Liver Innervation in Health and Disease: Neuroimmune–Neurovascular Interface and Future Therapeutic Implications
Abstract
1. Neuroanatomy of the Liver
2. Neural Regulation of Liver Functions
2.1. Parasympathetic Innervation and Regeneration
2.2. Sympathetic Innervation
2.3. Sensory Innervation
2.4. Thyroid Hormone (T3) and Hepatic Neural Influence
2.5. Neurovascular Interactions in the Liver
3. Hepatic Innervation in Specific Diseases
3.1. Liver Fibrosis/Cirrhosis
3.2. MASLD/MASH
3.3. ALD
3.3.1. Central Nervous System (CNS) Injury in AUD/ALD
3.3.2. Peripheral and Autonomic Nervous System (PNS/ANS) Injury in AUD/ALD
3.4. Autoimmune Liver Diseases
3.5. Hepatic Malignancies
4. Emerging Research and Therapeutic Approaches
4.1. Vagus Nerve Stimulation (VNS)
4.2. Liver Organoids
4.3. Digital Twins
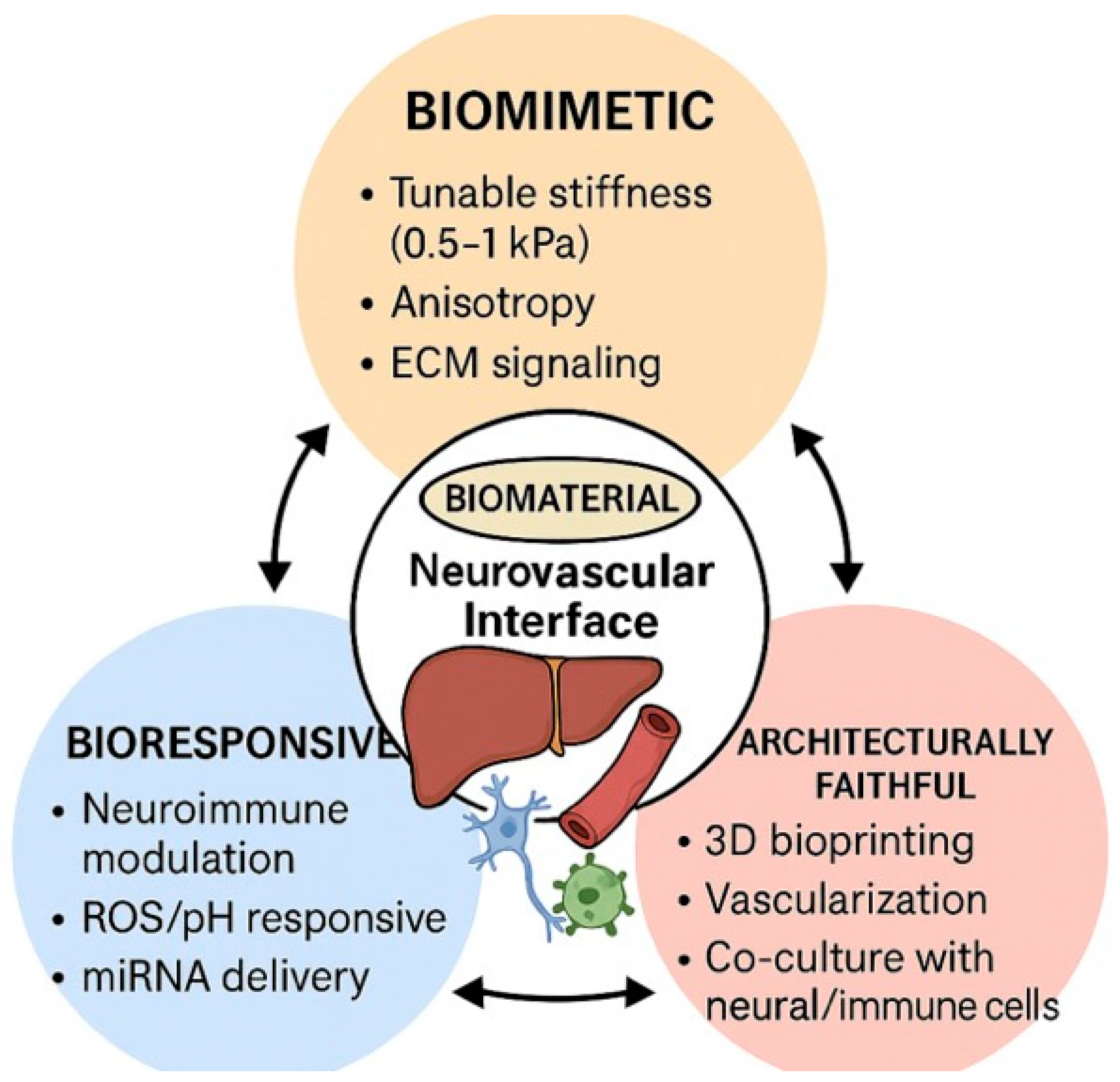
4.4. Biomaterials for Liver Tissue Engineering: Enabling Models and Therapies Targeting the Neuroimmune-Vascular Interface
5. Research Gaps and Future Directions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| AIH | Autoimmune Hepatitis |
| ALD | Alcohol-Associated Liver Disease |
| ALI | Acute Liver Injury |
| ALN | Alcohol related peripheral neuropathy |
| AR | Adrenergic Receptor |
| AUD | Alcohol Use Disorder |
| BBB | Blood–brain barrier |
| BDL | Bile Duct Ligation |
| BMAL1 | Brain and Muscle ARNT-Like 1 |
| CCL2 | Chemokine (C-C motif) Ligand 2 |
| CCL4 | Carbon Tetrachloride |
| CD8 | Cluster of Differentiation 8 |
| CGRP | Calcitonin Gene-Related Peptide |
| CLR | Calcitonin Receptor-Like Receptor |
| CNS | Central Nervous System |
| COVID | Coronavirus Disease |
| DILI | Drug-Induced Liver Injury |
| DMV | Dorsal Motor Nucleus of the Vagus |
| DNA | Deoxyribonucleic Acid |
| DVC | Dorsal Vagal Complex |
| ECM | Extracellular Matrix |
| EPO-R | Erythropoietin Receptor |
| ER | Endoplasmic Reticulum |
| ERK1/2 | Extracellular Signal-Regulated Kinases 1/2 |
| FGF21 | Fibroblast Growth Factor 21 |
| GDNF | Glial Cell Line-Derived Neurotrophic Factor |
| GLP-1 | Glucagon-Like Peptide 1 |
| HCC | Hepatocellular Carcinoma |
| HLA-G | Human Leukocyte Antigen G |
| HPA | Hypothalamic–Pituitary–Adrenal Axis |
| HPC | Hepatic Progenitor Cell |
| HSC | Hepatic Stellate Cell |
| HT | Hydroxytryptamine (Serotonin) |
| HVN | Hepatic Vagus Nerve |
| IL | Interleukin |
| iPSCs | induced Pluripotent Stem Cells |
| MASH | Metabolic Dysfunction-Associated Steatohepatitis |
| MASLD | Metabolic Dysfunction-Associated Steatotic Liver Disease |
| MHC | Major Histocompatibility Complex |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| NASH | Non-Alcoholic Steatohepatitis |
| NE | Norepinephrine |
| NF-κB | Nuclear Factor Kappa B |
| NGF | Nerve Growth Factor |
| NTF3 | Neurotrophin-3 |
| NTRK3 | Neurotrophic Tyrosine Kinase Receptor 3 |
| PBP | Peribiliary Plexus |
| PCNA | Proliferating Cell Nuclear Antigen |
| PDTs | Patient Digital Twins |
| PH | Partial Hepatectomy |
| PLC | Phospholipase C |
| PLGA | Poly(lactic-co-glycolic acid) |
| PVN | Paraventricular Nucleus |
| RAMP1 | Receptor Activity-Modifying Protein 1 |
| REV-ERBα/β | Nuclear Receptors Regulating Circadian Rhythm |
| ROS | Reactive Oxygen Species |
| RYGB | Roux-en-Y Gastric Bypass |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SFO | Subfornical Organ |
| SMAD | Mothers Against Decapentaplegic Homolog |
| STAT5 | Signal Transducer and Activator of Transcription 5 |
| TAZ | Transcriptional Coactivator with PDZ-Binding Motif |
| TE | Tissue Engineering |
| TGF-β | Transforming Growth Factor Beta |
| TGR5 | G Protein-Coupled Bile Acid Receptor |
| TH | Tyrosine Hydroxylase |
| VEGF | Vascular Endothelial Growth Factor |
| VEGF-C/D | Vascular Endothelial Growth Factor C/D |
| VEGFR3 | Vascular Endothelial Growth Factor Receptor 3 |
| VIP | Vasoactive Intestinal Peptide |
| VLDL | Very-Low-Density Lipoprotein |
| VNS | Vagus Nerve Stimulation |
| YAP | Yes-Associated Protein |
References
- Mizuno, K.; Ueno, Y. Autonomic Nervous System and the Liver. Hepatol. Res. 2017, 47, 160–165. [Google Scholar] [CrossRef]
- Kamimura, K.; Inoue, R.; Nagoya, T.; Sakai, N.; Goto, R.; Ko, M.; Niwa, Y.; Terai, S. Autonomic Nervous System Network and Liver Regeneration. World J. Gastroenterol. 2018, 24, 1616–1621. [Google Scholar] [CrossRef]
- Mikhail, Y.; Saleh, A.L. Intrinsic Nerve Fibers in the Liver Parenchyma. Anat. Rec. 1961, 141, 317–323. [Google Scholar] [CrossRef]
- McCuskey, R.S. Anatomy of Efferent Hepatic Nerves. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2004, 280A, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Li, J.; Wang, X.; Tang, D.; Chen, R. Neuroimmune Modulation in Liver Pathophysiology. J. Neuroinflammation 2024, 21, 188. [Google Scholar] [CrossRef]
- Trucas, M.; Kowalik, M.A.; Boi, M.; Serra, M.P.; Perra, A.; Quartu, M. The Density of Hepatic Autonomic Innervation Differs between Compensatory and Direct Hyperplasia Rat Models. J. Peripher. Nerv. Syst. 2023, 28, 98–107. [Google Scholar] [CrossRef]
- Adori, C.; Daraio, T.; Kuiper, R.; Barde, S.; Horvathova, L.; Yoshitake, T.; Ihnatko, R.; Valladolid-Acebes, I.; Vercruysse, P.; Wellendorf, A.M.; et al. Disorganization and Degeneration of Liver Sympathetic Innervations in Nonalcoholic Fatty Liver Disease Revealed by 3D Imaging. Sci. Adv. 2021, 7, eabg5733. [Google Scholar] [CrossRef] [PubMed]
- Mravec, B.; Szantova, M. Liver Neurobiology: Regulation of Liver Functions by the Nervous System. Semin. Liver Dis. 2025, 45, 420–437. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Guo, Y.; Jiang, W.; Wang, Y. Advanced Technologies for the Study of Neuronal Cross-Organ Regulation: A Narrative Review. Adv. Technol. Neurosci. 2024, 1, 166–176. [Google Scholar] [CrossRef]
