Ectopic Expression of Neurod1 Is Sufficient for Functional Recovery following a Sensory–Motor Cortical Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Endothelin-1 Stroke
2.3. Adeno-Associated Virus (AAV) Injections
2.4. Tissue Processing, Immunohistochemistry, Imaging, and Quantification
2.5. Lesion Volume Analysis
2.6. Astrocyte Sorting and ImageStream Analysis
2.7. Foot Fault Analysis
2.8. Gait Analysis
2.9. Data Acquisition and Statistical Analysis
3. Results
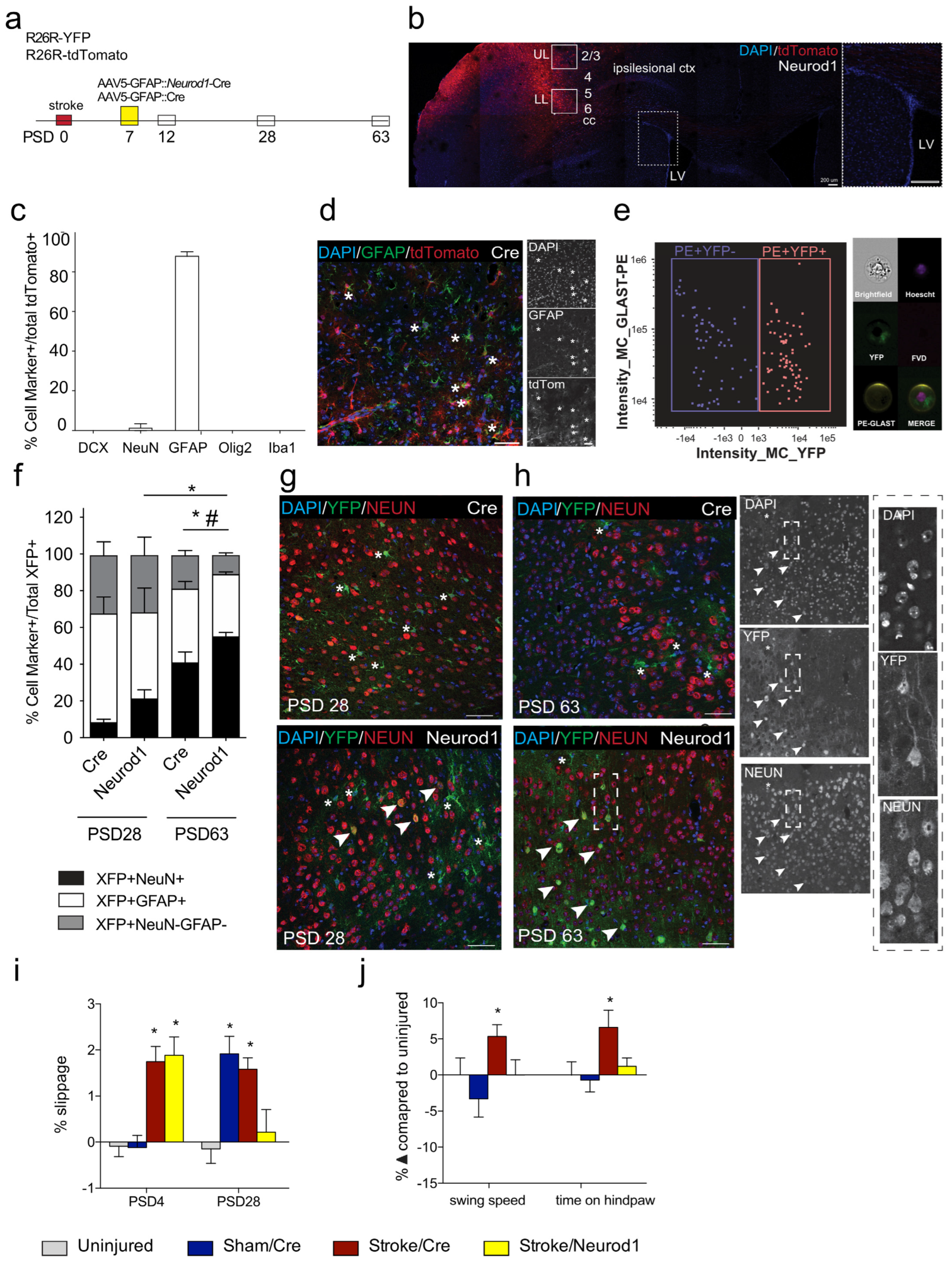
3.1. Transduced Cells Persist Long Term
3.2. Functional Recovery Is Observed following Ectopic Expression of Neurod1
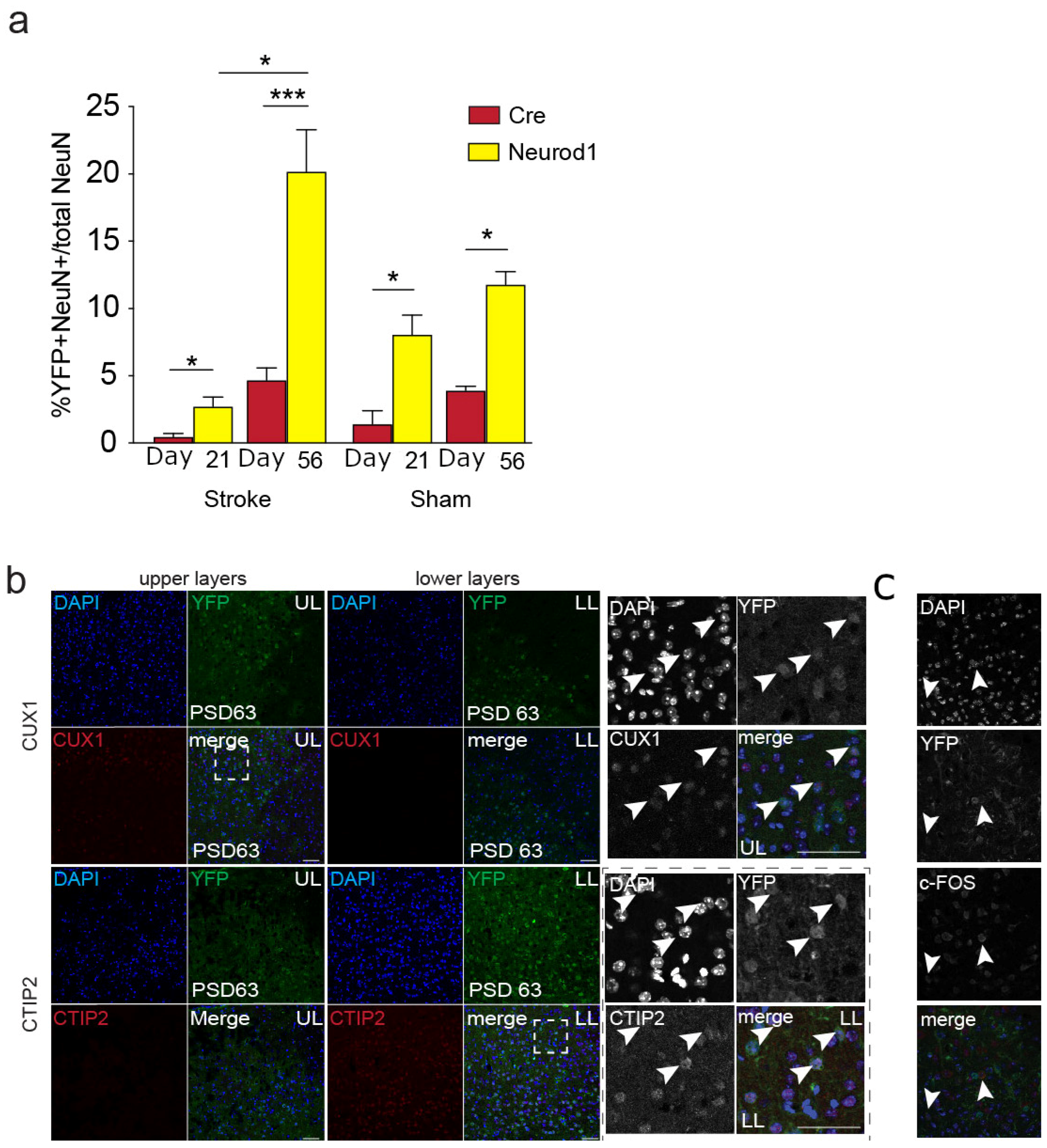
3.3. The Percent of Transduced Neurons in the Perilesional Cortex Increases over Time
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Circulation 2020, 141, E139–E596. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, E153–E639. [Google Scholar] [CrossRef] [PubMed]
- Burvill, P.; Johnson, G.; Jamrozik, K.; Anderson, C.; Stewart-Wynn, E. Risk Factors for Post-Stroke Depression. Int. J. Geriatr. Psychiatry 1997, 12, 219–226. [Google Scholar] [CrossRef]
- Krueger, H.; Koot, J.; Hall, R.; O’Callaghan, C.; Bayley, M.; Corbett, D. Prevalence of Individuals Experiencing the Effects of Stroke in Canada: Trends and Projections. Stroke 2015, 46, 2226–2231. [Google Scholar] [CrossRef] [PubMed]
- Venna, V.R.; Li, J.; Hammond, M.D.; Mancini, N.S.; McCullough, L.D. Chronic Metformin Treatment Improves Post-Stroke Angiogenesis and Recovery after Experimental Stroke. Eur. J. Neurosci. 2014, 39, 2129–2138. [Google Scholar] [CrossRef]
- Dadwal, P.; Mahmud, N.; Sinai, L.; Azimi, A.; Fatt, M.; Wondisford, F.E.; Miller, F.D.; Morshead, C.M. Activating Endogenous Neural Precursor Cells Using Metformin Leads to Neural Repair and Functional Recovery in a Model of Childhood Brain Injury. Stem Cell Rep. 2015, 5, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Nusrat, L.; Livingston-Thomas, J.M.; Raguthevan, V.; Adams, K.; Vonderwalde, I.; Corbett, D.; Morshead, C.M. Cyclosporin A-Mediated Activation of Endogenous Neural Precursor Cells Promotes Cognitive Recovery in a Mouse Model of Stroke. Front. Aging Neurosci. 2018, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Boese, A.C.; Le, Q.-S.E.; Pham, D.; Hamblin, M.H.; Lee, J.-P. Neural Stem Cell Therapy for Subacute and Chronic Ischemic Stroke. Stem Cell Res. Ther. 2018, 9, 154. [Google Scholar] [CrossRef]
