A2-Astrocyte Activation by Short-Term Hypoxia Rescues α-Synuclein Pre-Formed-Fibril-Induced Neuronal Cell Death
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal
2.2. Primary Cortical Neuronal Culture
2.3. Primary Astrocyte Culture
2.4. Preparation of α-Syn PFF
2.5. Preparation of Hypoxia
2.6. qPCR Analysis
2.7. TUNEL Staining/Assay
2.8. Cell Viability Assay
2.9. AlamarBlue Assay
2.10. Immunofluorescence Analysis
2.11. Western Blot Analysis
2.12. Statistics
3. Results
3.1. Long-Term Hypoxia Induced Cell Death of Astrocytes
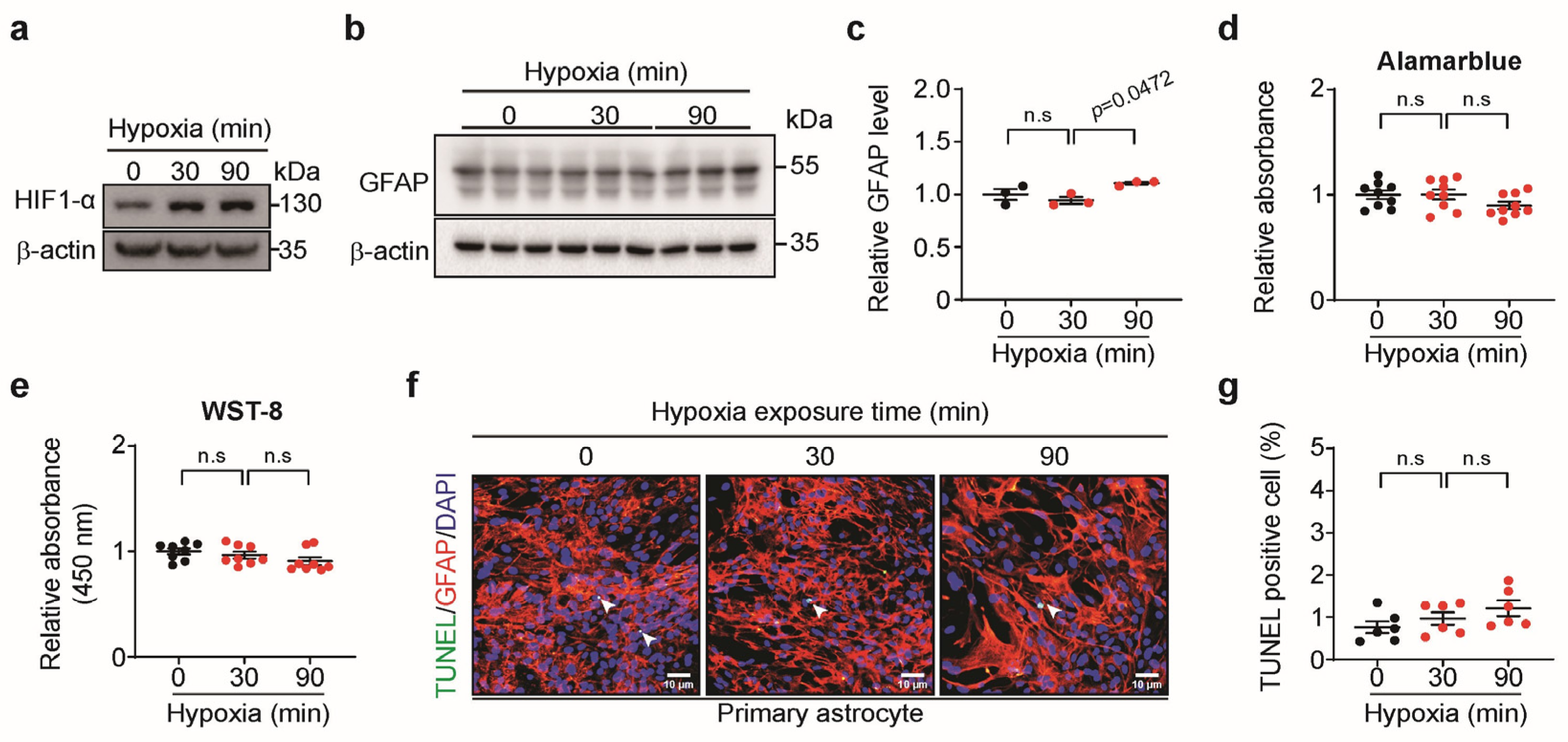
3.2. Short-Term Hypoxia Activated Astrocytes Without Cell Death
3.3. Short-Term Hypoxia Had a Protective Effect on Pre-Formed Fibril (PFF)-Treated Neurons
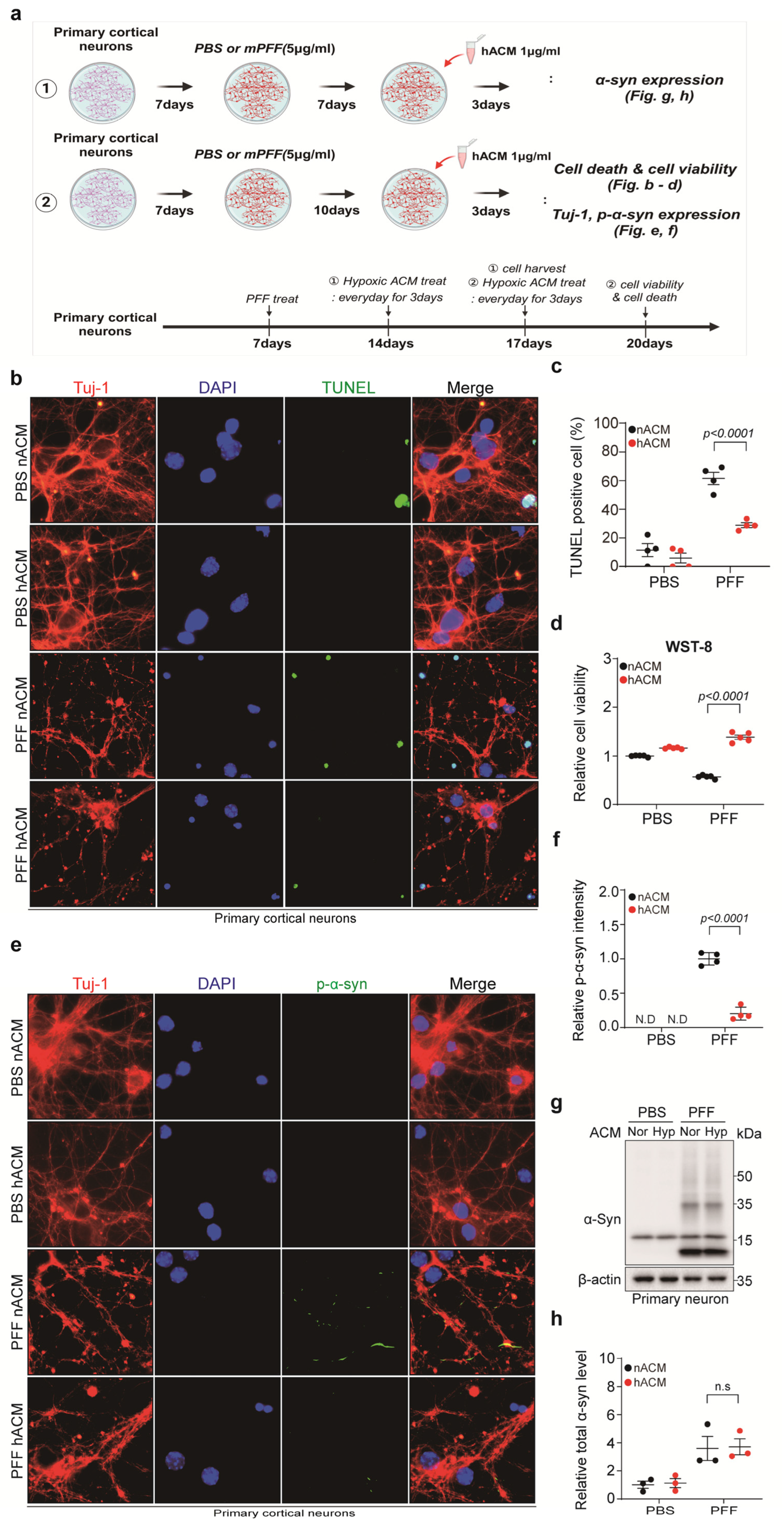
3.4. Hypoxia-Exposed Astrocyte-Conditioned Medium (hACM) Rescued PFF-Treated Neuronal Cell Death
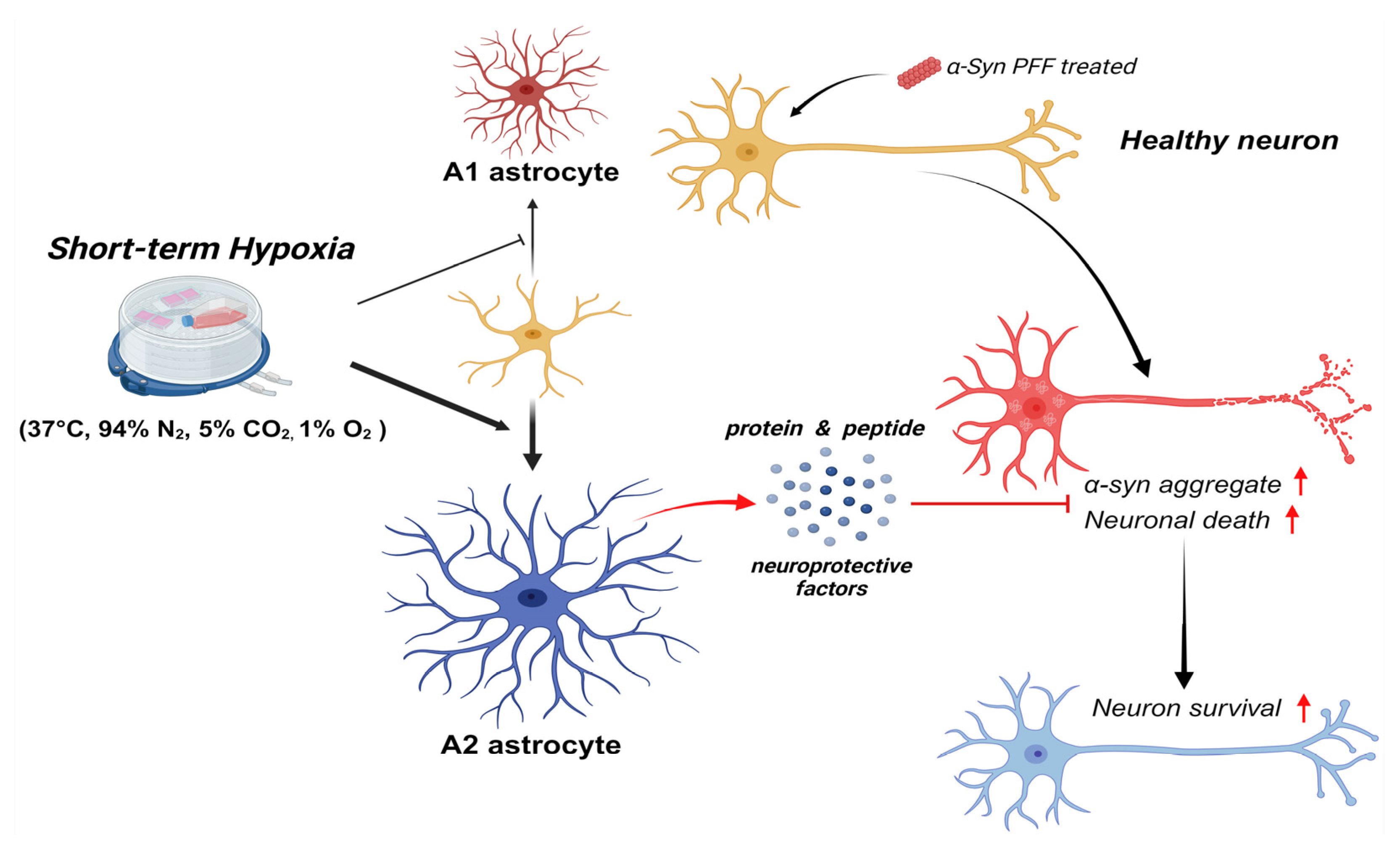
3.5. Short-Term Hypoxia Provided a Neuro-Protective Effect by Promoting A2 Astrocytes and Suppressing A1 Astrocytes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jankovic, J.; Tan, E.K. Parkinson’s disease: Etiopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry 2020, 91, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, J.; Syed, M.M.K.; Lashuel, H.A.; Millet, G.P. Hypoxia Conditioning as a Promising Therapeutic Target in Parkinson’s Disease? Mov. Disord. 2021, 36, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Garcia Ruiz, P.J.; Luquin Piudo, R.; Martinez Castrillo, J.C. On Disease Modifying and Neuroprotective Treatments for Parkinson’s Disease: Physical Exercise. Front. Neurol. 2022, 13, 938686. [Google Scholar] [CrossRef]
- Sidoryk-Wegrzynowicz, M.; Wegrzynowicz, M.; Lee, E.; Bowman, A.B.; Aschner, M. Role of astrocytes in brain function and disease. Toxicol. Pathol. 2011, 39, 115–123. [Google Scholar] [CrossRef]
