Unveiling the Role of CCL3: A Driver of CIPN in Colon Cancer Patients?
Abstract
1. Introduction
1.1. Definition and Function of Chemokines
1.2. CIPN and the Role of Chemokines
2. CCL3 and Cancer
2.1. Positive Correlation of CCL3 Increase with Tumor Growth
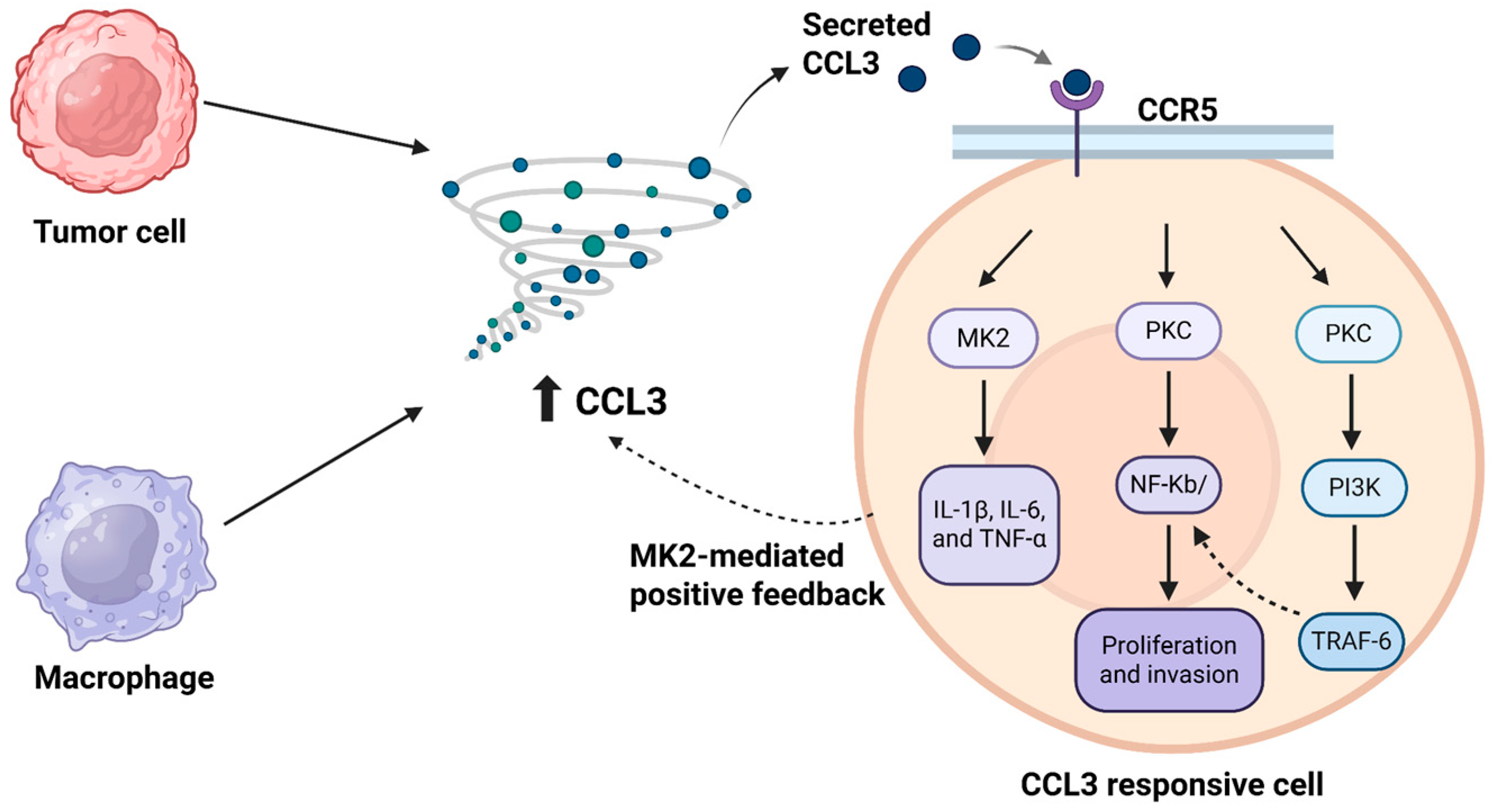
2.1.1. CCL3/CCR5-Driven Crosstalk in the Tumor Microenvironment
2.1.2. The Role of p53 in CCL3 Regulation
2.1.3. Chemokine-Chemokine Receptor Axis as a Treatment Option for Cancer
2.2. Negative Correlation Between CCL3 and Tumor Growth
3. CCL3 and Neuropathic Pain
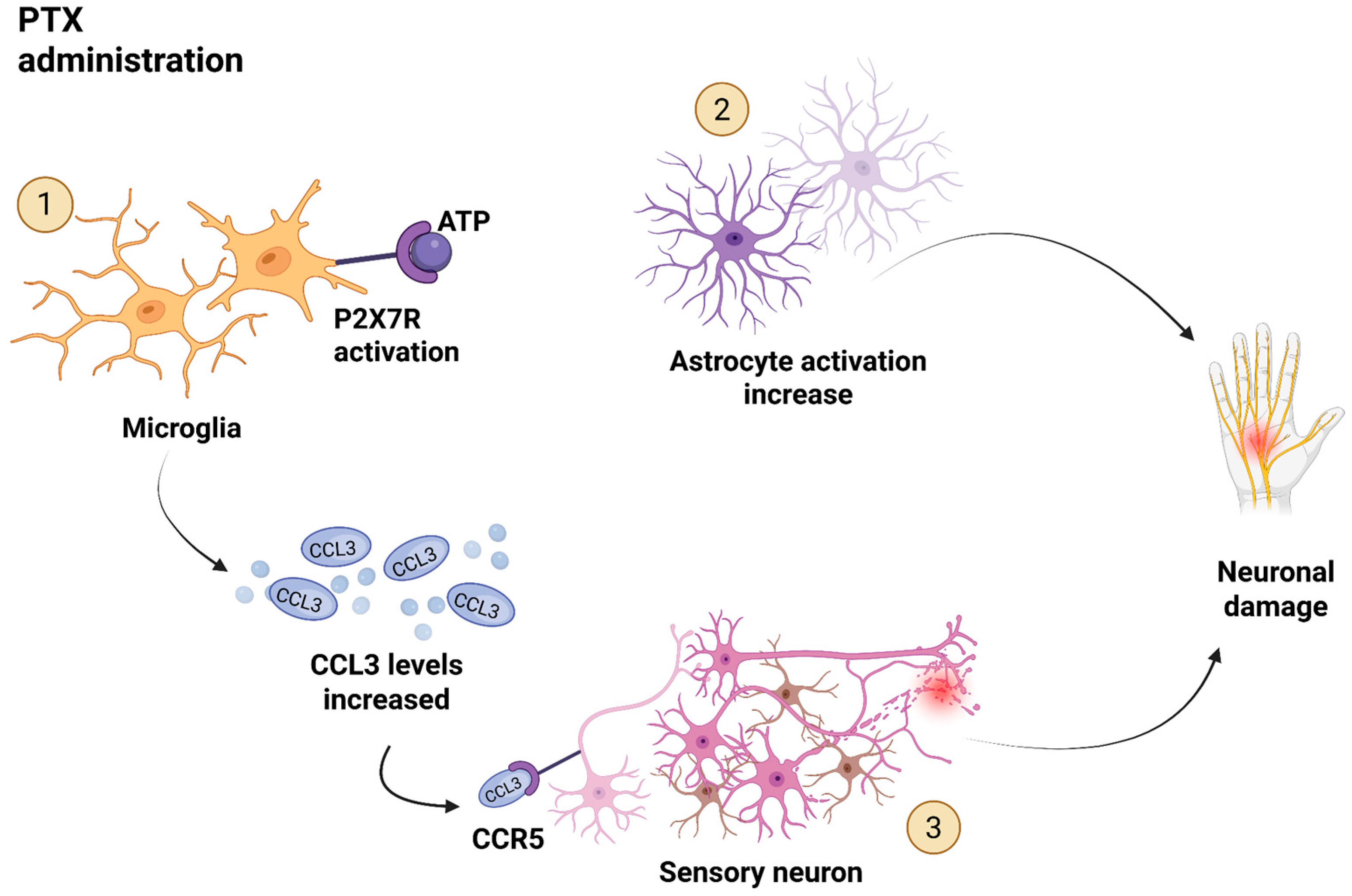
3.1. CCL3 and CIPN
3.2. CCL3 in Other Models or Forms of Neuropathic Pain
3.2.1. CCL3-Producing Cells and the Development of Neuropathy
3.2.2. CCL3 Influences Opioid and Transient Receptor Potential Vanilloid 1 (TRPV1) Receptor
3.2.3. Epigenetic Regulation of CCL3 via Macrophages
4. Summary
Link Between Colon Cancer and CIPN, with a Focus on CCL3
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
List of Abbreviations
| A438079 | (compound) selective P2X7 receptor antagonist |
| AOM | azoxymethane |
| AOM/DSS | azoxymethane + dextran sulfate sodium (colitis-associated cancer model) |
| ATF-3 | activating transcription factor 3 |
| CAF(s) | cancer-associated fibroblast(s) |
| CCK-8 | cell counting kit-8 |
| CCR1 | C-C chemokine receptor type 1 |
| CCR2 | C-C chemokine receptor type 2 |
| CCR5 | C-C chemokine receptor type 5 |
| CCL | CC-chemokine ligand (general) |
| CCL2 | C-C motif chemokine ligand 2 (MCP-1) |
| CCL3 | C-C motif chemokine ligand 3 (MIP-1α) |
| CCL4 | C-C motif chemokine ligand 4 (MIP-1β) |
| CCL5/RANTES | C-C motif chemokine ligand 5 (RANTES) |
| CCI | chronic constriction injury |
| CIK | cytokine-induced killer (cells) |
| CIPN | chemotherapy-induced peripheral neuropathy |
| CNS | central nervous system |
| CRC | colorectal cancer |
| CSF | cerebrospinal fluid |
| CXCL8 | chemokine (C-X-C motif) ligand 8 (IL-8) |
| CXCL9 | chemokine (C-X-C motif) ligand 9 |
| CXCL10 | chemokine (C-X-C motif) ligand 10 |
| DC/DCs | dendritic cell(s) |
| DPN | diabetic peripheral neuropathy |
| DRG | dorsal root ganglion (neurons/tissue) |
| DSS | dextran sulfate sodium |
| ECM | extracellular matrix |
| EGF | epidermal growth factor |
| HB-EGF | heparin-binding epidermal growth factor |
| HCT116 | HCT116 (human CRC cell line) |