- Vonderwalde, I.; Azimi, A.; Rolvink, G.; Ahlfors, J.; Shoichet, M.; Morshead, C. Transplantation of Directly Reprogrammed Human Neural Precursor Cells Following Stroke Promotes Synaptogenesis and Functional Recovery. Transl. Stroke Res. 2020, 11, 93–107. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Li, H.; Chen, G. In Vivo Reprogramming for CNS Repair: Regenerating Neurons from Endogenous Glial Cells. Neuron 2016, 91, 728–738. [Google Scholar] [CrossRef]
- Bajohr, J.; Faiz, M. Direct Lineage Reprogramming in the CNS. Adv. Exp. Med. Biol. 2020, 1212, 31–48. [Google Scholar] [CrossRef]
- Graf, T.; Enver, T. Forcing Cells to Change Lineages. Nature 2009, 462, 587–594. [Google Scholar] [CrossRef]
- Heinrich, C.; Blum, R.; Gascón, S.; Masserdotti, G.; Tripathi, P.; Sánchez, R.; Tiedt, S.; Schroeder, T.; Götz, M.; Berninger, B. Directing Astroglia from the Cerebral Cortex into Subtype Specific Functional Neurons. PLoS Biol. 2010, 8, e1000373. [Google Scholar] [CrossRef]
- Karow, M.; Sánchez, R.; Schichor, C.; Masserdotti, G.; Ortega, F.; Heinrich, C.; Gascón, S.; Khan, M.; Lie, D.; Dellavalle, A.; et al. Reprogramming of Pericyte-Derived Cells of the Adult Human Brain into Induced Neuronal Cells. Cell Stem Cell 2012, 11, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Du, Y.; Deng, H. Direct Lineage Reprogramming: Strategies, Mechanisms, and Applications. Cell Stem Cell 2015, 16, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Masserdotti, G.; Gascón, S.; Götz, M. Direct Neuronal Reprogramming: Learning from and for Development. Development 2016, 143, 2494–2510. [Google Scholar] [CrossRef] [PubMed]
- Gascón, S.; Masserdotti, G.; Russo, G.; Götz, M. Direct Neuronal Reprogramming: Achievements, Hurdles, and New Roads to Success. Cell Stem Cell 2017, 21, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Nakashima, K. Clarifying the Ability of NeuroD1 to Convert Mouse Microglia into Neurons. Neuron 2021, 109, 3912–3913. [Google Scholar] [CrossRef] [PubMed]
