Programmed-Cell-Death-Related Signature Reveals Immune Microenvironment Characteristics and Predicts Therapeutic Response in Diffuse Large B Cell Lymphoma
Abstract
1. Introduction
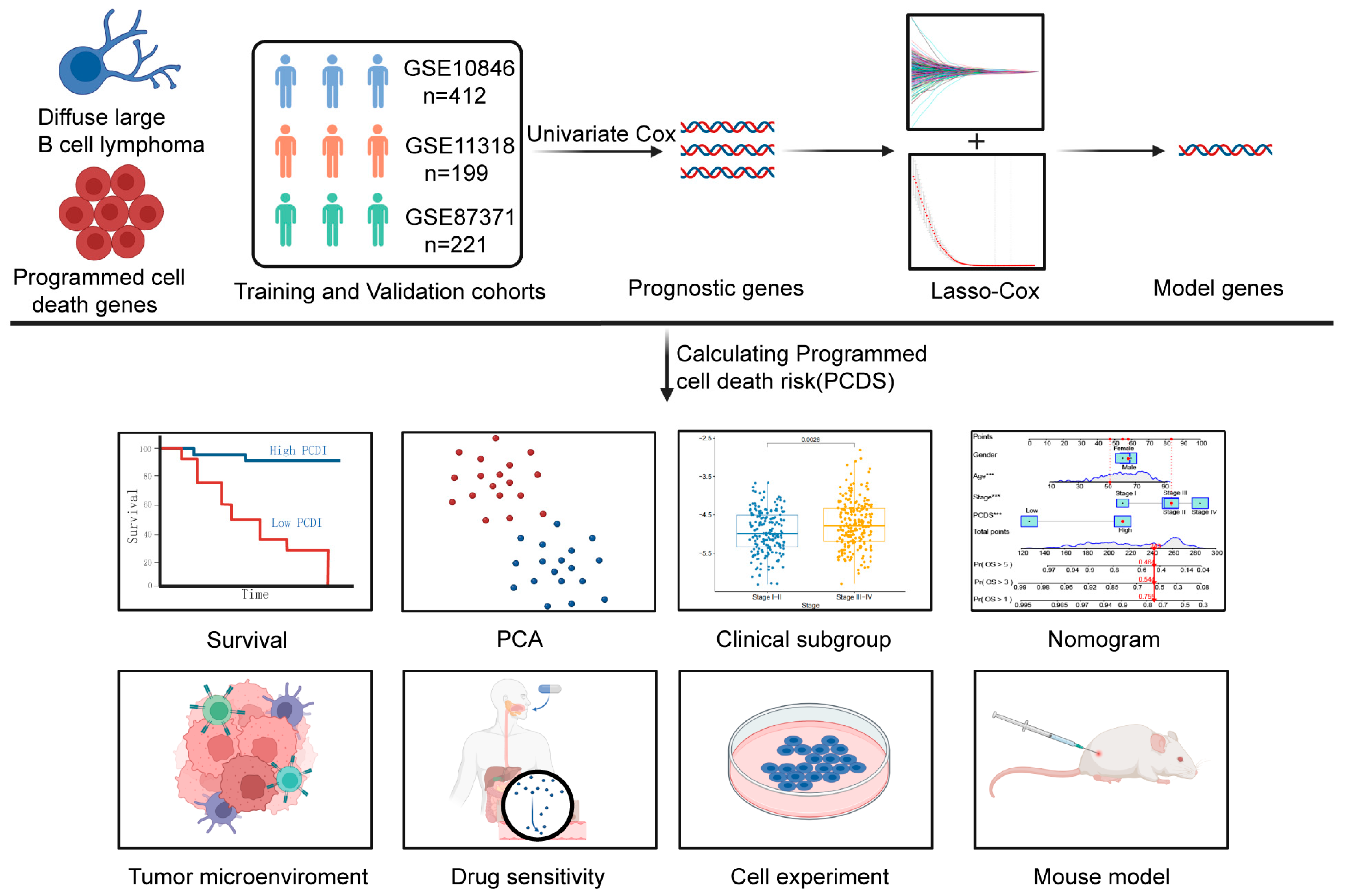
2. Materials and Methods
2.1. Data Collection
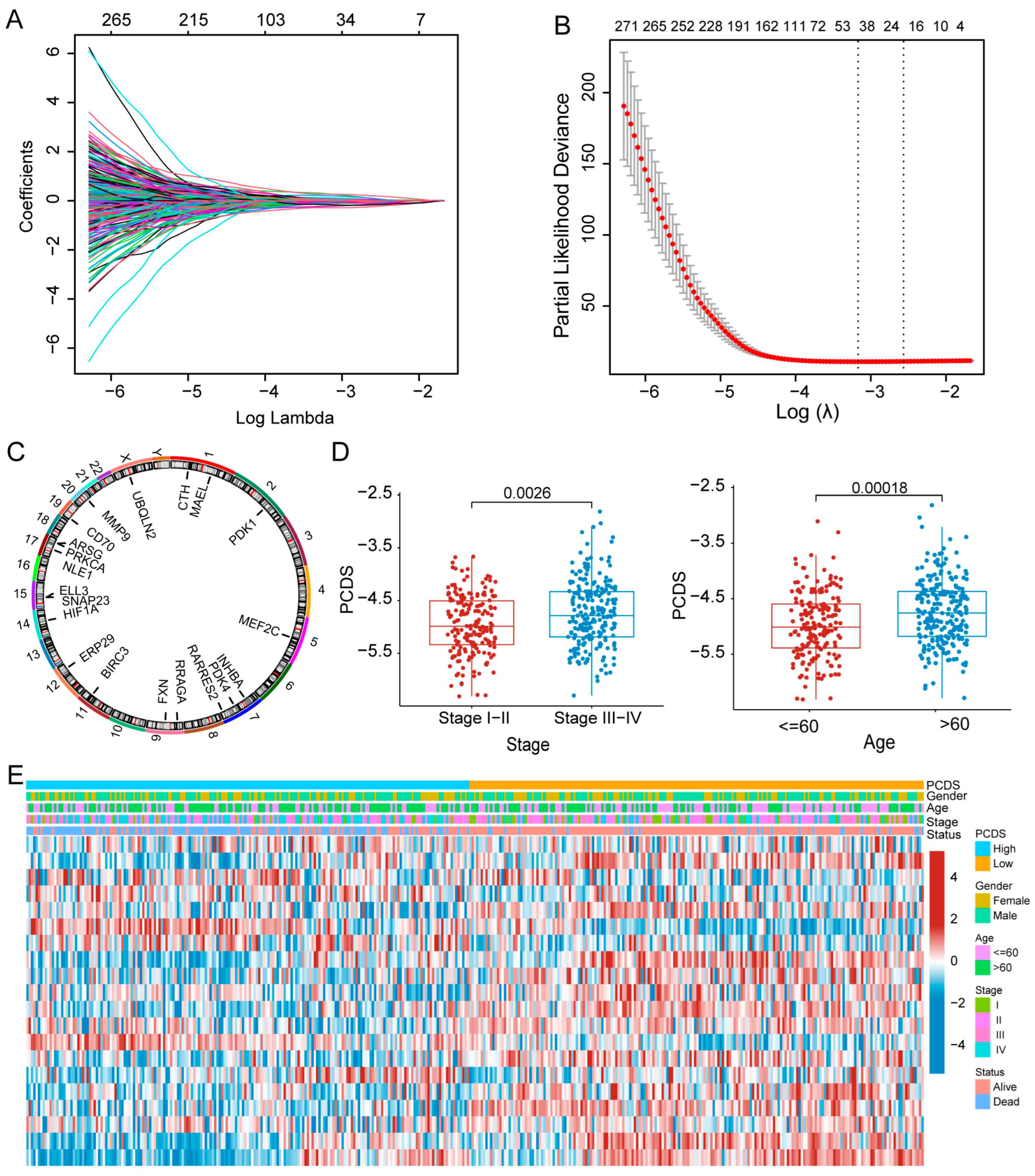
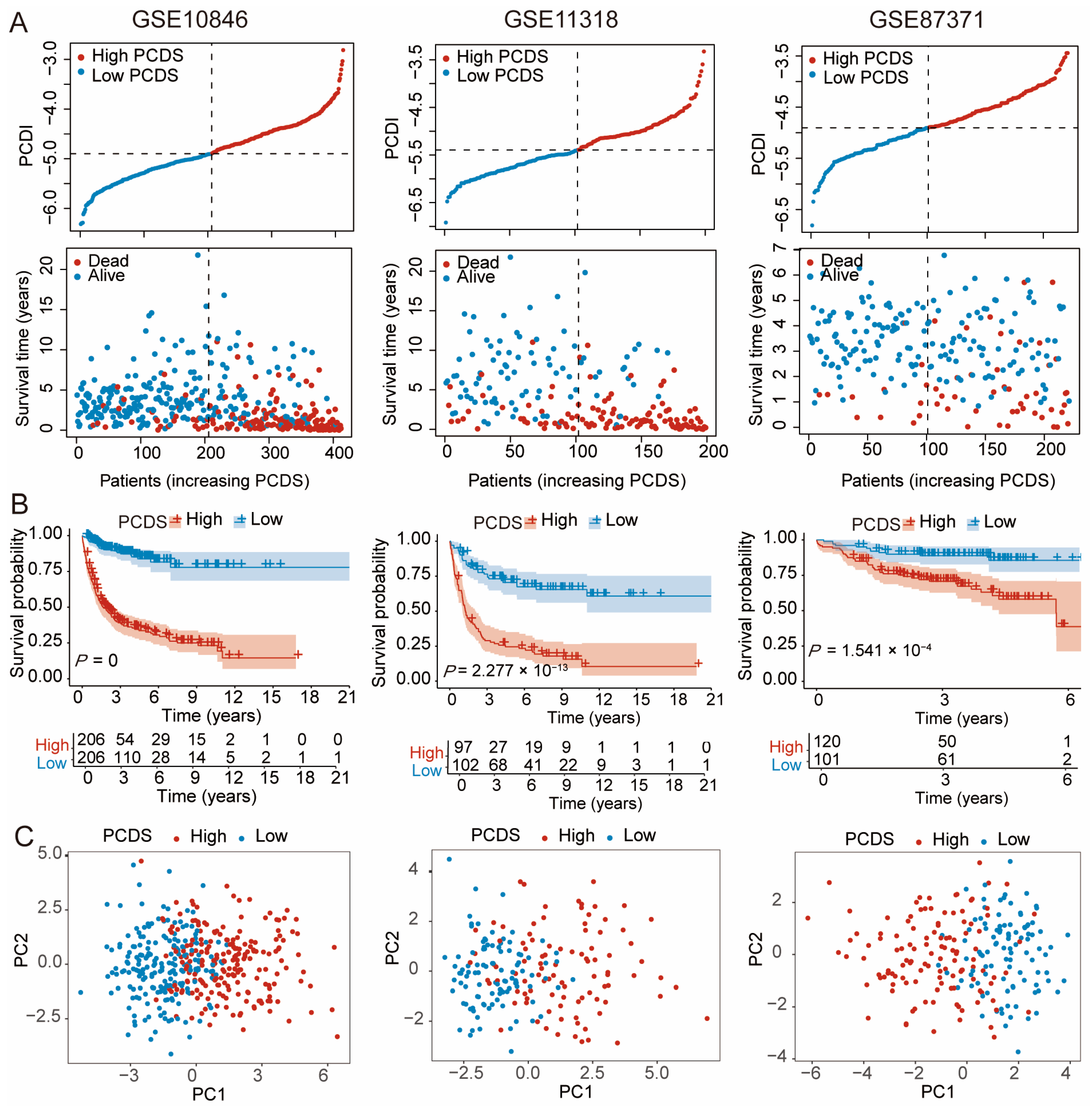
2.2. A Prognosis Model Development and Validation
2.3. Gene Set Enrichment Analysis
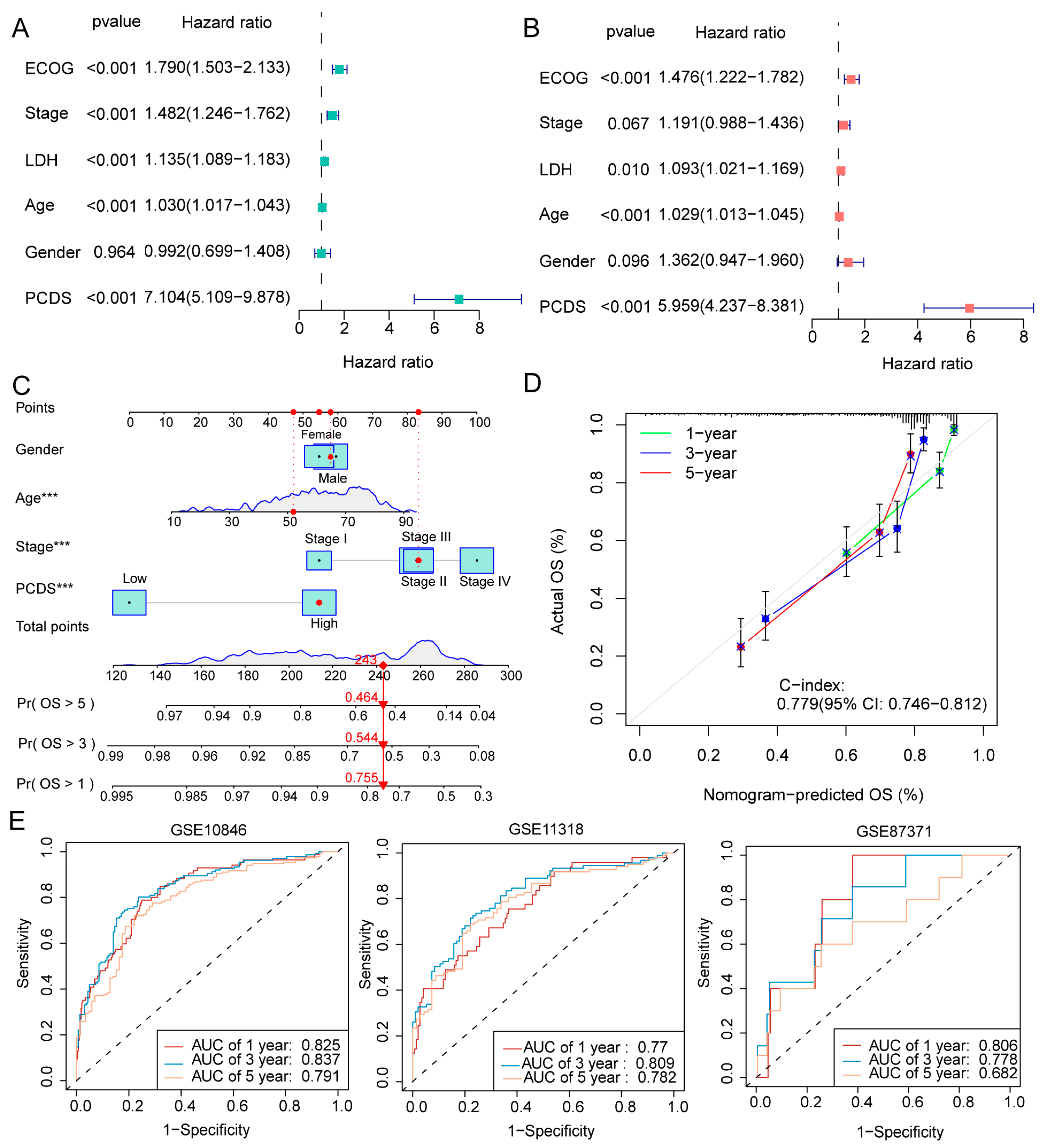
2.4. Nomogram Construction and Validation
2.5. Drug Sensitivity and TME Analysis
2.6. Cell Culture
2.7. Knockdown of the Key Model Gene CTH
2.8. Tumor Xenograft
2.9. Statistical Analysis
3. Results
3.1. Construction of a Prognostic Model for DLBCL Patients
3.2. Validation of the PCDS-Based Prognostic Model
3.3. Development and Evaluation of the Nomogram-Based Survival Model
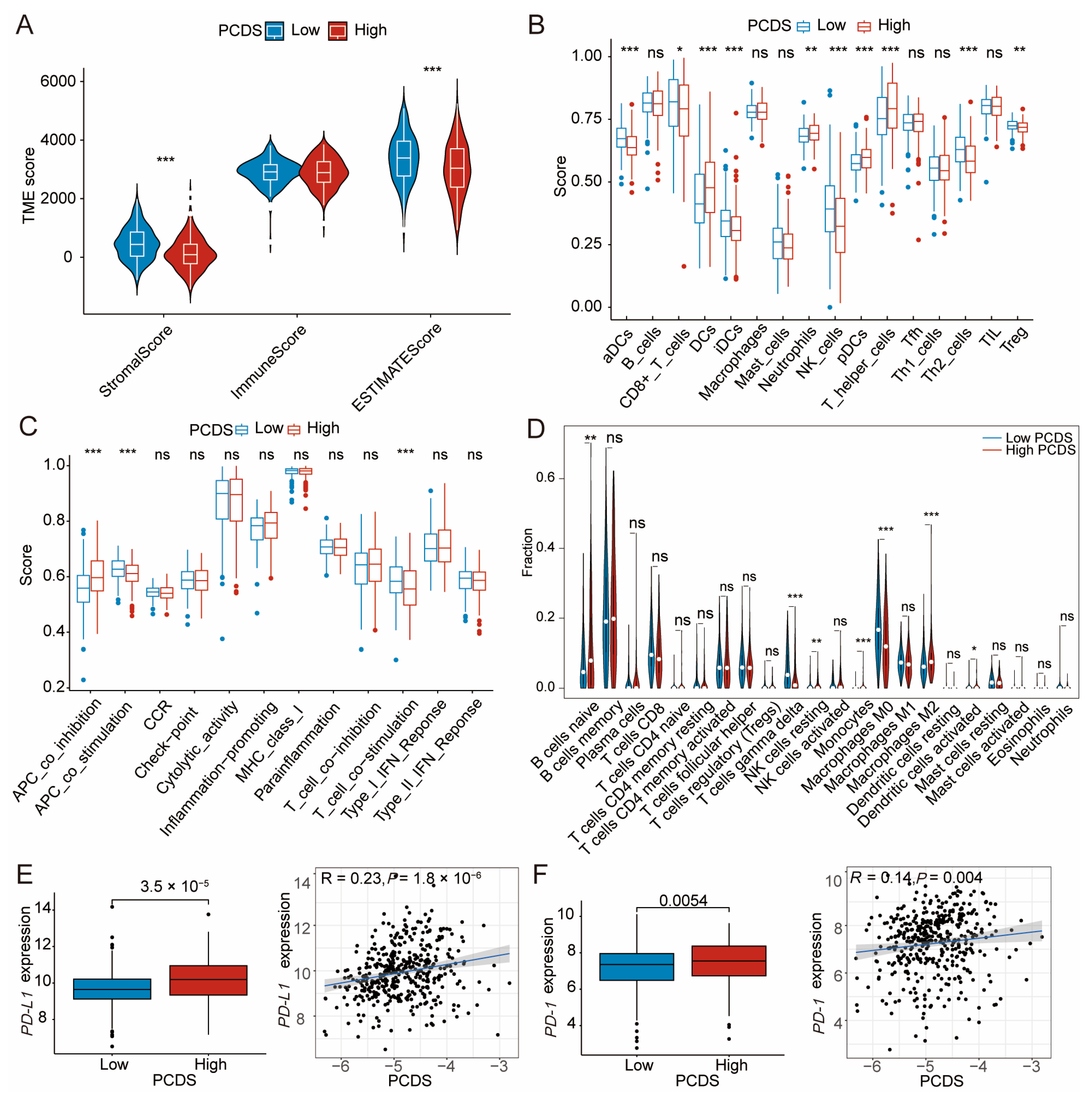
3.4. TME Dissection Based on ESTIMATE, ssGSEA, and CIBERSORT Analyses
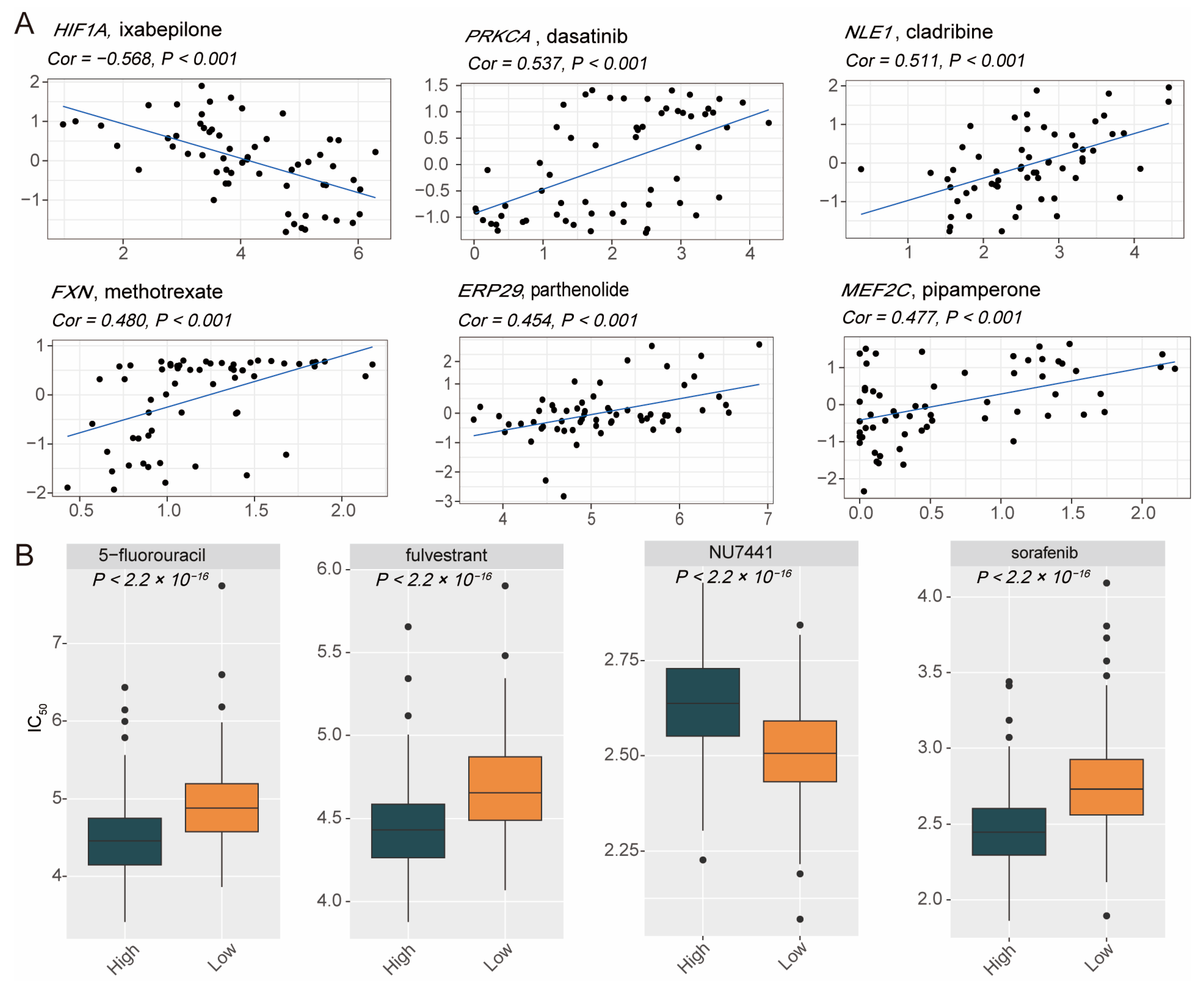
3.5. Heterogeneity of Drug Sensitivity Based on PCDS Values
3.6. Functional Effects of CTH Knockdown on DLBCL Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABC | Activated B cell-like |
| ATCC | American Type Culture Collection |
| Annexin V-FITC | Annexin V conjugated with fluorescein isothiocyanate |
| AUC | Area under the curve |
| aDCs | Activated dendritic cell |
| APC | Antigen-presenting cell |
| CI | Confidence interval |
| C-index | Concordance index |
