Clostridioides difficile Infection in Special Populations: Focus on Inflammatory Bowel Disease—A Narrative Review from Pathogenesis to Management
Abstract
1. Introduction
2. Methodology
3. Epidemiology of CDI in IBD Patients
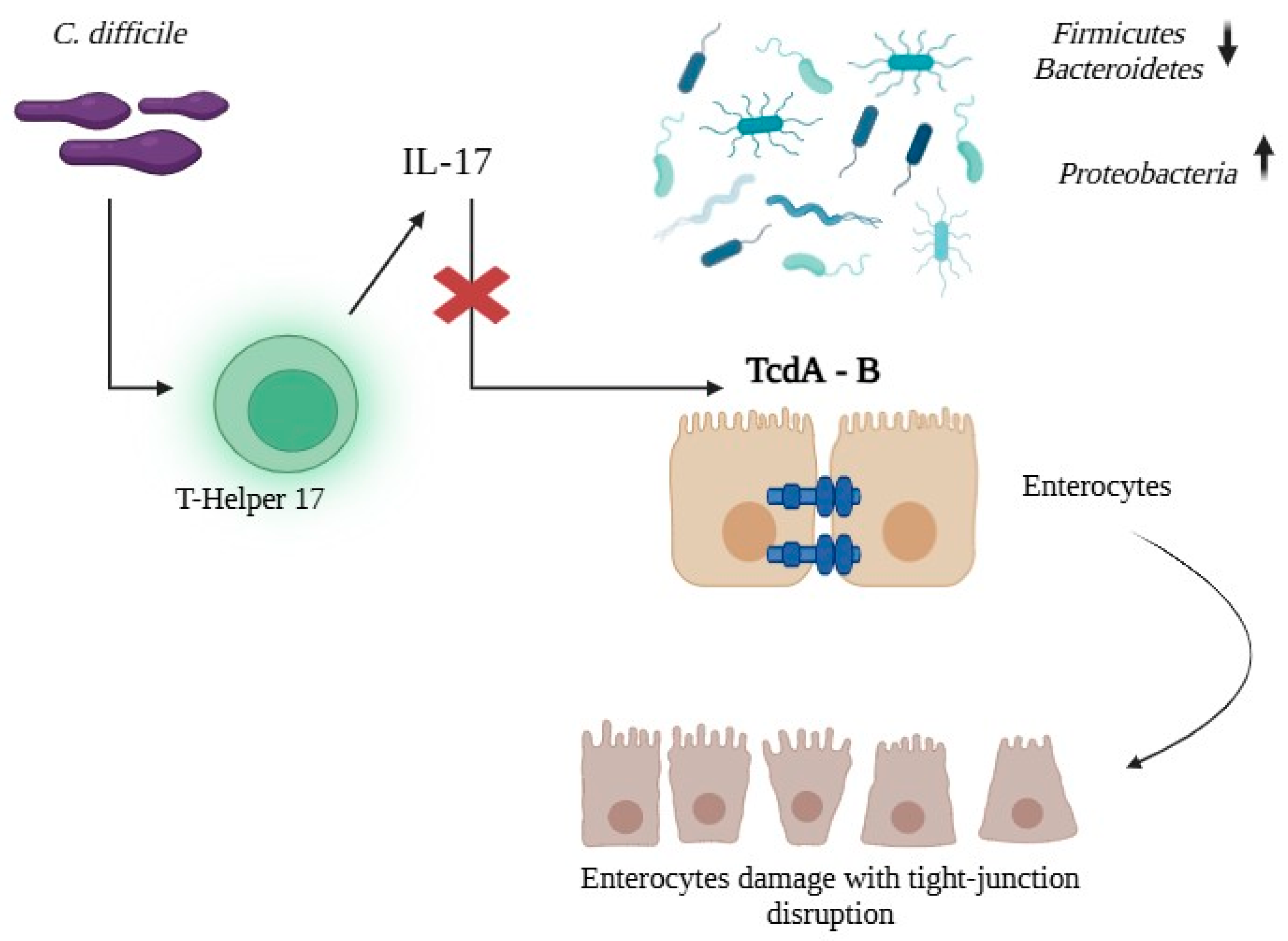
4. Pathogenesis
5. Risk Factors for CDI in IBD Patients
| Author, Year | Study Design | Population | IBD Type | Risk Factors for CDI (Main Results) |
|---|---|---|---|---|
| Schneeweiss et al., 2009 [42] | Population-based cohort | Adults with IBD (n = 10,662) | UC and CD | Corticosteroids (three-fold risk, RR 3.38; dose/duration independent) |
| Regnault et al., 2014 [22] | Retrospective cohort | Hospitalized IBD flares (n = 813) | UC and CD | Recent intake NSAIDs (OR 3.8) independent predictor of CDI (within 2 months prior admission) |
| Zhang et al., 2016 [41] | Retrospective cohort | Hospitalized IBD (n = 646) | UC and CD | CD: fistula (OR 2.48), antibiotic use (OR 5.11), infliximab use (OR 2.22); synergy when antibiotics + infliximab (risk increased 10.2-fold, p < 0.001. UC: infliximab use (OR 2.60) |
| Razik et al., 2016 [13] | Retrospective cohort | Hospitalized rCDI (n = 503, IBD = 110) | UC and CD | rCDI: 5-ASA (HR 2.15), non-ileal CD (HR 2.85). IBD 33% higher rates rCDI versus non-IBD |
| Balram et al., 2019 [36] | Systematic review & meta-analysis | Pooled IBD studies (n = 38,336 IBD with CDI, n = 1,199,752 IBD without CDI; n = 22 observational studies) | UC and CD | Colonic involvement in CD (OR 2.76); antibiotics ≤ 30 days (OR 1.85); biologics (OR 1.65). Higher colectomy risk (OR 2.22) |
| Chen et al., 2019 [38] | Prospective cohort | Hospitalized IBD (n = 230) | UC and CD | UC: Longer disease, hospitalization within previous 3 months, proton pump inhibitor use within 1 month, severe disease activity (p < 0.05) Crohn’s disease: Moderate disease activity (p = 0.03) Increased surgery and colectomy rate |
| Voth et al., 2021 [49] | Retrospective cohort | IBD with CDI (n = 137) | UC and CD | Overweight BMI (OR 2.85); statin use (OR 5.66) linked to severe/complicated CDI |
| Sandborn et al., 2021 [47] | Pooled phase 2/3 trials Ustekinumab | IBD receiving Ustekinumab (n = 2574) | UC and CD | No significant increase in CDI vs placebo over 1 year |
| Song et al., 2023 [24] | Nationwide population-based cohort | N = 54,836 IBD vs n = 109,178 controls | UC and CD | Age (aHR 1.02/yr), female (aHR 1.46), Charlson Comorbidity Index ≥ 3 (aHR 1.50), 5-ASA (aHR 1.64), immunomodulators (aHR 1.83), biologics (aHR 2.51), long-term steroids > 90 days (aHR 1.40) Risk of CDI 7 times higher in IBD vs non-IBD |
| Loftus et al., 2023 [48] | Pooled RCTs + long-term extension Tofacitinib | IBD receiving tofacitinib (n = 1157) | UC | Low overall CDI incidence rate (0.31; no significant excess vs placebo) |
| Jakubowska et al., 2024 [40] | Single-center retrospective | Hospitalized IBD (n = 204) | UC and CD | Low BMI (p < 0.001); Broad-spectrum antibiotics (OR 4.86), steroids (OR 3.62), azathioprine (OR 3.39) |
| Chen et al., 2024 [46] | Systematic review & meta-analysis Vedolizumab | IBD receiving vedolizumab (n = 41,862; 30 studies) | UC and CD | No additional risk of CDI with vedolizumab CDI rates are higher in UC than CD (RR 2.25) |
| Martínez-Lozano et al., 2025 [39] | Retrospective cohort | IBD ± rheumatologic diseases (n = 1866, IBD n = 1041) | UC and CD | IBD (OR 18.29), UC (OR 2.00), ≥3 different biologic agents received (OR 3.09) |
| Vitikainen et al., 2025 [37] | Nationwide registry | IBD with CDI (n = 279) | UC and CD | Higher IBD activity (p < 0.001), short disease duration (<2 years; p < 0.001), UC and colonic CD (p = 0.001), systemic corticosteroid use (within 3 months, p < 0.001), hospitalization (previous 3 months, p < 0.001), antibiotic use (p < 0.001) and proton pump inhibitors (p < 0.001) |
| Amakye et al., 2025 [25] | Systematic review & meta-analysis | 796,244 IBD (28 studies) | UC and CD | Male sex (OR 1.18), older age (OR 1.06); highest prevalence observed in Asia (11%) |
6. Diagnosis
6.1. Clinical Assessment
6.2. Blood and Stool Examinations
6.3. Endoscopy and Imaging
7. Treatment
7.1. IBD Management in CDI
7.2. Antibiotics
7.3. Fecal Microbiota Transplantation (FMT)
7.4. Monoclonal Antibodies
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alexiou, S.; Diakou, A.; Kachrimanidou, M. The Role of Clostridioides difficile Within the One Health Framework: A Review. Microorganisms 2025, 13, 429. [Google Scholar] [CrossRef]
- Dubberke, E.R.; Olsen, M.A. Burden of Clostridium difficile on the Healthcare System. Clin. Infect. Dis. 2012, 55, S88–S92. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Olsen, M.A.; Dubberke, E.R. The Morbidity, Mortality, and Costs Associated with Clostridium difficile Infection. Infect. Dis. Clin. N. Am. 2015, 29, 123–134. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. European Surveillance of Clostridioides (Clostridium) difficile Infections: Surveillance Protocol Version 2.4; Publications Office: Luxembourg, 2019. [Google Scholar]
- Kordus, S.L.; Thomas, A.K.; Lacy, D.B. Clostridioides difficile Toxins: Mechanisms of Action and Antitoxin Therapeutics. Nat. Rev. Microbiol. 2022, 20, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Eeuwijk, J.; Ferreira, G.; Yarzabal, J.P.; Robert-Du Ry Van Beest Holle, M. A Systematic Literature Review on Risk Factors for and Timing of Clostridioides difficile Infection in the United States. Infect. Dis. Ther. 2024, 13, 273–298. [Google Scholar] [CrossRef]