- Kwon, E.; Joung, H.-Y.; Liu, S.-M.; Chua, S.C.; Schwartz, G.J.; Jo, Y.-H. Optogenetic Stimulation of the Liver-Projecting Melanocortinergic Pathway Promotes Hepatic Glucose Production. Nat. Commun. 2020, 11, 6295. [Google Scholar] [CrossRef]
- Miller, B.M.; Oderberg, I.M.; Goessling, W. Hepatic Nervous System in Development, Regeneration, and Disease. Hepatology 2021, 74, 3513–3522. [Google Scholar] [CrossRef] [PubMed]
- Kruepunga, N.; Hakvoort, T.B.M.; Hikspoors, J.P.J.M.; Köhler, S.E.; Lamers, W.H. Anatomy of Rodent and Human Livers: What Are the Differences? Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2019, 1865, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Shiojiri, N.; Hirose, H.; Ota, N.; Sekiguchi, J.; Matsubara, S.; Kawakami, H. Changes of Biliary Cilia, Smooth Muscle Tissue Distribution, Innervation and Extracellular Matrices during Morphological Evolution of Hepatic Architectures in Vertebrates. Ann. Anat. Anat. Anz. 2023, 250, 152148. [Google Scholar] [CrossRef]
- Liu, W.; Kämpfe Nordström, C.; Danckwardt-Lillieström, N.; Rask-Andersen, H. Human Inner Ear Immune Activity: A Super-Resolution Immunohistochemistry Study. Front. Neurol. 2019, 10, 728. [Google Scholar] [CrossRef]
- Markus, A.M.A.; Köckerling, F.; Neuhuber, W.L. Close Anatomical Relationships between Nerve Fibers and MHC Class II-Expressing Dendritic Cells in the Rat Liver and Extrahepatic Bile Duct. Histochem. Cell Biol. 1998, 109, 409–415. [Google Scholar] [CrossRef]
- Winau, F.; Hegasy, G.; Weiskirchen, R.; Weber, S.; Cassan, C.; Sieling, P.A.; Modlin, R.L.; Liblau, R.S.; Gressner, A.M.; Kaufmann, S.H.E. Ito Cells Are Liver-Resident Antigen-Presenting Cells for Activating T Cell Responses. Immunity 2007, 26, 117–129. [Google Scholar] [CrossRef]
- Lin, Y.; Nosaka, S.; Amakata, Y.; Maeda, T. Comparative Study of the Mammalian Liver Innervation: An Immunohistochemical Study of Protein Gene Product 9.5, Dopamine β-Hydroxylase and Tyrosine Hydroxylase. Comp. Biochem. Physiol. A Physiol. 1995, 110, 289–298. [Google Scholar] [CrossRef]
- Amenta, F.; Cavallotti, C.; Ferrante, F.; Tonelli, F. Cholinergic Nerves in the Human Liver. Histochem. J. 1981, 13, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.W.; Song, H.J.; Back, S.J.; Kim, T.H.; Cho, S.H.; Han, J.-Y.; Yoo, K.; Lee, Y.S.; Chung, K.W. Decreased Hepatic Nerve Fiber Innervation in Patients with Liver Cirrhosis. Gut Liver 2007, 1, 165–170. [Google Scholar] [CrossRef]
- Ohata, M. Electron Microscope Study on the Innervation of Guinea Pig Liver—Proposal of Sensory Nerve Terminals in the Hepatic Parenchyme. Arch. Histol. Jpn. 1984, 47, 149–178. [Google Scholar] [CrossRef]
- Ito, Y.; Magari, S.; Sakanaka, M. Immunoelectron-Microscopic Localization of Peptidergic Nerve Fibers around Lymphatic Capillaries in the Rat Liver. Arch. Histol. Cytol. 1990, 53, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-H.; Shim, Y.-R.; Choi, S.E.; Kim, M.-H.; Lee, G.; You, H.J.; Choi, W.-M.; Yang, K.; Ryu, T.; Kim, K.; et al. Catecholamine Induces Kupffer Cell Apoptosis via Growth Differentiation Factor 15 in Alcohol-Associated Liver Disease. Exp. Mol. Med. 2023, 55, 158–170. [Google Scholar] [CrossRef]
- Zanchi, A.; Reidy, J.; Feldman, H.J.; Qualter, J.; Gouw, A.S.; Osbeck, J.; Kofman, A.; Balabaud, C.; Bioulac-Sage, P.; Tiniakos, D.G.; et al. Innervation of the Proximal Human Biliary Tree. Virchows Arch. 2020, 477, 385–392. [Google Scholar] [CrossRef]
- Jeong, J.; Tanaka, M.; Iwakiri, Y. Hepatic Lymphatic Vascular System in Health and Disease. J. Hepatol. 2022, 77, 206–218. [Google Scholar] [CrossRef]
- Ito, Y.; Takahashi, T.; Tadokoro, F.; Hayashi, K.; Iino, Z.; Sato, K.; Akira, K. Regeneration of the Hepatic Nerves Following Surgical Denervation of the Liver in Dogs. Liver 1998, 18, 20–26. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, S.; Prakash, S.S. Compensated Liver Cirrhosis: Natural Course and Disease-Modifying Strategies. World J. Methodol. 2023, 13, 179–193. [Google Scholar] [CrossRef]
- Tolaymat, M.; Sundel, M.H.; Alizadeh, M.; Xie, G.; Raufman, J.-P. Potential Role for Combined Subtype-Selective Targeting of M1 and M3 Muscarinic Receptors in Gastrointestinal and Liver Diseases. Front. Pharmacol. 2021, 12, 786105. [Google Scholar] [CrossRef]
- Elchaninov, A.; Vishnyakova, P.; Glinkina, V.; Fatkhudinov, T.; Sukhikh, G. Liver Regeneration as a Model for Studying Cellular Plasticity in Mammals: The Roles of Hepatocytes and Cholangiocytes. Cells 2025, 14, 1129. [Google Scholar] [CrossRef]
- Schuman, R.F.; Hunter, K.W. Secretion of Acetylcholinesterase by a Mouse Hepatocyte X Rat Liver Cell Hybrid Culture. Vitr. Cell. Dev. Biol. 1986, 22, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Nishio, T.; Taura, K.; Iwaisako, K.; Koyama, Y.; Tanabe, K.; Yamamoto, G.; Okuda, Y.; Ikeno, Y.; Yoshino, K.; Kasai, Y.; et al. Hepatic Vagus Nerve Regulates Kupffer Cell Activation via A7 Nicotinic Acetylcholine Receptor in Nonalcoholic Steatohepatitis. J. Gastroenterol. 2017, 52, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Hajiasgharzadeh, K.; Baradaran, B. Cholinergic Anti-Inflammatory Pathway and the Liver. Adv. Pharm. Bull. 2017, 7, 507–513. [Google Scholar] [CrossRef]
- Mravec, B.; Szantova, M. The Role of the Nervous System in Liver Diseases. Hepatol. Res. 2024, 54, 970–980. [Google Scholar] [CrossRef]
- Keever, K.R.; Cui, K.; Casteel, J.L.; Singh, S.; Hoover, D.B.; Williams, D.L.; Pavlov, V.A.; Yakubenko, V.P. Cholinergic Signaling via the A7 Nicotinic Acetylcholine Receptor Regulates the Migration of Monocyte-Derived Macrophages during Acute Inflammation. J. Neuroinflammation 2024, 21, 3. [Google Scholar] [CrossRef]