- Ghazale, H.; Park, E.J.; Vasan, L.; Mester, J.; Saleh, F.; Trevisiol, A.; Zinyk, D.; Chinchalongporn, V.; Liu, M.; Fleming, T.; et al. Ascl1 Phospho-Site Mutations Enhance Neuronal Conversion of Adult Cortical Astrocytes In Vivo. Front. Neurosci. 2022, 16, 917071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, B.; Cananzi, S.; Han, C.; Wang, L.L.; Zou, Y.; Fu, Y.X.; Hon, G.C.; Zhang, C.L. A Single Factor Elicits Multilineage Reprogramming of Astrocytes in the Adult Mouse Striatum. Proc. Natl. Acad. Sci. USA 2022, 119, e2107339119. [Google Scholar] [CrossRef] [PubMed]
- Talifu, Z.; Liu, J.Y.; Pan, Y.Z.; Ke, H.; Zhang, C.J.; Xu, X.; Gao, F.; Yu, Y.; Du, L.J.; Li, J.J. In Vivo Astrocyte-to-Neuron Reprogramming for Central Nervous System Regeneration: A Narrative Review. Neural Regen. Res. 2023, 18, 750–755. [Google Scholar] [CrossRef]
- Grande, A.; Sumiyoshi, K.; López-Juárez, A.; Howard, J.; Sakthivel, B.; Aronow, B.; Campbell, K.; Nakafuku, M. Environmental Impact on Direct Neuronal Reprogramming In Vivo in the Adult Brain. Nat. Commun. 2013, 4, 2373. [Google Scholar] [CrossRef]
- Chanda, S.; Ang, C.E.; Davila, J.; Pak, C.; Mall, M.; Lee, Q.Y.; Ahlenius, H.; Jung, S.W.; Südhof, T.C.; Wernig, M. Generation of Induced Neuronal Cells by the Single Reprogramming Factor ASCL1. Stem Cell Rep. 2014, 3, 282–296. [Google Scholar] [CrossRef]
- Puls, B.; Ding, Y.; Zhang, F.; Pan, M.; Lei, Z.; Pei, Z.; Jiang, M.; Bai, Y.; Forsyth, C.; Metzger, M.; et al. Regeneration of Functional Neurons After Spinal Cord Injury via in Situ NeuroD1-Mediated Astrocyte-to-Neuron Conversion. Front. Cell Dev. Biol. 2020, 8, 591883. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.J.; Yang, F.H.; Li, W.; Wang, T.; Lin, Y.; Feng, J.; Chen, N.H.; Jiang, M.; Wang, J.H.; Hu, X.T.; et al. In Vivo Neuroregeneration to Treat Ischemic Stroke Through NeuroD1 AAV-Based Gene Therapy in Adult Non-Human Primates. Front. Cell Dev. Biol. 2020, 8, 590008. [Google Scholar] [CrossRef]
- Vasan, L.; Park, E.; David, L.A.; Fleming, T.; Schuurmans, C. Direct Neuronal Reprogramming: Bridging the Gap Between Basic Science and Clinical Application. Front. Cell Dev. Biol. 2021, 9, 681087. [Google Scholar] [CrossRef]
- Mattugini, N.; Bocchi, R.; Scheuss, V.; Russo, G.L.; Torper, O.; Lao, C.L.; Götz, M. Inducing Different Neuronal Subtypes from Astrocytes in the Injured Mouse Cerebral Cortex. Neuron 2019, 103, 1086–1095.e5. [Google Scholar] [CrossRef]
