miR-21-5p Alleviates Retinal Ischemia–Reperfusion Injury by Inhibiting M1 Polarization of Microglia via Suppression of STAT3 Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Rat Model of AIH
2.2. Intravitreal Injection
2.3. Tissue Preparation
2.4. Cell Culture and Transfection
2.5. Oxygen–Glucose Deprivation/Reperfusion (OGD/R) and Conditioned Medium Transfer
2.6. Real-Time Quantitative PCR (qRT-PCR)
2.7. Hematoxylin and Eosin (H&E) Staining
2.8. Immunofluorescence
2.8.1. Immunofluorescence of Retinal Paraffin Sections
2.8.2. Cellular Immunofluorescence
2.9. TUNEL Staining
2.10. PI Staining
2.11. Western Blotting (WB)
2.12. Dual-Luciferase Reporter Assay
2.13. Statistical Analysis
3. Results
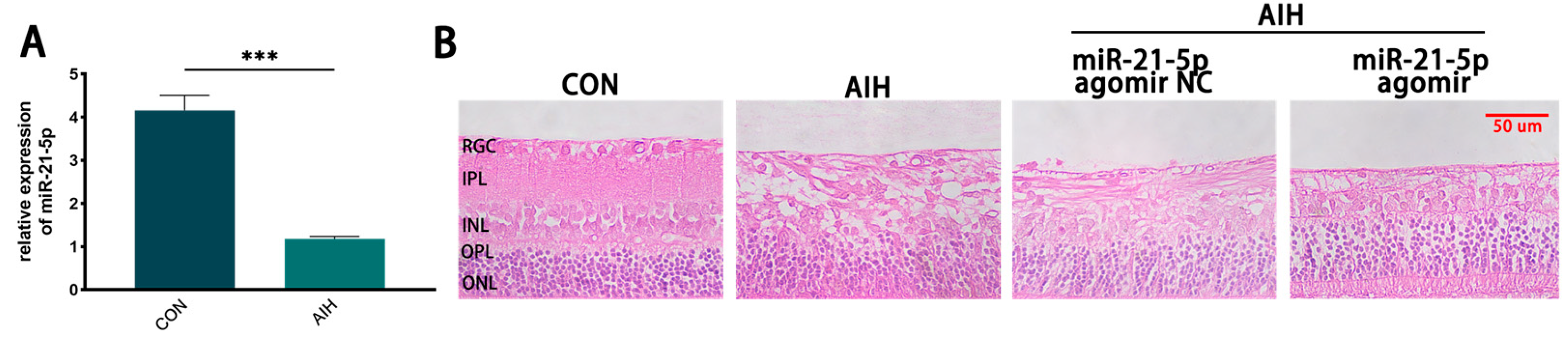
3.1. miR-21-5p Ameliorated AIH-Induced Retinal Damage In Vivo
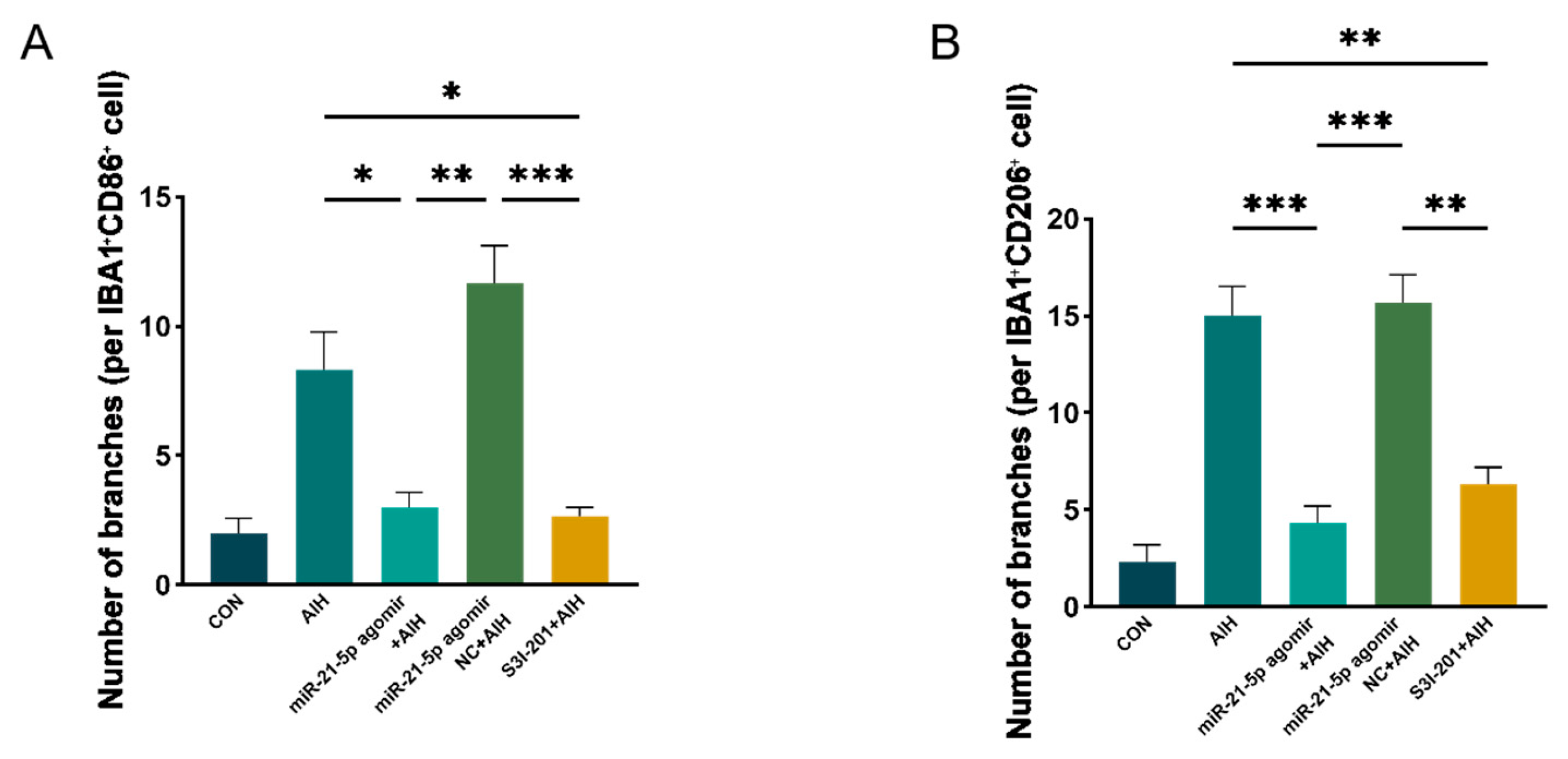
3.2. Overexpression of miR-21-5p Inhibits M1 Polarization of RM Cultured In Vitro
3.3. MCM from miR-21-5p-Overexpressing Microglia Attenuated OGD/R-Induced R28 Death
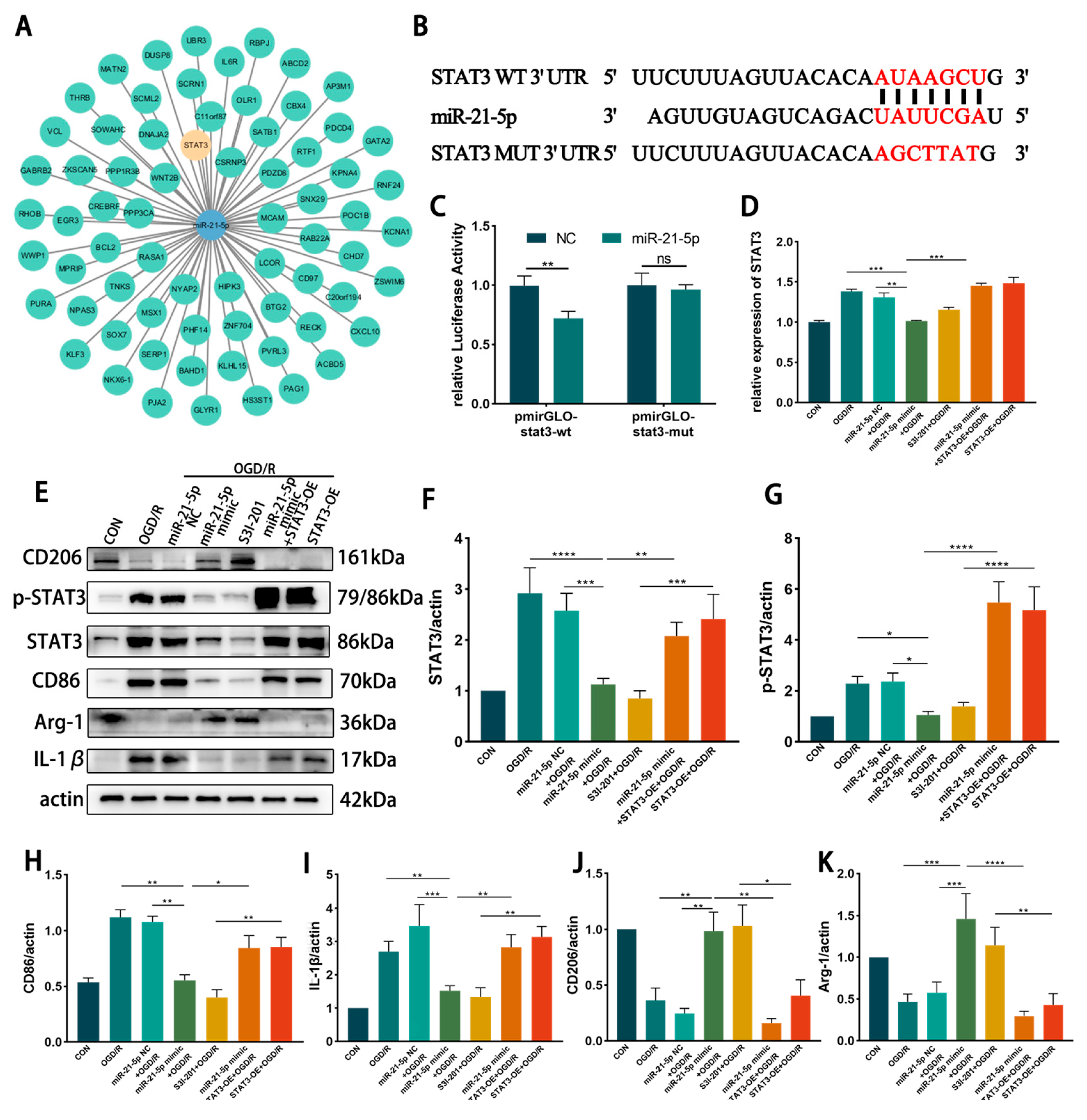
3.4. miR-21-5p Downregulates STAT3 Expression to Inhibit RM M1 Polarization
3.4.1. STAT3 Is the Direct Target Gene of miR-21-5p
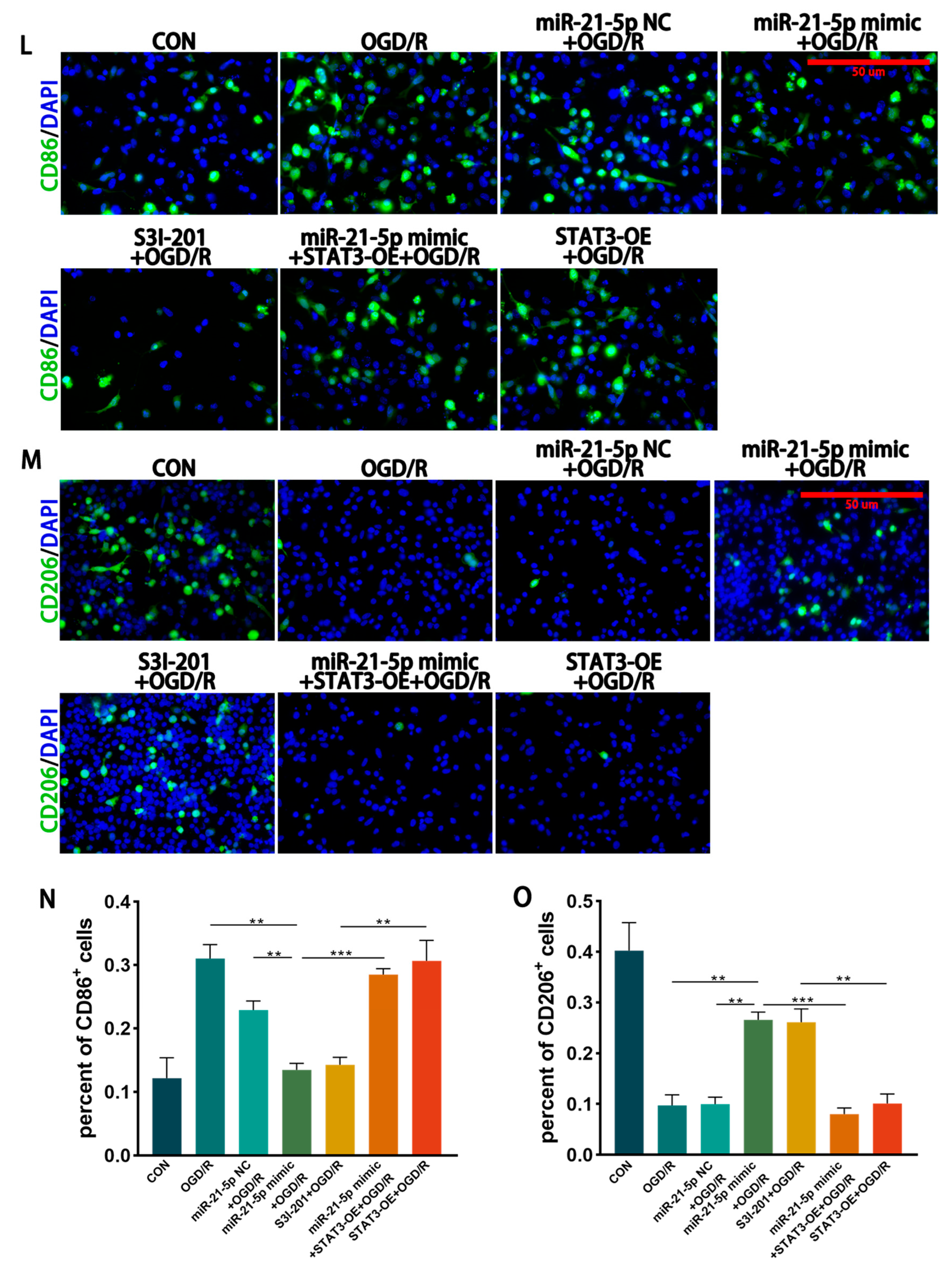
3.4.2. Inhibition of STAT3 Reduces M1 Polarization of RM Cultured In Vitro
3.4.3. miR-21-5p Downregulates STAT3 Levels to Inhibit M1 Polarization of RM
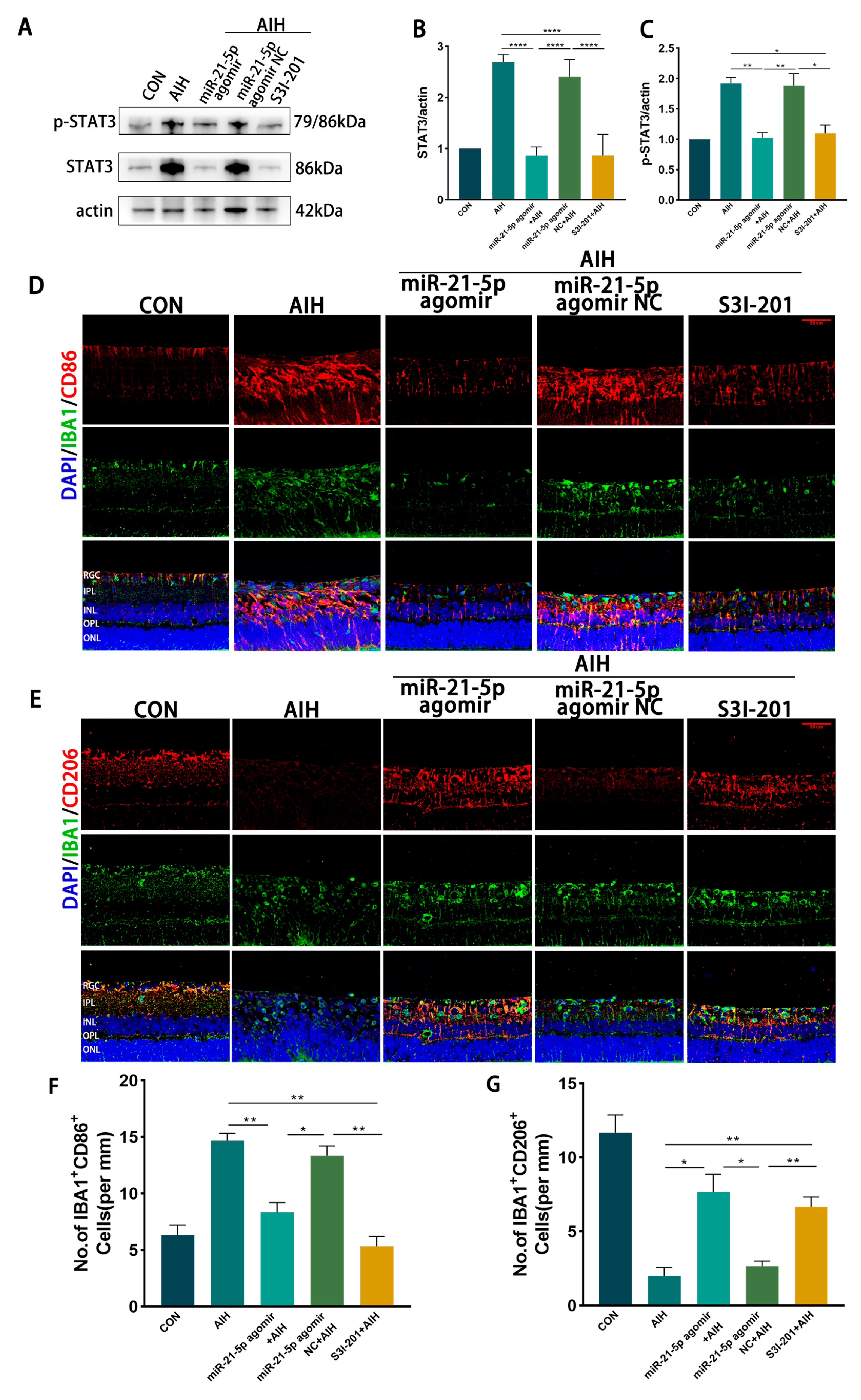
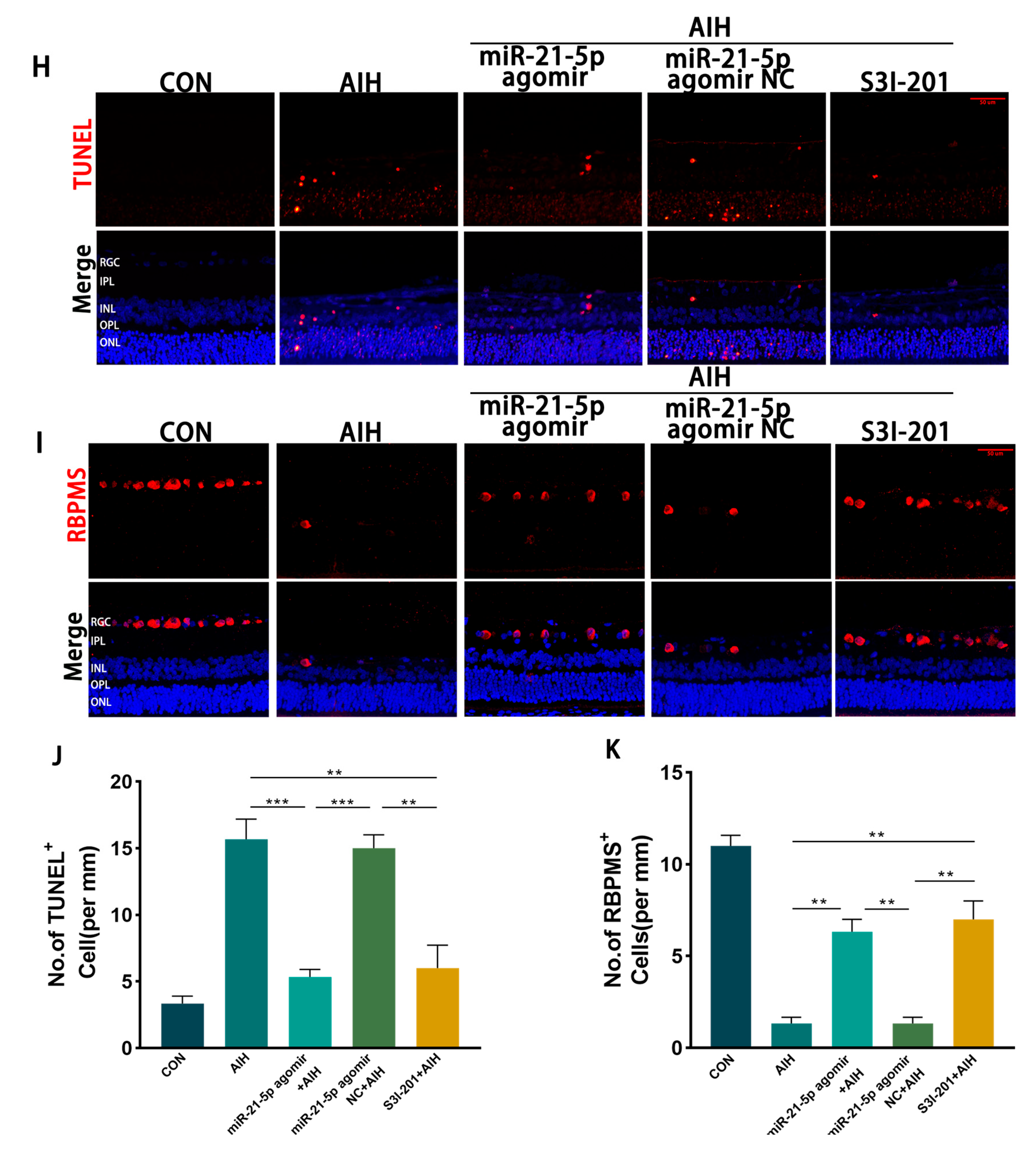
3.5. miR-21-5p Down-Regulation of STAT3 Levels Inhibits M1 Polarization and Reduces Apoptosis of RGCs in Retinal Microglia of AIH Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| I/R | Ischemia-reperfusion |
| RGCs | Retinal ganglion cells |
| AIH | Acute intraocular hypertension |
| OGD/R | Oxygen and glucose deprivation/reperfusion |
| MCM | Microglial-conditioned media |
| RM | Rat Microglia |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| Arg-1 | Arginase-1 |
| IL-1β | Interleukin-1 beta |
| LPS | Lipopolysaccharide |
| TLR | Toll-like receptor |
| IFN-γ | Interferon-gamma |
| ROS | Reactive Oxygen Species |
| ECM | Extracellular Matrix |
| TNF-α | Tumor Necrosis Factor-alpha |
| 3′-UTR | 3′ Untranslated Region |
| TM | Trabecular Meshwork |
Appendix A


References
- Jayaram, H.; Kolko, M.; Friedman, D.S.; Gazzard, G. Glaucoma: Now and Beyond. Lancet 2023, 402, 1788–1801. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Fang, W.; Hu, F.; Zhou, X.; Cheng, Y.; Jiang, C. A High-Salt Diet Aggravates Retinal Ischaemia/Reperfusion Injury. Exp. Eye Res. 2019, 188, 107784. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Zhao, G.-L.; Cheng, S.; Wang, Z.; Yang, X.-L. Activation of Retinal Glial Cells Contributes to the Degeneration of Ganglion Cells in Experimental Glaucoma. Prog. Retin. Eye Res. 2023, 93, 101169. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Su, W.; Zhang, Y.; Li, Z.; Deng, C.; Li, J.; Jiang, N.; Huang, S.; Long, E.; Zhuo, Y. LncRNA H19 Initiates Microglial Pyroptosis and Neuronal Death in Retinal Ischemia/Reperfusion Injury. Cell Death Differ. 2020, 27, 176–191. [Google Scholar] [CrossRef]