| CCR | Cytokine–cytokine receptor interaction |
| DLBCL | Diffuse large B cell lymphoma |
| DAMPs | Damage-associated molecular patterns |
| ECOG | Eastern Cooperative Oncology Group |
| EdU | 5-Ethynyl-2′-deoxyuridine |
| GCB | Germinal center B cell-like |
| GSCA | Gene Set Cancer Analysis |
| GSEA | Gene set enrichment analysis |
| HRs | Hazard ratios |
| IC50 | Half maximal inhibitory concentration |
| IFN | Interferon |
| LDH | Lactate dehydrogenase |
| IPI | International Prognostic Index |
| OS | Overall survival |
| PCD | Programmed cell death |
| PCDS | Programmed cell death score |
| PCA | Principal component analysis |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed cell death ligand 1 |
| PI | Propidium iodide |
| ROC | Receiver operating characteristic |
| STR | Short tandem repeat |
| TME | Tumor microenvironment |
References
- Shi, Y.; Xu, Y.; Shen, H.; Jin, J.; Tong, H.; Xie, W. Advances in biology, diagnosis and treatment of DLBCL. Ann. Hematol. 2024, 103, 3315–3334. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.P.; Huo, Y.J.; Zhao, W.L. All roads lead to targeted diffuse large B-cell lymphoma approaches. Cancer Cell 2022, 40, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Nie, L.; Zhang, Y.; Yan, Y.; Wang, C.; Colic, M.; Olszewski, K.; Horbath, A.; Chen, X.; Lei, G.; et al. Actin cytoskeleton vulnerability to disulfide stress mediates disulfidptosis. Nat. Cell Biol. 2023, 25, 404–414. [Google Scholar] [CrossRef]
