacillota/Bacteroidota (B/B) ratio in their fecal microbiota, regardless of dietary intake [34,35]. However, this microbial signature is not consistently observed, likely due to confounding factors that influence GM composition, including fasting status, dietary pattern, antibiotic use, age, geographic location, exercise habits, genetic background, and methodological or clinical variables [36,37]. In this context, a decreased B/B ratio was reported in overweight and obese individuals without dietary restrictions [38], suggesting that body mass index (BMI)-related differences in GM composition may not follow a universal pattern [39]. These discrepancies may stem from interpretive bias caused by methodological differences in sample processing and DNA sequencing. Alternatively, they could reflect insufficient characterization of the study participants, especially the omission of lifestyle-related factors known to affect GM composition and diversity [40].
In some cases, differences in GM have been detected at the genus and family levels within the same cohort, without significant variation at the phylum level. For instance, a recent study in Korean adolescents found that individuals with normal weight had higher levels of Bacteroides/Bacteroidaceae, whereas obese participants exhibited increased levels of Prevotella/Prevotellaceae. However, the relative abundance of Bacillota, Bacteroidota, and Pseudomonadota, as well as the B/B ratio, did not differ significantly between groups [37]. Although there is a general consensus on the increase in Bacillota [41], other studies suggest that obesity risk may be more closely linked to reductions in Bifidobacterium spp. (Actinomycetota) [35] or Akkermansia muciniphila (Verrucomicrobiota) [42], rather than changes in the B/B ratio.
Other studies have revealed marked differences in GM bacterial composition among individuals with obesity. Kasai et al. [43] found that certain bacterial species, including Blautia hydrogenotrophica, Blautia (formerly Ruminococcus) obeum, Coprococcus catus, Eubacterium ventriosum, and Ruminococcus bromii, were linked to obesity in a Japanese population. In turn, Gao et al. [44] reported that in individuals with obesity, beneficial bacteria such as Bifidobacterium, Faecalibacterium, and butyrate-producing Ruminococcaceae are significantly reduced, whereas potential opportunistic pathogens, including Escherichia/Shigella and Fusobacterium, are increased. In addition, Crovesy et al. [45] conducted a systematic review of 32 studies and reported that individuals with obesity exhibit an increased abundance of members of the phyla Bacillota, Fusobacteriota, and Pseudomonadota, as well as the species Limosilactobacillus (formerly Lactobacillus) reuteri. Conversely, a decline has been observed in the abundance of the phylum Bacteroidota and species such as A. muciniphila, Faecalibacterium prausnitzii, Methanobrevibacter smithii, Lactiplantibacillus (formerly Lactobacillus) plantarum, and Lacticaseibacillus (formerly Lactobacillus) paracasei.
In a review conducted by Cani et al. [46], it was reported that members of the genera Clostridium, Lactobacillus, and Ruminococcus are elevated in obese patients, while F. prausnitzii is more prevalent in healthy individuals and reduced in those with obesity. Similarly, Duan et al. [30] observed significant differences in GM composition between obese patients and control subjects. At the phylum level, Bacillota, Bacteroidota, Actinomycetota, and Fusobacteriota showed significant disparities between the groups. At the genus level, 16 major genera exhibited notable differences, with Prevotella, Megamonas, Fusobacterium, and Blautia markedly increased in obese patients. Conversely, the remaining 12 genera, specifically Faecalibacterium, Lachnospiracea_incertae_sedis, Clostridium XIVa, Coprococcus, Gemmiger, Ruminococcus, Parabacteroides, Bifidobacterium, Clostridium IV, Alistipes, Oscillibacter, and Barnesiella, demonstrated reduced prevalence. At the species level, nine species showed significant differences, with an increased abundance of Megamonas funiformis, Segatella (formerly Prevotella) copri, and Fusobacterium mortiferum in obese subjects, whereas Bacteroides uniformis, F. prausnitzii, Fusicatenibacter saccharivorans, Barnesiella intestinihominis, Parabacteroides distasonis, and Alistipes putredinis were decreased.
Palmas et al. [47] conducted a study characterizing the GM signatures of overweight and obese individuals compared to normal-weight controls in Sardinia, Italy. Their findings revealed a significant reduction in the relative abundance of multiple Bacteroidota taxa within the microbial communities of obese patients, including members of the families Flavobacteriaceae, Porphyromonadaceae, and Sphingobacteriaceae, as well as the genera Bacteroides, Flavobacterium, Parabacteroides, Pedobacter, and Rikenella. However, several Bacillota taxa exhibited a marked increase in the same subjects, including members of the families Gemellaceae, Lachnospiraceae, Paenibacillaceae, Streptococcaceae, and Thermicanaceae and the genera Acidaminococcus, Eubacterium, Gemella, Megamonas, Megasphaera, Mitsuokella, Ruminococcus, Streptococcus, Thermicanus, and Veillonella. Body fat (BF) percentage and waist circumference (WC) showed a negative correlation with the abundance of Bacteroidota taxa. In contrast, Bacillota taxa showed a positive correlation with BF and a negative correlation with muscle mass and/or physical activity levels. Furthermore, the obese group exhibited an increased relative abundance of several bacterial taxa within the Enterobacteriaceae family, known for their endotoxic activity, compared to normal-weight controls.
In a systematic review, Xu et al. [48] identified bacterial taxa specifically associated with obesity and metabolic disorders across Western and Eastern populations. The phylum Pseudomonadota was the most frequently reported in obese individuals, while the genera Faecalibacterium, Akkermansia, and Alistipes were negatively correlated with obesity. In turn, Ruminococcus and Prevotella were identified as obesity-related genera in studies conducted in Western regions. Conversely, these genera were found to be lean-related in studies conducted in Eastern regions. Moreover, the genera Roseburia and Bifidobacterium were associated with a lean BMI in the Eastern population, while Lactobacillus was associated with obesity in the Western population. Yan et al. [49] employed whole-genome shotgun sequencing to reveal that the GM of patients with high visceral fatty areas (VFAs) exhibited a distinct microbial composition compared to those with low VFAs, characterized by an increased abundance of 18 bacterial species in the high-VFA group and 9 species that were more prevalent in the low-VFA group. A total of 16 gut microbial species exhibited strong correlations with VFA, with E. coli showing the highest association, followed by Bifidobacterium longum, Dialister succinatiphilus, Eubacterium hallii, Escherichia unclassified, Mitsuokella unclassified, Ruminococcus gnavus, and Mediterraneibacter (formerly Ruminococcus) torques. Furthermore, only two species (i.e., E. hallii and Solobacterium moorei) were found to be positively linked with BMI, while two species (i.e., D. succinatiphilus and Mitsuokella unclassified) demonstrated a positive correlation with WC. Figure 1 provides a summary of GM alterations in patients with obesity, according to several studies [30,43,44,45,46,47,48].
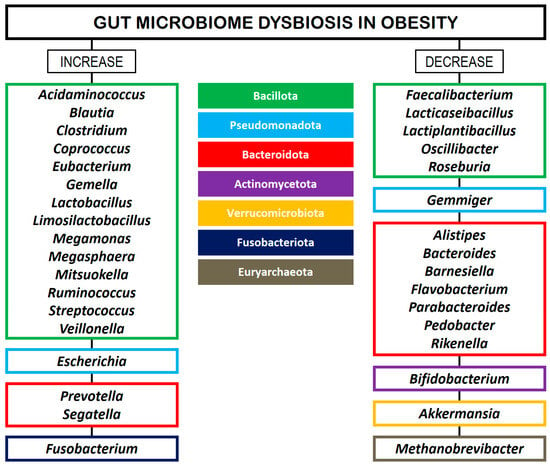
Figure 1.
GM dysbiosis in patients with obesity. The left column shows the major bacterial genera that are increased in abundance relative to controls. The right column shows the major bacterial genera that are decreased in abundance relative to controls. The bacterial phyla are color-coded in the middle column.
3. Gut Microbiome-Induced Mechanisms in Obesity
Various mechanisms have been proposed to explain the association between GM composition and the pathogenesis of obesity [50]. The first mechanism involves the microbial fermentation of non-digestible carbohydrates into metabolic byproducts, primarily short-chain fatty acids (SCFAs) such as acetate, butyrate, and propionate [51,52]. SCFAs are produced through anaerobic fermentation and function as ligands for G-protein-coupled receptors (GPRs). Acetate binds GPR43, butyrate binds GPR41, and propionate binds both. In states of dysbiosis and obesity, the expression of these receptors may be downregulated, contributing to hepatic lipogenesis and disruptions in energy homeostasis [53,54]. On the other hand, SCFAs have also been shown to promote fat oxidation and energy expenditure while inhibiting lipolysis. These effects support thermogenesis in brown adipose tissue and the browning of white adipose tissue [55], as well as enhanced insulin sensitivity [56]. Moreover, SCFAs have been implicated in the upregulation of peroxisome proliferator-activated receptors (PPARs), which play a key role in adipogenesis regulation [57].
A human pilot study demonstrated that oral butyrate supplementation positively influences glucose metabolism in lean individuals but not in those with metabolic syndrome, likely due to altered SCFA handling in insulin-resistant subjects [58]. Similarly, Li et al. [59] reported that butyrate can suppress appetite and activate brown adipose tissue through the gut–brain neural axis. Additional studies have identified associations between SCFA levels and improved insulin sensitivity, as well as regulatory effects on adipose tissue development, lipid storage, and substrate metabolism in the liver and skeletal muscle [60]. SCFAs that are not absorbed or metabolized by intestinal epithelial cells are transported to the liver via the portal vein, where they serve as substrates for gluconeogenesis, lipogenesis, and cholesterologenesis [61]. In addition, SCFAs have been shown to inhibit histone deacetylase activity, thereby influencing epigenetic modifications such as histone acetylation and methylation [62]. These changes can modulate the expression of genes involved in lipid metabolism and contribute to the restoration of chromatin structure and function [63].
According to Iqbal et al. [3], SCFAs exhibit a dual role in obesity, exerting both anti-obesogenic and pro-obesogenic effects. (i) SCFAs promote the secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY), hormones that suppress appetite and reduce caloric intake. (ii) They also activate the AMP-activated protein kinase (AMPK) pathway, enhancing fatty acid oxidation and decreasing fat accumulation. (iii) In contrast, in the context of dysbiosis, SCFAs have been shown to downregulate fasting-induced adipose factor (FIAF) expression in enterocytes, thereby increasing lipoprotein lipase (LPL) activity and promoting lipid storage in adipocytes. (iv) Excessive SCFA production, particularly acetate, can be utilized by the liver for fatty acid synthesis, contributing to lipid accumulation and increased energy extraction. A schematic representation of the dual role of SCFAs in obesity is shown in Figure 2 (modified from Iqbal et al. [3]).
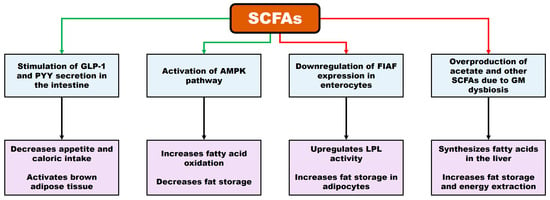
Figure 2.
Mechanisms involved in the dual role of SCFAs in obesity. Green arrows: activation processes. (i) SCFAs promote the secretion of hormones such as glucagon-like peptide-1 (GLP-1) and peptide YY (PYY), reducing appetite and, consequently, caloric intake. They also activate brown adipose tissue. (ii) SCFAs stimulate the AMP-activated protein kinase (AMPK) pathway, enhancing fatty acid oxidation and decreasing fat accumulation. Red arrows: inhibition processes. (i) SCFAs downregulate fasting-induced adipose factor (FIAF) expression in enterocytes, increasing lipoprotein lipase (LPL) activity and promoting lipid storage in adipocytes. (ii) Excessive SCFA production induces fatty acid synthesis in the liver, contributing to fat accumulation and increased energy extraction.
The second mechanism involves the capacity of the GM to modulate circulating lipopolysaccharide (LPS) levels by impairing epithelial barrier integrity, thereby initiating endotoxemia and the onset of moderate chronic systemic inflammation [64,65]. Once in the bloodstream, LPS binds to LPS-binding protein (LBP), which facilitates its recognition by the CD14 receptor. This interaction subsequently activates adipose tissue macrophages through the Toll-like receptor 4 (TLR4) pathway. LPS-TLR4 binding has been shown to upregulate genes involved in immune responses via the nuclear factor-kappa B (NF-κB) signaling pathway, promoting the secretion of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) [66,67]. This persistent low-grade inflammation, termed metabolic inflammation, impairs insulin signaling in peripheral tissues and contributes to systemic insulin resistance, which is a hallmark of obesity and metabolic syndrome [3]. Moreover, these inflammatory processes can drive genetic and epigenetic modifications that elevate the risk of obesity-related comorbidities [63]. In murine models, a single acute injection of LPS has been shown to enhance glucose disposal and glucose-stimulated insulin secretion through activation of the GLP-1 pathway [68].
The third mechanism is based on the hypothesis that the GM can modulate host genes implicated in energy storage and utilization, favoring fat accumulation [69]. In this context, Li et al. [70] conducted a comprehensive metagenomic analysis of 192 patients and reported functional gene variations in Bacteroides and Prevotella related to amino acid and carbohydrate metabolism. In addition, the authors identified a human genetic variant (rs878394) in the lysophospholipase-like 1 (LYPLAL1) gene, which is associated with BF distribution, insulin sensitivity, and the relative abundance of Prevotella. The GM has also been shown to regulate appetite and satiety through vagal nerve activation and immune–neuroendocrine signaling pathways [71]. Notably, lower levels of anorexigenic hormones involved in appetite suppression, such as GLP-1 and PYY, have been consistently observed in individuals with obesity [72].
The GM also plays a pivotal role in modulating bile acid (BA) metabolism, thereby influencing liver triglyceride (LT) levels and glucose homeostasis through the activation of the farnesoid X receptor (FXR) [73]. FXR activation in both the liver and intestines has been shown to inhibit hepatic lipogenesis, improve insulin sensitivity, and enhance energy expenditure, ultimately contributing to the mitigation of obesity risk. In addition, BAs have been implicated in preserving intestinal barrier integrity, thereby limiting LPS translocation into the systemic circulation and reducing the onset of chronic low-grade inflammation, which constitutes a key contributor to the pathogenesis of obesity and metabolic syndrome [74].
Furthermore, the GM may inhibit the expression of the FIAF gene, thereby increasing LPL activity and promoting lipid accumulation in white adipose tissue [75]. FIAF, which constitutes a protein produced by adipose tissue, the gastrointestinal tract, the liver, and skeletal muscle in response to fasting, plays a pivotal role in lipid metabolism by inhibiting LPL, which reduces triglyceride uptake in adipose and muscle tissues [76]. This inhibitory effect limits fatty acid uptake, potentially preventing excessive fat storage and serving as a mechanism to attenuate obesity [77].
4. Impact of Diet on Obesity via the Gut Microbiome
In the context of obesity management, short-term weight loss is primarily driven by calorie restriction rather than diet composition, with the most effective diet being the one that the individual can adhere to [78]. However, beyond caloric intake, dietary quality plays a pivotal role in obesity management and related health outcomes, with patterns such as the Mediterranean diet, vegetarian diets, ketogenic diets, and dairy-inclusive approaches showing benefits in weight control, metabolic health, and inflammation reduction [79,80,81,82,83,84,85,86,87]. Moreover, dietary quality is crucial in obesity-related conditions such as asthma, where high-fat, low-fiber diets are linked to increased airway inflammation and impaired medication responsiveness [20]. The Mediterranean diet, characterized by consumption of high-quality plant-based foods and moderate fat intake, demonstrates weight-loss effects comparable to low-fat and low-carbohydrate diets, while offering added cardiometabolic benefits and long-term sustainability when combined with energy restriction and physical activity [79,80]. Vegetarian diets are also associated with lower BMI and favorable weight outcomes, with key contributors including fiber content, lower caloric density, microbiota regulation, and the release of gastrointestinal appetite-regulating hormones [81,82,83]. Ketogenic diets, via mechanisms such as appetite suppression, reduced lipogenesis, increased lipolysis, and enhanced fat oxidation, have shown short- to medium-term benefits in terms of weight loss and cardiometabolic health in obese patients, although careful monitoring and a gradual transition to a normal diet are essential for safety and adherence [84,85]. In addition, dairy-inclusive, energy-restricted diets may enhance fat loss while preserving lean mass, with growing evidence supporting the benefits of whole-fat and fermented dairy products for body composition and cardiometabolic health [86,87].
Regarding diet outcomes, the GM has become a central focus in recent research due to its substantial contribution to obesity and related comorbidities. GM dysbiosis has been demonstrated to impact adiposity and glucose metabolism, thereby promoting obesity. Furthermore, modulating the GM through dietary strategies has emerged as a promising therapeutic approach in obesity management [5,6]. A high-calorie diet rich in fats and sugars, typical of the Western dietary pattern, combined with a sedentary lifestyle, has been identified as a major risk factor for obesity and its related metabolic disturbances [88]. Such dietary and lifestyle patterns induce the accumulation of adipose tissue, leading to an increase in visceral and subcutaneous fat. Once adipose tissue reaches its storage capacity, excess energy such as triglycerides leads to an increase in blood lipids and ectopic fat accumulation [89]. This progressive adiposity is accompanied by enhanced adipogenesis and the release of pro-inflammatory cytokines and adipokines, which are central to the pathogenesis of obesity-related disorders [90]. In contrast, the Mediterranean diet has been widely studied for its demonstrated efficacy in ameliorating chronic conditions, including T2D and metabolic syndrome [91,92]. These positive effects are largely attributable to the high intake of plant-based foods rich in dietary fiber and antioxidant compounds, such as terpenes and flavonoids, which enhance the production of SCFAs by the GM [93,94]. Consistently, elevated levels of SCFAs have also been observed in individuals following vegetarian diets [95].
