Abstract
In clinical practice, it is found that autoimmune thyroid disease often additionally occurs with systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). In addition, several studies showed that eye-specific autoimmune diseases may have a strong relationship with systemic autoimmune diseases. We focused on Graves’ disease (GD) with ocular conditions, also known as Graves’ ophthalmopathy (GO), trying to find out the potential genetic background related to GO, RA, and SLE. There were 40 GO cases and 40 healthy controls enrolled in this study. The association between single-nucleotide polymorphisms (SNPs) of the co-stimulatory molecule genes and GO was analyzed using a chi-square test. It showed that rs11571315, rs733618, rs4553808, rs11571316, rs16840252, and rs11571319 of CTLA4, rs3181098 of CD28, rs36084323 and rs10204525 of PDCD1, and rs11889352 and rs4675379 of ICOS were significantly associated with GO based on genotype analysis and/or allele analysis (p < 0.05). After summarizing the GO data and the previously published SLE and RA data, it was found that rs11571315, rs733618, rs4553808, rs16840252, rs11571319, and rs36084323 were shared in these three diseases. Furthermore, the bio-function was confirmed by dual-luciferase reporter assay. It was shown that rs733618 T > C and rs4553808 A > G significantly decreased the transcriptional activity (both p < 0.001). This study is the first to confirm that these three diseases share genetically predisposing factors, and our results support the proposal that rs733618 T > C and rs4553808 A > G have bio-functional effects on the transcriptional activity of the CTLA4 gene.
1. Introduction
Systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and Graves’ disease (GD) have many common characteristics, including their prevalence in women and the production of autoantibodies due to the over-activation of autoreactive T cells and the over-proliferation of B cells []. Additionally, it was found that thyroid dysfunction is common in SLE and RA [,]. Many patients begin treatment for thyroid dysfunction before they are diagnosed with lupus or RA, and vice versa []. Furthermore, studies found that there was serological overlap among SLE, RA, and autoimmune thyroid disease (AITD), such as thyroid autoantibodies (ThyAb), thyroid-stimulating hormone (TSH), triiodothyronine (T3), thyroxine (T4), free triiodothyronine (fT3), free thyroxine (fT4), and so on [,,]. Moreover, it was found that treating GD with methoxazole or propylthiouracil could induce the development of SLE []. Furthermore, hydroxychloroquine is a commonly used medication for RA and SLE to help control the symptoms []. These findings indicated a possible common mechanism among SLE, RA, and GD.
In recent years, more and more studies have shown that SLE is associated with AITD, including GD and Hashimoto’s thyroiditis []. In addition, a recent study indicated that GD was associated with an increased risk of SLE, which suggested that there may be an inseparable relationship between AITD and lupus disorders []. In a prospective study in 1987, abnormal thyroid function was found frequently in SLE patients [], which indicates that the association between AITD and SLE has been reported for more than 50 years. Furthermore, Wu et al. showed that the pathogeneses of RA and GD were interrelated []. Although AITD has been reported individually with SLE and RA for many years, the common pathogenesis is still not well understood.
It is clinically shown that about one-third of SLE patients will have ocular complications []. Eye symptoms may relate to systemic disease activity and can be used as an initial manifestation of SLE []. There are two major types of AITD: Graves’ disease and Hashimoto’s autoimmune thyroiditis. Eye involvement in GD has been named Graves’ ophthalmopathy (GO). GO is characterized by swelling of the orbital tissue in GD patients. A genetic factor is believed to be a risk factor for GO. According to statistics, 50% of GO patients have a family history []. Furthermore, compared with GD patients without ocular symptoms, GO patients had a higher frequency of catching other autoimmune diseases []. This suggests that eye-specific autoimmune diseases may have a stronger relationship with systemic autoimmune diseases than other AITDs. Therefore, we focused on the association between GO and other autoimmune diseases in this study. When we set out, there was no study on the correlation between GO, RA, and SLE, so we sought to determine the potential pathogenesis related to these three diseases.
SLE is a systemic autoimmune disease, which is mainly caused by the loss of immune tolerance and immune imbalance led by genetic factors []. GD is also an autoimmune disease, which is caused by the excessive secretion of thyroid hormone due to the production of thyrotropin receptor antibody (TRAb), and a genetic factor is one of the risk factors for the pathogenesis of GD []. In addition, RA is also an autoimmune disease associated with genetic susceptibility. The heritability of RA is up to 50–60%, which indicates that a genetic factor plays a vital role in the pathogenesis of RA []. Although the pathogenesis of SLE, RA, and GO is still unclear, genetic factors are considered to be the key query point. We previously studied the association between SNPs of the co-stimulation molecule genes and SLE [] and RA []. In this study, we determine the common SNPs in these three autoimmune diseases by consolidating the SNP analysis data of SLE, RA, and GO and further verify the biological function of the SNP with statistical significance.
2. Materials and Methods
2.1. Inclusion Criteria
The diagnosis of GO is made when 2 of the following 3 signs of the disease are present: (1) Circulating thyroid antibodies or a dysthyroid state. (2) Typical ocular signs. (3) Fusiform enlargement of extraocular muscles. The inclusion criteria of the healthy control group were those without autoimmune diseases, immune abnormalities, or using immunosuppressive drugs. A total of 100 volunteers were recruited as control cases in the same IRB, and the same number of control cases was taken from those for SNP analysis.
2.2. Selection of Candidate SNPs
Because these autoimmune diseases are caused by abnormal immune regulation, we explored the SNPs of the co-stimulatory molecule genes, which are involved in the regulation of T-cell activation, including CTLA4, CD28, PDCD1, TNFSF4, and ICOS. Previously, only the CTLA4 gene polymorphism and its correlation were analyzed in GO patients []. In this study, the GO sample size was increased, and we took the candidate SNPs in the previously published association study between SLE/RA and the genetic polymorphisms of the co-stimulatory system [,] as the candidate SNPs of GO, to explore the association between these SNPs and GO. Please refer to ref. [,] for the primers and PCR programs used.
