Abstract
During pregnancy, the placenta undergoes a natural aging process, which is considered normal. However, it has been hypothesized that an abnormally accelerated and premature aging of the placenta may contribute to placenta-related health issues. Placental senescence has been linked to several obstetric complications, including abnormal fetal growth, preeclampsia, preterm birth, and stillbirth, with stillbirth being the most challenging. A systematic search was conducted on Pubmed, Embase, and Scopus databases. Twenty-two full-text articles were identified for the final synthesis. Of these, 15 presented original research and 7 presented narrative reviews. There is a paucity of evidence in the literature on the role of placental aging in late small for gestational age (SGA), fetal growth restriction (FGR), and stillbirth. For future research, guidelines for both planning and reporting research must be implemented. The inclusion criteria should include clear differentiation between early and late SGA and FGR. As for stillbirths, only those with no other known cause of stillbirth should be included in the studies. This means excluding stillbirths due to congenital defects, infections, placental abruption, and maternal conditions affecting feto-maternal hemodynamics.
1. Introduction
As pregnancy progresses, the placenta undergoes a natural process of aging, which is considered a physiological occurrence. It has been hypothesized that an abnormally accelerated and premature aging of the placenta may contribute to placenta-related health issues [1]. Placental senescence has been identified as a pathological factor that can lead to various obstetric complications, including abnormal fetal growth, preeclampsia, preterm birth, and stillbirth. The most difficult of these complications is late fetal growth restriction and term fetal death, which can result in stillbirth [2,3]. Abnormal growth in the third trimester is classified into two types: small for gestational age (SGA) fetuses and fetal growth restriction (FGR) [4]. One differs from the other by the presence of signs of placental insufficiency, which include estimated fetal weight below the third percentile, weight between the third and tenth centile, or weight crossing centiles but with the presence of abnormal Doppler parameters [5]. SGA and FGR both carry an increased risk of stillbirth, but most term deaths are found in normally grown fetuses [6]. The majority of SGA neonates are born at term, but fetuses with estimated weight below the tenth centile are 2–3 times more often found among stillbirths [6]. Studies have shown a complex association between small size and stillbirth, as stillborn neonates tend to lose around 20–25% of their body weight while in the uterus [7]. Although being small is considered a risk factor for stillbirth, the majority of stillbirths that occur at term involve fetuses that have grown normally. Only 30–40% of stillbirths that occur after 32 weeks of gestation are classified as being below the tenth percentile for fetal size [8,9,10,11]. For this reason, despite different management strategies for term pregnancies, late stillbirth remains a challenge of perinatal medicine [8,12].
At term, most stillbirths occur in fetuses that have grown normally and are classified as “unexplained,” which often leads to the assumption that these events are unpreventable [11,13], although it has been hypothesized that underlying placental pathology is the key to understanding these deaths [8]. Unfortunately, not all symptoms present early enough to both the mother and medical provider for early intervention to be made. By definition, fetal growth restriction is not achieving programmed growth potential [14,15]. There is growing evidence that a proportion of appropriate for gestational age (AGA) neonates are indeed growth restricted [16]. This correlation could help decipher “unexplained” late stillbirths.
Aging is primarily characterized by a gradual decline in cellular, tissue, and organ function, resulting in the accumulation of senescent cells in mitotic tissues. The placenta, which is programmed to function for the duration of the pregnancy, also undergoes these processes [17]. These cells, which have started the aging process, disrupt the normal function of tissues by affecting neighboring cells, breaking down the extracellular matrix, and reducing the tissues’ regenerative capacity. This decline is due to a reduction in the number of stem and progenitor cells [18].
At the morphological level, senescence is associated with several characteristic features. These include cellular and tissue atrophy, reduced cell proliferation, altered cell morphology, and the accumulation of cellular debris. In many tissues, such as the skin, there is a decline in the number and activity of specialized cells such as fibroblasts and melanocytes, leading to the appearance of wrinkles, thinning, and loss of elasticity [17,18].
At the molecular level, senescence involves various mechanisms, including alterations in gene expression, telomere shortening, genomic instability, epigenetic modifications, mitochondrial dysfunction, and cellular senescence-associated secretory phenotype (SASP). Hormones play a crucial role in modulating these processes and influencing the pace of senescence [19].
One of the well-studied hormonal systems relating to senescence is the hypothalamic–pituitary–adrenal (HPA) axis. The HPA axis regulates the production and release of cortisol, a hormone involved in stress response. With age, the HPA axis becomes dysregulated, leading to altered cortisol levels. Elevated cortisol levels can accelerate the aging process, affecting multiple organ systems and contributing to the development of age-related diseases [20,21].
Fetal growth restriction and its consequences, including fetal death, varies in severity depending on the degree of abnormal placental development. It has been hypothesized that the changes observed in late placentation abnormalities may not be as significant, but the role of maternal morbidity and environmental stressors cannot be ignored. These stressors may trigger early placental senescence, contributing to the development of these pregnancy complications.
This review aimed to present the current understanding of the role of placental aging in late SGA, fetal growth restriction, and term stillbirth, and identify future research directions in this area.
