Abstract
Non-alcoholic fatty liver disease (NAFLD) and arterial hypertension (AH) are widespread noncommunicable diseases in the global population. Since hypertension and NAFLD are diseases associated with metabolic syndrome, they are often comorbid. In fact, many contemporary published studies confirm the association of these diseases with each other, regardless of whether other metabolic factors, such as obesity, dyslipidemia, and type 2 diabetes mellites, are present. This narrative review considers the features of the association between NAFLD and AH, as well as possible pathophysiological mechanisms.
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) and arterial hypertension (AH) are common noncommunicable diseases in the global population. To date, the impact of NAFLD extends far beyond the liver; its association with an increased risk of cardiovascular disease (CVD), both in isolation and as part of the metabolic syndrome, has been proven [1]. The relationship between CVD and NAFLD is also explained by the commonality of risk factors underlying the development of these diseases. In addition to already known risk factors (abdominal obesity [2], high blood cholesterol [3], metabolic disorders [4], etc.), data have been obtained on other factors that can trigger the development and progression of both NAFLD and CVD [5]. Among these, we should mention an increase in serum levels of uric acid [6], C-reactive protein, interleukin (IL)-6 [7], fibrinogen, von Willebrand factor, and plasminogen activator inhibitor-1 [8], as well as an augmented thickness of epicardial fat [9] and intima-media complex [10]. AH is included in the criteria for metabolic syndrome [11]. It accompanies NAFLD quite frequently and is a major risk factor for CVD [12].
Although NAFLD is not included in the criteria for metabolic syndrome [13,14], a growing body of evidence indicates an association between AH and NAFLD and supports the concept that NAFLD may be considered as the hepatic manifestation of metabolic syndrome [5]. In our narrative review, we consider the research results, features, and possible pathophysiological mechanisms explaining this association.
2. Methods
The objective of this literature review was to identify evidence that demonstrates the relationship between the development and progression of NAFLD and arterial hypertension with a focus on the most recent data (published within the past 5 years, 2018–2023). Papers published before 2018 were included only when they provided information critical for the topic of the present literature review. In this regard, we searched the PubMed electronic database on 25 May 2023 using the following PubMed filters:
- Article type: Classical Article, Clinical Study, Clinical Trial, Comparative Study, Con- trolled Clinical Trial, Multicenter Study, Meta-Analysis, Observational Study, and Randomized Controlled Trial, Preprint;
- Species: Humans, Other Animals;
- Article language: English;
- Age: Adult;
- Publication date: 5 years.
Articles outside of the PubMed search were also included, if relevant.
Articles were included in the full-text narrative review in cases where they reported on disease burden, epidemiology, and pathogenic mechanisms of the relationship between NAFLD and arterial hypertension.
3. Prognoses of Patients with NAFLD and AH
Regardless of common risk factors, NAFLD is associated with a higher probability of developing CVD and their complications. The latter include atherosclerotic manifestations (thickening of the intima-media, endothelial dysfunction, and increased arterial stiffness), left ventricular hypertrophy, calcification of the aortic valve and coronary arteries [15,16]. The comorbidity of NAFLD and AH significantly increases the risk of atherosclerosis compared to what is observed in the presence of only one of these diseases [17].
Epidemiological studies suggested that the simultaneous presence of NAFLD and AH, regardless of other factors, significantly aggravates the prognosis of patients and increases the likelihood of cardiovascular complications [18]. A large study involving a Chinese cohort established that patients with comorbid AH and NAFLD had a higher risk of CVD mortality vs. those with NAFLD alone. In addition, the risk of death was higher in patients with NAFLD and uncontrolled AH (hazard ratio [HR] 2.36, 95% confidence interval [CI] 1.36–4.10, p < 0.01). After adjusting for confounding factors (presence of diabetes mellitus, body mass index [BMI], age, gender, and smoking status), statistically significantly higher mortality rate was still observed [19]. Also, during a 13-year follow-up period for over 70,000 patients with NAFLD, it was noted that the presence of high normal blood pressure (BP) (in the range of 120/80–139/89 mmHg) increased the risk of developing CVD and CVD-related mortality 1.5-fold when compared with the group with normal BP [20]. Hence, even early-onset hypertension in individuals with NAFLD aggravates the prognosis of patients.
A prospective study of 271,906 patients with NAFLD established that the likelihood of NAFLD progressing to cirrhosis or developing hepatocellular carcinoma increased with each additional component of the metabolic syndrome: e.g., in the presence of AH, the risk increased by 59% [21]. This may be explained by the fact that frequent co-occurrence of metabolic conditions and their interplay may contribute to liver disease progression and hepatocarcinogenesis through the influence on common pathophysiological mechanisms, such as oxidative stress, lipotoxicity, cytokine disbalance, etc. [22]. However, interpreting negative impact of the comorbidity of these diseases seems problematic due to the high prevalence of concomitant confounding factors, such as obesity, diabetes mellitus, and hyperlipidemia. There is evidence that the presence of visceral fat rather than an excess of total body weight per se predicts the risk of progression of steatosis to steatohepatitis and liver fibrosis [23]. For instance, it is known that, among Asian people, NAFLD is most common in patients with normal BMI and simultaneous visceral obesity (high waist circumference) [24].
It should be noted that the association between the presence of NAFLD and AH in thin patients was observed as well [24]. The results of a Chinese patient cohort study were published in 2023. They demonstrated that, even though patients had normal body weight (BMI < 25 kg/m2), the AH was the main cause of NAFLD development with an overall HR of 2.05 (95% CI 1.87–2.25), and five different statistical methods were used for the HR calculation to assess the relationship [25]. Therefore, it can be concluded that the relationship between NAFLD and AH exists regardless of obesity (BMI ≥ 30 kg/m2 for the Caucasian population [26] and BMI ≥ 25 kg/m2 for the Asian population [27]) and overweight (BMI ≥ 25 kg/m2 for the Caucasian population [26] and BMI ≥ 23 kg/m2 for the Asian population [27]), and the combination of these two may have a more pronounced negative impact on the patient prognosis vs. the individual effect of each of them.
3.1. NAFLD as Independent Risk Factor for AH Development
Published studies demonstrated a correlation between the presence and severity of NAFLD and the incidence of AH (Table 1). In the Korean population, hepatic steatosis detected by ultrasound imaging was an independent risk factor for AH [28]. This association persisted even after adjusting for multiple confounding factors, including changes in BMI (HR 1.36, 95% CI 1.10–1.67, p = 0.004) [29].

Table 1.
NAFLD as an independent factor of arterial hypertension.
The fatty liver index (FLI), as a screening tool for steatosis, may also be an indirect marker of AH risk. Longitudinal studies demonstrated an increase in the risk of developing AH proportionately to the FLI increase, while FLI > 60 was considered a poor prognostic factor for the development of AH [32,35,36]. Even after adjusting for conventional risk factors and gender differences, the correlation persisted [36]. E. Siafi et al. (2023) discovered that FLI had an independent prognostic value for the development of cardiovascular events in hypertensive patients not receiving antihypertensive therapy [38]. Besides, J.H. Huh et al. (2015) confirmed that FLI was a predictor of the AH onset regardless of the presence of insulin resistance or systemic inflammation [31]. Since three of the four FLI variables (BMI, waist circumference, and serum triglyceride level) are criteria for metabolic syndrome, it is not clear whether the hepatic steatosis diagnosed by this index rather than metabolic disorders per se causes the development of AH. It is worth noting that the lack of direct measurement of intrahepatic fat content was the main limitation of the above studies, which must be considered when interpreting their results.
There is evidence that an increased content of liver fat (detected by magnetic resonance imaging) correlated with a higher risk of AH (HR 2.16, p = 0.025) [33]. Interestingly, with regression of steatosis, the risk of developing AH was comparable to that in the group of patients with a healthy liver [29,34]. However, patients with FLI < 60 had more consistent BP values within the normal range vs. patients with FLI ≥ 60 [38]. These findings suggest that improving liver health may reduce the risk of developing AH over time.
Fatty liver index was also investigated as a possible predictor for incident T2DM. For instance, D. Yadav et al. (2016) showed that, in a fully adjusted model that included baseline fasting blood glucose, baseline SBP, HDL, and homeostasis model assessment of insulin resistance (HOMA-IR), the ORs (95% CI) for new-onset T2DM in patients with FLI values of 30–59 and FLI values of ≥60 over 2.6 years were 1.87 (1.05–3.33) and 2.84 (1.40–5.75), respectively [39]. Similar results were published by I.H. Seo et al. (2022), who demonstrated that, after adjusting for potentially confounding factors, such as age, sex, waist circumference, alcohol intake, physical activity, mean arterial pressure, family history of diabetes, and HOMA-IR, the HRs of incident T2DM were 1.89 (95% CI 1.66–2.14) and 2.98 (95% CI 2.58–3.43) for the FLI values of 30–59 and of ≥60, respectively [40]. Moreover, E. García-Escobar et al. (2021) determined that the inclusion of FLI in the basic diabetes risk models (focused on the conventional risk factors for T2DM development, namely age, sex, fasting glucose level, and family history of T2DM in combination with HOMA-IR values or without them) allowed for correct reclassification of an additional 6.6% and 7% cases, respectively, in the overall study population [41]. In their study, D.J. Cuthbertson et al. (2021) clearly indicated that, apart from being a potential prognostic marker of NAFLD and T2DM, elevated FLI was also associated with the development of incident prediabetes in overweight and obese patients [42]. Possible mechanisms underlining the relationship between FLI and T2DM include hepatic insulin resistance and hepatic inflammation, as well as disturbed regulation of lipid and glucose metabolism in the settings of abnormal bile acid signaling [15,40].
3.2. AH as Independent Risk Factor for NAFLD Development
While NAFLD can influence the development of AH, an inverse relationship is observed as well. There is increasing evidence that AH is a predictor of the development and progression of NAFLD (Table 2). It was established that, in the group of patients with AH, the prevalence of hepatic steatosis confirmed by the results of liver elastography was almost twice higher, while the risk of developing steatosis increased proportionately to the severity of AH [43]. In the Chinese cohort (n = 2049), a history of AH increased the risk of NAFLD nearly 1.5-fold [44]. In a Brazilian study (n = 5362), the HR was 1.8 [45]. However, timely control of AH with the achievement of target values (BP < 140/90 mmHg) reduced both the likelihood of developing NAFLD by over 40% and the likelihood of liver fibrosis progression [45].

Table 2.
Arterial hypertension as an independent risk factor for NAFLD.
In addition to predicting the onset of NAFLD, AH is also a risk factor for the progression of steatosis to steatohepatitis and advanced liver fibrosis [48]. It was demonstrated that, regardless of age, BMI, and the presence of diabetes mellitus, AH was an independent predictor of liver fibrosis based on noninvasive procedures (elastography and calculated indices: fibrosis-4 score [FIB-4], NAFLD fibrosis score [NFS], aspartate aminotransferase to platelet ratio index [APRI]) [44,49].
Besides, based on the analysis of serial liver biopsies, AH was established as an independent risk factor for predicting the progression of liver fibrosis over a mean follow-up period of 6.4 years [47]. The results of a meta-analysis based on seven published studies confirmed that the presence of AH at the time of the initial biopsy was associated with the development of progressive fibrosis in patients with NAFLD: odds ratio (OR) 1.94, 95% CI 1.00–3.74 [50].
3.3. Bidirectional Relationship
Despite insufficient data to determine whether NAFLD is a consequence or a cause of AH, a strong bidirectional relationship between them has been confirmed by numerous studies [18,45].
Based on the results of the Dongfeng–Tongji cohort study, it can be concluded that the development and persistence of NAFLD over a 5-year follow-up period in patients without obesity and diabetes mellitus was associated with an increased risk of AH onset (OR 1.49 and 1.50, respectively). In fact, the development and persistence of AH predicted the onset of NAFLD (OR 1.45 and 1.61, correspondingly) [34]. Another prospective cohort study by J. Ma et al. (2017) with a 6-year follow-up period demonstrated a similar bidirectional relationship between signs of hepatic steatosis (according to computed tomography) and the risk of developing AH, which persisted even after adjusting for BMI and visceral adipose tissue volume [51]. The existence of a bidirectional association between NAFLD and AH regardless of common cardiometabolic risk factors was supported by the results of meta-analyses as well. A systematic review and meta-analysis of 390,348 patients detected an association between NAFLD and an increased risk of developing AH (HR 1.66, 95% CI 1.38–2.01, p < 0.001) [37]. Another meta-analysis (based on 11 studies) also revealed a strong bidirectional relationship. Thus, the presence of NAFLD was associated with a 1.55-fold increase in the risk of developing AH (95% CI 1.29–1.87; n = 46,487). On the other hand, the presence of AH was associated with a 1.63-fold increase in the risk of NAFLD (95% CI 1.41–1.88; n = 25,260) [52].