- Xu, X.; Lai, Y.; Hua, Z.C. Apoptosis and apoptotic body: Disease message and therapeutic target potentials. Biosci. Rep. 2019, 39, BSR20180992. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, Y.; Qi, Z.; Li, X.; Zhao, Y. Ferroptosis: CD8(+)T cells’ blade to destroy tumor cells or poison for self-destruction. Cell Death Discov. 2025, 11, 128. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, Q.; Li, X.; Wang, J.; Guo, L.; Huang, L.; Gao, W. Nanoformula Design for Inducing Non-Apoptotic Cell Death Regulation: A Powerful Booster for Cancer Immunotherapy. Adv. Healthc. Mater. 2025, 14, e2403493. [Google Scholar] [CrossRef]
- Wang, S.; Guo, S.; Guo, J.; Du, Q.; Wu, C.; Wu, Y.; Zhang, Y. Cell death pathways: Molecular mechanisms and therapeutic targets for cancer. MedComm (2020) 2024, 5, e693. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, Y.; Zong, L.; Zhang, W.; Liu, R.; Xing, Q.; Liu, Z.; Yan, Q.; Li, W.; Lei, H.; et al. The multifaceted roles of GSDME-mediated pyroptosis in cancer: Therapeutic strategies and persisting obstacles. Cell Death Dis. 2023, 14, 836. [Google Scholar] [CrossRef]
- Zheng, N.; Fang, J.; Xue, G.; Wang, Z.; Li, X.; Zhou, M.; Jin, G.; Rahman, M.M.; McFadden, G.; Lu, Y. Induction of tumor cell autosis by myxoma virus-infected CAR-T and TCR-T cells to overcome primary and acquired resistance. Cancer Cell 2022, 40, 973–985.e977. [Google Scholar] [CrossRef] [PubMed]