- Qin, Q.; Yu, N.; Gu, Y.; Ke, W.; Zhang, Q.; Liu, X.; Wang, K.; Chen, M. Inhibiting Multiple Forms of Cell Death Optimizes Ganglion Cells Survival after Retinal Ischemia Reperfusion Injury. Cell Death Dis. 2022, 13, 507. [Google Scholar] [CrossRef]
- Borst, K.; Dumas, A.A.; Prinz, M. Microglia: Immune and Non-Immune Functions. Immunity 2021, 54, 2194–2208. [Google Scholar] [CrossRef]
- Salkar, A.; Wall, R.V.; Basavarajappa, D.; Chitranshi, N.; Parilla, G.E.; Mirzaei, M.; Yan, P.; Graham, S.; You, Y. Glial Cell Activation and Immune Responses in Glaucoma: A Systematic Review of Human Postmortem Studies of the Retina and Optic Nerve. Aging Dis. 2024, 15, 2069–2083. [Google Scholar] [CrossRef]
- Fu, S.-P.; Chen, S.-Y.; Pang, Q.-M.; Zhang, M.; Wu, X.-C.; Wan, X.; Wan, W.-H.; Ao, J.; Zhang, T. Advances in the Research of the Role of Macrophage/Microglia Polarization-Mediated Inflammatory Response in Spinal Cord Injury. Front. Immunol. 2022, 13, 1014013. [Google Scholar] [CrossRef]
- Hou, X.; Qu, X.; Chen, W.; Sang, X.; Ye, Y.; Wang, C.; Guo, Y.; Shi, H.; Yang, C.; Zhu, K.; et al. CD36 Deletion Prevents White Matter Injury by Modulating Microglia Polarization through the Traf5-MAPK Signal Pathway. J. Neuroinflamm. 2024, 21, 148. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Kumari, S.; Dhapola, R.; Sharma, P.; Beura, S.K.; Singh, S.K.; Vellingiri, B.; HariKrishnaReddy, D. Shedding Light on Microglial Dysregulation in Alzheimer’s Disease: Exploring Molecular Mechanisms and Therapeutic Avenues. Inflammopharmacology 2025, 33, 679–702. [Google Scholar] [CrossRef]
- Long, Y.; Li, X.-Q.; Deng, J.; Ye, Q.-B.; Li, D.; Ma, Y.; Wu, Y.-Y.; Hu, Y.; He, X.-F.; Wen, J.; et al. Modulating the Polarization Phenotype of Microglia—A Valuable Strategy for Central Nervous System Diseases. Ageing Res. Rev. 2024, 93, 102160. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Zhan, H.; Li, S.; Zeng, K.; Xu, C.; Zou, Y.; Xie, Y.; Zhan, Z.; Yin, S.; et al. Targeting the HSP47-Collagen Axis Inhibits Brain Metastasis by Reversing M2 Microglial Polarization and Restoring Anti-Tumor Immunity. Cell Rep. Med. 2024, 5, 101533. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Yang, J.; Liu, Y.; Zhao, H.; Li, M.; Yang, D.; Xie, Q. Huanglian Wendan Decoction Improves Insomnia in Rats by Regulating BDNF/TrkB Signaling Pathway Through Gut Microbiota-Mediated SCFAs and Affecting Microglia Polarization. Mol. Neurobiol. 2025, 62, 1047–1066. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Zhu, X.; Wang, C.; Jiang, Q.; Yu, S.; Song, G.; Liu, Q.; Gong, P. Indole-3-Carbinol (I3C) Reduces Apoptosis and Improves Neurological Function after Cerebral Ischemia-Reperfusion Injury by Modulating Microglia Inflammation. Sci. Rep. 2024, 14, 3145. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Yang, B.; Liu, W.; Tan, C.; Chen, H.; Wang, X. Emerging Role of Non-Coding RNAs in Neuroinflammation Mediated by Microglia and Astrocytes. J. Neuroinflamm. 2023, 20, 173. [Google Scholar] [CrossRef]