2. Materials and Methods
This systematic review is registered in OSF Registries (doi.org/10.17605/OSF.IO/SJ93D). It was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement guidelines [22].
2.1. Search Strategy
Medline, Web of Science, Cochrane, Embase, and Scopus were searched using the search strategy presented in Table 1.

Table 1.
Search strategy.
2.2. Inclusion and Exclusion Criteria
No time limits were set. All peer-reviewed types of publications in English were included, except conference abstracts.
The original research was limited to studies conducted on placentas from pregnancies complicated by late SGA, FGR, or stillbirth. Review articles were limited to those that presented data relevant to pregnancies complicated by late SGA, FGR, or stillbirth.
2.3. Study Selection
Sixty full-text articles were assessed for eligibility. The study titles and abstracts were screened according to the following inclusion/exclusion criteria. The references cited in the found articles were also searched in order to identify other published articles on the topic. The study selection process is depicted in the PRISMA flow chart (Figure 1).
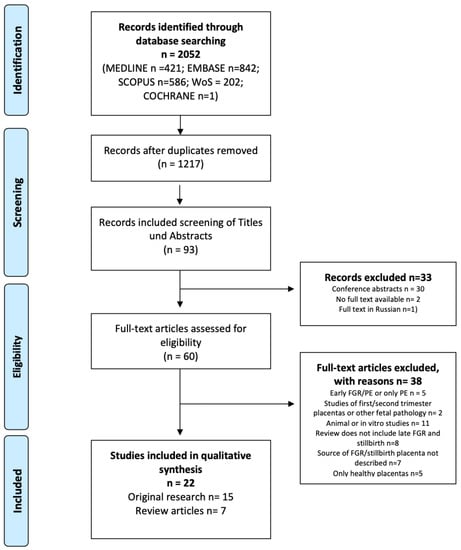
Figure 1.
PRISMA flow chart.
2.4. Process of Data Collection
The search was performed on 10 January 2023. The retrieved records were downloaded and imported into Zotero’s virtual library, which was accessed by the evaluation researchers. After the selection of articles for full-text analysis, they were also deposited in Zotero. The process of synthesizing the results was done using text documents stored in protected files in the virtual workspace. The study was completed on 31 March 2023.
2.5. Evaluation of Risk of Bias
The Newcastle—Ottawa quality assessment scale was used to assess the risk of bias in all the original research [23]; for observational studies, two reviewers independently assessed the risk of bias in each study using the aforementioned scale. The SANRA scale was used for the quality assessment of narrative review articles [24].
2.6. Synthesis Methods
The most important data from the publication were extracted and presented in a systematic approach; see Table 2 and Table 3. For the original research, four columns were developed; these included the publication author(s), publication year, population, and key points. The key points column presents relevant information on late SGA, FGR, and stillbirth from the research. For the narrative reviews, a parallel table was prepared that included the publication author(s), publication year, type of review, SANRA quality assessment, and key points. The data in this review were synthesized using thematic analysis and grouping similar information.

Table 2.
Original research studies included in the final synthesis.

Table 3.
Review articles included in the final synthesis.
3. Results
3.1. Characteristics of the Studies
For the final synthesis, we identified 22 full-text articles. Of these, 15 presented original research and 7 presented narrative reviews.
None of the narrative reviews specifically synthesized knowledge regarding late SGA, FGR, and stillbirth. Only one review concentrated solely on the role of placental aging in unexplained stillbirths at term. The review articles scored between 6 and 10 points in the qualitative assessment of narrative reviews. They primarily lacked justification for performing the review, did not state the hypothesis, and failed to describe the search strategies. Six original studies received the highest possible quality rating. The remaining original research studies presented several sources of bias (Table 4). First of all, there were no unified definitions of SGA, FGR, or stillbirth. The studies had mixed early and late FGR groups. We included studies with a subgroup of pregnancies with an average gestational age above 32 weeks, but this was not always easy to identify from the presented demographic data. The control groups were not always well described. Indeed, the control groups were matched by gestational age, but the reasons for preterm delivery were not specified. As for stillbirths, none of the studies described exclusion criteria for the known causes of stillbirths or the relationship of stillbirth with birthweight (SGA, AGA, LGA). The list of articles and the key points of the presented research are described in Table 2 and Table 3. This has made comparison of the results and synthetical analyses very difficult.

Table 4.
Newcastle—Ottawa quality assessment scale for the observational studies.
3.2. Synthesis of the Results
The following areas of research were identified in the original studies. Placental AURK expression decreases with gestational age, and AURKC is reduced in the placentas from pregnancies complicated by severe early-onset FGR. Additionally, the mtDNA copy number is increased in FGR and SGA placentas, and telomere length attrition is associated with stillbirth. Placentas from FGR cases exhibit accelerated aging, decreased telomerase activity, and shorter telomeres. FGR is associated with decreased activity of mTORC1 and mTORC2, increased expression of P53 mRNA and protein, and increased apoptosis (Figure 1). Furthermore, alpha klotho levels in cord blood are lower in cases of SGA, indicating a potential link between alpha klotho and the accelerated maturation of placental villi. These findings suggest that alterations in placental gene expression, mitochondrial function, telomere length, and protein expression may contribute to FGR and placental aging.
