Survivin Expression in Placentas with Intrauterine Growth Restriction
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Placental Tissue Samples
2.2. Tissue Selection and Regional Standardization
- Macroscopic Examination: Each placenta was sectioned to identify the central (near cord insertion) and peripheral (marginal) zones, basal plate (maternal interface), and chorionic plate (fetal surface).
- Coring Strategy: Two 2 mm cores were extracted per placenta:
- Core 1: Central region (within 2 cm of umbilical cord insertion).
- Core 2: Peripheral region (1 cm from the placental margin).
2.3. TMA Construction and Immunohistochemical Semi Quantification
2.4. Analysis of Results
2.5. Statistical Analysis
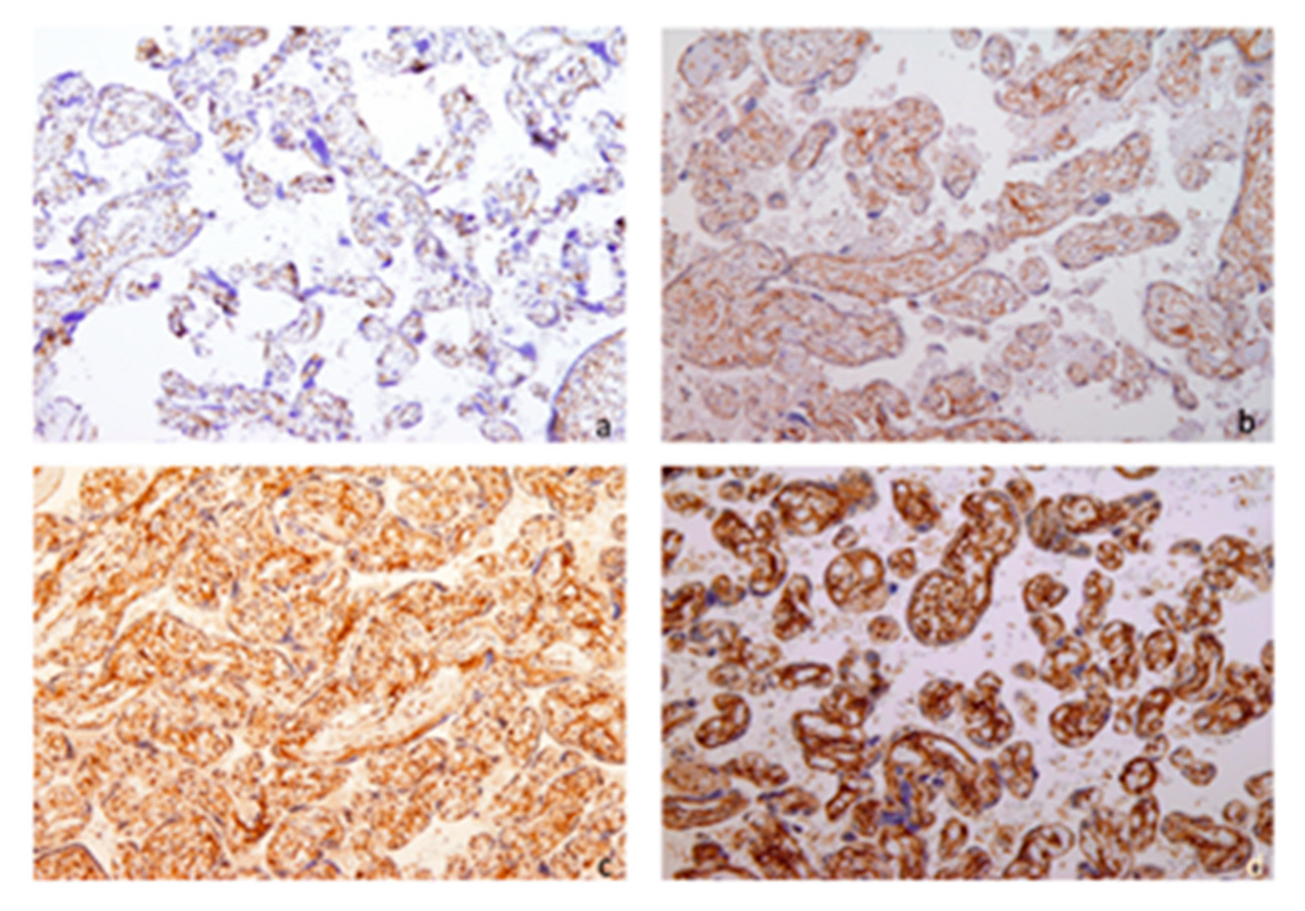
3. Results
Demographic Data of Study Population
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IUGR | intrauterine growth restriction |
| IAP | inhibitor of apoptosis |
| TMA | tissue microarray |
| PCP | positive cell proportion |
| IRS | immunoreactive score |
| HE | hematoxylin-eosin |
| IHC | immunohistochemistry |
| SD | standard deviation |
| STB | syncytiotrophoblast |
| LGA | large for gestational age |
| AGA | appropriate for gestational age |
| SGA | small for gestational age |
| VEGF | vascular endothelial growth factor |
| CPR | cerebroplacental ratio |
References
- Wu, M.; He, J.; Chen, Y.; Wan, F.; Tang, H.; Yin, C.; He, H.; Yu, H.; Yuan, C. Biomarkers for Diagnosing and Treating Fetal Growth Restriction. Curr. Med. Chem. 2024, 31, 4461–4478. [Google Scholar] [CrossRef] [PubMed]
- Stepan, H.; Hund, M.; Andraczek, T. Combining Biomarkers to Predict Pregnancy Complications and Redefine Preeclampsia: The Angiogenic-Placental Syndrome. Hypertension 2020, 75, 918–926. [Google Scholar] [CrossRef]
- Lestari, B.; Fukushima, T.; Utomo, R.Y.; Wahyuningsih, M.S.H. Apoptotic and non-apoptotic roles of caspases in placenta physiology and pathology. Placenta 2024, 151, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Laurini, R.; Laurin, J.; Marsál, K. Placental histology and fetal blood flow in intrauterine growth retardation. Acta Obstet. Gynecol. Scand. 1994, 73, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Straszewski-Chavez, S.L.; Abrahams, V.M.; Mor, G. The role of apoptosis in the regulation of trophoblast survival and differentiation during pregnancy. Endocr. Rev. 2005, 26, 877–897. [Google Scholar] [CrossRef] [PubMed]
