Essential Oils Produce Developmental Toxicity in Zebrafish Embryos and Cause Behavior Changes in Zebrafish Larvae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Preparation of Stock Solutions
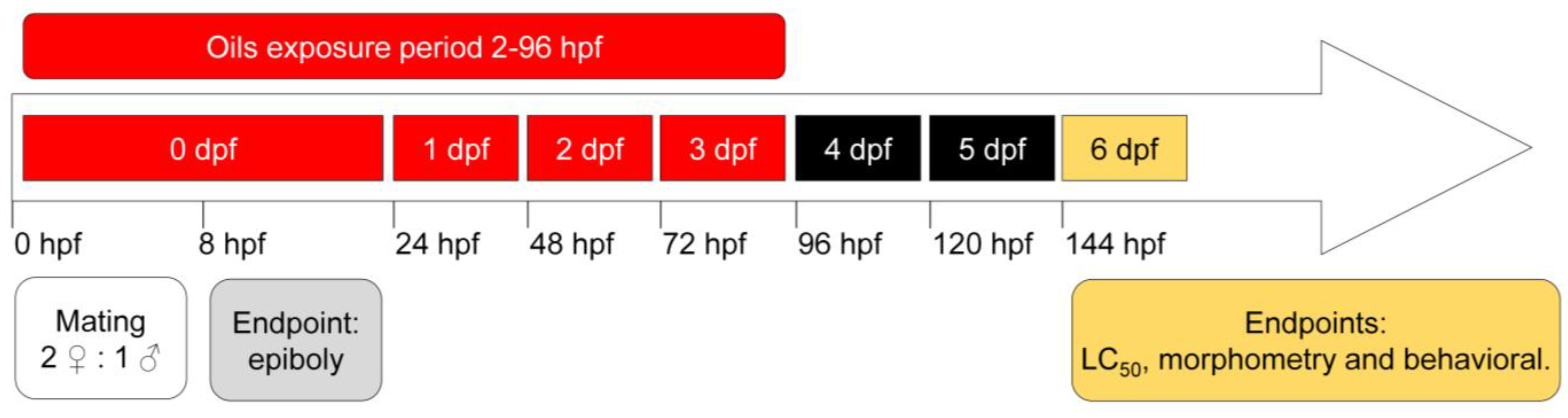