- Herrero-Navarro, Á.; Puche-Aroca, L.; Moreno-Juan, V.; Sempere-Ferràndez, A.; Espinosa, A.; Susín, R.; Torres-Masjoan, L.; Leyva-Díaz, E.; Karow, M.; Figueres-Oñate, M.; et al. Astrocytes and Neurons Share Region-Specific Transcriptional Signatures That Confer Regional Identity to Neuronal Reprogramming. Sci. Adv. 2021, 7, eabe8978. [Google Scholar] [CrossRef]
- Pfisterer, U.; Kirkeby, A.; Torper, O.; Wood, J.; Nelander, J.; Dufour, A.; Björklund, A.; Lindvall, O.; Jakobsson, J.; Parmar, M. Direct Conversion of Human Fibroblasts to Dopaminergic Neurons. Proc. Natl. Acad. Sci. USA 2011, 108, 10343–10348. [Google Scholar] [CrossRef]
- Heinrich, C.; Götz, M.; Berninger, B. Reprogramming of Postnatal Astroglia of the Mouse Neocortex into Functional, Synapse-Forming Neurons. Methods Mol. Biol. 2012, 814, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Miao, Q.; Yuan, J.; Han, S.; Zhang, P.; Li, S.; Rao, Z.; Zhao, W.; Ye, Q.; Geng, J.; et al. Ascl1 Converts Dorsal Midbrain Astrocytes into Functional Neurons In Vivo. J. Neurosci. 2015, 35, 9336–9355. [Google Scholar] [CrossRef]
- Vadodaria, K.; Mertens, J.; Paquola, A.; Bardy, C.; Li, X.; Jappelli, R.; Fung, L.; Marchetto, M.; Hamm, M.; Gorris, M.; et al. Generation of Functional Human Serotonergic Neurons from Fibroblasts. Mol. Psychiatry 2016, 21, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Serrano, C.; Zhong, X.; Ma, S.; Zou, Y.; Zhang, C.L. Revisiting Astrocyte to Neuron Conversion with Lineage Tracing In Vivo. Cell 2021, 184, 5465–5481.e16. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhou, J.; Wang, L.L.; Zhang, C.L.; Chen, B. New AAV Tools Fail to Detect Neurod1-Mediated Neuronal Conversion of Müller Glia and Astrocytes In Vivo. EBioMedicine 2023, 90, 1–19. [Google Scholar] [CrossRef]
- Xiang, Z.; Xu, L.; Liu, M.; Wang, Q.; Li, W.; Lei, W.; Chen, G. Lineage Tracing of Direct Astrocyte-to-Neuron Conversion in the Mouse Cortex. Neural Regen. Res. 2021, 16, 750–756. [Google Scholar] [CrossRef]
- Götz, M.; Bocchi, R. Neuronal Replacement: Concepts, Achievements, and Call for Caution. Curr. Opin. Neurobiol. 2021, 69, 185–192. [Google Scholar] [CrossRef]
- Barker, R.A.; Götz, M.; Parmar, M. New Approaches for Brain Repair-from Rescue to Reprogramming. Nature 2018, 557, 329–334. [Google Scholar] [CrossRef]