2.3. DNA Extraction and Sequencing
The genomic DNA was extracted from 200 µL of peripheral blood using a QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany). Then, the concentration and purity of the extracted DNA were measured using a UV spectrometer before polymerase chain reaction (PCR). PCR was carried out in a total volume of 25 μL containing 50 ng of DNA, 7.5 µL of Hotstar Taq DNA Polymerase (Qiagen, Hilden, Germany) or 2X Tag polymerase, 1 µL each of forward and reverse primer (10 μΜ), and 14.5 µL of ddH2O. The primer pairs of each gene region and the PCR programs were the same as in the previous study [,]. After verifying the DNA fragments produced by PCR through gel electrophoresis, the Big Dye Terminator Cycle Sequencing kit (Thermo Fisher, Waltham, MA, USA) and the ABI PRISM genetic analyzer (Thermo Fisher, Waltham, MA, USA) were used for direct sequencing according to the manufacturer’s instructions.
2.4. Promoter–Reporter Construction
First, we found a sample from the included cases with the Crs11571315Trs733618Ars4553808Crs16840252 haplotype, which we used as the wild type. The promoter region of the CTLA4 gene in this sample was amplified by using the primer with HindIII and SacI restriction enzyme cleavage sites. The sequence of promoter fragments was confirmed by using ABI PRISM 3730 DNA analyzer (Applied Biosystems, Foster City, CA, USA). Then, the fragments were transferred into competent cells (Top 10 or DH5α) through the TOPO TA Cloning Kit (Invitrogen, Carlsbad, CA, USA). After culturing the competent cells, the plasmid DNA was extracted by X-gal, and the sequence of plasmid DNA was checked using direct sequencing. This plasmid DNA was used as the template for creating a single SNP variation via site-directed mutagenesis PCR (Quick Change Site-Directed Mutagenesis Kit, Stratagene, La Jolla, CA, USA). The pairs of primers are shown in Table 1.

Table 1.
The pairs of primers used for promoter–reporter construction with a single SNP variation.
2.5. Cell Culture and Transient Transfections
We routinely cultured 1 × 106 K562 cells in 90% RPMI 1640 medium supplemented with 10% fetal bovine serum, penicillin (50 U/mL), and streptomycin (50 μg/mL) for follow-up experiments. The promoter–reporter constructs were transferred to the pNL1.1 [Nluc] expression vector (Promega, Madison, WI, USA) with NanoLuc luciferase. Similarly, these vectors were transferred into competent cells and confirmed by direct sequencing. Next, 1 μg of the pNL1.1 NanoLuc expression vector with the wild-type sequence or single SNP variation and 1 μg of the pGL 4.5 firefly expression vector (Promega) were transfected into 400 μL (2.5 × 105) K562 cells together by using Lipofectamine 2000 (Invitrogen) and cultured, with pGL 4.5 used as the internal control to exclude bias in the transfection efficiency.
2.6. Dual-Luciferase Reporter Assay
After culturing for 24 h, these cells were detected using a Luciferase Assay System (Nano-Glo® Dual-Luciferase® Reporter Assay System, Promega) according to the manufacturer’s protocol. Each promoter–reporter assay was conducted 5–6 times in parallel. The luminescence of NanoLuc luciferase was divided by Firefly luciferase to exclude bias. In addition, the value of the wild type was referenced as 1 to compare the relative light units (RLUs) of each SNP variation.
2.7. Statistical Analysis
Before all analyses, the genotype contributions of all genes in the control group were analyzed using the Hardy–Weinberg equilibrium (HWE) to confirm that the included control group was representative of the entire population. Then, the allele and genotype contributions were analyzed using the chi-square test or Fisher’s exact test when the expected value of more than 20% of the cells was less than 5, given the odds ratio (OR) with a 95% confidence interval (CI). Among them, the lower-frequency allele was known as the minor allele, which was used to assess the effect of people with a minor allele on disease development. For multiple comparisons, the false discovery rate (FDR) Q-values were calculated to evaluate the expected proportion of type I errors. The haploid blocks were identified by linkage disequilibrium (LD) analysis, which was defined according to the definition proposed previously by Gabriel et al. []. We deleted the haplotypes with frequencies of less than 0.01. ANOVA and Tukey’s honestly significant difference test were used to analyze the difference between the RLU of the wild type and the vector with a single SNP variation. The statistically significant differences were considered as p < 0.05.
3. Results
3.1. Study Subjects
The GO cases (45.5 ± 15.2 years old) comprised 18 males (45%) and 22 females (55%), totaling 40 cases. The control group (37.6 ± 6.8 years old) for GO contained 7 males (18%) and 33 females (72%).
3.2. Hardy–Weinberg Equilibrium Test
First, the genotype frequencies of every SNP from the control group were analyzed using the Hardy–Weinberg equilibrium (HWE) to eliminate statistical errors. It was found that most SNPs satisfied the HWE; only rs3181096 of CD28 and rs10932035 and rs11571305 of ICOS deviated from the HWE (Table 2). Therefore, these three SNPs were excluded from the subsequent SNP analysis and discussion.

Table 2.
The HWE analysis in the control group and the allele frequencies in cases and controls.