- Kahlson, M.A.; Dixon, S.J. Copper-induced cell death. Science 2022, 375, 1231–1232. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Huang, W.; Mei, J.; Bhushan, S.; Wu, X.; Yan, C.; Zheng, H.; Yang, Y. Advances in novel cell death mechanisms in breast cancer: Intersecting perspectives on ferroptosis, cuproptosis, disulfidptosis, and pyroptosis. Mol. Cancer 2025, 24, 224. [Google Scholar] [CrossRef]
- Jaillon, S.; Ponzetta, A.; Di Mitri, D.; Santoni, A.; Bonecchi, R.; Mantovani, A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat. Rev. Cancer 2020, 20, 485–503. [Google Scholar] [CrossRef] [PubMed]
- Huntington, N.D.; Cursons, J.; Rautela, J. The cancer-natural killer cell immunity cycle. Nat. Rev. Cancer 2020, 20, 437–454. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef]
- Jhunjhunwala, S.; Hammer, C.; Delamarre, L. Antigen presentation in cancer: Insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer 2021, 21, 298–312. [Google Scholar] [CrossRef]
- Wight, J.C.; Chong, G.; Grigg, A.P.; Hawkes, E.A. Prognostication of diffuse large B-cell lymphoma in the molecular era: Moving beyond the IPI. Blood Rev. 2018, 32, 400–415. [Google Scholar] [CrossRef]
- Wright, G.W.; Huang, D.W.; Phelan, J.D.; Coulibaly, Z.A.; Roulland, S.; Young, R.M.; Wang, J.Q.; Schmitz, R.; Morin, R.D.; Tang, J.; et al. A Probabilistic Classification Tool for Genetic Subtypes of Diffuse Large B Cell Lymphoma with Therapeutic Implications. Cancer Cell 2020, 37, 551–568.e514. [Google Scholar] [CrossRef]