The review articles highlighted various mechanisms that may contribute to abnormal growth. Telomere erosion and cellular senescence in the placenta were found to contribute to FGR, with shorter telomeres observed in FGR placentas compared to controls. Placental mTOR is blocked when maternal folate concentrations are low, leading to decreased placental amino acid transport and fetal nutrient unavailability. FGR coexists with increased expression of DNA damage biomarkers, reduction of telomere length and telomerase activity, upregulation of senescence-associated markers, and oxidative DNA damage. Placental apoptosis may result from placental hypoxia, reactive oxygen species, or a reduction of growth factors, and understanding cell turnover pathways may provide a novel therapeutic approach. Finally, changes in the late gestation placenta contribute to unexplained antepartum stillbirths, and genes that produce aging affect fewer pregnancies, with polymorphisms in genes that produce these effects remaining in the population (Figure 2).
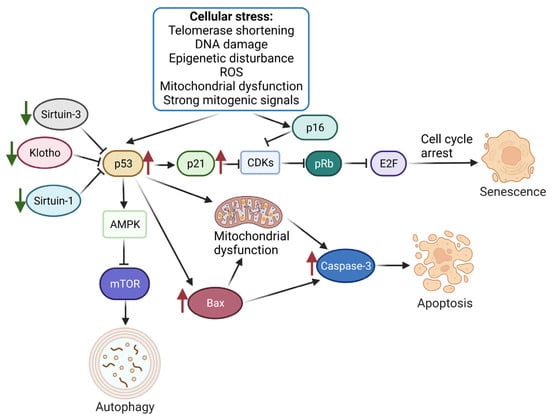
Figure 2.
Molecular mechanisms associated with the placental aging process.Cell stress stimulation triggers the activation of the p53/p21 and p16 pathways, leading to CDK inhibition and the subsequent inhibition of Rb phosphorylation. Rb, which binds to E2F to promote DNA replication, is inactivated, resulting in cell replication arrest at the G1/S phase and triggering cellular senescence. Regulatory factors, such as Sirtuin-1/Sirtuin-3 and Klotho, play a role in controlling this process. p53 also stimulates autophagy by regulating AMPK and mTOR. Moreover, the increased expression of p53 during placental aging leads to apoptosis by inducing mitochondrial dysfunction and upregulating the expression of Bax and Caspase-3. Green arrows indicate proteins downregulated during placental aging, while red indicates upregulated proteins. Created with BioRender (24 May 2023). Klotho—Klotho protein involved in aging; Sirtuin-1/Sirtuin-3—proteins responsible for mitochondrial hemostasis; E2F—a group of transcription factors; CDKs—Cyclin-dependent kinases; AMPK—5′ adenosine monophosphate-activated protein kinase.
4. Discussion
The presented research is the first that addresses the problem of late SGA, FGR, and stillbirth in a systematic review. SGA and FGR are two primary forms of prenatally diagnosed abnormal growth [3]. FGR is characterized by alterations in fetoplacental Doppler and poses a greater risk of intrauterine deterioration and mortality compared to SGA. SGA fetuses are often referred to as constitutionally small and have a near-normal perinatal outcome [3,4]. Both SGA and FGR are associated with suboptimal neurodevelopmental outcomes and intrauterine cardiovascular programming [44]. Placental dysfunction has been most commonly associated with abnormal fetal growth, but only 25% of pregnancies complicated by FGR show abnormalities on histopathological examinations in late forms [3]. For this reason, new approaches need to be researched to detect subtle changes in placental development.
An interesting hypothesis has been made by Smith et al. in their review of placental aging in unexplained stillbirth [42]. These authors refer to Guarente’s definition of aging “as the increase in the likelihood of death occurring with advancing time.” Of course, this definition is meant for mature individuals. Still, suppose we treated a developed placenta as an entity with a programmed life span. In this case, the definition brings new light to the discussion [45]. Analysis of the risk of stillbirths has numerously linked it to gestational age, meaning the older the pregnancy (meaning placenta), the higher the risk of unexplained intrauterine death of an otherwise healthy fetus [46]. Smith et al. hypothesize that etiologies associated with unexplained intrauterine death, such as infarction, hemorrhagic, or placental thrombotic events, are indeed associated with placental aging, as is grown-up human death from cardiac or cerebrovascular disease [42]. Similarly, in late SGA and FGR, exposure to stressors after completed implantation could result in progressive placental aging, abnormal growth, subtle adaptive changes, death, or intrauterine programming, resulting in a higher risk of abnormal development, and metabolic and cardiovascular diseases [3,41,44,47].
