Abstract
Osteonecrosis of the femoral head (ONFH) is a disabling disease characterized by the disruption of the blood supply to the femoral head, leading to the apoptosis and necrosis of bone cells and subsequent joint collapse. Total hip arthroplasty is not optimal since most patients are young. Multiple risk factors contribute to osteonecrosis, including glucocorticoid (GC) usage, excessive alcohol intake, hypercholesterolemia, and smoking. Continuous stimulation by many variables causes a chronic inflammatory milieu, with clinical repercussions including endothelial dysfunction, leading to thrombosis, coagulopathy, and poor angiogenesis. Immune cells are the primary regulators of inflammation. Innate and adaptive immune cells interact with endothelial cells to hinder the regeneration and repair of bone lesions. An in-depth examination of the pathological drivers of ONFH reveals that endothelial dysfunction may be a major cause of osteonecrosis. Understanding the involvement of endothelial dysfunction in the chronic inflammation of osteonecrosis could aid in the development of possible therapies. This review summarizes the role of endothelial cells in osteonecrosis and further explains the pathophysiological mechanism of endothelial dysfunction in this disease from the perspective of inflammation to provide new ideas for the treatment of osteonecrosis.
1. Introduction
Osteonecrosis of the femoral head is a debilitating illness caused by the gradual elimination of bone cells and bone marrow caused by insufficient blood flow to the injured subchondral bone [1]. There are approximately 20,000–30,000 new cases of osteonecrosis in the United States every year and approximately 150,000 cases in China [2,3]. Numerous risk factors, such as the use of glucocorticoids and excessive alcohol consumption, as well as certain disease states, like sickle cell disease and systemic lupus erythematosus, and treatment approaches, like radiation, chemotherapy, and hip surgery, can cause osteonecrosis to progress [4,5,6,7]. Hip preservation surgery (such as core decompression, bone grafting, and osteotomy) and a combination of medications (such as anticoagulants, fibrinolysis-enhancing drugs, vasoactive agents, and lipid-lowering therapies) can be utilized to treat early ONFH [8,9,10,11]. However, more than 80% of patients ultimately require total joint arthroplasty (THA) [12]. Although THA considerably improves patients’ lifestyles, it is not regarded as the best treatment for ONFH [13]. Consequently, it is critical to comprehend the mechanism underlying femoral head necrosis and ascertain its etiology.
Although there are many factors involved in ONFH, such as stem cell differentiation imbalance, bone remodeling dysfunction, and increased intramedullary pressure, its pathogenesis remains unclear [14]. Among these pathologies, endothelial dysfunction appears to be the most convincing [15]. Endothelial dysfunction is commonly defined as any alternations affecting the endothelium’s capacity to maintain vascular homeostasis. Subsequent alternations in hemodynamics and decreased vasodilation capability contribute to insufficient blood flow and oxygen starvation, causing ischemic injury and skeletal lesions in the femoral head, which worsen the disease by triggering a proinflammatory reaction [16]. Immune cells are attracted to the impacted region by various inflammatory mediators and chemokines, where they continuously generate harmful proteases, resulting in an excess of reactive oxygen species and greater injury to the endothelium [17]. Furthermore, endothelial dysfunction drives coagulation abnormalities in injured circulatory systems, promotes hypofibrinolysis, and impedes the clearance of metabolic wastes and the delivery of essential nutrients, which thus restrict endothelial repair and revascularization [18,19].
This review focuses primarily on the mechanisms involved in endothelial dysfunction in the pathological process of femoral head necrosis. Decreased angiogenesis, coagulation abnormalities, and chronic inflammation are all significant etiological contributors. There may be significant therapeutic promise in encouraging angiogenesis or focusing on endothelial cell repair.
2. Endothelial Cells Orchestrate Angiogenesis–Osteogenesis Coupling
Endothelial cells primarily line the inner surfaces of bone microvessels. A variety of physiological processes, including the blood vessel–tissue barrier, hemofiltration, the maintenance of vasomotor rhythm, the regulation of nutrient transport, and the coordination of immune effects, are involved [20,21]. Their role as a highly active metabolic and endocrine organ that secretes a range of molecules to maintain balance has come to light in recent years [22]. Vascular endothelial cells are critical for skeletal development because they coordinate multiple mechanisms. The blood vessels provide the instructional vasculature niche required for bone regeneration by serving as frameworks for cells that produce bone and matrix mineralization [23]. Endothelial cells are triggered in response to various extracellular stimuli, which help to sustain osteoprogenitor cells in the bone marrow by secreting chemicals in an endocrine way. Angiocrine mediators, along with angiokines, are substances generated by the diverse vasculature network of bones. They specifically target osteoprogenitors and osteolineage cells within the actively renewing callus. (Table 1). For example, the activation of MAPK/ERK, SMAD, Wnt/β-catenin, and cAMP signaling is facilitated by the secretion of exosomes and cytokines derived from endothelial cells, which foster the formation of osteoblasts and chondrocytes [24,25,26,27,28,29]. By modulating histone lactylation, endothelial cell-derived metabolites like lactate stimulate the osteogenic differentiation of mesenchymal stem cells [30]. This endothelial cell drives the bone remodeling process and boosts the maturation of osteoprogenitors, thereby helping to orchestrate bone–vascular interactions [31].

Table 1.
Vascular endothelial cell-derived factors orchestrate bone homeostasis.
Based on their functional characteristics and temporal arrangement, endothelial cells in mammalian bones are divided into three main types: type H, type L, and type E. Type-H endothelial cells, which highly express CD31 and endomucin (EMCN), are mostly found in the metaphyseal region, where bone metabolism is active, and they are surrounded by osteoprogenitor cells that help the bone formation process [35]. Type-L endothelial cells, which have low expression of CD31 and EMCN, are located mainly in the diaphysis and mediate the hematopoietic process [23]. However, type-E endothelial cells exist mainly transiently in the late embryonic and early postnatal development stages to promote bone formation. These cells behave similarly to type-H blood vessels, but with the stronger expression of CD31 and the weaker expression of EMCN [36]. Localizing to regions with active bone metabolism, type-H endothelial cells are encircled by osteoprogenitors expressing runx2+, collagen 1α+, and osterix, in addition to perivascular cells expressing platelet-derived growth factor receptor-β (PDGFR-β) and neuronglial antigen 2 (NG2) [31]. These cells exhibit a strong positive correlation with osteolineage cells and synergistically promote the osteogenic process.
As precursors of endothelial cells, endothelial progenitor cells (EPCs) are derived mainly from peripheral blood and bone marrow [37]. They play a pivotal role in disorders related to endothelial damage by proliferating, migrating, and differentiating into endothelial cells, in addition to secreting a range of cytokines and vascular growth factors [38]. Generally, EPCs are separated, based on their morphological traits and culture duration, into two categories: early myeloid angiogenic cells and late endothelial colony-forming cells [39]. The former mainly express cell surface markers, including CD45, CD31, and CD14, and secrete a large amount of cytokines but have a weak ability to proliferate; the latter mainly express cell surface markers, including CD31, CD105, CD146, VE-cadherin, and von Willebrand factor, and have a relatively strong ability to proliferate and differentiate into mature endothelial cells [40]. Damaged EPCs are often associated with adverse cardiovascular outcomes. Several studies have revealed that patients with GC-induced osteonecrosis of the femoral head (GIONFH) had a decrease in the number and function of circulating EPCs, as indicated by increased injury migration ability, reduced angiogenesis, and increased senescence [41,42,43]. Local hypoxia after ischemia stimulates the HIF-1α/VEGF pathway, which promotes the migration of bone marrow-derived EPCs and induces endothelial cell proliferation, migration, and differentiation [44]. Therefore, it is vital to research the causes and effects of aberrant angiogenesis or endothelial damage during ischemia, as well as the risk of postischemic osteonecrosis.
3. Endothelial Dysfunction Promotes Coagulopathy
The pathological characteristic of osteonecrosis caused by vascular ischemia is significantly impacted by coagulopathy [45]. Inadequate fibrinolysis and excessive thrombophilia are the primary causes of this. Both acquired and hereditary thrombophilia/hypofibrinolysis can result in osteonecrosis of the femoral head and jaw at all ages [46,47]. Extreme thrombophilia, also known as hypercoagulability, is a coagulation disorder in which a great number of blood clots form on the walls of circulating blood vessels. Fibrinolysis is the decomposition of a thrombosis or clot, and it is tightly controlled by tissue factor plasminogen inhibitor (TFPI), plasminogen activators, plasminogen activator inhibitor-1 (PAI-1), and plasmin [48]. A healthy endothelium constitutes an anticoagulant and antithrombotic protective layer and secretes an abundance of substances that directly regulate platelet activity, including nitric oxide (NO), prostaglandins, and ectonucleoside triphosphate diphosphate hydrolase-1 (E-NTPDase1) [49,50]. Endothelial cells express TFPI, which limits the effects of tissue factor (TF) and inhibits the TF-mediated overactivation of clotting factors VII and X [51]. The surfaces of endothelial cells bind to antithrombin, which effectively inhibits platelet activity. Endothelial cells generate thrombomodulin (TM) which binds to thrombin, inducing an alteration in structure that increases its affinity for protein C/S and inhibits clotting factors Va and VIIIa [52]. In addition, endothelial-derived tissue plasminogen activator (t-PA) and urokinase-type plasminogen activator (u-PA) can interact with PAI-1 to switch plasminogen into plasmin, thereby facilitating the degradation of fibrin [53].