- Kowalewski, B.; Lübke, T.; Kollmann, K.; Braulke, T.; Reinheckel, T.; Dierks, T.; Damme, M. Molecular characterization of arylsulfatase G: Expression, processing, glycosylation, transport, and activity. J. Biol. Chem. 2014, 289, 27992–28005. [Google Scholar] [CrossRef]
- Peleli, M.; Antoniadou, I.; Rodrigues-Junior, D.M.; Savvoulidou, O.; Caja, L.; Katsouda, A.; Ketelhuth, D.F.J.; Stubbe, J.; Madsen, K.; Moustakas, A.; et al. Cystathionine gamma-lyase (CTH) inhibition attenuates glioblastoma formation. Redox Biol. 2023, 64, 102773. [Google Scholar] [CrossRef]
- Kim, J.W.; Tchernyshyov, I.; Semenza, G.L.; Dang, C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006, 3, 177–185. [Google Scholar] [CrossRef]
- Jiang, Q.; Zheng, N.; Bu, L.; Zhang, X.; Zhang, X.; Wu, Y.; Su, Y.; Wang, L.; Zhang, X.; Ren, S.; et al. SPOP-mediated ubiquitination and degradation of PDK1 suppresses AKT kinase activity and oncogenic functions. Mol. Cancer 2021, 20, 100. [Google Scholar] [CrossRef]
- Dou, X.; Fu, Q.; Long, Q.; Liu, S.; Zou, Y.; Fu, D.; Xu, Q.; Jiang, Z.; Ren, X.; Zhang, G.; et al. PDK4-dependent hypercatabolism and lactate production of senescent cells promotes cancer malignancy. Nat. Metab. 2023, 5, 1887–1910. [Google Scholar] [CrossRef]
- Ma, W.Q.; Sun, X.J.; Zhu, Y.; Liu, N.F. PDK4 promotes vascular calcification by interfering with autophagic activity and metabolic reprogramming. Cell Death Dis. 2020, 11, 991. [Google Scholar] [CrossRef]