High-calorie diets have been shown to modulate GM composition by increasing the abundance of Bacillota members while concurrently decreasing Bacteroidota abundance [96,97]. This shift in the B/B ratio has been particularly noted in obese animal models carrying leptin gene mutations, in contrast to their lean counterparts without such mutations, indicating a link between obesity and altered microbial diversity [98]. Despite the existence of numerous studies documenting variations in the B/B ratio among obese adults, other evidence suggests that obesity is more broadly characterized by an increased prevalence of Actinomycetota and Bacillota phyla, along with a decreased prevalence of species within the Bacteroidota and Verrucomicrobiota phyla, including A. muciniphila, as well as a reduction in F. prausnitzii [99,100].
Several human studies have shown that a high-fat diet can reduce the abundance of Bacteroides spp., Bifidobacterium spp., Clostridium coccoides, and Eubacterium rectale in individuals with obesity. However, these alterations can be reversed via dietary interventions [32,101,102]. Thingholm et al. [103] observed that the abundance of L. reuteri in the GM of obese patients was substantially elevated compared to other bacterial genera such as Akkermansia, Alistipes, Faecalibacterium, and Oscillibacter, as well as the archaeal species M. smithii, which tended to decrease. In addition, increased sugar intake has been linked to GM dysbiosis, characterized by a rise in the relative abundance of intestinal Pseudomonadota and a reduction in Bacteroidota, contributing to pro-inflammatory effects and impaired epithelial barrier function [104]. An in vivo study in obese adolescents undergoing a calorie-restricted diet combined with increased physical activity demonstrated that weight-loss interventions induced alterations in GM composition. As observed in corresponding animal studies, these changes, which include increased inflammation and intestinal permeability, resulted in endotoxemia, enhanced fat accumulation, and steatosis [105].
Dietary fiber intake plays a pivotal role in maintaining host health and regulating body weight (BW). Adoption of a vegetarian diet, characterized by a high intake of indigestible fibers, has been shown to decrease the β-diversity of the human GM. This decrease is linked to an increased abundance of the genera Prevotella and Faecalibacterium, alongside a reduced prevalence of Bacteroides [95]. While high Prevotella abundances have been demonstrated to promote weight loss in healthy adults with overweight [106], F. prausnitzii may play a pivotal role in obesity and related metabolic disorders, as it is frequently found in lower abundance in obese subjects and it is associated with reduced inflammation and enhanced gut barrier integrity and has even been shown to reduce appetite [107]. In addition, plant polyphenols increase the populations of Bifidobacterium and Lactobacillus, which exert anti-inflammatory effects, improve metabolic parameters, and confer cardiovascular protection in humans [108,109]. This modulation of the GM is particularly relevant in the context of obesity, as chronic low-grade inflammation and cardiovascular risk are common comorbidities associated with excessive adiposity [110]. Furthermore, fermented vegetables have been proposed as potential therapeutic agents in managing obesity, as they are particularly rich in Lactobacillus species, reduce cholesterol, inhibit adipogenesis, and have anti-diabetic properties [111].
Ketogenic diets are normocaloric, high-fat, and very low-carbohydrate dietary patterns that induce a state of ketosis [112]. This state leads to a reduction in insulin levels due to carbohydrate restriction, thereby suppressing lipogenesis and fat storage, while depleting glucose reserves. Although ketogenic diets are generally normocaloric, they are frequently modified to very-low-calorie versions in certain applications, further enhancing fat loss and other metabolic benefits [108]. The therapeutic effects of ketogenic diets in obese patients are likely mediated by their capacity to augment fatty acid oxidation and lipolysis while suppressing de novo lipogenesis, as well as by modulating hepatic glucose metabolism through decreased circulating insulin and increased glucagon levels, which together inhibit gluconeogenesis and promote elevated resting energy expenditure [112]. Recent studies have emphasized the pivotal involvement of the GM in mediating the effects of the ketogenic diets [113,114]. In human subjects, these dietary patterns have been shown to alter GM composition by decreasing the abundance of the phylum Bacillota and increasing the abundance of members belonging to the phylum Bacteroidota [115,116]. At the genus level, decreases in the abundance of Bifidobacterium and Lachnobacterium, as well as increases in Akkermansia and Slackia, have been reported [108,117]. Specifically, A. muciniphila may play a significant role in obesity and related metabolic disorders, as it can help to prevent weight gain, reducing visceral fat and improving glucose homeostasis [118]. Moreover, Gong et al. [119] noted that Bifidobacterium substantially decreased in visceral obesity, with serum uric acid potentially acting as a mediator between decreased Bifidobacterium and increased visceral adipose tissue, which suggests that supplementation with this microorganism might be a crucial complement for ketogenic diets in order to promote visceral adipose tissue reduction.
5. The Role of Prebiotics in Obesity
Prebiotics are typically polysaccharides defined as substances that induce specific changes in the composition and/or function of the GM, thereby conferring health benefits to the host [120]. To qualify as prebiotics, these substances must meet three criteria: (i) resistance to digestion by host enzymes, gastric acid, and bile; (ii) the ability to selectively stimulate the growth and/or activity of beneficial commensal microbiota; and (iii) fermentability by the GM [121].
Administration of various prebiotics has been shown to alter metabolic functions and GM composition, leading to increased levels of the anorexigenic gastrointestinal peptides GLP-1 and PYY, alongside decreased concentrations of the orexigenic hormone ghrelin [122]. Specifically, prebiotics such as inulin, fructans, and oligofructose promote the proliferation of beneficial bacteria, including lactobacilli and bifidobacteria [123]. Preclinical studies have linked Bifidobacterium species to reduced FM, lower BW gain, and diminished inflammation and metabolic endotoxemia [124]. In addition, prebiotic supplementation has been reported to increase the abundance of A. muciniphila, a species positively associated with improved insulin sensitivity, reduced FM gain, lower systemic inflammation, decreased metabolic endotoxemia, and regulation of energy homeostasis [42,125,126]. These findings in animals have been corroborated by clinical evidence demonstrating that oligofructose intake modulates GLP-1, ghrelin, and PYY levels in humans, thereby reducing hunger sensations and postprandial glucose fluctuations [122,127,128]. In a seminal study, Genta et al. [129] reported that consumption of oligofructose-rich syrup derived from yacon (Smallanthus sonchifolius) roots increased satiety and resulted in reductions in BW, BMI, and WC among obese premenopausal women. This intervention also decreased low-density lipoprotein (LDL) cholesterol and fasting insulin levels. Similarly, supplementation with inulin-type fructans in obese women significantly decreased BW, WC, BMI, homeostasis model assessment for insulin resistance (HOMA-IR), and fasting insulin, while improving satiety compared to the placebo group [130]. Table 1 summarizes the effects of various prebiotics administered in both animal models and human studies.

Table 1.
Effects of prebiotics on animals and humans with obesity.
6. Microbiome-Based Approaches in Obesity Management
The GM offers a novel perspective on potential therapies by linking inflammation, energy homeostasis, metabolism, and obesity. Several reviews have extensively discussed microbiota modulation strategies [5,148], proposing various microbial approaches to restore gut dysbiosis associated with obesity. Among these, probiotics and synbiotics remain the primary treatment modalities, while FMT has recently shown promising efficacy.
6.1. Probiotics and Synbiotics
Probiotics are defined as live microorganisms which, when administered in adequate amounts, confer health benefits to the host [149]. Probiotics have the capacity to modulate the GM, influencing energy and lipid metabolism [150], which can lead to reduced insulin resistance and improved satiety [151]. Several studies have reported that probiotics may aid weight loss by inhibiting adipogenesis and lowering fasting blood glucose levels in obese individuals [109]. In addition, probiotics contribute to cardiovascular health by reducing cholesterol concentrations [152]. They also enhance gut barrier integrity and immunomodulatory functions, while exhibiting antibacterial properties [153]. Furthermore, probiotics can rapidly colonize the gut, promoting lipid metabolism and facilitating the breakdown and elimination of visceral fat [154]. Systematic reviews of randomized controlled trials in overweight and obese populations have concluded that high-dose probiotic supplementation represents a promising intervention, with moderate but significant reductions in BMI as the most commonly reported outcome [155,156,157,158].
The role of specific beneficial gut microbial species in the development and management of obesity has garnered considerable scientific interest [159]. Bifidobacterium and Lactobacillus are among the most commonly studied probiotics in both animal models and obese human subjects due to their notable antibiotic resistance and low pathogenic potential [155,160,161]. Probiotics within the Lactobacillaceae family have demonstrated significant efficacy in reducing adipose tissue mass while enhancing lipid metabolism, primarily via the stimulation of fatty acid oxidation and inhibition of LPL activity [162]. Sanchis-Chordà et al. [163] investigated the effects of the probiotic Bifidobacterium pseudocatenulatum on cardiometabolic risk factors, inflammatory cytokines, and GM composition in obese children with insulin resistance, reporting a significant reduction in BMI post-intervention. Furthermore, probiotic administration has been shown to modulate GM composition, particularly affecting the abundance of Rikenellaceae family members, with a notable predominance of the genus Alistipes.
In a randomized trial involving overweight or obese insulin-resistant individuals, administration of pasteurized A. muciniphila for three months resulted in a modest reduction in BW alongside a significant improvement in insulin sensitivity [164]. Previously, A. muciniphila abundance was linked to fasting glucose levels, visceral and subcutaneous adiposity, and weight management in a 6-week calorie-restriction intervention involving 49 overweight and obese participants [165]. Conversely, a reduction in F. prausnitzii has been associated with a decreased capacity to counteract obesity-related inflammatory processes [166]. This species contributes to the restoration of intestinal barrier integrity and is currently being explored for its therapeutic potential [167]. Similarly, Alistipes and Roseburia genera have shown promise as therapeutic targets in the treatment of obesity and metabolic disorders [168].
Contrary to the generally health-promoting, anti-obesogenic effects attributed to probiotics, some studies have reported negligible or even obesogenic outcomes associated with their use [169,170]. These conflicting results may stem from variations in probiotic strains, as well as differences in host factors such as age and baseline BW. Consequently, there is a clear need for more rigorous and comprehensive randomized controlled trials to definitively determine the efficacy of probiotics in obesity management across diverse populations.
Synbiotics, defined as the combination of prebiotics and probiotics, have been shown to exert synergistic effects that exceed those of their components alone [171,172]. Their primary advantage lies in enhancing probiotic survival and viability within the gastrointestinal tract. In addition, synbiotics play a pivotal role in modulating gut metabolic activity by promoting microbiota growth, maintaining intestinal integrity, and inhibiting pathogenic species [161]. Moreover, they also increase levels of carbon disulfides, ketones, methyl acetates, and SCFAs, which may confer health benefits to the host [173].
The therapeutic potential of synbiotics in managing obesity, T2D, and associated metabolic disorders has garnered considerable attention in recent research [174]. Notably, Rajkumar et al. [175] demonstrated that co-supplementation with omega-3 fatty acids and a high-dose probiotic mixture comprising Bifidobacterium, Lactobacillus, and Streptococcus species led to significant improvements in plasma lipid concentrations, GM composition, insulin sensitivity, and inflammatory markers in overweight subjects. While probiotic supplementation alone induced favorable microbial shifts, omega-3 fatty acid administration without probiotics did not produce comparable effects. Despite these promising findings, current evidence regarding synbiotic interventions remains limited, and variables such as the timing of administration appear to critically influence therapeutic outcomes. Table 2 presents a summary of recent clinical studies investigating the effects of probiotics and synbiotics on obesity.

Table 2.
Effects of probiotics and synbiotics in humans with obesity.
6.2. Fecal Microbiota Transplantation
Standard FMT in humans entails the transfer of intestinal microbiota from a donor to a recipient via various delivery methods, including oral fecal capsules, colonoscopy, nasogastric or nasojejunal tubes, enemas, sigmoidoscopy, or rectal tubes [192]. Successful FMT is characterized by the sustained establishment of a donor-like microbiome in the recipient for at least 3 months post-transplantation [193].
This procedure has demonstrated transferable behavioral phenotypes and established links between GM composition and metabolic disorders [194], with emerging evidence suggesting potential applications in obesity management [195]. The exploration of FMT for obesity and its related comorbidities was driven by the ability of GM interventions to modulate glucose metabolism, enhance SCFA production, and reduce systemic inflammation [196]. Notably, Kootte et al. [197] reported that FMT administration significantly improved insulin responsiveness in individuals with metabolic syndrome. Similarly, Vrieze et al. [198] observed a marked increase in insulin sensitivity 6 weeks post-FMT compared to baseline levels in metabolic syndrome patients.
Kang and Cai [199] proposed several mechanisms underlying the effects of FMT on obesity: (i) FMT alters GM composition, influencing bacterial bile salt hydrolase (BSH) activity, which decreases levels of tauro-β-muricholic acid (TβMCA), which constitutes an antagonist of the FXR, thereby reducing BW gain, plasma cholesterol, and LTs; (ii) FMT may suppress pathogen growth by stimulating antimicrobial peptide secretion and strengthening the intestinal barrier through maintenance of tight junctions and increased mucin production; (iii) SCFAs produced post-FMT activate GPR-43, promoting secretion of GLP-1 and GLP-2, which regulate fasting glycemia and insulin sensitivity; and (iv) FMT may modulate immune responses via TLR-4 on macrophages and monocytes, triggering NF-κB, mitogen-activated protein kinase (MAPK), and signal transducer and activator of transcription (STAT) pathways, thereby influencing inflammatory cytokine secretion and immune cell proliferation and differentiation. Figure 3 presents potential mechanisms underlying the relationship between FMT and obesity (according to Kang and Cai [199]).
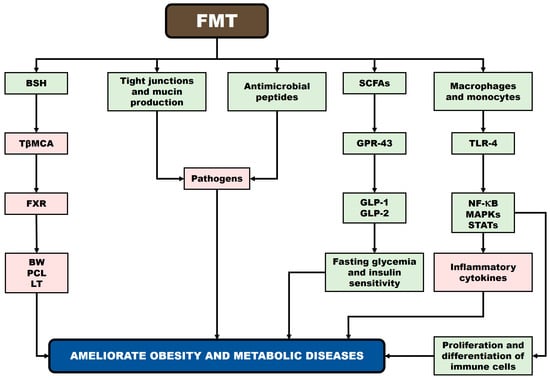
Figure 3.
Potential mechanisms underlying the relationship between FMT and obesity. Rectangles in green: increase. Rectangles in red: decrease. BSH: bile salt hydrolase; TβMCA: tauro-β-muricholic acid; FXR: farnesoid X receptor; BW: body weight; PCL: plasma cholesterol; LTs: liver triglycerides; SCFAs: short-chain fatty acids; GPR43: G-protein-coupled receptor 43; GLP-1 and GLP-2: glucagon-like peptides 1 and 2; TLR4: Toll-like receptor 4; NF-κB: nuclear factor-kappa B; MAPK: mitogen-activated protein kinase; and STAT: signal transducer and activator of transcription.
Regarding obesity interventions, the Gut Bugs Trial was conducted to assess the efficacy of FMT in improving metabolic function and treating obesity in 87 children and adolescents affected by this condition [200]. The primary outcome was the change in BMI six weeks post-FMT, administered via orally encapsulated fecal microbiota from same-sex donors, with a follow-up period of 26 weeks. While the intervention significantly reduced abdominal adiposity, no improvements were observed in insulin sensitivity, lipid profiles, BMI, liver function, blood pressure, inflammatory markers, or overall gut health. Mild adverse effects occurred, predominantly loose stools in 10% of participants, but no serious adverse events were reported. Complementing these findings, Zecheng et al. [201] demonstrated through a meta-analysis and systematic review that FMT improves blood glucose metabolism, insulin resistance, blood pressure, cholesterol levels, SCFA production, and inflammatory status in overweight individuals.
Allegretti et al. [202] conducted a double-blind trial involving 22 obese patients without diabetes, nonalcoholic steatohepatitis, or metabolic syndrome. Participants received oral FMT capsules (30 capsules at week 4 and week 8) derived from a single lean donor, with follow-up extending to week 26. The treatment was well tolerated and resulted in sustained alterations in GM composition and BA profiles resembling those of the donor. However, no significant differences in mean BMI were observed between groups at week 12. Similarly, Yu et al. [203] performed a 12-week double-blind, randomized, placebo-controlled pilot trial at a US academic center, enrolling 24 obese adults with mild-to-moderate insulin resistance who received FMT capsules from a healthy lean donor for six weeks. Although FMT led to GM engraftment in most recipients lasting at least 12 weeks, no clinically meaningful metabolic improvements were detected during the study period.
In turn, Hartstra et al. [204] demonstrated that FMT from Roux-en-Y gastric bypass donors modulate the microbiota–gut–brain axis in individuals with obesity. Recipients exhibited alterations in dopamine and serotonin transporter levels alongside shifts in GM composition, highlighting the potential of FMT to target the microbiota–gut–brain axis as a therapeutic approach for obesity [205]. Despite its ability to improve metabolic parameters, FMT has not been consistently associated with weight reduction in obese patients [206]. In addition, autologous FMT, where patients receive their own “healthy” microbiota collected prior to surgery during recovery, has emerged as a promising strategy to restore individualized GM composition [207].
Despite its currently limited clinical application, optimized FMT interventions remain a key focus of ongoing research. One promising strategy involves pretreatment with broad-spectrum antibiotic cocktails, which reduce the recipient’s native gut microbiota, thereby creating a less competitive environment and enhancing the engraftment and efficacy of transplanted microbes [207]. However, the specific effects of FMT on the microbiota–gut–brain axis components require further elucidation. Recently, Borrego-Ruiz and Borrego [208] outlined several critical questions for future investigation. First, the underlying mechanisms of FMT’s therapeutic efficacy remain unclear, partly due to the complex composition of FMT material, which includes viable and non-viable bacteria, as well as other microorganisms comprising the virome and mycobiome. Second, while FMT contains numerous metabolites (e.g., Bas and SCFAs) and proteins, the precise components responsible for its clinical effects are yet to be identified. Third, advances in FMT technology have introduced novel methods such as Washed Microbiota Transplantation (WMT) and spore transplantation. In this respect, controlled comparative studies and long-term safety evaluations of these approaches are still lacking.
