Optimizing Nephron Performance: The Old, the New, and the New–Old Diuretic Therapies
Abstract
1. Introduction
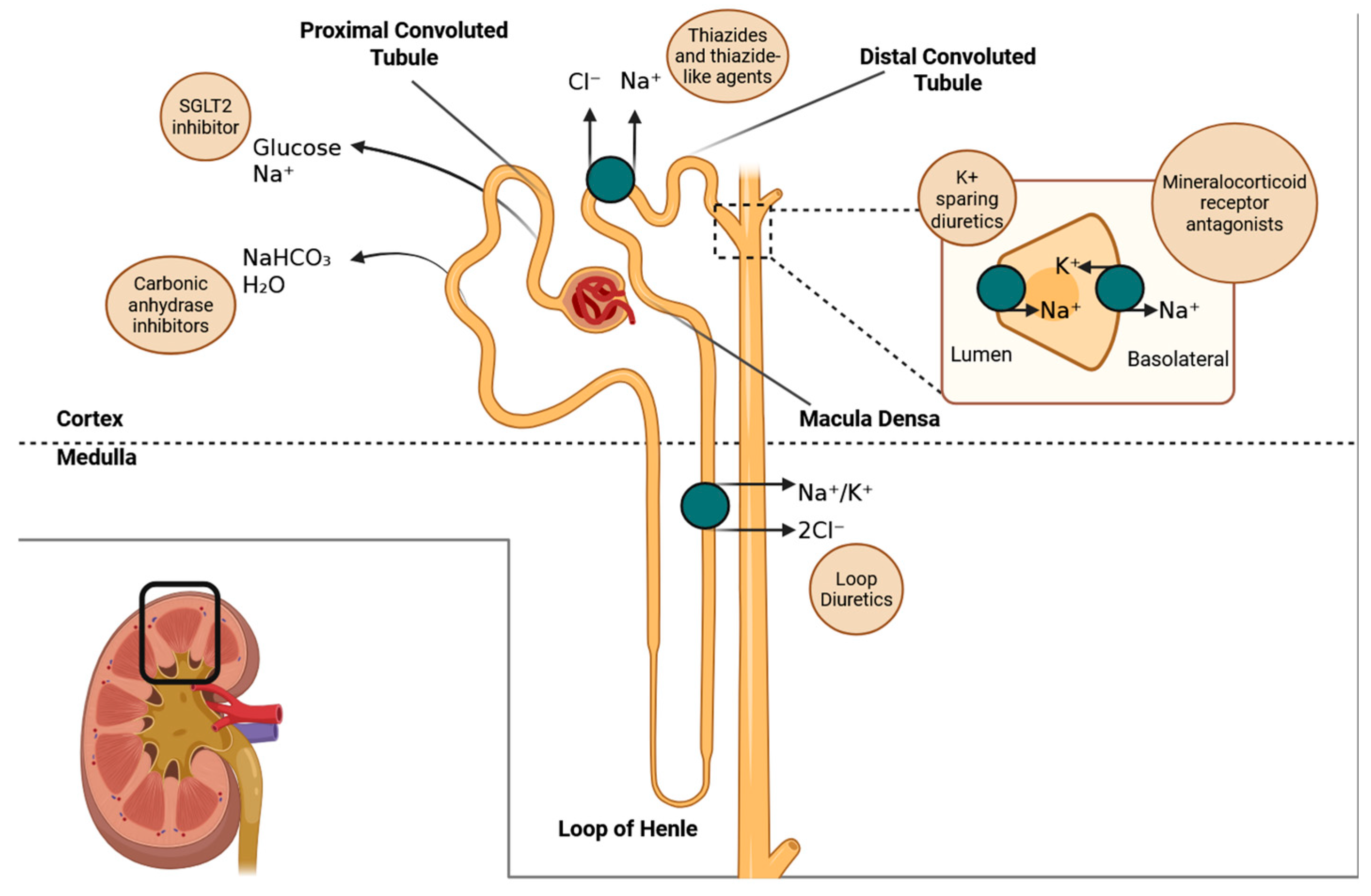
- Thiazide diuretics, such as hydrochlorothiazide and indapamide, function primarily in the distal convoluted tubule of the kidneys. They inhibit the sodium/chloride co-transporter (NCC), which leads to a reduction in the reabsorption of sodium and chloride. As a result, this causes a decrease in extracellular volume and lowers arterial pressure. Over time, their antihypertensive effect is further enhanced by a reduction in vascular resistance.