3.3. Allele and Genotype Analysis
The allele frequencies are shown in Table 2. The GO-associated SNPs are shown in Table 3 and the complete data are shown in Supplementary Table S1. In addition, the Q-values were calculated to evaluate the proportion of significant tests that will result in false positives (Supplementary Table S1). In the CTLA4 gene, six SNPs had statistical significance: rs11571315, rs733618, rs4553808, rs11571316, rs16840252, and rs11571319. The genotypes of rs11571315 were significantly different between GO cases and healthy controls (CC vs. CT vs. TT, p = 0.006, Q = 0.0720). Compared to TT, the CT genotype had 0.327 times lower odds (95% CI = 0.123–0.870, p = 0.023, Q = 0.1712) and it had 0.077 times lower the odds of CC genotype exposure (95% CI = 0.009–0.682, p = 0.015, Q = 0.1675). In addition, when people had at least one C-allele (CT + CC), they had lower odds of GO (OR = 0.257, 95% CI = 0.101–0.652, p = 0.004, Q = 0.0766). In allele analysis, the C-allele of rs11571315 had 0.291 times lower odds of GO (95% CI = 0.138–0.612, p = 0.001). In other words, the T-allele in rs11571315 was a risk allele of GO. The genotypes of rs733618 had significant differences between GO cases and controls (CC vs. CT vs. TT, p = 0.011, Q = 0.0977). Compared to CC, the TT genotype (OR = 0.135, 95% CI = 0.034–0.545, p = 0.003) and genotype with at least one T-allele (CT + TT, OR = 0.297, 95% CI = 0.094–0.934, p = 0.0032) had lower odds of GO. Moreover, people with TT in rs733618 had lower odds of GO than those with CT and CC (OR = 0.239, 95% CI = 0.082–0.696, p = 0.007, Q = 0.1173). In allele analysis, the T-allele of rs733618 had lower odds of GO (OR = 0.378, 95% CI = 0.199–0.717, p = 0.003). The genotypes of rs4553808 had statistical significance (AA vs. AG, p = 0.019, Q = 0.0977). In this SNP, there were only two genotypes, AA and AG, found in the population included in this study. Compared to AA, people with AG had 0.214 times lower odds (OR = 0.214, 95% CI = 0.055–0.838, p = 0.019, Q = 0.1675). In allele analysis, the G-allele of rs4553808 had lower odds of GO (OR = 0.244, 95% CI = 0.065–0.912, p = 0.025). The genotypes of rs11571316 were near statistical significance (GG vs. AG vs. AA, p = 0.056, Q = 0.1833). Compared to GG, people with the AG genotype had 0.321 times lower odds of GO (95% CI = 0.112–0.916, p = 0.030, Q = 0.1787) and people with at least one A-allele (AG + AA) had 0.306 times lower odds of GO (95% CI = 0.113–0.826, p = 0.017, Q = 0.1675). In allele analysis, the A-allele of rs11571316 had lower odds of GO (OR = 0.356, 95% CI = 0.152–0.836, p = 0.015). The genotypes of rs16840252 were significantly different between GO cases and controls (CC vs. CT, p = 0.019, Q = 0.0977). In this SNP, there were only two genotypes, CC and CT, found in the population included in this study. Compared to CC, the CT genotype had lower odds of GO (OR = 0.214, 95% CI = 0.055–0.838, p = 0.019, Q = 0.0977). In allele analysis, the T-allele of rs16840252 had lower odds of GO (OR = 0.244, 95% CI = 0.065–0.912, p = 0.025). The genotypes of rs11571319 located in 3′UTR of CTLA4 were significantly different between cases and controls (GG vs. AG vs. AA, p < 0.001, Q = 0.0178). Compared to GG, people with the AG genotype (OR = 0.123, 95% CI = 0.042–0.360, p < 0.001, Q = 0.0302) or at least one A-allele (OR = 0.118, 95% CI = 0.040–0.344, p < 0.001, Q = 0.0302) had lower odds of GO. In allele analysis, the A-allele of rs11571319 had lower odds of GO (OR = 0.178, 95% CI = 0.069–0.464, p < 0.001).

Table 3.
The significant SNPs associated with GO.
Regarding the CD28 gene, there were two SNPs associated with GO, rs3181097 and rs3181098. The genotypes of rs3181097 were significantly different between cases and controls (GG vs. AG vs. AA, p < 0.001, Q = 0.0178). Compared to AA, people with the GG genotype (OR = 0.027, 95% CI = 0.003–0.249, p < 0.001, Q = 0.0302) or at least one G-allele (OR = 0.041, 95% CI = 0.005–0.331, p < 0.001, Q = 0.0302) had lower odds of GO. In allele analysis, the G-allele of rs3181097 had lower odds of GO (OR = 0.301, 95% CI = 0.157–0.578, p < 0.001). The genotypes of rs3181098 were close to being statistically significant (GG vs. AG vs. AA, p = 0.055). Moreover, the genotype frequencies of AG + GG and AA were significantly different between GO cases and controls (GG + AG vs. AA, p = 0.026, Q = 0.1742). Because there were no GO cases with the AA genotype, the odds ratio is not shown.
Regarding the PDCD1 gene, two SNPs had statistical significance, rs36084323 and rs10204525. Compared to the TT genotype, people with the CC genotype in rs36084323 had higher odds of GO (OR = 4.125, 95% CI = 1.057–16.097, p = 0.037, Q = 0.1983). Compared to the TT + CT genotype, people with the CC genotype in rs10204525 had higher odds of GO (OR = 2.688, 95% CI = 1.076–6.715, p = 0.032, Q = 0.1787). In allele analysis, the C-allele of rs36084323 and rs10204525 had a higher risk of catching GO (OR = 2.048, 95% CI = 1.061–3.955, p = 0.032 and OR = 2.427, 95% CI = 1.172–5.023, p = 0.015, respectively).
