Abstract
Background: Osteoporosis (OP) is a chronic inflammatory bone disease characterized by reduced bone structure and strength, leading to increased fracture risk. Effective therapies targeting both bone and cartilage are limited. This study compared the therapeutic effects of extracorporeal shockwave therapy (ESWT), bisphosphonate (Aclasta), and human Wharton jelly-derived mesenchymal stem cells (WJMSCs) in a rat model of OP. Methods: Female rats were assigned to four groups: Sham (no surgery or treatment), OP (bilateral ovariectomy, OVX), ESWT (OVX + ESWT on both tibias at 0.25 mJ/mm2, 1500 impulses per tibia), Aclasta (OVX + zoledronic acid 0.1 mg/kg via tail vein injection), and WJMSC (OVX + 2 × 10⁶ WJMSCs). Pathological changes, bone microarchitecture (by micro-CT), serum cytokines (by ELISA), and tissue-specific molecular markers (by immunohistochemistry) were evaluated. Results: All treatments improved bone density, preserved cartilage, and modulated cytokines (IL31, IL33, VEGF, and BMP2), with Aclasta showing the greatest improvements in bone parameters and cartilage preservation. ESWT and WJMSC also demonstrated significant effects, with ESWT highlighting non-invasive chondroprotective potential. Conclusions: Aclasta provided the best overall therapeutic response, particularly in bone regeneration. However, ESWT and WJMSC also showed comparable chondroprotective effects. ESWT emerges as a promising non-invasive alternative for OP management when pharmacological or cell-based therapies are not feasible.
1. Introduction
Osteoporosis (OP) is a chronic, bone metabolic disease that causes the bones of humans to become less dense and more brittle []. Osteoporosis lowers quality of life, especially in the elderly people and postmenopausal women, as it results in 8.9 million fractures worldwide [,]. Various factors, such as aging, estrogen deficiency, medications, smoking, inflammation, and heredity for osteoporosis, adversely impact bone health []. As a result, improving bone health is thought to be a crucial issue for those who are more susceptible to osteoporosis. To stop or reverse bone loss, many medical and pharmaceutical interventions have been developed [,]. For example, weight-bearing aerobics exercise, resistance training, aquatic exercise, and mechanical stress are the physical approaches to decrease the rate of bone loss and improve bone mineral density [,,]. The physical methods need to include regular daily exercise or stimulation through the use of mechanical devices and important supplements including calcium, vitamin D, and dietary nutrients for bone recovery. Osteoporosis drugs are safe, consistently very successful, and work more swiftly to treat the condition. Current pharmacological treatments for osteoporosis include anti-resorptive (bisphosphonates and denosumab), bone-forming (PTH analogues and PTHrP analogues), and bone-sparing (Wnt antagonists) treatments [,,]. The pathology of osteoporosis is caused by an imbalance in the cellular functions of bone resorption by osteoclasts and bone formation by osteoblasts, resulting in reduced bone mass and increased fracture risk []. Maintaining the balance of bone modeling and remodeling is critical, and this is achieved through the contributions of various cell types, including osteocytes, osteoclasts, and osteoblasts [].
Bisphosphonates, such as zoledronic acid, are widely used as anti-resorptive agents in the management of OP []. They function by inhibiting osteoclast-mediated bone resorption, thereby reducing bone turnover and increasing BMD. Zoledronic acid has been demonstrated to significantly reduce the risk of vertebral, hip, and other fractures in patients with OP. In addition to its effects on bone metabolism, zoledronic acid may have a protective role in preserving joint cartilage by reducing inflammation and promoting subchondral bone health []. Despite these benefits, bisphosphonates primarily target bone resorption and may not fully address the regenerative aspects of bone and cartilage.
In contrast, human Wharton Jelly-derived mesenchymal stem cells (WJMSCs) offer a regenerative approach to treating OP due to their ability to differentiate into osteoblasts and chondrocytes, as well as their anti-inflammatory and immunomodulatory properties []. WJMSCs, harvested from the umbilical cord matrix, have been shown to promote bone regeneration, enhance osteogenic differentiation, and modulate cytokine levels, making them a potential therapeutic option for both bone and cartilage repair. WJMSCs, are known for their low immunogenicity due to their lack of expression of MHC class II molecules and co-stimulatory molecules such as CD40, CD80, and CD86, which is different than other MSCs [,,]. WJMSCs can be efficiently isolated and cultured, demonstrating a high proliferation rate while maintaining their stemness over multiple in vitro passages. They are characterized by hypoimmunogenicity, nontumorigenic properties, and multipotency, with lower heterogeneity compared to MSCs derived from adult tissues [,]. Further, WJMSCs regulate the host immune response by suppressing the activation of diverse immune cells []. These distinctive characteristics make allogeneic WJMSCs a reliable and safe option for applications in stem cell therapy and tissue engineering. These properties enable WJMSCs to evade immune recognition and reduce the likelihood of eliciting an immune response in xenogeneic models and a clinical trial [,]. Preclinical studies have demonstrated that WJMSCs can improve bone healing, reduce inflammation, and protect joint cartilage from degradation, highlighting their dual potential in addressing both bone loss and cartilage damage associated with OP []. Furthermore, the immunomodulatory effect of WJMSCs may help to balance the pro-inflammatory cytokines involved in OP, such as the IL-31 and IL-33 axis [].
Extracorporeal shockwave therapy (ESWT) is a noninvasive, drug- and cell-free method commonly used to treat musculoskeletal disorders []. ESWT has demonstrated potential in promoting bone regeneration, enhancing BMD, and protecting joint cartilage in various preclinical studies [,]. ESWT exerts local effects on bone strength and systemic effects on bone turnover by modulating molecular pathways, including BMP2 expression and the TGF-β/SMAD2 pathway [,]. In particular, site-specific regions of bone can be treated with ESWT, providing targeted therapeutic opportunities for the management of osteoporosis. Moreover, ESWT treatment can lead to an increase in the systemic concentration of osteogenesis factors and bone turnover markers in serum [,]. These results suggest that ESWT has not only local effects on bone strength, bone mineral density (BMD), and bone volume but also systemic effects on osteoporosis. In addition, ESWT has been reported to stimulate tissue regeneration through mechanotransduction []. Therefore, ESWT has the potential to be a promising noninvasive therapy for osteoporosis by promoting new bone formation and improving bone strength and density, not only locally but also systemically.
Previous research on OP treatments has limitations. Bisphosphonates, though effective in reducing bone resorption, lack the ability to regenerate bone or repair cartilage comprehensively. WJMSCs, while promising for bone and cartilage repair, involve invasive cell preparation and delivery processes, making them less accessible for widespread clinical application. On the other hand, the long-term effects for ESWT remain unclear, with inconsistent findings on its efficacy for different skeletal sites.
Therefore, this study not only evaluates the comparative efficacy of ESWT, bisphosphonate (zoledronic acid), and WJMSC treatments but also demonstrates the potential systemic effects of the three treatments in an OVX rat model of OP. In addition, the IL-33/IL-31 axis is known to play a significant role in the development of osteoporosis []. By focusing on the IL-31/IL-33 axis and its role in OP pathology, we investigated the levels of IL31 and IL33 and the pathological changes associated with OP after treatments in an ovariectomized (OVX) rat model. Furthermore, the study compared the efficacy of ESWT with bisphosphonate (zoledronic acid) and Wharton jelly-derived mesenchymal stem cell (WJMSC) treatments for OP, capable of simultaneously addressing bone loss and cartilage degeneration. This study provides new insights into the relative advantages and mechanisms of these therapies, aiming to bridge the limitations of existing OP treatments.