- Vangeison, G.; Rempe, D.A. The Janus-faced effects of hypoxia on astrocyte function. Neuroscientist 2009, 15, 579–588. [Google Scholar] [CrossRef]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic analysis of reactive astrogliosis. J. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef]
- Lee, B.; Choi, H.N.; Che, Y.H.; Ko, M.; Seong, H.M.; Jo, M.G.; Kim, S.H.; Song, C.; Yoon, S.; Choi, J.; et al. SARS-CoV-2 infection exacerbates the cellular pathology of Parkinson’s disease in human dopaminergic neurons and a mouse model. Cell Rep. Med. 2024, 5, 101570. [Google Scholar] [CrossRef]
- Burtscher, J.; Mallet, R.T.; Burtscher, M.; Millet, G.P. Hypoxia and brain aging: Neurodegeneration or neuroprotection? Ageing Res. Rev. 2021, 68, 101343. [Google Scholar] [CrossRef]
- Zhu, J.; Aja, S.; Kim, E.K.; Park, M.J.; Ramamurthy, S.; Jia, J.; Hu, X.; Geng, P.; Ronnett, G.V. Physiological oxygen level is critical for modeling neuronal metabolism in vitro. J. Neurosci. Res. 2012, 90, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.W.; Kim, Y.S.; Park, J.Y.; Chu, G.E.; Yang, Y.C.; Choi, B.Y.; Cho, W.G. Hypoxia-induced apoptosis of astrocytes is mediated by reduction of Dicer and activation of caspase-1. Cell Biol. Int. 2020, 44, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.A.; Adamson, D.C. Neuronal-astrocyte metabolic interactions: Understanding the transition into abnormal astrocytoma metabolism. J. Neuropathol. Exp. Neurol. 2011, 70, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Janssen Daalen, J.M.; Koopman, W.J.H.; Saris, C.G.J.; Meinders, M.J.; Thijssen, D.H.J.; Bloem, B.R. The Hypoxia Response Pathway: A Potential Intervention Target in Parkinson’s Disease? Mov. Disord. 2024, 39, 273–293. [Google Scholar] [CrossRef]