- Cui, Y.; Qi, Y.; Ding, L.; Ding, S.; Han, Z.; Wang, Y.; Du, P. miRNA Dosage Control in Development and Human Disease. Trends Cell Biol. 2024, 34, 31–47. [Google Scholar] [CrossRef]
- Diener, C.; Keller, A.; Meese, E. Emerging Concepts of miRNA Therapeutics: From Cells to Clinic. Trends Genet. 2022, 38, 613–626. [Google Scholar] [CrossRef]
- Li, D.; Huang, S.; Zhu, J.; Hu, T.; Han, Z.; Zhang, S.; Zhao, J.; Chen, F.; Lei, P. Exosomes from MiR-21-5p-Increased Neurons Play a Role in Neuroprotection by Suppressing Rab11a-Mediated Neuronal Autophagy In Vitro After Traumatic Brain Injury. Med. Sci. Monit. 2019, 25, 1871–1885. [Google Scholar] [CrossRef]
- Gao, X.; Xiong, Y.; Li, Q.; Han, M.; Shan, D.; Yang, G.; Zhang, S.; Xin, D.; Zhao, R.; Wang, Z.; et al. Extracellular Vesicle-Mediated Transfer of miR-21-5p from Mesenchymal Stromal Cells to Neurons Alleviates Early Brain Injury to Improve Cognitive Function via the PTEN/Akt Pathway after Subarachnoid Hemorrhage. Cell Death Dis. 2020, 11, 363. [Google Scholar] [CrossRef]
- Cong, M.; Hu, J.-J.; Yu, Y.; Li, X.-L.; Sun, X.-T.; Wang, L.-T.; Wu, X.; Zhu, L.-J.; Yang, X.-J.; He, Q.-R.; et al. miRNA-21-5p Is an Important Contributor to the Promotion of Injured Peripheral Nerve Regeneration Using Hypoxia-Pretreated Bone Marrow-Derived Neural Crest Cells. Neural Regen. Res. 2025, 20, 277–290. [Google Scholar] [CrossRef]
- Leinders, M.; Üçeyler, N.; Thomann, A.; Sommer, C. Aberrant microRNA Expression in Patients with Painful Peripheral Neuropathies. J. Neurol. Sci. 2017, 380, 242–249. [Google Scholar] [CrossRef]
- Qu, Y.; Cai, R.; Li, Q.; Wang, H.; Lu, L. Neuroinflammation Signatures in Dorsal Root Ganglia Following Chronic Constriction Injury. Heliyon 2024, 10, e31481. [Google Scholar] [CrossRef]
- Han, B.; Zhang, R.; Li, L.; Hu, C.; Li, M.; Liu, J.; Sun, X.; Fan, W.; Xie, J.; Lei, Y. Reduction-Responsive Polymeric Micelles for Trans-Corneal Targeted Delivery of microRNA-21-5p and Glaucoma-Specific Gene Therapy. J. Mater. Chem. B 2023, 11, 10433–10445. [Google Scholar] [CrossRef]
- Lin, B.; Li, Y.; Jiang, N.; Huang, S.; Su, W.; Zhuo, Y. Interleukin-35 Suppresses Pyroptosis and Protects against Neuronal Death in Retinal Ischaemia/Reperfusion Injury. Exp. Eye Res. 2022, 220, 109109. [Google Scholar] [CrossRef]