2. Materials and Methods
2.1. The Animals
All of the animals were treated humanely according to the protocol for the Care and Use of Laboratory Animals, published by the National Institute of Health. All animals were housed under 23 ± 1 °C and had a 12 h light and dark cycle in the Center for Laboratory Animals. The Center for Laboratory Animals was provided for the veterinarians to take care the animals. This study was subjected to the approval of the Institutional Animal Care and Use Committee (IACUC) at the hospital (Approval No: 2018112102) and the work has been reported in line with ARRIVE guidelines []. The sample size was calculated using statistical G*Power (version 3.1.9.7.) analysis to show that eight rats in each group would provide sufficient statistical power (0.8) to detect a 10% difference in the experiment [].
2.2. Wharton Jelly-Derived Mesenchymal Stem Cells and Cell Identification
Human Wharton jelly-derived mesenchymal stem cells (WJMSC) were purchased from Cellular Engineering Technologies Inc. (Coralville, IA, USA) (Item code: HMSC.WJ-100). The WJMSC were cultured with DMEM complete medium (Gibco, Grand Island, NY, USA) and were identified by surface markers with CD14, CD44, CD105, CD133, and CD166 using a flow cytometer as in our previous study (Supplemental Figure S1) (BD LSRII, Franklin Lakes, NJ, USA) []. The dosage and multiple administration of WJMSC cells (2 × 106 cells) were modified as in our previous study, and treatments were administered by tail intravenous injection at 6 and 10 weeks post-surgery (Figure 1A) [].
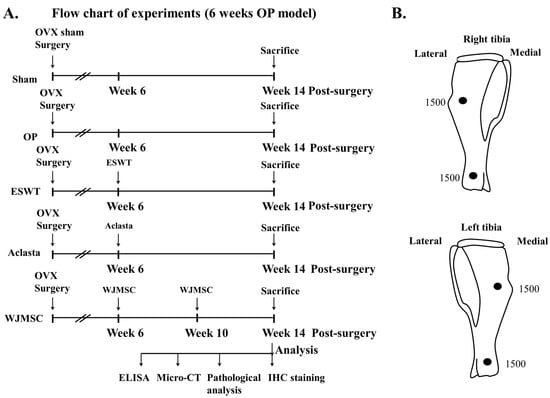
Figure 1.
The study design and application of ESWT. (A) Flowchart displaying the experiments and timing for knee surgery, shockwave application, Aclasta treatment, WJMSC treatments, and sacrifice of the animals. Eight rats were used for each group. (B) Focused ESWT application (0.25 mJ/mm2, 4 Hz, 1500 impulses for each location) on right and left tibia at 0.5 cm below the skin of rats at each location, indicated as black circles. OP group = osteoporosis, OVX = ovariectomy, ESWT group = extracorporeal shockwave therapy, WJMSC group = Wharton Jelly-derived mesenchymal stem cell treatment, micro-CT = micro computed tomography, IHC = immunohistochemistry, ELISA = enzyme-linked immunosorbent assay.
2.3. Establishment of the Animal Model and ESWT Application
The study design was shown in Figure 1A. In the experiment, forty 10-week old female Sprague-Dawley (SD) rats weighing approximately 200 to 250 g were randomly assigned to each group. The source of shockwaves was a DUOLITH SD1 (Storz Medical AG, Tägerwilen, Switzerland). The shockwaves were applied at two locations of the left tibia and right tibia at 0.5 cm below the skin surface post-surgery at 6 weeks (Figure 1B). Computer-generated randomization was used to assign the animals into 4 groups (8 rats for each group): The Sham group was the sham control without surgery or shockwaves. The OP group received bilateral ovariectomy (OVX), but no ESWT, and served as an osteoporotic animal model. The ESWT group included OVX rats that received a total of 3000 impulses (1500 impulses for each point per tibia) of shockwaves at 0.25 mJ/mm2 energy flux density, 4 Hz, on the bone of both tibias as shown in Figure 1B. The Aclasta group included OVX rats that received Aclasta (zoledronic acid, 0.1 mg/kg) by intravenous tail injection. The WJMSC group included OVX rats that received WJMSC cells (2 × 106 cells) by intravenous tail injection at 6 and 10 weeks post-surgery. After surgery, ampicillin (25 mg/kg) and ketorolac (1 mg/kg/day) were administered for five days to prevent infection and relieve pain. All animals were sacrificed at 14 weeks post-surgery (Figure 1A). The evaluating parameters included pathological analysis, micro-CT scanning, and immunohistochemical analysis.
2.4. Sample Collection
The rats were sacrificed at 14 weeks post-surgery. The samples were used for pathological and immunohistochemical analysis. Following the disarticulation of the femurs, tibias, knee joints, and spine (T5 to T9), the recovered regions were cleaned and washed with physiologic saline. The gross appearances of the specimens were recorded using a digital camera and the distal section of each specimen was fixed in 4% paraformaldehyde in phosphate buffered saline (PBS) for 24 h. The samples were then decalcified with 20% EDTA (pH 7.4) for 3 weeks at 4 °C and the medium was changed every 3 days. The serum of the rats was prepared after sacrifice at 14 weeks post-surgery and stored at −80 °C until use.
2.5. Micro-CT Scan
Cortical and trabecular bone morphometric parameters were determined in the distal femur, tibia, and spine using a micro-CT scan with filter A1 0.5 mm, exposure 270 ms, isotopic pixel size 18 × 18 × 18 μm, X-ray voltage 50 Kv, and 500 μA (SkyScan, 1076, Kartuizersweg 3B 2550 Kontich, Belgium). The bone volume, trabecular thickness, trabecular number, and bone mineral density were measured and computer analyzed.
2.6. Histomorphological Examination and Femoral and Tibial Bone Histopathology
The knee and spine samples were subjected to histomorphological examination. The harvested samples were fixed in 4% PBS-buffered formaldehyde at 4 °C for 7 days, and decalcified in 10% PBS-buffered EDTA at 4 °C for 14 days. Decalcified samples were fixed and subjected to paraffin wax embedding and dissection into 5 μm thick sections. The samples were stained with hematoxylin–eosin (HE) and safranin-O. The damage of the cartilage was graded histologically using OARSI scores for the assessments of cartilage structure, cartilage cells, and tidemark integrity. For bone histopathology, femur and tibia bones were fixed in 4% PBS-buffered formaldehyde and stored in decal for 24 h. The femur and tibia bones were sectioned into multiple segments and embedded in paraffin wax blocks. These blocks were subsequently cut using a microtome and stained with hematoxylin and eosin (H&E). After staining, the sections were mounted and examined for histopathological changes.