- Mineralocorticoid receptor antagonists, such as spironolactone, eplerenone, and finerenone, work by blocking aldosterone from binding to its receptor in the collecting duct of the kidneys. This action prevents the transcription of sodium channels and sodium/potassium-ATPase pumps, which leads to reduced sodium retention and decreased potassium excretion. Additionally, finerenone has effects in non-renal tissues, particularly in the heart, where it influences gene expression related to fibrosis and inflammation.
- Gliflozins, SGLT2 inhibitors such as dapagliflozin and empagliflozin, work by blocking the SGLT2 transporter located in the proximal tubule of the kidneys, which prevents the reabsorption of glucose and sodium. As a result, this action causes osmotic diuresis (increased urine output due to the presence of certain substances in the urine) and natriuresis (excretion of sodium in the urine). This process restores tubuloglomerular feedback, reduces glomerular hyperfiltration, and ultimately lowers glomerular pressure and wall stress. Additionally, these medications have metabolic and anti-inflammatory effects that contribute to cardiovascular and renal protection.
- Acetazolamide works primarily in the proximal tubule of the kidney, where it inhibits the enzyme carbonic anhydrase. This enzyme is responsible for converting carbonic acid into water and carbon dioxide (CO2). By inhibiting this process, acetazolamide decreases the availability of protons (H+), which impairs the Na+/H+ exchanger. As a result, bicarbonate reabsorption is reduced, leading to urine alkalinization and a mild diuretic effect. This medication is particularly beneficial for enhancing diuresis in patients experiencing fluid overload, especially when used in combination with loop diuretics.
| Drug Class | Primary Site of Action | Mechanism of Action | Pharmacological Effects |
|---|---|---|---|
| Thiazides | Distal convoluted tubule | Inhibit Na+/Cl− co-transporter (NCC) | ↓ Sodium reabsorption ↓ Intravascular volume ↓ Cardiac preload |
| Mineralocorticoid receptor antagonists (spironolactone, finerenone) | Collecting duct and cardiomyocytes | Block aldosterone binding at mineralocorticoid receptors | ↓ Sodium reabsorption ↓ Inflammation ↓ Fibrosis |
| Gliflozins (SGLT2 inhibitors) | Proximal convoluted tubule | Inhibit sodium-glucose co-transporter 2 (SGLT2) | ↓ Glomerular pressure ↓ Wall stress Improved metabolic and hemodynamic profile |
| Acetazolamide | Proximal convoluted tubule | Inhibit carbonic anhydrase → ↓ H+ availability for Na+/H+ exchanger | ↓ Bicarbonate reabsorption ↑ Urine alkalinization Mild diuresis |
2. The Old: Established Diuretics
3. The New: Recently Introduced Agents
4. The New–Old: Rediscovered Utility of Traditional Agents
5. Clinical Integration and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cirillo, M.; Marcarelli, F.; Mele, A.A.; Romano, M.; Lombardi, C.; Bilancio, G. Parallel-Group 8-Week Study on Chlorthalidone Effects in Hypertensives with Low Kidney Function. Hypertension 2014, 63, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Sinha, A.D.; Cramer, A.E.; Balmes-Fenwick, M.; Dickinson, J.H.; Ouyang, F.; Tu, W. Chlorthalidone for Hypertension in Advanced Chronic Kidney Disease. N. Engl. J. Med. 2021, 385, 2507–2519. [Google Scholar] [CrossRef]