- Siddle, M.; Gallego Durán, R.; Goel, D.; Renquist, B.J.; Holt, M.K.; Hadjihambi, A. Mechanistic Insights into the Liver–Brain Axis during Chronic Liver Disease. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 1–23. [Google Scholar] [CrossRef]
- Li, Y.; Ding, S.; Wang, Y. Targeting the Cholinergic Anti-Inflammatory Pathway: An Innovative Strategy for Treating Diseases. Mol. Biol. Rep. 2025, 52, 199. [Google Scholar] [CrossRef] [PubMed]
- Woodie, L.N.; Melink, L.C.; Midha, M.; de Araújo, A.M.; Geisler, C.E.; Alberto, A.J.; Krusen, B.M.; Zundell, D.M.; de Lartigue, G.; Hayes, M.R.; et al. Hepatic Vagal Afferents Convey Clock-Dependent Signals to Regulate Circadian Food Intake. Science 2024, 386, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Kandilis, A.N.; Koskinas, J.; Vlachos, I.; Skaltsas, S.; Karandrea, D.; Karakitsos, P.; Pantopoulou, A.; Palaiologou, M.; Nikiteas, N.; Tiniakos, D.G.; et al. Liver Regeneration: Immunohistochemichal Study of Intrinsic Hepatic Innervation after Partial Hepatectomy in Rats. BMC Gastroenterol. 2014, 14, 202. [Google Scholar] [CrossRef]
- Fukuda, Y.; Imoto, M.; Koyama, Y.; Miyazawa, Y.; Hayakawa, T. Demonstration of Noradrenaline-Immunoreactive Nerve Fibres in the Liver. J. Int. Med. Res. 1996, 24, 466–472. [Google Scholar] [CrossRef]
- Oben, J.A.; Diehl, A.M. Sympathetic Nervous System Regulation of Liver Repair. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2004, 280A, 874–883. [Google Scholar] [CrossRef]
- Luo, Q.; Liu, P.; Dong, Y.; Qin, T. The Role of the Hepatic Autonomic Nervous System. Clin. Mol. Hepatol. 2023, 29, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Gilboa, D.; Haim-Ohana, Y.; Deshet-Unger, N.; Ben-Califa, N.; Hiram-Bab, S.; Reuveni, D.; Zigmond, E.; Gassmann, M.; Gabet, Y.; Varol, C.; et al. Erythropoietin Enhances Kupffer Cell Number and Activity in the Challenged Liver. Sci. Rep. 2017, 7, 10379. [Google Scholar] [CrossRef]
- Kumar, R.; Lin, S.; Mehta, G.; Mesquita, M.D.; Calvao, J.A.F.; Sheikh, M.F.; Agarwal, B.; Mookerjee, R.P.; Jalan, R. Non-selective Beta-blocker Is Associated with Reduced Mortality in Critically Ill Patients with Cirrhosis: A Real-world Study. Aliment. Pharmacol. Ther. 2024, 60, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Sauerbruch, T.; Hennenberg, M.; Trebicka, J.; Schierwagen, R. Beta-Blockers in Patients with Liver Cirrhosis: Pragmatism or Perfection? Front. Med. 2023, 9, 1100966. [Google Scholar] [CrossRef]
- Chavan, S.S.; Pavlov, V.A.; Tracey, K.J. Mechanisms and Therapeutic Relevance of Neuro-Immune Communication. Immunity 2017, 46, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.F.; Hay, D.L. CGRP Physiology, Pharmacology, and Therapeutic Targets: Migraine and Beyond. Physiol. Rev. 2023, 103, 1565–1644. [Google Scholar] [CrossRef]
- Laschinger, M.; Wang, Y.; Holzmann, G.; Wang, B.; Stöß, C.; Lu, M.; Brugger, M.; Schneider, A.; Knolle, P.; Wohlleber, D.; et al. The CGRP Receptor Component RAMP1 Links Sensory Innervation with YAP Activity in the Regenerating Liver. FASEB J. 2020, 34, 8125–8138. [Google Scholar] [CrossRef]
- Lu, L.; Finegold, M.J.; Johnson, R.L. Hippo Pathway Coactivators Yap and Taz Are Required to Coordinate Mammalian Liver Regeneration. Exp. Mol. Med. 2018, 50, e423. [Google Scholar] [CrossRef] [PubMed]
- Anil, M.H.; Forbes, J.M. Neural Control and Neurosensory Functions of the Liver. Proc. Nutr. Soc. 1987, 46, 125–133. [Google Scholar] [CrossRef]
- Berthoud, H. Anatomy and Function of Sensory Hepatic Nerves. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2004, 280A, 827–835. [Google Scholar] [CrossRef]
- Perra, A.; Kowalik, M.A.; Cabras, L.; Runfola, M.; Sestito, S.; Migliore, C.; Giordano, S.; Chiellini, G.; Rapposelli, S.; Columbano, A. Potential Role of Two Novel Agonists of Thyroid Hormone Receptor-β on Liver Regeneration. Cell Prolif. 2020, 53, e12808. [Google Scholar] [CrossRef]
- Hönes, G.S.; Kerp, H.; Hoppe, C.; Kowalczyk, M.; Zwanziger, D.; Baba, H.A.; Führer, D.; Moeller, L.C. Canonical Thyroid Hormone Receptor β Action Stimulates Hepatocyte Proliferation in Male Mice. Endocrinology 2022, 163, bqac003. [Google Scholar] [CrossRef]
- Columbano, A.; Simbula, M.; Pibiri, M.; Perra, A.; Deidda, M.; Locker, J.; Pisanu, A.; Uccheddu, A.; Ledda-Columbano, G.M. Triiodothyronine Stimulates Hepatocyte Proliferation in Two Models of Impaired Liver Regeneration. Cell Prolif. 2008, 41, 521–531. [Google Scholar] [CrossRef]
- Tang, Q.; Zeng, M.; Chen, L.; Fu, N. Targeting Thyroid Hormone/Thyroid Hormone Receptor Axis: An Attractive Therapy Strategy in Liver Diseases. Front. Pharmacol. 2022, 13, 871100. [Google Scholar] [CrossRef]
- Fava, G.; Glaser, S.; Phinizy, J.; Francis, H.; Marucci, L.; Benedetti, A.; Tafettani, S.; Venter, J.; Baumann, B.; Reichenbach, R.; et al. 1097 Thyroid Hormone Inhibits CAMP Dependent Proliferation of Cholangiocytes from Bile Duct Ligated Rats by a IP/Ca/PKC-Dependent Mechanism. Hepatology 2003, 38, 684–685. [Google Scholar] [CrossRef]
- Fava, G.; Ueno, Y.; Glaser, S.; Francis, H.; DeMorrow, S.; Marucci, L.; Marzioni, M.; Benedetti, A.; Venter, J.; Vaculin, B.; et al. Thyroid Hormone Inhibits Biliary Growth in Bile Duct-Ligated Rats by PLC/IP3/Ca2+-Dependent Downregulation of SRC/ERK1/2. Am. J. Physiol. Cell Physiol. 2007, 292, C1467–C1475. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Assis, L.V.M.; Harder, L.; Lacerda, J.T.; Parsons, R.; Kaehler, M.; Cascorbi, I.; Nagel, I.; Rawashdeh, O.; Mittag, J.; Oster, H. Rewiring of Liver Diurnal Transcriptome Rhythms by Triiodothyronine (T3) Supplementation. Elife 2022, 11, e79405. [Google Scholar] [CrossRef]
- Ritter, M.J.; Amano, I.; Hollenberg, A.N. Thyroid Hormone Signaling and the Liver. Hepatology 2020, 72, 742–752. [Google Scholar] [CrossRef]