A significant correlation between AH and NAFLD emphasizes the need to identify risk groups for adverse outcomes, especially when such groups have anthropometric parameters within the norm. In this context, serum uric acid (SUA) level seems to be relevant. Indeed, in their study, J. He et al. (2022) demonstrated that SUA cut-off values of ≥478 µmol/L and ≥423.5 µmol/L were able to identify severe steatosis in men and women, respectively. Moreover, authors showed that higher SUA levels did predict severe steatosis even in lean patients with NAFLD [53]. Similar results were obtained in a 4-year prospective cohort study including 2832 subjects (33.97% had NAFLD at baseline). According to its results, elevated SUA levels may be used as an independent predictor of NAFLD [54]. Potential mechanisms mediating the relationship between NAFLD and elevated SUA level include insulin resistance, endothelial dysfunction, and systemic inflammation [55]. In addition, uric acid is able to induce oxidative stress, a decrease in endothelial nitric oxide availability, and an increase in both plasma renin activity and intrakidney angiotensin activity, thus leading to kidney vasoconstriction, ischemia, and salt-sensitive hypertension [56]. Elevated SUA levels are present in 25–60% of subjects with untreated essential hypertension, but also can serve as a prognostic factor for AH development [57,58]. It worth noting that there are several medications that are used “off label” and can be beneficial with regard to SUA level reduction, e.g., pioglitazone, losartan, and atorvastatin [55]. Of them, atorvastatin and losartan can be used for the treatment of both NAFLD and AH, thus emphasizing the possible commonality of the pathogenesis of these diseases and an important role of uric acid for their development and progression.
The other molecule that can unite two diseases is bilirubin. Serum bilirubin is a liver functional test that is widely used to assess liver function. It is important in the diagnosis and prognosis of patients with liver disorders, and it is included in all prognostic scores for liver diseases. In this context, the elevation of serum bilirubin is regarded as a marker of unfavorable outcomes. However, there is evidence indicating the association of a low level of bilirubin with increased risk of diseases, particularly cardiovascular disorders [59]. For instance, it was demonstrated that serum bilirubin level may be regarded as a marker for the assessment of left ventricular hypertrophy in patients with AH; hypertensive patients with left ventricular hypertrophy had lower bilirubin levels compared to those without left ventricular hypertrophy (p = 0.008) [60]. Similar results were obtained in the study by E.M. Bakirci et al. (2021), indicating that serum bilirubin levels may have a protective effect on the left atrium remodeling process in newly diagnosed hypertensive individuals [61].
At the same time, in the study by S.K. Kunutsor et al. (2017), it was shown that the hazard ratio for AH per 1-SD increase in total bilirubin level compared to baseline comprised 0.86 (95% CI, 0.81–0.92; p < 0.001). After adjustment for established risk factors (such as smoking status, alcohol consumption, history of diabetes mellitus, parental history of hypertension, body mass index, systolic blood pressure, total cholesterol, and estimated glomerular filtration rate) the hazard ratio was further attenuated to 0.94 (95% CI, 0.88–0.99; p = 0.040) [59]. In addition, there is evidence that elevated serum bilirubin may contribute to the development and progression of AH in newborns [62], as well as serve as a risk factor for death in patients with pulmonary artery hypertension [63]. Unfortunately, due to its bidirectional effect on the risk of cardiovascular disease, including AH, serum bilirubin does not appear to be suitable as a hypertension biomarker [64].
It is thought that protective effects of bilirubin in AH may be mainly attributed to its significant antioxidant properties, namely preventing vitamin A and polyunsaturated fatty acids from oxidation, as well as to its ability to scavenge hydrogen peroxide radicals [64]. Moreover, it was shown that bilirubin could reduce superoxide production via direct inhibition of NAD(P)H oxidases in the vasculature, which was associated with an increase in the bioavailability of nitric oxide [65]. According to the results of animal studies, elevated serum bilirubin level was protective against excessive vasoconstriction mediated by tubuloglomerular feedback and was able to attenuate vasoconstriction in response to high levels of vasoconstrictors such as angiotensin II [66]. The other possible mechanisms of the antihypertensive action of bilirubin include regulation of the endothelin production and signaling (via influence on the preproendothelin gene transcription), as well as the improvement in calcium handling in vascular smooth muscle cells [67].
Although both conjugated and unconjugated bilirubin display antioxidant effects, there is relatively little evidence in favor of the potential beneficial effects of conjugated bilirubin in vivo for the following reasons: (1) serum conjugated bilirubin concentrations remain markedly below those of unconjugated bilirubin, and (2) conjugated bilirubin in general increases in the course of hepatobiliary disease, which may hamper the evaluation of any possible protective effects [68].
It is alarming that the combination of AH and NAFLD can remain undetected for a long time, and the rate of disease progression in this case varies from one patient to another and depends on multiple pathogenetic factors. In the literature, the term “silent killer” is used for each of these diseases since they have a long, asymptomatic course that causes delays in diagnosis and treatment which can lead to serious complications and death [69].
4. Mechanisms of the Relationship between NAFLD and AH
Despite the presence of bidirectional relationship between NAFLD and AH, it is often difficult to establish which of the two conditions occurs first. However, the existence of common mechanisms determines the high risk of developing one of them in cases where the other is already present. The mechanisms of the relationship between NAFLD and AH are mostly known (Figure 1) [70], but they continue to be explored in more depth. Common links in pathogenesis that may explain the potential pathophysiological relationship between NAFLD and AH include:
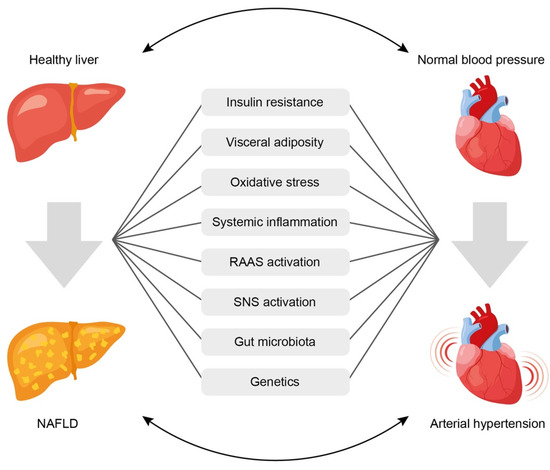
Figure 1.
Common pathophysiological links underlining the association between arterial hypertension and non-alcoholic fatty liver disease. See text for explanations. NAFLD = non-alcoholic fatty liver disease; RAAS = renin-angiotensin-aldosterone system; SNS = sympathetic nervous system.
- (1)
- Insulin resistance,
- (2)
- Systemic inflammation,
- (3)
- Activation of the renin-angiotensin-aldosterone system (RAAS),
- (4)
- Oxidative stress,
- (5)
- Activation of the sympathetic nervous system,
- (6)
- Gut microbiota disbalance,
- (7)
- Genetic factors.
4.1. Insulin Resistance
Uncontrolled AH leads to a decrease in peripheral circulation, which contributes to a reduction in the sensitivity of peripheral tissues to insulin and, consequently, to hyperinsulinemia. The latter stimulates the proliferation of smooth muscle cells and vascular fibroblasts, which leads to a narrowing of their lumen and an increase in total peripheral vascular resistance (TPVR). These vascular effects of insulin are mediated through the mitogen-activated protein kinase (MAPK) pathway, which promotes secretion of the vasoconstrictor endothelin-1 (ET-1). The other pathway that may be involved in the vascular deleterious effects of insulin is the phosphatidylinositol 3-kinase (PI3K)-dependent insulin signaling required for metabolic actions of insulin. It was shown that the impairment of PI3K/protein kinase B (Akt) pathway consistent with insulin resistance caused by glucotoxicity, lipotoxicity, or inflammation predicted diminished NO production and increased ET-1 secretion characteristic of diabetes and endothelial dysfunction [71]. Moreover, according to experimental data, hyperinsulinemia increases the activity of the central sections of the sympathetic nervous system, accompanied by augmented sympathetic stimulation of the heart, blood vessels, and kidneys [72].
There is evidence that compensatory hyperinsulinemia contributes to the activation of the RAAS and the melanocortin system of the brain, which play a pivotal role in the onset of AH [73]. Upon activation of the RAAS, a cascade of reactions occurs, including the production of angiotensin II, which causes spasm of smooth muscles of arterioles, an increase in hydrostatic pressure in the glomeruli, along with activation of aldosterone synthesis and higher sodium reabsorption in the kidneys. This ultimately leads to an increase in circulating blood volume and elevated BP. The kidneys are also a target organ for hyperinsulinemia. Insulin receptors are expressed on renal tubular cells and podocytes. Under physiological conditions, insulin induces vasodilation by increasing endothelial nitric oxide production through activation of the PI3K/ Akt pathway. In insulin resistance, this pathway is disrupted, and insulin triggers the MAPK pathway, which promotes renal vasoconstriction [18,72]. Besides this, under conditions of hyperinsulinemia, the activity of transmembrane ion-exchange Na+, K+ and Ca2+ ATPases is disrupted, while sodium reabsorption in the nephron tubules increases, which leads to fluid retention and the development of hypervolemia, as well as an increase in the sodium content in the blood vessel walls and their spasm [72].
4.2. Visceral Obesity
Visceral adipose tissue is an independent endocrine organ. Adipocytes secrete hormonally active molecules called adipokines (tumor necrosis factor α [TNF-α], IL-6, leptin, etc.), which enhance the pathological effects of insulin. TNF-α and IL-6 trigger the processes of cytotoxic inflammatory responses and chronic persistent inflammation both in the liver and in the vascular walls [74]. Adipocytes are also capable of synthesizing angiotensin II, thereby regulating BP levels [75].
It is known that adipokines can influence the RAAS. An increase in leptin levels against the background of a high-fat diet in rats caused the activation of the RAAS and the production of proinflammatory cytokines in the brain, which led to a progressive increase in BP [76]. Accordingly, L. D’Elia et al. (2021) showed that leptin values greater than 2.9 ng/mL were associated with a twofold increased risk of developing arterial stiffening in a sample of adult men without baseline arterial stiffening and antihypertensive treatment at baseline during an 8-year follow-up. These results were independent of body weight and BP [77]. Altered adipokine profile characterized by an increase in leptin concentration and a decrease in adiponectin levels was also described in NAFLD, thus indicating an additional common pathophysiological link between NAFLD and AH [78]. In addition to the effects described at the beginning of this paragraph, unfavorable action of leptin on BP level may be also attributed to the following mechanisms: reduction in NO bioavailability and regulation of ET-1 expression, as well as stimulation of endothelial cell growth and cardiovascular smooth muscle cell proliferation, increase in sympathetic nerve activity, and impairment in sodium handling [79,80].
An excess of free fatty acids (FFAs) released by adipose tissue under conditions of insulin resistance contributes to the formation of ectopic fat deposits in the form of perivascular and perirenal fat depots. In the insulin-resistant state, perivascular fat cells increase in size and number, and secrete reduced levels of regenerative factors, such as hepatocyte growth factor, and increased levels of proinflammatory factors, such as IL-6, TNF-α and monocyte chemoattractive factor 1 MCP-1. These proinflammatory factors inhibit the PI3K/Akt axis of insulin signal transduction, increase endothelial permeability and enhance insulin resistance [72]. This promotes the remodeling of the vascular wall with an increase in arterial stiffness and a resulting increase in pulse pressure [72,75,81]. Perirenal fat, along with inflicting mechanical pressure, is lipotoxic and can impair renal function in a paracrine manner, which results in RAAS activation and increased sodium reabsorption [75].
4.3. Oxidative Stress and Biologically Active Substances
Excessive release of FFAs from adipose tissue is lipotoxic to the liver. Against the background of mitochondrial dysfunction, the processes of lipid peroxidation are triggered with the formation of free radicals. An increase in free radical activity is the major process leading to the release of proinflammatory profibrogenic cytokines and hepatokines (fetuin-A, fibroblast growth factor 21 [FGF-21], and selenoprotein P) [82].