Since aging is a physiological process, the key in this discussion is to differentiate physiological from pathological cellular senescence and placental aging. The senescence of trophoblast cells is expected to progress with normally advancing pregnancy and, therefore, results in placental aging. Cellular senescence markers, including SA-ß-gal, the increased expression of p16 and p21 (CDK inhibitors), and p53 (tumor suppressor), have been identified in the syncytiotrophoblast of full-term pregnancies in various studies [1,2,41]. p16, 21, and 53 are responsible for keeping pRB (retinoma tumor suppressor protein) in its active state [33]. pRB suppresses transcription factors that are essential for cell proliferation [1,2,41]. The progression of the G1 and S phases of the cell cycle is controlled by E2F target genes. Silencing of E2F target genes by pRB results in the accumulation of reorganized heterochromatin structures in senescent cell nuclei. This marker is called senescence-associated heterochromatin foci (SAHF). Although these are all physiological processes, several factors have been identified that may trigger premature, accelerated senescence. These triggering factors are primarily stress-related, and they are of oxidative, mitochondrial, or endoplasmic origin [26,48]. The response to stressing factors will depend on the level of stress encountered. Low levels of stress induce adaptive responses and upregulate antioxidant capacities and cell turnover. Mild stress levels trigger adaptive responses that enhance cell turnover and increase antioxidant capabilities. However, moderate stress levels can impede stem cell function and reduce proliferation, while high stress levels can severely disrupt cell function. In such cases, pro-inflammatory cytokines and antiangiogenic factors are released, which can lead to abnormal placentation and hastened trophoblast senescence [48].
Oxidative stress damage of the syncytiotrophoblast is associated with mammalian target of rapamycin complex (mTORC1) activation and telomere shortening [49,50,51]. Both of them have been related to the genesis of obstetric complications. Senescent cells present increased levels of mTORC1, a serine-threonine kinase responsible for the induction of anabolism and the inhibition of catabolism by blocking autophagy [39]. The latter is an essential process in the cell recycling system. The inhibition of mTORC1 by rapamycin delays progression into senescence, prevents permanent loss of proliferative capacity, and allows re-entry into the cell cycle of arrest cells [1,39,41].
Critically short telomeres have also been described as a factor that initiates aging [34,40]. They form protective caps at DNA ends, protecting them from breaks and degradation [2,28]. With every consecutive cell division, telomeres progressively shorten and lose their protective ability [2]. Once they reach a critical minimum length, they expose the DNA ends and initiate a DNA response, leading to the activation of the cellular senescence pathway. Telomere shortening has also been associated with environmental stressors such as hyperglycemia, hypoxia, and the aforementioned oxidative stress [32,34,36,41,42].
During the final stages of pregnancy, the decidual cells and placental membranes of the mother also exhibit signs of aging, which may have a significant impact on the signaling pathways necessary for the onset of labor at term [1,3,35,37,52]. Evidence suggests that the expression of certain markers such as p53, p21, IL-6, IL-8, and SA-ß-gal in these tissues is elevated during term labor [53,54]. Pathological early secretion of senescence inflammatory signals (IL-1beta, IL-6, and IL-8) has been observed in premature rupture of membranes (PROM) and preterm birth (PTB) [52,55,56].
This systematic review provides a comprehensive and rigorous synthesis of evidence. We have included both original research and available narrative reviews and assessed the scientific value of both using adequate tools for analysis. Systematic reviews are dependent on the quality and availability of primary studies, and biases and limitations within those studies can affect the reliability of the review findings. Future research will need to include further analysis of the triggering factors, the time of their incidence, and the resulting impact of the degree of placental aging on pregnancy outcomes. To achieve this, a more systematic approach to defining both study and control groups needs to be incorporated into the research.
Our systematic literature review shows that the most significant limitation of the analyzed studies is the definition of fetal growth restriction and differentiating this from SGA. Late FGR and SGA are by definition growth abnormalities that develop after 32 weeks of gestation [5].
The only study that specifically made this differentiation is Paules et al. In their research, both SGA and FGR pregnancies presented signs of accelerated placental senescence, including lower telomerase activity, shorter telomeres, and reduced SIRT1 RNA expression together with increased P53 RNA expression [3,26,33]. The FGR cases showed signs of apoptosis, with increased levels of CASP3 RNA, expression of SIRT1 RNA, telomerase activity, and telomere length [3,26,33,37]. The caspase-3 activity showed a significant linear increase as the condition’s severity worsened [3]. In this study, both the control and study groups had an ultrasound assessment at 32 weeks, and Doppler parameters were included in the feto-maternal characteristics section of the study. The control group was well-defined but lacked information on the number of spontaneous vaginal deliveries, inductions of labor, or planned cesarean section. Since placental aging has a role in labor initiation, this is critical information for interpreting the results [2,41,42,43].