2.2. Zebrafish Husbandry, Embryo Collection, and Essential-Oil Exposure
2.3. Measurement of Epiboly
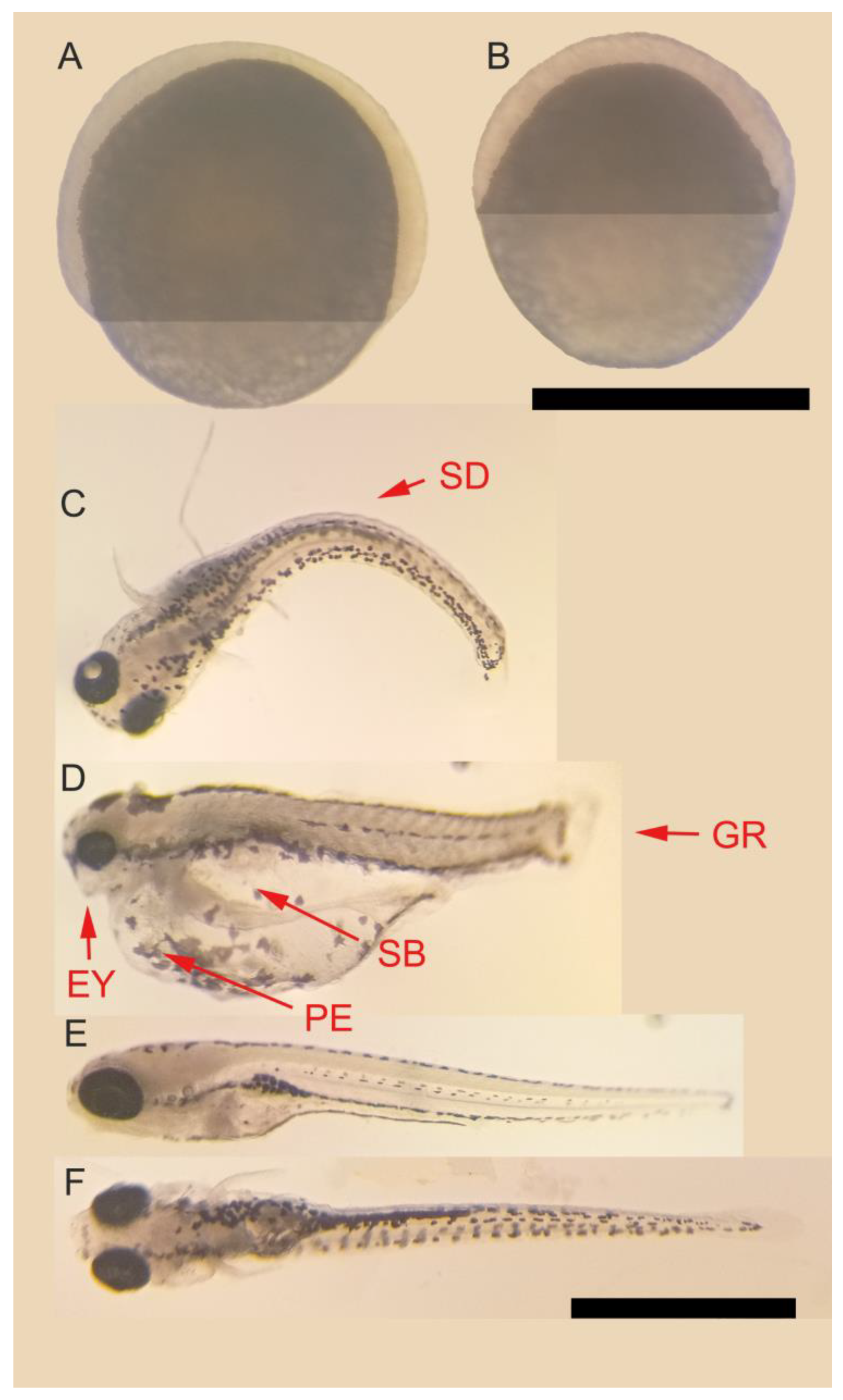
2.4. Analysis of Embryonic Development
2.5. Morphometry