- Mullens, W.; Dauw, J.; Martens, P.; Verbrugge, F.H.; Nijst, P.; Meekers, E.; Tartaglia, K.; Chenot, F.; Moubayed, S.; Dierckx, R.; et al. Acetazolamide in Acute Decompensated Heart Failure with Volume Overload. N. Engl. J. Med. 2022, 387, 1185–1195. [Google Scholar] [CrossRef]
- Moser, M. Fifty Years of Thiazide Diuretic Therapy for Hypertension. Arch. Intern. Med. 2009, 169, 1851. [Google Scholar] [CrossRef] [PubMed]
- Verel, D.; Rahman, F.; Stentiford, N.H.; Saynor, R. A clinical trial of frusemide. Lancet 1964, 284, 1088–1089. [Google Scholar] [CrossRef] [PubMed]
- Bolte, E.; Verdy, M.; Marc-Aurele, J.; Brouillet, J.; Beauregard, P.; Genest, J. Studies on new diuretic compounds: Spirolactone and chlorothiazide. Can. Med. Assoc. J. 1958, 79, 881–888. [Google Scholar]
- Georgopoulos, A.J.; Dustan, H.; Page, I.H. Spironolactone in Hypertensive Patients. Arch. Intern. Med. 1961, 108, 389. [Google Scholar] [CrossRef]
- Bull, M.B.; Laragh, J.H. Amiloride: A potassium-sparing natriuretic agent. Circulation 1968, 37, 45–53. [Google Scholar] [CrossRef]
- Freis, E.D.; Wanko, A.; Wilson, I.M.; Parrish, A.E. Treatment of Essential Hypertension with Chlorothiazide (Diuril). J. Am. Med. Assoc. 1958, 166, 137. [Google Scholar] [CrossRef]
- Ernst, M.E.; Fravel, M.A. Thiazide and the Thiazide-Like Diuretics: Review of Hydrochlorothiazide, Chlorthalidone, and Indapamide. Am. J. Hypertens. 2022, 35, 573–586. [Google Scholar] [CrossRef]
- Martins, V.M.; Ziegelmann, P.K.; Ferrari, F.; Bottino, L.G.; Lucca, M.B.; Corrêa, H.L.R.; Blum, G.B.; Helal, L.; Fuchs, S.C.; Fuchs, F.D. Thiazide diuretics alone or combined with potassium-sparing diuretics to treat hypertension: A systematic review and network meta-analysis of randomized controlled trials. J. Hypertens. 2023, 41, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Veterans Administration Cooperative Study Group on Anti-hypertensive Agents. Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA 1967, 202, 1028–1034. [Google Scholar] [CrossRef]
- Veterans Administration Cooperative Study Group on Anti-hypertensive Agents. Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA 1970, 213, 1143–1152. [Google Scholar] [CrossRef]
- MRC Working Party. Medical Research Council trial of treatment of hypertension in older adults: Principal results. BMJ 1992, 304, 405–412. [Google Scholar] [CrossRef] [PubMed]
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991, 265, 3255–3264. [Google Scholar] [CrossRef]
- Beckett, N.S.; Peters, R.; Fletcher, A.E.; Staessen, J.A.; Liu, L.; Dumitrascu, D.; Stoyanovsky, V.; Antikainen, R.L.; Nikitin, Y.; Anderson, C.; et al. Treatment of Hypertension in Patients 80 Years of Age or Older. N. Engl. J. Med. 2008, 358, 1887–1898. [Google Scholar] [CrossRef]
- PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6105 individuals with previous stroke or transient ischaemic attack. Lancet 2001, 358, 1033–1041. [Google Scholar] [CrossRef]
- Brown, M.J.; Palmer, C.R.; Castaigne, A.; de Leeuw, P.W.; Mancia, G.; Rosenthal, T.; Ruilope, L.M. Morbidity and mortality in patients randomised to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT). Lancet 2000, 356, 366–372. [Google Scholar] [CrossRef]
- The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major Outcomes in High-Risk Hypertensive Patients Randomized to Angiotensin-Converting Enzyme Inhibitor or Calcium Channel Blocker vs Diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002, 288, 2981–2997. [Google Scholar] [CrossRef]
