In Vitro Activity of Eravacycline against Carbapenemase-Producing Gram-Negative Bacilli Clinical Isolates in Central Poland
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhanel, G.G.; Cheung, D.; Adam, H.; Zelenitsky, S.; Golden, A.; Schweizer, F.; Gorityala, B.; Lagacé-Wiens, P.R.; Walkty, A.; Gin, A.S.; et al. Review of Eravacycline, a Novel Fluorocycline Antibacterial Agent. Drugs 2016, 76, 567–588. [Google Scholar] [CrossRef]
- XERAVA (Eravacycline) for Injection, for Intravenous Use—Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211109lbl.pdf (accessed on 6 May 2023).
- EUCAST Breakpoint Tables for Interpretation of MICs and Zone Diameters, Ver. 13.0. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_13.0_Breakpoint_Tables.pdf (accessed on 26 April 2023).
- Brauncajs, M.; Bielec, F.; Macieja, A.; Pastuszak-Lewandoska, D. Carbapenem-Resistant Gram-Negative Fermenting and Non-Fermenting Rods Isolated from Hospital Patients in Poland-What Are They Susceptible to? Biomedicines 2022, 10, 3049. [Google Scholar] [CrossRef]
- Connors, K.P.; Housman, S.T.; Pope, J.S.; Russomanno, J.; Salerno, E.; Shore, E.; Redican, S.; Nicolau, D.P. Phase I, open-label, safety and pharmacokinetic study to assess bronchopulmonary disposition of intravenous eravacycline in healthy men and women. Antimicrob. Agents Chemother. 2014, 58, 2113–2118. [Google Scholar] [CrossRef]
- Seifert, H.; Stefanik, D.; Sutcliffe, J.A.; Higgins, P.G. In-vitro activity of the novel fluorocycline eravacycline against carbapenem non-susceptible Acinetobacter baumannii. Int. J. Antimicrob. Agents 2018, 51, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Baxter, M.R.; Adam, H.J.; Sutcliffe, J.; Karlowsky, J.A. In vitro activity of eravacycline against 2213 Gram-negative and 2424 Gram-positive bacterial pathogens isolated in Canadian hospital laboratories: CANWARD surveillance study 2014-2015. Diagn. Microbiol. Infect. Dis. 2018, 91, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Livermore, D.M.; Mushtaq, S.; Warner, M.; Woodford, N. In Vitro Activity of Eravacycline against Carbapenem-Resistant Enterobacteriaceae and Acinetobacter baumannii. Antimicrob. Agents Chemother. 2016, 60, 3840–3844. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J. Eravacycline: A Review in Complicated Intra-Abdominal Infections. Drugs 2019, 79, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Dalhoff, A. Global fluoroquinolone resistance epidemiology and implictions for clinical use. Interdiscip. Perspect. Infect. Dis. 2012, 2012, 976273. [Google Scholar] [CrossRef]
- Okuniewicz, R.; Moos, Ł.; Brzoza, Z. Small Intestinal Bacterial Overgrowth Syndrome. Postepy Mikrobiol. 2021, 60, 203–210. [Google Scholar] [CrossRef]
- Tang, K.; Zhao, H. Quinolone Antibiotics: Resistance and Therapy. Infect. Drug Resist. 2023, 16, 811–820. [Google Scholar] [CrossRef]
- Efficacy and Safety Study of Eravacycline Compared with Levofloxacin in Complicated Urinary Tract Infections. Available online: https://clinicaltrials.gov/ct2/show/record/NCT01978938 (accessed on 26 April 2023).