The largest studied cohort of unexplained stillbirths was published by Ferrari et al. The study group was well-defined, and an initial workup of stillbirth etiologies was performed. Stillbirths were stratified according to birth weight, but only AGA and SGA were defined [28]. Previous studies suggest that large for gestational age is also a risk factor for stillbirth. This would have been an interesting addition to the study [57]. The stillbirths were also stratified as having occurred before and after 34 weeks [28]. It seems a logical strategy to differentiate between unexplained stillbirths related to early vs. late placentation or placental development abnormalities, as this has been done in the study of another placentation-related pathology, preeclampsia [58]. Unfortunately, there is no consensus regarding research on unexplained stillbirths [11,42,46,59]. By definition, stillbirth is death after 22 weeks of gestation or a neonatal weight of 500 g, but WHO recommends reporting only cases after 28 weeks and weight above 1000 g [42]. ACOG recommends stratification of the risk of recurrence and management depending on whether the previous stillbirth occurred before or after 32 weeks of gestation [59]. Many risk factors may contribute to stillbirth [46,59]. The biological pathways are unclear, making it difficult to identify pregnancies with a potential high-risk status or plan an appropriate intervention to reduce the risk [60]. Ferrari et al. tested placenta telomere length reduction as a surrogate for placental premature senescence [28]. They reported that unexplained stillbirth is associated with placental telomere attrition. Interestingly they had two control groups of premature and term deliveries. Premature deliveries included deliveries related to premature rupture of membranes. Telomere lengths in preterm PROM (pPROM) were shorter than in preterm birth with intact membranes and mimicked those present in stillbirth cases, which suggests a possible common pathophysiologic pathway of stillbirth pPROM [1,28].
Indeed, the studies described above specifically show the importance of differentiating between pathological and physiological senescence [40,41,42,43]. They also show that there is a spectrum of abnormal placental aging, which progresses with the severity of the disease, as we hypothesized at the beginning of this review. The delineation between the seriousness of the discussed pathologies could depend on the type and dose of risk exposure and the extent of oxidative stress [28].
5. Conclusions
The review revealed an inconsistency in definitions related to abnormal fetal growth, as well as in research methodologies. However, several original studies have identified mechanisms that contribute to abnormal growth. These mechanisms include decreased placental AURK expression with gestational age, increased mtDNA copy number in FGR and SGA placentas, and decreased activity of mTORC1 and mTORC2 in FGR cases. Additionally, alterations in placental gene expression, mitochondrial function, telomere length, and protein expression may contribute to FGR and placental aging. Future research should implement clear inclusion criteria, differentiate between early and late cases, and have a well-described control group to minimize bias and improve our understanding of the role of placental aging in these conditions.
Author Contributions
Conceptualization, J.M., S.F., A.K., D.S., A.C.-P., E.K., M.B.-J., D.B., K.S., M.R., A.B., A.T. and S.K.; methodology, J.M., A.K. and D.S; software, J.M., S.F., A.K., J.B. and D.S.; formal analysis, J.M., S.F., A.K. and D.S.; investigation, A.K.; resources, J.M., A.K. and D.S.; data curation, J.M., A.K. and D.S.; writing—original draft preparation, J.M., S.F., A.K. and D.S.; writing—review and editing, A.C.-P., E.K., M.B.-J., D.B., K.S., M.R., A.B., A.T., J.B. and S.K; visualization, A.K., S.F., J.B. and D.S.; supervision, D.B., A.T. and S.K.; project administration, D.S.; funding acquisition, A.K. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The APC is funded by the Centre of Postgraduate Medical Education, Warsaw, Poland.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sultana, Z.; Maiti, K.; Dedman, L.; Smith, R. Is There a Role for Placental Senescence in the Genesis of Obstetric Complications and Fetal Growth Restriction? Am. J. Obstet. Gynecol. 2018, 218, S762–S773. [Google Scholar] [CrossRef] [PubMed]