2.7. Immunohistochemistry
Decalcified sections of the knees and spines were probed with primary antibodies against inflammatory-related factors (IL31, 1:50, PAS-114390, Invitrogen, Waltham, MA, USA, and IL33, 1:100, orb 6205, Biorbyt, Cambridge, UK; receptor ST2, 1:50, PA5-23316, Invitrogen, Waltham, MA, USA) and growth factor (BMP2) (1:100, ab6285, Abcam, Cambridge, MA, USA) overnight at 4 °C. Detection was achieved by using a DAB kit (Sigma, Cream Ridge, NJ, USA) followed by counterstaining with hematoxylin. All images of each specimen were captured by using a cool CCD camera (Media Cybernetics, Silver Spring, MD, USA). Images were analyzed by manual counting and confirmed by using an image-pro Plus 6.0 Image-analysis software (Media Cybernetics, Silver Spring, MD, USA).
2.8. Enzyme-Linked Immunosorbent Assay
The expression of IL31 (MBS2702983, MyBioSource.com., San Diego, CA, USA), IL33 (M3300, R&D System, Minneapolis, MN, USA), bone morphogenetic protein 2 (BMP2) (DBP200, R&D System, Minneapolis, MN, USA), and vascular endothelial growth factor (VEGF) (RRV00, R&D System, Minneapolis, MN, USA) in the serum of rats was measured by enzyme-linked immunosorbent assay (ELISA) kits.
2.9. Statistical Analysis
The statistical analyses were performed using SPSS software (version 17.0, SPSS Inc., Chicago, IL, USA). The data are presented as mean ± standard deviation (SD). Differences between groups were evaluated using one-way analysis of variance (ANOVA) followed by a post-hoc Tukey–Kramer test to determine statistical significance. In order to compare between groups, statistical tests were employed, with significance levels set at p < 0.05, p < 0.01, and p < 0.001.
3. Results
3.1. ESWT, Aclasta, and WJMSC Modulated the Expression of IL31, IL33, VEGF, and BMP2 in the Serum of OVX Rats
The study design was shown in Figure 1, which involved the application of ESWT on both tibias to stimulate the release of growth factors for bone regeneration, protect the cartilage in joint and spine, and reduce inflammation. We also compared the effects of ESWT, Aclasta (zoledronic acid), and WJMSC treatments on OVX rats.
To assess the efficacy of the treatments, we measured the levels of serum IL31, IL33, VEGF, and BMP2 using ELISA. After treatments, we observed a significant decrease in serum IL31 and IL33 levels, while VEGF and BMP2 levels increased significantly as compared with the OP group (* p < 0.05, ** p < 0.01, and *** p < 0.001) (Table 1). These results indicated that the treatments had differential modulatory effects, with Aclasta demonstrating superior inhibition of IL31 and IL33 expression and greater enhancement of VEGF and BMP2 levels in OVX rats.

Table 1.
The serum levels of IL31, IL33, VEGF, and BMP2 in the OVX rat from Sham, OP, ESWT, Aclasta, and WJMSC groups by ELISA at 14 weeks post-surgery.
3.2. Micro-CT Analysis Shows Bone Recovery After ESWT, Aclasta, and WJMSC Treatments in the OVX Rats
In the experiment, micro-CT images were used to visualize the bone structures (lower limbs and spine) of Sham, OP, ESWT, Aclasta, and WJMSC groups (Figure 2). The images of the OP group showed that OVX rats had induced a loss of cancellous bone from the transverse and sagittal views of micro-CT as compared with the Sham group at 6 weeks. After treatments, ESWT, Aclasta, and WJMSC induced bone regeneration in both femurs, tibias, and vertebral bone (Figure 2A,B; red rectangle line, region of interest, ROI). The image results show that ESWT, Aclasta, and WJMSC improved bone regeneration and Aclasta was better than ESWT and WJMSC.
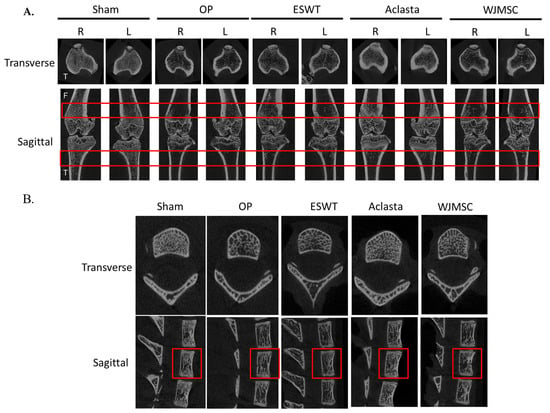
Figure 2.
Micro-CT scan of proximal femur, tibia, and spine in different groups. (A) The results displayed photomicrographs of the knee in transverse (tibia, up) and sagittal (femur and tibia, below) views from micro-CT. (B) The photomicrographs of the spine (T6 to T8) in transverse and sagittal views. The region of the red line was the region of interest in vertebral bone (T7). F indicated the femur and T indicated the tibia. R was the right lower limb and L was the left lower limb. Eight rats were used for each group. OP group was osteoporosis, OVX was ovariectomy, ESWT group was extracorporeal shockwave therapy, and WJMSC group was Wharton jelly-derived mesenchymal stem cell treatment.
Next, we were further analyzed the ROI regions for bone volume over total volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and bone mineral density (BMD) (Table 2). In the ESWT, Aclasta, and WJMSC groups, the BV/TV, Tb.Th, Tb.N, and BMD of right and left femurs and tibias as well as spine in the OVX rats were significantly improved as compared with the OP group (* p < 0.05, ** p < 0.01, and *** p < 0.001) (Figure 2 and Table 1). Further, the Aclasta group showed greater improvements in BV/TV, Tb.Th, Tb.N, and BMD compared to the ESWT and WJMSC groups (# p < 0.05 and ### p < 0.001). The results demonstrated that the treatment administered in Aclasta had better regeneration effects than ESWT and WJMSC in terms of improving the bone recovery of both lower limbs and vertebral bone in OVX rats.

Table 2.
The micro-CT analysis of both lower limbs and spine in Sham, OP, ESWT, Aclasta, and WJMSC groups.
3.3. ESWT, Aclasta, and WJMSC Protected the Degeneration of Articular Cartilage, Cancellous Bone, Epiphyseal Plate, Vertebral Cartilage, and Bone in OVX Rats
ESWT, Aclasta (zoledronic acid), and WJMSC treatments were compared the chondroprotective effects on the articular cartilage of the knees in OVX rats. The pathological changes of hyaline cartilage were revealed by H&E stain and safranin-O stain in Sham, OP, ESWT, Aclasta, and WJMSC groups (Figure 3). After treatment, the hyaline cartilages were improved in ESWT, Aclasta, and WJMSC groups as compared with Sham and OP groups (Figure 3B, arrow head). In addition, the epiphyseal plate (black arrow) and region of primary spongiosa (red triangle) became thin and lost the cancellous bone (black triangle) nearby in the OP group (Figure 3B). The epiphyseal plate and cancellous bone were regenerated in ESWT, Aclasta, and WJMSC groups as compared with the OP and Sham groups (Figure 3 and Table 2). The primary spongiosa of the Aclasta group was thicker than in the Sham, ESWT, and WJMSC groups (Figure 3B, red triangle). Furthermore, in the spine of the OP group, there was a loss of vertebral cartilage (arrowhead) and cancellous bone (black triangle). However, following treatments with ESWT, Aclasta, and WJMSC, all of these strictures improved (Figure 3C). Based on our findings, it appears that treatments with ESWT, Aclasta, and WJMSC had a protective effect on various structures including the articular cartilage, cancellous bone, epiphyseal plate, vertebral cartilage, and bone in OVX rats.