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J. Crohn’s Colitis 2022, 16, 2–17. [Google Scholar] [CrossRef]
- Gordon, H.; Minozzi, S.; Kopylov, U.; Verstockt, B.; Chaparro, M.; Buskens, C.; Warusavitarne, J.; Agrawal, M.; Allocca, M.; Atreya, R.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohn’s Colitis 2024, 18, 1531–1555. [Google Scholar] [CrossRef]
- Van Prehn, J.; Reigadas, E.; Vogelzang, E.H.; Bouza, E.; Hristea, A.; Guery, B.; Krutova, M.; Norén, T.; Allerberger, F.; Coia, J.E.; et al. European Society of Clinical Microbiology and Infectious Diseases: 2021 Update on the Treatment Guidance Document for Clostridioides difficile Infection in Adults. Clin. Microbiol. Infect. 2021, 27, S1–S21. [Google Scholar] [CrossRef]
- Johnson, S.; Lavergne, V.; Skinner, A.M.; Gonzales-Luna, A.J.; Garey, K.W.; Kelly, C.P.; Wilcox, M.H. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused Update Guidelines on Management of Clostridioides difficile Infection in Adults. Clin. Infect. Dis. 2021, 73, e1029–e1044. [Google Scholar] [CrossRef]
- Palacios Argueta, P.; Salazar, M.; Attar, B.; Simons-Linares, R.; Shen, B. 90-Day Specific Readmission for Clostridium difficile Infection After Hospitalization with an Inflammatory Bowel Disease Flare: Outcomes and Risk Factors. Inflamm. Bowel Dis. 2021, 27, 530–537. [Google Scholar] [CrossRef]
- Ricciardi, R.; Ogilvie, J.W.; Roberts, P.L.; Marcello, P.W.; Concannon, T.W.; Baxter, N.N. Epidemiology of Clostridium difficile Colitis in Hospitalized Patients with Inflammatory Bowel Diseases. Dis. Colon Rectum 2009, 52, 40–45. [Google Scholar] [CrossRef]
- Razik, R.; Rumman, A.; Bahreini, Z.; McGeer, A.; Nguyen, G.C. Recurrence of Clostridium difficile Infection in Patients with Inflammatory Bowel Disease: The RECIDIVISM Study. Am. J. Gastroenterol. 2016, 111, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; McGinley, E.L.; Saeian, K.; Binion, D.G. Temporal Trends in Disease Outcomes Related to Clostridium difficile Infection in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2011, 17, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, W.; Marya, N.; Fahed, J.; Leslie, G.; Patel, K.; Cave, D.R. Clostridium difficile in Inflammatory Bowel Disease: A Retrospective Study. Gastroenterol. Res. Pract. 2017, 2017, 4803262. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.C.; Kaplan, G.G.; Harris, M.L.; Brant, S.R. A National Survey of the Prevalence and Impact of Clostridium difficile Infection Among Hospitalized Inflammatory Bowel Disease Patients. Am. J. Gastroenterol. 2008, 103, 1443–1450. [Google Scholar] [CrossRef]
- Saffouri, G.; Gupta, A.; Loftus, E.V.; Baddour, L.M.; Pardi, D.S.; Khanna, S. The Incidence and Outcomes from Clostridium difficile Infection in Hospitalized Adults with Inflammatory Bowel Disease. Scand. J. Gastroenterol. 2017, 52, 1240–1247. [Google Scholar] [CrossRef]
- Singh, H.; Nugent, Z.; Yu, B.N.; Lix, L.M.; Targownik, L.E.; Bernstein, C.N. Higher Incidence of Clostridium difficile Infection Among Individuals with Inflammatory Bowel Disease. Gastroenterology 2017, 153, 430–438.e2. [Google Scholar] [CrossRef]
- Hourigan, S.K.; Oliva-Hemker, M.; Hutfless, S. The Prevalence of Clostridium difficile Infection in Pediatric and Adult Patients with Inflammatory Bowel Disease. Dig. Dis. Sci. 2014, 59, 2222–2227. [Google Scholar] [CrossRef]
- Spartz, E.J.; DeDecker, L.C.; Fansiwala, K.M.; Noorian, S.; Roney, A.R.; Hakimian, S.; Sauk, J.S.; Chen, P.; Limketkai, B.N. Recent Trends and Risk Factors Associated with Clostridioides difficile Infections in Hospitalized Patients with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2024, 59, 89–99. [Google Scholar] [CrossRef]
- Joshi, N.M.; Marks, I.H.; Crowson, R.; Ball, D.; Rampton, D.S. Incidence and Outcome of Clostridium difficile Infection in Hospitalized Patients with Inflammatory Bowel Disease in the UK. J. Crohn’s Colitis 2017, 11, 70–76. [Google Scholar] [CrossRef]
- Regnault, H.; Bourrier, A.; Lalande, V.; Nion-Larmurier, I.; Sokol, H.; Seksik, P.; Barbut, F.; Cosnes, J.; Beaugerie, L. Prevalence and Risk Factors of Clostridium difficile Infection in Patients Hospitalized for Flare of Inflammatory Bowel Disease: A Retrospective Assessment. Dig. Liver Dis. 2014, 46, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, H.; Xu, T.; Xiao, M.; Tang, H.; Wu, D.; Tan, B.; Li, J.; Yang, H.; Lv, H.; et al. Case–Control Study of Inflammatory Bowel Disease Patients with and without Clostridium difficile Infection and Poor Outcomes in Patients Coinfected with C. difficile and Cytomegalovirus. Dig. Dis. Sci. 2018, 63, 3074–3083. [Google Scholar] [CrossRef] [PubMed]
- Song, E.M.; Choi, A.; Kim, S.; Jung, S.H. The Prevalence and Risk Factors of Clostridioides difficile Infection in Inflammatory Bowel Disease: 10-Year South Korean Experience Based on the National Database. J. Korean Med. Sci. 2023, 38, e359. [Google Scholar] [CrossRef] [PubMed]
- Amakye, D.; Ssentongo, P.; Patel, S.; Dalessio, S.; Kochhar, S.; Momin, A.; Clarke, K. Global Patterns of Clostridioides difficile Infection in Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis of Prevalence, Epidemiology, and Risk Factors. Crohn’s Colitis 360 2025, 7, otaf024. [Google Scholar] [CrossRef]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the Intestinal Microbiome in Inflammatory Bowel Disease and Treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef]