- Volpicelli-Daley, L.A.; Luk, K.C.; Lee, V.M. Addition of exogenous α-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous α-synuclein to Lewy body and Lewy neurite-like aggregates. Nat. Protoc. 2014, 9, 2135–2146. [Google Scholar] [CrossRef]
- Bengoa-Vergniory, N.; Roberts, R.F.; Wade-Martins, R.; Alegre-Abarrategui, J. Alpha-synuclein oligomers: A new hope. Acta Neuropathol. 2017, 134, 819–838. [Google Scholar] [CrossRef]
- Hinkle, J.T.; Dawson, V.L.; Dawson, T.M. The A1 astrocyte paradigm: New avenues for pharmacological intervention in neurodegeneration. Mov. Disord. 2019, 34, 959–969. [Google Scholar] [CrossRef]
- Yun, S.P.; Kam, T.I.; Panicker, N.; Kim, S.; Oh, Y.; Park, J.S.; Kwon, S.H.; Park, Y.J.; Karuppagounder, S.S.; Park, H.; et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat. Med. 2018, 24, 931–938. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Fan, Y.-Y.; Huo, J. A1/A2 astrocytes in central nervous system injuries and diseases: Angels or devils? Neurochem. Int. 2021, 148, 105080. [Google Scholar] [CrossRef]
- Burbulla, L.F.; Song, P.; Mazzulli, J.R.; Zampese, E.; Wong, Y.C.; Jeon, S.; Santos, D.P.; Blanz, J.; Obermaier, C.D.; Strojny, C.; et al. Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science 2017, 357, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C.; Trushina, E.; Morland, C.; Prigione, A.; Casadesus, G.; Andrews, Z.B.; Beal, M.F.; Bergersen, L.H.; Brinton, R.D.; de la Monte, S.; et al. Brain energy rescue: An emerging therapeutic concept for neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 2020, 19, 609–633. [Google Scholar] [CrossRef] [PubMed]
