Prognosis and Risk Stratification of Patients with Advanced Heart Failure Followed-Up on an Outpatient Clinic
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
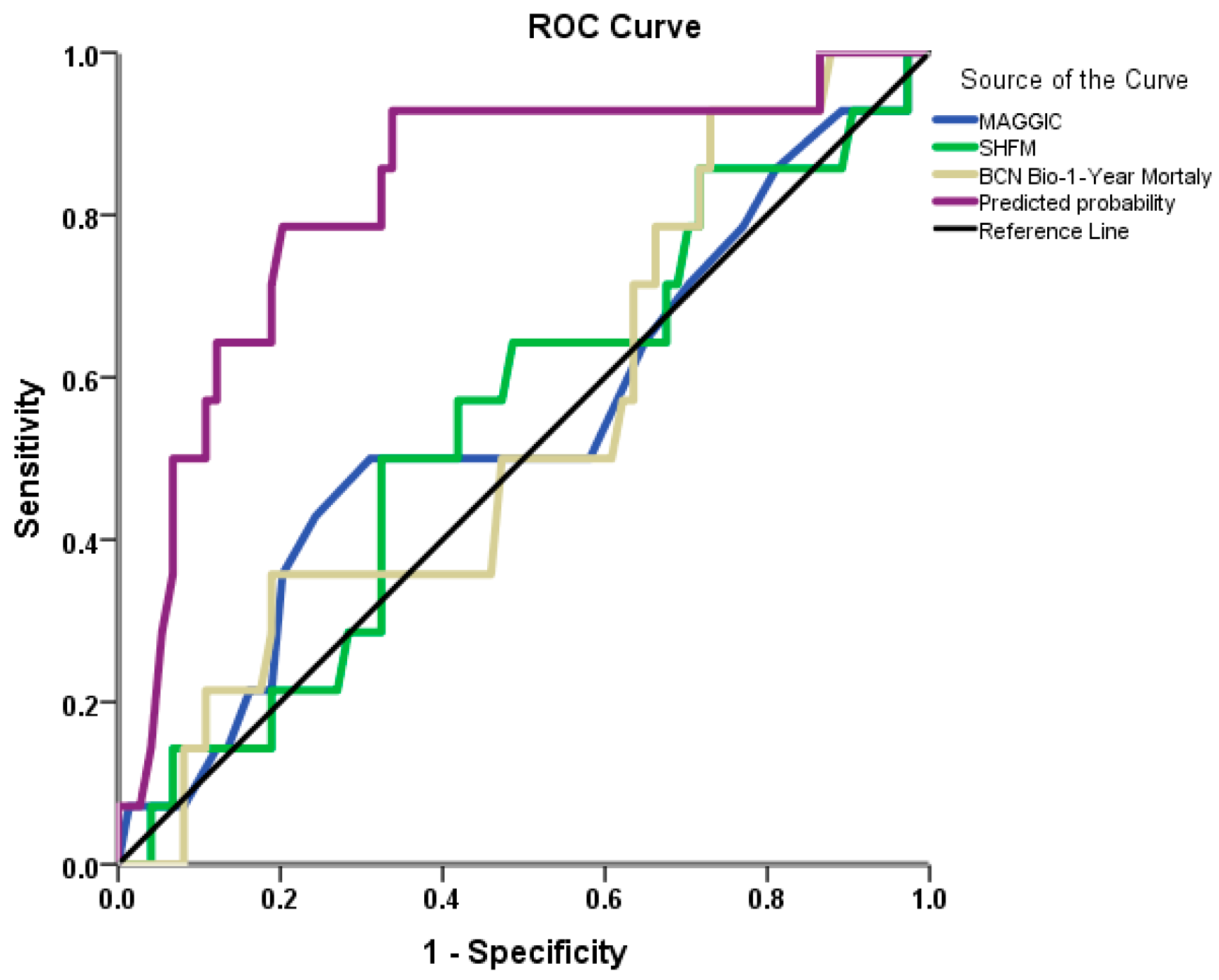
3.1. Mortality at 1-Year Follow-Up Analysis
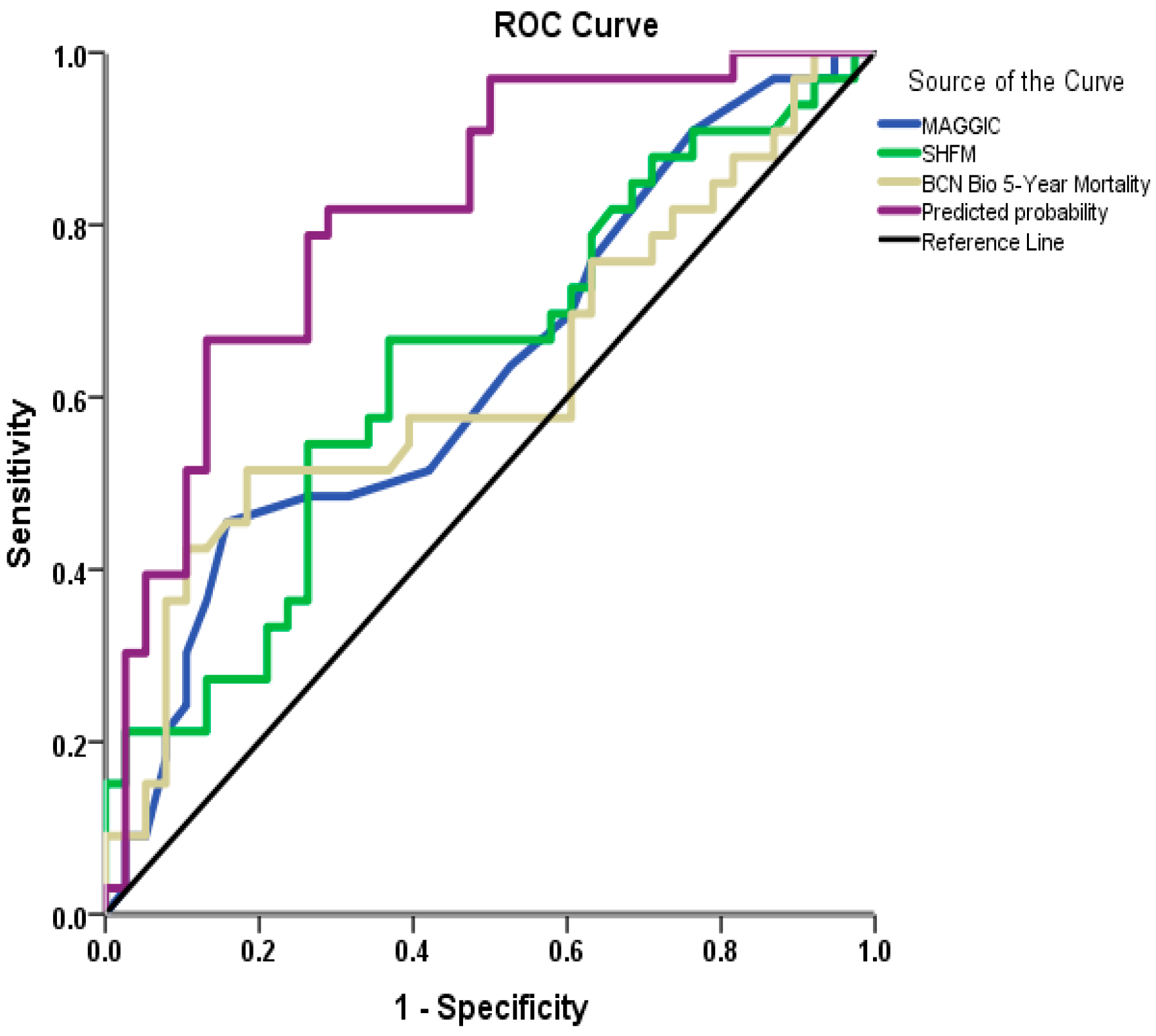
3.2. Mortality at 30-Month Follow-Up Analysis
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | American College of Cardiology |
| ACE | Angiotensin-converting enzyme inhibitor |
| AdvHF | Advanced heart failure |
| AHA | American Heart Association |
| ARB | Angiotensin II receptor blocker |
| AF | Atrial fibrillation |
| BB | Beta blocker |
| BCN Bio | Barcelona bio-heart failure risk calculator |
| BMI | Body mass index |
| BNP | Brain natriuretic peptide |
| CAD | Coronary artery disease |
| COPD | Chronic obstructive pulmonary disease |
| CRTD | Cardiac resynchronization therapy defibrillator |
| CRTP | Cardiac resynchronization therapy pacemaker |
| DBP | Diastolic blood pressure |
| DM | Diabetes mellitus |
| eGFR | Estimated glomerular filtration rate |
| GDMT | Guideline-directed medical therapy |
| HB | Hemoglobin |
| HCT | Hydrochlorothiazide |
| HF | Heart failure |
| HFpEF | Heart failure with preserved ejection fraction |
| HFrEF | Heart failure with reduced ejection fraction |
| HTx | Heart transplantation |
| ICD | Implantable cardioverter defibrillator |
| IV | Intravenous |
| LVAD | Left ventricular assist device |
| LVEF | Left ventricle ejection fraction |
| MAGGIC | Meta-Analysis Global Group in Chronic Heart Failure Risk Score |
| MRA | Mineralocorticoid receptor antagonist |
| NYHA | New York Heart Association |
| PC | Palliative care |
| PCM | Pacemaker |
| PP | Pulse pressure |
| SBP | Systolic blood pressure |
| SGLT2I | Sodium-glucose co-transporter 2 inhibitor |
| SHFM | Seattle Heart Failure Model |
| TNI | Troponin-I |
References
- Ponikowski, P.; Anker, S.D.; AlHabib, K.F.; Cowie, M.R.; Force, T.L.; Hu, S.; Jaarsma, T.; Krum, H.; Rastogi, V.; Rohde, L.E.; et al. Heart failure: Preventing disease and death worldwide. ESC Heart Fail. 2014, 1, 4–25. [Google Scholar] [CrossRef]
- Schmidt, M.; Ulrichsen, S.P.; Pedersen, L.; Bøtker, H.E.; Sørensen, H.T. Thirty-year trends in heart failure hospitalization and mortality rates and the prognostic impact of comorbidity: A Danish nationwide cohort study. Eur. J. Heart Fail. 2016, 18, 490–499. [Google Scholar] [CrossRef]
- Severino, P.; Mather, P.J.; Pucci, M.; D’Amato, A.; Mariani, M.V.; Infusino, F.; Birtolo, L.I.; Maestrini, V.; Mancone, M.; Fedele, F. Advanced Heart Failure and End-Stage Heart Failure: Does a Difference Exist. Diagnostics 2019, 9, 170. [Google Scholar] [CrossRef]
- Crespo-Leiro, M.G.; Metra, M.; Lund, L.H.; Milicic, D.; Costanzo, M.R.; Filippatos, G.; Gustafsson, F.; Tsui, S.; Barge-Caballero, E.; De Jonge, N.; et al. Advanced heart failure: A position statement of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 1505–1535. [Google Scholar] [CrossRef]