- Maiti, K.; Sultana, Z.; Aitken, R.J.; Morris, J.; Park, F.; Andrew, B.; Riley, S.C.; Smith, R. Evidence That Fetal Death Is Associated with Placental Aging. Am. J. Obstet. Gynecol. 2017, 217, 441.e1–441.e14. [Google Scholar] [CrossRef] [PubMed]
- Paules, C.; Dantas, A.P.; Miranda, J.; Crovetto, F.; Eixarch, E.; Rodriguez-Sureda, V.; Dominguez, C.; Casu, G.; Rovira, C.; Nadal, A.; et al. Premature Placental Aging in Term Small-for-Gestational-Age and Growth-Restricted Fetuses. Ultrasound Obstet. Gynecol. 2019, 53, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Figueras, F.; Caradeux, J.; Crispi, F.; Eixarch, E.; Peguero, A.; Gratacos, E. Diagnosis and Surveillance of Late-Onset Fetal Growth Restriction. Am. J. Obstet. Gynecol. 2018, 218, S790–S802.e1. [Google Scholar] [CrossRef] [PubMed]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus Definition of Fetal Growth Restriction: A Delphi Procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef]
- Coutinho, C.M.; Melchiorre, K.; Thilaganathan, B. Stillbirth at Term: Does Size Really Matter? Int. J. Gynaecol. Obstet. 2020, 150, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Man, J.; Hutchinson, J.C.; Ashworth, M.; Heazell, A.E.; Levine, S.; Sebire, N.J. Effects of Intrauterine Retention and Postmortem Interval on Body Weight Following Intrauterine Death: Implications for Assessment of Fetal Growth Restriction at Autopsy. Ultrasound Obstet. Gynecol. 2016, 48, 574–578. [Google Scholar] [CrossRef]
- Gardosi, J. Counterpoint. Am. J. Obstet. Gynecol. 2019, 220, 74–82. [Google Scholar] [CrossRef]
- Poon, L.C.Y.; Volpe, N.; Muto, B.; Syngelaki, A.; Nicolaides, K.H. Birthweight with Gestation and Maternal Characteristics in Live Births and Stillbirths. FDT 2012, 32, 156–165. [Google Scholar] [CrossRef]
- Poon, L.C.Y.; Tan, M.Y.; Yerlikaya, G.; Syngelaki, A.; Nicolaides, K.H. Birth Weight in Live Births and Stillbirths. Ultrasound Obstet. Gynecol. 2016, 48, 602–606. [Google Scholar] [CrossRef]
- Gardosi, J.; Kady, S.M.; McGeown, P.; Francis, A.; Tonks, A. Classification of Stillbirth by Relevant Condition at Death (ReCoDe): Population Based Cohort Study. BMJ 2005, 331, 1113–1117. [Google Scholar] [CrossRef] [PubMed]
- McCowan, L.M.; Figueras, F.; Anderson, N.H. Evidence-Based National Guidelines for the Management of Suspected Fetal Growth Restriction: Comparison, Consensus, and Controversy. Am. J. Obstet. Gynecol. 2018, 218, S855–S868. [Google Scholar] [CrossRef] [PubMed]
- Ego, A.; Zeitlin, J.; Batailler, P.; Cornec, S.; Fondeur, A.; Baran-Marszak, M.; Jouk, P.-S.; Debillon, T.; Cans, C. Stillbirth Classification in Population-Based Data and Role of Fetal Growth Restriction: The Example of RECODE. BMC Pregnancy Childbirth 2013, 13, 182. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 134: Fetal Growth Restriction. Obstet. Gynecol. 2013, 121, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Torbe, A.; Borowski, D.; Breborowicz, G.; Czajkowski, K.; Huras, H.; Kajdy, A.; Kalinka, J.; Kosinska-Kaczynska, K.; Leszczynska-Gorzelak, B.; et al. Polish Society of Gynecologists and Obstetricians Recommendations on Diagnosis and Management of Fetal Growth Restriction. Ginekol. Pol. 2020, 91, 10. [Google Scholar] [CrossRef]
- McLaughlin, E.J.; Hiscock, R.J.; Robinson, A.J.; Hui, L.; Tong, S.; Dane, K.M.; Middleton, A.L.; Walker, S.P.; MacDonald, T.M. Appropriate-for-Gestational-Age Infants Who Exhibit Reduced Antenatal Growth Velocity Display Postnatal Catch-up Growth. PLoS ONE 2020, 15, e0238700. [Google Scholar] [CrossRef]
- Fedarko, N.S. The Biology of Aging and Frailty. Clin. Geriatr. Med. 2011, 27, 27–37. [Google Scholar] [CrossRef]
- Burton, D.G.A. Cellular Senescence, Ageing and Disease. AGE 2009, 31, 1–9. [Google Scholar] [CrossRef]
- Kajdy, A.; Modzelewski, J.; Cymbaluk-Płoska, A.; Kwiatkowska, E.; Bednarek-Jędrzejek, M.; Borowski, D.; Stefańska, K.; Rabijewski, M.; Torbé, A.; Kwiatkowski, S. Molecular Pathways of Cellular Senescence and Placental Aging in Late Fetal Growth Restriction and Stillbirth. Int. J. Mol. Sci. 2021, 22, 4186. [Google Scholar] [CrossRef]
- Ruffaner-Hanson, C.; Noor, S.; Sun, M.S.; Solomon, E.; Marquez, L.E.; Rodriguez, D.E.; Allan, A.M.; Caldwell, K.K.; Bakhireva, L.N.; Milligan, E.D. The Maternal-Placental-Fetal Interface: Adaptations of the HPA Axis and Immune Mediators Following Maternal Stress and Prenatal Alcohol Exposure. Exp. Neurol. 2022, 355, 114121. [Google Scholar] [CrossRef]