- Li, M.; Zhang, T.; Li, P.; Luan, Z.; Liu, J.; Wang, Y.; Zhang, Y.; Liu, Y.; Wang, Y. IL-4-Primed Human Umbilical Cord Mesenchymal Stem Cells-Derived Extracellular Vesicles Facilitate Recovery in Spinal Cord Injury via the miR-21-5p/PDCD4-Mediated Shifting of Macrophage M1/M2 Polarization. Life Sci. 2025, 364, 123441. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Wu, E.; Chen, H.; Yao, J.; Wang, J.; Zhou, Y.; Bai, Y.; Wang, S.; Shen, C.; Li, Y.; et al. MSCs-Exosomes Can Promote Macrophage M2 Polarization via Exosomal miR-21-5p through Mesenteric Injection: A Promising Way to Attenuate Murine Colitis. J. Crohns Colitis 2024, 18, jjae110. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Jia, F.; Zhang, P.; Sun, X.; Qiao, Y.; Chen, X.; Wang, Y.; Chen, J.; Lei, Y. A miRNA Stabilizing Polydopamine Nano-Platform for Intraocular Delivery of miR-21-5p in Glaucoma Therapy. J. Mater. Chem. B 2021, 9, 3335–3345. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Tang, L.; Yuan, Z.; Zhang, Y. The Effects of STAT3 Acetylation on the Transcriptional Activity and Tumorigenesis. Biochem. Pharmacol. 2025, 240, 117076. [Google Scholar] [CrossRef]
- Tošić, I.; Frank, D.A. STAT3 as a Mediator of Oncogenic Cellular Metabolism: Pathogenic and Therapeutic Implications. Neoplasia 2021, 23, 1167–1178. [Google Scholar] [CrossRef]
- Osaki, Y.; Manolopoulou, M.; Ivanova, A.V.; Vartanian, N.; Mignemi, M.P.; Kern, J.; Chen, J.; Yang, H.; Fogo, A.B.; Zhang, M.; et al. Blocking Cell Cycle Progression through CDK4/6 Protects against Chronic Kidney Disease. JCI Insight 2022, 7, e158754. [Google Scholar] [CrossRef]
- Jian, Y.; Zheng, Q.; Hu, S.; Jian, Y. siRNA Nanodelivery Systems in the Treatment of Skin Diseases: Research Progress and Clinical Translation Prospects. Eur. J. Pharmacol. 2025, 1005, 178075. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Chen, Q.; He, Y.; Zhou, H.; Wan, H.; Yang, J. Formononetin Protects against Inflammation Associated with Cerebral Ischemia-Reperfusion Injury in Rats by Targeting the JAK2/STAT3 Signaling Pathway. Biomed. Pharmacother. 2022, 149, 112836. [Google Scholar] [CrossRef]
- Yue, P.; Zhu, Y.; Brotherton-Pleiss, C.; Fu, W.; Verma, N.; Chen, J.; Nakamura, K.; Chen, W.; Chen, Y.; Alonso-Valenteen, F.; et al. Novel Potent Azetidine-Based Compounds Irreversibly Inhibit Stat3 Activation and Induce Antitumor Response against Human Breast Tumor Growth In Vivo. Cancer Lett. 2022, 534, 215613. [Google Scholar] [CrossRef]
- Chen, Y.; Zhai, N.; Zhu, Y.; Yue, P.; Verma, N.; Brotherton-Pleiss, C.; Fu, W.; Nakamura, K.; Chen, W.; Kawakami, J.; et al. Azetidine Ring, Salicylic Acid, and Salicylic Acid Bioisosteres as Determinants of the Binding Characteristics of Novel Potent Compounds to Stat3. Bioorg. Med. Chem. Lett. 2024, 97, 129565. [Google Scholar] [CrossRef]