- Peled, Y.; Ducharme, A.; Kittleson, M.; Bansal, N.; Stehlik, J.; Amdani, S.; Saeed, D.; Cheng, R.; Clarke, B.; Dobbels, F.; et al. International Society for Heart and Lung Transplantation Guidelines for the Evaluation and Care of Cardiac Transplant Candidates-2024. J. Heart Lung Transpl. 2024, 43, 1529–1628.e54. [Google Scholar] [CrossRef]
- Garascia, A.; Palazzini, M.; Tedeschi, A.; Sacco, A.; Oliva, F.; Gentile, P. Advanced heart failure: From definitions to therapeutic options. Eur. Heart J. Suppl. J. Eur. Soc. Cardiol. 2023, 25 (Suppl. C), C283–C291. [Google Scholar] [CrossRef] [PubMed]
- Strangl, F.; Ullrich, A.; Oechsle, K.; Bokemeyer, C.; Blankenberg, S.; Knappe, D.; Reichenspurner, H.; Bernhardt, A.M.; Barten, M.J.; Rybczynski, M. Assessing palliative care need in left ventricular assist device patients and heart transplant recipients. Interact. Cardiovasc. Thorac. Surg. 2020, 31, 874–880. [Google Scholar] [CrossRef]
- Conti, N.; Gatti, M.; Raschi, E.; Diemberger, I.; Potena, L. Evidence and Current Use of Levosimendan in the Treatment of Heart Failure: Filling the Gap. Drug Des. Dev. Ther. 2021, 15, 3391–3409. [Google Scholar] [CrossRef] [PubMed]
- Setoguchi, S.; Stevenson, L.W.; Schneeweiss, S. Repeated hospitalizations predict mortality in the community population with heart failure. Am. Heart J. 2007, 154, 260–266. [Google Scholar] [CrossRef]
- Jiang, G.Y.; Lee, C.; Kearing, S.A.; Wadhera, R.K.; Gavin, M.C.; Wasfy, J.H.; Zeitler, E.P. IV Diuresis in Alternative Treatment Settings for the Management of Heart Failure: Implications for Mortality, Hospitalizations and Cost. J. Card. Fail. 2024, 30, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Papp, Z.; Csapó, K.; Pollesello, P.; Haikala, H.; Edes, I. Pharmacological mechanisms contributing to the clinical efficacy of levosimendan. Cardiovasc. Drug Rev. 2005, 23, 71–98. [Google Scholar] [CrossRef]
- Silva-Cardoso, J.; Ferreira, J.; Oliveira-Soares, A.; Martins-de-Campos, J.; Fonseca, C.; Lousada, N.; Ilídio-Moreira, J.; Rabaçal, C.; Damasceno, A.; Amorim, S.; et al. Effectiveness and safety of levosimendan in clinical practice. Rev. Port. Cardiol. 2009, 28, 143–154. [Google Scholar]
- Papp, Z.; Agostoni, P.; Alvarez, J.; Bettex, D.; Bouchez, S.; Brito, D.; Černý, V.; Comin-Colet, J.; Crespo-Leiro, M.G.; Delgado, J.F.; et al. Levosimendan Efficacy and Safety: 20 years of SIMDAX in Clinical Use. Card. Fail. Rev. 2020, 6, e19. [Google Scholar] [CrossRef]
- ter Maaten, J.M.; Valente, M.A.; Damman, K.; Hillege, H.L.; Navis, G.; Voors, A.A. Diuretic response in acute heart failure-pathophysiology, evaluation, and therapy. Nature reviews. Cardiology 2015, 12, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Tanner, G.; Williams, L. Intravenous diuretic day-care treatment for patients with heart failure. Clin. Med. 2012, 12, 133–136. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Levy, W.C.; Mozaffarian, D.; Linker, D.T.; Sutradhar, S.C.; Anker, S.D.; Cropp, A.B.; Anand, I.; Maggioni, A.; Burton, P.; Sullivan, M.D.; et al. The Seattle Heart Failure Model: Prediction of survival in heart failure. Circulation 2006, 113, 1424–1433. [Google Scholar] [CrossRef] [PubMed]