- Kilani, R.T.; Mackova, M.; Davidge, S.T.; Guilbert, L.J. Effect of oxygen levels in villous trophoblast apoptosis. Placenta 2003, 24, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Madazli, R.; Benian, A.; Ilvan, S.; Calay, Z. Placental apoptosis and adhesion molecules expression in the placenta and the maternal placental bed of pregnancies complicated by fetal growth restriction with and without pre-eclampsia. J. Obstet. Gynaecol. 2006, 26, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Murthi, P.; Kee, M.W.; Gude, N.M.; Brennecke, S.P.; Kalionis, B. Fetal growth restriction is associated with increased apoptosis in the chorionic trophoblast cells of human fetal membranes. Placenta 2005, 26, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, G.; Breborowicz, A.; Brązert, M.; Maczkiewicz, M.; Kobelski, M.; Dubiel, M.; Gudmundsson, S. Evaluation of Third Trimester Uterine Artery Flow Velocity Indices in Relationship to Perinatal Complications. J. Matern.-Fetal Neonatal Med. 2006, 19, 551–555. [Google Scholar] [CrossRef]
- La Verde, M.; Torella, M.; Ronsini, C.; Riemma, G.; Cobellis, L.; Marrapodi, M.M.; Capristo, C.; Rapisarda, A.M.C.; Morlando, M.; De Franciscis, P. The association between fetal Doppler and uterine artery blood volume flow in term pregnancies: A pilot study. Ultraschall Med. 2024, 45, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Muschol-Steinmetz, C.; Friemel, A.; Kreis, N.N.; Reinhard, J.; Yuan, J.; Louwen, F. Function of survivin in trophoblastic cells of the placenta. PLoS ONE 2013, 8, e73337. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karowicz-Bilińska, A.; Kowalska-Koprek, U.; Estemberg, D.; Sikora-Szubert, A. Evaluation of tissue metalloproteinase inhibitor TIMP-1 and Survivin levels during third trimester pregnancy—A preliminary report. Ginekol Pol. 2017, 88, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, N.; Bakirci, I.T.; Sahin, B.; Bolluk, G.; Can, E.; Dedeakayogullari, H. Assessment of serum survivin in women with placenta previa and accreta spectrum: A cross-sectional study. Sci. Rep. 2025, 15, 4735. [Google Scholar] [CrossRef] [PubMed]
- Lees, C.C.; Romero, R.; Stampalija, T.; Dall’Asta, A.; DeVore, G.A.; Prefumo, F.; Frusca, T.; Visser, G.H.A.; Hobbins, J.C.; Baschat, A.A.; et al. Clinical Opinion: The diagnosis and management of suspected fetal growth restriction: An evidence-based approach. Am. J. Obstet. Gynecol. 2022, 226, 366–378. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsikouras, P.; Antsaklis, P.; Nikolettos, K.; Kotanidou, S.; Kritsotaki, N.; Bothou, A.; Andreou, S.; Nalmpanti, T.; Chalkia, K.; Spanakis, V.; et al. Diagnosis, Prevention, and Management of Fetal Growth Restriction (FGR). J Pers Med. 2024, 14, 698. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cizkova, K.; Foltynkova, T.; Gachechiladze, M.; Tauber, Z. Comparative Analysis of Immunohistochemical Staining Intensity Determined by Light Microscopy, ImageJ and QuPath in Placental Hofbauer Cells. Acta Histochem Cytochem. 2021, 54, 21–29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blechschmidt, K.; Mylonas, I.; Mayr, D.; Schiessl, B.; Schulze, S.; Becker, K.-F.; Jeschke, U. Expression of E-cadherin and its repressor Snail in placental tissue of normal, preeclamptic and HELLP pregnancies. Virchows Archiv. 2006, 450, 95–202. [Google Scholar] [CrossRef]
- Chen, B.; Longtine, M.S.; Sadovsky, Y.; Nelson, D.M. Hypoxia downregulates p53 but induces apoptosis and enhances expression of BAD in cultures of human syncytiotrophoblasts. Am. J. Physiol. Cell Physiol. 2010, 299, C968–C976. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Q.; Greene, M.I. Survivin as a Therapeutic Target for the Treatment of Human Cancer. Cancers 2024, 16, 1705. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frøen, J.F.; Gardosi, J.O.; Thurmann, A.; Francis, A.; Stray-Pedersen, B. Restricted fetal growth in sudden intrauterine unexplained death. Acta Obstet. Gynecol. Scand. 2004, 83, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Backe, B.; Nakling, J. Effectiveness of antenatal care: A population based study. Br. J. Obstet. Gynaecol. 1993, 100, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, C.L.; Walker, S.P.; Lappas, M.; Tong, S. Circulating RNA coding genes regulating apoptosis in maternal blood in severe early onset fetal growth restriction and pre-eclampsia. J. Perinatol. 2013, 33, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Li, C.F.; Gou, W.L.; Li, X.L.; Wang, S.L.; Yang, T.; Chen, Q. Reduced expression of survivin, the inhibitor of apoptosis protein correlates with severity of preeclampsia. Placenta 2012, 33, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Lehner, R.; Bobak, J.; Kim, N.W.; Shroyer, A.L.; Kenneth, R. Localization of Telomerase hTERT Protein and Survivin in Placenta: Relation to Placental Development and Hydatidiform Mole. Obstet. Gynecol. 2001, 97, 965–970. [Google Scholar] [CrossRef]
- Malamitsi-Puchner, A.; Baka, S.; Boutsikou, M.; Liosi, S.; Gourgiotis, D.; Protonotariou, E.; Hassiakos, D.; Briana, D.D. Cord blood survivin concentrations in human full-term normal and complicated pregnancies. In Vivo 2009, 23, 139–142. [Google Scholar] [PubMed]
- Aplin, J.D.; Jones, C.J.P. Cell dynamics in human villous trophoblast. Hum. Reprod. Update 2021, 20, 904–922. [Google Scholar] [CrossRef] [PubMed]
