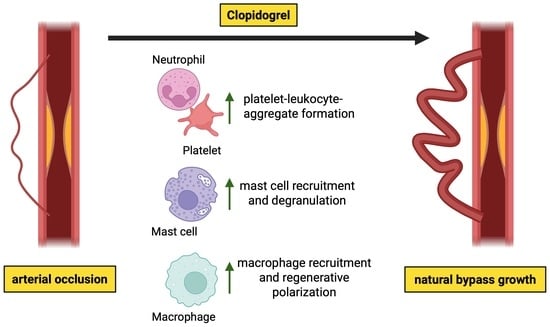
P2Y12-Inhibitor Clopidogrel Promotes Collateral Artery Growth in a Murine Hindlimb Model of Arteriogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model and Drug Treatment
2.2. Femoral Artery Ligation (FAL)
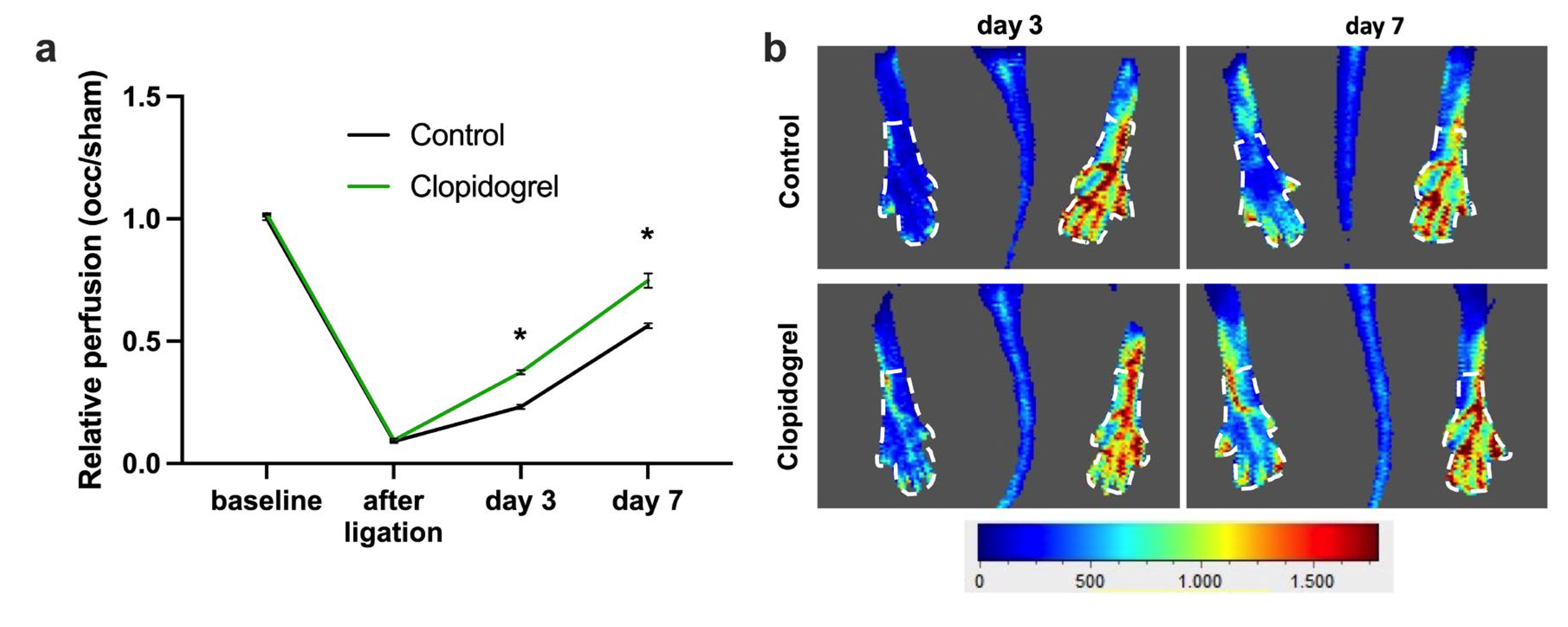
2.3. Laser-Doppler Perfusion Imaging
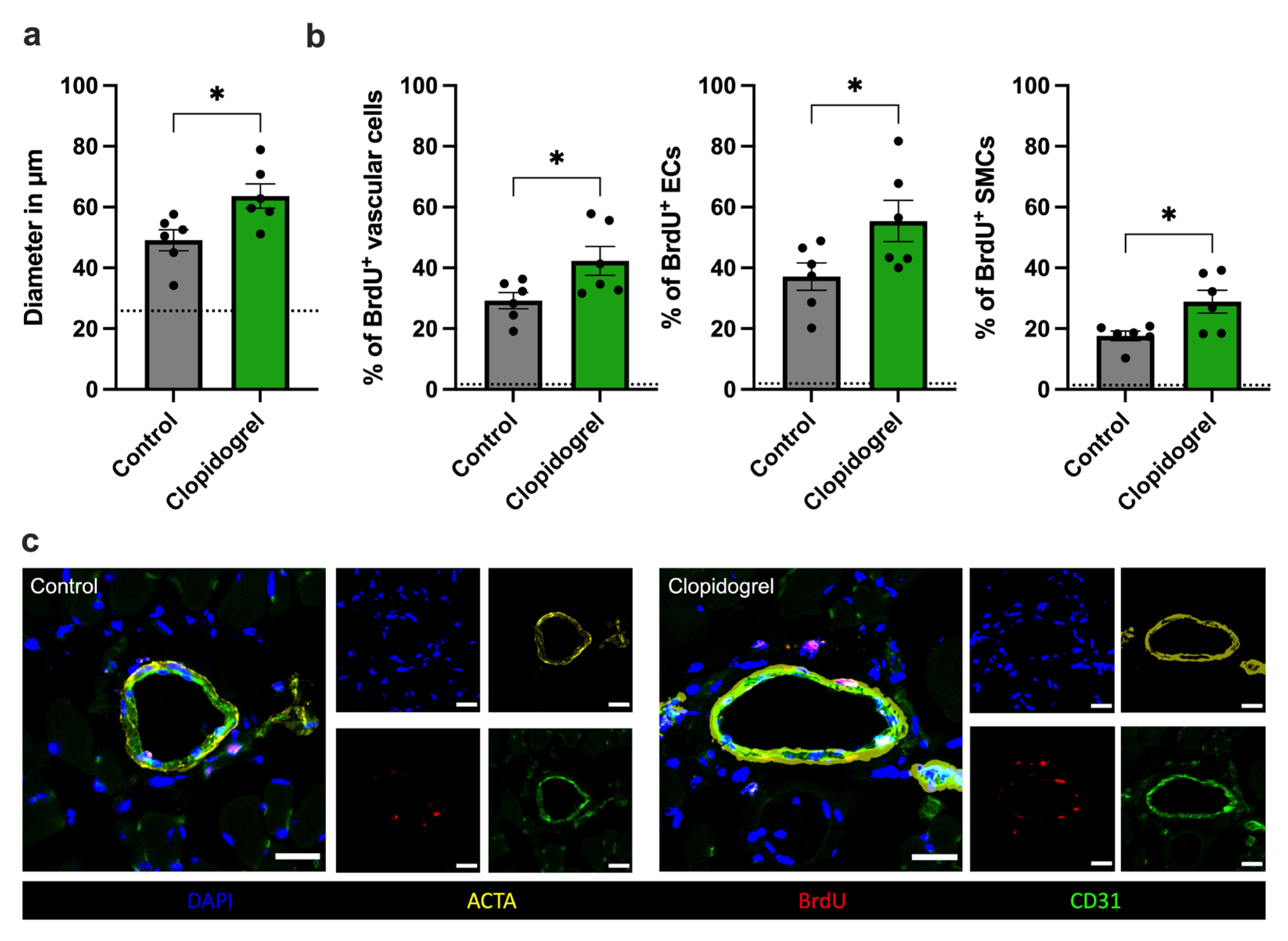
2.4. Immunofluorescence and Histological Staining
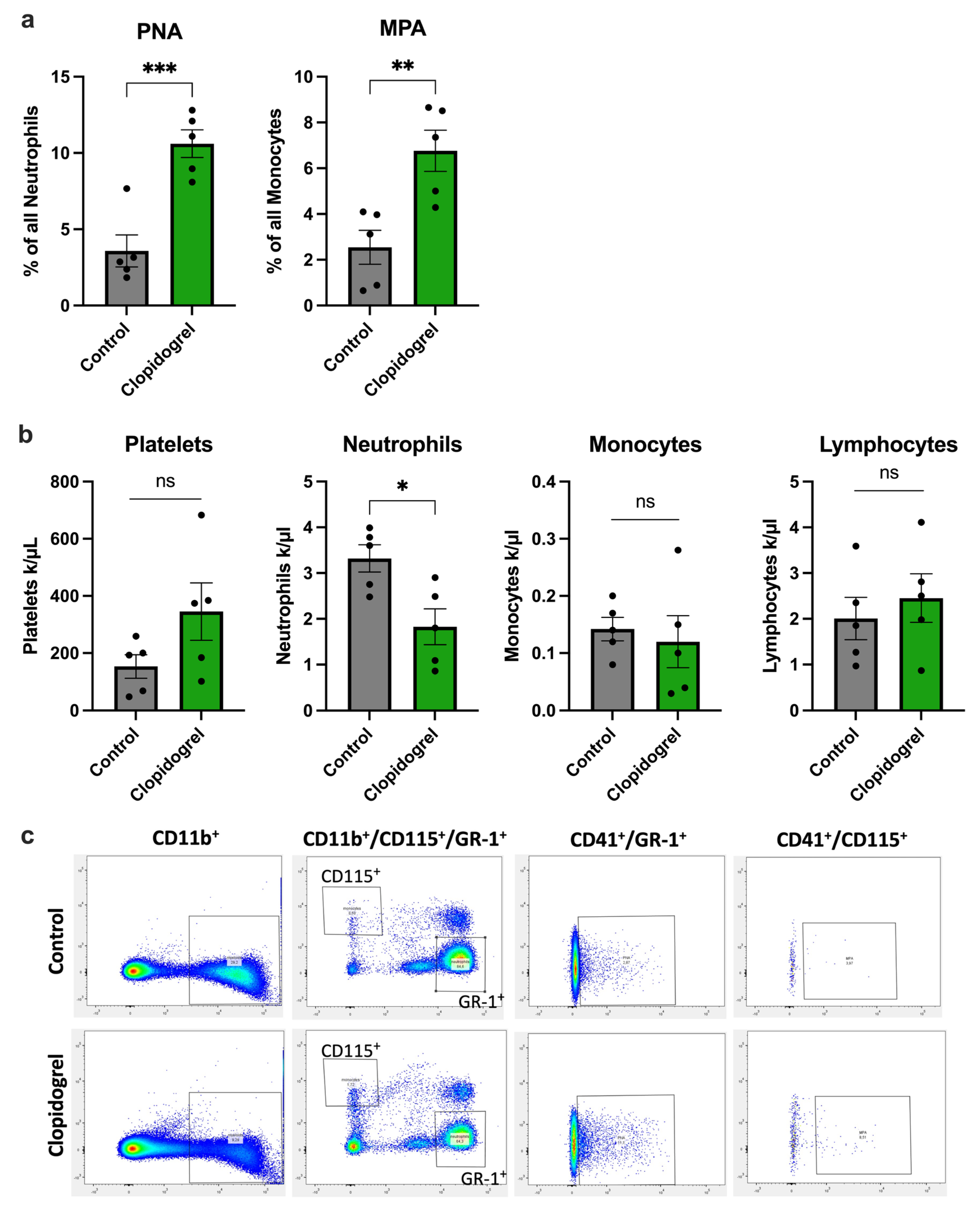
2.5. Flow-Cytometry
2.6. Differential Blood Analysis
2.7. Statistical Analyses
3. Results
3.1. P2Y12 Inhibition by Clopidogrel Boosts Perfusion Recovery After Femoral Artery Ligation
3.2. Clopidogrel Treatment Enhances Vascular Cell Proliferation In Vivo
3.3. Clopidogrel Promotes Platelet-Leukocyte-Aggregate Formation During Arteriogenesis
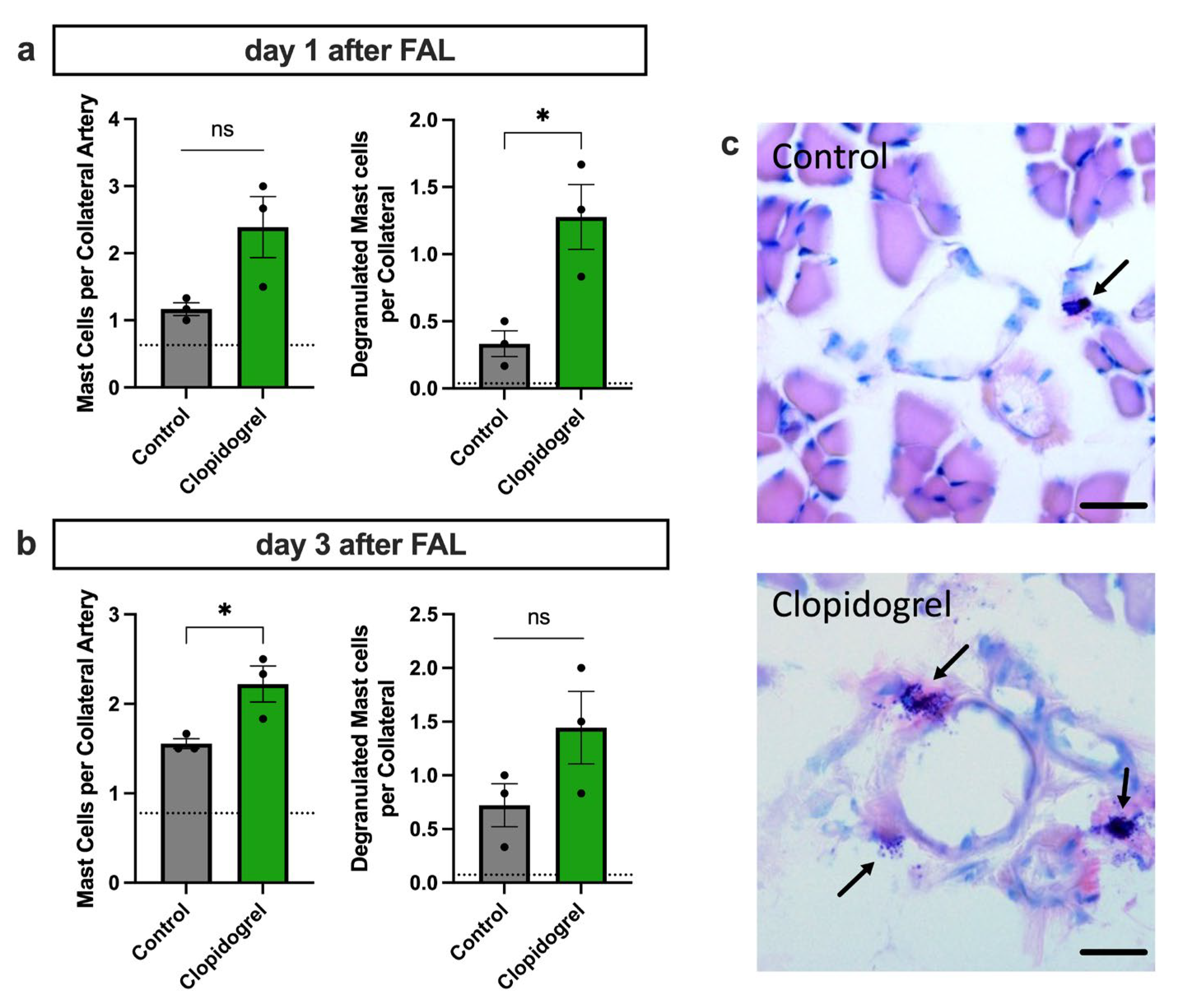
3.4. Enhanced Perivascular Mast Cell Recruitment and Degranulation upon Clopidogrel Treatment in Arteriogenesis
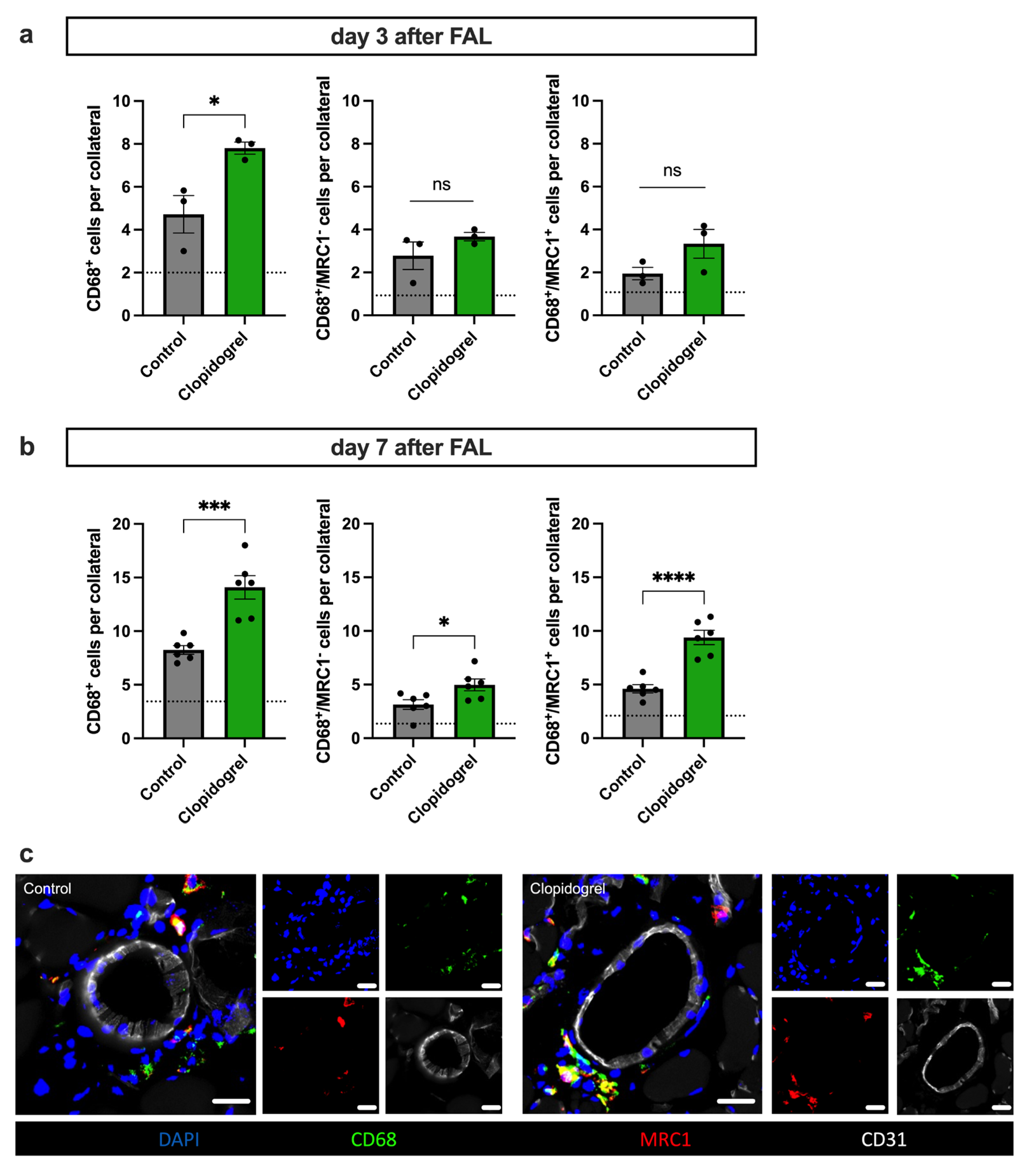
3.5. Clopidogrel Boosts Regenerative Inflammation Around Growing Collateral Arteries
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTA2 | Anti-alpha smooth muscle actin |
| ADP | Adenosine diphosphate |
| BrdU | Bromodeoxyuridine |
| BSA | Bovine serum albumin |
| FAL | Femoral artery ligation |
| GPIIb/IIIa | Glycoprotein IIb/IIIa integrin receptor |
| MCP-1 | Monocyte chemoattractant protein 1 |
| MPA | Monocyte-platelet-aggregate |
| MRC1 | Mannose receptor C type 1 |
| P2Y12 | Purinergic receptor P2Y subtype 12 |
| PAI-1 | Plasminogen activator inhibitor-1 |
| PBS | Phosphate-buffered saline |
| PDGF | Platelet derived growth factor |
| PFA | Paraformaldehyde |
| PKG | Protein kinase G |
| PLA | Platelet-leukocyte-aggregate |
| PNA | Platelet-neutrophil-aggregate |
| ROS | Reactive oxygen species |
| SDF-1α | Stromal-derived factor 1 alpha |
| SMC | Smooth muscle cell |
| TGF-ß | Transforming growth factor ß |
| uPA | Urokinase-type plasminogen activator |
| VASP | Vasodilator-stimulated phosphoprotein |
| VEGF | Vascular endothelial growth factor |
| VWF | Von Willebrand factor |
References