- Pocock, S.J.; Ariti, C.A.; McMurray, J.J.; Maggioni, A.; Køber, L.; Squire, I.B.; Swedberg, K.; Dobson, J.; Poppe, K.K.; Whalley, G.A.; et al. Predicting survival in heart failure: A risk score based on 39,372 patients from 30 studies. Eur. Heart J. 2013, 34, 1404–1413. [Google Scholar] [CrossRef]
- Lupón, J.; de Antonio, M.; Vila, J.; Peñafiel, J.; Galán, A.; Zamora, E.; Urrutia, A.; Bayes-Genis, A. Development of a novel heart failure risk tool: The barcelona bio-heart failure risk calculator (BCN bio-HF calculator). PLoS ONE 2014, 9, e85466. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.A.; McNeil, B.J. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983, 148, 839–843. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Roger, V.L.; Killian, J.M.; Weston, S.A.; Schulte, P.J.; Subramaniam, A.V.; Blecker, S.B.; Redfield, M.M. Advanced Heart Failure Epidemiology and Outcomes: A Population-Based Study. JACC Heart Fail. 2021, 9, 722–732. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cesario, D.; Clark, J.; Maisel, A. Beneficial effects of intermittent home administration of the inotrope/vasodilator milrinone in patients with end-stage congestive heart failure: A preliminary study. Am. Heart J. 1998, 135, 121–129. [Google Scholar] [CrossRef]
- Levine, B.S. Intermittent positive inotrope infusion in the management of end-stage, low-output heart failure. J. Cardiovasc. Nurs. 2000, 14, 76–93. [Google Scholar] [CrossRef]
- Nieminen, M.S.; Fruhwald, S.; Heunks, L.M.; Suominen, P.K.; Gordon, A.C.; Kivikko, M.; Pollesello, P. Levosimendan: Current data, clinical use and future development. Heart Lung Vessel. 2013, 5, 227–245. [Google Scholar] [PubMed]
- Comín-Colet, J.; Manito, N.; Segovia-Cubero, J.; Delgado, J.F.; García-Pinilla, J.M.; Almenar, L.; Crespo-Leiro, M.G.; Sionis, A.; Blasco, T.; Pascual-Figal, D.; et al. Efficacy and safety of intermittent intravenous outpatient administration of levosimendan in patients with advanced heart failure: The LION-HEART multicentre randomized trial. Eur. J. Heart Fail. 2018, 20, 1128–1136. [Google Scholar] [CrossRef]
- Follath, F.; Cleland, J.G.; Just, H.; Papp, J.G.; Scholz, H.; Peuhkurinen, K.; Harjola, V.P.; Mitrovic, V.; Abdalla, M.; Sandell, E.P.; et al. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): A randomised double-blind trial. Lancet 2002, 360, 196–202. [Google Scholar] [CrossRef]
- Schumann, J.; Henrich, E.C.; Strobl, H.; Prondzinsky, R.; Weiche, S.; Thiele, H.; Werdan, K.; Frantz, S.; Unverzagt, S. Inotropic agents and vasodilator strategies for the treatment of cardiogenic shock or low cardiac output syndrome. Cochrane Database Syst. Rev. 2018, 2018, CD009669. [Google Scholar] [CrossRef]