Some authors revealed the relationship between NAFLD and the biomarker of systemic endothelial dysfunction, E-selectin. E-selectin is a cell adhesion molecule expressed on endothelial cells; it is produced when they are damaged by cytokines. It was shown that the extent of NAFLD histological activity correlated with the level of E-selectin expression in the liver and the concentration of E-selectin in blood plasma [83]. Also, it was established that patients with AH and NAFLD had more pronounced endothelial function disorders, according to the values of arterial stiffness, compared with patients with isolated AH [84]. NAFLD is characterized by a change in the profile of hepatokines, which are biologically active substances secreted by hepatocytes. It was confirmed that they can participate in the development of systemic insulin resistance both directly (by influencing the insulin signaling pathway) and indirectly (by regulating lipid and glucose metabolism) [82]. For instance, in the study by T.W. Jung et al. (2013), it was demonstrated that fetuin-A might directly cause insulin resistance and modulate inflammatory reactions via stimulation of triacylglycerol accumulation in hepatocytes [85]. Accordingly, a significant decrease in circulating fetuin-A levels after 12 weeks of caloric restriction was accompanied by improvements in visceral fat area, blood pressure, lipid profiles, and glucose levels [86].
Regarding FGF-21, it was shown that circulating FGF-21 positively correlated with the brachial–ankle pulse wave velocity reflecting arterial stiffness. This suggests that FGF-21 might also be secreted by endothelial cells (not only hepatocytes) in response to stress, and that its elevated levels may be a signal of endothelial cell injury [87].
Regarding selenoprotein P, most published studies focus on the investigation of its role in the development and progression of pulmonary hypertension. Its effects in this condition are explained by the promotion of cell proliferation and apoptosis resistance through increased oxidative stress and mitochondrial dysfunction, which in turn are associated with activated hypoxia-inducible factor-1α and dysregulated glutathione metabolism [88,89].
The development of oxidative stress and the presence of systemic inflammation, accompanied by the circulation of TNF-α, IL-6, and advanced glycation end products, are key components of endothelial dysfunction. They can lead to both micro- and macroangiopathy and can contribute to progressive pathological remodeling of the vascular wall [81,90,91]. Elevated production of proinflammatory cytokines is a crucial mechanism involved in the progression of AH. TNF-α and IL-6 can regulate the expression of RAAS components, especially the production of angiotensinogen, which causes vasoconstriction, affects renal hemodynamics, and ultimately leads to an increased BP [92].
The presence of hepatic steatosis can negatively affect the regulation of the cardiovascular function via the autonomic nervous system. In patients with NAFLD, autonomic dysfunction was revealed in the form of an increased sympathetic activity and an impaired response to parasympathetic signals. Accordingly, the heart rate increased, and cardiac output decreased, which caused an additional impact on the cardiovascular system [93].
4.4. Gut Microbiota
An altered composition of the intestinal microbiota and a change in the permeability of the intestinal mucosa play an important role in the pathogenesis of both NAFLD and AH. The mechanisms of the gut microbiota impact on the development and progression of NAFLD and AH are caused by complex interactions between the gut microbiota and the host organism. Likely factors include metabolism of choline, bile acids, and amino acids resulting in synthesis of vasoactive hormones, such as trimethylamine (TMA) and trimethylamine N-oxide (TMAO), uremic toxins (indoxyl sulfate and p-cresol sulfate), as well as production of short-chain fatty acids (SCFAs) and ethanol by intestinal microorganisms [94,95]. Next, we discuss the role of SCFAs, TMA, and intestinal permeability in the pathogenesis of both AH and NAFLD.
4.4.1. Short-Chain Fatty Acids
In patients with AH and NAFLD, a reduction in the bacterial diversity of the intestinal microbiota and a decrease in the production of SCFA were revealed. The most important and biologically active SCFAs include acetate, propionate, and butyrate; they are made from dietary fiber in the intestines. Regarding their role in the pathogenesis of AH, animal studies suggested that SCFAs may have both hypotensive and hypertensive effects depending on the receptors they bind to [95,96]. For instance, propionate-induced activation of the G protein-coupled receptor (GPR) 42 in vascular endothelium caused a decrease in BP in Olfr78−/− mice [97]. In contrast, binding of propionate or acetate to the olfactory receptor (Olfr 78 in mice and OR51E2 in humans) increased BP, possibly due to their effect on vascular smooth muscle cells in renal afferent arterioles and peripheral blood vessels, as well as renin production [98]. The opposite effect of SCFAs on BP can be explained by the different sensitivity of Olfr78 and GPR41 to SCFAs. Indeed, GPR41 receptors are activated by basal serum concentrations of SCFAs ranging from 0.1 to 0.9 mmol, resulting in vasodilation and a reduction in BP. However, only higher serum levels of SCFA can activate Olfr78, thereby increasing renin production and BP values [98].
Moreover, by acting on GPR expressed in the sympathetic ganglia, SCFAs can directly regulate the sympathetic nervous system. They can also activate vagal afferent neurons and directly affect the function of the central nervous system. Such mechanisms of SCFA action on the central and peripheral nervous system lead to a decrease in BP [99].
The other mechanism of BP regulation by SCFAs includes their impact on the immune pathways, including intestinal and immune homeostasis, inflammatory cell biology and inflammatory response [99]. For instance, it was shown that butyrate was able to decrease the IL-6 and TNF-α levels caused by angiotensin II in vitro and induce regulatory T cells differentiation in vivo and in vitro. In addition, this SCFA could reduce IL-17 levels in patients with hypertension [100,101]. The anti-inflammatory effect of SCFAs, especially butyrate, may be also mediated by histone deacetylase inhibition in vascular endothelial cells, thereby contributing to the prevention of vascular inflammation [102].
Potential mechanisms of protective action of SCFAs on the liver include reduction of fat accumulation by promoting lipolysis, fatty acid oxidation, inhibition of fatty acid synthesis [103,104], maintenance of intestinal barrier function [105], regulation of intestinal motility [106], and suppression of inflammation and steatosis in the liver [107,108].
Since both NAFLD and AH are believed to be associated with metabolic disorders, the described protective effects of SCFAs, which are important for the pathogenesis of NAFLD, may also play a role in the development and progression of AH.
4.4.2. Trimethylamine and Trimethylamine N-oxide
Despite many studies examining the role of TMA and TMAO in the development and progression of NAFLD, there is no consensus regarding their effects on this liver disease. Some studies suggest that TMAO contributes to liver damage and disease progression [109,110,111,112], while others argue that TMA and TMAO may have a protective effect via improving lipid metabolism disorders, endoplasmic reticulum stress, and reducing cell death under lipid overload conditions [113,114,115]. The presented data suggest that the effect of TMAO may vary and strongly depends on the levels of other substrates in plasma, such as cholesterol [116]. Similarly, the role of TMA/TMAO in the development of AH remains uncertain. Some published data imply a vasoconstrictive mechanism of action of TMA/TMAO at high doses [117,118], while at low concentrations, these substances are thought to reduce diastolic dysfunction and cardiac fibrosis [119]. These data were confirmed by the results of meta-analysis published by X. Ge et al. (2020), indicating that, in comparison with low circulating TMAO concentrations, high TMAO concentrations were associated with a higher prevalence of hypertension (risk ratio [RR]: 1.12; 95% CI: 1.06, 1.17; p < 0.0001) [120].
Several potential mechanisms by which TMAO promotes hypertension include [121]: (1) enhanced angiotensin II-induced vasoconstriction and acute pressor response. This mechanism is implemented through the activation of the protein kinase RNA-like endoplasmic reticulum kinase (PERK) pathway, leading to apoptosis, inflammation, and vascular injury [122,123]; (2) the upregulation of scavenger receptors on the surface of macrophages and the stimulation of foam cell formation, atherosclerosis, vascular constriction and arterial stiffening [122,123]; (3) the disturbance of the reverse transport of cholesterol from extrahepatic organs and tissues into the liver resulting in increased oxidized-low density lipoprotein deposition in peripheral tissues, which contributes to atherosclerosis progression and increases the risk of CVD [124]; (4) increased production of proinflammatory cytokines, such as IL-1β, IL-18, TNF-α in combination with decreased production of anti-inflammatory cytokines, in particular IL-10 [123,125]; (5) participation in the development of renal dysfunction [126], and (6) the induction of cardiac dysfunction at high serum concentrations [127] by facilitating cardiac mitochondrial dysfunction, myocardial hypertrophy, and fibrosis [128].
4.4.3. Increased Intestinal Permeability
With increased intestinal permeability, gram-negative bacterial lipopolysaccharides reach the portal circulation and stimulate the development of systemic inflammation through toll-like receptors [129,130,131]. Moreover, it was shown that high circulating endotoxin levels resulted in the release of pro-inflammatory cytokines, including IL-1β, IL-12, IL-6, and TNF-α [132]. At the same time, high circulating levels of pro-inflammatory cytokines may contribute to intestinal junction disassembly and increase in gut permeability [133]. Indeed, an increase in BP was associated with a decrease in the level of tight junction proteins (occludin, tight junction protein 1 and claudin 4) in the rat model of AH compared with the control group [134]. The negative impact of arterial hypertension on gut barrier permeability may be explained by several possible mechanisms. The first one is the decreased intestinal blood flow due to changes in arterioles, particularly, thickening of the walls and narrowing of the lumens. This may lead to intestinal mucosa damage and barrier impairment. The second mechanism involves gut microbiota dysbiosis characterized by decreased microbial richness and diversity, reduced SCFAs production, and overgrowth of opportunistic pathogens [135].
According to the results of a meta-analysis conducted by J. Luther et al. (2015), almost 39.1% of NAFLD patients included in the analysis (n = 128) had signs of increased intestinal permeability vs. 6.8% in the control group of healthy individuals [136]. These data were further supported by another meta-analysis showing that impaired intestinal permeability in patients with NAFLD was associated with the grade of hepatic steatosis [137]. Similarly to patients with AH, it was assumed that patients with NAFLD (and especially with cirrhosis associated with NASH) had qualitative and quantitative changes in tight junction proteins [138,139]. Increased translocation of bacterial products was believed to lead to inflammation and fibrogenesis in the liver through toll-like receptor 4 stimulation. However, the relationship between intestinal permeability and fibrosis stage in NAFLD has not yet been established [140].
4.5. Genetic Factors
In addition to external factors, the role of genetic factors in the development of NAFLD and AH have been investigated. In the study of overlapping genes, 13 common genes for NAFLD and AH were identified. Besides this, four more common genes have been identified: leptin (LEP), adiponectin (ADIPOQ), aryl hydrocarbon receptor (AHR), and transforming growth factor beta-1 (TGFB1) genes, the expression of which is typical for patients with AH, NAFLD, liver fibrosis, and the presence of systemic inflammation [141]. In addition, C. Ma et al. (2021) showed that hypertension genes were more adjacent to NAFLD genes than random genes in the protein–protein interaction network, indicating a strong association between these two diseases [141].
According to expression information obtained from Gene cards database, both RNA-sequencing and microarray data indicated that RAS constituents, including a classical angiotensin-converting enzyme (ACE)/angiotensin II/type 1 angiotensin receptor axis and an alternative angiotensin-converting enzyme 2/angiotensin 1–7/Mas axis, were expressed in normal liver, heart and kidney. It is well-established that angiotensin II promotes insulin resistance, de novo lipogenesis, and pro-inflammatory cytokine production, and triggers liver inflammation and fibrogenesis [142], while active angiotensin 1–7 signal inhibits liver lipogenesis, fatty acid oxidation, inflammation, and fibrosis [143].
Another gene that seems to be related to both liver physiology and hypertension-NAFLD interaction is the aldehyde dehydrogenase (ALDH1A1, alternatively known as retinaldehyde dehydrogenase 1 or RALDH1) gene, encoding the enzyme which catalyzes the second and irreversible step of retinaldehyde oxidation to vitamin A (retinoic acid) [141]. According to the study by G. Zhong et al. (2019), the expression of ALDH1A1 was significantly higher in the livers of NASH patients than in the livers of healthy volunteers [144]. Moreover, analysis of the Oncomine database demonstrated that the expression of ALDH1A1 was significantly upregulated in the hepatocellular carcinoma tissues than in the normal tissues [145]. It is considered that ALDH1A1 might play an important role in the detoxification of lipid-derived aldehydes, including 4-hydroxy-2-nonenal and acrolein, which are known for their ability to mediate oxidative stress [146]. Additionally, through binding to either retinoic acid receptors (RARs) or retinoid X receptors (RXRs), retinoic acid may exert opposite effects on lipid metabolism in the liver: RARs binding leads to the suppression of hepatic non-esterified free fatty acids and triglyceride accumulation, while RXR-mediated signaling may cause hepatic lipid accumulation [147].