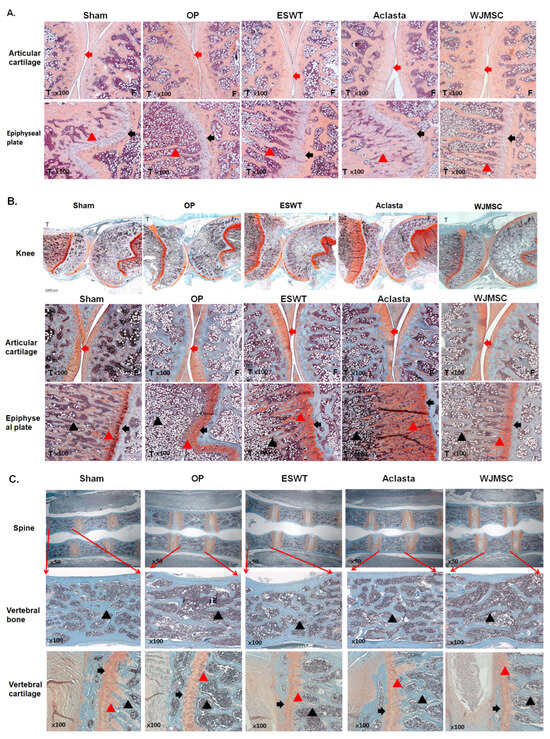
Figure 3.
The microphotographs of the left knees and spine. (A) The hematoxylin and eosin staining showed the cartilage change in the articular cartilage and epiphyseal plate of the left tibia (×100 magnification) in different groups. (B) Safranin-O staining showing the articular cartilage of the left knee (1000 μm) and epiphyseal plate of the left tibia (×100 magnification). (C) Safranin-O staining showing the spine (×50 magnification), vertebral bone (×100 magnification), and vertebral cartilage (×100 magnification). Eight rats were used for each group. The red arrowhead indicates cartilage. The black arrowhead indicated epiphyseal plate. The black triangle indicated cancellous bone. The red triangle indicated primary spongiosa. The tibia indicated by T and the femur indicated by F. Osteoporosis indicated by OP group. Extracorporeal shockwave therapy indicated ESWT group and Wharton jelly-derived mesenchymal stem cell treatment indicated WJMSC group.
3.4. ESWT, Aclasta, and WJMSC Modulated the Expression of Bone Remodeling-Related Cytokines in the Articular Cartilage and Epiphyseal Plate of OVX Rats
The cytokines IL31 and IL33 are reported to play a role in bone remodeling and osteoporosis. We showed the expression of IL31, IL33, and IL33 receptor ST2 in the articular cartilage and epiphyseal plate of the left tibia after treatments in OVX rats. The expression of IL31 and IL33 was induced in the articular cartilage and hypertrophic cartilage of the epiphyseal plate in the OP group (red arrow) and reduced in ESWT, Aclasta, and WJMSC groups (*** p < 0.001) (Figure 4A,B). There were no significant differences in the expression of IL31 and IL33 among the ESWT, Aclasta, and WJMSC groups. The expression of ST2 in the articular cartilage of the left femur and tibia showed contrasting patterns compared to IL31 and IL33. In the OP group, ST2 expression was reduced, whereas it was significantly induced in ESWT, Aclasta, and WJMSC groups (*** p < 0.001) (Figure 4C). The expression of ST2 did not show any significant differences among the ESWT, Aclasta, and WJMSC treatment groups.
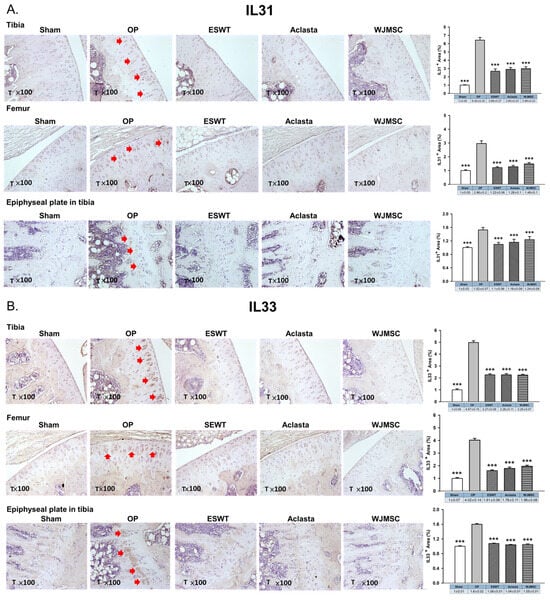
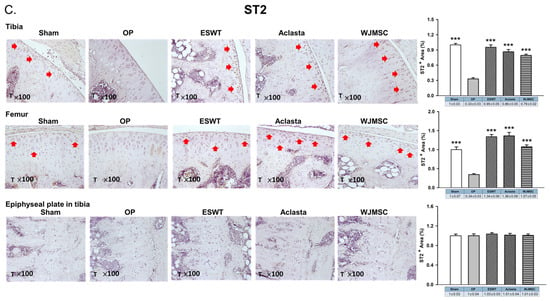
Figure 4.
Immunohistochemical analysis for (A) IL31, (B) IL33, and (C) ST2 in the articular cartilage (×100 magnification) of the left knee and epiphyseal plate of the left tibia in Sham, OP, ESWT, Aclasta, and WJMSC groups (right). The expression levels are assessed following the treatments (left). *** p < 0.001 as compared with the OP group. Eight rats were used for each group. Osteoporosis indicated OP group. Extracorporeal shockwave therapy indicated ESWT group and Wharton jelly-derived mesenchymal stem cell treatment indicated WJMSC group. T represented tibia. Red arrowhead indicated the expression of proteins.
3.5. ESWT, Aclasta, and WJMSC Stabilised BMP2 Expression to Protect Articular Cartilage, Cancellous Bone, and the Epiphyseal Plate in the OVX Rat Knee
In the analysis of the left knee, we observed that the expression of BMP2 was reduced in the OP group and was significantly induced to the same level of Sham group following treatments in the articular cartilage of the left tibia and femur (*** p < 0.001) (Figure 5). In the epiphyseal plate, the expression of BMP2 was obviously increased in the region of the primary spongiosa of metaphysis in the ESWT, Aclasta, and WJMSC groups to stimulate endochondral bone formation in OVX rats. The results may suggest that ESWT, Aclasta, and WJMSC maintained the level of BMP2 in articular cartilage to protect the extracellular matrix proteins and stimulated the expression in the primary spongiosa of metaphysis to promote the regeneration of endochondral bone and cancellous bone in OVX rats.
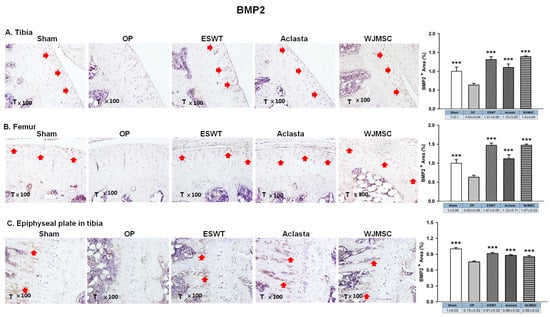
Figure 5.