- Dobarro, D.; Donoso-Trenado, V.; Solé-González, E.; Moliner-Abós, C.; Garcia-Pinilla, J.M.; Lopez-Fernandez, S.; Ruiz-Bustillo, S.; Diez-Lopez, C.; Castrodeza, J.; Méndez-Fernández, A.B.; et al. Intermittent inotropic support with levosimendan in advanced heart failure as destination therapy: The LEVO-D registry. ESC Heart Fail. 2023, 10, 1193–1204. [Google Scholar] [CrossRef]
- Buckley, L.F.; Carter, D.M.; Matta, L.; Cheng, J.W.; Stevens, C.; Belenkiy, R.M.; Burpee, L.J.; Young, M.A.; Weiffenbach, C.S.; Smallwood, J.A.; et al. Intravenous Diuretic Therapy for the Management of Heart Failure and Volume Overload in a Multidisciplinary Outpatient Unit. JACC Heart Fail. 2016, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ryder, M.; Murphy, N.F.; McCaffrey, D.; O’Loughlin, C.; Ledwidge, M.; McDonald, K. Outpatient intravenous diuretic therapy; potential for marked reduction in hospitalisations for acute decompensated heart failure. Eur. J. Heart Fail. 2008, 10, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Zhang, M.; Bell, M.; Tarolli, K.; Donalson, E.; Vaughn, J.; Hickey, G.W. Outpatient Intravenous Diuretic Clinic: An Effective Strategy for Management of Volume Overload and Reducing Immediate Hospital Admissions. J. Clin. Med. Res. 2021, 13, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, Z.; Liu, J.; Chong, Y.; Wu, B. Prognostic value of the albumin-bilirubin score in critically ill patients with heart failure. Ann. Palliat. Med. 2021, 10, 12727–12741. [Google Scholar] [CrossRef]
- Scrutinio, D.; Guida, P.; Ammirati, E.; Oliva, F.; Passantino, A. Risk scores did not reliably predict individual risk of mortality for patients with decompensated heart failure. J. Clin. Epidemiol. 2025, 125, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Averbuch, T.; Zafari, A.; Islam, S.; Lee, S.F.; Sankaranarayanan, R.; Greene, S.J.; Mamas, M.A.; Pandey, A.; Van Spall, H.G. Comparative performance of risk prediction indices for mortality or readmission following heart failure hospitalization. ESC Heart Fail. 2025, 12, 1227–1236. [Google Scholar] [CrossRef]
- Blumer, V.; Mentz, R.J.; Sun, J.L.; Butler, J.; Metra, M.; Voors, A.A.; Hernandez, A.F.; O’Connor, C.M.; Greene, S.J. Prognostic Role of Prior Heart Failure Hospitalization Among Patients Hospitalized for Worsening Chronic Heart Failure. Circ. Heart Fail. 2021, 14, e007871. [Google Scholar] [CrossRef]
- Iliadis, C.; Spieker, M.; Kavsur, R.; Metze, C.; Hellmich, M.; Horn, P.; Westenfeld, R.; Tiyerili, V.; Becher, M.U.; Kelm, M.; et al. “Get with the Guidelines Heart Failure Risk Score” for mortality prediction in patients undergoing MitraClip. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2021, 110, 1871–1880. [Google Scholar] [CrossRef]
- Scrutinio, D.; Ammirati, E.; Passantino, A.; Guida, P.; D’Angelo, L.; Oliva, F.; Ciccone, M.M.; Iacoviello, M.; Dentamaro, I.; Santoro, D.; et al. Predicting short-term mortality in advanced decompensated heart failure—Role of the updated acute decompensated heart failure/N-terminal pro-B-type natriuretic Peptide risk score. Circ. J. Off. J. Jpn. Circ. Soc. 2015, 79, 1076–1083. [Google Scholar] [CrossRef]