In addition to releasing molecules that regulate coagulation and fibrinolysis, endothelial cells have autocrine and paracrine functions which produce vasoactive substances, such as NO, adenosine diphosphate (ADP), platelet-activating factor (PAF), endothelin 1 (ET-1), thrombin, and thromboxane A2 (TXA2) [54]. These substances are secreted to control vasoconstriction and platelet activation during clotting circumstances. After 24 h of GC administration, Lu et al. found that ET-1 receptor and PAI-1 expression increased dramatically, whereas NO, PGI2 synthase, PGE synthase, the PGE receptor, and ET-1 expression dropped markedly [55]. Therefore, vasoconstriction and thrombosis are promoted after GC-induced damage to endothelial cells. Reduced vasodilator release and increased vasoconstrictor production are key features of endothelial dysfunction, which is a major factor in coagulopathy and the development of ONFH.
Following vascular injury, endothelial cells are involved in all significant hemostatic processes and confine thrombosis to particular regions where it is necessary to restore vascular integrity. Subendothelial collagen is exposed to circulating platelets and forms junctional complexes. Endothelial- and platelet-derived von Willebrand factor (vWF) further strengthens this connection and promotes platelet activation and aggregation [56]. Patients with primary ONFH exhibit lupus anticoagulants, anticardiolipin antibodies, the factor V Leiden mutation, the prothrombin gene G20210A mutation, the methylenetetrahydrofolate reductase (MTHFR) C677T gene polymorphism, and P1A1/A2 polymorphism in glycoprotein IIIa, along with decreased concentrations of proteins C/S and anti-thrombin III in comparison to healthy individuals [45,57,58,59,60,61]. These aberrant alterations can lead to coagulation disorders in osteonecrosis of the femoral head following one flaw or a combination of multiple flaws. Endothelial dysfunction and injury resulting from many risk factors, including oxidative stress, endoplasmic reticulum stress, apoptosis, and necrosis, work in concert with coagulation abnormalities to exacerbate osteonecrosis in secondary ONFH [45,62]. By augmenting PAI-1 levels and diminishing tPA levels, GCs provoke an increase in coagulation and reduce fibrinolytic activity [63,64]. In patients and animal models of GIONFH, a great deal of fibrinogen and lipoprotein imply a hypercoagulable environment at the site of bone lesions [65,66]. A great deal of fibrinogen and lipoprotein results in activated platelets and retarded thrombolysis, further supporting the notion that thrombophilia and low fibrinolysis are closely associated with disease progression in osteonecrosis [67]. Thrombosis development increases intraosseous venous pressure and decreases arterial blood flow, resulting in hypoxia-induced ischemic damage in the bone vasculature. Immune activation and inflammatory responses simultaneously act on the endothelium to address the ensuing damage and orchestrate the healing process (Figure 1).
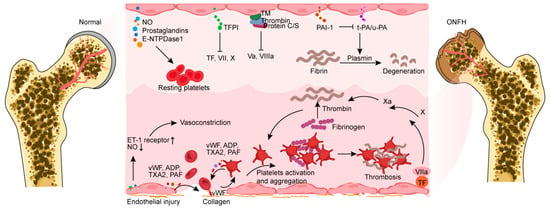
Figure 1.
Endothelial cells share several molecules that target platelets or coagulation factors, forming an anticoagulant and antithrombotic barrier surface. Vasoconstriction occurs because of endothelial injury exposing subendothelial collagen and an imbalance of vasoactive chemicals. Collagen and platelets combine to form a connection complex with endothelial-derived vWF, which facilitates platelet activation. The factors (vWF, ADP, TXA2, and PAF) produced from endothelial cells exhibit the capacity to directly activate platelets and promote their aggregation. Activated platelets can then produce these compounds to aid in the coagulation process. Furthermore, endothelial injury exposes and binds tissue factor, which combines with factor VIIa to promote fibrin formation. NO, nitric oxide; E-NTPDase1, ectonucleoside triphosphate diphosphate hydrolase-1; TFPI, tissue factor plasminogen inhibitor; TF, tissue factor; TM, thrombomodulin; PAI-1, plasminogen activator inhibitor-1; t-PA, tissue plasminogen activator; u-PA, urokinase-type plasminogen activator; ET-1, endothelin 1; vWF, von Willebrand factor; ADP, adenosine diphosphate; TXA2, thromboxane A2; PAF, platelet-activating factor; ONFH, osteonecrosis of the femoral head.
4. Endothelial Injury and Inflammation
4.1. Endothelial Injury Promotes Inflammatory Activation
The vascular endothelium actively participates in maintaining vascular homeostasis by balancing vasoconstrictor and vasodilator substances, anticoagulant and procoagulant substances, inflammatory and anti-inflammatory molecules, oxidants and antioxidants, and profibrinolytic and antifibrinolytic substances [68]. Endothelial dysfunction occurs as a reaction to different biochemical and physiological triggers, such as abnormal hemodynamic forces, excessive intramedullary compression, oxidative stress, intracellular injury, hyperlipidemia, harmful chemicals, and bacterial and viral infections [69,70,71]. Tiny crystals of cholesterol, monocytes, and lymphocytes reach the blood vessels and provoke an inflammation reaction that aids in the emergence of fatty streaks, leading to plaque deposition, progression, and collapse [72]. Plaque collapse promotes thrombosis, which, in conjunction with the blood clotting process, leads to atherosclerosis and vascular ischemia. Endothelial dysfunction is identified as both an evaluation and a predictive indicator for the advancement of atherosclerotic plaques throughout their stages of beginning and progressing, with the most adverse result being plaque collapse [73].
Multiple risks contribute to the production of reactive oxygen species (ROS) in the lining of vessels, such as glucocorticoids, drinking alcohol, hypercholesterolemia, diabetes, and hypertension [74,75,76]. Endothelium activity is impeded by ROS as they increase oxidative stress [77]. ROS accumulation in blood vessels leads to the production of harmful peroxynitrate and dysfunctional superoxide, disrupting the paracrine regulation of circulatory tone, antithrombosis, and vascular smooth muscle proliferation in endothelial cells [78]. Consequently, endothelial dysfunction brought on by oxidative stress propagates vasospasms, atherosclerosis, and vessel inflammation [79].
Inflammatory activation is crucial in initiating vasculopathy, which deteriorates due to both the synergistic effect on inflammation and endothelial injury [80]. Endothelial cells react to injury by releasing pro-inflammatory molecules, including interleukin 1β (IL-1β), interferons, tumor necrosis factor α (TNF-α), colony-stimulating factors, and vascular adhesion molecule 1 (VCAM-1) [81]. These molecules motivate monocytes and neutrophils to migrate to the activated endothelium surface and further promote inflammation, resulting in acute and chronic alternations in vascular permeability and vascular swelling [82]. Endothelial injury coincides with inflammation, revealing a hazardous process, including the mobilization of monocyte/macrophage cells through the circulatory system to the lesion, microcholesterol crystal translocation, lipid-containing cell blebbing, and cytokine/chemokine production [83]. These strongly promote the development of the plaque skeleton, leading to the rupture of structurally unstable plaques, the release of large amounts of thrombogenic substances into the lumen, and the triggering of atherosclerotic obstruction [84]. Even when the plaque is stable, endothelial apoptosis caused by plaque surface lesions can promote thrombosis [85].
4.2. Continuous Activation of Inflammation Hinders the Repair of Osteonecrosis
The initial goal of inflammation is to eliminate unwanted stimuli or infections and facilitate tissue healing. The inflammatory response facilitates the recruitment of factors that remove necrotic bone tissue and intramedullary tissue [86]. These proinflammatory factors derived from damaged blood vessels recruit macrophages, neutrophils, and other immune cells to remove harmful stimuli and relieve inflammation [87,88]. Normally, the resolution of inflammation facilitates tissue regeneration. However, in osteonecrosis, endothelial dysfunction coordinates inflammation and stimulates local immune cells to secrete inflammatory factors, inducing continued inflammation and hindering bone repair [89]. Vascular debris formed by a damaged endothelium and unstable plaques drives macrophages and neutrophils to secrete inflammatory factors, promoting the formation of immune thrombosis [90]. The injured endothelium, activated platelet-derived factors, and disrupted extracellular matrix serve as sources of damage-associated molecular patterns (DAMPs) [91]. Various immune cells, such as phagocytes, antigen-presenting cells, monocytes, and neutrophils, sense these stimuli through recognition receptors (PRRs) and further activate the inflammatory cascade and promote immune thrombosis, which in turn further destroys the injured endothelium [92].