- SPRINT Research Group; Lewis, C.E.; Fine, L.J.; Beddhu, S.; Cheung, A.K.; Cushman, W.C.; Cutler, J.A.; Evans, G.W.; Johnson, K.C.; Kitzman, D.W.; et al. Final Report of a Trial of Intensive versus Standard Blood-Pressure Control. N. Engl. J. Med. 2021, 384, 1921–1930. [Google Scholar]
- The SPRINT Research Group. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N. Engl. J. Med. 2015, 373, 2103–2116. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E. Gliflozins in the Management of Cardiovascular Disease. N. Engl. J. Med. 2022, 386, 2024–2034. [Google Scholar] [CrossRef] [PubMed]
- Komoroski, B.; Vachharajani, N.; Boulton, D.; Kornhauser, D.; Geraldes, M.; Li, L.; Pfister, M. Dapagliflozin, a Novel SGLT2 Inhibitor, Induces Dose-Dependent Glucosuria in Healthy Subjects. Clin. Pharmacol. Ther. 2009, 85, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Komoroski, B.; Vachharajani, N.; Feng, Y.; Li, L.; Kornhauser, D.; Pfister, M. Dapagliflozin, a Novel, Selective SGLT2 Inhibitor, Improved Glycemic Control Over 2 Weeks in Patients With Type 2 Diabetes Mellitus. Clin. Pharmacol. Ther. 2009, 85, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J.; Gross, J.L.; Pieters, A.; Bastien, A.; List, J.F. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: A randomised, double-blind, placebo-controlled trial. Lancet 2010, 375, 2223–2233. [Google Scholar] [CrossRef]
- Ridderstråle, M.; Andersen, K.R.; Zeller, C.; Kim, G.; Woerle, H.J.; Broedl, U.C. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: A 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014, 2, 691–700. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Wheeler, D.C.; Stefánsson, B.V.; Jongs, N.; Chertow, G.M.; Greene, T.; Hou, F.F.; McMurray, J.J.V.; Correa-Rotter, R.; Rossing, P.; Toto, R.D.; et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: A prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021, 9, 22–31. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-LaRocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Rawat, S.; Ho, K.L.; Wagg, C.S.; Zhang, L.; Teoh, H.; Dyck, J.E.; Uddin, G.M.; Oudit, G.Y.; Mayoux, E.; et al. Empagliflozin Increases Cardiac Energy Production in Diabetes. JACC Basic Transl. Sci. 2018, 3, 575–587. [Google Scholar] [CrossRef]
- McCullough, P.A.; Kluger, A.Y.; Tecson, K.M.; Barbin, C.M.; Lee, A.Y.; Lerma, E.V.; Rosol, Z.P.; Kluger, S.L.; Rangaswami, J. Inhibition of the Sodium–Proton Antiporter (Exchanger) is a Plausible Mechanism of Potential Benefit and Harm for Drugs Designed to Block Sodium Glucose Co-transporter 2. Rev. Cardiovasc. Med. 2018, 19, 51–63. [Google Scholar] [CrossRef]
- Philippaert, K.; Kalyaanamoorthy, S.; Fatehi, M.; Long, W.; Soni, S.; Byrne, N.J.; Barr, A.; Singh, J.; Wong, J.; Palechuk, T.; et al. Cardiac Late Sodium Channel Current Is a Molecular Target for the Sodium/Glucose Cotransporter 2 Inhibitor Empagliflozin. Circulation 2021, 143, 2188–2204. [Google Scholar] [CrossRef]
- Byrne, N.J.; Matsumura, N.; Maayah, Z.H.; Ferdaoussi, M.; Takahara, S.; Darwesh, A.M.; Levasseur, J.L.; Jahng, J.W.S.; Vos, D.; Parajuli, N.; et al. Empagliflozin Blunts Worsening Cardiac Dysfunction Associated With Reduced NLRP3 (Nucleotide-Binding Domain-Like Receptor Protein 3) Inflammasome Activation in Heart Failure. Circ. Heart Fail. 2020, 13, e006277. [Google Scholar] [CrossRef]