- Codina, P.; Lupón, J.; Borrellas, A.; Spitaleri, G.; Cediel, G.; Domingo, M.; Simpson, J.; Levy, W.C.; Santiago-Vacas, E.; Zamora, E.; et al. Head-to-head comparison of contemporary heart failure risk scores. Eur. J. Heart Fail. 2021, 23, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Lagu, T.; Pekow, P.S.; Shieh, M.S.; Stefan, M.; Pack, Q.R.; Kashef, M.A.; Atreya, A.R.; Valania, G.; Slawsky, M.T.; Lindenauer, P.K. Validation and Comparison of Seven Mortality Prediction Models for Hospitalized Patients With Acute Decompensated Heart Failure. Circ. Heart Fail. 2016, 9, e002912. [Google Scholar] [CrossRef]
- Subramaniam, A.; van Houten, H.; Redfield, M.M.; Sangaralingham, L.R.; Savitz, S.T.; Glasgow, A.; Schulte, P.J.; LeMond, L.M.; Dunlay, S.M. Advanced Heart Failure Characteristics and Outcomes in Commercially Insured U.S. Adults. JACC Heart Fail. 2023, 11, 1595–1606. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Allen, L.A.; Fonarow, G.C.; Liang, L.; Schulte, P.J.; Masoudi, F.A.; Rumsfeld, J.S.; Ho, P.M.; Eapen, Z.J.; Hernandez, A.F.; Heidenreich, P.A.; et al. Medication initiation burden required to comply with heart failure guideline recommendations and hospital quality measures. Circulation 2009, 120, 1342–1349. [Google Scholar] [CrossRef]
- Tromp, J.; Ouwerkerk, W.; Cleland, J.G.F.; Angermann, C.; Dahlstrom, U.; Teng, K.T.H.; Bamadhaj, S.; Ertl, G.; Hassanein, M.; Perrone, S.V.; et al. Global differences in HF: The importance of region in patient characteristics, management and outcomes. Curr. Heart Fail. Rep. 2020, 17, 234–244. [Google Scholar] [CrossRef]
- Canepa, M.; Fonseca, C.; Chioncel, O.; Laroche, C.; Crespo-Leiro, M.G.; Coats, A.J.S.; Mebazaa, A.; Piepoli, M.F.; Tavazzi, L.; Maggioni, A.P.; et al. Performance of Prognostic Risk Scores in Chronic Heart Failure Patients Enrolled in the European Society of Cardiology Heart Failure Long-Term Registry. JACC Heart Fail. 2018, 6, 452–462. [Google Scholar] [CrossRef]
- Khoury, J.; Ghersin, I.; Braun, E.; Elias, A.; Aronson, D.; Azzam, Z.S.; Bahouth, F. Adherence to Guidelines in Heart Failure, Is It Valid for Elderly Patients? Isr. Med. Assoc. J. 2022, 24, 757–762. [Google Scholar]
- Fuchida, A.; Suzuki, S.; Motoki, H.; Kanzaki, Y.; Maruyama, T.; Hashizume, N.; Kozuka, A.; Yahikozawa, K.; Kuwahara, K. Prognostic significance of diastolic blood pressure in patients with heart failure with preserved ejection fraction. Heart Vessels 2021, 36, 1159–1165. [Google Scholar] [CrossRef]
- Bae, E.; Rocco, M.V.; Lee, J.; Park, J.Y.; Kim, Y.C.; Yoo, K.D.; Kim, E.Y.; Park, D.J.; Lim, C.S.; Kim, Y.S.; et al. Impact of DBP on all-cause and cardiovascular mortality: Results from the National Health and Nutrition Examination survey, 1999–2014. J. Hypertens. 2022, 40, 108–116. [Google Scholar] [CrossRef]
- Rodriguez, M.; Hernandez, M.; Cheungpasitporn, W.; Kashani, K.B.; Riaz, I.; Rangaswami, J.; Herzog, E.; Guglin, M.; Krittanawong, C. Hyponatremia in Heart Failure: Pathogenesis and Management. Curr. Cardiol. Rev. 2019, 15, 252–261. [Google Scholar] [CrossRef]