It was also revealed that polymorphism of the angiotensin II receptor type 1 (AGTR1) gene was indicative of the occurrence of NAFLD and AH in patients without obesity, insulin resistance, or hyperlipidemia. Some studies demonstrated that carriers of the C allele of the AGTR1 gene exhibited an elevated release of FFAs from adipose tissue and an imbalance of adipokines with a predominance of proinflammatory adipokines and chemokines over anti-inflammatory adiponectin [148].
Understanding the shared genes and biological mechanisms of AH and NAFLD can help to develop combined preventive strategies, explore novel therapeutic approaches against NAFLD, and design the most suitable treatment plan for patients with comorbidity of AH and NAFLD.
4.6. Influence of Gender on the Development of AH and NAFLD
It is well known that the frequency and prevalence of AH and NAFLD depend on the gender and age of the patient.
Most current guidelines for the management of AH patients agree that its prevalence among men and women of different age groups is different [149,150]. This may be due to various biological and physiological factors, as well as their interaction [151]. Similar to NAFLD, the prevalence of AH in the age group under 50 years is significantly higher in men than in women, while after reaching 50 years of age, the prevalence of AH in women is approximately the same as it is in men, or even slightly higher [149,150,152]. The likely biological factors determining the unequal prevalence of AH in men and women are the effects of sex hormones and differences in the sex chromosomes [153], while probable behavioral factors are high values of body mass index [154], smoking [155] and low physical activity [156].
A twelve-year Japanese study established that the prevalence of fatty liver in men averaged 26% vs. 13% in women. Also, it was shown that the frequency of NAFLD in women increases with age to a greater extent than in men, and the maximum differences were recorded in the age group of 70–79 years. On the contrary, the incidence of this pathology in men was similar in all age groups [157,158,159]. Another study from South China reported that the prevalence of NAFLD in the age group under 50 years old was significantly higher in men compared with women (22.4% vs. 7.1%, respectively, p < 0.001). However, this pattern was reversed in the age group of 50 years and older (20.6% vs. 27.6%, respectively, p < 0.05) [158,160].
There is strong evidence suggesting an important role of sex hormones in the onset of NAFLD. A meta-analysis showed that an increase in serum testosterone levels in women was also observed in polycystic ovary syndrome (PCOS), which is one of the conditions associated with NAFLD; this was accompanied by an increased risk of developing NAFLD in women. Hypogonadism associated with PCOS was shown to double the risk of developing NAFLD and contribute to an increase in obesity, insulin resistance, and metabolic syndrome [161,162,163]. Meanwhile, a decrease in serum testosterone levels is associated with the risk of hepatic steatosis in men [164]. However, a few studies suggested some protective effect of androgens regarding the development of hepatic steatosis. They can promote exocytosis of very-low-density lipoproteins (VLDL), inhibition of de novo lipogenesis, maintenance of carbohydrate metabolism, and fatty acid beta-oxidation [165,166].
Estrogens play a protective role against the development of hepatic steatosis in both men and women. This effect is implemented due to the ability of estrogens to increase insulin sensitivity, reduce the synthesis of triglycerides, and activate free fatty acid oxidation in the liver, as well as improve mitochondrial function and reduce the severity of inflammation in the liver tissue [165,167,168,169]. Estrogens are also involved in maintaining cholesterol metabolism in the liver by means of ensuring the synthesis of lipoproteins, secretion of VLDL, increased formation of high-density lipoproteins (HDL) and elimination of oxidized low-density lipoproteins (LDL).
In addition to the well-known role of sex hormones in the development of metabolic syndrome and related disorders, some studies elucidated that sex chromosomes per se may be responsible for the sexual dimorphism seen in NAFLD and metabolic syndrome. Sex chromosomes independent of gonadal status in a mouse model were associated with simple steatosis and NAFLD [170]. A study by Chen et al. (2016) demonstrated that the sex chromosome complement, XX or XY, affected adiposity and weight gain, because XX mice exhibited higher adiposity than XY mice even after a gonadectomy was performed to eliminate the effect of sex hormones [171]. It has been argued that increased adiposity was due to the presence of an extra X chromosome rather than the absence of a Y chromosome, as shown by genetic studies in mice with XO and XXY chromosomes. The established mechanism involved certain genes avoiding X chromosome inactivation and exhibiting increased levels of expression in the adipose tissue and liver of XX mice rather than XY mice [158,171].
With regard to the combination of NAFLD and AH, the following age- and gender-related characteristics have been described. First, women with NAFLD had a higher prevalence of essential hypertension (75.8%) [172]. Second, women with AH were more likely to develop NAFLD within 5 years [34]. Female gender is associated with a greater likelihood of NAFLD progression with the development of advanced liver fibrosis [173], especially at the age of over 50 years, which can lead to the development of pathogenetic mechanisms of AH, viz., an increase in arterial wall stiffness and endothelial dysfunction [174].
Gender-based differences in the prevalence of AH appear multifactorial as well, and their causes are not fully understood. In recent years, differences in the activity of the sympathetic nervous system and arterial wall stiffness were widely discussed, in the genesis of which sex hormones may play a certain role [175]. It should be noted that dysfunction of the autonomic nervous system may be more important for the development of AH in women than in men [176]. Furthermore, the age-related increase in autonomic nervous system activity is more pronounced in women than in men and does not depend on body mass index or menopause [177,178]. It is well known that the risk and incidence of AH in premenopausal women is lower than in men, but after 65 years of age, the prevalence of AH becomes higher in women [179].
Androgens and estrogens are able to regulate BP by influencing the RAAS. Androgens stimulate the RAAS, which leads to an elevated BP [180], while ovarian hormones have the opposite effect via reducing the activity of renin and ACE in blood plasma [181]. The effect of sex hormones on renal sodium reabsorption and vascular resistance may also explain differences between men and women in BP control [180,182]. Estrogens are likely to have the ability to maintain normal endothelial function by stimulating nitric oxide (NO) production including positive effects on arterial wall structure and function, which, in turn, help reduce vascular wall stiffness. Moreover, they reduce the effects of the sympathetic nervous system [179,183].
5. Conclusions
Accumulated evidence suggests a significant bidirectional relationship between hypertension and non-alcoholic fatty liver disease, which can be independent of other metabolic syndrome components and may be explained by the existence of pathophysiological mechanisms that are common for both diseases. Considering the poorer prognosis in the comorbidity of AH and NAFLD demonstrated in clinical studies, active detection of AH in patients with NAFLD, as well as screening patients with AH for NAFLD, seems crucial. Considering AH as a predictive marker of NAFLD, and vice versa, may help predict risks of CVD development and mortality in these patient groups. Timely detection and treatment of AH can serve as a potentially important aspect of preventing the development and progression of NAFLD.
Author Contributions
Conceptualization, O.M.D. and A.F.S.; methodology, O.M.D.; investigation, J.A.G. and A.F.S.; data curation, A.R.K.; writing—original draft preparation, J.A.G., E.O.L. and A.F.S.; writing—review and editing, A.Y.E., A.F.S. and A.R.K.; supervision, A.R.K.; funding acquisition, O.M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Science Foundation (Project No. 23-45-10030) as part of the scientific work “Prevalence and factors, associated with the musculoskeletal disorders in young and middle-aged patients with arterial hypertension and non-alcoholic fatty liver disease in Russian and Belarusian populations” performed at the National Medical Research Center for Therapy and Preventive Medicine in 2023–2025.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ACE | angiotensin-converting enzyme |
| ADIPOQ | adiponectin |
| AGTR1 | angiotensin II receptor type 1 |
| AH | arterial hypertension |
| AHR | aryl hydrocarbon receptor |
| Akt | protein kinase B |
| BMI | body mass index |
| BP | blood pressure |
| CI | confidence interval |
| CVD | cardiovascular disease |
| DBP | diastolic blood pressure |
| ET-1 | endothelin-1 |
| FFAs | free fatty acids |
| FGF-21 | fibroblast growth factor 21 |
| FIB-4 | fibrosis-4 index |
| FLI | fatty liver index |
| GPR | G protein-coupled receptor |
| HDL | high-density lipoproteins |
| HFF | hepatic fat fraction |
| HOMA-IR | homeostasis model assessment of insulin resistance |
| HR | hazard ratio |
| hsCRP | high-sensitivity C-reactive protein |
| IL | interleukin |
| IR | insulin resistance |
| LDL | low-density lipoproteins |
| LEP | leptin |
| MAPK | mitogen-activated protein kinase |
| MRI | magnetic resonance imagining |
| N/A | not applicable |
| NAFLD | non-alcoholic fatty liver disease |
| NFS | NAFLD fibrosis score |
| NO | nitric oxide |
| Olfr | olfactory receptor |
| OR | odds ratio |
| PCOS | polycystic ovary syndrome |
| PERK | protein kinase RNA- like endoplasmic reticulum kinase |
| PI3K | phosphatidylinositol 3-kinase |
| RAAS | renin-angiotensin-aldosterone system |
| RR | risk ratio |
| SBP | systolic blood pressure |
| SCFAs | short-chain fatty acids |
| SD | standard deviation |
| SUA | serum uric acid |
| T2DM | type 2 diabetes mellites |
| TGFB1 | transforming growth factor beta-1 |
| TMA | trimethylamine |
| TMAO | trimethylamine N-oxide |
| TNF-α | tumor necrosis factor-α |
| US | ultrasound |
| VLDL | very-low-density lipoproteins |
References
- Wu, S.; Wu, F.; Ding, Y.; Hou, J.; Bi, J.; Zhang, Z. Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 33386. [Google Scholar] [CrossRef]