| HMGB1 | high mobility group box 1 protein |
| IFN-γ | interferon-gamma |
| IL-1β | interleukin-1 beta |
| IL-4 | interleukin-4 |
| IL-6 | interleukin-6 |
| IHC | immunohistochemistry |
| L3TU | CCL3-secreting CT26 cell line (engineered tumor cells used in cited studies) |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| MIP-1α | macrophage inflammatory protein-1 alpha (synonym of CCL3) |
| MK2 | MAPK-activated protein kinase 2 |
| MMP9 | matrix metalloproteinase 9 |
| MOR | µ-opioid receptor (mu-opioid receptor) |
| MSC(s) | mesenchymal stem cell(s) |
| NF-κB (p65) | nuclear factor kappa B (p65 subunit often referred to) |
| NCI | National Cancer Institute |
| NK | natural killer (cell) |
| OXA | oxaliplatin |
| p38 | p38 mitogen-activated protein kinase (often referred to as p38 MAPK) |
| p53 | tumor suppressor protein p53 (TP53) |
| PI3K | phosphatidylinositol 3-kinase (phosphoinositide 3-kinase) |
| PKC | protein kinase C |
| PNI | perineural invasion (contextually used as perineural invasion/peripheral nerve involvement) |
| P2X7R | P2X7 receptor (purinergic P2X7 receptor) |
| PAK1 | p21-activated kinase 1 |
| PD-1 | programmed cell death protein 1 (PD-1) |
| Phospho-STAT3/pSTAT3 | phosphorylated STAT3 (signal transducer and activator of transcription 3) |
| PKC | protein kinase C (already listed) |
| PTX | paclitaxel |
| qRT-PCR | quantitative reverse-transcription PCR |
| RAP-103 | multi-chemokine receptor antagonist peptide RAP-103 |
| rCCL3 | recombinant CCL3 |
| RT-PCR | reverse-transcription PCR (or RT-PCR) |
| SMA/α-SMA | (α-)smooth muscle actin (marker of myofibroblasts) |
| SN38 | active metabolite of irinotecan |
| SNI | spared nerve injury |
| STZ | streptozotocin |
| TCGA | The Cancer Genome Atlas |
| TDLN | tumor-draining lymph node(s) |
| TME | tumor microenvironment |
| TNF-α | tumor necrosis factor alpha |
| TRAF-6/TRAF6 | TNF receptor-associated factor 6 |
| TRPV1 | transient receptor potential vanilloid 1 |
| VEGF | vascular endothelial growth factor |
| WTTU | wild-type tumor (cell line in cited experiments) |
| GPCR(s) | G protein-coupled receptor(s) |
| PGRN | progranulin |
| TACC2 | transforming acidic coiled-coil containing protein 2 |
References
- Colorectal Cancer—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/colorect.html (accessed on 19 June 2025).