- Sharif, N.; Calzolari, F.; Berninger, B. Direct In Vitro Reprogramming of Astrocytes into Induced Neurons. Methods Mol. Biol. 2021, 2352, 13–29. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, L.; Wu, Z.; Chen, Y.; Wang, F.; Chen, G. In Vivo Direct Reprogramming of Reactive Glial Cells into Functional Neurons after Brain Injury and in an Alzheimer’s Disease Model. Cell Stem Cell 2014, 14, 188–202. [Google Scholar] [CrossRef]
- Rivetti di Val Cervo, P.; Romanov, R.; Spigolon, G.; Masini, D.; Martín-Montañez, E.; Toledo, E.; La Manno, G.; Feyder, M.; Pifl, C.; Ng, Y.; et al. Induction of Functional Dopamine Neurons from Human Astrocytes In Vitro and Mouse Astrocytes in a Parkinson’s Disease Model. Nat. Biotechnol. 2017, 35, 444–452. [Google Scholar] [CrossRef]
- Chen, Y.C.; Ma, N.X.; Pei, Z.F.; Wu, Z.; Do-Monte, F.H.; Keefe, S.; Yellin, E.; Chen, M.S.; Yin, J.C.; Lee, G.; et al. A NeuroD1 AAV-Based Gene Therapy for Functional Brain Repair after Ischemic Injury through In Vivo Astrocyte-to-Neuron Conversion. Mol. Ther. 2020, 28, 217–234. [Google Scholar] [CrossRef] [PubMed]
- Lapchak, P.A.; Zhang, J.H.; Noble-Haeusslein, L.J. RIGOR Guidelines: Escalating STAIR and STEPS for Effective Translational Research. Transl. Stroke Res. 2013, 4, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Roome, R.B.; Bartlett, R.F.; Jeffers, M.; Xiong, J.; Corbett, D.; Vanderluit, J.L. A Reproducible Endothelin-1 Model of Forelimb Motor Cortex Stroke in the Mouse. J. Neurosci. Methods 2014, 233, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Horie, N.; Maag, A.-L.; Hamilton, S.A.; Shichinohe, H.; Bliss, T.M.; Steinberg, G.K. Mouse Model of Focal Cerebral Ischemia Using Endothelin-1. J. Neurosci. Methods 2008, 173, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Merienne, N.; Le Douce, J.; Faivre, E.; Déglon, N.; Bonvento, G. Efficient Gene Delivery and Selective Transduction of Astrocytes in the Mammalian Brain Using Viral Vectors. Front. Cell Neurosci. 2013, 7, 54254. [Google Scholar] [CrossRef]
- Srinivas, S.; Watanabe, T.; Lin, C.S.; William, C.M.; Tanabe, Y.; Jessell, T.M.; Costantini, F. Cre Reporter Strains Produced by Targeted Insertion of EYFP and ECFP into the ROSA26 Locus. BMC Dev. Biol. 2001, 1, 4. [Google Scholar] [CrossRef]
- Tennant, K.A.; Jones, T.A. Sensorimotor Behavioral Effects of Endothelin-1 Induced Small Cortical Infarcts in C57BL/6 Mice. J. Neurosci. Methods 2009, 181, 18–26. [Google Scholar] [CrossRef]