- Rodríguez, C.; Romero, E.; Garrido-Sanchez, L.; Alcaín-Martínez, G.; Andrade, R.; Taminiau, B.; Daube, G.; García-Fuentes, E. Microbiota Insights in Clostridium difficile Infection and Inflammatory Bowel Disease. Gut Microbes 2020, 12, 1725220. [Google Scholar] [CrossRef]
- Allegretti, J.R.; Kearney, S.; Li, N.; Bogart, E.; Bullock, K.; Gerber, G.K.; Bry, L.; Clish, C.B.; Alm, E.; Korzenik, J.R. Recurrent Clostridium difficile Infection Associates with Distinct Bile Acid and Microbiome Profiles. Aliment. Pharmacol. Ther. 2016, 43, 1142–1153. [Google Scholar] [CrossRef]
- Barbara, G.; Barbaro, M.R.; Fuschi, D.; Palombo, M.; Falangone, F.; Cremon, C.; Marasco, G.; Stanghellini, V. Inflammatory and Microbiota-Related Regulation of the Intestinal Epithelial Barrier. Front. Nutr. 2021, 8, 718356. [Google Scholar] [CrossRef]
- Dong, D.; Su, T.; Chen, W.; Wang, D.; Xue, Y.; Lu, Q.; Jiang, C.; Ni, Q.; Mao, E.; Peng, Y. Clostridioides difficile Aggravates Dextran Sulfate Solution (DSS)-Induced Colitis by Shaping the Gut Microbiota and Promoting Neutrophil Recruitment. Gut Microbes 2023, 15, 2192478. [Google Scholar] [CrossRef]
- Cook, L.; Wong, M.Q.; Rees, W.D.; Schick, A.; Lisko, D.J.; Lunken, G.R.; Wang, X.; Peters, H.; Oliveira, L.; Lau, T.; et al. Dysregulated Immunity to Clostridioides difficile in IBD Patients Without a History of Recognized Infection. Inflamm. Bowel Dis. 2024, 30, 820–828. [Google Scholar] [CrossRef]
- Kelly, C.P.; Kyne, L. The Host Immune Response to Clostridium difficile. J. Med. Microbiol. 2011, 60, 1070–1079. [Google Scholar] [CrossRef]
- Bibbò, S.; Lopetuso, L.R.; Ianiro, G.; Di Rienzo, T.; Gasbarrini, A.; Cammarota, G. Role of Microbiota and Innate Immunity in Recurrent Clostridium difficile Infection. J. Immunol. Res. 2014, 2014, 462740. [Google Scholar] [CrossRef]
- Bassotti, G.; Fruganti, A.; Stracci, F.; Marconi, P.; Fettucciari, K. Cytotoxic Synergism of Clostridioides difficile Toxin B with Proinflammatory Cytokines in Subjects with Inflammatory Bowel Diseases. World J. Gastroenterol. 2023, 29, 582–596. [Google Scholar] [CrossRef]
- Huang, J.; Kelly, C.P.; Bakirtzi, K.; Villafuerte Gálvez, J.A.; Lyras, D.; Mileto, S.J.; Larcombe, S.; Xu, H.; Yang, X.; Shields, K.S.; et al. Clostridium difficile Toxins Induce VEGF-A and Vascular Permeability to Promote Disease Pathogenesis. Nat. Microbiol. 2018, 4, 269–279. [Google Scholar] [CrossRef]
- Balram, B.; Battat, R.; Al-Khoury, A.; D’Aoust, J.; Afif, W.; Bitton, A.; Lakatos, P.L.; Bessissow, T. Risk Factors Associated with Clostridium difficile Infection in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. J. Crohn’s Colitis 2019, 13, 27–38. [Google Scholar] [CrossRef]
- Vitikainen, K.; Kase, M.; Meriranta, L.; Molander, P.; Af Björkesten, C.-G.; Anttila, V.-J.; Arkkila, P. Higher Disease Activity of Inflammatory Bowel Disease Predisposes to Clostridioides difficile Infection. Ther. Adv. Gastroenterol. 2025, 18, 17562848251318292. [Google Scholar] [CrossRef]
- Chen, X.L.; Deng, J.; Chen, X.; Wan, S.S.; Wang, Y.; Cao, Q. High Incidence and Morbidity of Clostridium difficile Infection among Hospitalized Patients with Inflammatory Bowel Disease: A Prospective Observational Cohort Study. J. Dig. Dis. 2019, 20, 460–466. [Google Scholar] [CrossRef]
- Martínez-Lozano, H.; Saralegui-Gonzalez, P.; Reigadas, E.; Fueyo-Peláez, P.R.; García-García, A.; Miranda-Bautista, J.; Alcalá, L.; Nieto, J.C.; Lobato-Matilla, M.E.; Marín-Jiménez, I.; et al. Risk Factors for Clostridioides difficile Infection among Patients Diagnosed with Inflammatory Intestinal and Rheumatological Diseases in the Biologic Era. BMC Gastroenterol. 2025, 25, 70. [Google Scholar] [CrossRef]
- Jakubowska, A.; Szydlarska, D.; Rydzewska, G. Analysis of Risk Factors of Clostridioides difficile Infection in Patients with Inflammatory Bowel Disease. Przegląd Gastroenterol. 2024, 19, 277–283. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, Q.-Y.; Fei, J.-X.; Zhang, Y.; Lin, M.-Y.; Jiang, S.-H.; Wang, P.; Chen, Y. Clostridium difficile Infection Worsen Outcome of Hospitalized Patients with Inflammatory Bowel Disease. Sci. Rep. 2016, 6, 29791. [Google Scholar] [CrossRef]
- Schneeweiss, S.; Korzenik, J.; Solomon, D.H.; Canning, C.; Lee, J.; Bressler, B. Infliximab and Other Immunomodulating Drugs in Patients with Inflammatory Bowel Disease and the Risk of Serious Bacterial Infections. Aliment. Pharmacol. Ther. 2009, 30, 253–264. [Google Scholar] [CrossRef]
- Bossuyt, P.; Verhaegen, J.; Van Assche, G.; Rutgeerts, P.; Vermeire, S. Increasing Incidence of Clostridium difficile-Associated Diarrhea in Inflammatory Bowel Disease. J. Crohn’s Colitis 2009, 3, 4–7. [Google Scholar] [CrossRef]
- Issa, M.; Vijayapal, A.; Graham, M.B.; Beaulieu, D.B.; Otterson, M.F.; Lundeen, S.; Skaros, S.; Weber, L.R.; Komorowski, R.A.; Knox, J.F.; et al. Impact of Clostridium difficile on Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2007, 5, 345–351. [Google Scholar] [CrossRef]