- Bonhof, C.S.; Van De Poll-Franse, L.V.; Wasowicz, D.K.; Beerepoot, L.V.; Vreugdenhil, G.; Mols, F. The course of peripheral neuropathy and its association with health-related quality of life among colorectal cancer patients. J. Cancer Surviv. 2021, 15, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.J.; Sedhom, R.; Gupta, A. Chemotherapy-Induced Peripheral Neuropathy. JAMA Oncol. 2019, 5, 750. [Google Scholar] [CrossRef]
- Vitale, M.G.; Barbato, C.; Crispo, A.; Habetswallner, F.; De Martino, B.M.; Riccardi, F.; Maione, A.; Eisenwagen, S.; Vitale, G.; Cartenì, G. Zeoxanmulti trial: A Randomized, Double-Blinded, Placebo-Controlled Trial of Oral PMA-zeolite to prevent Chemotherapy-Induced Side Effects, in particular, Peripheral Neuropathy. Molecules 2020, 25, 2297. [Google Scholar] [CrossRef]
- Molassiotis, A.; Cheng, H.L.; Lopez, V.; Au, J.S.K.; Chan, A.; Bandla, A.; Leung, K.T.; Li, Y.C.; Wong, K.H.; Suen, L.K.; et al. Are we mis-estimating chemotherapy-induced peripheral neuropathy? Analysis of assessment methodologies from a prospective, multinational, longitudinal cohort study of patients receiving neurotoxic chemotherapy. BMC Cancer 2019, 19, 132. [Google Scholar] [CrossRef] [PubMed]
- Denlinger, C.S.; Barsevick, A.M. The Challenges of Colorectal Cancer Survivorship. J. Natl. Compr. Cancer Netw. 2009, 7, 883. [Google Scholar] [CrossRef]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–573. [Google Scholar] [CrossRef]
- Barker, C.E.; Thompson, S.; O’Boyle, G.; Lortat-Jacob, H.; Sheerin, N.S.; Ali, S.; Kirby, J.A. CCL2 nitration is a negative regulator of chemokine-mediated inflammation. Sci. Rep. 2017, 7, 44384. [Google Scholar] [CrossRef]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018, 285, 2944. [Google Scholar] [CrossRef]
- Definition of Chemokine—NCI Dictionary of Cancer Terms—NCI. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/chemokine (accessed on 9 February 2023).
- Jing, H.; Yen, J.H.; Ganea, D. A novel signaling pathway mediates the inhibition of CCL3/4 expression by prostaglandin E2. J. Biol. Chem. 2004, 279, 55176–55186. [Google Scholar] [CrossRef] [PubMed]
- Strieter, R.M.; Burdick, M.D.; Gomperts, B.N.; Belperio, J.A.; Keane, M.P. CXC chemokines in angiogenesis. Cytokine Growth Factor. Rev. 2005, 16, 593–609. [Google Scholar] [CrossRef] [PubMed]
- López-Cotarelo, P.; Gómez-Moreira, C.; Criado-García, O.; Sánchez, L.; Rodríguez-Fernández, J.L. Beyond Chemoattraction: Multifunctionality of Chemokine Receptors in Leukocytes. Trends Immunol. 2017, 38, 927–941. [Google Scholar] [CrossRef]
- Zlotnik, A.; Burkhardt, A.M.; Homey, B. Homeostatic chemokine receptors and organ-specific metastasis. Nat. Rev. Immunol. 2011, 11, 597–606. [Google Scholar] [CrossRef]
- Müller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M.E.; McClanahan, T.; Murphy, E.; Yuan, W.; Wagner, S.N.; et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410, 50–56. [Google Scholar] [CrossRef]
- Zlotnik, A.; Yoshie, O. Chemokines: A new classification system and their role in immunity. Immunity 2000, 12, 121–127. [Google Scholar] [CrossRef]
- Ma, X.; Su, J.; Zhao, S.; He, Y.; Li, S.; Yang, X.; Zhai, S.; Rong, S.; Zhang, X.; Xu, G.; et al. CCL3 Promotes Proliferation of Colorectal Cancer Related with TRAF6/NF-κB Molecular Pathway. Contrast Media Mol. Imaging 2022, 2022, 2387192. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente López, M.; Landskron, G.; Parada, D.; Dubois-Camacho, K.; Simian, D.; Martinez, M.; Romero, D.; Roa, J.C.; Chahuán, I.; Gutiérrez, R.; et al. The relationship between chemokines CCL2, CCL3, and CCL4 with the tumor microenvironment and tumor-associated macrophage markers in colorectal cancer. Tumor Biol. 2018, 40, 101042831881005. [Google Scholar] [CrossRef] [PubMed]
- Brandolini, L.; D’Angelo, M.; Antonosante, A.; Allegretti, M.; Cimini, A. Chemokine Signaling in Chemotherapy-Induced Neuropathic Pain. Int. J. Mol. Sci. 2019, 20, 2904. [Google Scholar] [CrossRef]
- Nomiyama, H.; Osada, N.; Yoshie, O. Systematic classification of vertebrate chemokines based on conserved synteny and evolutionary history. Genes Cells 2013, 18, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics 2022, 12, 2115–2132. [Google Scholar] [CrossRef]