- Wang, L.; Ostberg, O.; Wihlborg, A.K.; Brogren, H.; Jern, S.; Erlinge, D. Quantification of ADP and ATP receptor expression in human platelets. J. Thromb. Haemost. 2003, 1, 330–336. [Google Scholar] [CrossRef]
- Kim, S.; Kunapuli, S.P. P2Y12 receptor in platelet activation. Platelets 2011, 22, 56–60. [Google Scholar] [CrossRef]
- Wihlborg, A.-K.; Malmsjö, M.; Eyjolfsson, A.; Gustafsson, R.; Jacobson, K.; Erlinge, D. Extracellular nucleotides induce vasodilatation in human arteries via prostaglandins, nitric oxide and endothelium-derived hyperpolarising factor. Br. J. Pharmacol. 2003, 138, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.R.; Outteridge, S.N.; Ajjan, R.A.; Phoenix, F.; Sangha, G.K.; Faulkner, R.E.; Ecob, R.; Judge, H.M.; Khan, H.; West, L.E.; et al. Platelet P2Y12 Inhibitors Reduce Systemic Inflammation and Its Prothrombotic Effects in an Experimental Human Model. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2562–2570. [Google Scholar] [CrossRef]
- Puri, R.N.; Colman, R.W. ADP-induced platelet activation. Crit. Rev. Biochem. Mol. Biol. 1997, 32, 437–502. [Google Scholar] [CrossRef]
- Pultar, J.; Wadowski, P.P.; Panzer, S.; Gremmel, T. Oral antiplatelet agents in cardiovascular disease. Vasa 2019, 48, 291–302. [Google Scholar] [CrossRef]
- Patti, G.; Micieli, G.; Cimminiello, C.; Bolognese, L. The Role of Clopidogrel in 2020: A Reappraisal. Cardiovasc. Ther. 2020, 2020, 8703627. [Google Scholar] [CrossRef] [PubMed]
- Helisch, A.; Schaper, W. Arteriogenesis: The development and growth of collateral arteries. Microcirculation 2003, 10, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Deindl, E.; Buschmann, I.; Hoefer, I.E.; Podzuweit, T.; Boengler, K.; Vogel, S.; van Royen, N.; Fernandez, B.; Schaper, W. Role of ischemia and of hypoxia-inducible genes in arteriogenesis after femoral artery occlusion in the rabbit. Circ. Res. 2001, 89, 779–786. [Google Scholar] [CrossRef]
- Heil, M.; Eitenmüller, I.; Schmitz-Rixen, T.; Schaper, W. Arteriogenesis versus angiogenesis: Similarities and differences. J. Cell. Mol. Med. 2006, 10, 45–55. [Google Scholar] [CrossRef]
- Elbs, K.; Deindl, E. Die paradoxe arteriogene und thrombogene Rolle der Thrombozyten in der Arteriogenese. Gefässchirurgie 2025, 30, 177–182. [Google Scholar] [CrossRef]