4.2.1. Neutrophils and NETs Induce a Procoagulant Phenotype in the Endothelium
As the most plentiful white blood cells in the blood, neutrophils play an essential role in innate immunity [93]. Neutrophils typically have the lowest lifespan among white blood cells, which is critical for avoiding continued inflammation and tissue healing in patients with osteonecrosis [94]. A considerable portion of neutrophils depart from the bone marrow during inflammation. Accompanying this process are the secretion of inflammatory cytokines and molecules, the synthesis of reactive oxygen species, and the release of proteolytic enzymes [95]. Moreover, proinflammatory substances and other enzymes found in neutrophil extracellular traps (NETs), which are released by active neutrophils, cause endothelial dysfunction and apoptosis [90]. The externalization of extracellular matrix proteases during NET formation causes endothelial injury and glycocalyx shedding, which exposes adhesion receptors and TF, thereby stimulating platelet and leukocyte adhesion and further inflammation [95]. The ROS-generating enzymes NO synthase (NOS) and oxidized high-density lipoprotein are also expressed at high levels in NETs, where they reduce their ability to efflux cholesterol and prevent foam cell formation, which fosters plaque formation and coagulopathy [96]. Engelmann et al. evaluated the mechanisms of NET-mediated thrombosis and used the term “immunothrombosis” to describe this process [97]. By producing a cascade of favorable feedback that enhances thrombosis both in vivo and ex vivo, the scaffold formed by DNA fibers and histone networks in NETs attracts platelets, leukocytes, red blood cells, and plasma proteins [98]. Inflammation in injured endothelial cells results in a reaction secretion (e.g., adhesion molecules and pro-inflammatory factors) during the acute phase, which disseminates to the circulation [99]. Through interactions with vWF, extracellular DNA in NETs can intensify thrombosis and inflammatory processes [100]. NETs stimulate the intrinsic coagulation process and facilitate the connection among neutrophils with factor XII (FXII). TF expression and activity in endothelial cells can be increased by NETs, expanding the extrinsic coagulation pathway [101]. Moreover, endothelial-derived anticoagulants such as antithrombin (AT), activated protein C (APC), and TF pathway inhibitors are inhibited by NETs, which leads to thrombosis [102]. In patients with ONFH, the femoral head microvessels contain a considerable number of neutrophils and NETs [103]. Osteonecrosis was further promoted by the intravenous injection of neutrophils capable of forming NETs in rats [104]. Therefore, the efficient removal of activated neutrophils is a potential target for the treatment of osteonecrosis (Figure 2).
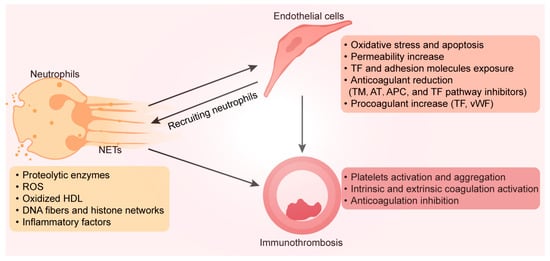
Figure 2.
Neutrophils stimulated by bone lesion sites can produce NETs, consisting of a mesh-like structure made of DNA and chromatin, filled with various bioactive compounds (such as proteolytic enzymes, ROS, oxidized HDL, and inflammatory factors). These substances stimulate endothelial cells to exhibit a procoagulant phenotype. Adhesion molecules and tissue factors on endothelial cell surfaces recruit more immune cells, intensify inflammation, and enhance platelet activation and aggregation. This NET-mediated activation of the coagulation pathway and anticoagulation inhibition is called immunothrombosis. NETs, neutrophil extracellular traps; ROS, reactive oxygen species; HDL, high-density lipoprotein; DNA, deoxyribonucleic acid; TF, tissue factor; TM, thrombomodulin; AT, antithrombin; APC, activated protein C; vWF, von Willebrand factor.
4.2.2. Endothelial Cell Junctions Coordinate Macrophage Activation
Macrophages play a crucial part in the inflammatory process by providing growth factors, cytokines, chemokines, and proteolytic enzymes. They also help combat infection, minimize tissue damage, and promote and support tissue remodeling [105]. Under healthy conditions, endothelial cells produce a range of anti-inflammatory mediators to prevent immune cell attachment to endothelial cells and vascular leakage [106]. Various adhesion molecules are released by endothelial cells in response to diverse stimuli in order to recruit monocytes to migrate towards the site of the lesion. This robust adherence results in polarization, which refers to alterations in morphology and phenotype [107]. Liu et al. reported that endothelial-derived extracellular vesicles promote the M1 polarization of macrophages and persistent inflammation by activating the TLR4/NF-κB signaling pathway, which may be a prerequisite for macrophage-induced chronic inflammation in GIONFH [108]. Endothelial cell adherent junctions are compromised, and endothelial cell connections become unstable when macrophages residing in bone lesions are stimulated by persistent inflammation associated with osteonecrosis to secrete inflammatory substances that disrupt these junctions [109,110]. The compromise of endothelial barrier function caused by this instability gives rise to the unregulated migration of monocytes and macrophages, as well as vascular leakage. Along with vascular abnormalities, endothelial cell conjunction defects can also cause vessel fragility and rupture, as well as the emergence of bleeding and edema [111]. Angioedema, cancer, diabetic microangiopathy, and allergic reactions are vascular conditions where a common characteristic is a breakdown of the barrier [112]. Endothelial connections facilitate attachment and initiate intracellular signals that determine cell location, limit development, and promote apoptosis [111]. They are crucial for maintaining vascular integrity and homeostasis.
4.2.3. Adaptive Immune Cells
Adaptive immune dysfunction plays an important role in osteonecrosis, but its effect on blood vessels has rarely been investigated. Lymphocytes, particularly T cells, have an essential role in atherosclerosis, suggesting that they may be involved in vascular damage in osteonecrosis [113]. Different T-cell types in the area of bone lesions modulate the inflammatory environment and endothelial homeostasis. For instance, mice with osteonecrosis showed a large decrease in the amount of circulating Treg cells, which can dramatically lower plaque inflammation; however, T-helper (Th) cells and cytotoxic T lymphocytes (CTLs) accelerated the development of osteonecrosis [114,115,116]. Regulatory T cells (Tregs) release many anti-inflammatory substances, including IL-4, IL-10, and TGF-β, which can relieve inflammation in bone lesion areas [117]. Osteonecrosis of the jaw and ONFH are associated with a large increase in circulating Th17 cells, which produce IL-17 to sustain persistent inflammation [115]. IL-17 may cause atheroma development and blood vessel injury by recruiting neutrophils. CTLs can cause endothelial toxicity or damage through the production of cytokines.
Abnormal B-cell function and quantitative changes are also associated with vascular deterioration in patients with osteonecrosis [116]. B cells play both protective and pathogenic roles in plaque formation [118]. Low-density lipoprotein (LDL)-neutralizing and oxidized LDL-neutralizing antibodies (mainly natural IgM antibodies) protect against atherosclerosis, while other antibodies (mostly IgGs) enhance the inflammatory response to LDLs via macrophages [119]. Anticardiolipin antibodies are present in 30–40% of nontrauma ONFH patients, which partially explains the abnormal B-cell function associated with vascular thrombosis and osteonecrosis through damage to endothelial cells [120,121]. Autoantibodies promote the formation of neutrophil NETs [95]. B cells exhibit improved antigen presentation, and Th cell-secreted cytokines, including interferon-γ, further induce B cells to generate autoantibodies and induce endothelium injury in ONFH [122]. Regulatory B cells (Bregs) restrict immune cells from expanding, especially T lymphocytes, by producing IL-10, IL-35, and TGF-β [123]. More development in favorable B-cell types may be advantageous for osteonecrosis treatment, yet this is a challenge that has to be explored further.
5. Conclusion and Future Perspectives: Targeting the Endothelium to Treat Osteonecrosis
Currently, the vast majority of patients with ONFH ultimately require total hip arthroplasty surgery. Joint preservation is still a better option than joint replacement for younger patients, even if total hip arthroplasty can greatly enhance a patient’s quality of life due to numerous problems, including hip dislocation, periprosthetic fractures, and prosthetic loosening [124]. There are many treatments for early ONFH, such as core decompression, extracorporeal shock wave therapy, vascularized bone transplantation, and stem cell therapy combined with vascularized bone transplantation [125,126,127]. These treatment strategies can significantly reduce the volume of bone lesions, delay the progression of osteonecrosis, and relieve pain, but they cannot completely cure osteonecrosis. Therefore, it is crucial to improve microcirculation disorders of the femoral head as early as possible and restore the blood supply. This study summarizes recent endothelial-targeted treatments for osteonecrosis, including alleviating endothelial dysfunction, improving coagulopathy, alleviating inflammation, and promoting angiogenesis (Table 2).