Regarding the ICOS gene, two SNPs had statistical significance, rs11889352 and rs4675379. The genotypes of rs11889352 were significantly different between cases and controls (AA vs. AT vs. TT, p = 0.045, Q = 0.1800). No matter whether comparing to the AA genotype or AG + AA, people with TT had higher odds of GO (AA vs. TT, OR = 10.733, 95% CI = 1.197–96.283, p = 0.020, Q = 0.1675; AA + AT vs. TT, OR = 8.531, 95% CI = 0.997–73.006, p = 0.029, Q = 0.1787). In allele analysis, the T-allele of rs11889352 had higher odds of GO (OR = 2.272, 95% CI = 1.135–4.546, p = 0.019). The genotypes of rs4675379 were significantly different between cases and controls (GG vs. CG vs. CC, p = 0.042, Q = 0.1800). Compared to the GG genotype, people with the CG (OR = 0.278, 95% CI = 0.090–0.859, p = 0.023, Q = 0.1712) or at least one C-allele (OR = 0.333, 95% CI = 0.111–1.001, p = 0.047) had lower odds of GO. However, the allele contributions of rs4675379 were not significantly different between cases and controls (p = 0.178).
3.4. Haplotype Analysis
In Figure 1, the color of the box indicates the degree of linkage disequilibrium (LD) between the two SNPs. The color gradually changes from white to red, indicating that LD is becoming stronger, and purple indicates that there is no LD. In LD analysis, it was found that CD28 (rs1879877/rs3181097/rs3181098), CTLA4 (rs62182595/rs16840252/rs5742909), and PDCD1 (rs2227981/rs2227982/rs6705653/rs41386349) each had one haplotype block, and ICOS had two haplotype blocks (rs11889352/rs11883722 and rs10932036/rs4404254/rs10932037/rs10932038). In haplotype analysis (Table 4), it was found that the five CTLA4 haplotypes (Ars62182595Trs16840252Crs5742909, Ars62182595Trs16840252Trs5742909, Ars62182595Crs16840252Crs5742909, Ars62182595Crs16840252Trs5742909, and Grs62182595Trs16840252Crs5742909) and one ICOS haplotype (Ars11889352Crs11883722) were associated with GO (all p = 0.034).
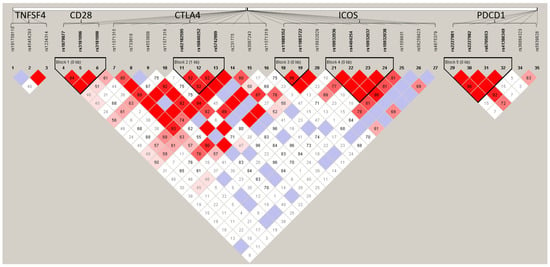
Figure 1.
The linkage disequilibrium (LD) plot of the target genes for GO cases and controls. The color gradually changes from white to red, indicating that LD is becoming stronger, and purple indicates that there is no LD.

Table 4.
The significant haplotypes associated with GO.
3.5. Transcriptional Activity Analysis
After integrating the data of SLE, RA, and GO, it was found that rs11571315, rs733618, rs4553808, rs16840252, and rs11571319 of CTLA4 and rs36084323 of PDCD1 had a significant statistical association in these three autoimmune diseases (Table 5). Then, the dual-luciferase reporter assay was used to explore the influence of SNP variation in the promoter region of the CTLA4 gene on transcriptional activity.

Table 5.
Common SNPs in SLE, RA, and GO.
The bio-function of the significant SNPs located in the CTLA4 promoter region was analyzed through dual-luciferase reporter assay. It was shown that rs733618 T > C and rs4553808 A > G had a significant effect on transcriptional activity, but rs11571315 C > T and rs16840252 C > T did not (Table 6 and Figure 2). The C-allele of rs733618 had 0.263 times lower transcriptional activity than the T-allele (p < 0.001), and the G-allele of rs4553808 reduced the transcriptional activity level to 0.245 times that of the A-allele (p < 0.001).

Table 6.
Analysis of the transcriptional activity of each common SNP variation in CTLA4 through dual-luciferase reporter assay.
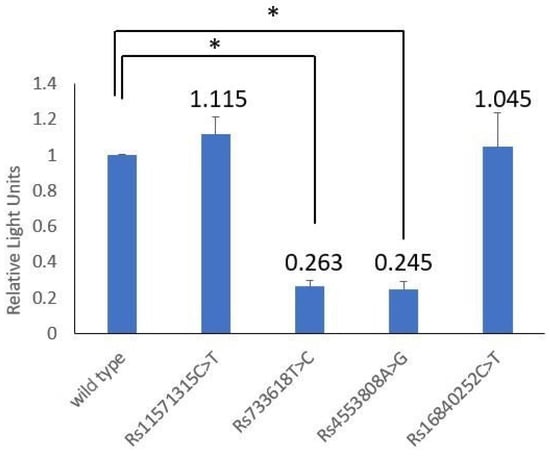
Figure 2.
Comparing the transcriptional activity levels of the reporter contractions with rs11571315 C > T, rs733618 T > C, rs4553808 A > G, rs16840252 C > T and wild type. “*” indicates statistical significance (p < 0.05).
4. Discussion
Previously, we found that rs733618 of CTLA4 was significantly associated with GO and rs16840252 had a strong tendency towards statistical significance based on the data from 22 GO cases and 20 healthy controls []. In this study, the sample size was increased to 40 GO cases and 40 healthy controls. In addition, the data about SLE and RA that were previously published [,] and the data on GO in this study were combined to find out the common SNPs among these three diseases.