- Grossman, T.H.; O’Brien, W.; Kerstein, K.O.; Sutcliffe, J.A. Eravacycline (TP-434) is active in vitro against biofilms formed by uropathogenic Escherichia coli. Antimicrob. Agents Chemother. 2015, 59, 2446–2449. [Google Scholar] [CrossRef]
- Zou, X.; Jin, S.; Chen, L.; Li, J.; Zhang, X.; Zhou, H.; Li, X.; Huang, H. Antibacterial Activity of Eravacycline Against Carbapenem-Resistant Gram-Negative Isolates in China: An in vitro Study. Infect. Drug Resist. 2023, 16, 2271–2279. [Google Scholar] [CrossRef]
- Morrissey, I.; Olesky, M.; Hawser, S.; Lob, S.H.; Karlowsky, J.A.; Corey, G.R.; Bassetti, M.; Fyfe, C. In Vitro Activity of Eravacycline against Gram-Negative Bacilli Isolated in Clinical Laboratories Worldwide from 2013 to 2017. Antimicrob. Agents Chemother. 2020, 64, e01699-19. [Google Scholar] [CrossRef]
- Abdallah, M.; Olafisoye, O.; Cortes, C.; Urban, C.; Landman, D.; Quale, J. Activity of eravacycline against Enterobacteriaceae and Acinetobacter baumannii, including multidrug-resistant isolates, from New York City. Antimicrob. Agents Chemother. 2015, 59, 1802–1805. [Google Scholar] [CrossRef]