- Hellerbrand, C. Hepatic Stellate Cells—The Pericytes in the Liver. Pflug. Arch. 2013, 465, 775–778. [Google Scholar] [CrossRef]
- Bioulac-Sage, P.; Lafon, M.E.; Saric, J.; Balabaud, C. Nerves and Perisinusoidal Cells in Human Liver. J. Hepatol. 1990, 10, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.; Sadiq, F.; Anstee, Q.M.; Levene, A.P.; Goldin, R.D.; Thursz, M.R. Thrombin and Factor Xa Link the Coagulation System with Liver Fibrosis. BMC Gastroenterol. 2018, 18, 60. [Google Scholar] [CrossRef]
- Yeo, X.Y.; Tan, L.Y.; Chae, W.R.; Lee, D.-Y.; Lee, Y.-A.; Wuestefeld, T.; Jung, S. Liver’s Influence on the Brain through the Action of Bile Acids. Front. Neurosci. 2023, 17, 1123967. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qiu, K.; Jiang, Y.; Huang, Y.; Zhang, Y.; Liao, Y. Metabolic Crosstalk between Liver and Brain: From Diseases to Mechanisms. Int. J. Mol. Sci. 2024, 25, 7621. [Google Scholar] [CrossRef]
- Carotti, S.; Morini, S.; Carpino, G.; Gaudio, E. Liver Histology. In Liver Diseases; Springer International Publishing: Cham, Switzerland, 2020; pp. 17–28. [Google Scholar]
- Fiederling, F.; Weschenfelder, M.; Fritz, M.; von Philipsborn, A.; Bastmeyer, M.; Weth, F. Ephrin-A/EphA Specific Co-Adaptation as a Novel Mechanism in Topographic Axon Guidance. Elife 2017, 6, e25533. [Google Scholar] [CrossRef]
- Takara, K.; Hayashi-Okada, Y.; Kidoya, H. Neurovascular Interactions in the Development of the Vasculature. Life 2022, 13, 42. [Google Scholar] [CrossRef]
- Liu, K.; Yang, L.; Wang, G.; Liu, J.; Zhao, X.; Wang, Y.; Li, J.; Yang, J. Metabolic Stress Drives Sympathetic Neuropathy within the Liver. Cell Metab. 2021, 33, 666–675.e4. [Google Scholar] [CrossRef]
- Minaya, D.M.; Di Lorenzo, P.M.; Hajnal, A.; Czaja, K. Roux-en-Y Gastric Bypass Surgery Triggers Rapid DNA Fragmentation in Vagal Afferent Neurons in Rats. Acta Neurobiol. Exp. 2019, 79, 432–444. [Google Scholar] [CrossRef]
- Orellana, E.R.; Nyland, J.E.; Horvath, N.; Hajnal, A. Vagotomy Increases Alcohol Intake in Female Rats in Diet Dependent Manner: Implications for Increased Alcohol Use Disorder after Roux-En-y Gastric Bypass Surgery. Physiol. Behav. 2021, 235, 113309. [Google Scholar] [CrossRef]
- Alpini, G.; Alvaro, D.; Marzioni, M.; LeSage, G.; LaRusso, N. The Pathophysiology of Biliary Epithelia; CRC Press: Boca Raton, FL, USA, 2020; ISBN 9780367813888. [Google Scholar]
- Saxena, R. Got Nerve? Autonomic Innervation of the Human Liver. Virchows Arch. 2020, 477, 383–384. [Google Scholar] [CrossRef]
- Abshagen, K.; Kuhla, A.; Genz, B.; Vollmar, B. Anatomy and Physiology of the Hepatic Circulation. In PanVascular Medicine; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2014; pp. 1–28. [Google Scholar]
- Lelou, E.; Corlu, A.; Nesseler, N.; Rauch, C.; Mallédant, Y.; Seguin, P.; Aninat, C. The Role of Catecholamines in Pathophysiological Liver Processes. Cells 2022, 11, 1021. [Google Scholar] [CrossRef] [PubMed]
- Adori, M.; Bhat, S.; Gramignoli, R.; Valladolid-Acebes, I.; Bengtsson, T.; Uhlèn, M.; Adori, C. Hepatic Innervations and Nonalcoholic Fatty Liver Disease. Semin. Liver Dis. 2023, 43, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Izumi, T.; Imai, J.; Yamamoto, J.; Kawana, Y.; Endo, A.; Sugawara, H.; Kohata, M.; Asai, Y.; Takahashi, K.; Kodama, S.; et al. Vagus-Macrophage-Hepatocyte Link Promotes Post-Injury Liver Regeneration and Whole-Body Survival through Hepatic FoxM1 Activation. Nat. Commun. 2018, 9, 5300. [Google Scholar] [CrossRef]
- Mandal, S.K.; Yadav, P.; Sheth, R.A. The Neuroimmune Axis and Its Therapeutic Potential for Primary Liver Cancer. Int. J. Mol. Sci. 2024, 25, 6237. [Google Scholar] [CrossRef]
- Lu, K. Cellular Pathogenesis of Hepatic Encephalopathy: An Update. Biomolecules 2023, 13, 396. [Google Scholar] [CrossRef]
- Le Guennec, L.; Mouri, S.; Thabut, D.; Weiss, N. Blood-Brain Barrier Dysfunction in Hepatic Encephalopathy: Pathophysiology, Diagnostic Assessment and Therapeutic Perspectives. Metab. Brain Dis. 2025, 40, 223. [Google Scholar] [CrossRef]
- Schwinghammer, U.A.; Melkonyan, M.M.; Hunanyan, L.; Tremmel, R.; Weiskirchen, R.; Borkham-Kamphorst, E.; Schaeffeler, E.; Seferyan, T.; Mikulits, W.; Yenkoyan, K.; et al. A2-Adrenergic Receptor in Liver Fibrosis: Implications for the Adrenoblocker Mesedin. Cells 2020, 9, 456. [Google Scholar] [CrossRef]
- Mizuno, K.; Haga, H.; Okumoto, K.; Hoshikawa, K.; Katsumi, T.; Nishina, T.; Saito, T.; Katagiri, H.; Ueno, Y. Intrahepatic Distribution of Nerve Fibers and Alterations Due to Fibrosis in Diseased Liver. PLoS ONE 2021, 16, e0249556. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Jiang, T.; Humaerhan, J.; Wang, M.; Ning, J.; Zhao, H.; Aji, T.; Shao, Y. Impact of Liver Sympathetic Nervous System on Liver Fibrosis and Regeneration After Bile Duct Ligation in Rats. J. Mol. Neurosci. 2024, 74, 4. [Google Scholar] [CrossRef] [PubMed]
- Mavila, N.; Siraganahalli Eshwaraiah, M.; Kennedy, J. Ductular Reactions in Liver Injury, Regeneration, and Disease Progression—An Overview. Cells 2024, 13, 579. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.L.; Sigala, B.; Soeda, J.; Cordero, P.; Nguyen, V.; McKee, C.; Mouraliderane, A.; Vinciguerra, M.; Oben, J.A. Acetylcholine Induces Fibrogenic Effects via M2/M3 Acetylcholine Receptors in Non-alcoholic Steatohepatitis and in Primary Human Hepatic Stellate Cells. J. Gastroenterol. Hepatol. 2016, 31, 475–483. [Google Scholar] [CrossRef]
- Airola, C.; Pallozzi, M.; Cerrito, L.; Santopaolo, F.; Stella, L.; Gasbarrini, A.; Ponziani, F.R. Microvascular Thrombosis and Liver Fibrosis Progression: Mechanisms and Clinical Applications. Cells 2023, 12, 1712. [Google Scholar] [CrossRef]