- Kolijn, D.; Pabel, S.; Tian, Y.; Lódi, M.; Herwig, M.; Carrizzo, A.; Zhazykbayeva, S.; Kovács, Á.; Fülöp, G.Á.; Falcão-Pires, I.; et al. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase Gα oxidation. Cardiovasc. Res. 2021, 117, 495–507. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, L.; Lu, Z.; Hu, Y.; Zhang, H.; Sun, F.; Li, Q.; He, C.; Shu, W.; Wang, L.; et al. Sodium-Glucose Cotransporter 2 Inhibitor Canagliflozin Antagonizes Salt-Sensitive Hypertension Through Modifying Transient Receptor Potential Channels 3 Mediated Vascular Calcium Handling. J. Am. Heart Assoc. 2022, 11, e027656. [Google Scholar] [CrossRef]
- Baker, W.L.; Smyth, L.R.; Riche, D.M.; Bourret, E.M.; Chamberlin, K.W.; White, W.B. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: A systematic review and meta-analysis. J. Am. Soc. Hypertens. 2014, 8, 262–275.e9. [Google Scholar] [CrossRef] [PubMed]
- Nassif, M.E.; Windsor, S.L.; Borlaug, B.A.; Kitzman, D.W.; Shah, S.J.; Tang, F.; Nassif, M.E.; Windsor, S.L.; Borlaug, B.A.; Kitzman, D.W.; et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: A multicenter randomized trial. Nat. Med. 2021, 27, 1954–1960. [Google Scholar] [CrossRef] [PubMed]
- Bode, B.; Stenlöf, K.; Harris, S.; Sullivan, D.; Fung, A.; Usiskin, K.; Meininger, G. Long-term efficacy and safety of canagliflozin over 104 weeks in patients aged 55-80 years with type 2 diabetes. Diabetes Obes. Metab. 2015, 17, 294–303. [Google Scholar] [CrossRef]
- Baker, W.L.; Buckley, L.F.; Kelly, M.S.; Bucheit, J.D.; Parod, E.D.; Brown, R.; Carbone, S.; Abbate, A.; Dixon, D.L. Effects of Sodium-Glucose Cotransporter 2 Inhibitors on 24-Hour Ambulatory Blood Pressure: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2017, 6, e005686. [Google Scholar] [CrossRef]
- Ferdinand, K.C.; Izzo, J.L.; Lee, J.; Meng, L.; George, J.; Salsali, A.; Seman, L. Antihyperglycemic and Blood Pressure Effects of Empagliflozin in Black Patients With Type 2 Diabetes Mellitus and Hypertension. Circulation 2019, 139, 2098–2109. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Vaduganathan, M.; Claggett, B.; Jhund, P.S.; Desai, A.S.; Henderson, A.D.; Lam, C.S.P.; Pitt, B.; Senni, M.; et al. Finerenone in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2024, 391, 1475–1485. [Google Scholar] [CrossRef]
- Schreiner, G.E. Chlorothiazide in renal disease. Ann. N. Y. Acad. Sci. 1958, 71, 420–429. [Google Scholar] [CrossRef]
- Reubi, F.C.; Cottier, P.T. Effects of reduced glomerular filtration rate on responsiveness to chlorothiazide and mercurial diuretics. Circulation 1961, 23, 200–210. [Google Scholar] [CrossRef]
- Dargie, H.J.; Allison, M.E.M.; Kennedy, A.C.; Gray, M.J.B. High Dosage Metolazone in Chronic Renal Failure. BMJ 1972, 4, 196–198. [Google Scholar] [CrossRef]
- Bennett, W.M.; Porter, G.A. Efficacy and safety of metolazone in renal failure and the nephrotic syndrome. J. Clin. Pharmacol. 1973, 13, 357–364. [Google Scholar] [CrossRef]