- Colombel, J.-F.; Sands, B.E.; Rutgeerts, P.; Sandborn, W.; Danese, S.; D’Haens, G.; Panaccione, R.; Loftus, E.V.; Sankoh, S.; Fox, I.; et al. The Safety of Vedolizumab for Ulcerative Colitis and Crohn’s Disease. Gut 2017, 66, 839–851. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Zhang, Y.; Zhang, H.; Chen, C.; Zhu, S.; Zhou, Y.; Zhao, H.; Zong, Y. Risk of Clostridioides difficile Infection in Inflammatory Bowel Disease Patients Undergoing Vedolizumab Treatment: A Systematic Review and Meta-Analysis. BMC Gastroenterol. 2024, 24, 377. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Danese, S.; O’Brien, C.D.; Ott, E.; Marano, C.; Baker, T.; Zhou, Y.; Volger, S.; Tikhonov, I.; et al. Safety of Ustekinumab in Inflammatory Bowel Disease: Pooled Safety Analysis of Results from Phase 2/3 Studies. Inflamm. Bowel Dis. 2021, 27, 994–1007. [Google Scholar] [CrossRef]
- Loftus, E.V.; Baumgart, D.C.; Gecse, K.; Kinnucan, J.A.; Connelly, S.B.; Salese, L.; Su, C.; Kwok, K.K.; Woolcott, J.C.; Armuzzi, A. Clostridium difficile Infection in Patients with Ulcerative Colitis Treated with Tofacitinib in the Ulcerative Colitis Program. Inflamm. Bowel Dis. 2023, 29, 744–751. [Google Scholar] [CrossRef]
- Voth, E.; Solanky, D.; Loftus, E.V.; Pardi, D.S.; Khanna, S. Novel Risk Factors and Outcomes in Inflammatory Bowel Disease Patients with Clostridioides difficile Infection. Ther. Adv. Gastroenterol. 2021, 14, 1756284821997792. [Google Scholar] [CrossRef]
- Lowe, S.C.; Sauk, J.S.; Limketkai, B.N.; Kwaan, M.R. Declining Rates of Surgery for Inflammatory Bowel Disease in the Era of Biologic Therapy. J. Gastrointest. Surg. 2021, 25, 211–219. [Google Scholar] [CrossRef]
- Bagdasarian, N.; Rao, K.; Malani, P.N. Diagnosis and Treatment of Clostridium difficile in Adults: A Systematic Review. JAMA 2015, 313, 398. [Google Scholar] [CrossRef]
- Spinelli, A.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Surgical Treatment. J. Crohn’s Colitis 2022, 16, 179–189. [Google Scholar] [CrossRef]
- Adamina, M.; Minozzi, S.; Warusavitarne, J.; Buskens, C.J.; Chaparro, M.; Verstockt, B.; Kopylov, U.; Yanai, H.; Vavricka, S.R.; Sigall-Boneh, R.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Surgical Treatment. J. Crohn’s Colitis 2024, 18, 1556–1582. [Google Scholar] [CrossRef]
- Seril, D.N.; Shen, B. Clostridium difficile Infection in the Postcolectomy Patient. Inflamm. Bowel Dis. 2014, 20, 2450–2469. [Google Scholar] [CrossRef]
- Kistangari, G.; Lopez, R.; Shen, B. Frequency and Risk Factors of Clostridium difficile Infection in Hospitalized Patients with Pouchitis: A Population-Based Study. Inflamm. Bowel Dis. 2017, 23, 661–671. [Google Scholar] [CrossRef]
- Barnes, E.L.; Agrawal, M.; Syal, G.; Ananthakrishnan, A.N.; Cohen, B.L.; Haydek, J.P.; Al Kazzi, E.S.; Eisenstein, S.; Hashash, J.G.; Sultan, S.S.; et al. AGA Clinical Practice Guideline on the Management of Pouchitis and Inflammatory Pouch Disorders. Gastroenterology 2024, 166, 59–85. [Google Scholar] [CrossRef]
- Li, Y.; Qian, J.; Queener, E.; Shen, B. Risk Factors and Outcome of PCR-Detected Clostridium difficile Infection in Ileal Pouch Patients. Inflamm. Bowel Dis. 2013, 19, 397–403. [Google Scholar] [CrossRef]
- Navaneethan, U.; Giannella, R.A. Thinking beyond the Colon-Small Bowel Involvement in Clostridium difficile Infection. Gut Pathog. 2009, 1, 7. [Google Scholar] [CrossRef]
- Crobach, M.J.T.; Baktash, A.; Duszenko, N.; Kuijper, E.J. Diagnostic Guidance for C. difficile Infections. In Updates on Clostridium difficile in Europe; Mastrantonio, P., Rupnik, M., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2018; Volume 1050, pp. 27–44. ISBN 978-3-319-72798-1. [Google Scholar]
- Senok, A.; Aldosari, K.; Alowaisheq, R.; Abid, O.; Alsuhaibani, K.; Khan, M.; Somily, A. Detection of Clostridium difficile Antigen and Toxin in Stool Specimens: Comparison of the C. difficile Quik Chek Complete Enzyme Immunoassay and GeneXpert C. difficile Polymerase Chain Reaction Assay. Saudi J. Gastroenterol. 2017, 23, 259. [Google Scholar] [CrossRef]
- Gateau, C.; Couturier, J.; Coia, J.; Barbut, F. How to: Diagnose Infection Caused by Clostridium difficile. Clin. Microbiol. Infect. 2018, 24, 463–468. [Google Scholar] [CrossRef]
- Prosty, C.; Hanula, R.; Katergi, K.; Longtin, Y.; McDonald, E.G.; Lee, T.C. Clinical Outcomes and Management of NAAT-Positive/Toxin-Negative Clostridioides difficile Infection: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2024, 78, 430–438. [Google Scholar] [CrossRef]
- Park, J.; Kim, S.; Im, J.P.; Lee, H.J.; Kim, J.S.; Park, H.; Han, Y.M.; Koh, S.-J. Clinical Outcome of Inflammatory Bowel Disease with Clostridioides difficile Polymerase Chain Reaction Toxin-Positive/Enzyme Immunoassay Toxin-Negative: A Retrospective Cohort Study. Dig. Dis. Sci. 2025, 70, 2794–2803. [Google Scholar] [CrossRef]
- Turner, D.P.; Thorburn, S.J.; Crowe, A.; Jardine, D.; Timmins, C. Trends in Clostridioides difficile Diagnosis before and after a Change in Testing Algorithm. J. Microbiol. Methods 2021, 184, 106189. [Google Scholar] [CrossRef]
- Dbeibo, L.; Lucky, C.W.; Fadel, W.F.; Sadowski, J.; Beeler, C.; Kelley, K.; Williams, J.; Webb, D.; Kara, A. Two-Step Algorithm-Based Clostridioides difficile Testing as a Tool for Antibiotic Stewardship. Clin. Microbiol. Infect. 2023, 29, 798.e1–798.e4. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Guzman-Perez, R.; Gainer, V.; Cai, T.; Churchill, S.; Kohane, I.; Plenge, R.M.; Murphy, S. Predictors of Severe Outcomes Associated with Clostridium difficile Infection in Patients with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2012, 35, 789–795. [Google Scholar] [CrossRef]
- Di Bella, S.; Di Masi, A.; Turla, S.; Ascenzi, P.; Gouliouris, T.; Petrosillo, N. The Protective Role of Albumin in Clostridium difficile Infection: A Step Toward Solving the Puzzle. Infect. Control Hosp. Epidemiol. 2015, 36, 1478–1480. [Google Scholar] [CrossRef]