- Kuang, M.; Lu, S.; Xie, Q.; Peng, N.; He, S.; Yu, C.; Qiu, J.; Sheng, G.; Zou, Y. Abdominal obesity phenotypes are associated with the risk of developing non-alcoholic fatty liver disease: Insights from the general population. BMC Gastroenterol. 2022, 1, 311. [Google Scholar] [CrossRef] [PubMed]
- Huh, Y.; Cho, Y.J.; Nam, G.E. Recent Epidemiology and Risk Factors of Nonalcoholic Fatty Liver Disease. J. Obes. Metab. Syndr. 2022, 1, 17–27. [Google Scholar] [CrossRef]
- Zarghamravanbakhsh, P.; Frenkel, M.; Poretsky, L. Metabolic causes and consequences of nonalcoholic fatty liver disease (NAFLD). Metabol. Open. 2021, 12, 100149, Erratum in Metabol. Open. 2023, 17, 100231. [Google Scholar] [CrossRef] [PubMed]
- Kasper, P.; Martin, A.; Lang, S.; Kütting, F.; Goeser, T.; Demir, M.; Steffen, H.M. NAFLD and cardiovascular diseases: A clinical review. Clin. Res. Cardiol. 2021, 7, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kang, K.; Sheol, H.; Shin, J.; Sim, Y.; Yang, T.; Hwang, J.; Lee, J.-M. The Association between Serum Uric Acid Levels and 10-Year Cardiovascular Disease Risk in Non-Alcoholic Fatty Liver Disease Patients. Int. J. Environ. Res. Public Health 2022, 19, 1042. [Google Scholar] [CrossRef]
- Duan, Y.; Pan, X.; Luo, J.; Xiao, X.; Li, J.; Bestman, P.L.; Luo, M. Association of Inflammatory Cytokines with Non-Alcoholic Fatty Liver Disease. Front. Immunol. 2022, 13, 880298. [Google Scholar] [CrossRef]
- Iglesias Morcillo, M.; Freuer, D.; Peters, A.; Heier, M.; Teupser, D.; Meisinger, C.; Linseisen, J. Association between fatty liver index and blood coagulation markers: A population-based study. Lipids Health Dis. 2023, 1, 83. [Google Scholar] [CrossRef]
- Emamat, H.; Tangestani, H.; Behrad Nasab, M.; Ghalandari, H.; Hekmatdoost, A. The association between epicardial adipose tissue and non-alcoholic fatty liver disease: A systematic review of existing human studies. EXCLI J. 2021, 20, 1096–1105. [Google Scholar] [CrossRef]
- Vu, H.; Khanh Tuong, T.T.; Hoang Lan, N.; Quoc Thang, T.; Bilgin, K.; Hoa, T.; Minh Duc, N.; The Dung, B. Association between nonalcoholic fatty liver disease and carotid intima-media thickness. Clin. Ter. 2023, 174, 42–47. [Google Scholar] [CrossRef]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 25, 3143–3421. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 17, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Corey, K.E.; Byrne, C.D. NAFLD, and cardiovascular and cardiac diseases: Factors influencing risk, prediction and treatment. Diabetes Metab. 2021, 47, 101215. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, Y.; Kim, S.U.; Kim, H.C. Metabolic dysfunction-associated fatty liver disease and incident cardiovascular disease risk: A nationwide cohort study. Clin. Gastroenterol. Hepatol. 2021, 19, 2138–2147. [Google Scholar] [CrossRef]
- Cattazzo, F.; Lombardi, R.; Mantovani, A.; Bevilacqua, M.; Zoncapè, M.; Iogna Prat, L.; Roccarina, D.; Fortuna, L.; Cespiati, A.; Sacerdoti, D.; et al. Subclinical and clinical atherosclerosis in non-alcoholic fatty liver disease is associated with the presence of hypertension. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2839–2847. [Google Scholar] [CrossRef]
- Lonardo, A.; Nascimbeni, F.; Mantovani, A.; Targher, G. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence? J. Hepatol. 2018, 68, 335–352. [Google Scholar] [CrossRef]
- Ng, C.H.; Wong, Z.Y.; Chew, N.W.S.; Chan, K.E.; Xiao, J.; Sayed, N.; Lim, W.H.; Tan, D.J.H.; Loke, R.W.K.; Tay, P.W.L.; et al. Hypertension is prevalent in non-alcoholic fatty liver disease and increases all-cause and cardiovascular mortality. Front. Cardiovasc. Med. 2022, 9, 942753. [Google Scholar] [CrossRef]
- Song, Q.; Liu, S.; Ling, Q.H.; Gao, Q.N.; Yang, R.X.; Chen, S.H.; Wu, S.; Chen, M.L.; Cai, J. Severity of nonalcoholic fatty liver disease is associated with cardiovascular outcomes in patients with prehypertension or hypertension: A community–based cohort study. Front. Endocrinol. 2022, 13, 942647. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.R.; Li, L.; Dai, J.; Natarajan, Y.; Yu, X.; Asch, S.M.; El-Serag, H.B. Effect of metabolic traits on the risk of cirrhosis and hepatocellular cancer in nonalcoholic fatty liver disease. Hepatology 2020, 71, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Welzel, T.M.; Graubard, B.I.; Zeuzem, S.; El-Serag, H.B.; Davila, J.A.; McGlynn, K.A. Metabolic syndrome increases the risk of primary liver cancer in the United States: A study in the SEER-Medicare database. Hepatology 2011, 2, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.J.; Kim, W.; Kim, D.; Yoon, J.H.; Lee, K.; Kim, J.H.; Cho, E.J.; Lee, J.H.; Kim, H.Y.; Kim, Y.J.; et al. Visceral obesity predicts significant fibrosis in patients with nonalcoholic fatty liver disease. Medicine 2015, 94, e2159. [Google Scholar] [CrossRef]
- Feng, R.N.; Du, S.S.; Wang, C.; Li, Y.C.; Liu, L.Y.; Guo, F.C.; Sun, C.H. Lean-non-alcoholic fatty liver disease increases risk for metabolic disorders in a normal weight Chinese population. World. J. Gastroenterol. 2014, 20, 17932–17940. [Google Scholar] [CrossRef]
- Yang, D.; Lan, J.; Cen, J.; Han, Y.; Hu, H. Association between hypertension and new-onset non-alcoholic fatty liver disease in chinese non-obese people: A longitudinal cohort study. Diabetes. Metab. Syndr. Obes. 2023, 16, 345–363. [Google Scholar] [CrossRef] [PubMed]
- WHO. Report of a WHO consultation. Obesity: Preventing and managing the global epidemic. World Health Organ. Tech. Rep. Ser. 2000, 894, 1–253. [Google Scholar]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 9403, 157–163. [Google Scholar] [CrossRef]
- Ryoo, J.H.; Suh, Y.J.; Shin, H.C.; Cho, Y.K.; Choi, J.M.; Park, S.K. Clinical association between non-alcoholic fatty liver disease and the development of hypertension. J. Gastroenterol. Hepatol. 2014, 29, 1926–1931. [Google Scholar] [CrossRef]
- Sung, K.C.; Wild, S.H.; Byrne, C.D. Development of new fatty liver, or resolution of existing fatty liver, over five years of follow-up, and risk of incident hypertension. J. Hepatol. 2014, 60, 1040–1045. [Google Scholar] [CrossRef]
- Vasunta, R.L.; Kesäniemi, Y.A.; Ylitalo, A.S.; Ukkola, O.H. High ambulatory blood pressure values associated with non-alcoholic fatty liver in middle-aged adults. J. Hypertens. 2012, 30, 2015–2019. [Google Scholar] [CrossRef]
- Huh, J.H.; Ahn, S.V.; Koh, S.B.; Choi, E.; Kim, J.Y.; Sung, K.C.; Kim, E.J.; Park, J.B. A prospective study of fatty liver index and incident hypertension: The KoGES-ARIRANG study. PLoS ONE 2015, 10, e0143560. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, F.; Gastaldelli, A.; Pihan-Le Bars, F.; Natali, A.; Roussel, R.; Petrie, J.; Tichet, J.; Marre, M.; Fromenty, B.; Balkau, B. Gamma-glutamyltransferase, fatty liver index and hepatic insulin resistance are associated with incident hypertension in two longitudinal studies. J. Hypertens. 2017, 35, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Lorbeer, R.; Bayerl, C.; Auweter, S.; Rospleszcz, S.; Lieb, W.; Meisinger, C.; Heier, M.; Peters, A.; Bamberg, F.; Hetterich, H. Association between MRI-derived hepatic fat fraction and blood pressure in participants without history of cardiovascular disease. J. Hypertens. 2017, 35, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Tang, Y.; Guo, X.; Zhu, X.; He, M.; Yuan, J.; Wang, Y.; Wei, S.; Chen, W.; Zhang, X.; et al. Bidirectional association between nonalcoholic fatty liver disease and hypertension from the Dongfeng-Tongji cohort study. J. Am. Soc. Hypertens. 2018, 12, 660–670. [Google Scholar] [CrossRef]
- Roh, J.H.; Park, J.H.; Lee, H.; Yoon, Y.H.; Kim, M.; Kim, Y.G.; Park, G.M.; Lee, J.H.; Seong, I.W. A close relationship between nonalcoholic fatty liver disease marker and new-onset hypertension in healthy Korean adults. Korean Circ. J. 2020, 50, 695–705. [Google Scholar] [CrossRef]
- Higashiura, Y.; Furuhashi, M.; Tanaka, M.; Takahashi, S.; Mori, K.; Miyamori, D.; Koyama, M.; Ohnishi, H.; Moniwa, N.; Numata, K.; et al. Elevated fatty liver index is independently associated with new onset of hypertension during a 10-year period in both male and female subjects. J. Am. Heart Assoc. 2021, 10, e021430. [Google Scholar] [CrossRef] [PubMed]
- Ciardullo, S.; Grassi, G.; Mancia, G.; Perseghin, G. Nonalcoholic fatty liver disease and risk of incident hypertension: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2022, 34, 365–371. [Google Scholar] [CrossRef]
- Siafi, E.; Andrikou, I.; Thomopoulos, C.; Konstantinidis, D.; Kakouri, N.; Tatakis, F.; Kariori, M.; Filippou, C.; Zamanis, I.; Manta, E.; et al. Fatty liver index and cardiovascular outcomes in never-treated hypertensive patients: A prospective cohort. Hypertens. Res. 2023, 46, 119–127. [Google Scholar] [CrossRef]
- Yadav, D.; Choi, E.; Ahn, S.V.; Koh, S.B.; Sung, K.C.; Kim, J.Y.; Huh, J.H. Fatty liver index as a simple predictor of incident diabetes from the KoGES-ARIRANG study. Medicine 2016, 31, e4447. [Google Scholar] [CrossRef]
- Seo, I.H.; Lee, H.S.; Lee, Y.J. Fatty liver index as a predictor for incident type 2 diabetes in community-dwelling adults: Longitudinal findings over 12 years. Cardiovasc. Diabetol. 2022, 1, 209. [Google Scholar] [CrossRef]
- García-Escobar, E.; Valdés, S.; Soriguer, F.; Vendrell, J.; Urrutia-Etxebarria, I.M.; Maldonado-Araque, C.; Ortega, E.; Ocón, P.; Montanya, E.; Menéndez, E.; et al. Fatty liver index as a predictor for type 2 diabetes in subjects with normoglycemia in a nationwide cohort study. Sci. Rep. 2021, 1, 16453. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, D.J.; Koskinen, J.; Brown, E.; Magnussen, C.G.; Hutri-Kähönen, N.; Sabin, M.; Tossavainen, P.; Jokinen, E.; Laitinen, T.; Viikari, J.; et al. Fatty liver index predicts incident risk of prediabetes, type 2 diabetes and non-alcoholic fatty liver disease (NAFLD). Ann. Med. 2021, 1, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zeng, Y.; Lin, C.; Chen, Z. Hypertension and non-alcoholic fatty liver disease proven by transient elastography. Hepatol. Res. 2016, 46, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Yu, H.; Zhong, X.; Tian, Y.; Cui, Z.; Quan, Z. Association between hypertension and nonalcoholic fatty liver disease: A cross-sectional and meta-analysis study. J. Hum. Hypertens. 2023, 37, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Aneni, E.C.; Oni, E.T.; Martin, S.S.; Blaha, M.J.; Agatston, A.S.; Feldman, T.; Veledar, E.; Conçeicao, R.D.; Carvalho, J.A.M.; Santos, R.D.; et al. Blood pressure is associated with the presence and severity of nonalcoholic fatty liver disease across the spectrum of cardiometabolic risk. J. Hypertens. 2015, 33, 1207–1214. [Google Scholar] [CrossRef]
- Tsuneto, A.; Hida, A.; Sera, N.; Imaizumi, M.; Ichimaru, S.; Nakashima, E.; Seto, S.; Maemura, K.; Akahoshi, M. Fatty liver incidence and predictive variables. Hypertens. Res. 2010, 33, 638–643. [Google Scholar] [CrossRef]