- Girerd, N.; Mewton, N.; Tartière, J.M.; Guijarro, D.; Jourdain, P.; Damy, T.; Lamblin, N.; Bayes-Génis, A.; Pellicori, P.; Januzzi, J.L.; et al. Practical outpatient management of worsening chronic heart failure. Eur. J. Heart Fail. 2022, 24, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Task Force of the Hellenic Heart Failure Clinics Network. How to develop a national heart failure clinics network: A consensus document of the Hellenic Heart Failure Association. ESC Heart Fail. 2020, 7, 15–25. [Google Scholar] [CrossRef] [PubMed]
- McAlister, F.A.; Stewart, S.; Ferrua, S.; McMurray, J.J. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: A systematic review of randomized trials. J. Am. Coll. Cardiol. 2004, 44, 810–819. [Google Scholar] [CrossRef] [PubMed]
| Total Population (n = 95) | Group 1 (n = 33) | Group 2 (n = 17) | Group 3 (n = 45) | p-Value | |
|---|---|---|---|---|---|
| Age (years) | 73 ± 10 | 72 ± 10 * | 80 ± 7 | 72 ± 10 * | 0.017 |
| Female gender, n (%) | 33 (38) | 6(18) | 12 (71) | 15 (33) | 0.001 |
| Body mass index (kg/m2) | 26.9 ± 5.7 | 26.2 ± 4.6 | 27.2 ± 5.9 | 27.3 ± 6.3 | 0.707 |
| Systolic BP (mmHg) | 110 ± 18 | 105 ± 15 | 118 ± 18 | 111 ± 20 | 0.062 |
| Diastolic BP (mmHg) | 68 ± 11 | 66 ± 11 | 67 ± 10 | 70 ± 10 | 0.179 |
| Pulse pressure (mmHg) | 42 ± 14 | 39 ± 10 * | 51 ± 16 | 41 ± 14 * | 0.007 |
| LVEF (%) | 30 (20, 50) | 25 (20, 30) | 50 (30, 55) | 40 (20, 55) | <0.001 |
| Smoking, n (%) | 15 (16) | 8 (24) | 0 (0) | 7 (16) | 0.084 |
| Hypertension, n (%) | 57 (60) | 21 (64) | 10 (59) | 26 (58) | 0.868 |
| Diabetes, n (%) | 39 (41) | 13 (39) | 6 (35) | 20 (44) | 0.785 |
| CAD, n (%) | 56 (59) | 25 (76) | 5 (29) | 26 (58) | 0.007 |
| COPD, n (%) | 39 (41) | 12 (36) | 6 (35) | 21 (47) | 0.572 |
| Atrial fibrillation, n (%) | 70 (73) | 23 (70) | 14 (82) | 33 (73) | 0.627 |
| Hemoglobin (g/dL) | 12.6 ± 2.0 | 13.1 ± 2.1 | 12.2 ± 2.1 | 12.4 ± 2.0 | 0.200 |
| Na+ (mmoL/L) | 137 ± 3 | 136 ± 3 | 138 ± 2 | 137 ± 3 | 0.268 |
| K+ (mmoL/L) | 4.22 ± 0.50 | 4.02 ± 0.44 ** | 4.00 ± 0.46 ** | 4.44 ± 0.48 | <0.001 |
| eGFR (ml/min/1.73 m2) | 47.6 ± 17.3 | 46.8 ± 18.1 | 49.1 ± 18.4 | 47.6 ± 16.7 | 0.909 |
| Troponin I (ng/mL) | 25.0 (14.2, 43.1) | 28.0 (16.8, 47.1) | 18.8 (14.3, 36.6) | 22.4 (11.9, 44.7) | 0.275 |
| BNP (pg/mL) | 589 (363, 897) | 626 (404, 907) | 425 (283, 769) | 645 (371, 1047) | 0.067 |
| Medications, n (%) | |||||
| B-blockers | 69 (73) | 25 (76) | 10 (59) | 34 (76) | 0.370 |
| SGLT2I | 52 (55) | 17 (52) | 10 (59) | 25 (56) | 0.876 |
| ACEi/ARB | 28 (30) | 3 (9) | 7 (41) | 18 (40) | 0.006 |
| MRA | 80 (84) | 32 (97) | 14 (82) | 34 (76) | 0.037 |
| Hydrochlorothiazide | 10 (11) | 4 (12) | 3 (18) | 3 (7) | 0.424 |
| Furosemide dose (mg) | 120 (80, 240) | 160 (110, 250) | 200 (120, 250) | 80 (40, 120) | <0.001 |
| Rhythm devices, n (%) | |||||
| PCM | 23 (24) | 3 (9) | 5 (29) | 15 (33) | |
| CRTP | 10 (11) | 0 (0) | 0 (0) | 10 (22) | |
| ICD | 22 (23) | 8 (24) | 1 (6) | 13 (29) | |
| CRTD | 23 (24) | 15 (46) | 1 (6) | 7 (16) | <0.001 |