- Chatterjee, M.; Huang, Z.; Zhang, W.; Jiang, L.; Hultenby, K.; Zhu, L.; Hu, H.; Nilsson, G.P.; Li, N. Distinct platelet packaging, release, and surface expression of proangiogenic and antiangiogenic factors on different platelet stimuli. Blood 2011, 117, 3907–3911. [Google Scholar] [CrossRef] [PubMed]
- Chillo, O.; Kleinert, E.C.; Lautz, T.; Lasch, M.; Pagel, J.I.; Heun, Y.; Troidl, K.; Fischer, S.; Caballero-Martinez, A.; Mauer, A.; et al. Perivascular Mast Cells Govern Shear Stress-Induced Arteriogenesis by Orchestrating Leukocyte Function. Cell Rep. 2016, 16, 2197–2207. [Google Scholar] [CrossRef]
- Geiger, J.; Brich, J.; Hönig-Liedl, P.; Eigenthaler, M.; Schanzenbächer, P.; Herbert, J.M.; Walter, U. Specific impairment of human platelet P2Y(AC) ADP receptor-mediated signaling by the antiplatelet drug clopidogrel. Arter. Thromb. Vasc. Biol. 1999, 19, 2007–2011. [Google Scholar] [CrossRef]
- Pampuch, A.; Cerletti, C.; de Gaetano, G. Comparison of VASP-phosphorylation assay to light-transmission aggregometry in assessing inhibition of the platelet ADP P2Y12 receptor. Thromb. Haemost. 2006, 96, 767–773. [Google Scholar] [CrossRef]
- Kuszynski, D.S.; Lauver, D.A. Pleiotropic effects of clopidogrel. Purinergic Signal. 2022, 18, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, A.; Chlopicki, S.; Olszanecki, R.; Jawien, J.; Lomnicka, M.; Dupin, J.P.; Gryglewski, R.J. Endothelial action of thienopyridines and thienopyrimidinones in the isolated guinea pig heart. Prostaglandins Leukot. Essent. Fat. Acids 2005, 72, 139–145. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Jiang, G.; Cheng, C.; Lv, Z.; Liu, Y.; Wang, F. Inhibition of Platelets by Clopidogrel Suppressed Ang II-Induced Vascular Inflammation, Oxidative Stress, and Remodeling. J. Am. Heart Assoc. 2018, 7, e009600. [Google Scholar] [CrossRef]
- Lieschke, F.; Zheng, Y.; Schaefer, J.H.; van Leyen, K.; Foerch, C. Measurement of Platelet Function in an Experimental Stroke Model With Aspirin and Clopidogrel Treatment. Front. Neurol. 2020, 11, 85. [Google Scholar] [CrossRef]
- Deindl, E.; Ziegelhöffer, T.; Kanse, S.M.; Fernandez, B.; Neubauer, E.; Carmeliet, P.; Preissner, K.T.; Schaper, W. Receptor-independent role of the urokinase-type plasminogen activator during arteriogenesis. Faseb J. 2003, 17, 1174–1176. [Google Scholar] [CrossRef]