- Abt, M.C.; McKenney, P.T.; Pamer, E.G. Clostridium difficile Colitis: Pathogenesis and Host Defence. Nat. Rev. Microbiol. 2016, 14, 609–620. [Google Scholar] [CrossRef]
- Ramachandran, I.; Sinha, R.; Rodgers, P. Pseudomembranous Colitis Revisited: Spectrum of Imaging Findings. Clin. Radiol. 2006, 61, 535–544. [Google Scholar] [CrossRef]
- Calméjane, L.; Laharie, D.; Kirchgesner, J.; Uzzan, M. Review Article: Updated Management of Acute Severe Ulcerative Colitis: From Steroids to Novel Medical Strategies. UEG J. 2023, 11, 722–732. [Google Scholar] [CrossRef]
- Kirkpatrick, I.D.C.; Greenberg, H.M. Evaluating the CT Diagnosis of Clostridium difficile Colitis: Should CT Guide Therapy? Am. J. Roentgenol. 2001, 176, 635–639. [Google Scholar] [CrossRef]
- Autenrieth, D.M.; Baumgart, D.C. Toxic Megacolon. Inflamm. Bowel Dis. 2012, 18, 584–591. [Google Scholar] [CrossRef]
- Khanna, S.; Shin, A.; Kelly, C.P. Management of Clostridium difficile Infection in Inflammatory Bowel Disease: Expert Review from the Clinical Practice Updates Committee of the AGA Institute. Clin. Gastroenterol. Hepatol. 2017, 15, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Solanky, D.; Pardi, D.S.; Loftus, E.V.; Khanna, S. Colon Surgery Risk with Corticosteroids Versus Immunomodulators or Biologics in Inflammatory Bowel Disease Patients with Clostridium difficile Infection. Inflamm. Bowel Dis. 2019, 25, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Ben-Horin, S.; Margalit, M.; Bossuyt, P.; Maul, J.; Shapira, Y.; Bojic, D.; Chermesh, I.; Al-Rifai, A.; Schoepfer, A.; Bosani, M.; et al. Combination Immunomodulator and Antibiotic Treatment in Patients with Inflammatory Bowel Disease and Clostridium difficile Infection. Clin. Gastroenterol. Hepatol. 2009, 7, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Bar-Yoseph, H.; Daoud, H.; Ben Hur, D.; Chowers, Y.; Waterman, M. Does Early Corticosteroid Therapy Affect Prognosis in IBD Patients Hospitalized with Clostridioides difficile Infection? Int. J. Colorectal Dis. 2020, 35, 513–519. [Google Scholar] [CrossRef]
- Lukin, D.J.; Lawlor, G.; Hudesman, D.P.; Durbin, L.; Axelrad, J.E.; Passi, M.; Cavaliere, K.; Coburn, E.; Loftus, M.; Jen, H.; et al. Escalation of Immunosuppressive Therapy for Inflammatory Bowel Disease Is Not Associated with Adverse Outcomes After Infection with Clostridium difficile. Inflamm. Bowel Dis. 2019, 25, 775–781. [Google Scholar] [CrossRef]
- Kucharzik, T.; Ellul, P.; Greuter, T.; Rahier, J.F.; Verstockt, B.; Abreu, C.; Albuquerque, A.; Allocca, M.; Esteve, M.; Farraye, F.A.; et al. ECCO Guidelines on the Prevention, Diagnosis, and Management of Infections in Inflammatory Bowel Disease. J. Crohn’s Colitis 2021, 15, 879–913. [Google Scholar] [CrossRef]
- Moran, G.W.; Gordon, M.; Sinopolou, V.; Radford, S.J.; Darie, A.-M.; Vuyyuru, S.K.; Alrubaiy, L.; Arebi, N.; Blackwell, J.; Butler, T.D.; et al. British Society of Gastroenterology Guidelines on Inflammatory Bowel Disease in Adults: 2025. Gut 2025, 74, s1–s101, Erratum in Gut 2025, 74, e20. [Google Scholar] [CrossRef]
- Johnson, S.; Louie, T.J.; Gerding, D.N.; Cornely, O.A.; Chasan-Taber, S.; Fitts, D.; Gelone, S.P.; Broom, C.; Davidson, D.M.; for the Polymer Alternative for CDI Treatment (PACT) investigators. Vancomycin, Metronidazole, or Tolevamer for Clostridium difficile Infection: Results from Two Multinational, Randomized, Controlled Trials. Clin. Infect. Dis. 2014, 59, 345–354. [Google Scholar] [CrossRef]
- Allegretti, J.R.; Marcus, J.; Storm, M.; Sitko, J.; Kennedy, K.; Gerber, G.K.; Bry, L. Clinical Predictors of Recurrence After Primary Clostridioides difficile Infection: A Prospective Cohort Study. Dig. Dis. Sci. 2020, 65, 1761–1766. [Google Scholar] [CrossRef]
- Vega, A.D.; Heil, E.L.; Blackman, A.L.; Banoub, M.; Kristie Johnson, J.; Leekha, S.; Claeys, K.C. Evaluation of Addition of Intravenous Metronidazole to Oral Vancomycin Therapy in Critically Ill Patients with Non-Fulminant Severe Clostridioides difficile Infection. Pharmacotherapy 2020, 40, 398–407. [Google Scholar] [CrossRef]
- Rokas, K.E.E.; Johnson, J.W.; Beardsley, J.R.; Ohl, C.A.; Luther, V.P.; Williamson, J.C. The Addition of Intravenous Metronidazole to Oral Vancomycin Is Associated with Improved Mortality in Critically Ill Patients with Clostridium difficile Infection. Clin. Infect. Dis. 2015, 61, 934–941. [Google Scholar] [CrossRef]
- Cornely, O.A.; Crook, D.W.; Esposito, R.; Poirier, A.; Somero, M.S.; Weiss, K.; Sears, P.; Gorbach, S. Fidaxomicin versus Vancomycin for Infection with Clostridium difficile in Europe, Canada, and the USA: A Double-Blind, Non-Inferiority, Randomised Controlled Trial. Lancet Infect. Dis. 2012, 12, 281–289. [Google Scholar] [CrossRef]
- Louie, T.J.; Miller, M.A.; Mullane, K.M.; Weiss, K.; Lentnek, A.; Golan, Y.; Gorbach, S.; Sears, P.; Shue, Y.-K. Fidaxomicin versus Vancomycin for Clostridium difficile Infection. N. Engl. J. Med. 2011, 364, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Kalocsai, K.; Fortuny, C.; Lazar, S.; Bosis, S.; Korczowski, B.; Petit, A.; Bradford, D.; Croos-Dabrera, R.; Incera, E.; et al. Safety and Efficacy of Fidaxomicin and Vancomycin in Children and Adolescents with Clostridioides (Clostridium) difficile Infection: A Phase 3, Multicenter, Randomized, Single-Blind Clinical Trial (SUNSHINE). Clin. Infect. Dis. 2020, 71, 2581–2588. [Google Scholar] [CrossRef] [PubMed]