- Amador-Martínez, I.; Aparicio-Trejo, O.E.; Bernabe-Yepes, B.; Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Sánchez-Lozada, L.G.; Pedraza-Chaverri, J.; Tapia, E. Mitochondrial Impairment: A Link for Inflammatory Responses Activation in the Cardiorenal Syndrome Type 4. Int. J. Mol. Sci. 2023, 24, 15875. [Google Scholar] [CrossRef]
- Starobova, H.; Vetter, I. Pathophysiology of chemotherapy-induced peripheral neuropathy. Front. Mol. Neurosci. 2017, 10, 174. [Google Scholar] [CrossRef]
- Lim, T.K.Y.; Rone, M.B.; Lee, S.; Antel, J.P.; Zhang, J. Mitochondrial and bioenergetic dysfunction in trauma-induced painful peripheral neuropathy. Mol. Pain 2015, 11. [Google Scholar] [CrossRef]
- Colvin, L.A. Chemotherapy-induced peripheral neuropathy: Where are we now? Pain 2019, 160, S1–S10. [Google Scholar] [CrossRef]
- Balogh, M.; Zhang, J.; Gaffney, C.M.; Kalakuntla, N.; Nguyen, N.T.; Trinh, R.T.; Aguilar, C.; Pham, H.V.; Milutinovic, B.; Nichols, J.M.; et al. Sensory neuron dysfunction in orthotopic mouse models of colon cancer. J. Neuroinflamm. 2022, 19, 204. [Google Scholar] [CrossRef]
- Lewandowska, P.; Szczuka, I.; Bednarz-Misa, I.; Szczęśniak-Sięga, B.M.; Neubauer, K.; Mierzchała-Pasierb, M.; Zawadzki, M.; Witkiewicz, W.; Krzystek-Korpacka, M. Modulating Properties of Piroxicam, Meloxicam and Oxicam Analogues against Macrophage-Associated Chemokines in Colorectal Cancer. Molecules 2021, 26, 7375. [Google Scholar] [CrossRef]
- Phinney, B.B.; Ray, A.L.; Peretti, A.S.; Jerman, S.J.; Grim, C.; Pinchuk, I.V.; Beswick, E.J. MK2 Regulates Macrophage Chemokine Activity and Recruitment to Promote Colon Tumor Growth. Front. Immunol. 2018, 9, 1857. [Google Scholar] [CrossRef] [PubMed]
- Duggirala, S.; Balasubramanian, V.; Seetharaman, A.; Murugan, S.; Roy, J.; Hassan, S.; Venkatraman, G.; Rayala, S.K. Transcriptome Analysis of Human Pancreatic Stellate Cells Co-cultured With PAK1-Modulated Cells Revealed the Role of Cytokine Pathway in Tumor Microenvironment. Pancreas 2025, 54, e414–e422. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, H.; Zhang, H.; Wang, B.; Ma, J. Cytokine profile of cerebrospinal fluid in pediatric patients with metastatic medulloblastoma. Heliyon 2024, 10. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Baba, T.; Shinagawa, K.; Matsushima, K.; Mukaida, N. Crucial involvement of the CCL3-CCR5 axis-mediated fibroblast accumulation in colitis-associated carcinogenesis in mice. Int. J. Cancer 2014, 135, 1297–1306. [Google Scholar] [CrossRef]
- Okayasu, I.; Hatakeyama, S.; Yamada, M.; Ohkusa, T.; Inagaki, Y.; Nakaya, R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990, 98, 694–702. [Google Scholar] [CrossRef]
- Okayasu, I.; Yamada, M.; Mikami, T.; Yoshida, T.; Kanno, J.; Ohkusa, T. Dysplasia and carcinoma development in a repeated dextran sulfate sodium-induced colitis model. J. Gastroenterol. Hepatol. 2002, 17, 1078–1083. [Google Scholar] [CrossRef]
- Neufert, C.; Becker, C.; Türeci, Ö.; Waldner, M.J.; Backert, I.; Floh, K.; Atreya, I.; Leppkes, M.; Jefremow, A.; Vieth, M.; et al. Tumor fibroblast-derived epiregulin promotes growth of colitis-associated neoplasms through ERK. J. Clin. Investig. 2013, 123, 1428–1443. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.-Y.; Matsushima, K.; Baba, T.; Mukaida, N. CCL3-CCR5 Axis Regulates Intratumoral Accumulation of Leukocytes and Fibroblasts and Promotes Angiogenesis in Murine Lung Metastasis Process. J. Immunol. 2008, 181, 6384–6393. [Google Scholar] [CrossRef]
- Tanabe, Y.; Sasaki, S.; Mukaida, N.; Baba, T. Blockade of the chemokine receptor, CCR5, reduces the growth of orthotopically injected colon cancer cells via limiting cancer-associated fibroblast accumulation. Oncotarget 2016, 7, 48335–48345. [Google Scholar] [CrossRef]
- Kobayashi, H.; Gieniec, K.A.; Lannagan, T.R.M.; Wang, T.; Asai, N.; Mizutani, Y.; Iida, T.; Ando, R.; Thomas, E.M.; Sakai, A.; et al. The origin and contribution of cancer-associated fibroblasts in colorectal carcinogenesis. Gastroenterology 2021, 162, 890. [Google Scholar] [CrossRef] [PubMed]