7. Current Therapeutic Tools for the Treatment of Obesity
7.1. Pharmacological Therapy
Pharmacological therapy for obesity is intended to support weight reduction and improve metabolic health when lifestyle modifications alone are insufficient. Current approved pharmacotherapies are reported to induce a 5–15% reduction in BW. Anti-obesity and glucose-lowering agents primarily act by promoting satiety (e.g., liraglutide, lorcaserin, pramlintide, setmelanotide, and sibutramine) or by leading to nutrient malabsorption (e.g., orlistat) [209].
Once pharmacotherapy has been initiated and the recommended or maximum tolerated dose is achieved, treatment efficacy should be reassessed after 3–4 months [210]. This evaluation should consider whether weight-loss goals have been met, as well as improvements in metabolic parameters, obesity-related comorbidities, and quality of life. Although currently there are no pharmacological treatment guidelines specifically designed for older adults with obesity, commonly used agents in clinical settings include pancreatic lipase inhibitors, GLP-1 receptor agonists, dopamine/norepinephrine reuptake inhibitors, and opioid receptor antagonists [210,211]. Given the increasing availability of anti-obesity medications, the therapeutic landscape is becoming progressively complex. Consequently, the selection of a specific pharmacological agent should not rely solely on efficacy, but must also consider patient preferences, comorbidities, tolerability, and potential drug–disease or drug–drug interactions [212].
Various studies have highlighted a potential therapeutic role for antibiotics, particularly vancomycin, in addressing obesity-related microbial dysbiosis by reducing TNF-α levels in murine models and enhancing insulin sensitivity in humans [213,214]. Beyond antibiotics, numerous non-antimicrobial pharmaceuticals, including hormones, antidepressants, and antihistamines, have been shown to modulate GM composition. Indeed, a broad survey identified 44 drug categories that significantly influence the GM, among them metformin, statins, and laxatives [215]. While current anti-obesity medications exert marked effects on GM composition, their specific interactions with the gut–brain axis remain insufficiently explored.
A range of unimolecular GLP-1R/GcgR dual agonists have been developed using the glucagon amino acid sequence as a basis. These glucagon-based chimeric peptides were generated through targeted amino acid substitutions designed to enhance potency at the GLP-1R and to achieve balanced co-agonism at both receptors. The therapeutic potential of GLP-1R/GcgR co-agonism in obesity management has been underscored by studies evaluating its effects in combination with leptin therapy [209]. GLP-1 receptor agonists have demonstrated substantial benefits in improving glycemic control, promoting weight loss, and mitigating metabolic complications. Emerging therapeutic strategies, including dual and triple incretin receptor agonists, are showing superior efficacy in the management of both diabetes and obesity. Nevertheless, important challenges remain, such as the need to optimize treatment outcomes, consider inter-individual variability, and enhance long-term adherence [216,217]. Notably, several studies have reported that this novel therapeutic approach exhibits lower efficacy than both retatrutide and tirzepatide in reducing BW and WC in individuals with obesity or overweight [218].
7.2. Bariatric Surgery
Bariatric surgery is widely acknowledged as a highly effective intervention for obesity and its associated comorbidities, particularly in cases where other conventional therapies have failed. Emerging evidence indicates that its metabolic benefits are, in part, mediated by alterations in GM composition. Post-surgical shifts in the GM include increased production of SCFAs, enhanced abundance of Akkermansia and members of the family Streptococcaceae, and a concomitant reduction in bacterial genera belonging to the family Bacteroidaceae. Regarding dietary changes, patients exhibit lower absolute carbohydrate intake in both the short and long term [219].
In addition to its immediate metabolic benefits, bariatric surgery exerts long-term effects on the GM, which may contribute to sustained weight loss and metabolic improvements [6,220,221,222]. These enduring effects are thought to result from multiple physiological changes post-surgery, including reduced gastric volume, increased luminal pH, and diminished efficiency in dietary energy extraction. Typically, these modifications lead to a reduction in Bacillota, a phylum whose high abundance is associated with obesity, and also to an increase in Pseudomonadota, which are linked to improvements in systemic inflammation, glucose homeostasis, and weight reduction. Moreover, there is often an observed rise in Enterobacteriaceae, a family negatively correlated with cholesterol levels and positively associated with weight loss [45,223,224,225].
A longitudinal study examining the fecal microbiome of obese patients before and after bariatric surgery revealed a post-operative increase in the abundance of the genera Butyricimonas, Parabacteroides, and Slackia. Conversely, genera such as Acinetobacter, Coprococcus, Lachnospira, Lactococcus, Megamonas, Oribacterium, and Phascolarctobacterium, which were predominant in non-surgical obese individuals, showed a marked decline following the intervention [220]. These microbial shifts are likely attributable to the post-surgical rise in intestinal pH and oxygen levels, which inhibit anaerobic bacteria and promote the proliferation of aerobic taxa such as Pseudomonadota [226]. Notably, patients who experienced greater weight loss and long-term weight maintenance exhibited a higher diversity in core microbiota. These individuals had increased relative abundance of genera such as Alkaliphilus, Butyrivibrio, Cetobacterium, Lachnospira, Pseudoalteromonas, and Sarcina [182].
7.3. Physical Activity
Physical activity is widely acknowledged as a fundamental component in the prevention and management of overweight and obesity in both children and adults, as it produces weight loss, improves fitness, reduces cardiometabolic risk, and enhances quality of life [227,228]. School-based interventions have been widely explored as platforms for promoting physical activity and healthy habits in children and adolescents. While short-term school interventions tend to improve knowledge regarding nutrition and physical activity, their behavioral impact remains modest unless supported by broader community involvement [229,230]. Multi-component strategies that integrate physical education, dietary counseling, and health promotion across school and community settings have shown more promising results, particularly among adolescents from disadvantaged backgrounds, improving adiposity markers and physical fitness without necessarily reducing BMI [231,232]. Notably, programs that incorporate circuit training, high-intensity interval training, or innovative approaches such as gamified platforms have demonstrated improvements in cardiometabolic risk factors, body composition, and muscular strength in children and adolescents with moderate to severe obesity [233,234,235,236]. Ultimately, for school-based interventions to effectively prevent obesity and foster lifelong physical activity habits, they must be longitudinal, centered on direct physical engagement, and designed to develop not only physical competence but also the motivation and knowledge that underpin physical literacy [237]. In adults with overweight or obesity, physical activity plays a key role in improving health beyond weight loss, contributing significantly to cardiovascular fitness, metabolic function, mental well-being, and reductions in all-cause mortality [238,239,240]. Both aerobic and resistance training, especially when combined, have been shown to improve cardiorespiratory fitness and muscle strength, with high-intensity interval training and aerobic-based programs offering the most notable cardiovascular benefits [241]. These effects are observed even without major changes in weight [240]. Interventions that use adaptive goals, mobile health technologies, and reward-based systems have proven effective in increasing physical activity and overcoming common barriers such as low motivation or self-efficacy [242,243,244].
Research has shown that physical activity can modulate the GM, resulting in improved metabolic and immune function, as evidenced in both animal models and human studies [245]. Distinct microbial compositions have been observed between individuals with normal weight and those with obesity. Post-exercise interventions reveal significant shifts in GM composition. In this respect, Bacteroides were found to be more dominant in obese individuals, whereas Faecalibacterium and Lachnospira were more prevalent among normal-weight individuals. Moreover, exercise-induced modifications in GM composition were shown to revert after the cessation of physical activity, suggesting that the microbiota is dynamically responsive to lifestyle interventions [246]. Similarly, Motiani et al. [247] conducted a study involving 27 sedentary individuals with obesity and reported that moderate-to-high-intensity physical activity resulted in a decreased B/B ratio. This shift was accompanied by an increased abundance of Bacteroides and a concomitant reduction in Blautia and Clostridium abundance.
7.4. Behavioral Therapies
Standard behavioral therapies for obesity include motivational interviewing and cognitive–behavioral therapy, both grounded in principles such as goal setting, self-monitoring, and stimulus control [248]. While motivational interviewing has been proposed as a useful tool in obesity management, its effectiveness remains uncertain, particularly given the lack of evidence supporting its impact on weight outcomes when added to behavioral weight management programs [249]. In addition, the resource-intensive nature of motivational interviewing, requiring substantial staff training and increased intervention time, limits its practicality in routine clinical settings [249]. In turn, cognitive–behavioral therapy has demonstrated moderate efficacy in promoting lifestyle change and weight reduction when compared to usual-care controls, suggesting its relative value in obesity interventions [250]. In this context, integrating components that address psychological well-being and health-related quality of life may enhance treatment outcomes, highlighting the importance of a more holistic approach to obesity care [251].
The GM may play a potential role in influencing the success of behavioral therapies. Through the microbiota–gut–brain axis, microbe-derived metabolites affecting neural pathways can influence appetite regulation, mood, and stress responses [252], factors that directly impact behavioral adherence and weight management. Therefore, integrating GM modulation strategies with behavioral therapies could enhance treatment efficacy for obesity by addressing certain psychological and physiological determinants.
8. Emerging Therapeutic Approaches for Obesity
8.1. Brown Adipocyte Thermogenesis
A promising therapeutic strategy for addressing obesity and its associated metabolic disorders is the stimulation of brown adipocyte thermogenesis. This mechanism enhances energy expenditure by promoting the browning of white adipose tissue, a process mediated in part by increased production of microbial metabolites such as acetate and lactate [253]. These metabolites have been shown to activate thermogenic pathways, thereby facilitating fat oxidation and energy dissipation. Nevertheless, a major challenge in advancing this approach lies in the identification and development of pharmacological agents capable of reliably inducing thermogenesis in humans, which holds the potential to markedly improve obesity treatment outcomes [254]. Moreover, critical questions remain regarding whether enhanced brown adipose tissue activity serves as a driver or a consequence of weight loss and the potential risks associated with sympathetic nervous system-mediated non-shivering thermogenesis [255].
8.2. Precision Nutrition
In recent years, precision nutrition has emerged as a promising strategy to adapt dietary recommendations based on individual factors such as genetics, epigenetics, the GM, and lifestyle. This personalized approach has demonstrated utility in managing metabolic disorders and obesity [256]. Central to this strategy is the identification of biological markers that are particular to each individual, allowing the design of more customized and effective nutritional interventions. Such targeted approaches are expected to improve the efficacy of both prevention and treatment. The current literature shows that weight-loss interventions yield better outcomes when genetic profiles are incorporated into the treatment plan [257]. For instance, patients with a history of unsuccessful weight loss who underwent nutrigenetic testing for 24 variants across 19 metabolism-related genes achieved significantly greater long-term weight loss and improved fasting glucose levels compared to non-tested controls [258].
A growing body of evidence highlights that imbalances in GM composition directly impact lipid metabolism, immune and inflammatory pathways, and appetite regulation [10,122]. Carvalho et al. [26] emphasized the interaction between the GM and diet, illustrating how dietary patterns such as Mediterranean and vegetarian fiber-rich diets enhance SCFA production and promote the growth of beneficial bacteria linked to weight regulation and reduced inflammation. Current data indicate that the abundance of taxa such as A. muciniphila and F. prausnitzii correlates strongly with metabolic health and responsiveness to weight-loss interventions [45]. As reported in the literature, diet remains the primary modulator of GM composition [95]. However, other factors such as genetic and epigenetic traits, body composition, maternal nutritional status, mode of delivery, physical activity, and drug consumption also play significant roles in shaping the GM [52]. Despite challenges such as cost and variability in individual response, the integration of omics technologies, artificial intelligence, and machine learning holds great promise for identifying specific microbial and genetic profiles, thereby enabling the development of personalized nutritional interventions optimized to meet individual metabolic needs.
8.3. Vagus Nerve Stimulation
Decreased vagus nerve activity has been implicated in the development of hemodynamic and metabolic dysfunction associated with obesity [27]. In high-fat diet-induced obesity, vagal regulation of hepatic glucose production is impaired, while pharmacological activation of cholinergic signaling pathways or direct vagus nerve stimulation suppresses appetite and promotes weight loss [259,260].
Clinical studies using implanted nerve stimulators have demonstrated that vagus nerve stimulation can induce significant weight reduction in obese patients. In this respect, Huerta et al. [27] applied peripheral focused ultrasound stimulation to the liver of C57BL/6J mice fed a high-fat, high-carbohydrate Western diet. Daily ultrasound treatment over eight weeks resulted in significant body weight loss and reductions in circulating lipid levels. In addition, the intervention also alleviated adipokine dysregulation. Moreover, hepatic ultrasound stimulation markedly decreased hepatic cytokine expression and leukocyte infiltration. These findings reveal the potential of hepatic-focused ultrasound as a novel, noninvasive therapeutic approach for obesity management.
9. Discussion
This review presents an updated synthesis of current evidence on the role of the GM in human obesity, including diet, prebiotics, probiotics, synbiotics, and FMT as therapeutic strategies for GM modulation. To our knowledge, no previous review has addressed the GM in human obesity in such a comprehensive and integrative manner, covering microbial alterations, underlying mechanisms, dietary influences, prebiotic modulation, and microbiome-targeted therapies, as well as current and emerging therapeutic approaches. While microbial-based interventions are emerging as promising tools for managing obesity and related pathologies, several challenges must be addressed to enhance their efficacy and reproducibility. One critical issue is the heterogeneity in patient responses, as individuals often exhibit varying outcomes despite receiving similar treatments, a discrepancy influenced by factors such as baseline microbiota composition and host-specific variables. Genetic differences among individuals may further modulate immune responses, nutrient metabolism, and host–microbiota interactions, thereby impacting therapeutic effectiveness. Moreover, a significant limitation lies in the insufficient mechanistic understanding of these interventions. For instance, the FMT interventions reviewed were generally performed in pilot trials with small sample sizes and heterogeneous study populations. In turn, clinically significant outcomes, such as weight loss and alterations in BMI, are typically assessed up to 12 months following an intervention [202,203]. However, the efficacy of a single donor in terms of inducing a sustained effect remains uncertain. In contrast, multiple FMTs did not produce substantial metabolic improvements in subjects with metabolic syndrome [204]. As noted, metabolic and microbiome responses among FMT recipients exhibited considerable variability, and the studies lacked sufficient statistical power to analyze outcomes by recipient subgroups or to evaluate donor-specific effects. Finally, the FMT protocols did not include dietary interventions, and the majority of participants consumed typical high-fat, low-fiber Western diets. Therefore, the influence of diet needs to be validated in larger randomized controlled trials encompassing participants from diverse ethnic backgrounds [261].
Although the beneficial effects of microbial therapies are increasingly being recognized, the precise molecular pathways, particularly those involving microbial metabolites such as SCFAs and BAs, and their interactions with host metabolic networks remain insufficiently elucidated. Integrating multi-omics technologies, including metagenomics, metabolomics, and proteomics, may offer comprehensive insights into the dynamic interplay between the GM, the immune system, and host metabolism. Ultimately, the development of an integrative conceptual framework that incorporates microbial metabolites, immune modulation, and systemic metabolic regulation is essential for optimizing microbiota-targeted interventions aimed at improving metabolic health.
It is increasingly evident that inter-individual variability in GM composition significantly influences responses to dietary interventions aimed at obesity management. Studies assessing fecal microbiota before and after dietary interventions have revealed the stability of the microbiome over time and demonstrated marked heterogeneity in microbial and physiological responses among individuals subjected to identical diets [262]. This variability means that two individuals adhering to the same diet may experience markedly different physiological and microbial outcomes. For instance, Hjorth et al. [263] demonstrated that individuals with Prevotella-dominant microbiomes exhibited greater BF loss on a Nordic diet compared to those with Bacteroides-dominant microbiota, underscoring the role of microbial profiles in modulating dietary responsiveness. These findings underscore the potential of precision nutrition approaches that customize dietary strategies based on individual microbiome characteristics to maximize metabolic benefits. Thus, dietary interventions for obesity should not overlook inter-individual heterogeneity in their design.
Precision nutrition aims to customize dietary interventions by predicting individual metabolic responses, typically involving three main strategies: (i) increasing fiber intake, (ii) restricting caloric intake, and (ii) supplementing with prebiotics and probiotics [264]. Although these resemble conventional approaches, precision nutrition differs in that it incorporates assessment of prior individual responses, such as shifts in GM composition or glycemic control, to design customized plans, as opposed to a standardized dietary model. The success of this approach is often evaluated through microbial markers (e.g., increases in Prevotella abundance), anthropometric outcomes (e.g., weight loss, BMI, and FM reduction), and metabolic indicators like glycemic responses [265]. For instance, Zeevi et al. [266] developed an algorithm that accurately predicted postprandial glucose responses based on individual microbiome and clinical data, demonstrating superior predictive capacity compared to traditional metrics such as the glycemic index. Despite these advances, the application of personalized nutrition within the context of the microbiota–gut–brain axis remains underexplored. There is a critical need for long-term studies to investigate how sustained dietary modifications affect the GM and associated biochemical pathways, particularly those influencing hunger hormones, inflammatory cytokines, and other neuroendocrine signals implicated in obesity and T2D.
Obesity has been linked to dysregulated glycolysis, impaired oxidative phosphorylation, increased production of reactive oxygen species, and mitochondrial fragmentation. Current therapeutic approaches aim to reduce cellular stress and restore metabolic homeostasis, primarily through antioxidants or by modulating mitochondrial dynamics. Considering this, mitochondria-targeted therapies hold promise as a novel strategy to correct metabolic dysfunction in affected individuals [267]. However, to date, interventions specifically targeting mitochondrial dynamics for obesity treatment have yielded limited success, highlighting a gap and an opportunity for further research in this area. Notably, one study demonstrated that a synthetic sphingolipid could modulate mitochondrial dynamics in mice fed a high-fat diet, leading to restoration of healthy body weight [268]. This finding suggests that physiological manipulation of mitochondrial function may represent a viable therapeutic option. Nevertheless, the complexity of mitochondrial involvement in obesity-related metabolic disturbances requires comprehensive investigation to elucidate precise mechanisms and optimize targeted treatments. Future research should focus on understanding the interplay between mitochondrial dynamics, energy metabolism, and systemic metabolic regulation to fully unlock the therapeutic potential of mitochondria-targeted interventions in obesity.