Immunohistochemical analysis for BMP2 in the articular cartilage of the (A) tibia, (B) femur, and (C) epiphyseal plate of the left tibia in Sham, OP, SW, Aclasta, and WJMSC groups (×100 magnification) (right). The expression level was assessed following the treatments (left). *** p < 0.001 as compared with the OP group. T represented tibia and Eight rats were used for each group. Extracorporeal shockwave therapy indicated ESWT group and Wharton jelly-derived mesenchymal stem cell treatment indicated WJMSC group. Red arrowhead indicated the expression of proteins.
3.6. ESWT, Aclasta, and WJMSC Modulated the Expression of IL31, IL33, ST2, and BMP2 in the Vertebral Cartilage of OVX Rats
The expression of IL31 and IL33 was significantly increased in the deep zone of vertebral cartilage in OVX rats, but was decreased in ESWT, Aclasta, and WJMSC groups (*** p < 0.001) (Figure 6). However, there was no notable difference in the level of ST2, the receptor for IL33, among all groups. Additionally, BMP2 expression was decreased in the deep zone of vertebral cartilage in OVX rats, but significantly increased in the ESWT, Aclasta, and WJMSC groups to promote endochondral bone formation (*** p < 0.001). These findings indicated that while ESWT, Aclasta, and WJMSC groups all led to the inhibition of IL31 and IL33 expression and the enhancement of BMP2 expression, no significant differences were observed between the treatment modalities.
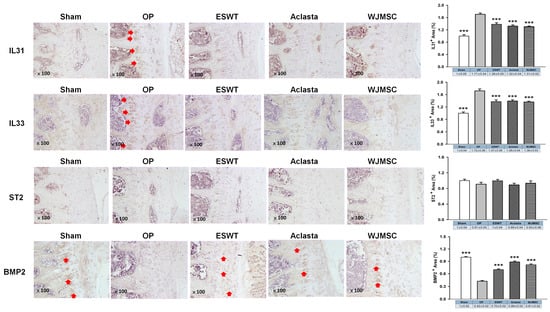
Figure 6.
The immunohistochemical analysis of the vertebral cartilage in OVX rats. The immunohistochemical staining (×100 magnification, left panel) and the expression levels (right) of IL31, IL33, ST2, and BMP2 in the vertebral cartilage of the spines for each group. *** p < 0.001 as compared with the OP group. Eight rats were used for each group. Extracorporeal shockwave therapy indicated ESWT group and Wharton jelly-derived mesenchymal stem cell treatment indicated WJMSC group. Red arrowhead indicated the expression of proteins.
4. Discussion
Our study demonstrated that all three treatments, ESWT, Aclasta, and WJMSCs, exerted chondroprotective and osteogenic effects in the ovariectomized (OVX) rat model of osteoporosis. Additionally, the expression of two pro-inflammatory cytokines (IL31 and IL33) was reduced and growth factor (BMP2) was enhanced in the articular cartilage, epiphyseal plate, and spinal cartilage by the widespread effect of the treatments. Interestingly, the levels of serum IL31, IL33, VEGF, and BMP2 were also modulated after treatments, suggesting a potential role for these factors in the pathogenesis and treatments of OP. Overall, these findings provide valuable insights into the potential mechanisms of action of ESWT, Aclasta, and WJMSC in treating OP and may help inform the development of novel therapies for this debilitating disease.
BMP2 and VEGF are significant growth factors for bone formation and bone healing [,]. BMP2 is a potent inducer of osteoblastic activity and can stimulate mesenchymal stem cells to differentiate into an osteoblastic lineage, promoting bone formation. Similarly, VEGF has been shown to enhance endochondral bone formation by promoting angiogenesis and the recruitment of chondroclasts to the hypertrophic cartilage, facilitating the replacement of the cartilaginous matrix with a bony callus, ultimately leading to intramembranous ossification []. Further, ESWT, Aclasta (zoledronic acid), and WJMSC have been reported to increase the levels of BMP2 and VEGF that are positively associated with bone formation [,,]. Our study findings suggest that all three treatments were effective in stimulating the serum levels of BMP2 and VEGF. This increase in BMP2 and VEGF levels may help to reduce bone loss and promote bone formation throughout the body in OVX rats (Figure 2 and Table 1). Subsequently, all treatments not only prevented bone loss, but also protected the cellular matrix protein in the articular cartilage, epiphyseal plate, and spinal cartilage (Figure 4 and Figure 6). These findings suggest that the treatments may have a beneficial effect on the preservation of bone and joint health. However, further investigations are required to elucidate the mechanisms underlying treatments aimed at promoting bone formation and protecting cartilage.
We know that Aclasta, zoledronic acid, is a type of bisphosphonate drug that is commonly used to treat osteoporosis, especially for postmenopausal women []. It works by inhibiting the activity of osteoclasts, the cells responsible for bone resorption, thereby reducing bone loss and improving bone density []. In addition, Wharton’s jelly-derived mesenchymal stem cells (WJMSCs) are a type of stem cell that is isolated from the umbilical cord. These stem cells have the ability to differentiate into various cell types, including osteoblasts. When transplanted into a patient, WJMSCs have been shown to improve bone density and promote bone regeneration, making them a potential therapeutic option for osteoporosis [,]. Further, ESWT is a well-known and safe physical technology for the bone diseases []. Clinical studies have been conducted to investigate the effectiveness of ESWT, zoledronic acid, and WJMSCs for osteoporosis treatment individually [,,]. In addition, zoledronic acid can effectively decrease the incidence of vertebral, hip, and non-vertebral fractures in individuals with osteoporosis. However, treatment with zoledronic acid may also carry the risk of serious side effects such as renal impairment, hypocalcemia, osteonecrosis of the jaw, and atrial fibrillation [,]. WJMSCs have been shown to improve bone mineral density and promote bone regeneration in clinical and animal models of osteoporosis; however, the complete side effects remain unknown []. Our study demonstrates that ESWT, Aclasta, and WJMSCs show comparable efficacy in promoting systemic bone regeneration in OVX rats, as evidenced by the data presented in Figure 1 and Table 2. The efficacy of Aclasta is better than ESWT and WJMSCs in bone regeneration (Table 2). In terms of overall efficacy, Aclasta appeared to have the greatest impact on systemic bone formation compared to ESWT and WJMSCs. However, all treatments are comparably effective in preventing bone loss across the skeleton. However, considering their comparable efficacy, it may be worthwhile to investigate the potential benefits of combining these treatments to achieve greater bone regeneration and to reduce side effects. ESWT combined with low dose Aclasta might be suggested for osteoporosis. Therefore, further research is necessary to evaluate the safety and efficacy of this combination therapy in human subjects.