- Proost, P.; Loos, T.; Mortier, A.; Schutyser, E.; Gouwy, M.; Noppen, S.; Dillen, C.; Ronsse, I.; Conings, R.; Struyf, S.; et al. Citrullination of CXCL8 by peptidylarginine deiminase alters receptor usage, prevents proteolysis, and dampens tissue inflammation. J. Exp. Med. 2008, 205, 2085–2097. [Google Scholar] [CrossRef]
- Nishikawa, G.; Kawada, K.; Nakagawa, J.; Toda, K.; Ogawa, R.; Inamoto, S.; Mizuno, R.; Itatani, Y.; Sakai, Y. Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression via CCR5. Cell Death Dis. 2019, 10, 264. [Google Scholar] [CrossRef]
- Pervaiz, A.; Zepp, M.; Georges, R.; Bergmann, F.; Mahmood, S.; Faiza, S.; Berger, M.R.; Adwan, H. Antineoplastic effects of targeting CCR5 and its therapeutic potential for colorectal cancer liver metastasis. J. Cancer Res. Clin. Oncol. 2021, 147, 73–91. [Google Scholar] [CrossRef]
- Noble, S.; Goa, K.L. Gemcitabine. A review of its pharmacology and clinical potential in non-small cell lung cancer and pancreatic cancer. Drugs 1997, 54, 447–472. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, A.; Ansari, S.; Berger, M.R.; Adwan, H. CCR5 blockage by maraviroc induces cytotoxic and apoptotic effects in colorectal cancer cells. Med. Oncol. 2015, 32, 158. [Google Scholar] [CrossRef] [PubMed]
- Leroy, B.; Girard, L.; Hollestelle, A.; Minna, J.D.; Gazdar, A.F.; Soussi, T. Analysis of TP53 Mutation Status in Human Cancer Cell Lines: A Reassessment. Hum. Mutat. 2014, 35, 756. [Google Scholar] [CrossRef]
- Pathak, S.; Meng, W.-J.; Nandy, S.K.; Ping, J.; Bisgin, A.; Helmfors, L.; Waldmann, P.; Sun, X.F. Radiation and SN38 treatments modulate the expression of microRNAs, cytokines and chemokines in colon cancer cells in a p53-directed manner. Oncotarget 2015, 6, 44758–44780. [Google Scholar] [CrossRef]
- Chabot, G.G. Clinical pharmacokinetics of irinotecan. Clin. Pharmacokinet. 1997, 33, 245–259. [Google Scholar] [CrossRef]
- Xi, Y.; Shalgi, R.; Fodstad, O.; Pilpel, Y.; Ju, J. Differentially regulated micro-RNAs and actively translated messenger RNA transcripts by tumor suppressor p53 in colon cancer. Clin. Cancer Res. 2006, 12, 2014–2024. [Google Scholar] [CrossRef]
- Hwang, H.W.; Mendell, J.T. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br. J. Cancer 2006, 94, 776–780. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Liang, J.; Li, D.; Fang, J.; Wang, L.; Wang, J.; Zhang, J.; Guo, Q.; Yan, X.; Tang, H. Application of the chemokine-chemokine receptor axis increases the tumor-targeted migration ability of cytokine-induced killer cells in patients with colorectal cancer. Oncol. Lett. 2020, 20, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Sasazuki, S.; Camargo, M.C.; Shimazu, T.; Charvat, H.; Yamaji, T.; Sawada, N.; Kemp, T.J.; Pfeiffer, R.M.; Hildesheim, A.; et al. Circulating inflammatory markers and colorectal cancer risk: A prospective case-cohort study in Japan. Int. J. Cancer 2018, 143, 2767–2776. [Google Scholar] [CrossRef] [PubMed]
- Pender, S.L.F.; Chance, V.; Whiting, C.V.; Buckley, M.; Edwards, M.; Pettipher, R.; MacDonald, T.T. Systemic administration of the chemokine macrophage inflammatory protein 1α exacerbates inflammatory bowel disease in a mouse model. Gut 2005, 54, 1114. [Google Scholar] [CrossRef]
- Allen, F.; Rauhe, P.; Askew, D.; Tong, A.A.; Nthale, J.; Eid, S.; Myers, J.T.; Tong, C.; Huang, A.Y. CCL3 Enhances Antitumor Immune Priming in the Lymph Node via IFNγ with Dependency on Natural Killer Cells. Front. Immunol. 2017, 8, 1390. [Google Scholar] [CrossRef]
- Allen, F.; Bobanga, I.D.; Rauhe, P.; Barkauskas, D.; Teich, N.; Tong, C.; Myers, J.; Huang, A.Y. CCL3 augments tumor rejection and enhances CD8+ T cell infiltration through NK and CD103+ dendritic cell recruitment via IFNγ. Oncoimmunology 2018, 7, e1393598. [Google Scholar] [CrossRef]
- Yuan, P.; Zhou, Y.; Wang, Z.; Gui, L.; Ma, B. Dendritic cell-targeting chemokines inhibit colorectal cancer progression. Explor. Target. Antitumor Ther. 2022, 3, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lu, X.; Cai, Q.; Liu, M.; Xia, T.; Hong, D.; Le, L.; Zhang, X.; Zhang, X. Loss of TACC2 impairs chemokine CCL3 and CCL4 expression and reduces response to anti-PD-1 therapy in soft tissue sarcoma. Mol. Cancer 2025, 24, 158. [Google Scholar] [CrossRef]