Table 2.
Targeting endothelial cells to treat osteonecrosis.
Type-H vascular endothelial cells couple the osteogenic and angiogenic processes in bone development, regeneration, and repair. The description of type-H vessels provides a deeper comprehension of the molecular and biological mechanisms behind the communication between endothelial cells and osteolineage cells. Bone loss diseases such as osteoporosis, fractures, and osteonecrosis are closely related to type-H vascular variations. Numerous treatment approaches aimed at type-H vessels have demonstrated noteworthy therapeutic outcomes in certain animal models of bone disease. Injecting recombinant PDGF-BB, for instance, targets type-H angiogenesis, hence promoting fracture healing and relieving osteoporosis. The pathophysiology of these disorders may vary in humans and animal models, as these findings have mostly focused on mouse disease models to date. More human ONFH specimens and animal models are required to elucidate the distribution of H-subtype blood vessels in the necrotic, sclerotic, and normal portions of ONFH because of the insidious character of early ONFH.
Early and consistent anticoagulant therapy for primary ONFH can delay osteonecrosis from progressing, lessen or eliminate discomfort, and enable the patient to resume full activity and range of motion. Nearly all patients experienced symptom relief, full function was restored, and femoral head collapse was avoided. Anticoagulation therapy can protect joints from progressive osteonecrosis, thereby reducing the need for total hip replacement. Although a prospective study noted that anticoagulation therapy may have little effect on patients with secondary osteonecrosis, anticoagulation therapy can still significantly alleviate GIONFH in some animal models.
Necrotic bone undermines local immune function, causes unchecked inflammation, produces a chronic inflammatory milieu, and impedes bone regeneration and repair along the course of osteonecrosis. At present, the important regulatory role of immune cells is mainly reflected in the abnormal repair process initiated by the femoral head after osteonecrosis occurs. It cannot be ruled out that immunological abnormalities and chronic inflammation mediated by endothelial dysfunction exist prior to the development of bone lesions, as endothelial dysfunction plays a significant role in the early stages of this disease. The structural deterioration or even collapse of the femoral head can result from abnormal healing brought on by chronic inflammation, which interferes with bone regeneration and repair. Thus, creating medications that are unique to the vasculature and targeting the endothelium are crucial for prospective osteonecrosis treatments.
Author Contributions
Conceptualization, W.S.; investigation, W.S. and X.L.; data curation, P.W., B.W. and S.G.; figures, W.S. and P.W.; writing—original draft preparation, W.S. and P.W.; writing—review and editing, Y.F.; visualization, Y.F.; supervision, Y.F.; funding acquisition, B.W., X.L. and Y.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (82102627, 82270935, 81974337), the Hubei Provincial Natural Science Foundation of China (2021CFB095), the Wuhan Knowledge Innovation Project (2022020801020468), and the Project of Scientific Research Plan of Wuhan Municipal Health Commission (WX21Q19).
Conflicts of Interest
The authors declare no competing interests.
References
- Chen, C.Y.; Rao, S.S.; Yue, T.; Tan, Y.J.; Yin, H.; Chen, L.J.; Luo, M.J.; Wang, Z.; Wang, Y.Y.; Hong, C.G.; et al. Glucocorticoid-induced loss of beneficial gut bacterial extracellular vesicles is associated with the pathogenesis of osteonecrosis. Sci. Adv. 2022, 8, eabg8335. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.W.; Yu, M.; Hu, K.; Wang, W.; Yang, L.; Wang, B.J.; Gao, X.H.; Guo, Y.M.; Xu, Y.Q.; Wei, Y.S.; et al. Prevalence of Nontraumatic Osteonecrosis of the Femoral Head and its Associated Risk Factors in the Chinese Population: Results from a Nationally Representative Survey. Chin. Med. J. 2015, 128, 2843–2850. [Google Scholar] [CrossRef] [PubMed]
- Petrigliano, F.A.; Lieberman, J.R. Osteonecrosis of the hip: Novel approaches to evaluation and treatment. Clin. Orthop. Relat. Res. 2007, 465, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Chen, H.; Kaufman, M.; Sverdlov, I.; Stein, E.M.; Park-Min, K.H. Glucocorticoid-induced osteonecrosis in systemic lupus erythematosus patients. Clin. Transl. Med. 2021, 11, e526. [Google Scholar] [CrossRef] [PubMed]
- Hines, J.T.; Jo, W.L.; Cui, Q.; Mont, M.A.; Koo, K.H.; Cheng, E.Y.; Goodman, S.B.; Ha, Y.C.; Hernigou, P.; Jones, L.C.; et al. Osteonecrosis of the Femoral Head: An Updated Review of ARCO on Pathogenesis, Staging and Treatment. J. Korean Med. Sci. 2021, 36, e177. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Jin, Y.; Chu, Y.; Wu, H.; Li, X.; Deng, Z.; Feng, S.; Chen, N.; Luo, Z.; Zheng, X.; et al. Single-cell transcriptome analysis reveals aberrant stromal cells and heterogeneous endothelial cells in alcohol-induced osteonecrosis of the femoral head. Commun. Biol. 2022, 5, 324. [Google Scholar] [CrossRef] [PubMed]
- Çolak, S.; Erdil, A.; Gevrek, F. Effects of systemic Anatolian propolis administration on a rat-irradiated osteoradionecrosis model. J. Appl. Oral Sci. 2023, 31, e20230231. [Google Scholar] [CrossRef]
- Glueck, C.J.; Freiberg, R.A.; Wang, P. Long-term Anticoagulation Prevents Progression of Stages I and II Primary Osteonecrosis of the Hip in Patients with Familial Thrombophilia. Orthopedics 2020, 43, e208–e214. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, P.; Yuan, B.; Li, L.; Bao, S. Pravastatin Protects against Avascular Necrosis of Femoral Head via Autophagy. Front. Physiol. 2018, 9, 307. [Google Scholar] [CrossRef]
- Wang, X.; Hu, L.; Wei, B.; Wang, J.; Hou, D.; Deng, X. Regenerative therapies for femoral head necrosis in the past two decades: A systematic review and network meta-analysis. Stem Cell Res. Ther. 2024, 15, 21. [Google Scholar] [CrossRef]
- Glueck, C.J.; Freiberg, R.A.; Wang, P. Medical treatment of osteonecrosis of the knee associated with thrombophilia-hypofibrinolysis. Orthopedics 2014, 37, e911–e916. [Google Scholar] [CrossRef]
- Johnson, A.J.; Mont, M.A.; Tsao, A.K.; Jones, L.C. Treatment of femoral head osteonecrosis in the United States: 16-year analysis of the Nationwide Inpatient Sample. Clin. Orthop. Relat. Res. 2014, 472, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Mont, M.A.; Salem, H.S.; Piuzzi, N.S.; Goodman, S.B.; Jones, L.C. Nontraumatic Osteonecrosis of the Femoral Head: Where Do We Stand Today?: A 5-Year Update. J. Bone Jt. Surg. Am. 2020, 102, 1084–1099. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Greenspan, A.; Gershwin, M.E. The pathogenesis, diagnosis and clinical manifestations of steroid-induced osteonecrosis. J. Autoimmun. 2020, 110, 102460. [Google Scholar] [CrossRef] [PubMed]
- Kerachian, M.A.; Harvey, E.J.; Cournoyer, D.; Chow, T.Y.; Séguin, C. Avascular necrosis of the femoral head: Vascular hypotheses. Endothelium 2006, 13, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Kavurma, M.M.; Bursill, C.; Stanley, C.P.; Passam, F.; Cartland, S.P.; Patel, S.; Loa, J.; Figtree, G.A.; Golledge, J.; Aitken, S.; et al. Endothelial cell dysfunction: Implications for the pathogenesis of peripheral artery disease. Front. Cardiovasc. Med. 2022, 9, 1054576. [Google Scholar] [CrossRef] [PubMed]
- Krüger-Genge, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [PubMed]