- Guery, B.; Menichetti, F.; Anttila, V.-J.; Adomakoh, N.; Aguado, J.M.; Bisnauthsing, K.; Georgopali, A.; Goldenberg, S.D.; Karas, A.; Kazeem, G.; et al. Extended-Pulsed Fidaxomicin versus Vancomycin for Clostridium difficile Infection in Patients 60 Years and Older (EXTEND): A Randomised, Controlled, Open-Label, Phase 3b/4 Trial. Lancet Infect. Dis. 2018, 18, 296–307. [Google Scholar] [CrossRef]
- Högenauer, C.; Mahida, Y.; Stallmach, A.; Marteau, P.; Rydzewska, G.; Ivashkin, V.; Gargalianos-Kakolyris, P.; Michon, I.; Adomakoh, N.; Georgopali, A.; et al. Pharmacokinetics and Safety of Fidaxomicin in Patients with Inflammatory Bowel Disease and Clostridium difficile Infection: An Open-Label Phase IIIb/IV Study (PROFILE). J. Antimicrob. Chemother. 2018, 73, 3430–3441. [Google Scholar] [CrossRef]
- Nerandzic, M.M.; Mullane, K.; Miller, M.A.; Babakhani, F.; Donskey, C.J. Reduced Acquisition and Overgrowth of Vancomycin-Resistant Enterococci and Candida Species in Patients Treated with Fidaxomicin Versus Vancomycin for Clostridium difficile Infection. Clin. Infect. Dis. 2012, 55, S121–S126. [Google Scholar] [CrossRef]
- Rubio-Terrés, C.; Aguado, J.M.; Almirante, B.; Cobo, J.; Grau, S.; Salavert, M.; González Antona Sánchez, E.; López Gutiérrez, C.; Rubio-Rodríguez, D. Extended-Pulsed Fidaxomicin versus Vancomycin in Patients 60 Years and Older with Clostridium difficile Infection: Cost-Effectiveness Analysis in Spain. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1105–1111. [Google Scholar] [CrossRef]
- Patel, D.; Senecal, J.; Spellberg, B.; Morris, A.M.; Saxinger, L.; Footer, B.W.; McDonald, E.G.; Lee, T.C. Fidaxomicin to Prevent Recurrent Clostridioides difficile: What Will It Cost in the USA and Canada? JAC-Antimicrob. Resist. 2022, 5, dlac138. [Google Scholar] [CrossRef]
- Dilnessa, T.; Getaneh, A.; Hailu, W.; Moges, F.; Gelaw, B. Prevalence and Antimicrobial Resistance Pattern of Clostridium difficile among Hospitalized Diarrheal Patients: A Systematic Review and Meta-Analysis. PLoS ONE 2022, 17, e0262597. [Google Scholar] [CrossRef]
- Toth, M.; Stewart, N.K.; Smith, C.; Vakulenko, S.B. Intrinsic Class D β-Lactamases of Clostridium difficile. mBio 2018, 9, e01803-18. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, B.K.; Edwards, A.N.; Anderson, S.E.; Woods, E.C.; McBride, S.M. Regulation and Anaerobic Function of the Clostridioides difficile β-Lactamase. Antimicrob. Agents Chemother. 2019, 64, e01496-19. [Google Scholar] [CrossRef] [PubMed]
- Darkoh, C.; Keita, K.; Odo, C.; Oyaro, M.; Brown, E.L.; Arias, C.A.; Hanson, B.M.; DuPont, H.L. Emergence of Clinical Clostridioides difficile Isolates with Decreased Susceptibility to Vancomycin. Clin. Infect. Dis. 2022, 74, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.V.S.; Pham, N.V.S.; Hays, R.A.; Bolick, D.T.; Goldbeck, S.M.; Poulter, M.D.; Hoang, S.C.; Shin, J.H.; Wu, M.; Warren, C.A. Influence of Binary Toxin Gene Detection and Decreased Susceptibility to Antibiotics among Clostridioides difficile Strains on Disease Severity: A Single-Center Study. Antimicrob. Agents Chemother. 2022, 66, e00489-22. [Google Scholar] [CrossRef]
- Putsathit, P.; Hong, S.; George, N.; Hemphill, C.; Huntington, P.G.; Korman, T.M.; Kotsanas, D.; Lahra, M.; McDougall, R.; McGlinchey, A.; et al. Antimicrobial Resistance Surveillance of Clostridioides difficile in Australia, 2015–2018. J. Antimicrob. Chemother. 2021, 76, 1815–1821. [Google Scholar] [CrossRef]
- Saha, S.; Kapoor, S.; Tariq, R.; Schuetz, A.N.; Tosh, P.K.; Pardi, D.S.; Khanna, S. Increasing Antibiotic Resistance in Clostridioides difficile: A Systematic Review and Meta-Analysis. Anaerobe 2019, 58, 35–46. [Google Scholar] [CrossRef]
- Olaitan, A.O.; Dureja, C.; Youngblom, M.A.; Topf, M.A.; Shen, W.-J.; Gonzales-Luna, A.J.; Deshpande, A.; Hevener, K.E.; Freeman, J.; Wilcox, M.H.; et al. Decoding a Cryptic Mechanism of Metronidazole Resistance among Globally Disseminated Fluoroquinolone-Resistant Clostridioides difficile. Nat. Commun. 2023, 14, 4130. [Google Scholar] [CrossRef]
- Boekhoud, I.M.; Hornung, B.V.H.; Sevilla, E.; Harmanus, C.; Bos-Sanders, I.M.J.G.; Terveer, E.M.; Bolea, R.; Corver, J.; Kuijper, E.J.; Smits, W.K. Plasmid-Mediated Metronidazole Resistance in Clostridioides difficile. Nat. Commun. 2020, 11, 598. [Google Scholar] [CrossRef]
- Smits, W.K.; Harmanus, C.; Sanders, I.M.J.G.; Bry, L.; Blackwell, G.A.; Ducarmon, Q.R.; De Oliveira Ferreira, E.; Kuijper, E.J. Sequence-Based Identification of Metronidazole-Resistant Clostridioides difficile Isolates. Emerg. Infect. Dis. 2022, 28, 2308–2311. [Google Scholar] [CrossRef]
- Deshpande, A.; Wu, X.; Huo, W.; Palmer, K.L.; Hurdle, J.G. Chromosomal Resistance to Metronidazole in Clostridioides difficile Can Be Mediated by Epistasis between Iron Homeostasis and Oxidoreductases. Antimicrob. Agents Chemother. 2020, 64, e00415-20. [Google Scholar] [CrossRef]
- Gargis, A.S.; Karlsson, M.; Paulick, A.L.; Anderson, K.F.; Adamczyk, M.; Vlachos, N.; Kent, A.G.; McAllister, G.; McKay, S.L.; Halpin, A.L.; et al. Reference Susceptibility Testing and Genomic Surveillance of Clostridioides difficile, United States, 2012–2017. Clin. Infect. Dis. 2023, 76, 890–896. [Google Scholar] [CrossRef]