Within the framework of this discussion, it is essential to acknowledge a key interconnection between obesity, mental health, and the GM, which serves as a central regulator of both metabolic and neuropsychological functions. Diet, as a major external factor, shapes the composition and metabolic activity of the GM, thereby influencing host health through modulation of immune development, nutrient metabolism, and bioactive molecule synthesis [93]. The GM is implicated in the etiology of various mental disorders, such as anxiety and depression, with substantial evidence linking its composition and functional dynamics to nervous system development and regulation [252]. Communication between the central nervous system and the GM occurs via immune, endocrine, and neural routes, including the vagal nerve, forming a complex gut–brain axis that impacts metabolic homeostasis and mental well-being [252]. Research indicates that obesity is associated with reductions in quality of life and increases in adverse mental health outcomes, underscoring a predominant influence of obesity on psychological distress [269,270,271]. Epidemiological data further indicate that individuals with obesity or overweight exhibit significantly diminished quality of life and heightened depression scores compared to normal-weight counterparts, with obesity exerting the most pronounced effect [269]. The high co-prevalence of obesity and mental illness poses a critical public health challenge, positioning the GM as a promising target for interventions aimed at improving metabolic and psychological outcomes. In recent years, the emerging field of nutritional psychiatry has begun to reshape clinical approaches, emphasizing the necessity for scientifically rigorous evaluation of dietary supplements and nutraceuticals due to their widespread use among individuals with and without mental health disorders [272]. Psychobiotics, a novel class of psychotropics comprising live microorganisms and bioactive substances, have demonstrated efficacy in ameliorating psychological conditions such as anxiety, stress, and depression, as well as in improving cognitive function, sleep quality, emotional regulation, and mood symptoms [273,274]. Given that diet constitutes a key element in obesity management, precision nutrition offers the opportunity not only to improve obesity-related metabolic dysfunctions but also to enhance mental health outcomes by modulating the GM. Concurrently, microbiome-based therapies, which have shown promise in managing obesity, could be strategically directed to address comorbid psychological conditions, particularly depression, which is highly prevalent among individuals with obesity. In this respect, a strictly vegetarian ketogenic diet has been proposed as a potential nutritional intervention to promote overall health, including improvements in both metabolic and mental health outcomes [108]. Considering the potential efficacy of vegetarian and ketogenic diets in obesity management, the implementation of a strictly vegetarian ketogenic dietary protocol, complemented by structured physical exercise and appropriately designed microbiome-based therapies, could constitute a promising, non-pharmacological, and non-surgical strategy for the effective treatment of obesity. This integrative approach may synergistically modulate metabolic regulation and the gut–brain axis, addressing the complex etiology of obesity and its associated mental health comorbidities. Furthermore, integrating components that address individual motivation, environmental factors, and long-term behavioral reinforcement may be key to improving maintenance outcomes.
Beyond its well-established medical implications, obesity often exposes individuals to stigma and discrimination across various social domains, including the workplace, education, and interpersonal relationships. Indeed, this pervasive stigma has become widely normalized and frequently goes unrecognized as a legitimate form of discrimination [275]. Addressing obesity stigma requires a coordinated societal response, beginning with dispelling common misconceptions about its causes and the rejection of simplistic notions of personal responsibility. A shift toward greater societal understanding and empathy may foster environments in which stigmatizing behaviors are challenged and ultimately eradicated [275]. In the healthcare setting, actionable strategies to mitigate stigma include shifting the clinical focus away from weight, adopting patient-centered communication, ensuring inclusive facilities and equipment, and improving access to specialized obesity care by removing administrative barriers [276]. In Spain, recent data reveal that individuals with obesity often report more aversion toward the condition than those of normal weight, and younger populations report higher frequencies of stigmatizing encounters [277]. These findings underscore the urgent need to confront negative societal beliefs and attitudes as an integral component of public health efforts. It is also noteworthy that even after bariatric surgery, patients continue to experience high levels of stigma, highlighting the persistence of weight-related prejudice despite clinical improvements [277]. While public visibility and advocacy from individuals living with obesity are essential to drive systemic changes and resource allocation, caution is warranted when individuals with self-induced overweight attempt to affiliate with this group. Although excessive weight due to unhealthy lifestyle choices remains a concern, equating it with medically defined obesity threatens the legitimacy of the condition and may overload limited healthcare resources intended for those truly affected. Sustainable progress will depend not only on individual and healthcare-level interventions but also on comprehensive public education campaigns and policy reforms that promote inclusivity and dismantle structural barriers contributing to obesity-related stigma. Ultimately, scientific progress driven by ongoing research must provide future approaches that effectively address obesity and mitigate its consequences, aiming to reduce its impact sufficiently to ensure an improved quality of life across affected demographics.
Lastly, it should be noted that obesity in adolescents is strongly associated with an increased likelihood of experiencing bullying compared to their healthy-weight peers [278]. The co-occurrence of obesity and bullying among children and adolescents remains a critical global concern, with evidence indicating a close relationship between these factors during key developmental stages [279]. While multiple strategies have been developed to manage and treat obesity, including cognitive and behavioral interventions, it seems that the role of certain emotional processes in the etiology and maintenance of this condition is not sufficiently considered. Bullying can provoke intense emotional responses such as humiliation, shame, and anger [280], which may contribute to maladaptive coping mechanisms, including emotional eating, which is defined as the tendency to consume food in response to negative emotions [281]. Since this phenomenon can exacerbate weight gain and complicate obesity management, integrating an assessment of the emotional experiences and coping strategies of individuals with obesity, particularly in the context of bullying and psychosocial stressors, could provide critical insights into underlying psychological mechanisms. Therefore, this approach would foster the development of more effective, comprehensive interventions addressing both the physiological and emotional dimensions of obesity.
Despite the comprehensive coverage of current findings, this review presents several limitations: (i) the heterogeneity in intervention types, including differences in intensity, duration, and methodology, as well as variability in outcome measures; (ii) sample sizes varied substantially, ranging from small pilot studies to large cohort trials; (iii) the uneven geographic distribution of the included studies, with limited representation from developing regions; (iv) inconsistencies in adverse event reporting and inadequate follow-up in several studies; (v) difficulties in translating microbiota-targeted therapies from controlled trials into routine clinical practice, including the lack of standardized operating procedures across diverse patient populations, variability in the quality and reproducibility of biological specimens, and infrastructural constraints, especially regarding advanced therapies such as FMT; (vi) limited clinical accessibility due to specialized equipment requirements for microbiota intervention preparation, storage, and delivery; (vii) burdens on healthcare providers and systems arising from the need for customized approaches in personalized microbiome-based therapies; (viii) high costs associated with microbiome analysis, therapeutic formulation, and delivery, particularly in resource-constrained settings.
Building on these identified limitations, it is also necessary to acknowledge that the clinical translation of emerging therapies faces several critical challenges, including the development of standardized treatment protocols, the assurance of patient safety, and the confirmation of long-term efficacy. These challenges must be considered in conjunction with future research priorities, such as clarifying inter-individual variability, elucidating the still opaque mechanisms of obesity and related diseases, and integrating multi-omics data to capture complex host–microbiome interactions. Furthermore, the precise mechanisms through which microbiome-targeted interventions exert clinical effects remain incompletely understood, limiting mechanistic insight and rational optimization. A central barrier to the development of standardized treatment processes lies in the heterogeneity of research findings, which highlights the need for consistent, evidence-based clinical protocols. The absence of standardized outcome measures further complicates comparisons across studies and the synthesis of results. Addressing this issue requires the establishment of clear clinical guidelines, robust quality control frameworks, and active regulatory oversight to ensure the reproducibility and reliability of new therapeutic and diagnostic approaches. Equally important is the identification and mitigation of adverse events before widespread adoption. This necessitates an exhaustive safety assessment pipeline, incorporating preclinical toxicology studies and rigorous post-market surveillance systems, to ensure that therapies are safe for long-term use. In addition to safety, a major challenge is to demonstrate sustained therapeutic efficacy. Short-term improvements must be substantiated by evidence of durable benefits, which can only be achieved through long-term follow-up studies tracking patient outcomes, disease recurrence, and the persistence of therapeutic effects. This is particularly important in chronic, multifactorial conditions such as obesity, where treatment success depends on stability over time. In this respect, many studies have relatively short intervention periods, which restricts evaluation of long-term efficacy and safety. Emerging innovations will gradually improve the scalability and reproducibility of microbiome-based therapies. Successful implementation may demonstrate their feasibility and clinical utility. Efforts should prioritize the standardization of methodologies, reductions in costs through technological innovation, and the generation of high-quality, large-scale clinical evidence to support widespread application.
10. Conclusions
The composition of the GM is altered in obesity and characterized by reduced microbial diversity and inconsistent shifts in dominant bacterial phyla, which collectively contribute to metabolic dysregulation. The GM contributes to obesity pathogenesis through multiple mechanisms, including SCFA-mediated regulation of energy homeostasis and insulin sensitivity, LPS-induced chronic inflammation, modulation of host metabolic and appetite-regulating genes, and alterations in BA signaling via FXR. In addition, GM-driven inhibition of FIAF promotes LPL activity and fat storage in adipose tissue.
Dietary patterns exert a profound influence on GM composition and function, with plant-based diets enhancing SCFA production and conferring protective effects against obesity and its comorbidities. Prebiotics modulate GM composition by promoting beneficial bacteria, such as Bifidobacterium and A. muciniphila, which are linked to improved metabolic outcomes, reduced inflammation, and enhanced insulin sensitivity. In addition, microbiome-based therapeutics, including probiotics, synbiotics, and FMT, have demonstrated potential in modulating key metabolic and inflammatory pathways associated with obesity. However, further research is needed to fully elucidate their mechanisms and optimize clinical applications.
As the scientific understanding of the human GM continues to advance, the integration of microbiome-based therapies into standard clinical practice is poised to become increasingly feasible and therapeutically transformative, particularly for obesity, a complex condition that demands innovative and customized interventions.
Author Contributions
Conceptualization, A.B.-R. and J.J.B.; Investigation, A.B.-R. and J.J.B.; Writing—Original Draft Preparation, A.B.-R. and J.J.B.; Writing—Review and Editing, A.B.-R.; Supervision, J.J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GM | Gut microbiome |
| T2D | Type 2 diabetes mellitus |
| FMT | Fecal microbiota transplantation |
| B/B | Bacillota/Bacteroidota |
| BMI | Body mass index |
| BF | Body fat |
| WC | Waist circumference |
| VFA | Visceral fatty area |
| SCFAs | Short-chain fatty acids |
| GPRs | G-protein-coupled receptors |
| PPARs | Peroxisome proliferator-activated receptors |
| GLP-1 | Glucagon-like peptide-1 |
| GLP-2 | Glucagon-like peptide-2 |
| PYY | Peptide YY |
| AMPK | AMP-activated protein kinase pathway |
| FIAF | Fasting-induced adipose factor |
| LPL | Lipoprotein lipase |
| LPS | Lipopolysaccharide |
| LBP | LPS-binding protein |
| TLR4 | Toll-like receptor 4 |
| NF-κB | Nuclear factor-κ B |
| TNF-α | Tumor necrosis factor-α |
| LYPLAL1 | Lysophospholipase-like 1 gene |
| BAs | Bile acids |
| LTs | Liver triglycerides |
| FXR | Farnesoid X receptor |
| BW | Body weight |
| FM | Fat mass |
| LDL | Low-density lipoprotein |
| HOMA-IR | Homeostasis model assessment |
| HFD | High-fat diet |
| MDD | Major depressive disorder |
| BSH | Bile salt hydrolase |
| TβMCA | Tauro-β-muricholic acid |
| MAPK | Mitogen-activated protein kinase |
| STAT | Signal transducer and activator of transcription |
| WMT | Washed Microbiota Transplantation |
References
- Sankararaman, S.; Noriega, K.; Velayuthan, S.; Sferra, T.; Martindale, R. Gut Microbiome and Its Impact on Obesity and Obesity-Related Disorders. Curr. Gastroenterol. Rep. 2023, 25, 31–44. [Google Scholar] [CrossRef]
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar]
- Iqbal, M.; Yu, Q.; Tang, J.; Xiang, J. Unraveling the gut microbiota’s role in obesity: Key metabolites, microbial species, and therapeutic insights. J. Bacteriol. 2025, 207, e0047924. [Google Scholar] [CrossRef]
- Romieu, I.; Dossus, L.; Barquera, S.; Blottière, H.M.; Franks, P.W.; Gunter, M.; Hwalla, N.; Hursting, S.D.; Leitzmann, M.; Margetts, B.; et al. Energy balance and obesity: What are the main drivers? Cancer Causes Control 2017, 28, 247–258. [Google Scholar] [CrossRef]
- Enache, R.M.; Profir, M.; Roşu, O.A.; Creţoiu, S.M.; Gaspar, B.S. The Role of Gut Microbiota in the Onset and Progression of Obesity and Associated Comorbidities. Int. J. Mol. Sci. 2024, 25, 12321. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Ni, Q.; Sun, W.; Li, L.; Feng, X. The links between gut microbiota and obesity and obesity related diseases. Biomed. Pharmacother. 2022, 147, 112678. [Google Scholar] [CrossRef]
- Jin, X.; Qiu, T.; Li, L.; Yu, R.; Chen, X.; Li, C.; Proud, C.G.; Jiang, T. Pathophysiology of obesity and its associated diseases. Acta Pharm. Sin. B 2023, 13, 2403–2424. [Google Scholar] [CrossRef] [PubMed]