- Craswell, P.W.; Ezzat, E.; Kopstein, J.; Varghese, Z.; Moorhead, J.F. Use of Metolazone, a New Diuretic, in Patients with Renal Disease. Nephron 1974, 12, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Paton, R.R.; Kane, R.E. Long-Term Diuretic Therapy with Metolazone of Renal Failure and the Nephrotic Syndrome. J. Clin. Pharmacol. 1977, 17, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Wollam, G.L.; Tarazi, R.C.; Bravo, E.L.; Dustan, H.P. Diuretic potency of combined hydrochlorothiazide and furosemide therapy in patients with azotemia. Am. J. Med. 1982, 72, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Leenen, F.H.; Smith, D.L.; Farkas, R.M.; Boer, W.H.; Reeves, R.A.; Marquez-Julio, A. Cardiovascular effects of indapamide in hypertensive patients with or without renal failure. A dose-response curve. Am. J. Med. 1988, 84, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Nanra, R.S. Double-blind trial of antihypertensive effect of chlorothiazide in severe renal failure. Lancet 1979, 314, 1258–1260. [Google Scholar] [CrossRef]
- Fliser, D.; Schröter, M.; Neubeck, M.; Ritz, E. Coadministration of thiazides increases the efficacy of loop diuretics even in patients with advanced renal failure. Kidney Int. 1994, 46, 482–488. [Google Scholar] [CrossRef]
- Knauf, H.; Mutschler, E. Diuretic Effectiveness of Hydrochlorothiazide and Furosemide Alone and in Combination in Chronic Renal Failure. J. Cardiovasc. Pharmacol. 1995, 26, 394–400. [Google Scholar] [CrossRef]
- Dussol, B.; Moussi-Frances, J.; Morange, S.; Somma-Delpero, C.; Mundler, O.; Berland, Y. A randomized trial of furosemide vs hydrochlorothiazide in patients with chronic renal failure and hypertension. Nephrol. Dial. Transplant. 2005, 20, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Dussol, B.; Moussi-Frances, J.; Morange, S.; Somma-Delpero, C.; Mundler, O.; Berland, Y. A Pilot Study Comparing Furosemide and Hydrochlorothiazide in Patients With Hypertension and Stage 4 or 5 Chronic Kidney Disease. J. Clin. Hypertens. 2012, 14, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Sinha, A.D.; Pappas, M.K.; Ammous, F. Chlorthalidone for Poorly Controlled Hypertension in Chronic Kidney Disease: An Interventional Pilot Study. Am. J. Nephrol. 2014, 39, 171–182. [Google Scholar] [CrossRef]
- Agarwal, R.; Sinha, A.D.; Tu, W. Chlorthalidone for Resistant Hypertension in Advanced Chronic Kidney Disease. Circulation 2022, 146, 718–720. [Google Scholar] [CrossRef] [PubMed]
- Piardi, D.S.; Butzke, M.; Mazzuca, A.C.M.; Gomes, B.S.; Alves, S.G.; Kotzian, B.J.; Ghisleni, E.C.; Giaretta, V.; Bellaver, P.; Varaschin, G.A.; et al. Effect of adding hydrochlorothiazide to usual treatment of patients with acute decompensated heart failure: A randomized clinical trial. Sci. Rep. 2021, 11, 16474. [Google Scholar]
- Trullàs, J.C.; Morales-Rull, J.L.; Casado, J.; Carrera-Izquierdo, M.; Sánchez-Marteles, M.; Conde-Martel, A.; Dávila-Ramos, M.F.; Llácer, P.; Salamanca-Bautista, P.; Pérez-Silvestre, J.; et al. Combining loop with thiazide diuretics for decompensated heart failure: The CLOROTIC trial. Eur. Heart J. 2023, 44, 411–421. [Google Scholar] [CrossRef]
| Trial Name | Population | Intervention | Comparator | Primary Outcome(s) | Main Result |
|---|---|---|---|---|---|
| SHEP (Systolic Hypertension in the Elderly Program, 1991) [15] | Older adults with isolated systolic hypertension | Chlorthalidone-based therapy | Placebo | Stroke | ↓ Stroke by 36%, ↓ heart failure by 54% |
| MRC Trial (Medical Research Council, 1992) [14] | Older hypertensives | HCTZ + amiloride | Placebo | Stroke, CHD | ↓ Stroke and CHD with diuretic combination |