- Zhang, W.; Qin, H.; Wang, G.; Zhang, J.; He, W.; Feng, C.; Wan, H.; Wang, F.; Guo, Z. Deciphering the potential role of PGRN in regulating CD8+ T cell antitumor immunity. Cell Death Discov. 2024, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Makker, P.G.S.; Duffy, S.S.; Lees, J.G.; Perera, C.J.; Tonkin, R.S.; Butovsky, O.; Park, S.B.; Goldstein, D.; Moalem-Taylor, G. Characterisation of Immune and Neuroinflammatory Changes Associated with Chemotherapy-Induced Peripheral Neuropathy. PLoS ONE 2017, 12, e0170814. [Google Scholar] [CrossRef]
- Ochi-ishi, R.; Nagata, K.; Inoue, T.; Tozaki-Saitoh, H.; Tsuda, M.; Inoue, K. Involvement of the chemokine CCL3 and the purinoceptor P2X7 in the spinal cord in paclitaxel-induced mechanical allodynia. Mol. Pain 2014, 10. [Google Scholar] [CrossRef]
- Zhang, R.; Li, N.; Zhao, M.; Tang, M.; Jiang, X.; Cai, X.; Ye, N.; Su, K.; Peng, J.; Zhang, X.; et al. From lead to clinic: A review of the structural design of P2X7R antagonists. Eur. J. Med. Chem. 2023, 251, 115234. [Google Scholar] [CrossRef] [PubMed]
- Rojewska, E.; Zychowska, M.; Piotrowska, A.; Kreiner, G.; Nalepa, I.; Mika, J. Involvement of Macrophage Inflammatory Protein-1 Family Members in the Development of Diabetic Neuropathy and Their Contribution to Effectiveness of Morphine. Front. Immunol. 2018, 9, 494. [Google Scholar] [CrossRef]
- Kou, Z.Z.; Wan, F.P.; Bai, Y.; Li, C.Y.; Hu, J.C.; Zhang, G.T.; Zhang, T.; Chen, T.; Wang, Y.Y.; Li, H.; et al. Decreased endomorphin-2 and µ-opioid receptor in the spinal cord are associated with painful diabetic neuropathy. Front. Mol. Neurosci. 2016, 9, 80. [Google Scholar] [CrossRef]
- Castany, S.; Carcolé, M.; Leánez, S.; Pol, O. The antinociceptive effects of a δ-opioid receptor agonist in mice with painful diabetic neuropathy: Involvement of heme oxygenase 1. Neurosci. Lett. 2016, 614, 49–54. [Google Scholar] [CrossRef]
- Zychowska, M.; Rojewska, E.; Kreiner, G.; Nalepa, I.; Przewlocka, B.; Mika, J. Minocycline influences the anti-inflammatory interleukins and enhances the effectiveness of morphine under mice diabetic neuropathy. J. Neuroimmunol. 2013, 262, 35–45. [Google Scholar] [CrossRef]
- Ruff, M.R.; Inan, S.; Shi, X.Q.; Meissler, J.J.; Adler, M.W.; Eisenstein, T.K.; Zhang, J. Potentiation of morphine antinociception and inhibition of diabetic neuropathic pain by the multi-chemokine receptor antagonist peptide RAP-103. Life Sci. 2022, 306, 120788. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Chen, D.; Lin, F.; Chen, M.; Yu, H.; Hou, L.; Li, C. Role of interleukin-4, the chemokine CCL3 and its receptor CCR5 in neuropathic pain. Mol. Immunol. 2016, 77, 184–192. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, S.; Liao, F.; Huang, Z.; Yang, X.; Zou, Y.; He, X.; Guo, Q.; Huang, C. A transcriptomic analysis of neuropathic pain in the anterior cingulate cortex after nerve injury. Bioengineered 2022, 13, 2058–2075. [Google Scholar] [CrossRef]
- Mert, T.; Metin, T.O.; Sahin, E.; Yaman, S.; Sahin, M. Neuroprotective and anti-neuropathic actions of pulsed magnetic fields with low frequencies in rats with chronic peripheral neuropathic pain. Brain Res. Bull. 2021, 177, 273–281. [Google Scholar] [CrossRef]
- Matsushita, K.; Tozaki-Saitoh, H.; Kojima, C.; Masuda, T.; Tsuda, M.; Inoue, K.; Hoka, S. Chemokine (C-C motif) receptor 5 is an important pathological regulator in the development and maintenance of neuropathic pain. Anesthesiology 2014, 120, 1491–1503. [Google Scholar] [CrossRef]
- Li, M.; Jiang, H.; Gu, K.; Sun, X.; Gu, J.; Li, C.; Wang, G. Lidocaine Alleviates Neuropathic Pain and Neuroinflammation by Inhibiting HMGB1 Expression to Mediate MIP-1α/CCR1 Pathway. J. Neuroimmune Pharmacol. 2021, 16, 318–333. [Google Scholar] [CrossRef]
- Anloague, A.; Sabol, H.M.; Kaur, J.; Khan, S.; Ashby, C.; Schinke, C.; Barnes, C.L.; Alturkmani, F.; Ambrogini, E.; Gundesen, M.T.; et al. A novel CCL3-HMGB1 signaling axis regulating osteocyte RANKL expression in multiple myeloma. Haematologica 2024, 110, 952. [Google Scholar] [CrossRef]