- Stefanović, D.; Samardžija, G.; Redžek, A.; Arnaut, M.; Nikin, Z.; Stefanović, M. Buffered Romanowsky-Giemsa method for formalin fixed, paraffin embedded sections: Taming a traditional stain. Biotech. Histochem. 2017, 92, 299–308. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Chandraratne, S.; von Bruehl, M.L.; Pagel, J.I.; Stark, K.; Kleinert, E.; Konrad, I.; Farschtschi, S.; Coletti, R.; Gartner, F.; Chillo, O.; et al. Critical role of platelet glycoprotein ibalpha in arterial remodeling. Arter. Thromb. Vasc. Biol. 2015, 35, 589–597. [Google Scholar] [CrossRef]
- Chatterjee, M.; Gawaz, M. Platelet-derived CXCL12 (SDF-1α): Basic mechanisms and clinical implications. J. Thromb. Haemost. 2013, 11, 1954–1967. [Google Scholar] [CrossRef]
- Gaidai, O.; Cao, Y.; Loginov, S. Global Cardiovascular Diseases Death Rate Prediction. Curr. Probl. Cardiol. 2023, 48, 101622. [Google Scholar] [CrossRef] [PubMed]
- Abou-Saleh, H.; Théorêt, J.F.; Yacoub, D.; Merhi, Y. Neutrophil P-selectin-glycoprotein-ligand-1 binding to platelet P-selectin enhances metalloproteinase 2 secretion and platelet-neutrophil aggregation. Thromb. Haemost. 2005, 94, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Choi, K.H.; Giacoppo, D.; Gragnano, F.; Kimura, T.; Watanabe, H.; Kim, H.S.; Kang, J.; Park, K.W.; Pettersen, A.; et al. Clopidogrel versus aspirin for secondary prevention of coronary artery disease: A systematic review and individual patient data meta-analysis. Lancet 2025, 406, 1091–1102. [Google Scholar] [CrossRef]
- Hoefer, I.E.; Grundmann, S.; Schirmer, S.; van Royen, N.; Meder, B.; Bode, C.; Piek, J.J.; Buschmann, I.R. Aspirin, but not clopidogrel, reduces collateral conductance in a rabbit model of femoral artery occlusion. J. Am. Coll. Cardiol. 2005, 46, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Duelsner, A.; Gatzke, N.; Glaser, J.; Hillmeister, P.; Li, M.; Lee, E.J.; Lehmann, K.; Urban, D.; Meyborg, H.; Stawowy, P.; et al. Acetylsalicylic acid, but not clopidogrel, inhibits therapeutically induced cerebral arteriogenesis in the hypoperfused rat brain. J. Cereb. Blood Flow. Metab. 2012, 32, 105–114. [Google Scholar] [CrossRef]