- Murphy, T.; Corbett, D. Plasticity during Stroke Recovery: From Synapse to Behaviour. Nat. Rev. Neurosci. 2009, 10, 861–872. [Google Scholar] [CrossRef]
- Ito, M.; Aswendt, M.; Lee, A.; Ishizaka, S.; Cao, Z.; Wang, E.; Levy, S.; Smerin, D.; McNab, J.; Zeineh, M.; et al. RNA-Sequencing Analysis Revealed a Distinct Motor Cortex Transcriptome in Spontaneously Recovered Mice After Stroke. Stroke 2018, 49, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.; Wang, Y.; Kim, S.; Hong, S.; Jeng, L.; Bilgen, M.; Liu, J. Assessing Gait Impairment Following Experimental Traumatic Brain Injury in Mice. J. Neurosci. Methods 2009, 176, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Cubelos, B.; Briz, C.G.; Esteban-Ortega, G.M.; Nieto, M. Cux1 and Cux2 Selectively Target Basal and Apical Dendritic Compartments of Layer II-III Cortical Neurons. Dev. Neurobiol. 2015, 75, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Arlotta, P.; Molyneaux, B.J.; Jabaudon, D.; Yoshida, Y.; Macklis, J.D. Ctip2 Controls the Differentiation of Medium Spiny Neurons and the Establishment of the Cellular Architecture of the Striatum. J. Neurosci. 2008, 28, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Torper, O.; Pfisterer, U.; Wolf, D.A.; Pereira, M.; Lau, S.; Jakobsson, J.; Björklund, A.; Grealish, S.; Parmar, M. Generation of Induced Neurons via Direct Conversion In Vivo. Proc. Natl. Acad. Sci. USA 2013, 110, 7038–7043. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Ma, L.; Liao, Z.; Le, Q.; Yu, J.; Liu, X.; Li, H.; Chen, Y.; Zheng, P.; Yang, Z.; et al. Astroglial β-Arrestin1-Mediated Nuclear Signaling Regulates the Expansion of Neural Precursor Cells in Adult Hippocampus. Sci. Rep. 2015, 5, 15506. [Google Scholar] [CrossRef] [PubMed]
- Dromerick, A.W.; Edwardson, M.A.; Edwards, D.F.; Giannetti, M.L.; Barth, J.; Brady, K.P.; Chan, E.; Tan, M.T.; Tamboli, I.; Chia, R.; et al. Critical Periods after Stroke Study: Translating Animal Stroke Recovery Experiments into a Clinical Trial. Front. Hum. Neurosci. 2015, 9, 136450. [Google Scholar] [CrossRef]
- De Boer, A.; Storm, A.; Gomez-Soler, M.; Smolders, S.; Rué, L.; Poppe, L.; B Pasquale, E.; Robberecht, W.; Lemmens, R. Environmental Enrichment during the Chronic Phase after Experimental Stroke Promotes Functional Recovery without Synergistic Effects of EphA4 Targeted Therapy. Hum. Mol. Genet. 2020, 29, 605–617. [Google Scholar] [CrossRef]