- Alexander, L.M.M.; Watters, J.; Reusch, J.A.; Maurin, M.; Nepon-Sixt, B.S.; Vrzalikova, K.; Alexandrow, M.G.; Murray, P.G.; Wright, K.L. Selective expression of the transcription elongation factor ELL3 in B cells prior to ELL2 drives proliferation and survival. Mol. Immunol. 2017, 91, 8–16. [Google Scholar] [CrossRef]
- Zhou, L.; Ou, S.; Liang, T.; Li, M.; Xiao, P.; Cheng, J.; Zhou, J.; Yuan, L. MAEL facilitates metabolic reprogramming and breast cancer progression by promoting the degradation of citrate synthase and fumarate hydratase via chaperone-mediated autophagy. FEBS J. 2023, 290, 3614–3628. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Hua, L.; Ye, Y.; Wang, D.; Li, C.; Xie, Q.; Wakimoto, H.; Gong, Y.; Ji, J. MEF2C silencing downregulates NF2 and E-cadherin and enhances Erastin-induced ferroptosis in meningioma. Neuro Oncol. 2021, 23, 2014–2027. [Google Scholar] [CrossRef] [PubMed]
- Huang, H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors 2018, 18, 3249. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Li, R.Z.; Sun, A.; Zhou, H.; Neher, E.; Yang, J.S.; Huang, J.M.; Zhang, Y.Z.; Jiang, Z.B.; Liang, T.L.; et al. Metabolomics and integrated network pharmacology analysis reveal Tricin as the active anti-cancer component of Weijing decoction by suppression of PRKCA and sphingolipid signaling. Pharmacol. Res. 2021, 171, 105574. [Google Scholar] [CrossRef]
- Zhang, D.; Richardson, D.R. Endoplasmic reticulum protein 29 (ERp29): An emerging role in cancer. Int. J. Biochem. Cell Biol. 2011, 43, 33–36. [Google Scholar] [CrossRef]