- Lauer, A.; Schlunk, F.; Van Cott, E.M.; Steinmetz, H.; Lo, E.H.; Foerch, C. Antiplatelet pretreatment does not increase hematoma volume in experimental intracerebral hemorrhage. J. Cereb. Blood Flow. Metab. 2011, 31, 1736–1742. [Google Scholar] [CrossRef]
- Kuszynski, D.S.; Christian, B.D.; Dorrance, A.M.; Lauver, D.A. Clopidogrel treatment inhibits P2Y(2)-Mediated constriction in the rabbit middle cerebral artery. Eur. J. Pharmacol. 2021, 911, 174545. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.X.; Qi, G.M.; Liu, O.; Li, T.T.; Yang, M.; Cui, W.; Zhang, W.M.; Qi, Y.F.; Du, J. Inhibition of platelet activation by clopidogrel prevents hypertension-induced cardiac inflammation and fibrosis. Cardiovasc. Drugs Ther. 2013, 27, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Kluever, A.K.; Braumandl, A.; Fischer, S.; Preissner, K.T.; Deindl, E. The Extraordinary Role of Extracellular RNA in Arteriogenesis, the Growth of Collateral Arteries. Int. J. Mol. Sci. 2019, 20, 6177. [Google Scholar] [CrossRef] [PubMed]
- Lasch, M.; Kleinert, E.C.; Meister, S.; Kumaraswami, K.; Buchheim, J.I.; Grantzow, T.; Lautz, T.; Salpisti, S.; Fischer, S.; Troidl, K.; et al. Extracellular RNA released due to shear stress controls natural bypass growth by mediating mechanotransduction in mice. Blood 2019, 134, 1469–1479. [Google Scholar] [CrossRef]
- Schrottmaier, W.C.; Kral, J.B.; Badrnya, S.; Assinger, A. Aspirin and P2Y12 Inhibitors in platelet-mediated activation of neutrophils and monocytes. Thromb. Haemost. 2015, 114, 478–489. [Google Scholar] [CrossRef]
- Evangelista, V.; Manarini, S.; Dell’Elba, G.; Martelli, N.; Napoleone, E.; Di Santo, A.; Lorenzet, P.S. Clopidogrel inhibits platelet-leukocyte adhesion and platelet-dependent leukocyte activation. Thromb. Haemost. 2005, 94, 568–577. [Google Scholar]
- De Servi, S.; Landi, A.; Savonitto, S. Clopidogrel induced reduction in neutrophil count: An overlooked beneficial effect? Eur. J. Intern. Med. 2024, 124, 32–34. [Google Scholar] [CrossRef]
- de Servi, S.; Ricevuti, G.; Mazzone, A.; Ghio, S.; Zito, A.; Raffaghello, S.; Specchia, G. Granulocyte function in coronary artery disease. Am. J. Cardiol. 1991, 68, 64b–68b. [Google Scholar] [CrossRef]