- Lavallee, K.L.; Zhang, X.C.; Schneider, S.; Margraf, J. Obesity and Mental Health: A Longitudinal, Cross-Cultural Examination in Germany and China. Front. Psychol. 2021, 12, 712567. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, M.; le Roux, C.W.; Docherty, N.G. Morbidity and mortality associated with obesity. Ann. Transl. Med. 2017, 5, 161. [Google Scholar] [CrossRef] [PubMed]
- Boulange, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef]
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef]
- Patra, D.; Banerjee, D.; Ramprasad, P.; Roy, S.; Pal, D.; Dasgupta, S. Recent insights of obesity-induced gut and adipose tissue dysbiosis in type 2 diabetes. Front. Mol. Biosci. 2023, 10, 1224982. [Google Scholar] [CrossRef] [PubMed]
- Peckmezian, T.; Garcia-Larsen, V.; Wilkins, K.; Mosli, R.H.; BinDhim, N.F.; John, G.K.; Yasir, M.; Azhar, E.I.; Mullin, G.E.; Alqahtani, S.A. Microbiome-Targeted Therapies as an Adjunct to Traditional Weight Loss Interventions: A Systematic Review and Meta-Analysis. Diabetes Metab. Syndr. Obes. 2022, 15, 3777–3798. [Google Scholar]
- Roomy, M.A.; Hussain, K.; Behbehani, H.M.; Abu-Farha, J.; Al-Harris, R.; Ambi, A.M.; Abdalla, M.A.; Al-Mulla, F.; Abu-Farha, M.; Abubaker, J. Therapeutic advances in obesity management: An overview of the therapeutic interventions. Front. Endocrinol. 2024, 15, 1364503. [Google Scholar] [CrossRef]
- Angelidi, A.M.; Belanger, M.J.; Kokkinos, A.; Koliaki, C.C.; Mantzoros, C.S. Novel Noninvasive Approaches to the Treatment of Obesity: From Pharmacotherapy to Gene Therapy. Endocr. Rev. 2022, 43, 507–557. [Google Scholar] [CrossRef]
- Williams, D.M.; Nawaz, A.; Evans, M. Drug Therapy in Obesity: A Review of Current and Emerging Treatments. Diabetes Ther. 2020, 11, 1199–1216. [Google Scholar] [CrossRef]
- Son, J.W.; Kim, S. Comprehensive Review of Current and Upcoming Anti-Obesity Drugs. Diabetes Metab. J. 2020, 44, 802–818. [Google Scholar] [CrossRef]
- Budny, A.; Janczy, A.; Szymanski, M.; Mika, A. Long-Term Follow-Up After Bariatric Surgery: Key to Successful Outcomes in Obesity Management. Nutrients 2024, 16, 4399. [Google Scholar] [CrossRef] [PubMed]
- Kushner, R.F. Weight loss strategies for treatment of obesity: Lifestyle management and pharmacotherapy. Prog. Cardiovasc. Dis. 2018, 61, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Kuder, M.M.; Nyenhuis, S.M. Optimizing lifestyle interventions in adult patients with comorbid asthma and obesity. Ther. Adv. Respir. Dis. 2020, 14, 1753466620906323. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K. Obesity: Lifestyle modification and behavior interventions. FP Essent. 2020, 492, 19–24. [Google Scholar]
- Yanovski, S.Z.; Yanovski, J.A. Approach to obesity treatment in primary care: A review. JAMA Intern. Med. 2024, 184, 818–829. [Google Scholar] [CrossRef] [PubMed]
- White, J.D.; Dewal, R.S.; Stanford, K.I. The beneficial effects of brown adipose tissue transplantation. Mol. Asp. Med. 2019, 68, 74–81. [Google Scholar] [CrossRef]
- Zhang, Z.; Mocanu, V.; Cai, C.; Dang, J.; Slater, L.; Deehan, E.C.; Walter, J.; Madsen, K.L. Impact of Fecal Microbiota Transplantation on Obesity and Metabolic Syndrome-A Systematic Review. Nutrients 2019, 11, 2291. [Google Scholar] [CrossRef] [PubMed]
- Allard, C.; Cota, D.; Quarta, C. Poly-Agonist Pharmacotherapies for Metabolic Diseases: Hopes and New Challenges. Drugs 2024, 84, 127–148. [Google Scholar] [CrossRef]
- Carvalho, L.M.; da Mota, J.C.N.L.; Ribeiro, A.A.; Carvalho, B.G.; Martinez, J.A.; Nicoletti, C.F. Precision nutrition for obesity management: A gut microbiota-centered weight-loss approach. Nutrition 2025, 140, 112892. [Google Scholar] [CrossRef]
- Huerta, T.S.; Devarajan, A.; Tsaava, T.; Rishi, A.; Cotero, V.; Puleo, C.; Ashe, J.; Coleman, T.R.; Chang, E.H.; Tracey, K.J.; et al. Targeted peripheral focused ultrasound stimulation attenuates obesity-induced metabolic and inflammatory dysfunctions. Sci. Rep. 2021, 11, 5083. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wang, Q.W.; Yang, X.Y.; Yang, W.; Li, D.R.; Jin, J.Y.; Zhang, H.C.; Zhang, X.F. GLP-1 receptor agonists for the treatment of obesity: Role as a promising approach. Front. Endocrinol. 2023, 14, 1085799. [Google Scholar] [CrossRef]
- Medina, D.A.; Li, T.; Thomson, P.; Artacho, A.; Pérez-Brocal, V.; Moya, A. Cross-Regional View of Functional and Taxonomic Microbiota Composition in Obesity and Post-Obesity Treatment Shows Country Specific Microbial Contribution. Front. Microbiol. 2019, 10, 2346. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Wang, Y.; Zhang, Q.; Zou, R.; Guo, M.; Zheng, H. Characteristics of gut microbiota in people with obesity. PLoS ONE 2021, 16, e0255446. [Google Scholar] [CrossRef]
- Kong, L.C.; Tap, J.; Aron-Wisnewsky, J.; Pelloux, V.; Basdevant, A.; Bouillot, J.L.; Zucker, J.D.; Doré, J.; Clément, K. Gut microbiota after gastric bypass in human obesity: Increased richness and associations of bacterial genera with adipose tissue genes. Am. J. Clin. Nutr. 2013, 98, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, Q.; Liu, Z.; Wang, Y.; Cao, C.; Jin, L.; Li, C.; Xiao, J.; Zhao, W. Alterations in the Gut Microbiota Composition in Obesity with and without Type 2 Diabetes: A Pilot Study. Diabetes Metab. Syndr. Obes. 2024, 17, 3965–3974. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, D.; Venkatesan, M.; Sabarathinam, S. Efficacy of microbiome-targeted interventions in obesity management—A comprehensive systematic review. Diabetes Metab. Syndr. 2025, 19, 103208. [Google Scholar]
- Dreyer, J.L.; Liebl, A.L. Early colonization of the gut microbiome and its relationship with obesity. Hum. Microbiome J. 2018, 10, 1–5. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar]
- Davis, C.D. The Gut Microbiome and Its Role in Obesity. Nutr. Today 2016, 51, 167–174. [Google Scholar] [CrossRef]
- Hu, H.J.; Park, S.G.; Jang, H.B.; Choi, M.K.; Park, K.H.; Kang, J.H.; Park, S.I.; Lee, H.J.; Cho, S.H. Obesity Alters the Microbial Community Profile in Korean Adolescents. PLoS ONE 2015, 10, e0134333. [Google Scholar]
- García-Gamboa, R.; Díaz-Torres, O.; Senés-Guerrero, C.; Gradilla-Hernández, M.S.; Moya, A.; Pérez-Brocal, V.; Gar-cia-Gonzalez, A.; González-Avila, M. Associations between bacterial and fungal communities in the human gut microbiota and their implications for nutritional status and body weight. Sci. Rep. 2024, 14, 5703. [Google Scholar] [CrossRef]
- Angelakis, E.; Armougom, F.; Million, M.; Raoult, D. The relationship between gut microbiota and weight gain in humans. Future Microbiol. 2012, 7, 91–109. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Abdallah Ismail, N.; Ragab, S.H.; Abd Elbaky, A.; Shoeib, A.R.; Alhosary, Y.; Fekry, D. Frequency of Firmicutes and Bacteroidetes in gut microbiota in obese and normal weight Egyptian children and adults. Arch. Med. Sci. 2011, 7, 501–507. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar]
- Kasai, C.; Sugimoto, K.; Moritani, I.; Tanaka, J.; Oya, Y.; Inoue, H.; Tameda, M.; Shiraki, K.; Ito, M.; Takei, Y.; et al. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 2015, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Zhu, C.; Li, H.; Yin, M.; Pan, C.; Huang, L.; Kong, C.; Wang, X.; Zhang, Y.; Qu, S.; et al. Dysbiosis Signatures of Gut Microbiota Along the Sequence from Healthy, Young Patients to Those with Overweight and Obesity. Obesity 2018, 26, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the Gut Microbiota of Adults with Obesity: A Systematic Review. Eur. J. Clin. Nutr. 2020, 74, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Moens de Hase, E.; Van Hul, M. Gut Microbiota and Host Metabolism: From Proof of Concept to Therapeutic Intervention. Microorganisms 2021, 9, 1302. [Google Scholar] [CrossRef]
- Palmas, V.; Pisanu, S.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Loviselli, A.; Manzin, A.; et al. Gut microbiota markers associated with obesity and overweight in Italian adults. Sci. Rep. 2021, 11, 5532. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, W.; Huang, W.; Lin, Y.; Chan, F.K.L.; Ng, S.C. Gut microbiota in patients with obesity and metabolic disorders—A systematic review. Genes Nutr. 2022, 17, 2. [Google Scholar]
- Yan, H.; Qin, Q.; Chen, J.; Yan, S.; Li, T.; Gao, X.; Yang, Y.; Li, A.; Ding, S. Gut Microbiome Alterations in Patients with Visceral Obesity Based on Quantitative Computed Tomography. Front. Cell. Infect. Microbiol. 2022, 11, 823262. [Google Scholar] [CrossRef]
- Tsukumo, D.M.; Carvalho, B.M.; Carvalho Filho, M.A.; Saad, M.J. Translational research into gut microbiota: New horizons on obesity treatment: Updated 2014. Arch. Endocrinol. Metab. 2015, 59, 154–160. [Google Scholar]
- Khan, M.J.; Gerasimidis, K.; Edwards, C.A.; Shaikh, M.G. Role of Gut Microbiota in the Aetiology of Obesity: Proposed Mechanisms and Review of the Literature. J. Obes. 2016, 2016, 7353642. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Kim, K.N.; Yao, Y.; Ju, S.Y. Short Chain Fatty Acids and Fecal Microbiota Abundance in Humans with Obesity: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 2512. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, S.; Nirmalkar, K.; Hoyo-Vadillo, C.; García-Espitia, M.; Ramírez-Sánchez, D.; García-Mena, J. Gut microbiome production of short-chain fatty acids and obesity in children. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 621–625. [Google Scholar] [CrossRef]
- Reynes, B.; Palou, M.; Rodriguez, A.M.; Palou, A. Regulation of Adaptive Thermogenesis and Browning by Prebiotics and Postbiotics. Front. Physiol. 2018, 9, 1908. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Warmbrunn, M.V.; Nieuwdorp, M.; Clément, K. Metabolism and Metabolic Disorders and the Microbiome: The Intestinal Microbiota Associated with Obesity, Lipid Metabolism, and Metabolic Health-Pathophysiology and Therapeutic Strategies. Gastroenterology 2021, 160, 573–599. [Google Scholar] [CrossRef] [PubMed]
- Lefterova, M.I.; Haakonsson, A.K.; Lazar, M.A.; Mandrup, S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol. Metab. 2014, 25, 293–302. [Google Scholar] [CrossRef]
- Bouter, K.; Bakker, G.J.; Levin, E.; Hartstra, A.V.; Kootte, R.S.; Udayappan, S.D.; Katiraei, S.; Bahler, L.; Gilijamse, P.W.; Tremaroli, V.; et al. Differential metabolic effects of oral butyrate treatment in lean versus metabolic syndrome subjects. Clin. Transl. Gastroenterol. 2018, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yi, C.X.; Katiraei, S.; Kooijman, S.; Zhou, E.; Chung, C.K.; Gao, Y.; van den Heuvel, J.K.; Meijer, O.C.; Berbée, J.F.P.; et al. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut 2018, 67, 1269–1279. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Pharmacogenomic and Pharmacomicrobiomic Aspects of Drugs of Abuse. Genes 2025, 16, 403. [Google Scholar] [CrossRef]
- Cuevas-Sierra, A.; Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez, J.A. Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv. Nutr. 2019, 10, S17–S30. [Google Scholar]
- Gomes, J.M.G.; Costa, J.A.; Alfenas, R.C.G. Metabolic endotoxemia and diabetes mellitus: A systematic review. Metabolism 2017, 68, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front. Immunol. 2020, 11, 571731. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Takeuchi, S.; Kubota, K.; Kobayashi, Y.; Kozakai, S.; Ukai, I.; Shichiku, A.; Okubo, M.; Numasaki, M.; Kanemitsu, Y.; et al. Lipopolysaccharide (LPS)-binding protein stimulates CD14-dependent Toll-like receptor 4 internalization and LPS-induced TBK1-IKKϵ-IRF3 axis activation. J. Biol. Chem. 2018, 293, 10186–10201. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Mandard, S.; Dray, C.; Deckert, V.; Valet, P.; Besnard, P.; Drucker, D.J.; Lagrost, L.; Grober, J. Lipopolysaccharides-mediated increase in glucose-stimulated insulin secretion: Involvement of the GLP-1 pathway. Diabetes 2014, 63, 471–482. [Google Scholar] [PubMed]
- Xiao, H.; Kang, S. The Role of the Gut Microbiome in Energy Balance with a Focus on the Gut-Adipose Tissue Axis. Front. Genet. 2020, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fu, R.; Yang, Y.; Horz, H.P.; Guan, Y.; Lu, Y.; Lou, H.; Tian, L.; Zheng, S.; Liu, H.; et al. A metagenomic approach to dissect the genetic composition of enterotypes in Han Chinese and two Muslim groups. Syst. Appl. Microbiol. 2018, 41, 1–12. [Google Scholar] [CrossRef]
- Mitev, K.; Taleski, V. Association between the Gut Microbiota and Obesity. Open Access Maced. J. Med. Sci. 2019, 7, 2050–2056. [Google Scholar] [CrossRef]
- Breton, J.; Galmiche, M.; Déchelotte, P. Dysbiotic Gut Bacteria in Obesity: An Overview of the Metabolic Mechanisms and Therapeutic Perspectives of Next-Generation Probiotics. Microorganisms 2022, 10, 452. [Google Scholar] [CrossRef]
- Jiao, Y.; Lu, Y.; Li, X.Y. Farnesoid X receptor: A master regulator of hepatic triglyceride and glucose homeostasis. Acta Pharmacol. Sin. 2015, 36, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, Y.; Dai, X.; Wang, B.; Zhang, J.; Cao, H. Polysaccharides: The Potential Prebiotics for Metabolic Associated Fatty Liver Disease (MAFLD). Nutrients 2023, 15, 3722. [Google Scholar] [CrossRef]
- Jian, Z.; Zeng, L.; Xu, T.; Sun, S.; Yan, S.; Zhao, S.; Su, Z.; Ge, C.; Zhang, Y.; Jia, J.; et al. The intestinal microbiome associated with lipid metabolism and obesity in humans and animals. J. Appl. Microbiol. 2022, 133, 2915–2930. [Google Scholar] [CrossRef] [PubMed]
- Mallick, R.; Basak, S.; Das, R.K.; Banerjee, A.; Paul, S.; Pathak, S.; Duttaroy, A.K. Fatty Acids and their Proteins in Adipose Tissue Inflammation. Cell Biochem. Biophys. 2024, 82, 35–51. [Google Scholar] [CrossRef]
- Wu, S.A.; Kersten, S.; Qi, L. Lipoprotein Lipase and Its Regulators: An Unfolding Story. Trends Endocrinol. Metab. 2021, 32, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.M.; Quigley, K.M.; Wadden, T.A. Dietary interventions for obesity: Clinical and mechanistic findings. J. Clin. Investig. 2021, 131, e140065. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Di Bella, G.; Cusumano, C.; Parisi, A.; Tagliaferri, F.; Ciriminna, S.; Barbagallo, M. Mediterranean diet in the management and prevention of obesity. Exp. Gerontol. 2023, 174, 112121. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Verde, L.; Sulu, C.; Katsiki, N.; Hassapidou, M.; Frias-Toral, E.; Cucalón, G.; Pazderska, A.; Yumuk, V.D.; Colao, A.; et al. Mediterranean diet and obesity-related disorders: What is the evidence? Curr. Obes. Rep. 2022, 11, 287–304. [Google Scholar] [CrossRef]
- Huang, R.Y.; Huang, C.C.; Hu, F.B.; Chavarro, J.E. Vegetarian diets and weight reduction: A meta-analysis of randomized controlled trials. J. Gen. Intern. Med. 2016, 31, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Turner-McGrievy, G.; Mandes, T.; Crimarco, A. A plant-based diet for overweight and obesity prevention and treatment. J. Geriatr. Cardiol. 2017, 14, 369–374. [Google Scholar]
- Zelaya, A.; Sinibaldi, E. Is vegetarianism a solution for obesity and NCDs? A review. Food Nutr. Sci. 2021, 12, 249–261. [Google Scholar] [CrossRef]
- Baylie, T.; Ayelgn, T.; Tiruneh, M.; Tesfa, K.H. Effect of ketogenic diet on obesity and other metabolic disorders: Narrative review. Diabetes Metab. Syndr. Obes. 2024, 17, 1391–1401. [Google Scholar] [CrossRef]
- Balestra, F.; Luca, M.; Panzetta, G.; Palieri, R.; Shahini, E.; Giannelli, G.; Pergola, G.; Scavo, M.P. Advancing Obesity Man-agement: The Very Low-Energy Ketogenic therapy (VLEKT) as an Evolution of the “Traditional” Ketogenic Diet. Curr. Obes. Rep. 2025, 14, 30. [Google Scholar]
- Mozaffarian, D. Dairy foods, obesity, and metabolic health: The role of the food matrix compared with single nutrients. Adv. Nutr. 2019, 10, 917S–923S. [Google Scholar] [CrossRef]
- Stonehouse, W.; Wycherley, T.; Luscombe-Marsh, N.; Taylor, P.; Brinkworth, G.; Riley, M. Dairy intake enhances body weight and composition changes during energy restriction in 18–50-year-old adults—A meta-analysis of randomized controlled trials. Nutrients 2016, 8, 394. [Google Scholar]