- Adelborg, K.; Larsen, J.B.; Hvas, A.M. Disseminated intravascular coagulation: Epidemiology, biomarkers, and management. Br. J. Haematol. 2021, 192, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef]
- Neubauer, K.; Zieger, B. Endothelial cells and coagulation. Cell Tissue Res. 2022, 387, 391–398. [Google Scholar] [CrossRef]
- Sabe, S.A.; Feng, J.; Sellke, F.W.; Abid, M.R. Mechanisms and clinical implications of endothelium-dependent vasomotor dysfunction in coronary microvasculature. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H819–H841. [Google Scholar] [CrossRef]
- Zhu, S.; Bennett, S.; Kuek, V.; Xiang, C.; Xu, H.; Rosen, V.; Xu, J. Endothelial cells produce angiocrine factors to regulate bone and cartilage via versatile mechanisms. Theranostics 2020, 10, 5957–5965. [Google Scholar] [CrossRef] [PubMed]
- Langen, U.H.; Pitulescu, M.E.; Kim, J.M.; Enriquez-Gasca, R.; Sivaraj, K.K.; Kusumbe, A.P.; Singh, A.; Di Russo, J.; Bixel, M.G.; Zhou, B.; et al. Cell-matrix signals specify bone endothelial cells during developmental osteogenesis. Nat. Cell Biol. 2017, 19, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, J.; Xie, X.; Chen, Y.; Zheng, Q.; He, J.; Lu, Q. Exosome-derived miR-5p-72106_14 in vascular endothelial cells regulates fate determination of BMSCs. Toxicol. Appl. Pharmacol. 2024, 482, 116793. [Google Scholar] [CrossRef]
- Wu, H.; Chen, G.; Zhang, G.; Lv, Q.; Gu, D.; Dai, M. Mechanism of vascular endothelial cell-derived exosomes modified with vascular endothelial growth factor in steroid-induced femoral head necrosis. Biomed. Mater. 2023, 18, 025017. [Google Scholar] [CrossRef] [PubMed]
- Salazar, V.S.; Gamer, L.W.; Rosen, V. BMP signalling in skeletal development, disease and repair. Nat. Rev. Endocrinol. 2016, 12, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Koh, J.M. Coupling factors involved in preserving bone balance. Cell. Mol. Life Sci. 2019, 76, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hao, J.; Duan, X.; Wu, N.; Zhou, Z.; Yang, F.; Li, J.; Zhao, Z.; Huang, S. The Role of Semaphorin 3A in Bone Remodeling. Front. Cell. Neurosci. 2017, 11, 40. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, Y.; Ding, Y.; Xie, W.; Li, H.; Li, S.; Li, Y.; Cai, M. Inhibition of PGE2 in Subchondral Bone Attenuates Osteoarthritis. Cells 2022, 11, 2760. [Google Scholar] [CrossRef]
- Wu, J.; Hu, M.; Jiang, H.; Ma, J.; Xie, C.; Zhang, Z.; Zhou, X.; Zhao, J.; Tao, Z.; Meng, Y.; et al. Endothelial Cell-Derived Lactate Triggers Bone Mesenchymal Stem Cell Histone Lactylation to Attenuate Osteoporosis. Adv. Sci. 2023, 10, e2301300. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Huang, X.; Gu, Y.; Li, S.; Yin, P.; Zhang, L.; Tang, P. Skeleton-vasculature chain reaction: A novel insight into the mystery of homeostasis. Bone Res. 2021, 9, 21. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, H.; Guo, Z.; Chang, Y.; Li, Z. Role of prostaglandin E2 in tissue repair and regeneration. Theranostics 2021, 11, 8836–8854. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.K.; Kusumbe, A.P.; Wang, L.; Adams, R.H. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 2014, 507, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Romeo, S.G.; Alawi, K.M.; Rodrigues, J.; Singh, A.; Kusumbe, A.P.; Ramasamy, S.K. Endothelial proteolytic activity and interaction with non-resorbing osteoclasts mediate bone elongation. Nat. Cell Biol. 2019, 21, 430–441. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, P.; Gu, Y.; Guo, Q.; Liu, Y. Type H vessels: Functions in bone development and diseases. Front. Cell Dev. Biol. 2023, 11, 1236545. [Google Scholar] [CrossRef]
- Kusumbe, A.P.; Ramasamy, S.K.; Adams, R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014, 507, 323–328. [Google Scholar] [CrossRef]
- Leone, A.M.; Valgimigli, M.; Giannico, M.B.; Zaccone, V.; Perfetti, M.; D’Amario, D.; Rebuzzi, A.G.; Crea, F. From bone marrow to the arterial wall: The ongoing tale of endothelial progenitor cells. Eur. Heart J. 2009, 30, 890–899. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Lyu, L.; Xiao, L.; Memon, A.A.; Yu, X.; Halim, A.; Patel, S.; Osman, A.; Yin, W.; et al. Integrin α5 Is Regulated by miR-218-5p in Endothelial Progenitor Cells. J. Am. Soc. Nephrol. 2022, 33, 565–582. [Google Scholar] [CrossRef] [PubMed]
- Mak, A.; Chan, J.K.Y. Endothelial function and endothelial progenitor cells in systemic lupus erythematosus. Nat. Rev. Rheumatol. 2022, 18, 286–300. [Google Scholar] [CrossRef]
- Salybekov, A.A.; Kobayashi, S.; Asahara, T. Characterization of Endothelial Progenitor Cell: Past, Present, and Future. Int. J. Mol. Sci. 2022, 23, 7697. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, S.H.; Xiao, B.J.; Xu, W.H.; Ye, S.N.; Xia, T.; Zheng, D.; Liu, X.Z.; Liao, Y.F. Decreased in the number and function of circulation endothelial progenitor cells in patients with avascular necrosis of the femoral head. Bone 2010, 46, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, S.; Feng, Y.; Wu, X.; Chen, D.; Yu, Q.; Wang, X.; Li, J.; Chen, J. Impairment of two types of circulating endothelial progenitor cells in patients with glucocorticoid-induced avascular osteonecrosis of the femoral head. Jt. Bone Spine 2013, 80, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Zhang, W.; Tan, Q.; Yao, C.; Lin, S. Impairment of circulating endothelial progenitor cells (EPCs) in patients with glucocorticoid-induced avascular necrosis of the femoral head and changes of EPCs after glucocorticoid treatment in vitro. J. Orthop. Surg. Res. 2019, 14, 226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.Y.; Pan, Z.W.; Li, Q.Q.; Sun, L.H.; Li, X.; Gong, M.Y.; Yang, X.W.; Wang, Y.Y.; Li, H.D.; et al. GDF11 promotes wound healing in diabetic mice via stimulating HIF-1ɑ-VEGF/SDF-1ɑ-mediated endothelial progenitor cell mobilization and neovascularization. Acta Pharmacol. Sin. 2023, 44, 999–1013. [Google Scholar] [CrossRef] [PubMed]
- Rezus, E.; Tamba, B.I.; Badescu, M.C.; Popescu, D.; Bratoiu, I.; Rezus, C. Osteonecrosis of the Femoral Head in Patients with Hypercoagulability-From Pathophysiology to Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 6801. [Google Scholar] [CrossRef] [PubMed]
- Badescu, M.C.; Rezus, E.; Ciocoiu, M.; Badulescu, O.V.; Butnariu, L.I.; Popescu, D.; Bratoiu, I.; Rezus, C. Osteonecrosis of the Jaws in Patients with Hereditary Thrombophilia/Hypofibrinolysis-From Pathophysiology to Therapeutic Implications. Int. J. Mol. Sci. 2022, 23, 640. [Google Scholar] [CrossRef] [PubMed]
- Glueck, C.J.; Freiberg, R.A.; Wang, P. Familial Thrombophilia Is Associated With Primary Multifocal Osteonecrosis: A Case-Control Study of 40 Patients. Orthopedics 2023, 46, 164–168. [Google Scholar] [CrossRef]
- Bodary, P.F.; Wickenheiser, K.J.; Eitzman, D.T. Recent advances in understanding endogenous fibrinolysis: Implications for molecular-based treatment of vascular disorders. Expert. Rev. Mol. Med. 2002, 4, 1–10. [Google Scholar] [CrossRef]
- Deaglio, S.; Robson, S.C. Ectonucleotidases as regulators of purinergic signaling in thrombosis, inflammation, and immunity. Adv. Pharmacol. 2011, 61, 301–332. [Google Scholar]
- Kazemi, N.; Bordbar, A.; Bavarsad, S.S.; Ghasemi, P.; Bakhshi, M.; Rezaeeyan, H. Molecular Insights into the Relationship between Platelet Activation and Endothelial Dysfunction: Molecular Approaches and Clinical Practice. Mol. Biotechnol. 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Wittig, J.; Drekolia, M.K.; Kyselova, A.; Delgado Lagos, F.; Bochenek, M.L.; Hu, J.; Schäfer, K.; Fleming, I.; Bibli, S.I. Endothelial-dependent S-Sulfhydration of tissue factor pathway inhibitor regulates blood coagulation. Redox Biol. 2023, 62, 102694. [Google Scholar] [CrossRef]