| Total Population (n = 92) | Group 1 (n = 33) | Group 2 (n = 15) | Group 3 (n = 44) | p-Value | |
|---|---|---|---|---|---|
| Actual mortality | 17 (19) | 10 (30) | 3 (20) | 4 (9) | 0.059 |
| MAGGIC score | 30 (27, 35) | 34 (27, 38) | 29 (27, 32) | 30 (26, 34) | 0.139 |
| MAGGIC 1-year mortality | 24.8 (19.1, 36.9) | 34.2 (19.1, 45.8) | 22.7 (19.1, 29.2) | 24.8 (17.5, 34.2) | 0.139 |
| SHFM score | 1.46 (0.98, 2.22) | 1.52 (1.14, 2.25) | 1.82 (0.99, 2.65) | 1.38 (0.80, 2.12) | 0.197 |
| SHFM 1-year survival | 84 (69, 90) | 83 (68, 88) | 78 (56, 90) | 85 (71, 91) | 0.203 |
| BCN 1-year mortality | 16.1 (8.2, 28.4) | 16.1 (8.5, 30.2) | 25.9 (16.0, 31.3) | 15.3 (6.2, 26.7) | 0.174 |
| Odds Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|
| Age/10-year increase | 1.30 | 0.74, 2.29 | 0.368 |
| Female | 0.34 | 0.09, 1.29 | 0.112 |
| BMI per 5 kg/m2 increase | 0.78 | 0.46, 1.31 | 0.343 |
| SBP per 10 mmHg increase | 0.81 | 0.58, 1.12 | 0.204 |
| DBP per 5 mmHg increase | 0.61 | 0.42, 0.87 | 0.007 |
| PP per 10 mmHg increase | 1.07 | 0.74, 1.57 | 0.710 |
| LVEF per 10% increase | 0.77 | 0.53, 1.11 | 0.163 |
| Smoking | 2.71 | 0.79, 9.34 | 0.115 |
| Hypertension | 0.67 | 0.23, 1.94 | 0.460 |
| Diabetes | 0.50 | 0.16, 1.57 | 0.236 |
| Coronary artery disease | 3.87 | 1.03, 14.59 | 0.046 |
| COPD | 1.78 | 0.62, 5.14 | 0.284 |
| Atrial fibrillation | 1.82 | 0.47, 6.97 | 0.385 |
| Hemoglobin per 1 g/dL increase | 1.00 | 0.77, 1.31 | 0.974 |
| Na+ per 1 mmoL/L increase | 0.82 | 0.68, 0.99 | 0.041 |
| K+ per 0.5 mmoL/L increase | 0.60 | 0.33, 1.08 | 0.09 |
| eGFR per 10 mL/min increase | 1.09 | 0.81, 1.47 | 0.555 |
| Troponin I > 25 ng/mL | 1.22 | 0.42, 3.52 | 0.711 |
| BNP > 600 pg/mL | 0.99 | 0.35, 2.88 | 0.995 |
| Medications | |||
| B-blockers | 1.26 | 0.37, 4.32 | 0.709 |
| SGLT2i | 1.60 | 0.54, 4.79 | 0.397 |
| ACEi/ARB | 0.46 | 0.12, 1.74 | 0.249 |
| MRA | 3.67 | 0.45, 30.05 | 0.225 |
| Hydrochlorothiazide | 0.52 | 0.06, 4.49 | 0.555 |
| Furosemide dose > 120 mg | 1.43 | 0.50, 4.12 | 0.505 |
| Odds Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|
| Diastolic blood pressure per 5 mmHg increase | 0.60 | 0.40, 0.91 | 0.015 |
| Coronary artery disease | 3.89 | 1.01, 16.95 | 0.05 |
| Na+ per 1 mmoL/L increase | 0.77 | 0.61, 0.97 | 0.024 |
| Total Population (n = 74) | Group 1 (n = 29) | Group 2 (n = 7) | Group 3 (n = 38) | p-Value | |
|---|---|---|---|---|---|
| Actual mortality | 36 (49) | 21 (72) | 5 (71) | 10 (26) | <0.001 |
| MAGGIC | 30 (27, 36) | 34 (27, 38) | 29 (27, 32) | 30 (26, 34) | 0.142 |
| MAGGIC 3-year mortality | 52.3 (42.7, 70.1) | 65.8 (42.7, 78,7) | 49.0 (42.7, 59.0) | 52.3 (39.7, 65.8) | 0.142 |
| SHFM | 1.46 (1.05, 2.11) | 1.52 (1.14, 2.07) | 2.06 (0.61, 2.44) | 1.39 (0.81, 2.16) | 0.591 |
| SHFM 5-year survival | 42 (19, 56) | 40 (20, 54) | 21 (10, 67) | 44 (18, 64) | 0.617 |
| BCN 5-year mortality | 67.5 (42.8, 91.2) | 67.6 (48.2, 89.9) | 85.6 (71.1, 91.1) | 65.8 (36.8, 92.3) | 0.619 |
| Odds Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|