- Shen, W.-J.; Deshpande, A.; Hevener, K.E.; Endres, B.T.; Garey, K.W.; Palmer, K.L.; Hurdle, J.G. Constitutive Expression of the Cryptic vanGCd Operon Promotes Vancomycin Resistance in Clostridioides difficile Clinical Isolates. J. Antimicrob. Chemother. 2020, 75, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Belitsky, B.R. VanG- and D-Ala-D-Ser-dependent Peptidoglycan Synthesis and Vancomycin Resistance in Clostridioides difficile. Mol. Microbiol. 2022, 118, 526–540. [Google Scholar] [CrossRef] [PubMed]
- Tijerina-Rodríguez, L.; Villarreal-Treviño, L.; Baines, S.D.; Morfín-Otero, R.; Camacho-Ortíz, A.; Flores-Treviño, S.; Maldonado-Garza, H.; Rodríguez-Noriega, E.; Garza-González, E. High Sporulation and Overexpression of Virulence Factors in Biofilms and Reduced Susceptibility to Vancomycin and Linezolid in Recurrent Clostridium [Clostridioides] difficile Infection Isolates. PLoS ONE 2019, 14, e0220671. [Google Scholar] [CrossRef]
- Buddle, J.E.; Thompson, L.M.; Williams, A.S.; Wright, R.C.T.; Durham, W.M.; Turner, C.E.; Chaudhuri, R.R.; Brockhurst, M.A.; Fagan, R.P. Identification of Pathways to High-Level Vancomycin Resistance in Clostridioides difficile That Incur High Fitness Costs in Key Pathogenicity Traits. PLoS Biol. 2024, 22, e3002741. [Google Scholar] [CrossRef]
- Thorpe, C.M.; McDermott, L.A.; Tran, M.K.; Chang, J.; Jenkins, S.G.; Goldstein, E.J.C.; Patel, R.; Forbes, B.A.; Johnson, S.; Gerding, D.N.; et al. U.S.-Based National Surveillance for Fidaxomicin Susceptibility of Clostridioides difficile-Associated Diarrheal Isolates from 2013 to 2016. Antimicrob. Agents Chemother. 2019, 63, e00391-19. [Google Scholar] [CrossRef]
- Redmond, S.N.; Cadnum, J.L.; Jencson, A.L.; Kaple, C.E.; Wilson, B.M.; Skinner, A.M.; Gargis, A.S.; Hwang, M.; Choi, H.; Chatterjee, P.; et al. Emergence and Spread of Clostridioides difficile Isolates with Reduced Fidaxomicin Susceptibility in an Acute Care Hospital. Clin. Infect. Dis. 2025, 80, 984–991. [Google Scholar] [CrossRef]
- Marchandin, H.; Anjou, C.; Poulen, G.; Freeman, J.; Wilcox, M.; Jean-Pierre, H.; Barbut, F. In Vivo Emergence of a Still Uncommon Resistance to Fidaxomicin in the Urgent Antimicrobial Resistance Threat Clostridioides difficile. J. Antimicrob. Chemother. 2023, 78, 1992–1999. [Google Scholar] [CrossRef]
- Le, T.M.; Eubank, T.A.; McKelvey, A.M.; Cao, X.; Hurdle, J.G.; Garey, K.W. Fidaxomicin Resistance in Clostridioides difficile: A Systematic Review and Predictive Modeling with RNA Polymerase Binding Sites. Antimicrob. Agents Chemother. 2024, 68, e01206-24. [Google Scholar] [CrossRef]
- Cao, X.; Boyaci, H.; Chen, J.; Bao, Y.; Landick, R.; Campbell, E.A. Basis of Narrow-Spectrum Activity of Fidaxomicin on Clostridioides difficile. Nature 2022, 604, 541–545. [Google Scholar] [CrossRef]
- Schwanbeck, J.; Riedel, T.; Laukien, F.; Schober, I.; Oehmig, I.; Zimmermann, O.; Overmann, J.; Groß, U.; Zautner, A.E.; Bohne, W. Characterization of a Clinical Clostridioides difficile Isolate with Markedly Reduced Fidaxomicin Susceptibility and a V1143D Mutation in rpoB. J. Antimicrob. Chemother. 2019, 74, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Baunwall, S.M.D.; Andreasen, S.E.; Hansen, M.M.; Kelsen, J.; Høyer, K.L.; Rågård, N.; Eriksen, L.L.; Støy, S.; Rubak, T.; Damsgaard, E.M.S.; et al. Faecal Microbiota Transplantation for First or Second Clostridioides difficile Infection (EarlyFMT): A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Gastroenterol. Hepatol. 2022, 7, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Mullish, B.H.; Quraishi, M.N.; Segal, J.P.; McCune, V.L.; Baxter, M.; Marsden, G.L.; Moore, D.J.; Colville, A.; Bhala, N.; Iqbal, T.H.; et al. The Use of Faecal Microbiota Transplant as Treatment for Recurrent or Refractory Clostridium difficile Infection and Other Potential Indications: Joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) Guidelines. Gut 2018, 67, 1920–1941. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Maida, M.; Burisch, J.; Simonelli, C.; Hold, G.; Ventimiglia, M.; Gasbarrini, A.; Cammarota, G. Efficacy of Different Faecal Microbiota Transplantation Protocols for Clostridium difficile Infection: A Systematic Review and Meta-analysis. UEG J. 2018, 6, 1232–1244. [Google Scholar] [CrossRef]
- Yadegar, A.; Bar-Yoseph, H.; Monaghan, T.M.; Pakpour, S.; Severino, A.; Kuijper, E.J.; Smits, W.K.; Terveer, E.M.; Neupane, S.; Nabavi-Rad, A.; et al. Fecal Microbiota Transplantation: Current Challenges and Future Landscapes. Clin. Microbiol. Rev. 2024, 37, e00060-22. [Google Scholar] [CrossRef]
- Van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; De Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.W.M.; Tijssen, J.G.P.; et al. Duodenal Infusion of Donor Feces for Recurrent Clostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef]
- Kelly, C.R.; Yen, E.F.; Grinspan, A.M.; Kahn, S.A.; Atreja, A.; Lewis, J.D.; Moore, T.A.; Rubin, D.T.; Kim, A.M.; Serra, S.; et al. Fecal Microbiota Transplantation Is Highly Effective in Real-World Practice: Initial Results from the FMT National Registry. Gastroenterology 2021, 160, 183–192.e3. [Google Scholar] [CrossRef]
- Baunwall, S.M.D.; Lee, M.M.; Eriksen, M.K.; Mullish, B.H.; Marchesi, J.R.; Dahlerup, J.F.; Hvas, C.L. Faecal Microbiota Transplantation for Recurrent Clostridioides difficile Infection: An Updated Systematic Review and Meta-Analysis. eClinicalMedicine 2020, 29–30, 100642. [Google Scholar] [CrossRef]
- Qazi, T.; Amaratunga, T.; Barnes, E.L.; Fischer, M.; Kassam, Z.; Allegretti, J.R. The Risk of Inflammatory Bowel Disease Flares after Fecal Microbiota Transplantation: Systematic Review and Meta-Analysis. Gut Microbes 2017, 8, 574–588. [Google Scholar] [CrossRef]