In 2019, we found that rs733618 of CTLA4 was significantly associated with GO based on data from 22 GO cases and 20 healthy controls, while rs16840252 had a strong tendency towards statistical significance []. Here, we increased the sample size to 40 GO cases and 40 healthy controls, and it was found that rs11571315, rs4553808, and rs11571319 of the CTLA4 gene were also associated with GO in addition to rs733618 and rs16840252. Most studies found that rs231775 of CTLA4 was associated with GO [], but our study did not. A meta-analysis showed that rs231775 was associated with GO, which was more significant in European populations than in Asian populations []. Thus, there may be differences between ethnic groups. In addition, other significant SNPs had only been reported related to GD rather than GO. For example, rs733618 was found to be associated with GD in the Taiwanese population []; rs11571315 was found to be associated with GD in the Chinese Han population []; and rs11571319 was associated with GD when combined with other SNPs into a haplotype []. It could be seen that CTLA4 was undoubtedly one of the susceptibility genes for GD or further development into GO; however, its variants associated with GD/GO varied widely across populations. Concerning our research about the correlation between GD and CTLA4 polymorphism, it was found that rs733618 T/C and rs231775 G/A were associated with GD []. In addition to rs733618, we also found that rs11571319 was associated with GO. Thus, rs11571319 may be a susceptibility SNP specific to GO, rather than GD.
According to the available information, there was no literature about the association between the SNPs of rs4553808, rs16840252, rs36084323, rs10204525, rs3181098, rs11889352, and rs4675379, and GD/GO. Although there was no literature associated with GO or GD, these SNPs were associated with other autoimmune diseases or cancers. It was found that rs4553808 was significantly correlated with Hashimoto’s thyroiditis disease, which is also a thyroid disease []; rs16840252 was related to the risk of colon cancer []; rs10204525 was related to Posner–Schlossman syndrome, an orbital disease, when it was integrated with other SNPs []; rs3181098 was associated with malignant melanoma and its metastasis-free survival rate reduction []; and rs4675379 was associated with coeliac disease []. It shows that these SNPs also have specific functions in immune regulation. However, rs11889352 has no relevant research at present. Meanwhile, it is known that the promoter activity of rs36084323 with the A-allele is lower than the G-allele, and it may cause various autoimmune thyroid diseases by affecting the expression of PD-1 on Treg cells, the expression of PD-1/PD-1 ligand (PD-L1) on thyroid, and the titers of thyroglobulin autoantibody []. Therefore, rs36084323 may be an important hub of thyroid disease.
After integrating the data of SLE, RA, and GO, it was found that several SNPs had intersections, including rs11571315, rs733618, rs4553808, rs16840252, and rs11571319 of CTLA4 and rs36084323 of PDCD1, which indicated that these three diseases had a partial genetic background. Thus, these SNPs may play an important role both in the pathogenesis of systemic autoimmune diseases (such as SLE and RA) and eye-specific autoimmune diseases (such as GO). Since they share many features, it was not surprising that they shared the same genetic predisposing factors. CTLA4 and PDCD1 are important negative regulators of T-cell activation []. As mentioned in the first paragraph of Section 4, these SNPs may also be susceptible to other autoimmune diseases and cancers. It shows that negative regulation of T cells may be more important than positive regulation in the pathogenic mechanism of autoimmune diseases. The haplotypes with statistical significance of SLE, RA, and GO contained rs62182595 and rs16840252 of CTLA4, leading us to surmise that these two SNPs may have an interaction with the key SNP that causes the disease. They were significant in SNP analysis, but it was not real pathogenic SNPs. This conjecture was verified in our functional analysis. The SNP variation of rs16840252 did not affect the transcriptional activity of the CTLA4 gene. In the functional analysis, it was found that rs733618 T > C and rs4553808 A > G significantly reduced the transcriptional activity. In addition, the transcriptional activity analysis of rs36084323 of PDCD1 was conducted in our previous study [], and it was found that rs36084323 C > T would decrease the transcription activity by 0.68 ± 0.07 times. In the SNP analysis, it was found that rs733618 T-allele and rs4553808 G-allele had a lower risk of SLE, RA, and GO. Theoretically, the decreased expression of CTLA4 contributes to autoimmune disease. Therefore, the higher gene expression level may explain the association of rs733618 T-allele with a lower risk of various autoimmune diseases. Our results proved that the rs733618 C-allele had lower transcriptional activity, which was the same finding as that of our research team [,,]. Moreover, an eQTL analysis by Cai et al. showed that rs733618 could function as a cis-eQTL to affect membrane CTLA4 or total CTLA4 expression in the hippocampus [], and cis-eQTL can affect the majority of human genes rather than specific tissue []. Thus, rs733618 seems to be a key SNP regulating CTLA4 expression level. In this study, it was found that the rs4553808 G-allele decreased the transcriptional activity of CTLA4, and Kaykhaei et al. also demonstrated that rs4553808 in the presence of the G-allele was the transcription factor binding sites of CCAAT-enhancer-binding protein β and glucocorticoid receptor [], thereby up- or down-regulating the transcription of CTLA4. However, the rs4553808 G-allele decreased the risk of SLE, RA, and GO, which was rather illogical. After integrating these results, we found that more than one SNP in a gene could regulate the gene transcriptional level at the same time, and we inferred that the final protein expression level should be the integration of these functional SNPs. Therefore, it was not enough to demonstrate that SNP affected the occurrence of diseases only by looking at the effect of specific sites on gene expression. It was found from our results that the allele changes in rs11571315 and rs16840252 would not affect their transcriptional activity. The single-tissue expression quantitative trait loci (eQTL) analysis showed that the allele variation of rs11571315 only influenced the expression level of CTLA4 in certain tissues, such as the esophagus, testis, heart, and artery [], which indicated that the gene expression changes caused by rs11571315 may be tissue-specific. At present, it has not been suggested that rs16840252 is functional, and it often had a strong LD with other susceptibility SNPs or was associated with disease susceptibility after being combined into a haplotype [,,,,,]. Therefore, it was speculated that rs16840252 was statistically significant in SNP analysis because of its strong linkage imbalance with susceptibility SNPs, or it will be functional after interacting with other SNPs. In addition, the mechanism of other diseases related to the meaningful SNPs found in functional analysis may also be due to their regulation of gene transcription activity.