- Sorrentino, P.; Terracciano, L.; D’Angelo, S.; Ferbo, U.; Bracigliano, A.; Vecchione, R. Predicting fibrosis worsening in obese patients with NASH through parenchymal fibronectin, HOMA-IR, and hypertension. Am. J. Gastroenterol. 2010, 105, 336–344. [Google Scholar] [CrossRef]
- Gawrieh, S.; Wilson, L.A.; Cummings, O.W.; Clark, J.M.; Loomba, R.; Hameed, B.; Abdelmalek, M.F.; Dasarathy, S.; Neuschwander-Tetri, B.A.; Kowdley, K.; et al. Histologic findings of advanced fibrosis and cirrhosis in patients with nonalcoholic fatty liver disease who have normal aminotransferase levels. Am. J. Gastroenterol. 2019, 114, 1626–1635. [Google Scholar] [CrossRef]
- Labenz, C.; Huber, Y.; Kalliga, E.; Nagel, M.; Ruckes, C.; Straub, B.K.; Galle, P.R.; Wörns, M.A.; Anstee, Q.M.; Schuppan, D.; et al. Predictors of advanced fibrosis in non-cirrhotic non-alcoholic fatty liver disease in Germany. Aliment. Pharmacol. Ther. 2018, 48, 1109–1116. [Google Scholar] [CrossRef]
- Singh, S.; Allen, A.M.; Wang, Z.; Prokop, L.J.; Murad, M.H.; Loomba, R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: A systematic review and meta-analysis of paired-biopsy studies. Clin. Gastroenterol. Hepatol. 2015, 13, 643–654. [Google Scholar] [CrossRef]
- Ma, J.; Hwang, S.J.; Pedley, A.; Massaro, J.M.; Hoffmann, U.; Chung, R.T.; Benjamin, E.J.; Levy, D.; Fox, C.S.; Long, M.T. Bi-directional analysis between fatty liver and cardiovascular disease risk factors. J. Hepatol. 2017, 66, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Peng, Y.; Chen, Z.; Li, H.; Liu, D.; Ye, X. Bidirectional association between hypertension and NAFLD: A systematic review and meta-analysis of observational studies. Int. J. Endocrinol. 2022, 2022, 8463640. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ye, J.; Sun, Y.; Feng, S.; Chen, Y.; Zhong, B. The Additive Values of the Classification of Higher Serum Uric Acid Levels as a Diagnostic Criteria for Metabolic-Associated Fatty Liver Disease. Nutrients 2022, 14, 3587. [Google Scholar] [CrossRef]
- Wei, F.; Li, J.; Chen, C.; Zhang, K.; Cao, L.; Wang, X.; Ma, J.; Feng, S.; Li, W.D. Higher Serum Uric Acid Level Predicts Non-alcoholic Fatty Liver Disease: A 4-Year Prospective Cohort Study. Front. Endocrinol. 2020, 11, 179. [Google Scholar] [CrossRef]
- Paschos, P.; Athyros, V.G.; Tsimperidis, A.; Katsoula, A.; Grammatikos, N.; Giouleme, O. Can Serum Uric Acid Lowering Therapy Contribute to the Prevention or Treatment of Nonalcoholic Fatty Liver Disease? Curr. Vasc. Pharmacol. 2018, 3, 269–275. [Google Scholar] [CrossRef]
- Sanchez-Lozada, L.G.; Rodriguez-Iturbe, B.; Kelley, E.E.; Nakagawa, T.; Madero, M.; Feig, D.I.; Borghi, C.; Piani, F.; Cara-Fuentes, G.; Bjornstad, P.; et al. Uric Acid and Hypertension: An Update With Recommendations. Am. J. Hypertens. 2020, 7, 583–594. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Giovannini, M.; Grandi, E.; D’Addato, S.; Borghi, C.; Brisighella Heart Study Group. Interaction between low-density lipoprotein-cholesterolaemia, serum uric level and incident hypertension: Data from the Brisighella Heart Study. J. Hypertens. 2019, 4, 728–731. [Google Scholar] [CrossRef]
- Piani, F.; Cicero, A.F.G.; Borghi, C. Uric Acid and Hypertension: Prognostic Role and Guide for Treatment. J. Clin. Med. 2021, 10, 448. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Bakker, S.J.; Gansevoort, R.T.; Chowdhury, R.; Dullaart, R.P. Circulating total bilirubin and risk of incident cardiovascular disease in the general population. Arterioscler. Thromb. Vasc. Biol. 2015, 3, 716–724. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, X.; Li, Z.; Li, L. Relationship between Serum Bilirubin and Left Ventricular Hypertrophy in Patients with Essential Hypertension. PLoS ONE 2015, 4, e0125275. [Google Scholar] [CrossRef]
- Bakirci, E.M.; Degirmenci, H.; Hamur, H.; Cosgun, M.S.; Coskun, R.; Gunduz, T.; Tan, M.; Dogan, M.O.; Tanriseven, H.I.; Cakir, M.; et al. Predictors of left atrial remodeling in newly diagnosed hypertensive patients: A speckle-tracking echocardiographic study. Int. J. Cardiovasc. Imaging 2021, 10, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zou, L.; He, Y.; Luo, L.; Dong, W.; Zhang, Y.; Lei, X. Associations between neonatal serum bilirubin and childhood hypertension. PLoS ONE 2019, 7, e0219942. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Takeda, Y.; Tomimoto, S.; Tani, T.; Narita, H.; Kimura, G. Bilirubin as a prognostic marker in patients with pulmonary arterial hypertension. BMC Pulm. Med. 2010, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Jiang, H.; Zhao, B.; Lin, Y.; Lin, S.; Chen, T.; Su, Y.; Zhang, Y.; Zhou, L.; Li, L.; et al. The association between bilirubin and hypertension among a Chinese ageing cohort: A prospective follow-up study. J. Transl. Med. 2022, 1, 108. [Google Scholar] [CrossRef]
- Lanone, S.; Bloc, S.; Foresti, R.; Almolki, A.; Taillé, C.; Callebert, J.; Conti, M.; Goven, D.; Aubier, M.; Dureuil, B.; et al. Bilirubin decreases nos2 expression via inhibition of NAD(P)H oxidase: Implications for protection against endotoxic shock in rats. FASEB J. 2005, 13, 1890–1892. [Google Scholar] [CrossRef]
- Vera, T.; Stec, D.E. Moderate hyperbilirubinemia improves renal hemodynamics in ANG II-dependent hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 4, R1044–R1049. [Google Scholar] [CrossRef]
- Stec, D.E.; Hosick, P.A.; Granger, J.P. Bilirubin, renal hemodynamics, and blood pressure. Front. Pharmacol. 2012, 3, 18. [Google Scholar] [CrossRef]
- Bulmer, A.C.; Bakrania, B.; Du Toit, E.F.; Boon, A.C.; Clark, P.J.; Powell, L.W.; Wagner, K.H.; Headrick, J.P. Bilirubin acts as a multipotent guardian of cardiovascular integrity: More than just a radical idea. Am. J. Physiol. Heart Circ. Physiol. 2018, 3, H429–H447. [Google Scholar] [CrossRef]
- Ilan, Y. Analogy between non-alcoholic steatohepatitis (NASH) and hypertension: A stepwise patient-tailored approach for NASH treatment. Ann. Gastroenterol. 2018, 3, 1296–1304. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Zhao, G.J.; Chen, Z.; She, Z.G.; Cai, J.; Li, H. Nonalcoholic fatty liver disease: An emerging driver of hypertension. Hypertension 2020, 75, 275–284. [Google Scholar] [CrossRef]
- Muniyappa, R.; Chen, H.; Montagnani, M.; Sherman, A.; Quon, M.J. Endothelial dysfunction due to selective insulin resistance in vascular endothelium: Insights from mechanistic modeling. Am. J. Physiol. Endocrinol. Metab. 2020, 3, E629–E646. [Google Scholar] [CrossRef] [PubMed]
- Artunc, F.; Schleicher, E.; Weigert, C.; Fritsche, A.; Stefan, N.; Häring, H.U. The impact of insulin resistance on the kidney and vasculature. Nat. Rev. Nephrol. 2016, 12, 721–737. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.A.; do Carmo, J.M.; Li, X.; Wang, Z.; Mouton, A.J.; Hall, J.E. Role of hyperinsulinemia and insulin resistance in hypertension: Metabolic syndrome revisited. Can. J. Cardiol. 2020, 36, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, D.; Georgiopoulos, G.; Katsi, V.; Kourek, C.; Tsioufis, C.; Alexopoulou, A.; Koutli, E.; Tousoulis, D. Non-alcoholic fatty liver disease and hypertension: Coprevalent or correlated? Eur. J. Gastroenterol. Hepatol. 2018, 30, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Yu, Y.; Zhang, Z.; Guo, F.; Beltz, T.G.; Thunhorst, R.L.; Felder, R.B.; Johnson, A.K. Leptin mediates high-fat diet sensitization of angiotensin II-elicited hypertension by upregulating the brain renin-angiotensin system and inflammation. Hypertension 2016, 67, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Catena, C.; Bernardi, S.; Sabato, N.; Grillo, A.; Ermani, M.; Sechi, L.A.; Fabris, B.; Carretta, R.; Fallo, F. Ambulatory arterial stiffness indices and non-alcoholic fatty liver disease in essential hypertension. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 389–393. [Google Scholar] [CrossRef]
- D’Elia, L.; Giaquinto, A.; Iacone, R.; Russo, O.; Strazzullo, P.; Galletti, F. Serum leptin is associated with increased pulse pressure and the development of arterial stiffening in adult men: Results of an eight-year follow-up study. Hypertens. Res. 2021, 11, 1444–1450. [Google Scholar] [CrossRef]
- Ferrara, D.; Montecucco, F.; Dallegri, F.; Carbone, F. Impact of different ectopic fat depots on cardiovascular and metabolic diseases. J. Cell Physiol. 2019, 12, 21630–21641. [Google Scholar] [CrossRef]
- Poetsch, M.S.; Strano, A.; Guan, K. Role of Leptin in Cardiovascular Diseases. Front. Endocrinol. 2020, 11, 354. [Google Scholar] [CrossRef]
- D’Elia, L.; Strazzullo, P. Excess Body Weight, Insulin Resistance and Isolated Systolic Hypertension: Potential Pathophysiological Links. High Blood Press. Cardiovasc. Prev. 2018, 1, 17–23. [Google Scholar] [CrossRef]
- Brandes, R.P. Endothelial dysfunction and hypertension. Hypertension 2014, 64, 924–928. [Google Scholar] [CrossRef]
- Meex, R.C.R.; Watt, M.J. Hepatokines: Linking nonalcoholic fatty liver disease and insulin resistance. Nat. Rev. Endocrinol. 2017, 13, 509–520. [Google Scholar] [CrossRef]
- Guzik, T.J.; Touyz, R.M. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension 2017, 70, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Statsenko, M.E.; Streltsova, A.M.; Turovets, M.I. Effect of non-alcoholic fatty liver disease on arterial stiffness and the risk of cardiovascular complications in patients with arterial hypertension. Russ. Arch. Intern. Med. 2020, 10, 296–304. [Google Scholar] [CrossRef]
- Jung, T.W.; Youn, B.S.; Choi, H.Y.; Lee, S.Y.; Hong, H.C.; Yang, S.J.; Yoo, H.J.; Kim, B.H.; Baik, S.H.; Choi, K.M. Salsalate and adiponectin ameliorate hepatic steatosis by inhibition of the hepatokine fetuin-A. Biochem. Pharmacol. 2013, 7, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Han, K.A.; Ahn, H.J.; Lee, S.Y.; Hwang, S.Y.; Kim, B.H.; Hong, H.C.; Choi, H.Y.; Yang, S.J.; Yoo, H.J.; et al. The effects of caloric restriction on fetuin-A and cardiovascular risk factors in rats and humans: A randomized controlled trial. Clin. Endocrinol. 2013, 3, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Hong, H.C.; Choi, H.Y.; Yoo, H.J.; Cho, G.J.; Hwang, T.G.; Baik, S.H.; Choi, D.S.; Kim, S.M.; Choi, K.M. Effects of a three-month combined exercise programme on fibroblast growth factor 21 and fetuin-A levels and arterial stiffness in obese women. Clin. Endocrinol. 2011, 4, 464–469. [Google Scholar] [CrossRef]
- Kikuchi, N.; Satoh, K.; Kurosawa, R.; Yaoita, N.; Elias-Al-Mamun, M.; Siddique, M.A.H.; Omura, J.; Satoh, T.; Nogi, M.; Sunamura, S.; et al. Selenoprotein P Promotes the Development of Pulmonary Arterial Hypertension: Possible Novel Therapeutic Target. Circulation 2018, 6, 600–623. [Google Scholar] [CrossRef]
- Sun, Q.; Hackler, J.; Hilger, J.; Gluschke, H.; Muric, A.; Simmons, S.; Schomburg, L.; Siegert, E. Selenium and Copper as Biomarkers for Pulmonary Arterial Hypertension in Systemic Sclerosis. Nutrients 2020, 12, 1894. [Google Scholar] [CrossRef]