Interleukin-33 (IL-33) and interleukin-31 (IL-31) are two cytokines that have been implicated in the pathogenesis of several bone-related disorders, including osteoporosis []. IL-33 is an alarmin cytokine, a member of the IL-1 family, and is produced by various cell types such as endothelial cells, fibroblasts, and osteoblasts. IL-33 may play a double-edged role in the regulation of bone remodeling by inducing the differentiation and activation of osteoclasts and osteoblasts [,]. IL-33 can stimulate the activity of immune cells that produce inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6) []. These cytokines can contribute to bone loss by increasing the activity of osteoclasts and suppressing the activity of osteoblasts []. On the other hand, IL-33 has been shown to enhance bone formation and prevent bone loss by modulating the expression of IL31, suggesting that it has a protective effect against osteoporosis in animal studies []. In addition, IL-31 is a cytokine produced by T-helper 2 (Th2) cells and has been implicated in the pathogenesis of several inflammatory, allergic, and autoimmune disorders []. Recent studies have suggested that IL-31 may play a role in bone metabolism by promoting osteoclastogenesis and bone resorption []. Studies have shown that IL33 and IL-31 can promote cartilage destruction by inducing the production of matrix metalloproteinases (MMPs) and inhibiting the production of tissue inhibitors of metalloproteinases (TIMPs) in chondrocytes [,]. In the experiments, the expression of inflammatory IL31 and IL33 is observed in the articular cartilage of the knee as well as in the hypertrophic zone of the growth plate and spine of OXV rats (Figure 4 and Figure 6). The serum IL-31 and IL-33 levels are increased in OVX rats compared to sham rats (Table 1). These results indicated that osteoporosis-induced IL31 and IL33 systemically disrupted the balance of cartilage homeostasis and endochondral bone formation. In addition, ESWT, Aclasta, and WJMSCs treatments equally reduced the expression of IL31 and IL33 to protect the loss of cartilage matrix in the joints and intervertebral disc. Cartilage protection was an important endpoint in this study, given the dual focus on osteoporosis and its effects on the articular cartilage, epiphyseal plate, and spinal cartilage. Histological analysis demonstrated that all treatments preserved the integrity of the cartilage matrix proteins, preventing the loss of collagen and proteoglycans (Figure 3). This suggests that the treatments not only reduce bone loss but also maintain cartilage homeostasis, which is critical in osteoporotic joints and intervertebral discs.
The limitations of the study are illustrated as follows. The OVX rat model is a commonly used animal model for studying osteoporosis; however, it does not fully elucidate the complexity and symptoms of human osteoporosis. Small animal experiments cannot fully represent the treatment of the human disease. The optimization of ESWT dosage should be further studied and refined in human trials, as the response to the treatments may differ between animals and humans. Moreover, the dosage of WJMSCs requires further optimization in large animal models or human clinical trials. Previous studies have demonstrated that a high dose of WJMSCs (10 × 10⁶ cells/kg, nearly double the dose used in our study) is safe for OVX rats []. However, the long-term safety of WJMSC treatment remains to be thoroughly evaluated in future investigations. Further demonstration of the effects of all three treatments is necessary to evaluate their efficacy in treating osteoporosis. Additionally, a more comprehensive analysis of cytokines and growth factors is needed to fully assess the therapeutic potential of these treatments in future clinical trials.
5. Conclusions
This study demonstrates that ESWT, Aclasta, and WJMSC treatments improve bone regeneration and prevent the degeneration of articular and vertebral cartilage in osteoporotic rats. The three therapies reduced IL31 and IL33 expression in articular and vertebral cartilage while inducing the IL33 receptor ST2 in articular cartilage. The levels of IL31 and IL33 in serum were decreased, while BMP2 and VEGF were increased, enhancing bone formation in limb and vertebral bones. BMP2 modulation in cartilage further supports their role in osteogenesis. In addition to the need for effective osteoporosis treatments with fewer side effects, our findings suggest that combining ESWT with lower doses of Aclasta or WJMSC may enhance bone regeneration while minimizing risks like renal impairment or osteonecrosis. However, the study has limitations. It is restricted to a preclinical OVX rat model, with unknown applicability to humans. The molecular pathways underlying cytokine modulation remain unclear, and the long-term safety of combination therapies needs validation. Future studies should confirm these results in large animal models or clinical trials, investigate the mechanisms of cytokine and growth factor regulation, and optimize dosing regimens to enhance safety and efficacy. Addressing these challenges will lead to the development of safer and more effective osteoporosis treatments for clinical use.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines12122823/s1, Supplemental Figure S1. The Human WJMSC surface markers are measured by flow cytometry. The positive markers are CD44 (99.71%), CD105 (99.66%) and CD166 (99.97%). The negative markers are CD14 and CD133.
Author Contributions
J.-H.C.: Conceptualization, Funding Acquisition, Investigation, Methodology, Supervision, Validation, Writing—original draft, Writing—review and editing. C.-W.C.: Methodology, Writing—original draft. W.-Y.C.: Data curation, Supervision, Validation, Writing—original draft. P.-C.C.: Methodology, Validation, Writing—original draft. K.-T.W.: Formal Analysis, Investigation, Writing—original draft. S.-W.J.: Formal Analysis, Data curation, Writing—original draft. S.-L.H.: Data curation, Methodology, Validation. Y.-N.W.: Methodology, Writing—original draft. H.-T.C.: Methodology, Validation, Writing—original draft, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded from Chang Gung Medical Foundation, Kaohsiung Chang Gung Memorial Hospital, grant number CMRPG8J1591.