- Papargyri, P.; Zapanti, E.; Salakos, N.; Papargyris, L.; Bargiota, A.; Mastorakos, G. Links between HPA Axis and Adipokines: Clinical Implications in Paradigms of Stress-Related Disorders. Expert. Rev. Endocrinol. Metab. 2018, 13, 317–332. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Lo, C.K.-L.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing Reviewers’ to Authors’ Assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A Scale for the Quality Assessment of Narrative Review Articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Beard, S.; Pritchard, N.; Binder, N.; Schindler, K.; De Alwis, N.; Kaitu’u-Lino, T.J.; Tong, S.; Hannan, N.J. Aurora Kinase MRNA Expression Is Reduced with Increasing Gestational Age and in Severe Early Onset Fetal Growth Restriction. Placenta 2020, 95, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Naha, R.; Anees, A.; Chakrabarty, S.; Naik, P.S.; Pandove, M.; Pandey, D.; Satyamoorthy, K. Placental Mitochondrial DNA Mutations and Copy Numbers in Intrauterine Growth Restricted (IUGR) Pregnancy. Mitochondrion 2020, 55, 85–94. [Google Scholar] [CrossRef]
- Franklin, A.D.; Saqibuddin, J.; Stephens, K.; Birkett, R.; Marsden, L.; Ernst, L.M.; Mestan, K.K. Cord Blood Alpha Klotho Is Decreased in Small for Gestational Age Preterm Infants with Placental Lesions of Accelerated Aging. Placenta 2019, 87, 1–7. [Google Scholar] [CrossRef]
- Ferrari, F.; Facchinetti, F.; Saade, G.; Menon, R. Placental Telomere Shortening in Stillbirth: A Sign of Premature Senescence? J. Matern.-Fetal Neonatal Med. 2016, 29, 1283–1288. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Rosario, F.J.; Shehab, M.A.; Powell, T.L.; Gupta, M.B.; Jansson, T. Increased Ubiquitination and Reduced Plasma Membrane Trafficking of Placental Amino Acid Transporter SNAT-2 in Human IUGR. Clin. Sci. 2015, 129, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Biron-Shental, T.; Sukenik-Halevy, R.; Sharon, Y.; Laish, I.; Fejgin, M.D.; Amiel, A. Telomere Shortening in Intra Uterine Growth Restriction Placentas. Early Hum. Dev. 2014, 90, 465–469. [Google Scholar] [CrossRef]
- Seidmann, L.; Suhan, T.; Unger, R.; Gerein, V.; Kirkpatrick, C.J. Imbalance of Expression of BFGF and PK1 Is Associated with Defective Maturation and Antenatal Placental Insufficiency. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Biron-Shental, T.; Kidron, D.; Sukenik-Halevy, R.; Goldberg-Bittman, L.; Sharony, R.; Fejgin, M.D.; Amiel, A. TERC Telomerase Subunit Gene Copy Number in Placentas from Pregnancies Complicated with Intrauterine Growth Restriction. Early Hum. Dev. 2011, 87, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Heazell, A.E.P.; Sharp, A.N.; Baker, P.N.; Crocker, I.P. Intra-Uterine Growth Restriction Is Associated with Increased Apoptosis and Altered Expression of Proteins in the P53 Pathway in Villous Trophoblast. Apoptosis 2011, 16, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Biron-Shental, T.; Sukenik-Halevy, R.; Sharon, Y.; Goldberg-Bittman, L.; Kidron, D.; Fejgin, M.D.; Amiel, A. Short Telomeres May Play a Role in Placental Dysfunction in Preeclampsia and Intrauterine Growth Restriction. Am. J. Obstet. Gynecol. 2010, 202, 381.e1–381.e7. [Google Scholar] [CrossRef]
- Biron-Shental, T.; Sukenik Halevy, R.; Goldberg-Bittman, L.; Kidron, D.; Fejgin, M.D.; Amiel, A. Telomeres Are Shorter in Placental Trophoblasts of Pregnancies Complicated with Intrauterine Growth Restriction (IUGR). Early Hum. Dev. 2010, 86, 451–456. [Google Scholar] [CrossRef]
- Davy, P.; Nagata, M.; Bullard, P.; Fogelson, N.S.; Allsopp, R. Fetal Growth Restriction Is Associated with Accelerated Telomere Shortening and Increased Expression of Cell Senescence Markers in the Placenta. Placenta 2009, 30, 539–542. [Google Scholar] [CrossRef]
- Kudo, T.; Izutsu, T.; Sato, T. Telomerase Activity and Apoptosis as Indicators of Ageing in Placenta with and without Intrauterine Growth Retardation. Placenta 2000, 21, 493–500. [Google Scholar] [CrossRef]
- Kohlrausch, F.B.; Keefe, D.L. Telomere Erosion as a Placental Clock: From Placental Pathologies to Adverse Pregnancy Outcomes. Placenta 2020, 97, 101–107. [Google Scholar] [CrossRef]
- Silva, E.; Rosario, F.J.; Powell, T.L.; Jansson, T. Mechanistic Target of Rapamycin Is a Novel Molecular Mechanism Linking Folate Availability and Cell Function. J. Nutr. 2017, 147, 1237–1242. [Google Scholar] [CrossRef]
- Biron-Shental, T.; Sadeh-Mestechkin, D.; Amiel, A. Telomere Homeostasis in IUGR Placentas—A Review. Placenta 2016, 39, 21–23. [Google Scholar] [CrossRef]