- Tariq, R.; Disbrow, M.B.; Dibaise, J.K.; Orenstein, R.; Saha, S.; Solanky, D.; Loftus, E.V.; Pardi, D.S.; Khanna, S. Efficacy of Fecal Microbiota Transplantation for Recurrent C. difficile Infection in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2020, 26, 1415–1420. [Google Scholar] [CrossRef]
- Hirten, R.P.; Grinspan, A.; Fu, S.-C.; Luo, Y.; Suarez-Farinas, M.; Rowland, J.; Contijoch, E.J.; Mogno, I.; Yang, N.; Luong, T.; et al. Microbial Engraftment and Efficacy of Fecal Microbiota Transplant for Clostridium difficile in Patients with and Without Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Newman, K.M.; Rank, K.M.; Vaughn, B.P.; Khoruts, A. Treatment of Recurrent Clostridium difficile Infection Using Fecal Microbiota Transplantation in Patients with Inflammatory Bowel Disease. Gut Microbes 2017, 8, 303–309. [Google Scholar] [CrossRef]
- DeFilipp, Z.; Bloom, P.P.; Torres Soto, M.; Mansour, M.K.; Sater, M.R.A.; Huntley, M.H.; Turbett, S.; Chung, R.T.; Chen, Y.-B.; Hohmann, E.L. Drug-Resistant E. Coli Bacteremia Transmitted by Fecal Microbiota Transplant. N. Engl. J. Med. 2019, 381, 2043–2050. [Google Scholar] [CrossRef]
- Quera, R.; Espinoza, R.; Estay, C.; Rivera, D. Bacteremia as an Adverse Event of Fecal Microbiota Transplantation in a Patient with Crohn’s Disease and Recurrent Clostridium difficile Infection. J. Crohn’s Colitis 2014, 8, 252–253. [Google Scholar] [CrossRef] [PubMed]
- Tixier, E.N.; Verheyen, E.; Luo, Y.; Grinspan, L.T.; Du, C.H.; Ungaro, R.C.; Walsh, S.; Grinspan, A.M. Systematic Review with Meta-Analysis: Fecal Microbiota Transplantation for Severe or Fulminant Clostridioides difficile. Dig. Dis. Sci. 2022, 67, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, J.R.; Feuerstadt, P.; Knapple, W.L.; Orenstein, R.; Pinton, P.; Sheh, A.; Khanna, S. Safety and Efficacy of Fecal Microbiota, Live-Jslm (REBYOTA®), for the Prevention of Recurrent Clostridioides difficile Infection in Participants with Inflammatory Bowel Disease in PUNCH CD3-OLS. Inflamm. Bowel Dis. 2025, 31, 2112–2122. [Google Scholar] [CrossRef]
- Ianiro, G.; Bibbò, S.; Porcari, S.; Settanni, C.R.; Giambò, F.; Curta, A.R.; Quaranta, G.; Scaldaferri, F.; Masucci, L.; Sanguinetti, M.; et al. Fecal Microbiota Transplantation for Recurrent C. difficile Infection in Patients with Inflammatory Bowel Disease: Experience of a Large-Volume European FMT Center. Gut Microbes 2021, 13, 1994834. [Google Scholar] [CrossRef]
- Johnson, S.; Gerding, D.N. Bezlotoxumab. Clin. Infect. Dis. 2019, 68, 699–704. [Google Scholar] [CrossRef]
- Wilcox, M.H.; Gerding, D.N.; Poxton, I.R.; Kelly, C.; Nathan, R.; Birch, T.; Cornely, O.A.; Rahav, G.; Bouza, E.; Lee, C.; et al. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. N. Engl. J. Med. 2017, 376, 305–317. [Google Scholar] [CrossRef]
- Gerding, D.N.; Kelly, C.P.; Rahav, G.; Lee, C.; Dubberke, E.R.; Kumar, P.N.; Yacyshyn, B.; Kao, D.; Eves, K.; Ellison, M.C.; et al. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection in Patients at Increased Risk for Recurrence. Clin. Infect. Dis. 2018, 67, 649–656. [Google Scholar] [CrossRef]
- Hernandez, L.D.; Kroh, H.K.; Hsieh, E.; Yang, X.; Beaumont, M.; Sheth, P.R.; DiNunzio, E.; Rutherford, S.A.; Ohi, M.D.; Ermakov, G.; et al. Epitopes and Mechanism of Action of the Clostridium difficile Toxin A-Neutralizing Antibody Actoxumab. J. Mol. Biol. 2017, 429, 1030–1044. [Google Scholar] [CrossRef]
- Posteraro, B.; Pea, F.; Masucci, L.; Posteraro, P.; Sanguinetti, M. Actoxumab + Bezlotoxumab Combination: What Promise for Clostridium difficile Treatment? Expert Opin. Biol. Ther. 2018, 18, 469–476. [Google Scholar] [CrossRef]
- Tkaczyk, C.; Dayao, D.; Girouard, D.; Godfrey, V.; Gamson, A.; Stanley, A.M.; Stepanov, O.; DiGiandomenico, A.; Sellman, B.R.; Tzipori, S. P-1055. Anti-Toxin B Neutralizing Monoclonal Antibody AZD5148 Provides Protection in a Clostridioides difficile Gnotobiotic Piglet Model. Open Forum Infect. Dis. 2025, 12, ofae631.1244. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seguiti, C.; Tettoni, E.; Pezzuto, E.; Gerardi, V.; Quadarella, A.; Cesaro, P.; Colombini, P. Clostridioides difficile Infection in Special Populations: Focus on Inflammatory Bowel Disease—A Narrative Review from Pathogenesis to Management. Biomedicines 2025, 13, 2702. https://doi.org/10.3390/biomedicines13112702
Seguiti C, Tettoni E, Pezzuto E, Gerardi V, Quadarella A, Cesaro P, Colombini P. Clostridioides difficile Infection in Special Populations: Focus on Inflammatory Bowel Disease—A Narrative Review from Pathogenesis to Management. Biomedicines. 2025; 13(11):2702. https://doi.org/10.3390/biomedicines13112702
Chicago/Turabian StyleSeguiti, Cristina, Enrico Tettoni, Edoardo Pezzuto, Viviana Gerardi, Alessandro Quadarella, Paola Cesaro, and Paolo Colombini. 2025. "Clostridioides difficile Infection in Special Populations: Focus on Inflammatory Bowel Disease—A Narrative Review from Pathogenesis to Management" Biomedicines 13, no. 11: 2702. https://doi.org/10.3390/biomedicines13112702
APA StyleSeguiti, C., Tettoni, E., Pezzuto, E., Gerardi, V., Quadarella, A., Cesaro, P., & Colombini, P. (2025). Clostridioides difficile Infection in Special Populations: Focus on Inflammatory Bowel Disease—A Narrative Review from Pathogenesis to Management. Biomedicines, 13(11), 2702. https://doi.org/10.3390/biomedicines13112702