Institutional Review Board Statement
This study was subjected to the approval of the Institutional Animal Care and Use Committee (IACUC) at KCGMH (Approval number: 2018112102).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We are grateful to the Department of Medical Research, Core Lab for Phenomics and Diagnostics, Center for Laboratory Animals, Kaohsiung Chang Gung Memorial Hospital, for supporting this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Barnsley, J.; Buckland, G.; Chan, P.E.; Ong, A.; Ramos, A.S.; Baxter, M.; Laskou, F.; Dennison, E.M.; Cooper, C.; Patel, H.P. Pathophysiology and treatment of osteoporosis: Challenges for clinical practice in older people. Aging Clin. Exp. Res. 2021, 33, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Society, N.A.M. Management of osteoporosis in postmenopausal women: The 2021 position statement of The North American Menopause Society. Menopause 2021, 28, 973–997. [Google Scholar] [CrossRef]
- Chitra, V.; Sharon, S.E. Diagnosis, Screening and Treatment of Osteoporosis—A Review. Biomed. Pharmacol. J. 2021, 14, 567–575. [Google Scholar] [CrossRef]
- Benedetti, M.G.; Furlini, G.; Zati, A.; Letizia Mauro, G. The Effectiveness of Physical Exercise on Bone Density in Osteoporotic Patients. BioMed Res. Int. 2018, 2018, 840531. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-S.; He, S.-H.; Xie, P.-Y.; Li, W.; Zhang, X.-X.; Li, T.-F.; Li, D.-F. Recent Progresses in the Treatment of Osteoporosis. Front. Pharmacol. 2021, 12, 717065. [Google Scholar] [CrossRef] [PubMed]
- Simas, V.; Hing, W.; Pope, R.; Climstein, M. Effects of water-based exercise on bone health of middle-aged and older adults: A systematic review and meta-analysis. Open Access J. Sports Med. 2017, 8, 39–60. [Google Scholar] [CrossRef]
- Inoue, S.; Hatakeyama, J.; Aoki, H.; Kuroki, H.; Niikura, T.; Oe, K.; Fukui, T.; Kuroda, R.; Akisue, T.; Moriyama, H. Utilization of Mechanical Stress to Treat Osteoporosis: The Effects of Electrical Stimulation, Radial Extracorporeal Shock Wave, and Ultrasound on Experimental Osteoporosis in Ovariectomized Rats. Calcif. Tissue Int. 2021, 109, 215–229. [Google Scholar] [CrossRef]
- Türker, M. Histological and biomechanical effects of zoledronate on fracture healing in an osteoporotic rat tibia model. Jt. Dis. Relat. Surg. 2016, 27, 9–15. [Google Scholar] [CrossRef]
- Kim, B.; Cho, Y.; Lim, W. Osteoporosis therapies and their mechanisms of action (Review). Exp. Ther. Med. 2021, 22, 1379. [Google Scholar] [CrossRef]
- Bi, H.; Chen, X.; Gao, S.; Yu, X.; Xiao, J.; Zhang, B.; Liu, X.; Dai, M. Key Triggers of Osteoclast-Related Diseases and Available Strategies for Targeted Therapies: A Review. Front. Med. 2017, 4, 234. [Google Scholar] [CrossRef]
- Foger-Samwald, U.; Dovjak, P.; Azizi-Semrad, U.; Kerschan-Schindl, K.; Pietschmann, P. Osteoporosis: Pathophysiology and therapeutic options. EXCLI J. 2020, 19, 1017–1037. [Google Scholar] [CrossRef] [PubMed]
- Dalle Carbonare, L.; Mirko, Z.; Adriano, G.; Maria Teresa, V. Safety and tolerability of zoledronic acid and other bisphosphonates in osteoporosis management. Drug Healthc. Patient Saf. 2010, 2, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Tawaratsumida, H.; Iuchi, T.; Masuda, Y.; Ide, T.; Maesako, S.; Miyazaki, T.; Ijuin, T.; Maeda, S.; Taniguchi, N. Zoledronate alleviates subchondral bone collapse and articular cartilage degeneration in a rat model of rotator cuff tear arthropathy. Osteoarthr. Cartil. 2024. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-W.; Staples, M.; Shinozuka, K.; Pantcheva, P.; Kang, S.-D.; Borlongan, C. Wharton’s Jelly-Derived Mesenchymal Stem Cells: Phenotypic Characterization and Optimizing Their Therapeutic Potential for Clinical Applications. Int. J. Mol. Sci. 2013, 14, 11692–11712. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Davies, L.C. Mesenchymal stromal cells and the innate immune response. Immunol. Lett. 2015, 168, 140–146. [Google Scholar] [CrossRef]
- Weiss, M.L.; Anderson, C.; Medicetty, S.; Seshareddy, K.B.; Weiss, R.J.; VanderWerff, I.; Troyer, D.; McIntosh, K.R. Immune Properties of Human Umbilical Cord Wharton’s Jelly-Derived Cells. Stem Cells 2008, 26, 2865–2874. [Google Scholar] [CrossRef]
- Li, X.; Bai, J.; Ji, X.; Li, R.; Xuan, Y.; Wang, Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int. J. Mol. Med. 2014, 34, 695–704. [Google Scholar] [CrossRef]
- Bongso, A.; Fong, C.-Y. The Therapeutic Potential, Challenges and Future Clinical Directions of Stem Cells from the Wharton’s Jelly of the Human Umbilical Cord. Stem Cell Rev. Rep. 2012, 9, 226–240. [Google Scholar] [CrossRef]
- Liau, L.L.; Ruszymah, B.H.I.; Ng, M.H.; Law, J.X. Characteristics and clinical applications of Wharton’s jelly-derived mesenchymal stromal cells. Curr. Res. Transl. Med. 2020, 68, 5–16. [Google Scholar] [CrossRef]
- De Windt, T.S.; Vonk, L.A.; Slaper-Cortenbach, I.C.M.; van den Broek, M.P.H.; Nizak, R.; van Rijen, M.H.P.; de Weger, R.A.; Dhert, W.J.A.; Saris, D.B.F. Allogeneic Mesenchymal Stem Cells Stimulate Cartilage Regeneration and Are Safe for Single-Stage Cartilage Repair in Humans upon Mixture with Recycled Autologous Chondrons. Stem Cells 2017, 35, 256–264. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, N.; Li, T.; Guo, J.; Wang, Z.; Yang, M.; Gao, L. Human umbilical cord Wharton’s jelly stem cells: Immune property genes assay and effect of transplantation on the immune cells of heart failure patients. Cell. Immunol. 2012, 276, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.-S.; Lu, C.-H.; Chu, K.-A.; Yeh, C.-C.; Chiang, T.-L.; Ko, T.-L.; Chiu, M.-M.; Chen, C.-F. Xenograft of Human Umbilical Mesenchymal Stem Cells from Wharton’s Jelly Differentiating into Osteocytes and Reducing Osteoclast Activity Reverses Osteoporosis in Ovariectomized Rats. Cell Transplant. 2018, 27, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Phetfong, J.; Sanvoranart, T.; Nartprayut, K.; Nimsanor, N.; Seenprachawong, K.; Prachayasittikul, V.; Supokawej, A. Osteoporosis: The current status of mesenchymal stem cell-based therapy. Cell. Mol. Biol. Lett. 2016, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- De Martinis, M.; Sirufo, M.M.; Suppa, M.; Ginaldi, L. IL-33/IL-31 Axis in Osteoporosis. Int. J. Mol. Sci. 2020, 21, 1239. [Google Scholar] [CrossRef]
- Moya, D.; Ramón, S.; Schaden, W.; Wang, C.-J.; Guiloff, L.; Cheng, J.-H. The Role of Extracorporeal Shockwave Treatment in Musculoskeletal Disorders. J. Bone Jt. Surg. 2018, 100, 251–263. [Google Scholar] [CrossRef]