- Stern, D.; Brett, J.; Harris, K.; Nawroth, P. Participation of endothelial cells in the protein C-protein S anticoagulant pathway: The synthesis and release of protein S. J. Cell Biol. 1986, 102, 1971–1978. [Google Scholar] [CrossRef]
- Duque, P.; Mora, L.; Levy, J.H.; Schöchl, H. Pathophysiological Response to Trauma-Induced Coagulopathy: A Comprehensive Review. Anesth. Analg. 2020, 130, 654–664. [Google Scholar] [CrossRef]
- Durand, M.J.; Gutterman, D.D. Diversity in mechanisms of endothelium-dependent vasodilation in health and disease. Microcirculation 2013, 20, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yu, Q.; Guo, W.; Hao, Y.; Sun, W.; Cheng, L. Effect of glucocorticoids on the function of microvascular endothelial cells in the human femoral head bone. Adv. Clin. Exp. Med. 2020, 29, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.C.; Santos, C.V.; Vieira Neto, L.; Gadelha, M.R. Adverse effects of glucocorticoids: Coagulopathy. Eur. J. Endocrinol. 2015, 173, M11–M21. [Google Scholar] [CrossRef] [PubMed]
- Glueck, C.J.; Freiberg, R.A.; Boriel, G.; Khan, Z.; Brar, A.; Padda, J.; Wang, P. The role of the factor V Leiden mutation in osteonecrosis of the hip. Clin. Appl. Thromb. Hemost. 2013, 19, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Zalavras, C.G.; Vartholomatos, G.; Dokou, E.; Malizos, K.N. Genetic background of osteonecrosis: Associated with thrombophilic mutations? Clin. Orthop. Relat. Res. 2004, 422, 251–255. [Google Scholar] [CrossRef]
- Shang, X.F.; Su, H.; Chang, W.W.; Wang, C.C.; Han, Q.; Xu, Z.W. Association between MTHFR C677T polymorphism and osteonecrosis of the femoral head: A meta-analysis. Mol. Biol. Rep. 2012, 39, 7089–7094. [Google Scholar] [CrossRef]
- Rathod, T.N.; Tayade, M.B.; Shetty, S.D.; Jadhav, P.; Sathe, A.H.; Mohanty, S.S. Association of Thrombophilic Factors in Pathogenesis of Osteonecrosis of Femoral Head in Indian Population. Indian J. Orthop. 2020, 54, 33–38. [Google Scholar] [CrossRef]
- Garcia, F.L.; Ramalli, E.L.; Picado, C.H. Coagulation disorders in patients with femoral head osteonecrosis. Acta Ortop. Bras. 2013, 21, 43–45. [Google Scholar] [CrossRef][Green Version]
- Gao, Y.; Zhu, H.; Wang, Q.; Feng, Y.; Zhang, C. Inhibition of PERK Signaling Prevents Against Glucocorticoid-induced Endotheliocyte Apoptosis and Osteonecrosis of the Femoral Head. Int. J. Biol. Sci. 2020, 16, 543–552. [Google Scholar] [CrossRef]
- Bouarab, C.; Roullot-Lacarrière, V.; Vallée, M.; Le Roux, A.; Guette, C.; Mennesson, M.; Marighetto, A.; Desmedt, A.; Piazza, P.V.; Revest, J.M. PAI-1 protein is a key molecular effector in the transition from normal to PTSD-like fear memory. Mol. Psychiatry 2021, 26, 4968–4981. [Google Scholar] [CrossRef]
- Kerachian, M.A.; Séguin, C.; Harvey, E.J. Glucocorticoids in osteonecrosis of the femoral head: A new understanding of the mechanisms of action. J. Steroid Biochem. Mol. Biol. 2009, 114, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Kim, K.H.; Lee, W.K.; Hong, W.; Won, H.; Kim, S.Y. Abnormal Lipid Profiles in Nontraumatic Osteonecrosis of the Femoral Head: A Comparison with Osteoarthritis Using Propensity Score Matching. J. Bone Jt. Surg. Am. 2022, 104, 19–24. [Google Scholar] [CrossRef]
- Liu, K.; Wang, K.; Wang, L.; Zhou, Z. Changes of lipid and bone metabolism in broilers with spontaneous femoral head necrosis. Poult. Sci. 2021, 100, 100808. [Google Scholar] [CrossRef] [PubMed]
- van der Meijden, P.E.J.; Heemskerk, J.W.M. Platelet biology and functions: New concepts and clinical perspectives. Nat. Rev. Cardiol. 2019, 16, 166–179. [Google Scholar] [CrossRef]
- Pacinella, G.; Ciaccio, A.M.; Tuttolomondo, A. Endothelial Dysfunction and Chronic Inflammation: The Cornerstones of Vascular Alterations in Age-Related Diseases. Int. J. Mol. Sci. 2022, 23, 15722. [Google Scholar] [CrossRef]
- Souilhol, C.; Serbanovic-Canic, J.; Fragiadaki, M.; Chico, T.J.; Ridger, V.; Roddie, H.; Evans, P.C. Endothelial responses to shear stress in atherosclerosis: A novel role for developmental genes. Nat. Rev. Cardiol. 2020, 17, 52–63. [Google Scholar] [CrossRef]
- Friedrich, E.E.; Hong, Z.; Xiong, S.; Zhong, M.; Di, A.; Rehman, J.; Komarova, Y.A.; Malik, A.B. Endothelial cell Piezo1 mediates pressure-induced lung vascular hyperpermeability via disruption of adherens junctions. Proc. Natl. Acad. Sci. USA 2019, 116, 12980–12985. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ji, W.; Yang, H.; Chen, S.; Zhang, W.; Duan, G. Endothelial activation and dysfunction in COVID-19: From basic mechanisms to potential therapeutic approaches. Signal Transduct. Target. Ther. 2020, 5, 293. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhou, Y.; Nabavi, S.M.; Sahebkar, A.; Little, P.J.; Xu, S.; Weng, J.; Ge, J. Mechanisms of Oxidized LDL-Mediated Endothelial Dysfunction and Its Consequences for the Development of Atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 925923. [Google Scholar] [CrossRef] [PubMed]
- Horio, E.; Kadomatsu, T.; Miyata, K.; Arai, Y.; Hosokawa, K.; Doi, Y.; Ninomiya, T.; Horiguchi, H.; Endo, M.; Tabata, M.; et al. Role of endothelial cell-derived angptl2 in vascular inflammation leading to endothelial dysfunction and atherosclerosis progression. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Nie, Z.; Sun, F.; Peng, H. Glucocorticoids induce femoral head necrosis in rats through the ROS/JNK/c-Jun pathway. FEBS Open Bio 2021, 11, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Deng, Y.; Deng, G.; Chen, P.; Wang, Y.; Wu, H.; Ji, Z.; Yao, Z.; Zhang, X.; Yu, B.; et al. High cholesterol induces apoptosis and autophagy through the ROS-activated AKT/FOXO1 pathway in tendon-derived stem cells. Stem Cell Res. Ther. 2020, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Haag, F.; Janicova, A.; Xu, B.; Powerski, M.; Fachet, M.; Bundkirchen, K.; Neunaber, C.; Marzi, I.; Relja, B.; Sturm, R. Reduced phagocytosis, ROS production and enhanced apoptosis of leukocytes upon alcohol drinking in healthy volunteers. Eur. J. Trauma Emerg. Surg. 2022, 48, 2689–2699. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.I.; Romano, M.; Basarali, M.K.; Elzagallaai, A.; Karaman, M.; Demir, Z.; Demir, M.F.; Akcay, F.; Seyrek, M.; Haksever, N.; et al. The Effect of Corrected Inflammation, Oxidative Stress and Endothelial Dysfunction on Fmd Levels in Patients with Selected Chronic Diseases: A Quasi-Experimental Study. Sci. Rep. 2020, 10, 9018. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liu, J.; Piao, H.; Zhu, Z.; Wei, R.; Liu, K. ROS-triggered endothelial cell death mechanisms: Focus on pyroptosis, parthanatos, and ferroptosis. Front. Immunol. 2022, 13, 1039241. [Google Scholar] [CrossRef]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid. Med. Cell. Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef]
- Balta, S. Endothelial Dysfunction and Inflammatory Markers of Vascular Disease. Curr. Vasc. Pharmacol. 2021, 19, 243–249. [Google Scholar] [CrossRef]