- Ye, Y.; Rao, Z.; Xie, X.; Liu, Y.; Qiu, L.; Liu, Q.; Weng, X.; Wang, C.; Bi, Y.; Zeng, T. Naoqing Formula Alleviates Cerebral Ischemia/Reperfusion Injury Induced Inflammatory Injury by Regulating Csf3 Mediated JAK/STAT Pathway and Macrophage Polarization. Phytomedicine 2025, 140, 156626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, Y.; Guo, L.; Zhang, Y.; Zhao, M.; Xue, F.; Tan, C.; Huang, J.; Chen, D. Melatonin Alleviates Pyroptosis of Retinal Neurons Following Acute Intraocular Hypertension. CNS Neurol. Disord. Drug Targets 2021, 20, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, S.; Wang, J.; Liu, Y.; Chen, Y.; Wen, T.; Fang, X.; Vidal-Sanz, M.; Jonas, J.B.; Zhang, X. MicroRNA-93/STAT3 Signalling Pathway Mediates Retinal Microglial Activation and Protects Retinal Ganglion Cells in an Acute Ocular Hypertension Model. Cell Death Dis. 2021, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Kotnala, A.; Anderson, D.M.G.; Patterson, N.H.; Cantrell, L.S.; Messinger, J.D.; Curcio, C.A.; Schey, K.L. Tissue Fixation Effects on Human Retinal Lipid Analysis by MALDI Imaging and LC-MS/MS Technologies. J. Mass. Spectrom. 2021, 56, e4798. [Google Scholar] [CrossRef]
- Xu, F.; Yue, Y.; Sun, D. Mechanism of the AMPK/SIRT1 Pathway in Gut Dysbiosis-Mediated Postoperative Cognitive Dysfunction in Aged Mice. Int. J. Neuropsychopharmacol. 2025, 28, pyaf066. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.-N.; Li, F.-F.; Zhang, Z.; Cui, L.-Y.; He, H.-Y.; Yan, X.; He, W.-B.; Sun, H.-S.; Feng, Z.-P.; et al. Neuronal Chemokine-like-Factor 1 (CKLF1) up-Regulation Promotes M1 Polarization of Microglia in Rat Brain after Stroke. Acta Pharmacol. Sin. 2022, 43, 1217–1230. [Google Scholar] [CrossRef]
- Qin, T.; Jiang, T.; Zhou, Z.; Wu, M.; Zhang, Y.; Yang, Z.; Zheng, Z.; Yi, J.; Wang, X.; Luo, M.; et al. Treg-Microglia Partnership in the Injured Spinal Cord Preserves Treg Cell Function and Regulates Microglial Cholesterol Metabolism. Neuron, 2025; in press. [Google Scholar] [CrossRef]
- Liu, H.; Dai, L.; Wang, M.; Feng, F.; Xiao, Y. Tunicamycin Induces Hepatic Stellate Cell Apoptosis Through Calpain-2/Ca2 +-Dependent Endoplasmic Reticulum Stress Pathway. Front. Cell Dev. Biol. 2021, 9, 684857. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; You, M.; Fan, C.; Rong, R.; Li, H.; Xia, X. Pathologically High Intraocular Pressure Induces Mitochondrial Dysfunction through Drp1 and Leads to Retinal Ganglion Cell PANoptosis in Glaucoma. Redox Biol. 2023, 62, 102687. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Zuo, Z.; Zhao, Y.; Ai, Y.; Zhang, L.; Li, L.; He, X.; Luo, J.; Xu, J.; Yang, X.; et al. Transcranial Focused Ultrasound Stimulation Alleviates NLRP3-Related Neuroinflammation Induced by Ischemic Stroke via Regulation of the Nespas/miR-383-3p/SHP2 Pathway. Int. Immunopharmacol. 2025, 144, 113680. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Huang, S.; Gao, H.; Han, Z.; Chen, F.; Zhang, S.; Wang, Z.; Kang, C.; Jiang, R.; Yue, S.; et al. miR-21-5p Alleviates Leakage of Injured Brain Microvascular Endothelial Barrier in Vitro through Suppressing Inflammation and Apoptosis. Brain Res. 2016, 1650, 31–40. [Google Scholar] [CrossRef]