- Pijnenborg, R.; Vercruysse, L.A.A.W. Hubrecht and the naming of the trophoblast. Placenta 2013, 34, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.L.; Cao, X.P.; Xiao, J.; Hu, Y.; Chen, M.; Raza, H.K.; Wang, H.Y.; He, X.; Gu, J.F.; Zhang, K.J. Overview of role of survivin in cancer: Expression, regulation, functions, and its potential as a therapeutic target. J. Drug Target 2024, 32, 23–240. [Google Scholar] [CrossRef] [PubMed]
- Siragusa, G.; Tomasello, L.; Giordano, C.; Pizzolanti, G. Survivin (BIRC5): Implications in cancer therapy. Life Sci. 2024, 350, 122788. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zou, L.; Guan, Y.; Jiang, L.; Liu, Y.; Zhang, X.; Huang, X.; Ren, H.; Li, Z.; Niu, H.; et al. Survivin as a potential biomarker in the diagnosis of bladder cancer: A systematic review and meta-analysis. Urol. Oncol. 2024, 42, 133–143. [Google Scholar] [CrossRef] [PubMed]
| IUGR N = 122 | Control Group N = 31 | p | |
|---|---|---|---|
| Maternal age (years) | 35.3 (5.7) * | 33.0 (4.0) | 0.042 |
| Parity (% primiparous) | 58.2 | 54.8 | |
| Gestational age at delivery (weeks) | 39.3 (1.1) | 39.7 (1.05) | 0.123 |
| Birth Weight (grams) | 2542 (255) | 3487 (402) | p < 0.001 |
| Male fetal gender (%) | 43.4 | 45.2 |
| Survivin | IUGR N (%) | Control Group N (%) | X2 Value | df | p |
|---|---|---|---|---|---|
| Staining intensity score * | 12.594 | 1 | <0.001 | ||
| 1 | 10 (8.2) | 10 (32.3) | |||
| 2 | 74 (60.7) | 15 (48.4) | |||
| 3 | 38 (31.1) | 6 (19.4) | |||
| Immunoreactive score ** | 12.783 | 1 | 0.002 | ||
| 4 | 10 (8.2) | 10 (32.3) | |||
| 8 | 74 (60.7) | 15 (48.4) | |||
| 12 | 38 (31.1) | 6 (19.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perković, P.; Štifter-Vretenar, S.; Perković, M.; Štefančić, M.; Holjević, E.; Dekanić, A.; Štimac, T. Survivin Expression in Placentas with Intrauterine Growth Restriction. Biomedicines 2025, 13, 1576. https://doi.org/10.3390/biomedicines13071576
Perković P, Štifter-Vretenar S, Perković M, Štefančić M, Holjević E, Dekanić A, Štimac T. Survivin Expression in Placentas with Intrauterine Growth Restriction. Biomedicines. 2025; 13(7):1576. https://doi.org/10.3390/biomedicines13071576
Chicago/Turabian StylePerković, Pavo, Sanja Štifter-Vretenar, Marina Perković, Marko Štefančić, Ena Holjević, Andrea Dekanić, and Tea Štimac. 2025. "Survivin Expression in Placentas with Intrauterine Growth Restriction" Biomedicines 13, no. 7: 1576. https://doi.org/10.3390/biomedicines13071576
APA StylePerković, P., Štifter-Vretenar, S., Perković, M., Štefančić, M., Holjević, E., Dekanić, A., & Štimac, T. (2025). Survivin Expression in Placentas with Intrauterine Growth Restriction. Biomedicines, 13(7), 1576. https://doi.org/10.3390/biomedicines13071576