In the future, large-scale and carefully designed research should be carried out, taking into account detailed environmental factors, to confirm this relationship in different populations, so as to further verify these associations, especially for gene–environment and gene–gene interactions, or researchers could select T cells with specific SNPs or haplotypes from patients to culture in vitro to test the CTLA4-mediated immunosuppression. In addition, functional analysis of the promoter SNP could verify that these SNPs affected the transcriptional function of the gene and were associated with the occurrence of the disease. rs733618 and rs4553808 were related and had a biological function in three autoimmune diseases at the same time, indicating that these two SNPs may play an important role in the mechanism of these autoimmune diseases, which could provide a new direction for their treatment. However, the allele frequencies of these common SNPs of CTLA4 had no statistical significance in RA but were only associated with RA in the heterozygous genotype [], which could indicate that the pathogenesis of RA caused by CTLA4 SNPs may be different from that of SLE and GO. Moreover, the human leukocyte antigen (HLA) gene is one of the SNPs that is closely understood in a broad sense. In immune-mediated diseases in particular, there have been reports of SNPs that were associated with autoimmune diseases. It is known that the HLA and its costimulatory system form a necessary part of the immune response. People with certain HLAs are more likely to develop certain autoimmune diseases. For example, the HLA-DR3 allele was a shared SNP for Sjögren syndrome, diabetes mellitus type 1, and SLE [,]. An animal study showed that HLA-DR3 was associated with the autoimmune response induced by the anti-Smith (Sm) antibody in SLE patients []. Thus, the bio-function of the functional SNPs should also be verified through animal studies.
The present study has some merits. Previously, GO was mostly discussed with RA, and this study is the first research to show that SLE, RA, and GO share a genetic background. In addition, since the genotype frequency distribution of the control group was evaluated via HWE analysis before the genotype and haplotype analysis, this indicates that our findings are less prone to bias. The sample size of GO was a limitation, though FDR was used to correct for multiple testing, which could have solved the problem that the sample size of GO cases was small, which may have given false-negative outcomes. In addition, we also used a dual-luciferase reporter assay to verify the bio-functional effect of common SNPs on transcriptional activity. However, some limitations of the study should be acknowledged. This study only included the Taiwanese population. Thus, based on ethnic differences, the findings may only apply to the Taiwanese population. Additionally, because the materials of the reporter assay used in the promoter–reporter cannot be shared with the 3’UTR-reporter, and the 3’UTR-reporter assay needs to consider the influence of microRNA [], only the promoter region was discussed in this study.
5. Conclusions
We found that there were six SNPs of genes that are involved in regulating T-cell activation that were common in SLE, RA, and GO. Furthermore, the bio-functional effect of the promoter SNPs on the transcriptional activity of the CTLA4 gene was verified by dual-luciferase reporter assay. This indicated that these SNPs had a functional effect on the pathogenesis of autoimmune disease rather than just an association. Additionally, T-cell activation can be considered as an upstream pathway of adaptive immunity. Therefore, it can be inferred from this result that these SNP mutations involved in the upstream pathway of adaptive immunity may be related to the regulation of immune response, especially since these SNPs were also associated with other immune-related diseases or cancers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines11092426/s1, Table S1: The complete data of genotype analysis in GO cases and healthy controls.
Author Contributions
D.-P.C. conceived and designed the experiments and reviewed the final draft. W.-T.L. and W.-T.W. performed the experiments and analyzed and interpreted data. Y.-C.C. and K.-H.Y. wrote the draft of the manuscript and provided samples. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a Ministry of Science and Technology grant (110-2320-B-182A-007) and Chang Gung Memorial Hospital grant (CMRPG3M0571) to Ding-Ping Chen. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Chang Gung Memorial Hospital. The approval IRB numbers were 202002097B0 and 201700691B0.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khoo, T.K.; Bahn, R.S. Pathogenesis of Graves’ ophthalmopathy: The role of autoantibodies. Thyroid 2007, 17, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Miao, H.B.; Lin, S.; Chen, Z. Association between rheumatoid arthritis and thyroid dysfunction: A meta-analysis and systematic review. Front. Endocrinol. 2022, 13, 1015516. [Google Scholar] [CrossRef]