- Fiorucci, S.; Antonelli, E.; Distrutti, E.; Severino, B.; Fiorentina, R.; Baldoni, M.; Caliendo, G.; Santagada, V.; Morelli, A.; Cirino, G. PAR1 Antagonism Protects against Experimental Liver Fibrosis. Role of Proteinase Receptors in Stellate Cell Activation. Hepatology 2004, 39, 365–375. [Google Scholar] [CrossRef]
- Hwang, J.; Okada, J.; Liu, L.; Pessin, J.E.; Schwartz, G.J.; Jo, Y.-H. The Development of Hepatic Steatosis Depends on the Presence of Liver-Innervating Parasympathetic Cholinergic Neurons in Mice Fed a High-Fat Diet. PLoS Biol. 2024, 22, e3002865. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Tabiatnejad, P.; Arestakesyan, H.; Young, C.N. Modulation of Liver Lipid Metabolic Pathways by Central Nervous System ER Stress. Am. J. Physiol. Endocrinol. Metab. 2025, 328, E833–E844. [Google Scholar] [CrossRef]
- Hwang, J.; Lee, S.; Okada, J.; Liu, L.; Pessin, J.E.; Chua, S.C.; Schwartz, G.J.; Jo, Y.-H. Liver-Innervating Vagal Sensory Neurons Are Indispensable for the Development of Hepatic Steatosis and Anxiety-like Behavior in Diet-Induced Obese Mice. Nat. Commun. 2025, 16, 991. [Google Scholar] [CrossRef] [PubMed]
- John, S.; Bhowmick, K.; Park, A.; Huang, H.; Yang, X.; Mishra, L. Recent Advances in Targeting Obesity, with a Focus on TGF-β Signaling and Vagus Nerve Innervation. Bioelectron. Med. 2025, 11, 10. [Google Scholar] [CrossRef]
- Crunkhorn, S. Stopping Stellate Cell Communication Reverses Fibrosis. Nat. Rev. Drug Discov. 2023, 22, 180. [Google Scholar] [CrossRef]
- Owaki, T.; Kamimura, K.; Ko, M.; Nagayama, I.; Nagoya, T.; Shibata, O.; Oda, C.; Morita, S.; Kimura, A.; Sato, T.; et al. Involvement of the Liver-Gut Peripheral Neural Axis in Nonalcoholic Fatty Liver Disease Pathologies via Hepatic HTR2A. Dis. Model. Mech. 2022, 15, dmm049612. [Google Scholar] [CrossRef] [PubMed]
- Dunne, N.; Ivers, J.-H. HPA Axis Function in Alcohol Use Disorder: A Systematic Review and Meta-Analysis. Addict. Neurosci. 2023, 8, 100114. [Google Scholar] [CrossRef]
- Mueller, B.; Figueroa, A.; Robinson-Papp, J. Structural and Functional Connections between the Autonomic Nervous System, Hypothalamic–Pituitary–Adrenal Axis, and the Immune System: A Context and Time Dependent Stress Response Network. Neurol. Sci. 2022, 43, 951–960. [Google Scholar] [CrossRef]
- Massey, V.L.; Arteel, G.E. Acute Alcohol-Induced Liver Injury. Front. Physiol. 2012, 3, 193. [Google Scholar] [CrossRef]
- Ruiter-Lopez, L.; Khan, M.A.S.; Wang, X.; Song, B.-J. Roles of Oxidative Stress and Autophagy in Alcohol-Mediated Brain Damage. Antioxidants 2025, 14, 302. [Google Scholar] [CrossRef]
- Jacob, A.; Wang, P. Alcohol Intoxication and Cognition: Implications on Mechanisms and Therapeutic Strategies. Front. Neurosci. 2020, 14, 102. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, T.; Gao, Z.; Liu, Y.; Wei, Z.; Wang, Y. Mechanisms of Alcohol Interference with Hippocampal Neurogenesis and Its Repair Strategies. Mol. Neurobiol. 2025, 62, 15410–15429. [Google Scholar] [CrossRef]
- Julian, T.; Glascow, N.; Syeed, R.; Zis, P. Alcohol-Related Peripheral Neuropathy: A Systematic Review and Meta-Analysis. J. Neurol. 2019, 266, 2907–2919. [Google Scholar] [CrossRef]
- Keller, B.N.; Hajnal, A.; Browning, K.N.; Arnold, A.C.; Silberman, Y. Involvement of the Dorsal Vagal Complex in Alcohol-Related Behaviors. Front. Behav. Neurosci. 2022, 16, 801825. [Google Scholar] [CrossRef]
- Julian, T.H.; Syeed, R.; Glascow, N.; Zis, P. Alcohol-Induced Autonomic Dysfunction: A Systematic Review. Clin. Auton. Res. 2020, 30, 29–41. [Google Scholar] [CrossRef]
- Covelli, C.; Sacchi, D.; Sarcognato, S.; Cazzagon, N.; Grillo, F.; Baciorri, F.; Fanni, D.; Cacciatore, M.; Maffeis, V.; Guido, M. Pathology of Autoimmune Hepatitis. Pathologica 2021, 113, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, M.C.; Araujo, L.P.; Maricato, J.T.; Nascimento, V.M.; Guereschi, M.G.; Rezende, R.M.; Quintana, F.J.; Basso, A.S. Norepinephrine Controls Effector T Cell Differentiation through Β2-Adrenergic Receptor–Mediated Inhibition of NF-ΚB and AP-1 in Dendritic Cells. J. Immunol. 2016, 196, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Sasaki-Tanaka, R.; Kimura, N.; Abe, H.; Yoshida, T.; Hayashi, K.; Sakamaki, A.; Yokoo, T.; Kamimura, H.; Tsuchiya, A.; et al. Pruritus in Chronic Cholestatic Liver Diseases, Especially in Primary Biliary Cholangitis: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 1883. [Google Scholar] [CrossRef]
- Hashimoto, T.; Okuno, S. Practical Guide for the Diagnosis and Treatment of Localized and Generalized Cutaneous Pruritus (Chronic Itch with No Underlying Pruritic Dermatosis). J. Dermatol. 2025, 52, 204–220. [Google Scholar] [CrossRef] [PubMed]
- Falvey, A. Vagus Nerve Stimulation and Inflammation: Expanding the Scope beyond Cytokines. Bioelectron. Med. 2022, 8, 19. [Google Scholar] [CrossRef]
- Rose, J.P.; Morgan, D.A.; Sullivan, A.I.; Fu, X.; Inigo-Vollmer, M.; Burgess, S.C.; Meyerholz, D.K.; Rahmouni, K.; Potthoff, M.J. FGF21 Reverses MASH through Coordinated Actions on the CNS and Liver. Cell Metab. 2025, 37, 1515–1529.e6. [Google Scholar] [CrossRef] [PubMed]
- Windell, D.; Magness, A.; Beyer, C.; Brears, H.T.; Larkin, S.; Hobson, K.; Aljabar, P.; Fleming, K.; Fryer, E.; Kendall, T.; et al. AI Portal Tract Detection and Characterisation for a Regional Analysis of Steatosis and Inflammation in MASLD, MASH, and AIH. medRxiv 2025. medRxiv:23.25326290. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.F.; Nawaz, Z.; Aamir, A.; Kumar, N.; Kumar, N.K. A Case Report of Metabolic Dysfunction-Associated Steatohepatitis (MASH) Presenting with High-Titre Anti-Nuclear Antibody and Systemic Autoimmune Features: Navigating Diagnostic Challenges in the Presence of Overlapping Phenotypes. SN Compr. Clin. Med. 2025, 7, 285. [Google Scholar] [CrossRef]