- Ge, X.; Han, Z.; Chen, F.; Wang, H.; Zhang, B.; Jiang, R.; Lei, P.; Zhang, J. MiR-21 Alleviates Secondary Blood-Brain Barrier Damage after Traumatic Brain Injury in Rats. Brain Res. 2015, 1603, 150–157. [Google Scholar] [CrossRef]
- Ge, X.-T.; Lei, P.; Wang, H.-C.; Zhang, A.-L.; Han, Z.-L.; Chen, X.; Li, S.-H.; Jiang, R.-C.; Kang, C.-S.; Zhang, J.-N. miR-21 Improves the Neurological Outcome after Traumatic Brain Injury in Rats. Sci. Rep. 2014, 4, 6718. [Google Scholar] [CrossRef]
- Wang, L.; Yin, Z.; Wang, F.; Han, Z.; Wang, Y.; Huang, S.; Hu, T.; Guo, M.; Lei, P. Hydrogen Exerts Neuroprotection by Activation of the miR-21/PI3K/AKT/GSK-3β Pathway in an in Vitro Model of Traumatic Brain Injury. J. Cell. Mol. Med. 2020, 24, 4061–4071. [Google Scholar] [CrossRef]
- Yan, H.; Huang, W.; Rao, J.; Yuan, J. miR-21 Regulates Ischemic Neuronal Injury via the P53/Bcl-2/Bax Signaling Pathway. Aging 2021, 13, 22242–22255. [Google Scholar] [CrossRef]
- Deng, C.-L.; Hu, C.-B.; Ling, S.-T.; Zhao, N.; Bao, L.-H.; Zhou, F.; Xiong, Y.-C.; Chen, T.; Sui, B.-D.; Yu, X.-R.; et al. Photoreceptor Protection by Mesenchymal Stem Cell Transplantation Identifies Exosomal MiR-21 as a Therapeutic for Retinal Degeneration. Cell Death Differ. 2021, 28, 1041–1061. [Google Scholar] [CrossRef]
- Mead, B.; Tomarev, S. The Role of miRNA in Retinal Ganglion Cell Health and Disease. Neural Regen. Res. 2022, 17, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, H.; Cepurna, W.O.; Johnson, E.C.; Morrison, J.C. MicroRNA Expression in the Glaucomatous Retina. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7971–7982. [Google Scholar] [CrossRef] [PubMed]
- Abcouwer, S.F.; Shanmugam, S.; Muthusamy, A.; Lin, C.-M.; Kong, D.; Hager, H.; Liu, X.; Antonetti, D.A. Inflammatory Resolution and Vascular Barrier Restoration after Retinal Ischemia Reperfusion Injury. J. Neuroinflamm. 2021, 18, 186. [Google Scholar] [CrossRef] [PubMed]
- Bou Ghanem, G.O.; Wareham, L.K.; Calkins, D.J. Addressing Neurodegeneration in Glaucoma: Mechanisms, Challenges, and Treatments. Prog. Retin. Eye Res. 2024, 100, 101261. [Google Scholar] [CrossRef]
- Cheng, B.; Liu, S.; Gao, L.; Xin, N.; Shang, Z.; Zhu, Z.; Yang, Y.; Ma, R.; Xu, Z.; Liu, J.; et al. Long-Term Minocycline Treatment Exhibits Enhanced Therapeutic Effects on Ischemic Stroke by Suppressing Inflammatory Phenotype of Microglia Through the EMB/MCT4/STING Pathway. CNS Neurosci. Ther. 2025, 31, e70328. [Google Scholar] [CrossRef]
- Sun, H.; Hao, Y.; Liu, H.; Gao, F. The Immunomodulatory Effects of GLP-1 Receptor Agonists in Neurogenerative Diseases and Ischemic Stroke Treatment. Front. Immunol. 2025, 16, 1525623. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, C.; Wang, Y.; Zhu, X.; Wu, L.; Chen, L.; Zhou, J.; Wang, F. METTL3/IGF2BP2/IκBα Axis Participates in Neuroinflammation in Alzheimer’s Disease by Regulating M1/M2 Polarization of Microglia. Neurochem. Int. 2025, 186, 105964. [Google Scholar] [CrossRef]