- Luk, C.; Haywood, N.J.; Bridge, K.I.; Kearney, M.T. Paracrine Role of the Endothelium in Metabolic Homeostasis in Health and Nutrient Excess. Front. Cardiovasc. Med. 2022, 9, 882923. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Cao, Y.; Zhang, S.; Sun, J.; Wang, Y.; Song, S.; Zhang, H. Targeting the Microenvironment of Vulnerable Atherosclerotic Plaques: An Emerging Diagnosis and Therapy Strategy for Atherosclerosis. Adv. Mater. 2022, 34, e2110660. [Google Scholar] [CrossRef] [PubMed]
- Durand, E.; Scoazec, A.; Lafont, A.; Boddaert, J.; Al Hajzen, A.; Addad, F.; Mirshahi, M.; Desnos, M.; Tedgui, A.; Mallat, Z. In vivo induction of endothelial apoptosis leads to vessel thrombosis and endothelial denudation: A clue to the understanding of the mechanisms of thrombotic plaque erosion. Circulation 2004, 109, 2503–2506. [Google Scholar] [CrossRef]
- Maruyama, M.; Rhee, C.; Utsunomiya, T.; Zhang, N.; Ueno, M.; Yao, Z.; Goodman, S.B. Modulation of the Inflammatory Response and Bone Healing. Front. Endocrinol. 2020, 11, 386. [Google Scholar] [CrossRef]
- Sharifiaghdam, M.; Shaabani, E.; Faridi-Majidi, R.; De Smedt, S.C.; Braeckmans, K.; Fraire, J.C. Macrophages as a therapeutic target to promote diabetic wound healing. Mol. Ther. 2022, 30, 2891–2908. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Zheng, J.; Yao, Z.; Xue, L.; Wang, D.; Tan, Z. The role of immune cells in modulating chronic inflammation and osteonecrosis. Front. Immunol. 2022, 13, 1064245. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Qu, M.; Li, W.; Wu, D.; Cata, J.P.; Miao, C. Neutrophil, neutrophil extracellular traps and endothelial cell dysfunction in sepsis. Clin. Transl. Med. 2023, 13, e1170. [Google Scholar] [CrossRef]
- Vazquez-Garza, E.; Jerjes-Sanchez, C.; Navarrete, A.; Joya-Harrison, J.; Rodriguez, D. Venous thromboembolism: Thrombosis, inflammation, and immunothrombosis for clinicians. J. Thromb. Thrombolysis 2017, 44, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Ntelis, K.; Solomou, E.E.; Sakkas, L.; Liossis, S.N.; Daoussis, D. The role of platelets in autoimmunity, vasculopathy, and fibrosis: Implications for systemic sclerosis. Semin. Arthritis Rheum. 2017, 47, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Savill, J.; Dransfield, I.; Gregory, C.; Haslett, C. A blast from the past: Clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2002, 2, 965–975. [Google Scholar] [CrossRef]
- Castanheira, F.V.S.; Kubes, P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood 2019, 133, 2178–2185. [Google Scholar] [CrossRef]
- Smith, C.K.; Vivekanandan-Giri, A.; Tang, C.; Knight, J.S.; Mathew, A.; Padilla, R.L.; Gillespie, B.W.; Carmona-Rivera, C.; Liu, X.; Subramanian, V.; et al. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: An additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol. 2014, 66, 2532–2544. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, B.; Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013, 13, 34–45. [Google Scholar] [CrossRef]
- Li, T.; Peng, R.; Wang, F.; Hua, L.; Liu, S.; Han, Z.; Pei, J.; Pei, S.; Zhao, Z.; Jiang, X.; et al. Lysophosphatidic acid promotes thrombus stability by inducing rapid formation of neutrophil extracellular traps: A new mechanism of thrombosis. J. Thromb. Haemost. 2020, 18, 1952–1964. [Google Scholar] [CrossRef]
- Fernández, S.; Moreno-Castaño, A.B.; Palomo, M.; Martinez-Sanchez, J.; Torramadé-Moix, S.; Téllez, A.; Ventosa, H.; Seguí, F.; Escolar, G.; Carreras, E.; et al. Distinctive Biomarker Features in the Endotheliopathy of COVID-19 and Septic Syndromes. Shock 2022, 57, 95–105. [Google Scholar] [CrossRef]
- Sandoval-Pérez, A.; Berger, R.M.L.; Garaizar, A.; Farr, S.E.; Brehm, M.A.; König, G.; Schneider, S.W.; Collepardo-Guevara, R.; Huck, V.; Rädler, J.O.; et al. DNA binds to a specific site of the adhesive blood-protein von Willebrand factor guided by electrostatic interactions. Nucleic Acids Res. 2020, 48, 7333–7344. [Google Scholar] [CrossRef]
- Skendros, P.; Mitsios, A.; Chrysanthopoulou, A.; Mastellos, D.C.; Metallidis, S.; Rafailidis, P.; Ntinopoulou, M.; Sertaridou, E.; Tsironidou, V.; Tsigalou, C.; et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J. Clin. Investig. 2020, 130, 6151–6157. [Google Scholar] [CrossRef]
- Döring, Y.; Libby, P.; Soehnlein, O. Neutrophil Extracellular Traps Participate in Cardiovascular Diseases: Recent Experimental and Clinical Insights. Circ. Res. 2020, 126, 1228–1241. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, X.; Lai, B.; Hu, D.; Lai, L.; Xu, J.; Chen, S.; Li, X. Correlational analysis between neutrophil granulocyte levels and osteonecrosis of the femoral head. BMC Musculoskelet. Disord. 2019, 20, 393. [Google Scholar] [CrossRef]
- Nonokawa, M.; Shimizu, T.; Yoshinari, M.; Hashimoto, Y.; Nakamura, Y.; Takahashi, D.; Asano, T.; Nishibata, Y.; Masuda, S.; Nakazawa, D.; et al. Association of Neutrophil Extracellular Traps with the Development of Idiopathic Osteonecrosis of the Femoral Head. Am. J. Pathol. 2020, 190, 2282–2289. [Google Scholar] [CrossRef]
- Vannella, K.M.; Wynn, T.A. Mechanisms of Organ Injury and Repair by Macrophages. Annu. Rev. Physiol. 2017, 79, 593–617. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kong, X.; You, Y.; Xiang, L.; Zhang, Y.; Wu, R.; Zhou, L.; Duan, L. S100A8-Mediated NLRP3 Inflammasome-Dependent Pyroptosis in Macrophages Facilitates Liver Fibrosis Progression. Cells 2022, 11, 3579. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Wang, Y.; Chen, Y.; Liu, Y.; Ma, M.; Ma, Z.; Wang, C.; Zeng, H.; Xue, L.; Yue, C.; et al. The Dynamic Feature of Macrophage M1/M2 Imbalance Facilitates the Progression of Non-Traumatic Osteonecrosis of the Femoral Head. Front. Bioeng. Biotechnol. 2022, 10, 912133. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Luo, N.S.; Ying, R.; Xie, Y.; Chen, J.Y.; Wang, X.Q.; Gu, Z.J.; Mai, J.T.; Liu, W.H.; Wu, M.X.; et al. Macrophage-derived foam cells impair endothelial barrier function by inducing endothelial-mesenchymal transition via CCL-4. Int. J. Mol. Med. 2017, 40, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Dejana, E.; Tournier-Lasserve, E.; Weinstein, B.M. The control of vascular integrity by endothelial cell junctions: Molecular basis and pathological implications. Dev. Cell 2009, 16, 209–221. [Google Scholar] [CrossRef]
- Wettschureck, N.; Strilic, B.; Offermanns, S. Passing the Vascular Barrier: Endothelial Signaling Processes Controlling Extravasation. Physiol. Rev. 2019, 99, 1467–1525. [Google Scholar] [CrossRef]
- Saigusa, R.; Winkels, H.; Ley, K. T cell subsets and functions in atherosclerosis. Nat. Rev. Cardiol. 2020, 17, 387–401. [Google Scholar] [CrossRef]
- Kikuiri, T.; Kim, I.; Yamaza, T.; Akiyama, K.; Zhang, Q.; Li, Y.; Chen, C.; Chen, W.; Wang, S.; Le, A.D.; et al. Cell-based immunotherapy with mesenchymal stem cells cures bisphosphonate-related osteonecrosis of the jaw-like disease in mice. J. Bone Miner. Res. 2010, 25, 1668–1679. [Google Scholar] [CrossRef]
- Zou, D.; Zhang, K.; Yang, Y.; Ren, Y.; Zhang, L.; Xiao, X.; Zhang, H.; Liu, S.; Li, J. Th17 and IL-17 exhibit higher levels in osteonecrosis of the femoral head and have a positive correlation with severity of pain. Endokrynol. Pol. 2018, 69, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Gong, S.; Shao, W.; Han, L.; Li, Z.; Zhang, Z.; Zheng, Y.; Ouyang, F.; Ma, Y.; Xu, W.; et al. Comprehensive analysis of pivotal biomarkers, immune cell infiltration and therapeutic drugs for steroid-induced osteonecrosis of the femoral head. Bioengineered 2021, 12, 5971–5984. [Google Scholar] [CrossRef] [PubMed]