- Baothman, O.A.; Zamzami, M.A.; Taher, I.; Abubaker, J.; Abu-Farha, M. The role of Gut Microbiota in the development of obesity and Diabetes. Lipids Health Dis. 2016, 15, 108. [Google Scholar] [CrossRef]
- Buitinga, M.; Veeraiah, P.; Haans, F.; Schrauwen-Hinderling, V.B. Ectopic lipid deposition in muscle and liver, quantified by proton magnetic resonance spectroscopy. Obesity 2023, 31, 2447–2459. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Jones, P.H.; Jacobson, T.A.; Cohen, D.E.; Orringer, C.E.; Kothari, S.; Azagury, D.E.; Morton, J.; Nguyen, N.T.; Westman, E.C.; et al. Lipids and bariatric procedures part 1 of 2: Scientific statement from the National Lipid Association, American Society for Metabolic and Bariatric Surgery, and Obesity Medicine Association: FULL REPORT. J. Clin. Lipidol. 2016, 10, 33–57. [Google Scholar] [CrossRef] [PubMed]
- Finicelli, M.; Di Salle, A.; Galderisi, U.; Peluso, G. The Mediterranean Diet: An Update of the Clinical Trials. Nutrients 2022, 14, 2956. [Google Scholar] [CrossRef]
- Haro, C.; García-Carpintero, S.; Rangel-Zúñiga, O.A.; Alcalá-Díaz, J.F.; Landa, B.B.; Clemente, J.C.; Pérez-Martínez, P.; López-Miranda, J.; Pérez-Jiménez, F.; Camargo, A. Consumption of Two Healthy Dietary Patterns Restored Microbiota Dysbiosis in Obese Patients with Metabolic Dysfunction. Mol. Nutr. Food Res. 2017, 61, 1700300. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Human gut microbiome, diet, and mental disorders. Int. Microbiol. 2025, 28, 1–15. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Influencia de la dieta vegetariana en el microbioma intestinal humano [Influence of the vegetarian diet on the human intestinal microbiome]. Nutr. Clín. Diet. Hosp. 2024, 44, 149–157. [Google Scholar] [CrossRef]
- Cornejo-Pareja, I.; Munoz-Garach, A.; Clemente-Postigo, M.; Tinahones, F.J. Importance of gut microbiota in obesity. Eur. J. Clin. Nutr. 2019, 72, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Salazar, N.; Ponce-Alonso, M.; Garriga, M.; Sánchez-Carrillo, S.; Hernández-Barranco, A.M.; Redruello, B.; Fernández, M.; Botella-Carretero, J.I.; Vega-Piñero, B.; Galeano, J.; et al. Fecal Metabolome and Bacterial Composition in Severe Obesity: Impact of Diet and Bariatric Surgery. Gut Microbes 2022, 14, 2106102. [Google Scholar] [CrossRef]
- Smoczek, M.; Vital, M.; Wedekind, D.; Basic, M.; Zschemisch, N.H.; Pieper, D.H.; Siebert, A.; Bleich, A.; Buettner, M. A combination of genetics and microbiota influences the severity of the obesity phenotype in diet-induced obesity. Sci. Rep. 2020, 10, 6118. [Google Scholar] [CrossRef]
- Chakraborti, C.K. New-found link between microbiota and obesity. World J. Gastrointest. Pathophysiol. 2015, 6, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, Y.; Wang, X.; Yang, R.; Zhu, X.; Zhang, Y.; Chen, C.; Yuan, H.; Yang, Z.; Sun, L. Gut bacteria Akkermansia is associated with reduced risk of obesity: Evidence from the American Gut Project. Nutr. Metab. 2020, 17, 90. [Google Scholar] [CrossRef]
- Lee, Y.K. Effects of diet on gut microbiota profile and the implications for health and disease. Biosci. Microbiota Food Health 2013, 32, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Senghor, B.; Sokhna, C.; Ruimy, R.; Lagier, J.C. Gut microbiota diversity according to dietary habits and geographical provenance. Human. Microbiome J. 2018, 7–8, 1–9. [Google Scholar] [CrossRef]
- Thingholm, L.B.; Ruhlemann, M.C.; Koch, M.; Fuqua, B.; Laucke, G.; Boehm, R.; Bang, C.; Franzosa, E.A.; Hubenthal, M.; Rahnavard, A.; et al. Obese Individuals with and without Type 2 Diabetes Show Different Gut Microbial Functional Capacity and Composition. Cell Host Microbe 2019, 26, 252–264.e210. [Google Scholar] [CrossRef] [PubMed]
- Satokari, R. High Intake of Sugar and the Balance between Pro- and Anti-Inflammatory Gut Bacteria. Nutrients 2020, 12, 1348. [Google Scholar] [CrossRef] [PubMed]
- Do, M.H.; Lee, E.; Oh, M.J.; Kim, Y.; Park, H.Y. High-Glucose or -Fructose Diet Cause Changes of the Gut Microbiota and Metabolic Disorders in Mice without Body Weight Change. Nutrients 2018, 10, 761. [Google Scholar] [CrossRef]
- Christensen, L.; Vuholm, S.; Roager, H.M.; Nielsen, D.S.; Krych, L.; Kristensen, M.; Astrup, A.; Hjorth, M.F. Prevotella abundance predicts weight loss success in healthy, overweight adults consuming a whole-grain diet ad libitum: A post hoc analysis of a 6-wk randomized controlled trial. J. Nutr. 2019, 149, 2174–2181. [Google Scholar] [CrossRef]
- Wang, H.; Yu, S.; Zhao, K.; Hu, T.; Wu, Z.; Liang, H.; Lin, X.; Cui, L.; Yao, J.; Liu, X.; et al. Faecalibacterium longum alleviates high-fat diet-induced obesity and protects the intestinal epithelial barrier in mice. Gut Microbes Rep. 2025, 2, 2459590. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A. Vegetarian and ketogenic diets: Their relationship with gut microbiome and mental health, and their clinical applications. Food Nutr. Chem. 2025, 3, 278. [Google Scholar] [CrossRef]
- Crovesy, L.; Ostrowski, M.; Ferreira, D.M.T.P.; Rosado, E.L.; Soares-Mota, M. Effect of Lactobacillus on body weight and body fat in overweight subjects: A systematic review of randomized controlled clinical trials. Int. J. Obes. 2017, 41, 1607–1614. [Google Scholar] [CrossRef]
- Battineni, G.; Sagaro, G.G.; Chintalapudi, N.; Amenta, F.; Tomassoni, D.; Tayebati, S.K. Impact of obesity-induced inflammation on cardiovascular diseases (CVD). Int. J. Mol. Sci. 2021, 22, 4798. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; González-Domenech, C.M.; Borrego, J.J. The Role of Fermented Vegetables as a Sustainable and Health-Promoting Nutritional Resource. Appl. Sci. 2024, 14, 10853. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Therapeutic effects of ketogenic diets on physiological and mental health. Explor. Foods Foodomics 2025, 3, 101079. [Google Scholar] [CrossRef]
- Attaye, I.; van Oppenraaij, S.; Warmbrunn, M.V.; Nieuwdorp, M. The Role of the Gut Microbiota on the Beneficial Effects of Ketogenic Diets. Nutrients 2021, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Rew, L.; Harris, M.D.; Goldie, J. The ketogenic diet: Its impact on human gut microbiota and potential consequent health outcomes: A systematic literature review. Gastroenterol. Hepatol. Bed Bench 2022, 15, 326–342. [Google Scholar]
- Basciani, S.; Camajani, E.; Contini, S.; Persichetti, A.; Risi, R.; Bertoldi, L.; Strigari, L.; Prossomariti, G.; Watanabe, M.; Mariani, S.; et al. Very-Low-Calorie Ketogenic Diets with Whey, Vegetable, or Animal Protein in Patients with Obesity: A Randomized Pilot Study. J. Clin. Endocrinol. Metab. 2020, 105, 2939–2949. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, S.; Zhou, Y.; Yu, L.; Zhang, L.; Wang, Y. Altered gut microbiome composition in children with refractory epilepsy after ketogenic diet. Epilepsy Res. 2018, 145, 163–168. [Google Scholar] [CrossRef]
- Güzey Akansel, M.; Baş, M.; Gençalp, C.; Kahrıman, M.; Şahin, E.; Öztürk, H.; Gür, G.; Gür, C. Effects of the Ketogenic Diet on Microbiota Composition and Short-Chain Fatty Acids in Women with Overweight/Obesity. Nutrients 2024, 16, 4374. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, N.; Tan, H.Y.; Li, S.; Zhang, C.; Feng, Y. Function of Akkermansia muciniphila in Obesity: Interactions with Lipid Metabolism, Immune Response and Gut Systems. Front. Microbiol. 2020, 11, 219. [Google Scholar] [CrossRef]
- Gong, H.; Gao, H.; Ren, Q.; He, J. The abundance of Bifidobacterium in relation to visceral obesity and serum uric acid. Sci. Rep. 2022, 12, 13073. [Google Scholar] [CrossRef] [PubMed]
- Hutkins, R.W.; Krumbeck, J.A.; Bindels, L.B.; Cani, P.D.; Fahey, G., Jr.; Goh, Y.J.; Hamaker, B.; Martens, E.C.; Mills, D.A.; Rastal, R.A.; et al. Prebiotics: Why definitions matter. Curr. Opin. Biotechnol. 2016, 37, 1–7. [Google Scholar] [CrossRef]
- Ali, S.; Hamayun, M.; Siraj, M.; Khan, S.A.; Kim, H.Y.; Lee, B. Recent advances in prebiotics: Classification, mechanisms, and health applications. Future Foods 2025, 12, 100680. [Google Scholar] [CrossRef]
- Yu, M.; Yu, B.; Chen, D. The effects of gut microbiota on appetite regulation and the underlying mechanisms. Gut Microbes 2024, 16, 2414796. [Google Scholar] [CrossRef]
- Danneskiold-Samsoe, N.B.; Barros, H.D.D.Q.; Santos, R.; Bicas, J.L.; Cazarin, C.B.B.; Madsen, L.; Kristiansen, K.; Pastore, G.M.; Brix, S.; Marostica, M.R. Interplay between food and gut microbiota in health and disease. Food Res. Int. 2019, 115, 23–31. [Google Scholar] [CrossRef]
- Mills, S.; Yang, B.; Smith, G.J.; Stanton, C.; Ross, R.P. Efficacy of Bifidobacterium longum alone or in multi-strain probiotic formulations during early life and beyond. Gut Microbes 2023, 15, 2186098. [Google Scholar] [CrossRef]
- Everard, A.; Lazarevic, V.; Gaïa, N.; Johansson, M.; Ståhlman, M.; Backhed, F.; Delzenne, N.M.; Schrenzel, J.; François, P.; Cani, P.D. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J. 2014, 8, 2116–2130. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Lecourt, E.; Dewulf, E.M.; Sohet, F.M.; Pachikian, B.D.; Naslain, D.; De Backer, F.; Neyrinck, A.M.; Delzenne, N.M. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am. J. Clin. Nutr. 2009, 90, 1236–1243. [Google Scholar] [CrossRef]
- Parnell, J.A.; Reimer, R.A. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am. J. Clin. Nutr. 2009, 89, 1751–1759. [Google Scholar] [CrossRef]
- Genta, S.; Cabrera, W.; Habib, N.; Pons, J.; Carillo, I.M.; Grau, A.; Sánchez, S. Yacon syrup: Beneficial effects on obesity and insulin resistance in humans. Clin. Nutr. 2009, 28, 182–187. [Google Scholar] [CrossRef]
- Salazar, N.; Dewulf, E.M.; Neyrinck, A.M.; Bindels, L.B.; Cani, P.D.; Mahillon, J.; de Vos, W.M.; Thissen, J.P.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; et al. Inulin-type fructans modulate intestinal Bifidobacterium species populations and decrease fecal short-chain fatty acids in obese women. Clin. Nutr. 2015, 34, 501–507. [Google Scholar] [CrossRef]
- Bai, R.; Cui, F.; Li, W.; Wang, Y.; Wang, Z.; Gao, Y.; Wang, N.; Xu, Q.; Hu, F.; Zhang, Y. Codonopsis pilosula oligosaccharides modulate the gut microbiota and change serum metabolomic profiles in high-fat diet-induced obese mice. Food Funct. 2022, 13, 8143–8157. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, K.; Guo, W.; Zhang, C.; Chen, H.; Xu, T.; Lu, Y.; Wu, Q.; Li, Y.; Chen, Y. Aspergillus niger fermented Tartary buckwheat ameliorates obesity and gut microbiota dysbiosis through the NLRP3/Caspase-1 signaling pathway in high-fat diet mice. J. Funct. Foods 2022, 95, 105171. [Google Scholar] [CrossRef]
- Li, Y.; Bai, D.; Lu, Y.; Chen, J.; Yang, H.; Mu, Y.; Xu, J.; Huang, X.; Li, L. The crude guava polysaccharides ameliorate high-fat diet-induced obesity in mice via reshaping gut microbiota. Int. J. Biol. Macromol. 2022, 213, 234–246. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, L.; Ke, H.; Jiang, S.; Zeng, M. Oyster (Crassostrea gigas) polysaccharide ameliorates obesity in association with modulation of lipid metabolism and gut microbiota in high-fat diet fed mice. Int. J. Biol. Macromol. 2022, 216, 916–926. [Google Scholar] [CrossRef]
- Mo, X.; Sun, Y.; Liang, X.; Li, L.; Hu, S.; Xu, Z.; Liu, S.; Zhang, Y.; Li, X.; Liu, L. Insoluble yeast β-glucan attenuates high-fat diet-induced obesity by regulating gut microbiota and its metabolites. Carbohydr. Polym. 2022, 281, 119046. [Google Scholar] [CrossRef]
- Oh, J.K.; Vasquez, R.; Kim, S.H.; Lee, J.H.; Kim, E.J.; Hong, S.K.; Kang, D.K. Neoagarooligosaccharides modulate gut microbiota and alleviate body weight gain and metabolic syndrome in high-fat diet-induced obese rats. J. Funct. Foods 2022, 88, 104869. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, Y.; Zhou, C.; Zhao, Q.; Zhong, H.; Zhu, X.; Fu, T.; Pan, L.; Shang, Q.; Yu, G. Dietary Polysaccharide from Enteromorpha clathrata Attenuates Obesity and Increases the Intestinal Abundance of Butyrate-Producing Bacterium, Eubacterium xylanophilum, in Mice Fed a High-Fat Diet. Polymers 2021, 13, 3286. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; van der Beek, C.M.; Hermes, G.D.A.; Goossens, G.H.; Jocken, J.W.E.; Holst, J.J.; van Eijk, H.M.; Venema, K.; Smidt, H.; Zoetendal, E.G.; et al. Supplementation of Diet with Galacto-oligosaccharides Increases Bifidobacteria, but Not Insulin Sensitivity, in Obese Prediabetic Individuals. Gastroenterology 2017, 153, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Edrisi, F.; Salehi, M.; Ahmadi, A.; Fararoei, M.; Rusta, F.; Mahmoodianfard, S. Effects of supplementation with rice husk powder and rice bran on inflammatory factors in overweight and obese adults following an energy-restricted diet: A randomized controlled trial. Eur. J. Nutr. 2018, 57, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.E.; Parnell, J.A.; Tunnicliffe, J.M.; Han, J.; Sturzenegger, T.; Reimer, R.A. Consuming yellow pea fiber reduces voluntary energy intake and body fat in overweight/obese adults in a 12-week randomized controlled trial. Clin. Nutr. 2017, 36, 126–133. [Google Scholar] [CrossRef]
- Machado, A.M.; da Silva, N.B.; Chaves, J.B.P.; Rita de Cássia, G.A. Consumption of yacon flour improves body composition and intestinal function in overweight adults: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. ESPEN 2019, 29, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Neyrinck, A.M.; Rodriguez, J.; Zhang, Z.; Seethaler, B.; Sánchez, C.R.; Roumain, M.; Hiel, S.; Bindels, L.B.; Cani, P.D.; Paquot, N.; et al. Prebiotic dietary fibre intervention improves fecal markers related to inflammation in obese patients: Results from the Food4Gut randomized placebo-controlled trial. Eur. J. Nutr. 2021, 60, 3159–3170. [Google Scholar] [CrossRef]
- Nicolucci, A.C.; Hume, M.P.; Martínez, I.; Mayengbam, S.; Walter, J.; Reimer, R.A. Prebiotics Reduce Body Fat and Alter Intestinal Microbiota in Children Who Are Overweight or with Obesity. Gastroenterology 2017, 153, 711–722. [Google Scholar] [CrossRef]
- Pol, K.; de Graaf, C.; Meyer, D.; Mars, M. The efficacy of daily snack replacement with oligofructose-enriched granola bars in overweight and obese adults: A 12-week randomised controlled trial. Br. J. Nutr. 2018, 119, 1076–1086. [Google Scholar] [CrossRef]
- Reimer, R.A.; Willis, H.J.; Tunnicliffe, J.M.; Park, H.; Madsen, K.L.; Soto-Vaca, A. Inulin-type fructans and whey protein both modulate appetite but only fructans alter gut microbiota in adults with overweight/obesity: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61, 1700484. [Google Scholar] [CrossRef]
- Vaghef-Mehrabany, E.; Ranjbar, F.; Asghari-Jafarabadi, M.; Hosseinpour-Arjmand, S.; Ebrahimi-Mameghani, M. Calorie restriction in combination with prebiotic supplementation in obese women with depression: Effects on metabolic and clinical response. Nutr. Neurosci. 2021, 24, 339–353. [Google Scholar] [CrossRef] [PubMed]
- van der Beek, C.M.; Canfora, E.E.; Kip, A.M.; Gorissen, S.H.M.; Olde Damink, S.W.M.; van Eijk, H.M.; Holst, J.J.; Blaak, E.E.; Dejong, C.H.C.; Lenaerts, K. The prebiotic inulin improves substrate metabolism and promotes short-chain fatty acid production in overweight to obese men. Metabolism 2018, 87, 25–35. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, L.; Yang, L.; Chu, H. The critical role of gut microbiota in obesity. Front. Endocrinol. 2022, 13, 1025706. [Google Scholar] [CrossRef]
- Morelli, L.; Capurso, L. FAO/WHO guidelines on probiotics: 10 years later. J. Clin. Gastroenterol. 2012, 46, S1–S2. [Google Scholar] [CrossRef]
- Aboseidah, A.; Osman, M.M.; Salha, D.; Desouky, S.G.; Nehal, K. Cholesterol reduction in vitro by novel probiotic lactic acid bacterial strains of Enterococcus isolated from healthy infant’s stool. Afr. J. Microbiol. Res. 2017, 11, 1434–1444. [Google Scholar]
- Li, H.Y.; Zhou, D.D.; Gan, R.Y.; Huang, S.Y.; Zhao, C.N.; Shang, A.; Xu, X.Y.; Li, H.B. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef]
- Sumitha, D.; Aboorva, D.; Daniel, J.C.; Megala, S.; Gayathri, P. Human gut microbiota as a potential source to treat obesity. IP Int. J. Med. Microbiol. Trop. Dis. 2024, 10, 95–100. [Google Scholar]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; et al. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef]