- AL-Homood, I.A.; Alkhathami, R.A.; Alenazi, S.K.; Mohammed, A.A. Thyroid dysfunction among patients with systemic lupus erythematosus in Saudi Arabia. Dr. Sulaiman Al Habib Med. J. 2022, 4, 169–173. [Google Scholar] [CrossRef]
- Chan, A.T.; Al-Saffar, Z.; Bucknall, R.C. Thyroid disease in systemic lupus erythematosus and rheumatoid arthritis. Rheumatology 2001, 40, 353–354. [Google Scholar] [CrossRef] [PubMed]
- Hijmans, W.; Doniach, D.; Roitt, I.M.; Holborow, E.J. Serological overlap between lupus erythematosus, rheumatoid arthritis, and thyroid autoimmune disease. Br. Med. J. 1961, 2, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Morand, E.F.; McCloud, P.I.; Littlejohn, G.O. Continuation of long term treatment with hydroxychloroquine in systemic lupus erythematosus and rheumatoid arthritis. Ann. Rheum. Dis. 1992, 1, 1318–1321. [Google Scholar] [CrossRef]
- Klionsky, Y.; Antonelli, M. Thyroid disease in lupus: An updated review. ACR Open Rheumatol. 2020, 2, 74–78. [Google Scholar] [CrossRef]
- Lee, C.; Chen, S.F.; Yang, Y.C.; Hsu, C.Y.; Shen, Y.C. Association between Graves’ disease and risk of incident systemic lupus erythematosus: A nationwide population-based cohort study. Int. J. Rheum. Dis. 2021, 24, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.W.; Moore, G.F.; Weintraub, B.D.; Steinberg, A.D. Prevalence of thyroid disease and abnormal thyroid function test results in patients with systemic lupus erythematosus. Arthritis Rheum. 1987, 30, 1124–1131. [Google Scholar] [CrossRef]
- Wu, D.; Xian, W.; Hong, S.; Liu, B.; Xiao, H.; Li, Y. Graves’ disease and rheumatoid arthritis: A bidirectional mendelian randomization study. Front. Endocrinol. 2021, 12, 702482. [Google Scholar] [CrossRef]
- Read, R.W. Clinical mini-review: Systemic lupus erythematosus and the eye. Ocul. Immunol. Inflamm. 2004, 12, 87–99. [Google Scholar] [CrossRef]
- Foster, C.S.; Vitale, A.T. Diagnosis & Treatment of Uveitis, 2nd ed.; Jaypee Brothers Medical Publishers Ltd.: New Delhi, India, 2013. [Google Scholar]
- Martin, L. The hereditary and familial aspects of exophthalmic goitre and nodular goitre. Q. J. Med. 1945, 14, 207–219. [Google Scholar] [PubMed]
- Ferrari, S.M.; Fallahi, P.; Ruffilli, I.; Elia, G.; Ragusa, F.; Benvenga, S.; Antonelli, A. The association of other autoimmune diseases in patients with Graves’ disease (with or without ophthalmopathy): Review of the literature and report of a large series. Autoimmun. Rev. 2019, 18, 287–292. [Google Scholar] [CrossRef]
- Choi, J.; Kim, S.T.; Craft, J. The pathogenesis of systemic lupus erythematosus-an update. Curr. Opin. Immunol. 2012, 24, 651–657. [Google Scholar] [CrossRef]
- Khan, M.S.; Lone, S.S.; Faiz, S.; Farooq, I.; Majid, S. Graves’ Disease: Pathophysiology, Genetics and Management; IntechOpen: London, UK, 2021; p. 67. [Google Scholar]
- Karami, J.; Aslani, S.; Jamshidi, A.; Garshasbi, M.; Mahmoudi, M. Genetic implications in the pathogenesis of rheumatoid arthritis; an updated review. Gene 2019, 702, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.P.; Lin, W.T.; Yu, K.H. Investigation of the association between the genetic polymorphisms of the co-stimulatory system and systemic lupus erythematosus. Front. Immunol. 2022, 13, 946456. [Google Scholar]
- Chen, D.P.; Wen, Y.H.; Lin, W.T.; Hsu, F.P.; Yu, K.H. Exploration of the association between the single nucleotide polymorphism of co-stimulatory system and rheumatoid arthritis. Front. Immunol. 2023, 14, 1123832. [Google Scholar] [CrossRef]
- Chen, D.P.; Chu, Y.C.; Wen, Y.H.; Lin, W.T.; Hour, A.L.; Wang, W.T. Investigation of the correlation between Graves’ ophthalmopathy and CTLA4 gene polymorphism. J. Clin. Med. 2019, 8, 1842. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S.B.; Schaffner, S.F.; Nguyen, H.; Moore, J.M.; Roy, J.; Blumenstiel, B.; Higgins, J.; Defelice, M. The structure of haplotype blocks in the human genome. Science 2002, 296, 2225–2229. [Google Scholar] [CrossRef]
- Du, P.; Ma, X.; Wang, C. Associations of CTLA4 gene polymorphisms with Graves’ ophthalmopathy: A meta-analysis. Int. J. Genom. 2014, 2014, 537969. [Google Scholar] [CrossRef]
- Chen, P.L.; Fann, C.S.; Chang, C.C.; Wu, I.L.; Chiu, W.Y.; Lin, C.Y.; Yang, W.-S.; Chang, T.-C. Family-based association study of cytotoxic T-lymphocyte antigen-4 with susceptibility to Graves’ disease in Han population of Taiwan. Genes Immun. 2008, 9, 87–92. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, S.X.; Pan, C.M.; Cao, H.M.; Han, B.; Shi, J.Y.; Liang, J.; Gao, G.Q.; Peng, Y.D.; Su, Q. Association of the CTLA4 gene with Graves’ disease in the Chinese Han population. PLoS ONE 2010, 5, e9821. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Z.; Liu, M.; Li, H.; Liang, C.; Li, W.; Bao, L.; Chen, M.; Wu, G. Correlation between CTLA-4 and CD40 gene polymorphisms and their interaction in graves’ disease in a Chinese Han population. BMC Med. Genet. 2018, 19, 171. [Google Scholar] [CrossRef]