- Lee, Y.; Messing, A.; Su, M.; Brenner, M. GFAP Promoter Elements Required for Region-Specific and Astrocyte-Specific Expression. Glia 2008, 56, 481–493. [Google Scholar] [CrossRef]
- Irie, T.; Matsuda, T.; Hayashi, Y.; Matsuda-Ito, K.; Kamiya, A.; Masuda, T.; Prinz, M.; Isobe, N.; Kira, J.I.; Nakashima, K. Direct Neuronal Conversion of Microglia/Macrophages Reinstates Neurological Function after Stroke. Proc. Natl. Acad. Sci. USA 2023, 120, e2307972120. [Google Scholar] [CrossRef]
- Bocchi, R.; Masserdotti, G.; Götz, M. Direct Neuronal Reprogramming: Fast Forward from New Concepts toward Therapeutic Approaches. Neuron 2022, 110, 366–393. [Google Scholar] [CrossRef]
- Rao, Y.; Du, S.; Yang, B.; Wang, Y.; Li, Y.; Li, R.; Zhou, T.; Du, X.; He, Y.; Wang, Y.; et al. NeuroD1 Induces Microglial Apoptosis and Cannot Induce Microglia-to-Neuron Cross-Lineage Reprogramming. Neuron 2021, 109, 4094–4108.e5. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, J.; Xu, L.; Foo, L.; Nouri, N.; Zhou, L.; Giffard, R.; Barres, B. Genomic Analysis of Reactive Astrogliosis. J. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef]
- Liddelow, S.; Guttenplan, K.; Clarke, L.; Bennett, F.; Bohlen, C.; Schirmer, L.; Bennett, M.; Münch, A.; Chung, W.; Peterson, T.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Kam, T.; Panicker, N.; Kim, S.; Oh, Y.; Park, J.; Kwon, S.; Park, Y.; Karuppagounder, S.; Park, H.; et al. Block of A1 Astrocyte Conversion by Microglia Is Neuroprotective in Models of Parkinson’s Disease. Nat. Med. 2018, 24, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lei, Z.; Guo, Z.; Pei, Z.; Chen, Y.; Zhang, F.; Cai, A.; Mok, G.; Lee, G.; Swaminathan, V.; et al. Development of Neuroregenerative Gene Therapy to Reverse Glial Scar Tissue Back to Neuron-Enriched Tissue. Front. Cell Neurosci. 2020, 1–19. [Google Scholar] [CrossRef]
- Chen, G.; Wernig, M.; Berninger, B.; Nakafuku, M.; Parmar, M.; Zhang, C.L. In Vivo Reprogramming for Brain and Spinal Cord Repair. eNeuro 2015, 2. [Google Scholar] [CrossRef]
- Lois, C.; Alvarez-Buylla, A. Long-Distance Neuronal Migration in the Adult Mammalian Brain. Science 1994, 264, 1145–1148. [Google Scholar] [CrossRef]
- Doetsch, F.; García-Verdugo, J.M.; Alvarez-Buylla, A. Regeneration of a Germinal Layer in the Adult Mammalian Brain. Proc. Natl. Acad. Sci. USA 1999, 96, 11619–11624. [Google Scholar] [CrossRef]