- Weiss, S.J.; Young, J.; LoBuglio, A.F.; Slivka, A.; Nimeh, N.F. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J. Clin. Investig. 1981, 68, 714–721. [Google Scholar] [CrossRef]
- Borges, P.A.; Waclawiak, I.; Georgii, J.L.; Fraga-Junior, V.D.S.; Barros, J.F.; Lemos, F.S.; Russo-Abrahão, T.; Saraiva, E.M.; Takiya, C.M.; Coutinho-Silva, R.; et al. Adenosine Diphosphate Improves Wound Healing in Diabetic Mice Through P2Y(12) Receptor Activation. Front. Immunol. 2021, 12, 651740. [Google Scholar] [CrossRef]
- Nassar, A.; Wagura, E.; Loukas, M. Mast cells and arteriogenesis: A systematic review. Cardiovasc. Pathol. 2025, 75, 107716. [Google Scholar] [CrossRef] [PubMed]
- Troidl, C.; Jung, G.; Troidl, K.; Hoffmann, J.; Mollmann, H.; Nef, H.; Schaper, W.; Hamm, C.W.; Schmitz-Rixen, T. The temporal and spatial distribution of macrophage subpopulations during arteriogenesis. Curr. Vasc. Pharmacol. 2013, 11, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Mantsounga, C.S.; Lee, C.; Neverson, J.; Sharma, S.; Healy, A.; Berus, J.M.; Parry, C.; Ceneri, N.M.; López-Giráldez, F.; Chun, H.J.; et al. Macrophage IL-1β promotes arteriogenesis by autocrine STAT3- and NF-κB-mediated transcription of pro-angiogenic VEGF-A. Cell Rep. 2022, 38, 110309. [Google Scholar] [CrossRef]
- Busti, C.; Falcinelli, E.; Momi, S.; Gresele, P. Matrix metalloproteinases and peripheral arterial disease. Intern. Emerg. Med. 2010, 5, 13–25. [Google Scholar] [CrossRef]
- Spiller, K.L.; Anfang, R.R.; Spiller, K.J.; Ng, J.; Nakazawa, K.R.; Daulton, J.W.; Vunjak-Novakovic, G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 2014, 35, 4477–4488. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tang, Y.; Zhong, Y.; Wei, B.; Huang, X.R.; Tang, P.M.; Xu, A.; Lan, H.Y. P2Y12 inhibitor clopidogrel inhibits renal fibrosis by blocking macrophage-to-myofibroblast transition. Mol. Ther. 2022, 30, 3017–3033. [Google Scholar] [CrossRef]
- Cerda, A.; Pavez, M.; Manriquez, V.; Luchessi, A.D.; Leal, P.; Benavente, F.; Fajardo, C.M.; Salazar, L.; Hirata, M.H.; Hirata, R.D.C. Effects of clopidogrel on inflammatory cytokines and adhesion molecules in human endothelial cells: Role of nitric oxide mediating pleiotropic effects. Cardiovasc. Ther. 2017, 35, e12261. [Google Scholar] [CrossRef]