- Trist, B.G.; Hare, D.J.; Double, K.L. Oxidative stress in the aging substantia nigra and the etiology of Parkinson’s disease. Aging Cell 2019, 18, e13031. [Google Scholar] [CrossRef] [PubMed]
- Skibinski, G.; Hwang, V.; Ando, D.M.; Daub, A.; Lee, A.K.; Ravisankar, A.; Modan, S.; Finucane, M.M.; Shaby, B.A.; Finkbeiner, S. Nrf2 mitigates LRRK2- and α-synuclein-induced neurodegeneration by modulating proteostasis. Proc. Natl. Acad. Sci. USA 2017, 114, 1165–1170. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, J.; Chang, Y.; ShiDu Yan, S.; Shi, H. Hypoxia inducible factor-1 as a target for neurodegenerative diseases. Curr. Med. Chem. 2011, 18, 4335–4343. [Google Scholar] [CrossRef]
- Lang, A.E.; Lozano, A.M. Parkinson’s disease. First of two parts. N. Engl. J. Med. 1998, 339, 1044–1053. [Google Scholar] [CrossRef]
- Lin, A.M.; Chen, C.F.; Ho, L.T. Neuroprotective effect of intermittent hypoxia on iron-induced oxidative injury in rat brain. Exp. Neurol. 2002, 176, 328–335. [Google Scholar] [CrossRef]
- Li, G.; Guan, Y.; Gu, Y.; Guo, M.; Ma, W.; Shao, Q.; Liu, J.; Ji, X. Intermittent hypoxic conditioning restores neurological dysfunction of mice induced by long-term hypoxia. CNS Neurosci. Ther. 2023, 29, 202–215. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]



| Antibodies | Company | Catalog No. | Concentration |
|---|---|---|---|
| p-α-synuclein (Ser129) | Biolegend (San Diego, CA, USA) | 825701 | IF 1:1000 |
| α-synuclein | BD Bioscience (Herlev, Denmark) | 610787 | WB 1:1000 |
| β-actin-HRP | Sigma-Aldrich (St. Louis, MO, USA) | A3854 | WB 1:20,000 |
| GFAP | EMD Millipore (Burlington, MA, USA) Dako (Santa Clara, CA, USA) | MAB360 Z033429 | WB 1:1000 IF 1:500 |
| Tuj-1 | Biolegend (San Diego, CA, USA) | 802001 | IF 1:1000 |
| HIF-1α | Cell Signaling (St. Louis, MO, USA) | 14179 | WB 1:500 |