- Auersperg, V.; Trieb, K. Extracorporeal shock wave therapy: An update. EFORT Open Rev. 2020, 5, 584–592. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Cheng, J.-H.; Wang, C.-J.; Ko, J.-Y.; Hsu, S.-L.; Hsu, T.-C. Shockwave Therapy Combined with Autologous Adipose-Derived Mesenchymal Stem Cells Is Better than with Human Umbilical Cord Wharton’s Jelly-Derived Mesenchymal Stem Cells on Knee Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 1217. [Google Scholar] [CrossRef]
- Li, B.; Wang, R.; Huang, X.; Ou, Y.; Jia, Z.; Lin, S.; Zhang, Y.; Xia, H.; Chen, B. Extracorporeal Shock Wave Therapy Promotes Osteogenic Differentiation in a Rabbit Osteoporosis Model. Front. Endocrinol. 2021, 12, 627718. [Google Scholar] [CrossRef]
- Wang, C.J.; Yang, Y.J.; Huang, C.C. The effects of shockwave on systemic concentrations of nitric oxide level, angiogenesis and osteogenesis factors in hip necrosis. Rheumatol. Int. 2011, 31, 871–877. [Google Scholar] [CrossRef]
- Wolfl, C.; Schuster, L.; Honer, B.; Englert, S.; Klein, R.; Hirche, C.; Munzberg, M.; Grutzner, P.A.; Kneser, U.; Harhaus, L. Influence of extracorporeal shock wave therapy (ESWT) on bone turnover markers in organisms with normal and low bone mineral density during fracture healing: A randomized clinical trial. GMS Interdiscip. Plast. Reconstr. Surg. DGPW 2017, 6, Doc17. [Google Scholar] [CrossRef]
- D’Agostino, M.C.; Craig, K.; Tibalt, E.; Respizzi, S. Shock wave as biological therapeutic tool: From mechanical stimulation to recovery and healing, through mechanotransduction. Int. J. Surg. 2015, 24, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Burguera, B.; Hofbauer, L.C.; Thomas, T.; Gori, F.; Evans, G.L.; Khosla, S.; Riggs, B.L.; Turner, R.T. Leptin Reduces Ovariectomy-Induced Bone Loss in Rats. Endocrinology 2001, 142, 3546–3553. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gronthos, S.; Huang, S.; Xu, L.; Sun, Y.; Lin, S.; Gu, W.; Liu, Y.; Zhang, J.; Chen, L.; Li, G. Systemic Administration of Allogeneic Mesenchymal Stem Cells Does Not Halt Osteoporotic Bone Loss in Ovariectomized Rats. PLoS ONE 2016, 11, e0163131. [Google Scholar] [CrossRef][Green Version]
- Liu, K.; Meng, C.-X.; Lv, Z.-Y.; Zhang, Y.-J.; Li, J.; Li, K.-Y.; Liu, F.-Z.; Zhang, B.; Cui, F.-Z. Enhancement of BMP-2 and VEGF carried by mineralized collagen for mandibular bone regeneration. Regen. Biomater. 2020, 7, 435–440. [Google Scholar] [CrossRef]
- Rady, A.A.M.; Hamdy, S.M.; Abdel-Hamid, M.A.; Hegazy, M.G.A.; Fathy, S.A.; Mostafa, A.A. The role of VEGF and BMP-2 in stimulation of bone healing with using hybrid bio-composite scaffolds coated implants in animal model. Bull. Natl. Res. Cent. 2020, 44, 131. [Google Scholar] [CrossRef]
- Grosso, A.; Burger, M.G.; Lunger, A.; Schaefer, D.J.; Banfi, A.; Di Maggio, N. It Takes Two to Tango: Coupling of Angiogenesis and Osteogenesis for Bone Regeneration. Front. Bioeng. Biotechnol. 2017, 5, 68. [Google Scholar] [CrossRef]
- Bellone, F.; Catalano, A.; Sottile, A.R.; Gaudio, A.; Loddo, S.; Corica, F.; Morabito, N. Early Changes of VEGF Levels After Zoledronic Acid in Women With Postmenopausal Osteoporosis: A Potential Role of Vitamin D. Front. Med. 2021, 8, 748438. [Google Scholar] [CrossRef]
- Simplicio, C.L.; Purita, J.; Murrell, W.; Santos, G.S.; dos Santos, R.G.; Lana, J.F.S.D. Extracorporeal shock wave therapy mechanisms in musculoskeletal regenerative medicine. J. Clin. Orthop. Trauma 2020, 11, S309–S318. [Google Scholar] [CrossRef]
- Kang, B.-J.; Ryu, H.-H.; Park, S.S.; Koyama, Y.; Kikuchi, M.; Woo, H.-M.; Kim, W.H.; Kweon, O.-K. Comparing the osteogenic potential of canine mesenchymal stem cells derived from adipose tissues, bone marrow, umbilical cord blood, and Wharton’s jelly for treating bone defects. J. Vet. Sci. 2012, 13, 299–310. [Google Scholar] [CrossRef]
- Wang, C. Efficacy and Safety of Zoledronic Acid for Treatment of Postmenopausal Osteoporosis: A Meta-Analysis of Randomized Controlled Trials. Am. J. Ther. 2017, 24, e544–e552. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhan, Y.; Yan, L.; Hao, D. How zoledronic acid improves osteoporosis by acting on osteoclasts. Front. Pharmacol. 2022, 13, 961941. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.; Kim, K.-T.; Kim, K.G.; Choi, U.-Y.; Kyung, J.W.; Sohn, S.; Lim, S.H.; Choi, H.; Ahn, T.-K.; Choi, H.J.; et al. Safety and efficacy of Wharton’s jelly-derived mesenchymal stem cells with teriparatide for osteoporotic vertebral fractures: A phase I/IIa study. Stem Cells Transl. Med. 2021, 10, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.S.; Yazid, M.D.; Sainik, N.Q.A.V.; Razali, R.A.; Saim, A.B.; Idrus, R.B.H. Osteogenic Induction of Wharton’s Jelly-Derived Mesenchymal Stem Cell for Bone Regeneration: A Systematic Review. Stem Cells Int. 2018, 2018, 2406462. [Google Scholar] [CrossRef]
- Shi, L.; Gao, F.; Sun, W.; Wang, B.; Guo, W.; Cheng, L.; Li, Z.; Wang, W. Short-term effects of extracorporeal shock wave therapy on bone mineral density in postmenopausal osteoporotic patients. Osteoporos. Int. 2017, 28, 2945–2953. [Google Scholar] [CrossRef]
- He, B.; Zhao, J.Q.; Zhang, M.Z.; Quan, Z.X. Zoledronic acid and fracture risk: A meta-analysis of 12 randomized controlled trials. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1564–1573. [Google Scholar] [CrossRef]
- Greear, E.L.; Bankole, A. Zoledronate. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Okragly, A.J.; Hamang, M.J.; Pena, E.A.; Baker, H.E.; Bullock, H.A.; Lucchesi, J.; Martin, A.P.; Ma, Y.L.; Benschop, R.J. Elevated levels of Interleukin (IL)-33 induce bone pathology but absence of IL-33 does not negatively impact normal bone homeostasis. Cytokine 2016, 79, 66–73. [Google Scholar] [CrossRef]
- Miller, A.M. Role of IL-33 in inflammation and disease. J. Inflamm. 2011, 8, 22. [Google Scholar] [CrossRef]
- Wang, T.; He, C. TNF-α and IL-6: The Link between Immune and Bone System. Curr. Drug Targets 2020, 21, 213–227. [Google Scholar] [CrossRef]
- Murdaca, G.; Greco, M.; Tonacci, A.; Negrini, S.; Borro, M.; Puppo, F.; Gangemi, S. IL-33/IL-31 Axis in Immune-Mediated and Allergic Diseases. Int. J. Mol. Sci. 2019, 20, 5856. [Google Scholar] [CrossRef]
- He, Z.; Song, Y.; Yi, Y.; Qiu, F.; Wang, J.; Li, J.; Jin, Q.; Sacitharan, P.K. Blockade of IL-33 signalling attenuates osteoarthritis. Clin. Transl. Immunol. 2020, 9, e1185. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.M.L.; Ng, A.M.H.; Mohd Yunus, M.H.; Hj Idrus, R.B.; Law, J.X.; Yazid, M.D.; Chin, K.-Y.; Shamsuddin, S.A.; Mohd Yusof, M.R.; Razali, R.A.; et al. Safety study of allogeneic mesenchymal stem cell therapy in animal model. Regen. Ther. 2022, 19, 158–165. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).