- Sutcliffe, J.A.; O’Brien, W.; Fyfe, C.; Grossman, T.H. Antibacterial activity of eravacycline (TP-434), a novel fluorocycline, against hospital and community pathogens. Antimicrob. Agents Chemother. 2013, 57, 5548–5558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, X.; Bush, K. In vitro susceptibility of β-lactamase-producing carbapenem-resistant Enterobacteriaceae (CRE) to eravacycline. J. Antibiot. 2016, 69, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Gilchrist, M.; Heard, K.; Hamilton, R.; Sneddon, J. Treating infections caused by carbapenemase-producing Enterobacterales (CPE): A pragmatic approach to antimicrobial stewardship on behalf of the UKCPA Pharmacy Infection Network (PIN). JAC Antimicrob. Resist. 2020, 2, dlaa075. [Google Scholar] [CrossRef]
- Walsh, T.R.; Toleman, M.A.; Poirel, L.; Nordmann, P. Metallo-beta-lactamases: The quiet before the storm? Clin. Microbiol. Rev. 2005, 18, 306–325. [Google Scholar] [CrossRef] [PubMed]
- Boyd, S.E.; Livermore, D.M.; Hooper, D.C.; Hope, W.W. Metallo-β-Lactamases: Structure, Function, Epidemiology, Treatment Options, and the Development Pipeline. Antimicrob. Agents Chemother. 2020, 64, e00397-20. [Google Scholar] [CrossRef]