| INSIGHT (International Nifedipine GITS Study: Intervention as a Goal in Hypertension Treatment, 2000) [18] | Hypertensives | Nifedipine GITS vs. hydrochlorothiazide + amiloride | Head to head | CVD outcomes | Similar total CVD; ↓ fatal MI with diuretic |
| PROGRESS (Perindopril Protection Against Recurrent Stroke Study, 2001) [17] | Stroke survivors | Perindopril ± indapamide | Placebo | Recurrent stroke | ↓ Stroke recurrence by 28% (43% with combo) |
| ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial, 2002) [19] | High-risk hypertensives (n ≈ 33,000) | Chlorthalidone vs. amlodipine or lisinopril | Head to head | CHD, stroke, heart failure | Chlorthalidone superior for heart failure; similar CHD/mortality |
| Treat Heart Attack Trial (ALLHAT) [19] | Same as above | See ALLHAT | See ALLHAT | Coronary heart disease | ↓ HF with diuretic, no superiority for CHD |
| HYVET (Hypertension in the Very Elderly Trial, 2008) [16] | Adults ≥80 years with hypertension | Indapamide ± perindopril | Placebo | Stroke, all-cause mortality | ↓ Stroke risk by 30%, ↓ all-cause mortality by 21% |
| SPRINT (Systolic BP Intervention Trial, 2015) [20] | High-risk hypertensives | SBP target <120 mmHg | SBP target <140 mmHg | CVD events, mortality | ↓ CVD events by 25%, ↓ mortality by 27% |
| Drug Class | Common Adverse Effects | Considerations in CKD |
|---|---|---|
| Thiazide diuretics | Hypokalemia, hyperuricemia, mild hyperglycemia, hyponatremia | Reduced efficacy at GFR <30 mL/min, risk of hyponatremia |
| Loop diuretics | Hypokalemia, hypomagnesemia, dehydration, ototoxicity (high doses) | Still effective in advanced CKD, monitor electrolytes closely |
| Mineralocorticoid receptor antagonists (e.g., spironolactone, finerenone) | Hyperkalemia, gynecomastia (spironolactone), risk of worsening kidney function in advanced CKD | Monitor potassium and eGFR; finerenone safer than spironolactone in some CKD contexts |
| SGLT2 inhibitors (gliflozins) | Genital mycotic infections, volume depletion, rare ketoacidosis, risk of urinary tract infections | Effective down to eGFR ~20–25 mL/min; monitor volume status and euglycemic DKA risk |
| Acetazolamide | Metabolic acidosis, hypokalemia, fatigue, paresthesia, renal stone formation (rare) | Use with caution; can cause acidosis in advanced CKD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuchs, F.D.; Procianoy, G.S.; Bottino, L.G.; Fuchs, S.C.; Whelton, P.K. Optimizing Nephron Performance: The Old, the New, and the New–Old Diuretic Therapies. Biomedicines 2025, 13, 1413. https://doi.org/10.3390/biomedicines13061413
Fuchs FD, Procianoy GS, Bottino LG, Fuchs SC, Whelton PK. Optimizing Nephron Performance: The Old, the New, and the New–Old Diuretic Therapies. Biomedicines. 2025; 13(6):1413. https://doi.org/10.3390/biomedicines13061413
Chicago/Turabian StyleFuchs, Flavio D., Guilherme S. Procianoy, Leonardo G. Bottino, Sandra C. Fuchs, and Paul K. Whelton. 2025. "Optimizing Nephron Performance: The Old, the New, and the New–Old Diuretic Therapies" Biomedicines 13, no. 6: 1413. https://doi.org/10.3390/biomedicines13061413
APA StyleFuchs, F. D., Procianoy, G. S., Bottino, L. G., Fuchs, S. C., & Whelton, P. K. (2025). Optimizing Nephron Performance: The Old, the New, and the New–Old Diuretic Therapies. Biomedicines, 13(6), 1413. https://doi.org/10.3390/biomedicines13061413