- Kaykhaei, M.; Moghadam, H.; Dabiri, S.; Salimi, S.; Jahantigh, D.; Tamandani, D.M.K.; Rasouli, A. Association of CTLA4 (rs4553808) and PTPN22 (rs2476601) gene polymorphisms with Hashimoto’s thyroiditis disease: A case-control study and an In-silico analysis. Meta Gene 2020, 24, 100693. [Google Scholar] [CrossRef]
- Zou, C.; Qiu, H.; Tang, W.; Wang, Y.; Lan, B.; Chen, Y. CTLA4 tagging polymorphisms and risk of colorectal cancer: A case-control study involving 2,306 subjects. Onco Targets Ther. 2018, 11, 4609–4619. [Google Scholar] [CrossRef]
- Huang, X.; Liu, X.; Ye, Y.; Zhang, T.; Mei, S.; Zhu, T.; Peng, S.; Cai, J.; Yan, Z.; Zeng, K.; et al. Polymorphisms and circulating plasma protein levels of immune checkpoints (CTLA-4 and PD-1) are associated with Posner-Schlossman syndrome in Southern Chinese. Front. Immunol. 2021, 12, 607966. [Google Scholar] [CrossRef] [PubMed]
- Bouwhuis, M.G.; Gast, A.; Figl, A.; Eggermont, A.M.; Hemminki, K.; Schadendorf, D.; Kumar, R. Polymorphisms in the CD28/CTLA4/ICOS genes: Role in malignant melanoma susceptibility and prognosis? Cancer Immunol. Immunother. 2010, 59, 303–312. [Google Scholar] [CrossRef]
- Haimila, K. Genetics of T Cell Co-Stimulatory Receptors: CD28, CTLA4, ICOS and PDCD1 in Immunity and Transplantation; Academic Dissertations from the Finnish Red Cross Blood Transfusion Service, no. 54; Finnish Red Cross Blood Service: Helsinki, Finland, 2009. [Google Scholar]
- Kawabata, M.; Inoue, N.; Watanabe, M.; Kobayashi, A.; Hidaka, Y.; Miyauchi, A.; Iwatani, Y. PD-1 gene polymorphisms and thyroid expression of PD-1 ligands differ between Graves’ and Hashimoto’s diseases. Autoimmunity 2021, 54, 450–459. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 pathways: Similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.P.; Wen, Y.H.; Wang, W.T.; Lin, W.T. Exploring the bio-functional effect of single nucleotide polymorphisms in the promoter region of the TNFSF4, CD28, and PDCD1 genes. J. Clin. Med. 2023, 12, 2157. [Google Scholar] [CrossRef]
- Ligers, A.; Teleshova, N.; Masterman, T.; Huang, W.X.; Hillert, J. CTLA-4 gene expression is influenced by promoter and exon 1 polymorphisms. Genes Immun. 2001, 2, 145–152. [Google Scholar] [CrossRef]
- Wang, X.B.; Zhao, X.; Giscombe, R.; Lefvert, A.K. A CTLA-4 gene polymorphism at position -318 in the promoter region affects the expression of protein. Genes Immun. 2002, 3, 233–234. [Google Scholar] [CrossRef]
- Anjos, S.M.; Tessier, M.C.; Polychronakos, C. Association of the cytotoxic T lymphocyte-associated antigen 4 gene with type 1 diabetes: Evidence for independent effects of two polymorphisms on the same haplotype block. J. Clin. Endocrinol. Metab. 2004, 89, 6257–6265. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cai, L.; Yang, X.H.; Kang, Y.N.; Wen, W.; Zhang, F.Q.; Yue, W.H.; Zhang, Q.; Chen, F.; Cao, W.; Yue, J.; et al. Association of the soluble CTLA4 with schizophrenia: An observational study. J. Bio-X Res. 2020, 3, 116–122. [Google Scholar] [CrossRef]
- GTEx consortium. Genetic effects on gene expression across human tissues. Nature 2017, 550, 204–213. [Google Scholar] [CrossRef]
- Yao, L.; Liu, B.; Jiang, L.; Zhou, L.; Liu, X. Association of cytotoxic T-lymphocyte antigen 4 gene with immune thrombocytopenia in Chinese Han children. Hematology 2019, 24, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, J.; Chen, Y.; Tang, W.; Liu, C.; Sun, Y.; Chen, J. Association of CTLA-4 tagging polymorphisms and haplotypes with hepatocellular carcinoma risk: A case-control study. Medicine 2019, 98, e16266. [Google Scholar] [CrossRef]
- Chen, D.P.; Chang, S.W.; Wang, P.N.; Lin, W.T.; Hsu, F.P.; Wang, W.T.; Tseng, C.P. The association between single-nucleotide polymorphisms of co-stimulatory genes within non-HLA region and the prognosis of leukemia patients with hematopoietic stem cell transplantation. Front. Immunol. 2021, 12, 730507. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Wang, Y.; Chen, S.; Lin, J.; Chen, B.; Yu, S.; Kang, M. Investigation of cytotoxic T-lymphocyte antigen 4 polymorphisms in gastric cardia adenocarcinoma. Scand. J. Immunol. 2016, 83, 212–218. [Google Scholar] [CrossRef]
- Kumar, V.; Abbas, A.K.; Fausto, N.; Mitchell, R.N. Robbins Basic Pathology, 8th ed.; Saunders Elsevier: Philadelphia, PA, USA, 2007. [Google Scholar]
- Xue, K.; Niu, W.Q.; Cui, Y. Association of HLA-DR3 and HLA-DR15 Polymorphisms with Risk of Systemic Lupus Erythematosus. Chin. Med. J. 2018, 131, 2844–2851. [Google Scholar]
- Chowdhary, V.R.; Dai, C.; Tilahun, A.Y.; Hanson, J.A.; Smart, M.K.; Grande, J.P.; Rajagopalan, G. A central role for hla-dr3 in anti-smith antibody responses and glomerulonephritis in a transgenic mouse model of spontaneous lupus. J. Immunol. 2015, 195, 4660–4667. [Google Scholar] [CrossRef] [PubMed]
- Rykova, E.; Ershov, N.; Damarov, I.; Merkulova, T. SNPs in 3’UTR miRNA target sequences associated with individual drug susceptibility. Int. J. Mol. Sci. 2022, 23, 13725. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).