- Zhang, J.; Mu, J.; Li, X.X.; Zhao, X. Relationship between probiotics and obesity: A review of recent research. Food Sci. Technol. 2022, 42, e30322. [Google Scholar] [CrossRef]
- Mazloom, K.; Siddiqi, I.; Covasa, M. Probiotics: How Effective Are They in the Fight against Obesity? Nutrients 2019, 11, 258. [Google Scholar] [CrossRef]
- Shirvani-Rad, S.; Tabatabaei-Malazy, O.; Mohseni, S.; Hasani-Ranjbar, S.; Soroush, A.R.; Hoseini-Tavassol, Z.; Ejtahed, H.S.; Larijani, B. Probiotics as a Complementary Therapy for Management of Obesity: A Systematic Review. Evid. Based Complement. Altern. Med. 2021, 2021, e6688450. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Kong, L.; Shan, M.; Lu, Z.; Lu, Y. Protective and ameliorating effects of probiotics against diet-induced obesity: A review. Food Res. Int. 2021, 147, 110490. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.B.; Xin, S.S.; Ding, L.N.; Ding, W.Y.; Hou, Y.L.; Liu, C.Q.; Zhang, X.D. The Potential Role of Probiotics in Controlling Overweight/Obesity and Associated Metabolic Parameters in Adults: A Systematic Review and Meta-Analysis. Evid. Based Complement. Altern. Med. 2019, 2019, 3862971. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. The microbiota-gut-brain-axis in obesity. Lancet Gastroenterol. Hepatol. 2017, 2, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Cerdo, T.; Garcia-Santos, J.A.; Bermudez, M.G.; Campoy, C. The Role of Probiotics and Prebiotics in the Prevention and Treatment of Obesity. Nutrients 2019, 11, 635. [Google Scholar] [CrossRef]
- Yarahmadi, A.; Afkhami, H.; Javadi, A.; Kashfi, M. Understanding the complex function of gut microbiota: Its impact on the pathogenesis of obesity and beyond: A comprehensive review. Diabetol. Metab. Syndr. 2024, 16, 308. [Google Scholar] [CrossRef]
- Gerard, P. Gut microbiota and obesity. Cell. Mol. Life Sci. 2016, 73, 147–162. [Google Scholar] [CrossRef]
- Sanchis-Chordà, J.; Del Pulgar, E.M.G.; Carrasco-Luna, J.; Benítez-Páez, A.; Sanz, Y.; Codoñer-Franch, P. Bifidobacterium pseudocatenulatum CECT 7765 supplementation improves inflammatory status in insulin-resistant obese children. Eur. J. Nutr. 2019, 58, 2789–2800. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-ofconcept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef]
- Indiani, C.M.D.S.P.; Rizzardi, K.F.; Castelo, P.M.; Ferraz, L.F.C.; Darrieux, M.; Parisotto, T.M. Childhood Obesity and Firmicutes/Bacteroidetes Ratio in the Gut Microbiota: A Systematic Review. Child. Obes. 2018, 14, 501–509. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Neculae, E.; Costea, C.F.; Ciocoiu, M.; Hurjui, L.L.; Tarniceriu, C.C.; Maranduca, M.A.; Lacatusu, C.M.; Floria, M.; et al. Role of Gut Microbiota on Onset and Progression of Microvascular Complications of Type 2 Diabetes (T2DM). Nutrients 2020, 12, 3719. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan Pillai, S.; Gagnon, C.A.; Foster, C.; Ashraf, A.P. Exploring the Gut Microbiota: Key Insights Into Its Role in Obesity, Metabolic Syndrome, and Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2024, 109, 2709–2719. [Google Scholar] [CrossRef]
- Dror, T.; Dickstein, Y.; Dubourg, G.; Paul, M. Microbiota manipulation for weight change. Microb. Pathog. 2017, 106, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Karlsson Videhult, F.; Andersson, Y.; Öhlund, I.; Stenlund, H.; Hernell, O.; West, C.E. Impact of probiotics during weaning on the metabolic and inflammatory profile: Follow-up at school age. Int. J. Food Sci. Nutr. 2015, 66, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Darb Emamie, A.; Rajabpour, M.; Ghanavati, R.; Asadolahi, P.; Farzi, S.; Sobouti, B.; Darbandi, A. The effects of probiotics, prebiotics and synbiotics on the reduction of IBD complications, a periodic review during 2009–2020. J. Appl. Microbiol. 2021, 130, 1823–1838. [Google Scholar] [CrossRef]
- Zhang, Y.; Hong, J.; Zhang, Y.; Gao, Y.; Liang, L. The effects of synbiotics surpass prebiotics in improving inflammatory biomarkers in children and adults: A systematic review, meta-analysis, and meta-evidence of data from 5207 participants in 90 randomized controlled trials. Pharmacol. Res. 2025, 218, 107832. [Google Scholar] [CrossRef]
- Shan, L.; Tyagi, A.; Shabbir, U.; Chen, X.; Vijayalakshmi, S.; Yan, P.; Oh, D.H. The Role of Gut Microbiota Modulation Strategies in Obesity: The Applications and Mechanisms. Fermentation 2022, 8, 376. [Google Scholar] [CrossRef]
- Rajkumar, H.; Mahmood, N.; Kumar, M.; Varikuti, S.R.; Challa, H.R.; Myakala, S.P. Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: A randomized, controlled trial. Mediators Inflamm. 2014, 2014, 348959. [Google Scholar]
- Banach, K.; Glibowski, P.; Jedut, P. The Effect of Probiotic Yogurt Containing Lactobacillus acidophilus LA-5 and Bifidobacterium lactis BB-12 on Selected Anthropometric Parameters in Obese Individuals on an Energy-Restricted Diet: A Randomized, Controlled Trial. Appl. Sci. 2020, 10, 17. [Google Scholar] [CrossRef]
- Kim, J.; Yun, J.M.; Kim, M.K.; Kwon, O.; Cho, B. Lactobacillus gasseri BNR17 supplementation reduces the visceral fat accumulation and waist circumference in obese adults: A randomized, double-blind, placebo-controlled trial. J. Med. Food. 2018, 21, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Minami, J.; Iwabuchi, N.; Tanaka, M.; Yamauchi, K.; Xiao, J.Z.; Abe, F.; Sakane, N. Effects of Bifidobacterium breve B-3 on body fat reductions in pre-obese adults: A randomized, double-blind, placebo-controlled trial. Biosci. Microbiota Food Health 2018, 37, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Narmaki, E.; Borazjani, M.; Ataie-Jafari, A.; Hariri, N.; Doost, A.H.; Qorbani, M.; Saidpour, A. The combined effects of probiotics and restricted calorie diet on the anthropometric indices, eating behavior, and hormone levels of obese women with food addiction: A randomized clinical trial. Nutr. Neurosci. 2020, 25, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Razmpoosh, E.; Zare, S.; Fallahzadeh, H.; Safi, S.; Nadjarzadeh, A. Effect of a low energy diet, containing a high protein, probiotic condensed yogurt, on biochemical and anthropometric measurements among women with overweight/obesity: A randomised controlled trial. Clin. Nutr. ESPEN 2020, 35, 194–200. [Google Scholar] [CrossRef]
- Zarrati, M.; Raji Lahiji, M.; Salehi, E.; Yazdani, B.; Razmpoosh, E.; Shokouhi Shoormasti, R.; Shidfar, F. Effects of Probiotic Yogurt on Serum Omentin-1, Adropin, and Nesfatin-1 Concentrations in Overweight and Obese Participants Under Low-Calorie Diet. Probiotics Antimicrob. Proteins 2019, 11, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Angelino, D.; Martina, A.; Rosi, A.; Veronesi, L.; Antonini, M.; Mennella, I.; Vitaglione, P.; Grioni, S.; Brighenti, F.; Zavaroni, I.; et al. Glucose- and Lipid-Related Biomarkers Are Affected in Healthy Obese or Hyperglycemic Adults Consuming a Whole-Grain Pasta Enriched in Prebiotics and Probiotics: A 12-Week Randomized Controlled Trial. J. Nutr. 2019, 149, 1714–1723. [Google Scholar] [CrossRef]
- Gutiérrez-Repiso, C.; Hernández-García, C.; García-Almeida, J.M.; Bellido, D.; Martín-Núñez, G.M.; Sánchez-Alcoholado, L.; Alcaide-Torres, J.; Sajoux, I.; Tinahones, F.J.; Moreno-Indias, I. Effect of Synbiotic Supplementation in a Very-Low-Calorie Ketogenic Diet on Weight Loss Achievement and Gut Microbiota: A Randomized Controlled Pilot Study. Mol. Nutr. Food Res. 2019, 63, e1900167. [Google Scholar] [CrossRef]
- Janczy, A.; Aleksandrowicz-Wrona, E.; Kochan, Z.; Malgorzewicz, S. Impact of diet and synbiotics on selected gut bacteria and intestinal permeability in individuals with excess body weight—A prospective, randomized study. Acta Biochim. Pol. 2020, 67, 571–578. [Google Scholar] [CrossRef]
- Kanazawa, A.; Aida, M.; Yoshida, Y.; Kaga, H.; Katahira, T.; Suzuki, L.; Tamaki, S.; Sato, J.; Goto, H.; Azuma, K.; et al. Effects of Synbiotic Supplementation on Chronic Inflammation and the Gut Microbiota in Obese Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Study. Nutrients 2021, 13, 558. [Google Scholar] [CrossRef] [PubMed]
- Kassaian, N.; Feizi, A.; Aminorroaya, A.; Jafari, P.; Ebrahimi, M.T.; Amini, M. The effects of probiotics and synbiotic supplementation on glucose and insulin metabolism in adults with prediabetes: A double-blind randomized clinical trial. Acta Diabetol. 2018, 55, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Krumbeck, J.A.; Rasmussen, H.E.; Hutkins, R.W.; Clarke, J.; Shawron, K.; Keshavarzian, A.; Walter, J. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome 2018, 6, 121. [Google Scholar] [CrossRef]
- Mohammadi-Sartang, M.; Mazloomi, S.M.; Fararouie, M.; Bedeltavana, A.; Famouri, M.; Mazloom, Z. Daily fortified-synbiotic yogurt consumption facilitates appetite control in overweight and obese adults with metabolic syndrome during a weight-loss program: A 10-week randomized controlled trial. Prog. Nutr. 2019, 21, 135–144. [Google Scholar]
- Perraudeau, F.; McMurdie, P.; Bullard, J.; Cheng, A.; Cutcliffe, C.; Deo, A.; Eid, J.; Gines, J.; Iyer, M.; Justice, N.; et al. Improvements to postprandial glucose control in subjects with type 2 diabetes: A multicenter, double blind, randomized placebo-controlled trial of a novel probiotic formulation. BMJ Open Diabetes Res. Care 2020, 8, e001319. [Google Scholar] [CrossRef]
- Sergeev, I.N.; Aljutaily, T.; Walton, G.; Huarte, E. Effects of Synbiotic Supplement on Human Gut Microbiota, Body Composition and Weight Loss in Obesity. Nutrients 2020, 12, 222. [Google Scholar] [CrossRef]
- Xavier-Santos, D.; Lima, E.D.; Simao, A.N.C.; Bedani, R.; Saad, I.; Marta, S. Effect of the consumption of a synbiotic diet mousse containing Lactobacillus acidophilus La-5 by individuals with metabolic syndrome: A randomized controlled trial. J. Funct. Foods 2018, 41, 55–61. [Google Scholar] [CrossRef]
- Kelly, C.R.; Kahn, S.; Kashyap, P.; Laine, L.; Rubin, D.; Atreja, A.; Moore, T.; Wu, G. Update on Fecal Microbiota Transplantation 2015: Indications, Methodologies, Mechanisms, and Outlook. Gastroenterology 2015, 149, 223–237. [Google Scholar] [CrossRef]
- Li, S.S.; Zhu, A.; Benes, V.; Costea, P.I.; Hercog, R.; Hildebrand, F.; Huerta-Cepas, J.; Nieuwdorp, M.; Salojärvi, J.; Voigt, A.Y.; et al. Durable coexistence of donor and recipient strains after fecal microbiota transplantation. Science 2016, 352, 586–589. [Google Scholar] [CrossRef]
- Zhang, F.; Cui, B.; He, X.; Nie, Y.; Wu, K.; Fan, D.; FMT-standardization Study Group. Microbiota transplantation: Concept, methodology and strategy for its modernization. Protein Cell 2018, 9, 462–473. [Google Scholar] [CrossRef]
- Hemachandra, S.; Rathnayake, S.N.; Jayamaha, A.A.; Francis, B.S.; Welmillage, D.; Kaur, D.N.; Zaw, H.K.; Zaw, L.T.; Chandra, H.A.; Abeysekera, M.E. Fecal Microbiota Transplantation as an Alternative Method in the Treatment of Obesity. Cureus 2025, 17, e76858. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Clément, K.; Nieuwdorp, M. Fecal Microbiota Transplantation: A Future Therapeutic Option for Obesity/Diabetes? Curr. Diab Rep. 2019, 19, 51. [Google Scholar] [CrossRef]
- Kootte, R.S.; Levin, E.; Salojärvi, J.; Smits, L.P.; Hartstra, A.V.; Udayappan, S.D.; Hermes, G.; Bouter, K.E.; Koopen, A.M.; Holst, J.J.; et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metabol. 2017, 26, 611–619.e6. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Cai, Y. Gut microbiota and obesity: Implications for fecal microbiota transplantation therapy. Hormones 2017, 16, 223–234. [Google Scholar] [CrossRef]
- Leong, K.S.W.; Jayasinghe, T.N.; Wilson, B.C.; Derraik, J.G.B.; Albert, B.B.; Chiavaroli, V.; Svirskis, D.M.; Beck, K.L.; Conlon, C.A.; Jiang, Y.; et al. Effects of Fecal Microbiome Transfer in Adolescents with Obesity: The Gut Bugs Randomized Controlled Trial. JAMA Netw. Open 2020, 3, e2030415. [Google Scholar] [CrossRef]
- Zecheng, L.; Donghai, L.; Runchuan, G.; Yuan, Q.; Qi, J.; Yijia, Z.; Shuaman, R.; Xiaoqi, L.; Yi, W.; Ni, M.; et al. Fecal microbiota transplantation in obesity metabolism: A meta-analysis and systematic review. Diabetes Res. Clin. Pract. 2023, 202, 110803. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, J.R.; Kassam, Z.; Mullish, B.H.; Chiang, A.; Carrellas, M.; Hurtado, J.; Marchesi, J.R.; McDonald, J.A.K.; Pechlivanis, A.; Barker, G.F.; et al. Effects of Fecal Microbiota Transplantation with Oral Capsules in Obese Patients. Clin. Gastroenterol. Hepatol. 2020, 18, 855–863.e2. [Google Scholar] [CrossRef]
- Yu, E.W.; Gao, L.; Stastka, P.; Cheney, M.C.; Mahabamunuge, J.; Torres Soto, M.; Ford, C.B.; Bryant, J.A.; Henn, M.R.; Hohmann, E.L. Fecal microbiota transplantation for the improvement of metabolism in obesity: The FMT-TRIM double-blind placebo-controlled pilot trial. PLoS Med. 2020, 17, e1003051. [Google Scholar] [CrossRef]
- Hartstra, A.V.; Schüppel, V.; Imangaliyev, S.; Schrantee, A.; Prodan, A.; Collard, D.; Levin, E.; Dallinga-Thie, G.; Ackermans, M.T.; Winkelmeijer, M.; et al. Infusion of donor feces affects the gut-brain axis in humans with metabolic syndrome. Mol. Metab. 2020, 42, 101076. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Lahtinen, P.; Juuti, A.; Luostarinen, M.; Niskanen, L.; Liukkonen, T.; Tillonen, J.; Kössi, J.; Ilvesmäki, V.; Viljakka, M.; Satokari, R.; et al. Effectiveness of Fecal Microbiota Transplantation for Weight Loss in Patients with Obesity Undergoing Bariatric Surgery: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2247226. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.; Sandhu, K.V.; Bastiaanssen, T.F.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Fecal Microbiota Transplantation as a Tool for Therapeutic Modulation of Neurological and Mental Disorders. SciBase Neurol. 2024, 2, 1018. [Google Scholar] [CrossRef]
- Sánchez-Garrido, M.A.; Brandt, S.J.; Clemmensen, C.; Müller, T.D.; DiMarchi, R.D.; Tschöp, M.H. GLP-1/glucagon receptor co-agonism for treatment of obesity. Diabetologia 2017, 60, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Sagredo Pérez, J.; Allo Miguel, G. Tratamiento farmacológico de la obesidad. Situación actual y nuevos tratamientos [Pharmacological treatment of obesity. Current situation and new treatments]. Aten. Primaria 2025, 57, 103074. [Google Scholar] [CrossRef] [PubMed]
- Žižka, O.; Haluzík, M.; Jude, E.B. Pharmacological treatment of obesity in older adults. Drugs Aging 2024, 41, 881–896. [Google Scholar] [CrossRef]
- Kosmalski, M.; Deska, K.; Bąk, B.; Różycka-Kosmalska, M.; Pietras, T. Pharmacological support for the treatment of obesity—Present and future. Healthcare 2023, 11, 433. [Google Scholar] [CrossRef]
- Reijnders, D.; Goossens, G.H.; Hermes, G.D.; Neis, E.P.; van der Beek, C.M.; Most, J.; Holst, J.J.; Lenaerts, K.; Kootte, R.S.; Nieuwdorp, M.; et al. Effects of Gut Microbiota Manipulation by Antibiotics on Host Metabolism in Obese Humans: A Randomized Double-Blind Placebo-Controlled Trial. Cell Metab. 2016, 24, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, A.; Out, C.; Fuentes, S.; Jonker, L.; Reuling, I.; Kootte, R.S.; van Nood, E.; Holleman, F.; Knaapen, M.; Romijn, J.A.; et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J. Hepatol. 2014, 60, 824–831. [Google Scholar] [CrossRef]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef]
- Alzahrani, A.M.; Alshobragi, G.A.; Alshehri, A.M.; Alzahrani, M.S.; Alshehri, H.A.; Alzhrani, R.M.; Basudan, S.; Alkatheeri, A.A.; Almutairi, S.A.; Alzahrani, Y.A. Molecular Pharmacology of Glucagon-Like Peptide 1-Based Therapies in the Man-agement of Type Two Diabetes Mellitus and Obesity. Integr. Pharm. Res. Pract. 2025, 14, 59–72. [Google Scholar]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like peptide-1 receptor: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 234. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Zheng, G.; Liang, Z.; Li, M.; Deng, W.; Cao, W. Seven glucagon-like peptide-1 receptor agonists and polyagonists for weight loss in patients with obesity or overweight: An updated systematic review and network meta-analysis of randomized controlled trials. Metabolism 2024, 161, 156038. [Google Scholar] [CrossRef] [PubMed]