- Kwiatkowski, K.; Piotrowska, A.; Rojewska, E.; Makuch, W.; Jurga, A.; Slusarczyk, J.; Trojan, E.; Basta-Kaim, A.; Mika, J. Beneficial properties of maraviroc on neuropathic pain development and opioid effectiveness in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 64, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, K.; Pawlik, K.; Ciapała, K.; Piotrowska, A.; Makuch, W.; Mika, J. Bidirectional Action of Cenicriviroc, a CCR2/CCR5 Antagonist, Results in Alleviation of Pain-Related Behaviors and Potentiation of Opioid Analgesia in Rats With Peripheral Neuropathy. Front. Immunol. 2020, 11, 615327. [Google Scholar] [CrossRef]
- Kiguchi, N.; Kobayashi, Y.; Maeda, T.; Saika, F.; Kishioka, S. CC-chemokine MIP-1α in the spinal cord contributes to nerve injury-induced neuropathic pain. Neurosci. Lett. 2010, 484, 17–21. [Google Scholar] [CrossRef]
- Dorf, M.E.; Berman, M.A.; Tanabe, S.; Heesen, M.; Luo, Y. Astrocytes express functional chemokine receptors. J. Neuroimmunol. 2000, 111, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Boddeke, E.W.G.M.; Meigel, I.; Frentzel, S.; Gourmala, N.G.; Harrison, J.K.; Buttini, M.; Spleiss, O.; Gebicke-Härter, P. Cultured rat microglia express functional β-chemokine receptors. J. Neuroimmunol. 1999, 98, 176–184. [Google Scholar] [CrossRef]
- Murphy, G.; Jia, X.C.; Song, Y.; Ong, E.; Shrivastava, R.; Bocchini, V.; Lee, Y.L.; Eng, L.F. Macrophage inflammatory protein 1-α mRNA expression in an immortalized microglial cell line and cortical astrocyte cultures. J. Neurosci. Res. 1995, 40, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Inan, S.; Cowan, A.; Sun, R.; Wang, J.M.; Rogers, T.J.; Caterina, M.; Oppenheim, J.J. A proinflammatory chemokine, CCL3, sensitizes the heat-and capsaicin-gated ion channel TRPV1. Proc. Natl. Acad. Sci. USA 2005, 102, 4536–4541. [Google Scholar] [CrossRef] [PubMed]
- Sukhotinsky, I.; Ben-Dor, E.; Raber, P.; Devor, M. Key role of the dorsal root ganglion in neuropathic tactile hypersensibility. Eur. J. Pain 2004, 8, 135–143. [Google Scholar] [CrossRef]
- Malek, N.; Pajak, A.; Kolosowska, N.; Kucharczyk, M.; Starowicz, K. The importance of TRPV1-sensitisation factors for the development of neuropathic pain. Mol. Cell. Neurosci. 2015, 65, 1–10. [Google Scholar] [CrossRef]
- Szabo, I.; Chen, X.-H.; Xin, L.; Adler, M.W.; ZHoward, O.M.; Oppenheim, J.J.; Rogers, T.J. Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proc. Natl. Acad. Sci. USA 2002, 99, 10276–10281. [Google Scholar]
- Kiguchi, N.; Kobayashi, Y.; Saika, F.; Kishioka, S. Epigenetic upregulation of CCL2 and CCL3 via histone modifications in infiltrating macrophages after peripheral nerve injury. Cytokine 2013, 64, 666–672. [Google Scholar] [CrossRef]
- Kiguchi, N.; Saika, F.; Kobayashi, Y.; Ko, M.C.; Kishioka, S. TC-2559, an α4β2 nicotinic acetylcholine receptor agonist, suppresses the expression of CCL3 and IL-1β through STAT3 inhibition in cultured murine macrophages. J. Pharmacol. Sci. 2015, 128, 83–86. [Google Scholar] [CrossRef]
- Samavati, L.; Rastogi, R.; Du, W.; Hüttemann, M.; Fite, A.; Franchi, L. STAT3 tyrosine phosphorylation is critical for interleukin 1 beta and interleukin-6 production in response to lipopolysaccharide and live bacteria. Mol. Immunol. 2009, 46, 1867–1877. [Google Scholar] [CrossRef]
- Saeed, R.W.; Varma, S.; Peng-Nemeroff, T.; Sherry, B.; Balakhaneh, D.; Huston, J.; Tracey, K.J.; Al-Abed, Y.; Metz, C.N. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J. Exp. Med. 2005, 201, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Marginean, E.C.; Gotfrit, J.; Marginean, H.; Yokom, D.W.; Bateman, J.J.; Daneshmand, M.; Sud, S.; Gown, A.M.; Jonker, D.; Asmis, T.; et al. Phosphorylated transducer and activator of transcription-3 (pSTAT3) immunohistochemical expression in paired primary and metastatic colorectal cancer. Transl. Oncol. 2021, 14, 100996. [Google Scholar] [CrossRef]
- Kiguchi, N.; Maeda, T.; Kobayashi, Y.; Fukazawa, Y.; Kishioka, S. Activation of Extracellular Signal-Regulated Kinase in Sciatic Nerve Contributes to Neuropathic Pain After Partial Sciatic Nerve Ligation in Mice. Anesth. Analg. 2009, 109, 1305–1311. [Google Scholar] [CrossRef]