- Camacho, V.; Matkins, V.R.; Patel, S.B.; Lever, J.M.; Yang, Z.; Ying, L.; Landuyt, A.E.; Dean, E.C.; George, J.F.; Yang, H.; et al. Bone marrow Tregs mediate stromal cell function and support hematopoiesis via IL-10. JCI Insight 2020, 5, e135681. [Google Scholar] [CrossRef] [PubMed]
- Mangge, H.; Prüller, F.; Schnedl, W.; Renner, W.; Almer, G. Beyond Macrophages and T Cells: B Cells and Immunoglobulins Determine the Fate of the Atherosclerotic Plaque. Int. J. Mol. Sci. 2020, 21, 4082. [Google Scholar] [CrossRef] [PubMed]
- Baumgarth, N. The double life of a B-1 cell: Self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 2011, 11, 34–46. [Google Scholar] [CrossRef]
- Okazaki, S.; Nishitani, Y.; Nagoya, S.; Kaya, M.; Yamashita, T.; Matsumoto, H. Femoral head osteonecrosis can be caused by disruption of the systemic immune response via the toll-like receptor 4 signalling pathway. Rheumatology 2009, 48, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Korompilias, A.V.; Gilkeson, G.S.; Ortel, T.L.; Seaber, A.V.; Urbaniak, J.R. Anticardiolipin antibodies and osteonecrosis of the femoral head. Clin. Orthop. Relat. Res. 1997, 345, 174–180. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, F.; Liu, Y.; Zhao, D.; Shan, Y.; Jiang, Y. A higher frequency of peripheral blood activated B cells in patients with non-traumatic osteonecrosis of the femoral head. Int. Immunopharmacol. 2014, 20, 95–100. [Google Scholar] [CrossRef]
- Ran, Z.; Luo, Y.-B.; Zeng, Q.-M.; Huan, Y. Regulatory B Cells and Its Role in Central Nervous System Inflammatory Demyelinating Diseases. Front. Immunol. 2020, 11, 1884. [Google Scholar] [CrossRef]
- Ng, M.K.; Gordon, A.M.; Piuzzi, N.S.; Wong, C.H.J.; Jones, L.C.; Mont, M.A. Trends in Surgical Management of Osteonecrosis of the Femoral Head: A 2010 to 2020 Nationwide Study. J. Arthroplast. 2023, 38, S51–S57. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Y.; Fan, X.; Xu, X.; Sun, W. Extracorporeal shockwave relieves endothelial injury and dysfunction in steroid-induced osteonecrosis of the femoral head via miR-135b targeting FOXO1: In vitro and in vivo studies. Aging 2022, 14, 410–429. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, Y.; Ma, C.; Gu, G.; Han, D.F. A Mini Review: Stem Cell Therapy for Osteonecrosis of the Femoral Head and Pharmacological Aspects. Curr. Pharm. Des. 2019, 25, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wang, B.; Tian, S.; Liu, B.; Qin, K.; Zhao, D. Retrospective Long-Term Follow-Up Survival Analysis of the Management of Osteonecrosis of the Femoral Head with Pedicled Vascularized Iliac Bone Graft Transfer. J. Arthroplast. 2019, 34, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hua, Z.; Liao, S.; Li, B.; Tang, S.; Huang, Q.; Wei, Z.; Lu, R.; Lin, C.; Ding, X. Prediction of the active compounds and mechanism of Biochanin A in the treatment of Legg-Calvé-Perthes disease based on network pharmacology and molecular docking. BMC Complement. Med. Ther. 2024, 24, 26. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, W.; Mao, Y.; Jiang, L.; Yu, J.; Zhu, X.; Fu, H.; Lin, Z.; Shen, H.; Pan, X.; et al. Morroniside-mediated mitigation of stem cell and endothelial cell dysfunction for the therapy of glucocorticoid-induced osteonecrosis of the femoral head. Int. Immunopharmacol. 2024, 127, 111421. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Zhang, J. Vitamin B(2) Prevents Glucocorticoid-Caused Damage of Blood Vessels in Osteonecrosis of the Femoral Head. Biomed. Res. Int. 2022, 2022, 4006184. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jie, S.; Li, W.; Li, H.; Ni, J.; Liu, C. miR-122-5p targets GREM2 to protect against glucocorticoid-induced endothelial damage through the BMP signaling pathway. Mol. Cell. Endocrinol. 2022, 544, 111541. [Google Scholar] [CrossRef]
- Kong, L.; Zuo, R.; Wang, M.; Wang, W.; Xu, J.; Chai, Y.; Guan, J.; Kang, Q. Silencing MicroRNA-137-3p, which Targets RUNX2 and CXCL12 Prevents Steroid-induced Osteonecrosis of the Femoral Head by Facilitating Osteogenesis and Angiogenesis. Int. J. Biol. Sci. 2020, 16, 655–670. [Google Scholar] [CrossRef]
- Shao, W.; Li, Z.; Wang, B.; Gong, S.; Wang, P.; Song, B.; Chen, Z.; Feng, Y. Dimethyloxalylglycine Attenuates Steroid-Associated Endothelial Progenitor Cell Impairment and Osteonecrosis of the Femoral Head by Regulating the HIF-1α Signaling Pathway. Biomedicines 2023, 11, 992. [Google Scholar] [CrossRef]
- Xu, X.; Fan, X.; Wu, X.; Xia, R.; Liang, J.; Gao, F.; Shu, J.; Yang, M.; Sun, W. Luteolin ameliorates necroptosis in Glucocorticoid-induced osteonecrosis of the femoral head via RIPK1/RIPK3/MLKL pathway based on network pharmacology analysis. Biochem. Biophys. Res. Commun. 2023, 661, 108–118. [Google Scholar] [CrossRef]
- Fan, X.; Xu, X.; Wu, X.; Xia, R.; Gao, F.; Zhang, Q.; Sun, W. The protective effect of DNA aptamer on osteonecrosis of the femoral head by alleviating TNF-α-mediated necroptosis via RIP1/RIP3/MLKL pathway. J. Orthop. Translat. 2022, 36, 44–51. [Google Scholar] [CrossRef]
- Cao, F.; Qin, K.R.; Kang, K.; Zheng, G.; Wang, W.; Zhang, X.; Zhao, D. Ginkgo biloba L. extract prevents steroid-induced necrosis of the femoral head by rescuing apoptosis and dysfunction in vascular endothelial cells via the PI3K/AKT/eNOS pathway. J. Ethnopharmacol. 2022, 296, 115476. [Google Scholar] [CrossRef]
- Jing, X.; Du, T.; Yang, X.; Zhang, W.; Wang, G.; Liu, X.; Li, T.; Jiang, Z. Desferoxamine protects against glucocorticoid-induced osteonecrosis of the femoral head via activating HIF-1α expression. J. Cell. Physiol. 2020, 235, 9864–9875. [Google Scholar] [CrossRef]
- Yao, X.; Yu, S.; Jing, X.; Guo, J.; Sun, K.; Guo, F.; Ye, Y. PTEN inhibitor VO-OHpic attenuates GC-associated endothelial progenitor cell dysfunction and osteonecrosis of the femoral head via activating Nrf2 signaling and inhibiting mitochondrial apoptosis pathway. Stem Cell Res. Ther. 2020, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yue, J.; Wang, W.; Liu, P.; Zuo, W.; Guo, W.; Zhang, Q. Icariin promotes angiogenesis in glucocorticoid-induced osteonecrosis of femoral heads: In vitro and in vivo studies. J. Cell. Mol. Med. 2019, 23, 7320–7330. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Yu, H.; Liu, P.; Wen, P.; Zhang, H.; Guo, W.; Zhang, Q. Preliminary study of icariin indicating prevention of steroid-induced osteonecrosis of femoral head by regulating abnormal expression of miRNA-335 and protecting the functions of bone microvascular endothelial cells in rats. Gene 2021, 766, 145128. [Google Scholar] [CrossRef] [PubMed]
- Haydock, M.M.; Elhamdani, S.; Alsharedi, M. Long-term direct oral anticoagulation in primary osteonecrosis with elevated plasminogen activation inhibitor. SAGE Open Med. Case Rep. 2019, 7, 2050313x19827747. [Google Scholar] [CrossRef] [PubMed]
- Li, C.G.; Shen, L.; Yang, Y.P.; Xu, X.J.; Shuai, B.; Ma, C. Effects of Modified Qing’e Pill on expression of adiponectin, bone morphogenetic protein 2 and coagulation-related factors in patients with nontraumatic osteonecrosis of femoral head. Chin. J. Integr. Med. 2017, 23, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.L.; Weng, X.S.; Wu, Z.H.; Guo, S.G. Effect of Resveratrol on Preventing Steroid-induced Osteonecrosis in a Rabbit Model. Chin. Med. J. 2016, 129, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Beytemur, O.; Dasci, M.F.; Gök Yurttaş, A.; Bayrak, B.Y.; Alagöz, E. The protective role of vitamins C and E in steroid-induced femoral head osteonecrosis: An experimental study in rats. Jt. Dis. Relat. Surg. 2024, 35, 72–84. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).