- Chen, S.; Li, Z.; Yang, L.; Xu, Z.; Liu, A.; He, Q.; Xiao, F.; Zhan, J. Cannabinoid Receptor-2 Alleviates Sepsis-Induced Neuroinflammation by Modulating Microglia M1/M2 Subset Polarization Through Inhibiting Nogo-B Expression. Mol. Neurobiol. 2025, 62, 9258–9270. [Google Scholar] [CrossRef]
- Xian, X.; Cai, L.-L.; Li, Y.; Wang, R.-C.; Xu, Y.-H.; Chen, Y.-J.; Xie, Y.-H.; Zhu, X.-L.; Li, Y.-F. Neuron Secrete Exosomes Containing miR-9-5p to Promote Polarization of M1 Microglia in Depression. J. Nanobiotechnol. 2022, 20, 122. [Google Scholar] [CrossRef]
- Liu, W.; Rong, Y.; Wang, J.; Zhou, Z.; Ge, X.; Ji, C.; Jiang, D.; Gong, F.; Li, L.; Chen, J.; et al. Exosome-Shuttled miR-216a-5p from Hypoxic Preconditioned Mesenchymal Stem Cells Repair Traumatic Spinal Cord Injury by Shifting Microglial M1/M2 Polarization. J. Neuroinflamm. 2020, 17, 47. [Google Scholar] [CrossRef]
- Yu, Z.; Wen, Y.; Jiang, N.; Li, Z.; Guan, J.; Zhang, Y.; Deng, C.; Zhao, L.; Zheng, S.G.; Zhu, Y.; et al. TNF-α Stimulation Enhances the Neuroprotective Effects of Gingival MSCs Derived Exosomes in Retinal Ischemia-Reperfusion Injury via the MEG3/miR-21a-5p Axis. Biomaterials 2022, 284, 121484. [Google Scholar] [CrossRef]
- Bai, X.; Bian, Z. MicroRNA-21 Is a Versatile Regulator and Potential Treatment Target in Central Nervous System Disorders. Front. Mol. Neurosci. 2022, 15, 842288. [Google Scholar] [CrossRef]
- Garcia, G.; Pinto, S.; Ferreira, S.; Lopes, D.; Serrador, M.J.; Fernandes, A.; Vaz, A.R.; Mendonça, A.d.; Edenhofer, F.; Malm, T.; et al. Emerging Role of miR-21-5p in Neuron-Glia Dysregulation and Exosome Transfer Using Multiple Models of Alzheimer’s Disease. Cells 2022, 11, 3377. [Google Scholar] [CrossRef]
- Hu, Z.; Li, L.; Li, M.; Zhang, X.; Zhang, Y.; Ran, J.; Li, L. miR-21-5p Inhibits Ferroptosis in Hepatocellular Carcinoma Cells by Regulating the AKT/mTOR Signaling Pathway through MELK. J. Immunol. Res. 2023, 2023, 8929525. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, F.; Zhao, C.; Song, J.; Hu, M.; Lv, Y.; Duan, Y.; Fang, W.; Ding, R.; Qiu, Y. miR-21-5p Prevents Doxorubicin-Induced Cardiomyopathy by Downregulating BTG2. Heliyon 2023, 9, e15451. [Google Scholar] [CrossRef] [PubMed]
- Vandewalle, V.; Essaghir, A.; Bollaert, E.; Lenglez, S.; Graux, C.; Schoemans, H.; Saussoy, P.; Michaux, L.; Valk, P.J.M.; Demoulin, J.-B.; et al. miR-15a-5p and miR-21-5p Contribute to Chemoresistance in Cytogenetically Normal Acute Myeloid Leukaemia by Targeting PDCD4, ARL2 and BTG2. J. Cell. Mol. Med. 2021, 25, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Li, Y.; Chen, Z.; Guo, J.; Yang, M.; Peng, Y. Exosomes Derived from Antler Mesenchymal Stem Cells Promote Wound Healing by miR-21-5p/STAT3 Axis. Int. J. Nanomed. 2024, 19, 11257–11273. [Google Scholar] [CrossRef]