- Beghini, M.; Metz, M.; Baumgartner, C.; Wolf, P.; Bastian, M.; Hackl, M.; Baumgartner-Parzer, S.; Marculescu, R.; Krebs, M.; Harreiter, J.; et al. Leptin Acutely Increases Hepatic Triglyceride Secretion in Patients with Lipodystrophy. Metabolism 2025, 169, 156261. [Google Scholar] [CrossRef]
- Lyu, Q.; Xue, W.; Liu, R.; Ma, Q.; Kasaragod, V.B.; Sun, S.; Li, Q.; Chen, Y.; Yuan, M.; Yang, Y.; et al. A Brain-to-Gut Signal Controls Intestinal Fat Absorption. Nature 2024, 634, 936–943. [Google Scholar] [CrossRef]
- Chew, V. Brain-to-Gut Control of Fat Absorption: Implications for Metabolic Liver Diseases. J. Hepatol. 2025, 82, 388–389. [Google Scholar] [CrossRef]
- Fanni, D.; Gerosa, C.; Serra, G.; Miglianti, M.; Coghe, F.; Van Eyken, P.; Faa, G.; La Nasa, G.; Guido, M. Autoimmune Liver Disease Triggered by SARS-CoV-2: A Case Report and Review of the Literature. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 1632–1638. [Google Scholar] [CrossRef]
- Littera, R.; Perra, A.; Miglianti, M.; Piras, I.S.; Mocci, S.; Lai, S.; Melis, M.; Zolfino, T.; Balestrieri, C.; Conti, M.; et al. The Double-Sided of Human Leukocyte Antigen-G Molecules in Type 1 Autoimmune Hepatitis. Front. Immunol. 2022, 13, 1007647. [Google Scholar] [CrossRef]
- Trucas, M.; Vincis, M.; Intini, C.; Johnston, D.; Diana, A.; Barry, D. The Potential Translational Utility of Embalmed Cadaveric Gastrointestinal Tract Specimens: A Proof-of-Concept Study. Transl. Res. Anat. 2025, 39, 100404. [Google Scholar] [CrossRef]
- Baiocchini, A.; Conti, M.; Fanni, D.; Ponziani, F.R.; Alagna, G.; Balsano, C.; Carotti, S.; Cossu, A.; Delle Monache, G.; Demma, S.; et al. Biopsy in Chronic Liver Disease: Proposal for a Shared Path between Clinicians and Pathologists. Pathologica 2025, 117, 136–144. [Google Scholar] [CrossRef]
- Tang, P.; Han, Z.; Zhao, Y.; Xu, T.; Zhang, Y.; Zhu, L.; Song, F.; Gao, C.; Gong, J.; Cheng, J.; et al. ALG3 as a Prognostic Biomarker and Mediator of PD-1 Blockade Resistance in Hepatocellular Carcinoma. Front. Immunol. 2025, 16, 1589153. [Google Scholar] [CrossRef]
- Bauer, K.C.; Ruf, B.; Myojin, Y.; Benmebarek, M.-R.; Ma, C.; Nur, A.; Qi, J.; Green, B.L.; Wabitsch, S.; Trehan, R.; et al. 988 Vagal-CD8+ T Cell Neuroimmune Axis Modulates Liver Cancer. In Proceedings of the Regular and Young Investigator Award Abstracts, San Diego, CA, USA, 1–5 November 2023; BMJ Publishing Group Ltd.: London, UK, 2023; p. A1095. [Google Scholar]
- Globig, A.-M.; Zhao, S.; Roginsky, J.; Maltez, V.I.; Guiza, J.; Avina-Ochoa, N.; Heeg, M.; Araujo Hoffmann, F.; Chaudhary, O.; Wang, J.; et al. The Β1-Adrenergic Receptor Links Sympathetic Nerves to T Cell Exhaustion. Nature 2023, 622, 383–392. [Google Scholar] [CrossRef]
- Bauer, K.C.; Ghabra, S.; Ma, C.; Chedester, L.; Greten, T.F. Liver Cancer Neuroscience: Regulating Liver Tumors via Selective Hepatic Vagotomy. Methods Protoc. 2024, 7, 99. [Google Scholar] [CrossRef]
- Khalifeh, J.M.; Zohny, Z.; MacEwan, M.; Stephen, M.; Johnston, W.; Gamble, P.; Zeng, Y.; Yan, Y.; Ray, W.Z. Electrical Stimulation and Bone Healing: A Review of Current Technology and Clinical Applications. IEEE Rev. Biomed. Eng. 2018, 11, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.; Meneses, J.; Garrudo, F.F.F.; Fernandes, S.R.; Alves, N.; Ferreira, F.C.; Pascoal-Faria, P. Direct Coupled Electrical Stimulation towards Improved Osteogenic Differentiation of Human Mesenchymal Stem/Stromal Cells: A Comparative Study of Different Protocols. Sci. Rep. 2024, 14, 5458. [Google Scholar] [CrossRef] [PubMed]
- Lerman, I.; Bu, Y.; Singh, R.; Silverman, H.A.; Bhardwaj, A.; Mann, A.J.; Widge, A.; Palin, J.; Puleo, C.; Lim, H. Next Generation Bioelectronic Medicine: Making the Case for Non-Invasive Closed-Loop Autonomic Neuromodulation. Bioelectron. Med. 2025, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kang, M.; Mamo, M.G.; Adisasmita, M.; Huch, M.; Choi, D. Liver Organoids: Current Advances and Future Applications for Hepatology. Clin. Mol. Hepatol. 2025, 31, S327–S348. [Google Scholar] [CrossRef]
- Gong, D.; Mo, J.; Zhai, M.; Zhou, F.; Wang, G.; Ma, S.; Dai, X.; Deng, X. Advances, Challenges and Future Applications of Liver Organoids in Experimental Regenerative Medicine. Front. Med. 2025, 11, 1521851. [Google Scholar] [CrossRef]
- Hu, Y.; Geng, Q.; Wang, L.; Wang, Y.; Huang, C.; Fan, Z.; Kong, D. Research Progress and Application of Liver Organoids for Disease Modeling and Regenerative Therapy. J. Mol. Med. 2024, 102, 859–874. [Google Scholar] [CrossRef]
- Fang, H.; Xu, H.; Yu, J.; Cao, H.; Li, L. Human Hepatobiliary Organoids: Recent Advances in Drug Toxicity Verification and Drug Screening. Biomolecules 2024, 14, 794. [Google Scholar] [CrossRef]
- Li, J.; Chu, J.; Lui, V.C.H.; Chen, S.; Chen, Y.; Tam, P.K.H. Bioengineering Liver Organoids for Diseases Modelling and Transplantation. Bioengineering 2022, 9, 796. [Google Scholar] [CrossRef] [PubMed]
- Dolciotti, C.; Righi, M.; Grecu, E.; Trucas, M.; Maxia, C.; Murtas, D.; Diana, A. The Translational Power of Alzheimer’s-Based Organoid Models in Personalized Medicine: An Integrated Biological and Digital Approach Embodying Patient Clinical History. Front. Cell Neurosci. 2025, 19, 1553642. [Google Scholar] [CrossRef]
- Camara Dit Pinto, S.; Cherkaoui, J.; Ghosh, D.; Cazaubon, V.; Benzeroual, K.E.; Levine, S.M.; Cherkaoui, M.; Sood, G.K.; Anandasabapathy, S.; Dhingra, S.; et al. A Virtual Scalable Model of the Hepatic Lobule for Acetaminophen Hepatotoxicity Prediction. NPJ Digit. Med. 2024, 7, 340. [Google Scholar] [CrossRef]
- Subramanian, K. Digital Twin for Drug Discovery and Development—The Virtual Liver. J. Indian Inst. Sci. 2020, 100, 653–662. [Google Scholar] [CrossRef]