| CyTM3 AffiniPure Donkey Anti-Rabbit IgG (H + L) | Jackson (West Grove, PA, USA) | 711-165-152 | IF 1:500 |
| Fluorescein (FITC) AffiniPure Donkey Anti-Mouse IgG (H + L) | Jackson | 715-095-151 | IF 1:500 |
| Goat anti-Mouse IgG-heavy- and light-chain Antibody HRP Conjugated | BETHYL (Montgomery, TX, USA) | A90-116P | WB 1:10,000 |
| Peroxidase-AffiniPure Goat Anti-Rabbit IgG (H + L) | Jackson | 111-035-144 | WB 1:10,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.N.; Kim, S.-H.; Jo, M.G.; Lee, B.; Kim, Y.J.; Lee, S.E.; Lee, J.H.; Seong, H.M.; Kim, S.J.; Park, S.W.; et al. A2-Astrocyte Activation by Short-Term Hypoxia Rescues α-Synuclein Pre-Formed-Fibril-Induced Neuronal Cell Death. Biomedicines 2025, 13, 604. https://doi.org/10.3390/biomedicines13030604
Choi HN, Kim S-H, Jo MG, Lee B, Kim YJ, Lee SE, Lee JH, Seong HM, Kim SJ, Park SW, et al. A2-Astrocyte Activation by Short-Term Hypoxia Rescues α-Synuclein Pre-Formed-Fibril-Induced Neuronal Cell Death. Biomedicines. 2025; 13(3):604. https://doi.org/10.3390/biomedicines13030604
Chicago/Turabian StyleChoi, Ha Nyeoung, Seon-Hee Kim, Min Gi Jo, Bina Lee, Young Jin Kim, So Eun Lee, Jeong Hyun Lee, Hye Min Seong, Seong Jae Kim, Sang Won Park, and et al. 2025. "A2-Astrocyte Activation by Short-Term Hypoxia Rescues α-Synuclein Pre-Formed-Fibril-Induced Neuronal Cell Death" Biomedicines 13, no. 3: 604. https://doi.org/10.3390/biomedicines13030604
APA StyleChoi, H. N., Kim, S.-H., Jo, M. G., Lee, B., Kim, Y. J., Lee, S. E., Lee, J. H., Seong, H. M., Kim, S. J., Park, S. W., Kim, H. J., Kang, H., Lee, C. H., Lee, M. Y., Yun, S. P., & Kim, M. (2025). A2-Astrocyte Activation by Short-Term Hypoxia Rescues α-Synuclein Pre-Formed-Fibril-Induced Neuronal Cell Death. Biomedicines, 13(3), 604. https://doi.org/10.3390/biomedicines13030604