- Nosalski, R.; McGinnigle, E.; Siedlinski, M.; Guzik, T.J. Novel immune mechanisms in hypertension and cardiovascular risk. Curr. Cardiovasc. Risk Rep. 2017, 11, 12. [Google Scholar] [CrossRef]
- Simons, N.; Bijnen, M.; Wouters, K.A.M.; Rensen, S.S.; Beulens, J.W.J.; van Greevenbroek, M.M.J.; ’t Hart, L.M.; Greve, J.W.M.; van der Kallen, C.J.H.; Schaper, N.C.; et al. The endothelial function biomarker soluble E-selectin is associated with nonalcoholic fatty liver disease. Liver Int. 2020, 40, 1079–1088. [Google Scholar] [CrossRef]
- Satou, R.; Penrose, H.; Navar, L.G. Inflammation as a regulator of the renin-angiotensin system and blood pressure. Curr. Hypertens. Rep. 2018, 20, 100. [Google Scholar] [CrossRef]
- Houghton, D.; Zalewski, P.; Hallsworth, K.; Cassidy, S.; Thoma, C.; Avery, L.; Slomko, J.; Hardy, T.; Burt, A.D.; Tiniakos, D.; et al. The degree of hepatic steatosis associates with impaired cardiac and autonomic function. J. Hepatol. 2019, 70, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Sharpton, S.R.; Ajmera, V.; Loomba, R. Emerging role of the gut microbiome in nonalcoholic fatty liver disease: From composition to function. Clin. Gastroenterol. Hepatol. 2019, 17, 296–306. [Google Scholar] [CrossRef]
- Tokarek, J.; Budny, E.; Saar, M.; Kućmierz, J.; Młynarska, E.; Rysz, J.; Franczyk, B. Does the Composition of Gut Microbiota Affect Hypertension? Molecular Mechanisms Involved in Increasing Blood Pressure. Int. J. Mol. Sci. 2023, 24, 1377. [Google Scholar] [CrossRef] [PubMed]
- Verhaar, B.J.H.; Prodan, A.; Nieuwdorp, M.; Muller, M. Gut Microbiota in Hypertension and Atherosclerosis: A Review. Nutrients 2020, 12, 2982. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, N.; Hori, D.; Flavahan, S.; Steppan, J.; Flavahan, N.A.; Berkowitz, D.E.; Pluznick, J.L. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol. Genom. 2016, 48, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, J.; Kasubuchi, M.; Nakajima, A.; Irie, J.; Itoh, H.; Kimura, I. The role of short-chain fatty acid on blood pressure regulation. Curr. Opin. Nephrol. Hypertens. 2016, 25, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, H.; Tu, X.; Gao, Z. The Role of Short-Chain Fatty Acids of Gut Microbiota Origin in Hypertension. Front. Microbiol. 2021, 12, 730809. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Q.; Lu, A.; Liu, X.; Zhang, L.; Xu, C.; Liu, X.; Li, H.; Yang, T. Sodium butyrate suppresses angiotensin II-induced hypertension by inhibition of renal (pro)renin receptor and intrarenal renin-angiotensin system. J. Hypertens. 2017, 9, 1899–1908. [Google Scholar] [CrossRef]
- Kim, S.; Goel, R.; Kumar, A.; Qi, Y.; Lobaton, G.; Hosaka, K.; Mohammed, M.; Handberg, E.M.; Richards, E.M.; Pepine, C.J.; et al. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin. Sci. 2018, 6, 701–718. [Google Scholar] [CrossRef] [PubMed]
- Chun, P. Therapeutic effects of histone deacetylase inhibitors on heart disease. Arch. Pharm. Res. 2020, 12, 1276–1296. [Google Scholar] [CrossRef]
- Ghosh, A.; Gao, L.; Thakur, A.; Siu, P.M.; Lai, C.W.K. Role of free fatty acids in endothelial dysfunction. J. Biomed. Sci. 2017, 24, 50. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fu, C.; Li, F. Acetate Affects the Process of Lipid Metabolism in Rabbit Liver, Skeletal Muscle and Adipose Tissue. Animals 2019, 9, 799. [Google Scholar] [CrossRef]
- Xie, L.; Alam, M.J.; Marques, F.Z.; Mackay, C.R. A major mechanism for immunomodulation: Dietary fibres and acid metabolites. Semin. Immunol. 2023, 66, 101737. [Google Scholar] [CrossRef]
- Mishima, Y.; Ishihara, S. Enteric Microbiota-Mediated Serotonergic Signaling in Pathogenesis of Irritable Bowel Syndrome. Int. J. Mol. Sci. 2021, 22, 10235. [Google Scholar] [CrossRef]
- Skelly, A.N.; Sato, Y.; Kearney, S.; Honda, K. Mining the microbiota for microbial and metabolite-based immunotherapies. Nat. Rev. Immunol. 2019, 19, 305–323. [Google Scholar] [CrossRef]
- Chen, M.; Hui, S.; Lang, H.; Zhou, M.; Zhang, Y.; Kang, C.; Zeng, X.; Zhang, Q.; Yi, L.; Mi, M. SIRT3 Deficiency Promotes High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease in Correlation with Impaired Intestinal Permeability through Gut Microbial Dysbiosis. Mol. Nutr. Food Res. 2019, 63, 1800612. [Google Scholar] [CrossRef]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Di Somma, C.; Laudisio, D.; Maisto, M.; de Alteriis, G.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine-N-oxide (TMAO) as Novel Potential Biomarker of Early Predictors of Metabolic Syndrome. Nutrients 2018, 10, 1971. [Google Scholar] [CrossRef] [PubMed]
- Aragonès, G.; Colom-Pellicer, M.; Aguilar, C.; Guiu-Jurado, E.; Martínez, S.; Sabench, F.; Antonio Porras, J.; Riesco, D.; Del Castillo, D.; Richart, C.; et al. Circulating microbiota-derived metabolites: A “liquid biopsy?”. Int. J. Obes. 2020, 44, 875–885. [Google Scholar] [CrossRef]
- Cordeiro, A.; Costa, R.; Andrade, N.; Silva, C.; Canabrava, N.; Pena, M.J.; Rodrigues, I.; Andrade, S.; Ramalho, A. Does adipose tissue inflammation drive the development of non-alcoholic fatty liver disease in obesity? Clin. Res. Hepatol. Gastroenterol. 2020, 44, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liu, Y.; Long, J.; Chen, S.; Liao, G.; Wu, S.; Li, C.; Wang, L.; Ling, W.; Zhu, H. Trimethylamine N-Oxide Aggravates Liver Steatosis through Modulation of Bile Acid Metabolism and Inhibition of Farnesoid X Receptor Signaling in Nonalcoholic Fatty Liver Disease. Mol. Nutr. Food Res. 2019, 63, 1900257. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.H.; Xin, F.Z.; Zhou, D.; Xue, Y.Q.; Liu, X.L.; Yang, R.X.; Pan, Q.; Fan, J.G. Trimethylamine N-oxide attenuates high-fat high-cholesterol diet-induced steatohepatitis by reducing hepatic cholesterol overload in rats. World. J. Gastroenterol. 2019, 25, 2450–2462. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 7341, 57–63. [Google Scholar] [CrossRef]
- Vu, V.; Kim, Y.; Cho, M. Effects of SCFAs and TMAO on non-alcoholic fatty liver disease indicating the therapeutic benefits of plant-based diet, and supplemental prebiotics, probiotics and synbiotics. Appl. Biol. Chem. 2023, 66, 11. [Google Scholar] [CrossRef]
- Maksymiuk, K.M.; Szudzik, M.; Gawryś-Kopczyńska, M.; Onyszkiewicz, M.; Samborowska, E.; Mogilnicka, I.; Ufnal, M. Trimethylamine, a gut bacteria metabolite and air pollutant, increases blood pressure and markers of kidney damage including proteinuria and KIM-1 in rats. J. Transl. Med. 2022, 20, 470. [Google Scholar] [CrossRef]
- Brunt, V.E.; Casso, A.G.; Gioscia-Ryan, R.A.; Sapinsley, Z.J.; Ziemba, B.P.; Clayton, Z.S.; Bazzoni, A.E.; VanDongen, N.S.; Richey, J.J.; Hutton, D.A.; et al. Gut Microbiome-Derived Metabolite Trimethylamine N-Oxide Induces Aortic Stiffening and Increases Systolic Blood Pressure With Aging in Mice and Humans. Hypertension 2021, 78, 499–511. [Google Scholar] [CrossRef]
- Huc, T.; Drapala, A.; Gawrys, M.; Konop, M.; Bielinska, K.; Zaorska, E.; Samborowska, E.; Wyczalkowska-Tomasik, A.; Pączek, L.; Dadlez, M.; et al. Chronic, low-dose TMAO treatment reduces diastolic dysfunction and heart fibrosis in hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, 1805–1820. [Google Scholar] [CrossRef]
- Ge, X.; Zheng, L.; Zhuang, R.; Yu, P.; Xu, Z.; Liu, G.; Xi, X.; Zhou, X.; Fan, H. The Gut Microbial Metabolite Trimethylamine N-Oxide and Hypertension Risk: A Systematic Review and Dose-Response Meta-analysis. Adv. Nutr. 2020, 1, 66–76. [Google Scholar] [CrossRef]
- Mutengo, K.H.; Masenga, S.K.; Mweemba, A.; Mutale, W.; Kirabo, A. Gut microbiota dependant trimethylamine N-oxide and hypertension. Front. Physiol. 2023, 14, 1075641. [Google Scholar] [CrossRef]
- Jiang, S.; Shui, Y.; Cui, Y.; Tang, C.; Wang, X.; Qiu, X.; Hu, W.; Fei, L.; Li, Y.; Zhang, S.; et al. Gut microbiota dependent trimethylamine N-oxide aggravates angiotensin II-induced hypertension. Redox Biol. 2021, 46, 102115. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, M. Trimethylamine N-Oxide Generated by the Gut Microbiota Is Associated with Vascular Inflammation: New Insights into Atherosclerosis. Mediat. Inflamm. 2020, 2020, 4634172. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qiu, J.; Lian, J.; Yang, X.; Zhou, J. Gut Metabolite Trimethylamine-N-Oxide in Atherosclerosis: From Mechanism to Therapy. Front. Cardiovasc. Med. 2021, 8, 723886. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lin, F.; Tang, R.; Bao, C.; Zhou, Q.; Ye, K.; Shen, Y.; Liu, C.; Hong, C.; Yang, K.; et al. Gut Microbial Metabolite Trimethylamine N-Oxide Aggravates Pulmonary Hypertension. Am. J. Respir. Cell Mol. Biol. 2022, 4, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, D.; Li, B.; Li, X.; Lai, X.; Lei, S.; Li, N.; Zhang, X. Relationship between Plasma Trimethylamine N-Oxide Levels and Renal Dysfunction in Patients with Hypertension. Kidney Blood Press. Res. 2021, 4, 421–432. [Google Scholar] [CrossRef]
- Naghipour, S.; Cox, A.J.; Peart, J.N.; Du Toit, E.F.; Headrick, J.P. Trimethylamine N-oxide: Heart of the microbiota-CVD nexus? Nutr. Res. Rev. 2021, 1, 125–146. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Z.; Yan, J.; Liu, H.; Liu, Q.; Deng, Y.; Ou, C.; Chen, M. Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab. Investig. 2019, 3, 346–357. [Google Scholar] [CrossRef]
- Ma, J.; Li, H. The role of gut microbiota in atherosclerosis and hypertension. Front. Pharmacol. 2018, 9, 1082. [Google Scholar] [CrossRef]
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut dysbiosis is linked to hypertension. Hypertension 2015, 65, 1331–1340. [Google Scholar] [CrossRef]
- Li, F.; Ye, J.; Shao, C.; Zhong, B. Compositional alterations of gut microbiota in nonalcoholic fatty liver disease patients: A systematic review and meta-analysis. Lipids Health Dis. 2021, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Ntlahla, E.E.; Mfengu, M.M.; Engwa, G.A.; Nkeh-Chungag, B.N.; Sewani-Rusike, C.R. Gut permeability is associated with hypertension and measures of obesity but not with Endothelial Dysfunction in South African youth. Afr. Health Sci. 2021, 3, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, C.; Fasano, A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers 2016, 4, e1251384. [Google Scholar] [CrossRef] [PubMed]
- Santisteban, M.M.; Qi, Y.; Zubcevic, J.; Kim, S.; Yang, T.; Shenoy, V.; Cole-Jeffrey, C.T.; Lobaton, G.O.; Stewart, D.C.; Rubiano, A.; et al. Hypertension-linked pathophysiological alterations in the gut. Circ. Res. 2017, 120, 312–323. [Google Scholar] [CrossRef]
- Li, C.; Xiao, P.; Lin, D.; Zhong, H.J.; Zhang, R.; Zhao, Z.G.; He, X.X. Risk Factors for Intestinal Barrier Impairment in Patients With Essential Hypertension. Front. Med. 2021, 7, 543698. [Google Scholar] [CrossRef]