- McCreary, E.K.; Heil, E.L.; Tamma, P.D. New Perspectives on Antimicrobial Agents: Cefiderocol. Antimicrob. Agents Chemother. 2021, 65, e0217120. [Google Scholar] [CrossRef]
- Syed, Y.Y. Cefiderocol: A Review in Serious Gram-Negative Bacterial Infections. Drugs 2021, 81, 1559–1571. [Google Scholar] [CrossRef] [PubMed]
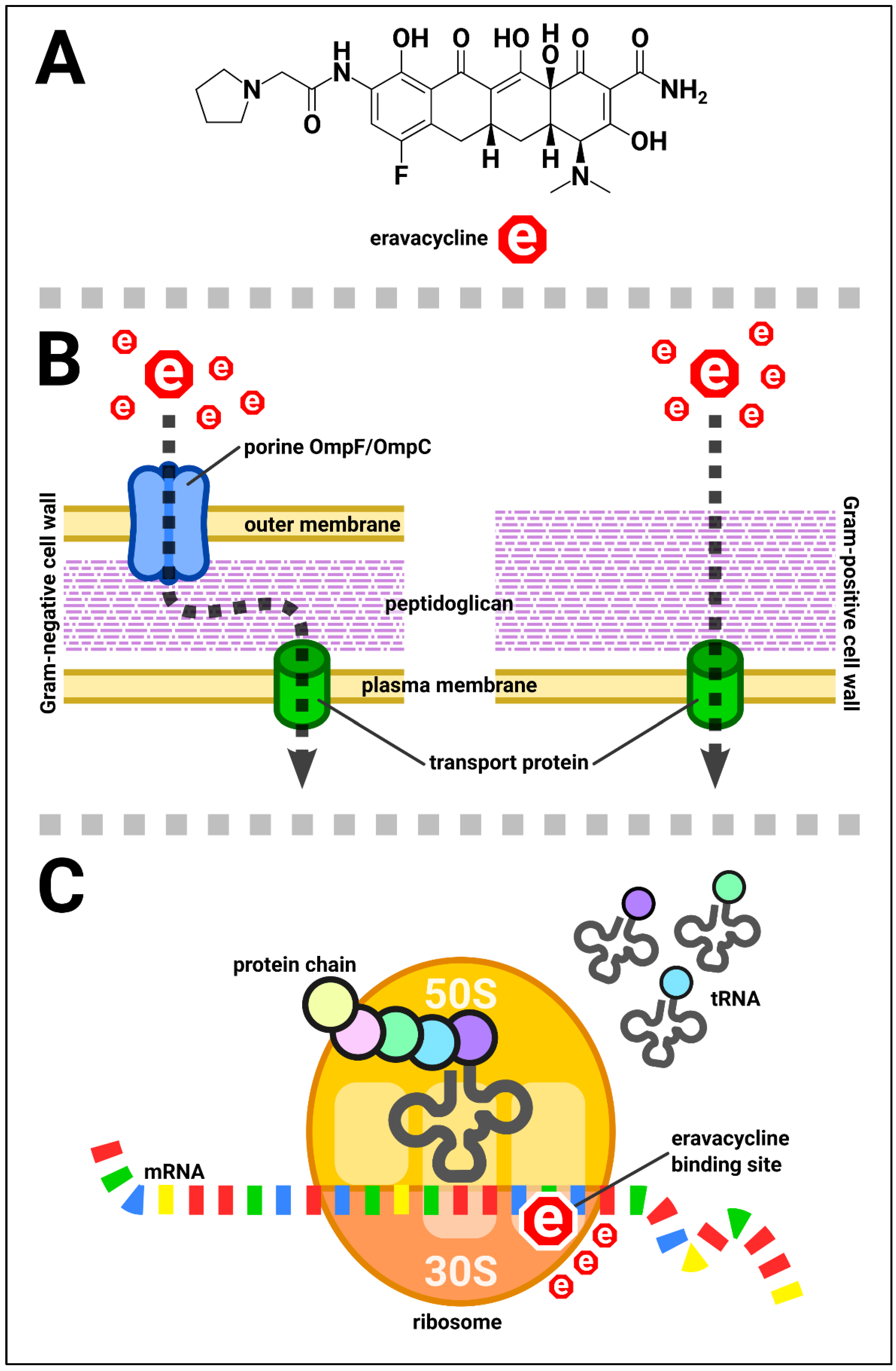
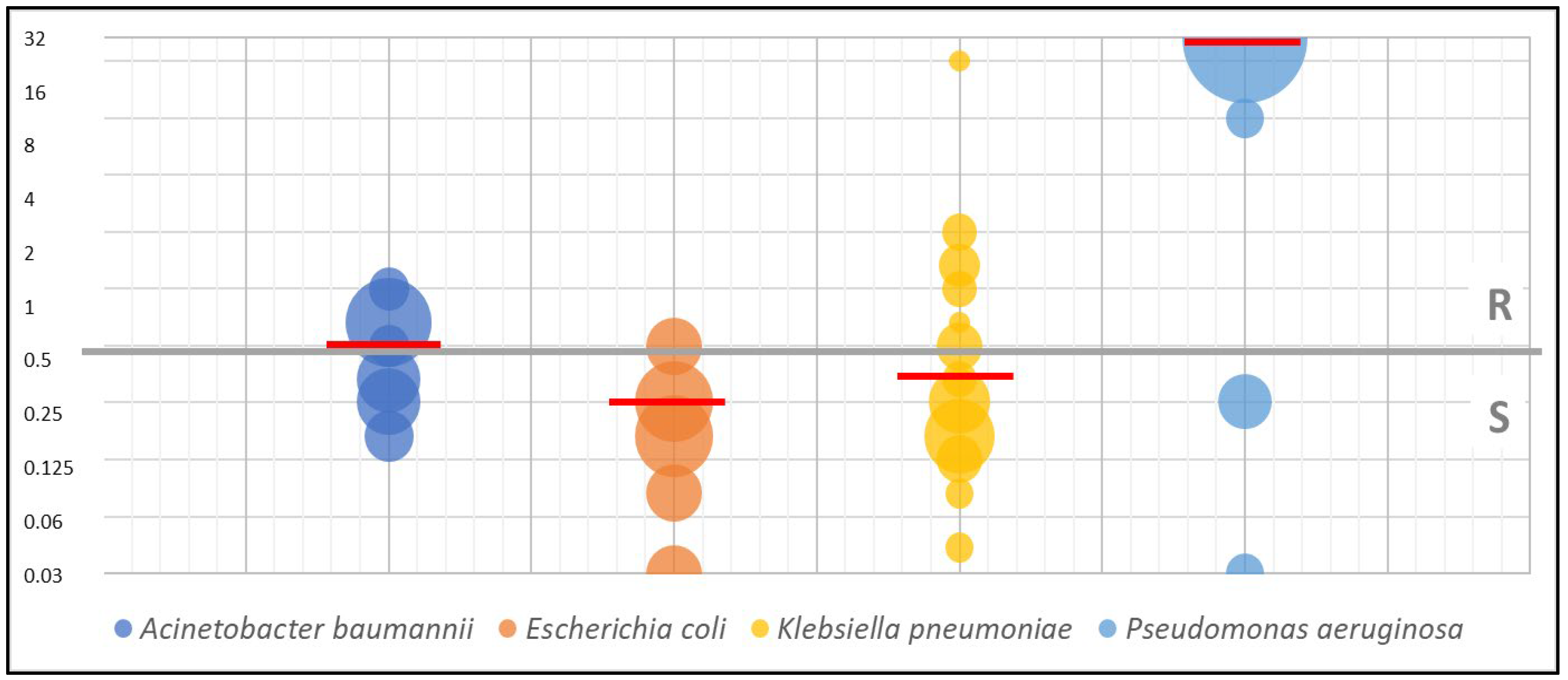
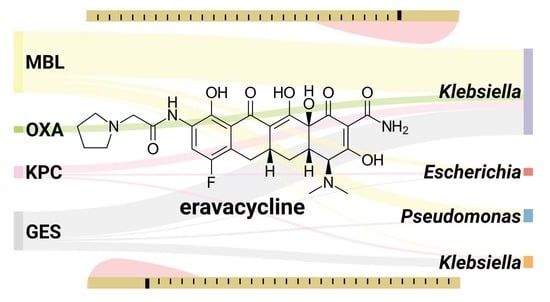
| Organism/Organisms’ Group Name | Breakpoint (S≤; R>) [mg/L] |
|---|---|
| Escherichia coli | 0.5 |
| Staphylococcus aureus | 0.25 |
| Enterococcus spp.; Viridans group streptococci | 0.125 |
| other Enterobacterales; other Staphylococcus spp.; other Streptococcus spp.; Acinetobacter spp.; Haemophilus influenzae; Neisseria spp.; Moraxella catarrhalis | Insufficient evidence that the organism or group is a good target for therapy with the agent. |
| Pseudomonas spp. | No breakpoints. Susceptibility testing is not recommended. |
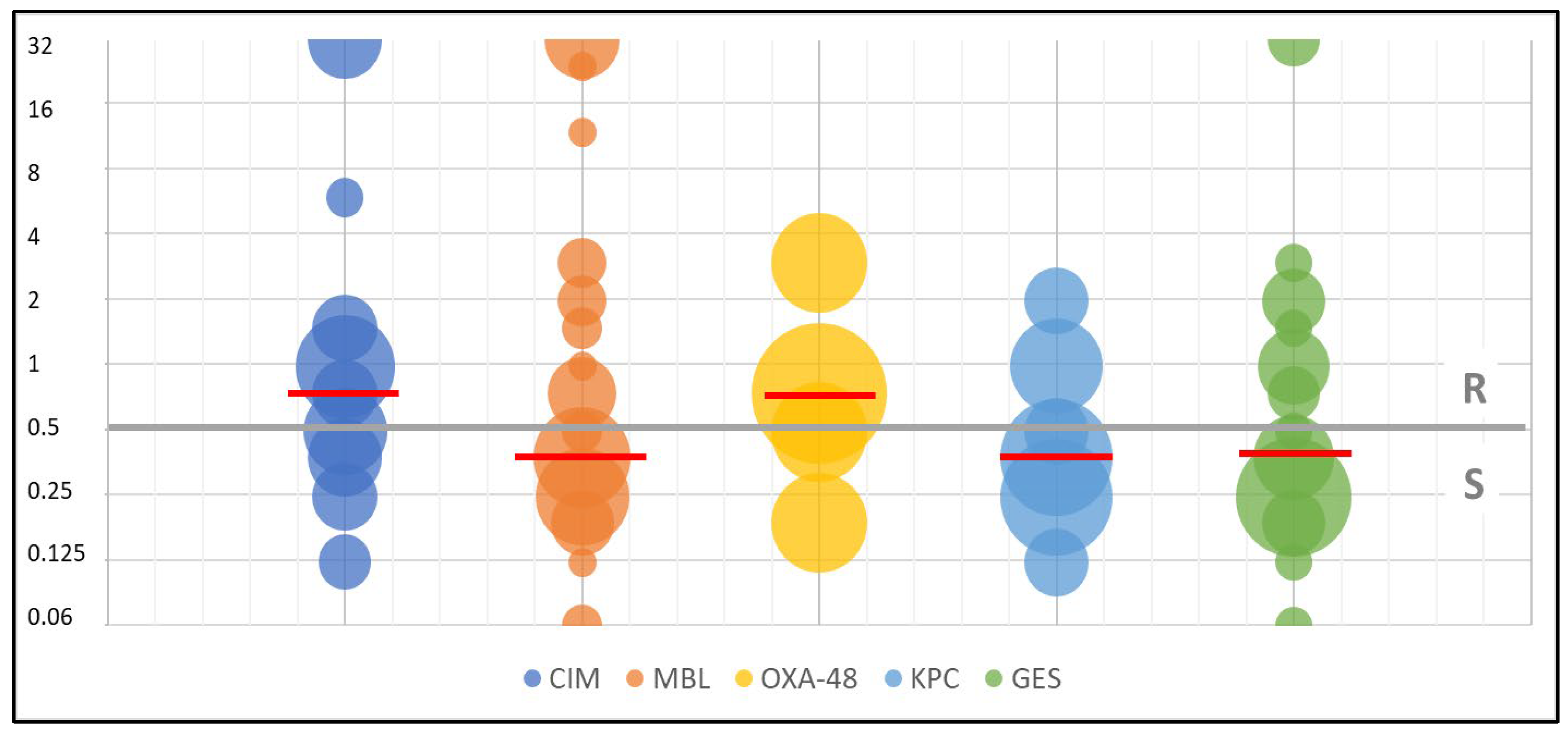
| Resistance Mechanism | N | MIC50 [mg/L] | MIC90 [mg/L] | MIC Range [mg/L] |
|---|---|---|---|---|
| CIM | 26 | 0.75 | 32 | 0.047→32 |
| MBL | 58 | 0.38 | 32 | 0.047→32 |
| OXA-48 | 6 | 0.5 | 3 | 0.19–3 |
| KPC | 11 | 0.38 | 1 | 0.125–2 |