- Abroumand Gholami, A.; Tahmasebi, F.; Haghir, H.; Babaloo, H. Targeting JAK/STAT Signaling Pathway by Curcumin: Implications for Spinal Cord Injury Neuroprotection. Inflammopharmacology 2025, 33, 4377–4395. [Google Scholar] [CrossRef]
- Li, H.-W.; Zeng, H.-S. Regulation of JAK/STAT Signal Pathway by miR-21 in the Pathogenesis of Juvenile Idiopathic Arthritis. World J. Pediatr. 2020, 16, 502–513. [Google Scholar] [CrossRef]
- Wang, X.; Ren, T.; Zhang, X.; Pan, T.; Peng, F.; Feng, J.; Sun, Q.; Song, N.N.; Ding, X.; Jia, P. MiR-21 Suppression in Macrophages Promotes M2-like Polarization and Attenuates Kidney Ischemia-Reperfusion Injury. FASEB J. 2024, 38, e70251. [Google Scholar] [CrossRef]
- Hashemi, M.; Abbaszadeh, S.; Rashidi, M.; Amini, N.; Talebi Anaraki, K.; Motahhary, M.; Khalilipouya, E.; Harif Nashtifani, A.; Shafiei, S.; Ramezani Farani, M.; et al. STAT3 as a Newly Emerging Target in Colorectal Cancer Therapy: Tumorigenesis, Therapy Response, and Pharmacological/Nanoplatform Strategies. Environ. Res. 2023, 233, 116458. [Google Scholar] [CrossRef]
- Hillmer, E.J.; Zhang, H.; Li, H.S.; Watowich, S.S. STAT3 Signaling in Immunity. Cytokine Growth Factor. Rev. 2016, 31, 1–15. [Google Scholar] [CrossRef]
- Nam, H.Y.; Nam, J.H.; Yoon, G.; Lee, J.-Y.; Nam, Y.; Kang, H.-J.; Cho, H.-J.; Kim, J.; Hoe, H.-S. Ibrutinib Suppresses LPS-Induced Neuroinflammatory Responses in BV2 Microglial Cells and Wild-Type Mice. J. Neuroinflamm. 2018, 15, 271. [Google Scholar] [CrossRef]
- Xin, D.-Q.; Zhao, Y.-J.; Li, T.-T.; Ke, H.-F.; Gai, C.-C.; Guo, X.-F.; Chen, W.-Q.; Liu, D.-X.; Wang, Z. The Delivery of miR-21a-5p by Extracellular Vesicles Induces Microglial Polarization via the STAT3 Pathway Following Hypoxia-Ischemia in Neonatal Mice. Neural Regen. Res. 2022, 17, 2238–2246. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, L.; Liao, J.; Tan, C.; Liu, C.; Shi, W.; Chen, D. miR-21-5p Alleviates Retinal Ischemia–Reperfusion Injury by Inhibiting M1 Polarization of Microglia via Suppression of STAT3 Signaling. Biomedicines 2025, 13, 2456. https://doi.org/10.3390/biomedicines13102456
Qin L, Liao J, Tan C, Liu C, Shi W, Chen D. miR-21-5p Alleviates Retinal Ischemia–Reperfusion Injury by Inhibiting M1 Polarization of Microglia via Suppression of STAT3 Signaling. Biomedicines. 2025; 13(10):2456. https://doi.org/10.3390/biomedicines13102456
Chicago/Turabian StyleQin, Liangshi, Junle Liao, Cheng Tan, Can Liu, Wenjia Shi, and Dan Chen. 2025. "miR-21-5p Alleviates Retinal Ischemia–Reperfusion Injury by Inhibiting M1 Polarization of Microglia via Suppression of STAT3 Signaling" Biomedicines 13, no. 10: 2456. https://doi.org/10.3390/biomedicines13102456
APA StyleQin, L., Liao, J., Tan, C., Liu, C., Shi, W., & Chen, D. (2025). miR-21-5p Alleviates Retinal Ischemia–Reperfusion Injury by Inhibiting M1 Polarization of Microglia via Suppression of STAT3 Signaling. Biomedicines, 13(10), 2456. https://doi.org/10.3390/biomedicines13102456