- Du, J.; Zhou, Y.; Li, Y.; Xia, J.; Chen, Y.; Chen, S.; Wang, X.; Sun, W.; Wang, T.; Ren, X.; et al. Identification of Frataxin as a regulator of ferroptosis. Redox Biol. 2020, 32, 101483. [Google Scholar] [CrossRef]
- Dolai, S.; Takahashi, T.; Qin, T.; Liang, T.; Xie, L.; Kang, F.; Miao, Y.F.; Xie, H.; Kang, Y.; Manuel, J.; et al. Pancreas-specific SNAP23 depletion prevents pancreatitis by attenuating pathological basolateral exocytosis and formation of trypsin-activating autolysosomes. Autophagy 2021, 17, 3068–3081. [Google Scholar] [CrossRef]
- Shah, P.P.; Saurabh, K.; Kurlawala, Z.; Vega, A.A.; Siskind, L.J.; Beverly, L.J. Towards a molecular understanding of the overlapping and distinct roles of UBQLN1 and UBQLN2 in lung cancer progression and metastasis. Neoplasia 2022, 25, 1–8. [Google Scholar] [CrossRef]
- Chen, J.H.; Huang, C.; Zhang, B.; Yin, S.; Liang, J.; Xu, C.; Huang, Y.; Cen, L.P.; Ng, T.K.; Zheng, C.; et al. Mutations of RagA GTPase in mTORC1 Pathway Are Associated with Autosomal Dominant Cataracts. PLoS Genet. 2016, 12, e1006090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.W.; Johmura, Y.; Suzuki, N.; Omori, S.; Migita, T.; Yamaguchi, K.; Hatakeyama, S.; Yamazaki, S.; Shimizu, E.; Imoto, S.; et al. Blocking PD-L1-PD-1 improves senescence surveillance and ageing phenotypes. Nature 2022, 611, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.H.; Chan, L.C.; Li, C.W.; Hsu, J.L.; Hung, M.C. Mechanisms Controlling PD-L1 Expression in Cancer. Mol. Cell 2019, 76, 359–370. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X.; et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer 2019, 18, 10. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, D.; Luo, K.; He, X.; Hu, X.; Zhai, Y.; Jiang, Y.; Wu, W.; Zhao, Z. Programmed-Cell-Death-Related Signature Reveals Immune Microenvironment Characteristics and Predicts Therapeutic Response in Diffuse Large B Cell Lymphoma. Biomedicines 2025, 13, 2320. https://doi.org/10.3390/biomedicines13102320
Xing D, Luo K, He X, Hu X, Zhai Y, Jiang Y, Wu W, Zhao Z. Programmed-Cell-Death-Related Signature Reveals Immune Microenvironment Characteristics and Predicts Therapeutic Response in Diffuse Large B Cell Lymphoma. Biomedicines. 2025; 13(10):2320. https://doi.org/10.3390/biomedicines13102320
Chicago/Turabian StyleXing, Donghui, Kaiping Luo, Xiang He, Xin Hu, Yixin Zhai, Yanan Jiang, Wenqi Wu, and Zhigang Zhao. 2025. "Programmed-Cell-Death-Related Signature Reveals Immune Microenvironment Characteristics and Predicts Therapeutic Response in Diffuse Large B Cell Lymphoma" Biomedicines 13, no. 10: 2320. https://doi.org/10.3390/biomedicines13102320
APA StyleXing, D., Luo, K., He, X., Hu, X., Zhai, Y., Jiang, Y., Wu, W., & Zhao, Z. (2025). Programmed-Cell-Death-Related Signature Reveals Immune Microenvironment Characteristics and Predicts Therapeutic Response in Diffuse Large B Cell Lymphoma. Biomedicines, 13(10), 2320. https://doi.org/10.3390/biomedicines13102320