- Miedel, M.T.; Schurdak, M.E.; Stern, A.M.; Soto-Gutierrez, A.; von Strobl, E.; Behari, J.; Taylor, D.L. Integrated Patient Digital and Biomimetic Twins for Precision Medicine: A Perspective. Semin. Liver Dis. 2025, 45, 458–475. [Google Scholar] [CrossRef] [PubMed]
- Aravindakshan, M.R.; Mandal, C.; Pothen, A.; Schaller, S.; Maass, C. DigiLoCS: A Leap Forward in Predictive Organ-on-Chip Simulations. PLoS ONE 2025, 20, e0314083. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Lawrence, M.C.; Testa, G.; Periwal, V. Donor-Specific Digital Twin for Living Donor Liver Transplant Recovery. Biol. Methods Protoc. 2025, 10, bpaf037. [Google Scholar] [CrossRef]
- Sel, K.; Hawkins-Daarud, A.; Chaudhuri, A.; Osman, D.; Bahai, A.; Paydarfar, D.; Willcox, K.; Chung, C.; Jafari, R. Survey and Perspective on Verification, Validation, and Uncertainty Quantification of Digital Twins for Precision Medicine. NPJ Digit. Med. 2025, 8, 40. [Google Scholar] [CrossRef]
- Wei, Z.; Lei, M.; Wang, Y.; Xie, Y.; Xie, X.; Lan, D.; Jia, Y.; Liu, J.; Ma, Y.; Cheng, B.; et al. Hydrogels with Tunable Mechanical Plasticity Regulate Endothelial Cell Outgrowth in Vasculogenesis and Angiogenesis. Nat. Commun. 2023, 14, 8307, Erratum in Nat Commun. 2024, 15, 3274. [Google Scholar] [CrossRef]
- Xue, T.; Zhang, J.; Li, F.; Chen, G.; Yi, K.; Chen, X.; Zhang, Y.; Xu, Y.; Wang, H.; Ju, E.; et al. Tunable Biomechanical Niches Regulate Hepatic Differentiation of Mesenchymal Stem Cells for Acute Liver Failure Therapy. Biomaterials 2026, 324, 123458. [Google Scholar] [CrossRef]
- Lin, C.-H.; Su, J.J.-M.; Lee, S.-Y.; Lin, Y.-M. Stiffness Modification of Photopolymerizable Gelatin-Methacrylate Hydrogels Influences Endothelial Differentiation of Human Mesenchymal Stem Cells. J. Tissue Eng. Regen. Med. 2018, 12, 2099–2111. [Google Scholar] [CrossRef]
- Kim, D.-H.; Ahn, J.; Kang, H.K.; Kim, M.-S.; Kim, N.-G.; Kook, M.G.; Choi, S.W.; Jeon, N.L.; Woo, H.-M.; Kang, K.-S. Development of Highly Functional Bioengineered Human Liver with Perfusable Vasculature. Biomaterials 2021, 265, 120417. [Google Scholar] [CrossRef]
- Li, K.; Tharwat, M.; Larson, E.L.; Felgendreff, P.; Hosseiniasl, S.M.; Rmilah, A.A.; Safwat, K.; Ross, J.J.; Nyberg, S.L. Re-Endothelialization of Decellularized Liver Scaffolds: A Step for Bioengineered Liver Transplantation. Front. Bioeng. Biotechnol. 2022, 10, 833163. [Google Scholar] [CrossRef] [PubMed]
- Hussein, K.H.; Park, K.-M.; Yu, L.; Kwak, H.-H.; Woo, H.-M. Decellularized Hepatic Extracellular Matrix Hydrogel Attenuates Hepatic Stellate Cell Activation and Liver Fibrosis. Mater. Sci. Eng. C 2020, 116, 111160. [Google Scholar] [CrossRef]
- Liu, H.; Puiggalí-Jou, A.; Chansoria, P.; Janiak, J.; Zenobi-Wong, M. Filamented Hydrogels as Tunable Conduits for Guiding Neurite Outgrowth. Mater. Today Bio 2025, 31, 101471. [Google Scholar] [CrossRef]
- Kim, K.; Yang, J.; Li, C.; Yang, C.-Y.; Hu, P.; Liu, Y.; Huang, Y.; Sun, X.; Chi, M.; Huang, C.; et al. Anisotropic Structure of Nanofiber Hydrogel Accelerates Diabetic Wound Healing via Triadic Synergy of Immune-Angiogenic-Neurogenic Microenvironments. Bioact. Mater. 2025, 47, 64–82. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, C.; Liu, Y.; Ding, Q.; Wang, T.; Li, H.; Wu, H.; Ma, T. Harnessing Electromagnetic Fields to Assist Bone Tissue Engineering. Stem Cell Res. Ther. 2023, 14, 7. [Google Scholar] [CrossRef]
- Kasahara, N.; Teratani, T.; Doi, J.; Yokota, S.; Shimodaira, K.; Kaneko, Y.; Ohzawa, H.; Sakuma, Y.; Sasanuma, H.; Fujimoto, Y.; et al. Controlled Release of Hydrogel-Encapsulated Mesenchymal Stem Cells-Conditioned Medium Promotes Functional Liver Regeneration after Hepatectomy in Metabolic Dysfunction-Associated Steatotic Liver Disease. Stem Cell Res. Ther. 2024, 15, 395. [Google Scholar] [CrossRef]
- Yang, H.; Wang, W.; Xiao, J.; Yang, R.; Feng, L.; Xu, H.; Xu, L.; Xing, Y. ROS-Responsive Injectable Hydrogels Loaded with Exosomes Carrying MiR-4500 Reverse Liver Fibrosis. Biomaterials 2025, 314, 122887. [Google Scholar] [CrossRef] [PubMed]
- Mays, E.A.; Ellis, E.B.; Hussain, Z.; Parajuli, P.; Sundararaghavan, H.G. Enzyme-Mediated Nerve Growth Factor Release from Nanofibers Using Gelatin Microspheres. Tissue Eng. Part A 2023, 29, 333–343. [Google Scholar] [CrossRef]
- Shen, H.; Xu, B.; Yang, C.; Xue, W.; You, Z.; Wu, X.; Ma, D.; Shao, D.; Leong, K.; Dai, J. A DAMP-Scavenging, IL-10-Releasing Hydrogel Promotes Neural Regeneration and Motor Function Recovery after Spinal Cord Injury. Biomaterials 2022, 280, 121279. [Google Scholar] [CrossRef]
- Lackington, W.A.; Kočí, Z.; Alekseeva, T.; Hibbitts, A.J.; Kneafsey, S.L.; Chen, G.; O’Brien, F.J. Controlling the Dose-Dependent, Synergistic and Temporal Effects of NGF and GDNF by Encapsulation in PLGA Microparticles for Use in Nerve Guidance Conduits for the Repair of Large Peripheral Nerve Defects. J. Control. Release 2019, 304, 51–64. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, Q.; Chen, M.; Wang, W.; He, B.; Jiang, Y.; Li, Y. MicroRNA-122 Inhibits Hepatic Stellate Cell Proliferation and Activation in Vitro and Represses Carbon Tetrachloride-Induced Liver Cirrhosis in Mice. Ann. Hepatol. 2022, 27, 100700. [Google Scholar] [CrossRef]
- Ji, D.; Wang, Q.; Zhao, Q.; Tong, H.; Yu, M.; Wang, M.; Lu, T.; Jiang, C. Co-Delivery of MiR-29b and Germacrone Based on Cyclic RGD-Modified Nanoparticles for Liver Fibrosis Therapy. J. Nanobiotechnol. 2020, 18, 86. [Google Scholar] [CrossRef]
- Jiang, S.; Kim, T.M.; Park, S.Y.; Jin, E.-J. ROS-Responsive MnO2 Mesoporous Hydrogel to Modulate Liver-Muscle Crosstalk and Mitigate NAFLD-Associated Sarcopenia via Exosomal MiR-582-5p Delivery. Theranostics 2025, 15, 4579–4592. [Google Scholar] [CrossRef]