| Age per 10-year increase | 1.23 | 0.77, 1.97 | 0.394 |
| Female | 0.49 | 0.18, 1.37 | 0.173 |
| BMI per 5 kg/m2 increase | 0.95 | 0.64, 1.40 | 0.794 |
| SBP per 10 mmHg increase | 0.74 | 0.56, 0.96 | 0.025 |
| DBP per 5 mmHg increase | 0.63 | 0.47, 0.85 | 0.002 |
| PP per 10 mmHg increase | 0.87 | 0.63, 1.22 | 0.429 |
| LVEF per 10% increase | 0.60 | 0.43, 0.84 | 0.003 |
| Smoking | 0.91 | 0.29, 2.82 | 0.863 |
| Hypertension | 1.30 | 0.50, 3.37 | 0.584 |
| Diabetes | 1.37 | 0.55, 3.45 | 0.502 |
| Coronary artery disease | 3.15 | 1.15, 8.66 | 0.026 |
| COPD | 1.10 | 0.44, 2.76 | 0.839 |
| Atrial fibrillation | 1.91 | 0.66, 5.59 | 0.236 |
| Hemoglobin per 1 g/dL increase | 0.97 | 0.77, 1.22 | 0.817 |
| Na per 1 mmoL/L increase | 0.81 | 0.68, 0.96 | 0.015 |
| K per 0.5 mmoL/L increase | 0.66 | 0.41, 1.09 | 0.100 |
| eGFR per 10 mL/min increase | 0.99 | 0.76, 1.30 | 0.944 |
| Troponin I > 25 ng/mL | 1.47 | 0.59, 3.70 | 0.412 |
| BNP > 600 pg/mL | 1.39 | 0.55, 3.52 | 0.487 |
| Medications | |||
| B-blockers | 0.93 | 0.32, 2.69 | 0.895 |
| SGLT2i | 2.41 | 0.95, 6.13 | 0.065 |
| ACEi/ARB | 0.33 | 0.12, 0.95 | 0.039 |
| MRA | 6.07 | 1.23, 30.03 | 0.027 |
| Hydrochlorothiazide | 1.64 | 0.26, 10.41 | 0.602 |
| Furosemide dose > 120 mg | 3.02 | 1.17, 8.02 | 0.022 |
| Odds Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|
| Diastolic blood pressure per 5 mmHg increase | 0.68 | 0.49, 0.94 | 0.018 |
| Left ventricular ejection fraction per 10% increase | 0.69 | 0.48, 0.99 | 0.042 |
| Na per 1 mmoL/L increase | 0.84 | 0.71, 1.00 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papaioannou, E.; Chatzipanteliadou, S.; Rammos, A.; Gkartzonikas, I.; Bechlioulis, A.; Stamou, I.; Bouratzis, V.; Lakkas, L.; Michalis, L.K.; Naka, K.K. Prognosis and Risk Stratification of Patients with Advanced Heart Failure Followed-Up on an Outpatient Clinic. Biomedicines 2025, 13, 2743. https://doi.org/10.3390/biomedicines13112743
Papaioannou E, Chatzipanteliadou S, Rammos A, Gkartzonikas I, Bechlioulis A, Stamou I, Bouratzis V, Lakkas L, Michalis LK, Naka KK. Prognosis and Risk Stratification of Patients with Advanced Heart Failure Followed-Up on an Outpatient Clinic. Biomedicines. 2025; 13(11):2743. https://doi.org/10.3390/biomedicines13112743
Chicago/Turabian StylePapaioannou, Eftychia, Stefania Chatzipanteliadou, Aidonis Rammos, Ilias Gkartzonikas, Aris Bechlioulis, Ilektra Stamou, Vasileios Bouratzis, Lampros Lakkas, Lampros K. Michalis, and Katerina K. Naka. 2025. "Prognosis and Risk Stratification of Patients with Advanced Heart Failure Followed-Up on an Outpatient Clinic" Biomedicines 13, no. 11: 2743. https://doi.org/10.3390/biomedicines13112743
APA StylePapaioannou, E., Chatzipanteliadou, S., Rammos, A., Gkartzonikas, I., Bechlioulis, A., Stamou, I., Bouratzis, V., Lakkas, L., Michalis, L. K., & Naka, K. K. (2025). Prognosis and Risk Stratification of Patients with Advanced Heart Failure Followed-Up on an Outpatient Clinic. Biomedicines, 13(11), 2743. https://doi.org/10.3390/biomedicines13112743