- Zhu, A.; Baur, C.; Götz, P.; Elbs, K.; Lasch, M.; Faro, A.; Preissner, K.T.; Deindl, E. The Complement System Is Essential for Arteriogenesis by Enhancing Sterile Inflammation as a Relevant Step in Collateral Artery Growth. Cells 2024, 13, 1405. [Google Scholar] [CrossRef]
- Zhao, L.; Gray, L.; Leonardi-Bee, J.; Weaver, C.S.; Heptinstall, S.; Bath, P.M. Effect of aspirin, clopidogrel and dipyridamole on soluble markers of vascular function in normal volunteers and patients with prior ischaemic stroke. Platelets 2006, 17, 100–104. [Google Scholar] [CrossRef]
- Thulasingam, S.; Krishnasamy, S.; Raj, C.D.; Lasch, M.; Vedantham, S.; Deindl, E. Insulin Treatment Forces Arteriogenesis in Diabetes Mellitus by Upregulation of the Early Growth Response-1 (Egr-1) Pathway in Mice. Int. J. Mol. Sci. 2019, 20, 3320. [Google Scholar] [CrossRef]
- Lopez, J.; Mark, J.; Duarte, G.J.; Shaban, M.; Sosa, F.; Mishra, R.; Jain, S.; Tran, A.; Khizar, A.; Karpel, D.; et al. Role of genetic polymorphisms in clopidogrel response variability: A systematic review. Open Heart 2023, 10, e002436. [Google Scholar] [CrossRef] [PubMed]
- Ancrenaz, V.; Daali, Y.; Fontana, P.; Besson, M.; Samer, C.; Dayer, P.; Desmeules, J. Impact of genetic polymorphisms and drug-drug interactions on clopidogrel and prasugrel response variability. Curr. Drug Metab. 2010, 11, 667–677. [Google Scholar] [CrossRef]
- Hall, H.M.; Banerjee, S.; McGuire, D.K. Variability of clopidogrel response in patients with type 2 diabetes mellitus. Diabetes Vasc. Dis. Res. 2011, 8, 245–253. [Google Scholar] [CrossRef]
- Cattaneo, M. Response variability to clopidogrel: Is tailored treatment, based on laboratory testing, the right solution? J. Thromb. Haemost. 2012, 10, 327–336. [Google Scholar] [CrossRef]
- Yusuf, S.; Zhao, F.; Mehta, S.R.; Chrolavicius, S.; Tognoni, G.; Fox, K.K. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N. Engl. J. Med. 2001, 345, 494–502. [Google Scholar] [CrossRef]