- Mardpour, S.; Ghanian, M.H.; Sadeghi-abandansari, H.; Mardpour, S.; Nazari, A.; Shekari, F.; Baharvand, H. Hydrogel-Mediated Sustained Systemic Delivery of Mesenchymal Stem Cell-Derived Extracellular Vesicles Improves Hepatic Regeneration in Chronic Liver Failure. ACS Appl. Mater. Interfaces 2019, 11, 37421–37433. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Sales, E.; Mohaddes, G.; Alipour, M.R. Hepatoprotection of Capsaicin in Alcoholic and Non-Alcoholic Fatty Liver Diseases. Arch. Physiol. Biochem. 2024, 130, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hao, L.; Yu, F.; Li, N.; Deng, J.; Zhang, J.; Xiong, S.; Hu, X. Capsaicin: A Spicy Way in Liver Disease. Front. Pharmacol. 2024, 15, 1451084. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Y.; Zhang, S.; Yang, H.; Mao, Y. 3D Bioprinted Liver Tissue and Disease Models: Current Advances and Future Perspectives. Biomater. Adv. 2023, 152, 213499. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, Z.; Wang, X. The Prospect of Hepatic Decellularized Extracellular Matrix as a Bioink for Liver 3D Bioprinting. Biomolecules 2024, 14, 1019. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, Y.; Wang, A.; Hu, Y.; Cao, Y.; Zheng, J.; Le, Y.; Liu, J. Innovations in 3D Bioprinting and Biomaterials for Liver Tissue Engineering: Paving the Way for Tissue-Engineered Liver. iLIVER 2024, 3, 100080. [Google Scholar] [CrossRef]
- Karschau, J.; Scholich, A.; Wise, J.; Morales-Navarrete, H.; Kalaidzidis, Y.; Zerial, M.; Friedrich, B.M. Resilience of Three-Dimensional Sinusoidal Networks in Liver Tissue. PLoS Comput. Biol. 2020, 16, e1007965. [Google Scholar] [CrossRef]
- Maji, S.; Lee, M.; Lee, J.; Lee, J.; Lee, H. Development of Lumen-Based Perfusable 3D Liver in Vitro Model Using Single-Step Bioprinting with Composite Bioinks. Mater. Today Bio 2023, 21, 100723. [Google Scholar] [CrossRef] [PubMed]
- Taymour, R.; Chicaiza-Cabezas, N.A.; Gelinsky, M.; Lode, A. Core–Shell Bioprinting of Vascularized in Vitro Liver Sinusoid Models. Biofabrication 2022, 14, 45019. [Google Scholar] [CrossRef]
- Kang, D.; Hong, G.; An, S.; Jang, I.; Yun, W.; Shim, J.; Jin, S. Bioprinting of Multiscaled Hepatic Lobules within a Highly Vascularized Construct. Small 2020, 16, 1905505. [Google Scholar] [CrossRef]
- Zhang, L.; Qiu, P.; Shen, S.; Zhang, C.; Qin, J.; Xu, Z.; Yixi, Z.; Lv, J.; Pu, H.; Lei, J.; et al. Polydopamine-Modified Hydrogel Nanofibers for Sustained SFRP2 Release: Synergistic Promotion of Angiogenesis and Nerve Regeneration. NPG Asia Mater. 2025, 17, 29. [Google Scholar] [CrossRef]
- Zheng, T.; Wu, L.; Sun, S.; Xu, J.; Han, Q.; Liu, Y.; Wu, R.; Li, G. Co-Culture of Schwann Cells and Endothelial Cells for Synergistically Regulating Dorsal Root Ganglion Behavior on Chitosan-Based Anisotropic Topology for Peripheral Nerve Regeneration. Burn. Trauma 2022, 10, tkac030. [Google Scholar] [CrossRef]
- Alakpa, E.V.; Bahrd, A.; Wiklund, K.; Andersson, M.; Novikov, L.N.; Ljungberg, C.; Kelk, P. Bioprinted Schwann and Mesenchymal Stem Cell Co-Cultures for Enhanced Spatial Control of Neurite Outgrowth. Gels 2023, 9, 172. [Google Scholar] [CrossRef] [PubMed]


| Disease | Sympathetic Role | Parasympathetic Role | Sensory/Other Notes |
|---|---|---|---|
| Liver Fibrosis | Targets sinusoids & HSCs; promotes fibrosis; α2-AR antagonists reduce HSC activation | Supports regeneration; cholinergic fibres around ductular reactions; ACh modulates HSC proliferation | CGRP from sensory fibres promotes regeneration |
| MASLD/MASH | Overactivity leads to metabolic dysregulation & inflammation; Endoplasmic Reticulum stress in CNS circuits leads to increased sympathetic tone | Vagal afferents regulate satiety & energy; dysfunction worsens MASLD/MASH | Gut–liver axis: serotonin (5-HT) signalling implicated |
| ALD | Sympathetic drive via HPA axis leads to Kupffer activation, fibrosis, NF-κB activation | Vagus modulates neuroimmune response; potential therapeutic target (VNS) | ROS & acetaldehyde sensitise the liver to immune-mediated damage |
| Autoimmune Liver Diseases | Norepinephrine disrupts immune tolerance; increased dendritic cell activation | Vagus promotes immune tolerance via α7nAChR; VNS reduces inflammation | Sensory fibres mediate pruritus via TGR5 & MRGPR signalling |
| Hepatic Malignancies | Promotes tumour growth & immune suppression via β1-AR | Anti-inflammatory, but may dampen anti-tumour immunity | Neuroimmune axis shapes tumour microenvironment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trucas, M.; Barry, D.; Conroy, M.J.; Vincis, M.; Diana, A.; Intini, C.; Gobbi, P.; Gerosa, C.; Fanni, D.; Perra, A. Liver Innervation in Health and Disease: Neuroimmune–Neurovascular Interface and Future Therapeutic Implications. Biomedicines 2025, 13, 3091. https://doi.org/10.3390/biomedicines13123091
Trucas M, Barry D, Conroy MJ, Vincis M, Diana A, Intini C, Gobbi P, Gerosa C, Fanni D, Perra A. Liver Innervation in Health and Disease: Neuroimmune–Neurovascular Interface and Future Therapeutic Implications. Biomedicines. 2025; 13(12):3091. https://doi.org/10.3390/biomedicines13123091
Chicago/Turabian StyleTrucas, Marcello, Denis Barry, Melissa J. Conroy, Michela Vincis, Andrea Diana, Claudio Intini, Pietro Gobbi, Clara Gerosa, Daniela Fanni, and Andrea Perra. 2025. "Liver Innervation in Health and Disease: Neuroimmune–Neurovascular Interface and Future Therapeutic Implications" Biomedicines 13, no. 12: 3091. https://doi.org/10.3390/biomedicines13123091
APA StyleTrucas, M., Barry, D., Conroy, M. J., Vincis, M., Diana, A., Intini, C., Gobbi, P., Gerosa, C., Fanni, D., & Perra, A. (2025). Liver Innervation in Health and Disease: Neuroimmune–Neurovascular Interface and Future Therapeutic Implications. Biomedicines, 13(12), 3091. https://doi.org/10.3390/biomedicines13123091