- Saika, F.; Kiguchi, N.; Kobayashi, Y.; Fukazawa, Y.; Kishioka, S. CC-chemokine ligand 4/macrophage inflammatory protein-1β participates in the induction of neuropathic pain after peripheral nerve injury. Eur. J. Pain 2012, 16, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Nibbs, R.J.B.; Yang, J.; Landau, N.R.; Mao, J.H.; Graham, G.J. LD78beta, a non-allelic variant of human MIP-1alpha (LD78alpha), has enhanced receptor interactions and potent HIV suppressive activity. J. Biol. Chem. 1999, 274, 17478–17483. [Google Scholar] [CrossRef] [PubMed]
- Colobran, R.; Pedrosa, E.; Carretero-Iglesia, L.; Juan, M. Copy number variation in chemokine superfamily: The complex scene of CCL3L–CCL4L genes in health and disease. Clin. Exp. Immunol. 2010, 162, 41–52. [Google Scholar] [CrossRef]
- Carpenter, D.; Mcintosh, R.S.; Pleass, R.J.; Armour, J.A.L. Functional effects of CCL3L1 copy number. Genes Immun. 2012, 13, 374. [Google Scholar] [CrossRef][Green Version]
- Regalado, C.R.; Balogh, M. MMP9: Link between neuropathy and colorectal cancer? Front. Mol. Biosci. 2024, 11, 1451611. [Google Scholar] [CrossRef]
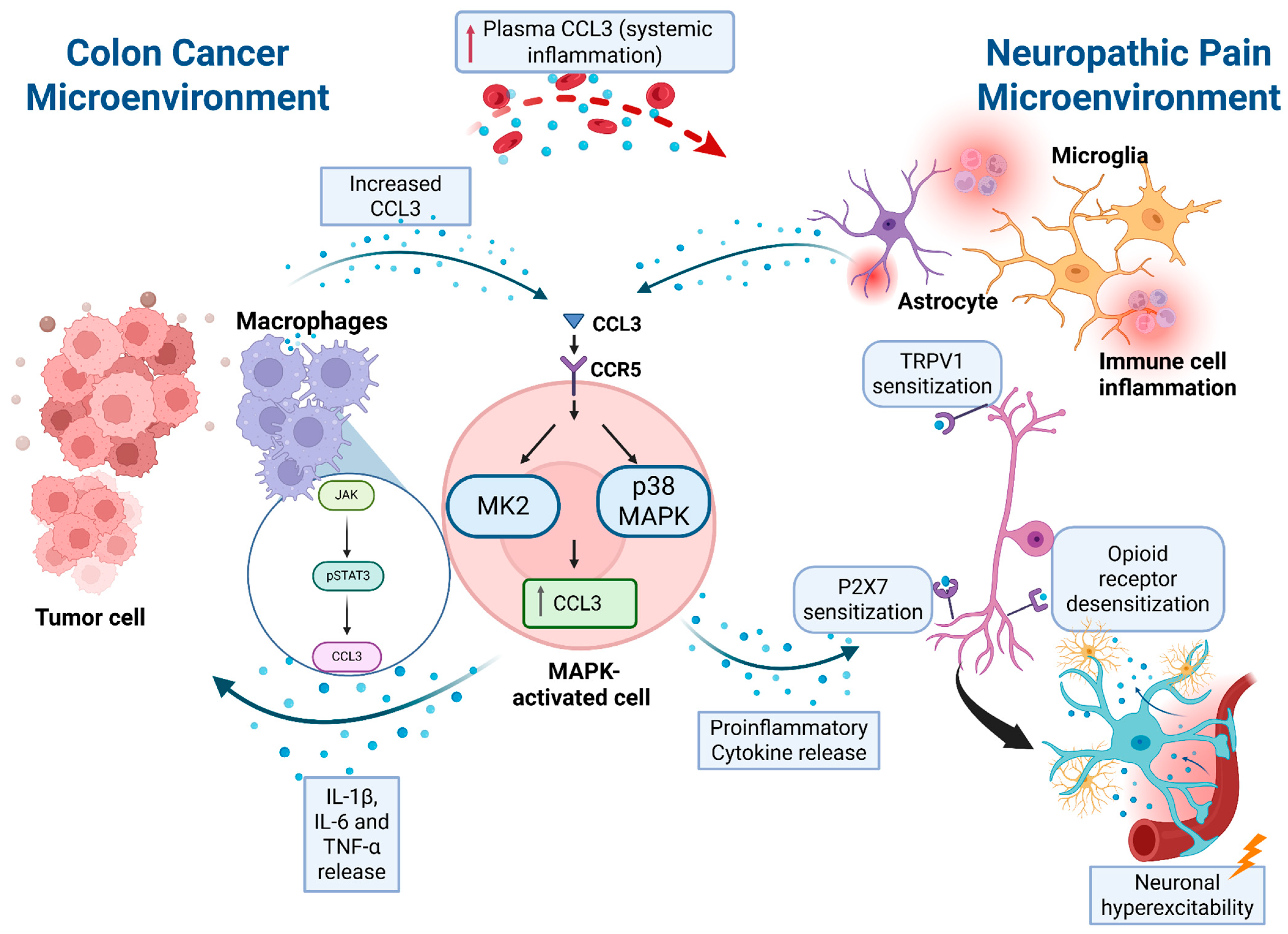
| Pathway | Role in CRC | Role in Neuropathy | Proposed Mechanism |
|---|---|---|---|
| TRAF6/NF-κB | Drives tumor development through TRAF6/NF-κB signaling. | Not directly implicated. | Upregulation of CCL3 via NF-κB contributes to tumor proliferation, with cross-talk to MAPK. |
| MAPK | Promotes tumor cell proliferation when activated in tumor cells and macrophages. | Activates microglia and astrocytes, leading to pro-inflammatory cytokine release and neuronal excitability. | CCL3 activates MAPK, creating a feedback loop that amplifies inflammation and pain sensitization |
| PI3K–AKT | Activated in CRC cells following chemokine stimulation. | Upregulated in models involving p53-dependent regulation of CCL3. | Enhances proliferation and survival signaling; links to miRNA regulation of CCL3 and VEGF. |
| Wnt | Identified as one of the pathways associated with CCL3 upregulation. | Found in conjunction with PI3K–AKT activation in neuropathic models. | Supports tumorigenesis and possibly influences CCL3 expression through p53–miRNA regulation. |
| p53 | Downregulation of p53 associated with increased CCL3. | CCL3 upregulation dependent on p53 status; absent in p53-null cells. | p53 status regulates CCL3 and VEGF expression, linking tumor stress responses to chemokine levels. |
| STAT3 | Considered a therapeutic target in CRC. | pSTAT3 in macrophages drives CCL3 and IL-1β expression, contributing to neuropathic pain | JAK/STAT3 activation upregulates CCL3; inhibition suppresses inflammation and pain. |
| TRPV1 | Not implicated in CRC. | Sensitization of nociceptive neurons via CCL3–CCR1 signaling increases TRPV1 activity. | CCL3 activates PLC–PKC pathway, phosphorylating TRPV1 and enhancing thermal hyperalgesia. |
| P2X7R | Not implicated in CRC. | Microglial P2X7R activation promotes CCL3 release and sustains CIPN. | P2X7R–CCL3 circuit amplifies microglial activation, lowering pain thresholds. |
| Opioid receptor desensitization | Not implicated in CRC. | Chemokines desensitize opioid receptors, reducing analgesic function | CCR1/CCL3 (and related chemokines) cross-desensitize opioid GPCRs, lowering pain thresholds. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luzac, I.; Regalado, C.R.; Balogh, M. Unveiling the Role of CCL3: A Driver of CIPN in Colon Cancer Patients? Biomedicines 2025, 13, 2512. https://doi.org/10.3390/biomedicines13102512
Luzac I, Regalado CR, Balogh M. Unveiling the Role of CCL3: A Driver of CIPN in Colon Cancer Patients? Biomedicines. 2025; 13(10):2512. https://doi.org/10.3390/biomedicines13102512
Chicago/Turabian StyleLuzac, Irene, Cynthia Rosa Regalado, and Mihály Balogh. 2025. "Unveiling the Role of CCL3: A Driver of CIPN in Colon Cancer Patients?" Biomedicines 13, no. 10: 2512. https://doi.org/10.3390/biomedicines13102512
APA StyleLuzac, I., Regalado, C. R., & Balogh, M. (2025). Unveiling the Role of CCL3: A Driver of CIPN in Colon Cancer Patients? Biomedicines, 13(10), 2512. https://doi.org/10.3390/biomedicines13102512