- Barcena, A.J.R.; Perez, J.V.D.; Bernardino, M.R.; San Valentin, E.M.D.; Damasco, J.A.; Klusman, C.; Martin, B.; Court, K.A.; Godin, B.; Canlas, G.; et al. Controlled Delivery of Rosuvastatin or Rapamycin through Electrospun Bismuth Nanoparticle-Infused Perivascular Wraps Promotes Arteriovenous Fistula Maturation. ACS Appl. Mater. Interfaces 2024, 16, 33159–33168. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Curneen, J.M.G.; McEvoy, J.W. Aspirin in the Modern Era of Cardiovascular Disease Prevention. Methodist Debakey Cardiovasc. J. 2021, 17, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Park, Y.H.; Lee, J.Y.; Jeong, J.O.; Kim, C.J.; Yun, K.H.; Lee, H.C.; Chang, K.; Park, M.W.; Bae, J.W.; et al. Efficacy and safety of clopidogrel versus aspirin monotherapy in patients at high risk of subsequent cardiovascular event after percutaneous coronary intervention (SMART-CHOICE 3): A randomised, open-label, multicentre trial. Lancet 2025, 405, 1252–1263. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbs, K.; Bobrowski, L.; Arnholdt, C.; Kübler, M.; Götz, P.; Rohrmoser, M.R.; Merkus, D.; Lasch, M.; Deindl, E. P2Y12-Inhibitor Clopidogrel Promotes Collateral Artery Growth in a Murine Hindlimb Model of Arteriogenesis. Biomedicines 2025, 13, 2790. https://doi.org/10.3390/biomedicines13112790
Elbs K, Bobrowski L, Arnholdt C, Kübler M, Götz P, Rohrmoser MR, Merkus D, Lasch M, Deindl E. P2Y12-Inhibitor Clopidogrel Promotes Collateral Artery Growth in a Murine Hindlimb Model of Arteriogenesis. Biomedicines. 2025; 13(11):2790. https://doi.org/10.3390/biomedicines13112790
Chicago/Turabian StyleElbs, Katharina, Lisa Bobrowski, Christoph Arnholdt, Matthias Kübler, Philipp Götz, Michael R. Rohrmoser, Daphne Merkus, Manuel Lasch, and Elisabeth Deindl. 2025. "P2Y12-Inhibitor Clopidogrel Promotes Collateral Artery Growth in a Murine Hindlimb Model of Arteriogenesis" Biomedicines 13, no. 11: 2790. https://doi.org/10.3390/biomedicines13112790
APA StyleElbs, K., Bobrowski, L., Arnholdt, C., Kübler, M., Götz, P., Rohrmoser, M. R., Merkus, D., Lasch, M., & Deindl, E. (2025). P2Y12-Inhibitor Clopidogrel Promotes Collateral Artery Growth in a Murine Hindlimb Model of Arteriogenesis. Biomedicines, 13(11), 2790. https://doi.org/10.3390/biomedicines13112790