- Mabey, J.G.; Chaston, J.M.; Castro, D.G.; Adams, T.D.; Hunt, S.C.; Davidson, L.E. Gut microbiota differs a decade after bariatric surgery relative to a nonsurgical comparison group. Surg. Obes. Relat. Dis. 2020, 16, 1304–1311. [Google Scholar] [CrossRef]
- Juárez-Fernández, M.; Román-Saguillo, S.; Porras, D.; García-Mediavilla, M.V.; Linares, P.; Ballesteros-Pomar, M.D.; Urioste-Fondo, A.; Álvarez-Cuenllas, B.; González-Gallego, J.; Sánchez-Campos, S.; et al. Long-Term Effects of Bariatric Surgery on Gut Microbiota Composition and Faecal Metabolome Related to Obesity Remission. Nutrients 2021, 13, 2519. [Google Scholar] [CrossRef]
- Neff, K.J.; Baud, G.; Raverdy, V.; Caiazzo, R.; Verkindt, H.; Noel, C.; le Roux, C.W.; Pattou, F. Renal Function and Remission of Hypertension After Bariatric Surgery: A 5-Year Prospective Cohort Study. Obes. Surg. 2017, 27, 613–619. [Google Scholar] [CrossRef]
- Rajabi, M.R.; Rezaei, M.; Abdollahi, A.; Gholi, Z.; Mokhber, S.; Mohammadi-Farsani, G.; Abdoli, D.; Mousavi, S.D.; Amini, H.; Ghandchi, M. Long-term systemic effects of metabolic bariatric surgery: A multidisciplinary perspective. Heliyon 2024, 10, e34339. [Google Scholar] [CrossRef]
- Debédat, J.; Clément, K.; Aron-Wisnewsky, J. Gut Microbiota Dysbiosis in Human Obesity: Impact of Bariatric Surgery. Curr. Obes. Rep. 2019, 8, 229–242. [Google Scholar] [CrossRef]
- Ilhan, Z.E.; DiBaise, J.K.; Dautel, S.E.; Isern, N.G.; Kim, Y.M.; Hoyt, D.W.; Schepmoes, A.A.; Brewer, H.M.; Weitz, K.K.; Metz, T.O.; et al. Temporospatial shifts in the human gut microbiome and metabolome after gastric bypass surgery. NPJ Biofilms Microbiomes 2020, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Shu, X.O.; Howard, E.F.; Long, J.; English, W.J.; Flynn, C.R. Fecal metagenomics and metabolomics reveal gut microbial changes after bariatric surgery. Surg. Obes. Relat. Dis. 2020, 16, 1772–1782. [Google Scholar] [CrossRef]
- Medina, D.A.; Pedreros, J.P.; Turiel, D.; Quezada, N.; Pimentel, F.; Escalona, A.; Garrido, D. Distinct patterns in the gut microbiota after surgical or medical therapy in obese patients. PeerJ 2017, 5, e3443. [Google Scholar] [CrossRef] [PubMed]
- Arufe-Giráldez, V.; Pereira Loureiro, J.; Groba González, M.B.; Nieto Riveiro, L.; Canosa Domínguez, N.M.; Miranda-Duro, M.D.C.; Concheiro Moscoso, P.; Rodríguez-Padín, R.; Roibal Pravio, J.; Lagos Rodríguez, M.; et al. Multi-Context Strategies and Opportunities for Increasing Levels of Physical Activity in Children and Young People: A Literature Review. Children 2024, 11, 1475. [Google Scholar] [CrossRef] [PubMed]
- Oppert, J.M.; Bellicha, A.; van Baak, M.A.; Battista, F.; Beaulieu, K.; Blundell, J.E.; Carraça, E.V.; Encantado, J.; Ermolao, A.; Pramono, A.; et al. Exercise training in the management of overweight and obesity in adults: Synthesis of the evidence and recommendations from the European Association for the Study of Obesity Physical Activity Working Group. Obes. Rev. 2021, 22, e13273. [Google Scholar] [CrossRef]
- Aperman-Itzhak, T.; Yom-Tov, A.; Vered, Z.; Waysberg, R.; Livne, I.; Eilat-Adar, S. School-based intervention to promote a healthy lifestyle and obesity prevention among fifth- and sixth-grade children. Am. J. Health Educ. 2018, 49, 289–295. [Google Scholar] [CrossRef]
- Lynch, B.A.; Gentile, N.; Maxson, J.; Quigg, S.; Swenson, L.; Kaufman, T. Elementary school-based obesity intervention using an educational curriculum. J. Prim. Care Community Health 2016, 7, 265–271. [Google Scholar] [CrossRef]
- Hollis, J.L.; Sutherland, R.; Campbell, L.; Morgan, P.J.; Lubans, D.R.; Nathan, N.; Wolfenden, L.; Okely, A.D.; Davies, L.; Williams, A.; et al. Effects of a ‘school-based’ physical activity intervention on adiposity in adolescents from economically disadvantaged communities: Secondary outcomes of the ‘Physical Activity 4 Everyone’ RCT. Int. J. Obes. 2016, 40, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Bhave, S.; Pandit, A.; Yeravdekar, R.; Madkaikar, V.; Chinchwade, T.; Shaikh, N.; Shaikh, T.; Naik, S.; Marley-Zagar, E.; Fall, C.H.D. Effectiveness of a 5-year school-based intervention programme to reduce adiposity and improve fitness and lifestyle in Indian children; the SYM-KEM study. Arch. Dis. Child. 2016, 101, 33–40. [Google Scholar] [CrossRef]
- Seo, Y.G.; Lim, H.; Kim, Y.; Ju, Y.S.; Lee, H.J.; Jang, H.B.; Park, S.I.; Park, K.H. The effect of a multidisciplinary lifestyle intervention on obesity status, body composition, physical fitness, and cardiometabolic risk markers in children and adolescents with obesity. Nutrients 2019, 11, 137. [Google Scholar] [CrossRef]
- Meng, C.; Yucheng, T.; Shu, L.; Yu, Z. Effects of school-based high-intensity interval training on body composition, cardiorespiratory fitness and cardiometabolic markers in adolescent boys with obesity: A randomized controlled trial. BMC Pediatr. 2022, 22, 112. [Google Scholar] [CrossRef]
- Sánchez-López, A.M.; Menor-Rodríguez, M.J.; Sánchez-García, J.C.; Aguilar-Cordero, M.J. Play as a method to reduce overweight and obesity in children: An RCT. Int. J. Environ. Res. Public. Health 2020, 17, 346. [Google Scholar] [CrossRef]
- Fang, Y.; Ma, Y.; Mo, D.; Zhang, S.; Xiang, M.; Zhang, Z. Methodology of an exercise intervention program using social incentives and gamification for obese children. BMC Public. Health 2019, 19, 686. [Google Scholar] [CrossRef]
- Yuksel, H.S.; Şahin, F.N.; Maksimovic, N.; Drid, P.; Bianco, A. School-based intervention programs for preventing obesity and promoting physical activity and fitness: A systematic review. Int. J. Environ. Res. Public. Health 2020, 17, 347. [Google Scholar] [CrossRef]
- Martínez-Vizcaíno, V.; Fernández-Rodríguez, R.; Reina-Gutiérrez, S.; Rodríguez-Gutiérrez, E.; Garrido-Miguel, M.; Núñez de Arenas-Arroyo, S.; Torres-Costoso, A. Physical activity is associated with lower mortality in adults with obesity: A systematic review with meta-analysis. BMC Public. Health 2024, 24, 1867. [Google Scholar] [CrossRef]
- Pojednic, R.; D’Arpino, E.; Halliday, I.; Bantham, A. The benefits of physical activity for people with obesity, independent of weight loss: A systematic review. Int. J. Environ. Res. Public. Health 2022, 19, 4981. [Google Scholar] [CrossRef] [PubMed]
- Raiman, L.; Amarnani, R.; Abdur-Rahman, M.; Marshall, A.; Mani-Babu, S. The role of physical activity in obesity: Let’s actively manage obesity. Clin. Med. 2023, 23, 311–317. [Google Scholar] [CrossRef] [PubMed]
- van Baak, M.A.; Pramono, A.; Battista, F.; Beaulieu, K.; Blundell, J.E.; Busetto, L.; Carraça, E.V.; Dicker, D.; Encantado, J.; Ermolao, A.; et al. Effect of different types of regular exercise on physical fitness in adults with overweight or obesity: Systematic review and meta-analyses. Obes. Rev. 2021, 22, e13239. [Google Scholar] [CrossRef]
- Curran, F.; Davis, M.E.; Murphy, K.; Tersigni, N.; King, A.; Ngo, N.; O’Donoghue, G. Correlates of physical activity and sedentary behavior in adults living with overweight and obesity: A systematic review. Obes. Rev. 2023, 24, e13615. [Google Scholar] [CrossRef] [PubMed]
- Domin, A.; Spruijt-Metz, D.; Theisen, D.; Ouzzahra, Y.; Vögele, C. Smartphone-Based Interventions for Physical Activity Promotion: Scoping Review of the Evidence Over the Last 10 Years. JMIR mHealth uHealth 2021, 9, e24308. [Google Scholar] [CrossRef]
- Sousa Basto, P.; Ferreira, P. Mobile applications, physical activity, and health promotion. BMC Health Serv. Res. 2025, 25, 359. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Pérez, D.; Bressa, C.; Bailén, M.; Hamed-Bousdar, S.; Naclerio, F.; Carmona, M.; Pérez, M.; González-Soltero, R.; Montalvo-Lominchar, M.G.; Carabaña, C.; et al. Effect of a Protein Supplement on the Gut Microbiota of Endurance Athletes: A Randomized, Controlled, Double-Blind Pilot Study. Nutrients 2018, 10, 337. [Google Scholar] [CrossRef]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef]
- Motiani, K.K.; Collado, M.C.; Eskelinen, J.J.; Virtanen, K.A.; Löyttyniemi, E.; Salminen, S.; Nuutila, P.; Kalliokoski, K.K.; Hannukainen, J.C. Exercise Training Modulates Gut Microbiota Profile and Improves Endotoxemia. Med. Sci. Sports Exerc. 2020, 52, 94–104. [Google Scholar] [CrossRef]
- Butryn, M.L.; Webb, V.; Wadden, T.A. Behavioral treatment of obesity. Psychiatr. Clin. N. Am. 2011, 34, 841–859. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulou, M.; Ferrey, A.E.; Harmer, G.; Goddard, L.; Kebbe, M.; Theodoulou, A.; Jebb, S.A.; Aveyard, P. Effectiveness of motivational interviewing in managing overweight and obesity: A systematic review and meta-analysis. Ann. Intern. Med. 2022, 175, 838–850. [Google Scholar] [CrossRef]
- Kurnik Mesarič, K.; Pajek, J.; Logar Zakrajšek, B.; Bogataj, Š.; Kodrič, J. Cognitive behavioral therapy for lifestyle changes in patients with obesity and type 2 diabetes: A systematic review and meta-analysis. Sci. Rep. 2023, 13, 12793. [Google Scholar] [CrossRef]
- Moraes, A.D.S.; Padovani, R.D.C.; La Scala Teixeira, C.V.; Cuesta, M.G.S.; Gil, S.D.S.; de Paula, B.; Dos Santos, G.M.; Gonçalves, R.T.; Dâmaso, A.R.; Oyama, L.M.; et al. Cognitive behavioral approach to treat obesity: A randomized clinical trial. Front. Nutr. 2021, 8, 611217. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. An updated overview on the relationship between human gut microbiome dysbiosis and psychiatric and psychological disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 128, 110861. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, J.; Dai, H.; Duan, Y.; An, Y.; Shi, L.; Lv, Y.; Li, H.; Wang, C.; Ma, Q.; et al. Brown and beige adipose tissue: A novel therapeutic strategy for obesity and type 2 diabetes mellitus. Adipocyte 2021, 10, 48–65. [Google Scholar] [CrossRef]
- Li, G.; Xie, C.; Lu, S.; Nichols, R.G.; Tian, Y.; Li, L.; Patel, D.; Ma, Y.; Brocker, C.N.; Yan, T.; et al. Intermittent Fasting Promotes White Adipose Browning and Decreases Obesity by Shaping the Gut Microbiota. Cell Metab. 2017, 26, 672–685.e674. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Song, Y.; Xie, H.; Dong, M. An update on brown adipose tissue and obesity intervention: Function, regulation and therapeutic implications. Front. Endocrinol. 2023, 13, 1065263. [Google Scholar] [CrossRef]
- Voruganti, V.S. Precision Nutrition: Recent Advances in Obesity. Physiology 2023, 38, 42–50. [Google Scholar] [CrossRef]
- Horne, J.R.; Gilliland, J.A.; O’Connor, C.P.; Seabrook, J.A.; Madill, J. Change in Weight, BMI, and Body Composition in a Population-Based Intervention Versus Genetic-Based Intervention: The NOW Trial. Obesity 2020, 28, 1419–1427. [Google Scholar] [CrossRef]
- Arkadianos, I.; Valdes, A.M.; Marinos, E.; Florou, A.; Gill, R.D.; Grimaldi, K.A. Improved weight management using genetic information to personalize a calorie controlled diet. Nutr. J. 2007, 6, 29. [Google Scholar] [CrossRef]
- Gruber, T.; Lechner, F.; Krieger, J.P.; García-Cáceres, C. Neuroendocrine gut-brain signaling in obesity. Trends Endocrinol. Metab. 2025, 36, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Kang, L.; Li, J.; Long, Y.; Wei, H.; Ferreira, C.A.; Jeffery, J.J.; Lin, Y.; Cai, W.; Wang, X. Effective weight control via an implanted self-powered vagus nerve stimulation device. Nat. Commun. 2018, 9, 5349. [Google Scholar] [CrossRef] [PubMed]
- Deschasaux, M.; Bouter, K.E.; Prodan, A.; Levin, E.; Groen, A.K.; Herrema, H. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat. Med. 2018, 24, 1526–1531. [Google Scholar] [CrossRef]
- Hughes, R.L.; Marco, M.L.; Hughes, J.P.; Keim, N.L.; Kable, M.E. The role of the gut microbiome in predicting response to diet and the development of precision nutrition models-Part I: Overview of current methods. Adv. Nutr. 2019, 10, 953–978. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, M.F.; Roager, H.M.; Larsen, T.M.; Poulsen, S.K.; Licht, T.R.; Bahl, M.I.; Zohar, Y.; Astrup, A. Pre-treatment microbial Prevotella-to-Bacteroides ratio determines body fat loss success during a 6-month randomized controlled diet intervention. Int. J. Obes. 2018, 42, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.L.; Kable, M.E.; Marco, M.; Keim, N.L. The role of the gut microbiome in predicting response to diet and the development of precision nutrition models. Part II: Results. Adv. Nutr. 2019, 10, 979–998. [Google Scholar] [CrossRef]
- Tebani, A.; Bekri, S. Paving the way to precision nutrition through metabolomics. Front. Nutr. 2019, 6, 41. [Google Scholar] [CrossRef]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized nutrition by prediction of glycemic responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef]
- Hartsoe, P.; Holguin, F.; Chu, H.W. Mitochondrial Dysfunction and Metabolic Reprogramming in Obesity and Asthma. Int. J. Mol. Sci. 2024, 25, 2944. [Google Scholar] [CrossRef]
- Jayashankar, V.; Selwan, E.; Hancock, S.E.; Verlande, A.; Goodson, M.O.; Eckenstein, K.H.; Milinkeviciute, G.; Hoover, B.M.; Chen, B.; Fleischman, A.G.; et al. Drug-like sphingolipid SH-BC-893 opposes ceramide-induced mitochondrial fission and corrects diet-induced obesity. EMBO Mol. Med. 2021, 13, e13086. [Google Scholar] [CrossRef]
- Calcaterra, V.; Rossi, V.; Magenes, V.C.; Baldassarre, P.; Grazi, R.; Loiodice, M.; Fabiano, V.; Zuccotti, G. Dietary habits, depression and obesity: An intricate relationship to explore in pediatric preventive strategies. Front. Pediatr. 2024, 12, 1368283. [Google Scholar] [CrossRef] [PubMed]
- Rindler, G.A.; Gries, A.; Freidl, W. Associations between overweight, obesity, and mental health: A retrospective study among European adults aged 50. Front. Public Health 2023, 11, 1206283. [Google Scholar] [CrossRef]
- Steptoe, A.; Frank, P. Obesity and psychological distress. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2023, 378, 20220225. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Nutritional psychiatry: A novel approach to the treatment of mental health disorders. Actas Esp. Psiquiatr. 2025, 53, 443–445. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Psychobiotics: A new perspective on the treatment of stress, anxiety, and depression. Ansiedad Estrés 2024, 30, 79–93. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Microbial therapeutic tools for human brain disorders: A current overview. Brain Disord. 2025, 19, 100262. [Google Scholar] [CrossRef]
- Westbury, S.; Oyebode, O.; van Rens, T.; Barber, T.M. Obesity stigma: Causes, consequences, and potential solutions. Curr. Obes. Rep. 2023, 12, 10–23. [Google Scholar] [CrossRef]
- Phelan, S.M.; Burgess, D.J.; Yeazel, M.W.; Hellerstedt, W.L.; Griffin, J.M.; van Ryn, M. Impact of weight bias and stigma on quality of care and outcomes for patients with obesity. Obes. Rev. 2015, 16, 319–326. [Google Scholar] [CrossRef]
- Sánchez, E.; Ciudin, A.; Sánchez, A.; Gutiérrez-Medina, S.; Valdés, N.; Flores, L.; Marí-Sanchis, A.; Goñi, F.; Sánchez, M.; Nicolau, J.; et al. Assessment of obesity stigma and discrimination among Spanish subjects with a wide weight range: The OBESTIGMA study. Front. Psychol. 2023, 14, 1209245. [Google Scholar] [CrossRef] [PubMed]
- Rupp, K.; McCoy, S.M. Bullying perpetration and victimization among adolescents with overweight and obesity in a nationally representative sample. Child. Obes. 2019, 15, 323–330. [Google Scholar] [CrossRef]
- Bacchini, D.; Licenziati, M.R.; Garrasi, A.; Corciulo, N.; Driul, D.; Tanas, R.; Fiumani, P.M.; Di Pietro, E.; Pesce, S.; Crinò, A.; et al. Bullying and victimization in overweight and obese outpatient children and adolescents: An Italian multicentric study. PLoS ONE 2015, 10, e0142715. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Fernández, S. Humiliation and its relationship with bullying victimization: A narrative review. Psychol. Soc. Educ. 2024, 16, 42–51. [Google Scholar] [CrossRef]
- Dakanalis, A.; Mentzelou, M.; Papadopoulou, S.K.; Papandreou, D.; Spanoudaki, M.; Vasios, G.K.; Pavlidou, E.; Mantzorou, M.; Giaginis, C. The association of emotional eating with overweight/obesity, depression, anxiety/stress, and dietary patterns: A review of the current clinical evidence. Nutrients 2023, 15, 1173. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).