- Imura, T.; Kornblum, H.; Sofroniew, M. The Predominant Neural Stem Cell Isolated from Postnatal and Adult Forebrain but Not Early Embryonic Forebrain Expresses GFAP. J. Neurosci. 2003, 23, 2824–2832. [Google Scholar] [CrossRef]
- Morshead, C.; Garcia, A.; Sofroniew, M.; van Der Kooy, D. The Ablation of Glial Fibrillary Acidic Protein-Positive Cells from the Adult Central Nervous System Results in the Loss of Forebrain Neural Stem Cells but Not Retinal Stem Cells. Eur. J. Neurosci. 2003, 18, 76–84. [Google Scholar] [CrossRef]
- Faiz, M.; Sachewsky, N.; Gascón, S.; Bang, K.; Morshead, C.; Nagy, A. Adult Neural Stem Cells from the Subventricular Zone Give Rise to Reactive Astrocytes in the Cortex after Stroke. Cell Stem Cell 2015, 17, 624–634. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Livingston, J.M.; Lee, T.T.; Enbar, T.; Daniele, E.; Phillips, C.M.; Krassikova, A.; Bang, K.W.A.; Kortebi, I.; Donville, B.W.; Ibragimov, O.S.; et al. Ectopic Expression of Neurod1 Is Sufficient for Functional Recovery following a Sensory–Motor Cortical Stroke. Biomedicines 2024, 12, 663. https://doi.org/10.3390/biomedicines12030663
Livingston JM, Lee TT, Enbar T, Daniele E, Phillips CM, Krassikova A, Bang KWA, Kortebi I, Donville BW, Ibragimov OS, et al. Ectopic Expression of Neurod1 Is Sufficient for Functional Recovery following a Sensory–Motor Cortical Stroke. Biomedicines. 2024; 12(3):663. https://doi.org/10.3390/biomedicines12030663
Chicago/Turabian StyleLivingston, Jessica M., Tina T. Lee, Tom Enbar, Emerson Daniele, Clara M. Phillips, Alexandra Krassikova, K. W. Annie Bang, Ines Kortebi, Brennan W. Donville, Omadyor S. Ibragimov, and et al. 2024. "Ectopic Expression of Neurod1 Is Sufficient for Functional Recovery following a Sensory–Motor Cortical Stroke" Biomedicines 12, no. 3: 663. https://doi.org/10.3390/biomedicines12030663
APA StyleLivingston, J. M., Lee, T. T., Enbar, T., Daniele, E., Phillips, C. M., Krassikova, A., Bang, K. W. A., Kortebi, I., Donville, B. W., Ibragimov, O. S., Sachewsky, N., Lozano Casasbuenas, D., Olfat, A., & Morshead, C. M. (2024). Ectopic Expression of Neurod1 Is Sufficient for Functional Recovery following a Sensory–Motor Cortical Stroke. Biomedicines, 12(3), 663. https://doi.org/10.3390/biomedicines12030663