| GES | 35 | 0.38 | 2 | 0.047→32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brauncajs, M.; Bielec, F.; Macieja, A.; Pastuszak-Lewandoska, D. In Vitro Activity of Eravacycline against Carbapenemase-Producing Gram-Negative Bacilli Clinical Isolates in Central Poland. Biomedicines 2023, 11, 1784. https://doi.org/10.3390/biomedicines11071784
Brauncajs M, Bielec F, Macieja A, Pastuszak-Lewandoska D. In Vitro Activity of Eravacycline against Carbapenemase-Producing Gram-Negative Bacilli Clinical Isolates in Central Poland. Biomedicines. 2023; 11(7):1784. https://doi.org/10.3390/biomedicines11071784
Chicago/Turabian StyleBrauncajs, Małgorzata, Filip Bielec, Anna Macieja, and Dorota Pastuszak-Lewandoska. 2023. "In Vitro Activity of Eravacycline against Carbapenemase-Producing Gram-Negative Bacilli Clinical Isolates in Central Poland" Biomedicines 11, no. 7: 1784. https://doi.org/10.3390/biomedicines11071784
APA StyleBrauncajs, M., Bielec, F., Macieja, A., & Pastuszak-Lewandoska, D. (2023). In Vitro Activity of Eravacycline against Carbapenemase-Producing Gram-Negative Bacilli Clinical Isolates in Central Poland. Biomedicines, 11(7), 1784. https://doi.org/10.3390/biomedicines11071784