- Sultana, Z.; Maiti, K.; Aitken, J.; Morris, J.; Dedman, L.; Smith, R. Oxidative Stress, Placental Ageing-Related Pathologies and Adverse Pregnancy Outcomes. Am. J. Reprod. Immunol. 2017, 77, e12653. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Maiti, K.; Aitken, R.J. Unexplained Antepartum Stillbirth: A Consequence of Placental Aging? Placenta 2013, 34, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Heazell, A.E.P.; Crocker, I.P. Live and Let Die—Regulation of Villous Trophoblast Apoptosis in Normal and Abnormal Pregnancies. Placenta 2008, 29, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Crispi, F.; Miranda, J.; Gratacós, E. Long-Term Cardiovascular Consequences of Fetal Growth Restriction: Biology, Clinical Implications, and Opportunities for Prevention of Adult Disease. Am. J. Obstet. Gynecol. 2018, 218, S869–S879. [Google Scholar] [CrossRef] [PubMed]
- Johnson, F.B.; Sinclair, D.A.; Guarente, L. Molecular Biology of Aging. Cell 1999, 96, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Muglu, J.; Rather, H.; Arroyo-Manzano, D.; Bhattacharya, S.; Balchin, I.; Khalil, A.; Thilaganathan, B.; Khan, K.S.; Zamora, J.; Thangaratinam, S. Risks of Stillbirth and Neonatal Death with Advancing Gestation at Term: A Systematic Review and Meta-Analysis of Cohort Studies of 15 Million Pregnancies. PLoS Med. 2019, 16, e1002838. [Google Scholar] [CrossRef]
- Crispi, F.; Figueras, F.; Cruz-Lemini, M.; Bartrons, J.; Bijnens, B.; Gratacos, E. Cardiovascular Programming in Children Born Small for Gestational Age and Relationship with Prenatal Signs of Severity. Am. J. Obstet. Gynecol. 2012, 207, 121.e1–121.e9. [Google Scholar] [CrossRef]
- Burton, G.J.; Yung, H.W.; Murray, A.J. Mitochondrial—Endoplasmic Reticulum Interactions in the Trophoblast: Stress and Senescence. Placenta 2017, 52, 146–155. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Rosario, F.J.; Powell, T.L.; Gupta, M.B.; Jansson, T. Inhibition of Placental Mammalian Target of Rapamycin Complex 2 (MTORC2) Signaling in Human IUGR. Reprod. Sci. 2015, 22, 376A. [Google Scholar]
- Rosario, F.J.; Dimasuay, K.G.; Kanai, Y.; Powell, T.L.; Jansson, T. Regulation of Amino Acid Transporter Trafficking by MTORC1 in Primary Human Trophoblast Cells Is Mediated by the Ubiquitin Ligase Nedd4-2. Clin. Sci. 2016, 130, 499–512. [Google Scholar] [CrossRef]
- Dimasuay, G.; Glazier, J.; Rogerson, S.; Jansson, T.; Boeuf, P. Inhibition of Placental MTORC1 Signalling May Explain Decreased Placental Nutrient Transport and Contribute to Restricted Fetal Growth in Placental Malaria. Placenta 2014, 35, A62. [Google Scholar] [CrossRef]
- Cox, L.S.; Redman, C. The Role of Cellular Senescence in Ageing of the Placenta. Placenta 2017, 52, 139–145. [Google Scholar] [CrossRef]
- Bonney, E.A.; Krebs, K.; Saade, G.; Kechichian, T.; Trivedi, J.; Huaizhi, Y.; Menon, R. Differential Senescence in Feto-Maternal Tissues during Mouse Pregnancy. Placenta 2016, 43, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Behnia, F.; Polettini, J.; Saade, G.R.; Campisi, J.; Velarde, M. Placental Membrane Aging and HMGB1 Signaling Associated with Human Parturition. Aging 2016, 8, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.M.; Chamley, L.; Keelan, J.A.; Mitchell, M.D. Cytokines of the Placenta and Extra-Placental Membranes: Roles and Regulation During Human Pregnancy and Parturition. Placenta 2002, 23, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Bonney, E.A.; Condon, J.; Mesiano, S.; Taylor, R.N. Novel Concepts on Pregnancy Clocks and Alarms: Redundancy and Synergy in Human Parturition. Hum. Reprod. Update 2016, 22, 535–560. [Google Scholar] [CrossRef]
- Carter, E.B.; Stockburger, J.; Tuuli, M.G.; Macones, G.A.; Odibo, A.O.; Trudell, A.S. Large for Gestational Age and Stillbirth: Is There a Role for Antenatal Testing? Ultrasound Obstet. Gynecol. 2019, 54, 334–337. [Google Scholar] [CrossRef]
- National Collaborating Centre for Women’s and Children’s Health (UK). Hypertension in Pregnancy: The Management of Hypertensive Disorders During Pregnancy; National Institute for Health and Clinical Excellence: Guidance; RCOG Press: London, UK, 2010. [Google Scholar]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 102: Management of Stillbirth. Obstet. Gynecol. 2009, 113, 748–761. [Google Scholar] [CrossRef]
- Reddy, U.M.; Goldenberg, R.; Silver, R.; Smith, G.C.S.; Pauli, R.M.; Wapner, R.J.; Gardosi, J.; Pinar, H.; Grafe, M.; Kupferminc, M.; et al. Stillbirth Classification—Developing an International Consensus for Research: Executive Summary of a National Institute of Child Health and Human Development Workshop. Obstet. Gynecol. 2009, 114, 901–914. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).