- Luther, J.; Garber, J.J.; Khalili, H.; Dave, M.; Bale, S.S.; Jindal, R.; Motola, D.L.; Luther, S.; Bohr, S.; Jeoung, S.W.; et al. Hepatic Injury in Nonalcoholic Steatohepatitis Contributes to Altered Intestinal Permeability. Cell Mol. Gastroenterol. Hepatol. 2015, 1, 222–232. [Google Scholar] [CrossRef]
- De Munck, T.J.I.; Xu, P.; Verwijs, H.J.A.; Masclee, A.A.M.; Jonkers, D.; Verbeek, J.; Koek, G.H. Intestinal permeability in human nonalcoholic fatty liver disease: A systematic review and meta-analysis. Liver Int. 2020, 40, 2906–2916. [Google Scholar] [CrossRef]
- Miele, L.; Valenza, V.; La Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Mascianà, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G.; et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009, 49, 1877–1887. [Google Scholar] [CrossRef]
- Muñoz, L.; Caparrós, E.; Albillos, A.; Francés, R. The shaping of gut immunity in cirrhosis. Front. Immunol. 2023, 14, 1139554. [Google Scholar] [CrossRef]
- Forlano, R.; Mullish, B.H.; Roberts, L.A.; Thursz, M.R.; Manousou, P. The Intestinal Barrier and Its Dysfunction in Patients with Metabolic Diseases and Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 662. [Google Scholar] [CrossRef]
- Ma, C.; Yan, K.; Wang, Z.; Zhang, Q.; Gao, L.; Xu, T.; Sai, J.; Cheng, F.; Du, Y. The association between hypertension and nonalcoholic fatty liver disease (NAFLD): Literature evidence and systems biology analysis. Bioengineered 2021, 12, 2187–2202. [Google Scholar] [CrossRef] [PubMed]
- Roderburg, C.; Krieg, S.; Krieg, A.; Demir, M.; Luedde, T.; Kostev, K.; Loosen, S.H. Non-alcoholic fatty liver disease (NAFLD) is associated with an increased incidence of chronic kidney disease (CKD). Eur. J. Med. Res. 2023, 1, 153. [Google Scholar] [CrossRef] [PubMed]
- Hartl, L.; Rumpf, B.; Domenig, O.; Simbrunner, B.; Paternostro, R.; Jachs, M.; Poglitsch, M.; Marculescu, R.; Trauner, M.; Reindl-Schwaighofer, R.; et al. The systemic and hepatic alternative renin-angiotensin system is activated in liver cirrhosis, linked to endothelial dysfunction and inflammation. Sci. Rep. 2023, 1, 953. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Kirkwood, J.; Won, K.J.; Tjota, N.; Jeong, H.; Isoherranen, N. Characterization of Vitamin A Metabolome in Human Livers With and Without Nonalcoholic Fatty Liver Disease. J. Pharmacol. Exp. Ther. 2019, 1, 92–103. [Google Scholar] [CrossRef]
- Yang, C.K.; Wang, X.K.; Liao, X.W.; Han, C.Y.; Yu, T.D.; Qin, W.; Zhu, G.Z.; Su, H.; Yu, L.; Liu, X.G.; et al. Aldehyde dehydrogenase 1 (ALDH1) isoform expression and potential clinical implications in hepatocellular carcinoma. PLoS ONE 2017, 8, e0182208. [Google Scholar] [CrossRef]
- Makia, N.L.; Bojang, P.; Falkner, K.C.; Conklin, D.J.; Prough, R.A. Murine hepatic aldehyde dehydrogenase 1a1 is a major contributor to oxidation of aldehydes formed by lipid peroxidation. Chem. Biol. Interact. 2011, 1–3, 278–287. [Google Scholar] [CrossRef]
- Saeed, A.; Dullaart, R.P.F.; Schreuder, T.C.M.A.; Blokzijl, H.; Faber, K.N. Disturbed Vitamin A Metabolism in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2017, 1, 29. [Google Scholar] [CrossRef]
- Musso, G.; Saba, F.; Cassader, M.; Paschetta, E.; De Michieli, F.; Pinach, S.; Framarin, L.; Berrutti, M.; Leone, N.; Parente, R.; et al. Angiotensin II type 1 receptor rs5186 gene variant predicts incident NAFLD and associated hypertension: Role of dietary fat-induced pro-inflammatory cell activation. Am. J. Gastroenterol. 2019, 114, 607–619. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104, Erratum in Eur. Heart J. 2019, 40, 475. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, 13–115, Erratum in Hypertension 2018, 71, 140–144. [Google Scholar] [CrossRef]
- Connelly, P.J.; Currie, G.; Delles, C. Sex Differences in the Prevalence, Outcomes and Management of Hypertension. Curr. Hypertens. Rep. 2022, 6, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Meinert, F.; Thomopoulos, C.; Kreutz, R. Sex and gender in hypertension guidelines. J. Hum. Hypertens. 2023, 8, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, K.; Ji, H. Sex differences in primary hypertension. Biol. Sex. Differ. 2012, 3, 7. [Google Scholar] [CrossRef]
- Khalid, F.; Siddique, A.; Siddiqui, J.A.; Panhwar, G.; Singh, S.; Anwar, A.; Hashmi, A.A. Correlation Between Body Mass Index and Blood Pressure Levels Among Hypertensive Patients: A Gender-Based Comparison. Cureus 2020, 12, e10974. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A. Associations between smoking and alcohol consumption with blood pressure in a middle-aged population. Tob. Induc. Dis. 2023, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Egan, B.M. Physical Activity and Hypertension: Knowing Is Not Enough; We Must Apply. Willing Is Not Enough; We Must Do-von Goethe. Hypertension 2017, 69, 404–406. [Google Scholar] [CrossRef]
- Kojima, S.; Watanabe, N.; Numata, M.; Ogawa, T.; Matsuzaki, S. Increase in the prevalence of fatty liver in Japan over the past 12 years: Analysis of clinical background. J. Gastroenterol. 2003, 38, 954–961. [Google Scholar] [CrossRef]
- Nagral, A.; Bangar, M.; Menezes, S.; Bhatia, S.; Butt, N.; Ghosh, J.; Manchanayake, J.H.; Mahtab, M.A.; Singh, S.P. Gender Differences in Nonalcoholic Fatty Liver Disease. Euroasian J. Hepatogastroenterol. 2022, 12 (Suppl. S1), S19–S25. [Google Scholar] [CrossRef]
- Martin-Grau, M.; Monleon, D. Sex dimorphism and metabolic profiles in management of metabolic-associated fatty liver disease. World J. Clin. Cases. 2023, 11, 1236–1244. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Li, Y.Y.; Nie, Y.Q.; Ma, J.X.; Lu, L.G.; Shi, S.L.; Chen, M.H.; Hu, P.J. Prevalence of fatty liver disease and its risk factors in the population of South China. World J. Gastroenterol. 2007, 13, 6419–6424. [Google Scholar] [CrossRef]
- Gariani, K.; Jornayvaz, F.R. Pathophysiology of NASH in endocrine diseases. Endocr. Connect. 2021, 10, R52–R65. [Google Scholar] [CrossRef] [PubMed]
- Singeap, A.M.; Stanciu, C.; Huiban, L.; Muzica, C.M.; Cuciureanu, T.; Girleanu, I.; Chiriac, S.; Zenovia, S.; Nastasa, R.; Sfarti, C.; et al. Association between Nonalcoholic Fatty Liver Disease and Endocrinopathies: Clinical Implications. Can. J. Gastroenterol. Hepatol. 2021, 2021, 6678142. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Ho, H.N. Hepatic manifestations of women with polycystic ovary syndrome. Best. Pract. Res. Clin. Obstet. Gynaecol. 2016, 37, 119–128. [Google Scholar] [CrossRef]
- Jaruvongvanich, V.; Sanguankeo, A.; Riangwiwat, T.; Upala, S. Testosterone, Sex Hormone-Binding Globulin and Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Ann. Hepatol. 2017, 16, 382–394. [Google Scholar] [CrossRef]
- Yang, M.; Ma, F.; Guan, M. Role of Steroid Hormones in the Pathogenesis of Nonalcoholic Fatty Liver Disease. Metabolites 2021, 11, 320. [Google Scholar] [CrossRef] [PubMed]
- Della Torre, S. Beyond the X Factor: Relevance of Sex Hormones in NAFLD Pathophysiology. Cells 2021, 10, 2502. [Google Scholar] [CrossRef]
- Varlamov, O.; Bethea, C.L.; Roberts, C.T., Jr. Sex-specific differences in lipid and glucose metabolism. Front. Endocrinol. 2015, 5, 241. [Google Scholar] [CrossRef]
- Ceccarelli, I.; Bioletti, L.; Peparini, S.; Solomita, E.; Ricci, C.; Casini, I.; Miceli, E.; Aloisi, A.M. Estrogens and phytoestrogens in body functions. Neurosci. Biobehav. Rev. 2022, 132, 648–663. [Google Scholar] [CrossRef]
- Von-Hafe, M.; Borges-Canha, M.; Vale, C.; Leite, A.R.; Sérgio Neves, J.; Carvalho, D.; Leite-Moreira, A. Nonalcoholic Fatty Liver Disease and Endocrine Axes-A Scoping Review. Metabolites 2022, 12, 298. [Google Scholar] [CrossRef]
- Link, J.C.; Chen, X.; Arnold, A.P.; Reue, K. Metabolic impact of sex chromosomes. Adipocyte 2013, 2, 74–79. [Google Scholar] [CrossRef]
- Chen, X.; McClusky, R.; Chen, J.; Beaven, S.W.; Tontonoz, P.; Arnold, A.P.; Reue, K. The number of x chromosomes causes sex differences in adiposity in mice. PLoS Genet. 2012, 8, e1002709. [Google Scholar] [CrossRef]
- Michopoulos, S.; Chouzouri, V.I.; Manios, E.D.; Grapsa, E.; Antoniou, Z.; Papadimitriou, C.A.; Zakopoulos, N.; Dimopoulos, A.M. Untreated newly diagnosed essential hypertension is associated with nonalcoholic fatty liver disease in a population of a hypertensive center. Clin. Exp. Gastroenterol. 2016, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, M.; Patel, P.; Dunn-Valadez, S.; Dao, C.; Khan, V.; Ali, H.; El-Serag, L.; Hernaez, R.; Sisson, A.; Thrift, A.P.; et al. Women Have a Lower Risk of Nonalcoholic Fatty Liver Disease but a Higher Risk of Progression vs Men: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2021, 19, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Villela-Nogueira, C.A.; Leite, N.C.; Cardoso, C.R.; Salles, G.F. NAFLD and Increased Aortic Stiffness: Parallel or Common Physiopathological Mechanisms? Int. J. Mol. Sci. 2016, 17, 460. [Google Scholar] [CrossRef] [PubMed]
- Cadeddu, C.; Franconi, F.; Cassisa, L.; Campesi, I.; Pepe, A.; Cugusi, L.; Maffei, S.; Gallina, S.; Sciomer, S.; Mercuro, G.; et al. Arterial hypertension in the female world: Pathophysiology and therapy. J. Cardiovasc. Med. 2016, 17, 229–236. [Google Scholar] [CrossRef]
- Sevre, K.; Lefrandt, J.D.; Nordby, G.; Os, I.; Mulder, M.; Gans, R.O.; Rostrup, M.; Smit, A.J. Autonomic function in hypertensive and normotensive subjects: The importance of gender. Hypertension 2001, 37, 1351–1356. [Google Scholar] [CrossRef]
- Narkiewicz, K.; Phillips, B.G.; Kato, M.; Hering, D.; Bieniaszewski, L.; Somers, V.K. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension 2005, 45, 522–525. [Google Scholar] [CrossRef]
- Matsukawa, T.; Sugiyama, Y.; Watanabe, T.; Kobayashi, F.; Mano, T. Gender difference in age-related changes in muscle sympathetic nerve activity in healthy subjects. Am. J. Physiol. 1998, 275, R1600–R1604. [Google Scholar] [CrossRef]
- Meloni, A.; Cadeddu, C.; Cugusi, L.; Donataccio, M.P.; Deidda, M.; Sciomer, S.; Gallina, S.; Vassalle, C.; Moscucci, F.; Mercuro, G.; et al. Gender Differences and Cardiometabolic Risk: The Importance of the Risk Factors. Int. J. Mol. Sci. 2023, 24, 1588. [Google Scholar] [CrossRef]
- Reckelhoff, J.F. Gender differences in the regulation of blood pressure. Hypertension 2001, 37, 1199–1208. [Google Scholar] [CrossRef]
- Oparil, S.; Miller, A.P. Gender and blood pressure. J. Clin. Hypertens. 2005, 7, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Barris, C.T.; Faulkner, J.L.; Belin de Chantemèle, E.J. Salt Sensitivity of Blood Pressure in Women. Hypertension 2023, 80, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Mercuro, G.; Podda, A.; Pitzalis, L.; Zoncu, S.; Mascia, M.; Melis, G.B.; Rosano, G.M. Evidence of a role of endogenous estrogen in the modulation of autonomic nervous system. Am. J. Cardiol. 2000, 85, 787–789. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).