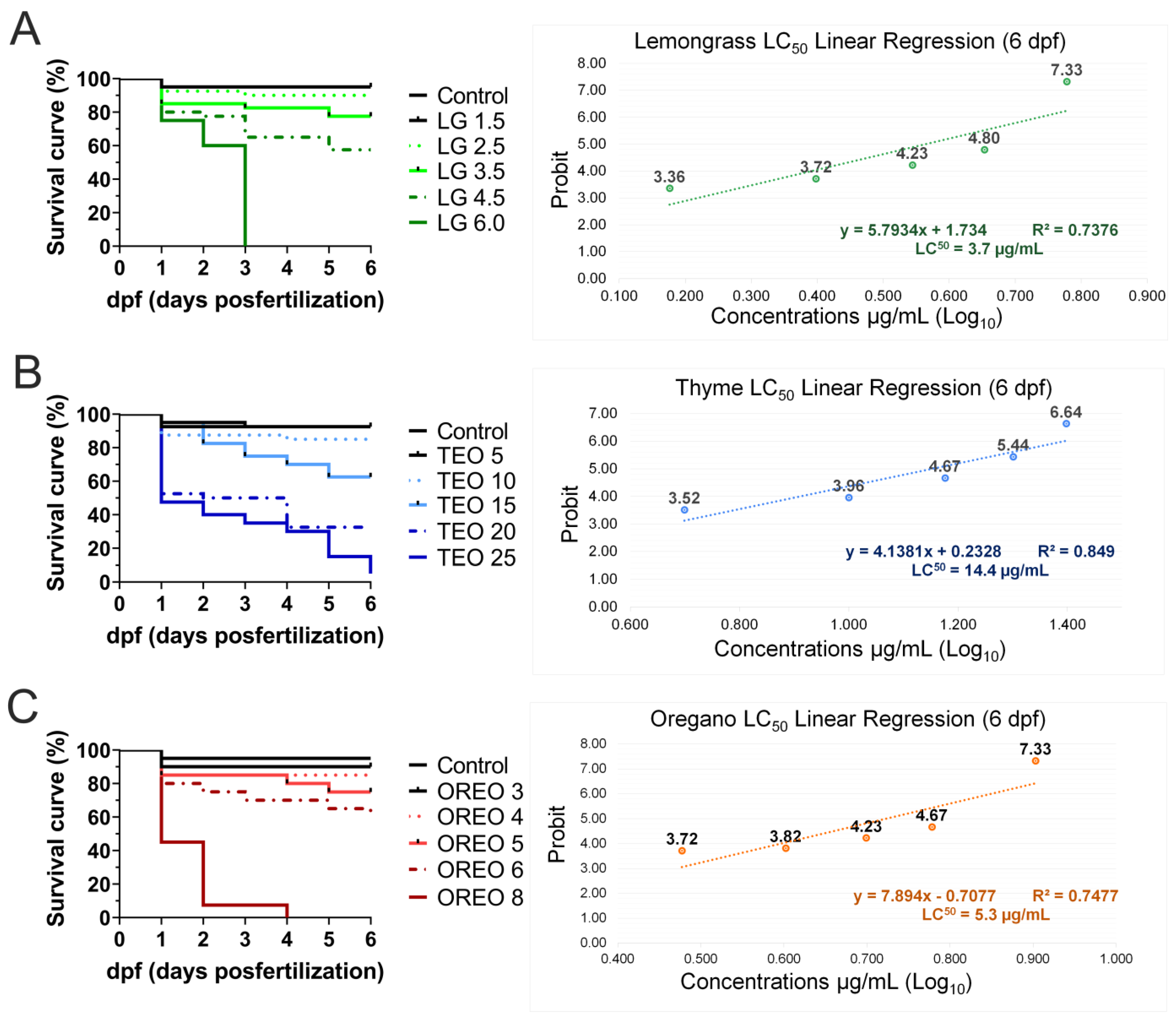
2.6. LC50 Determination
2.7. Animal Behavior
2.8. Statistical Analysis
3. Results
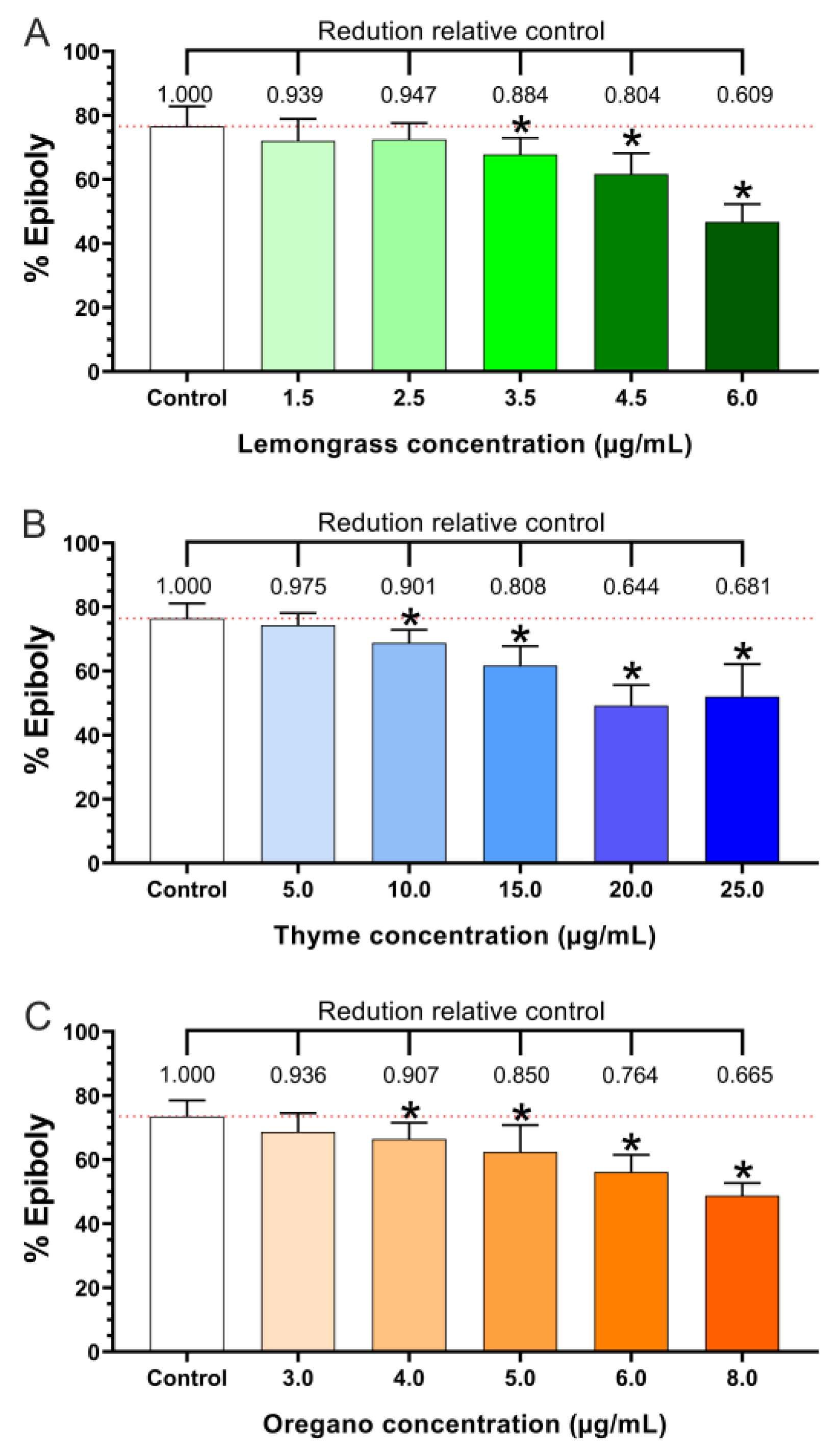
3.1. Epiboly Measurement
3.2. Embryonic Development and Mortality Analysis
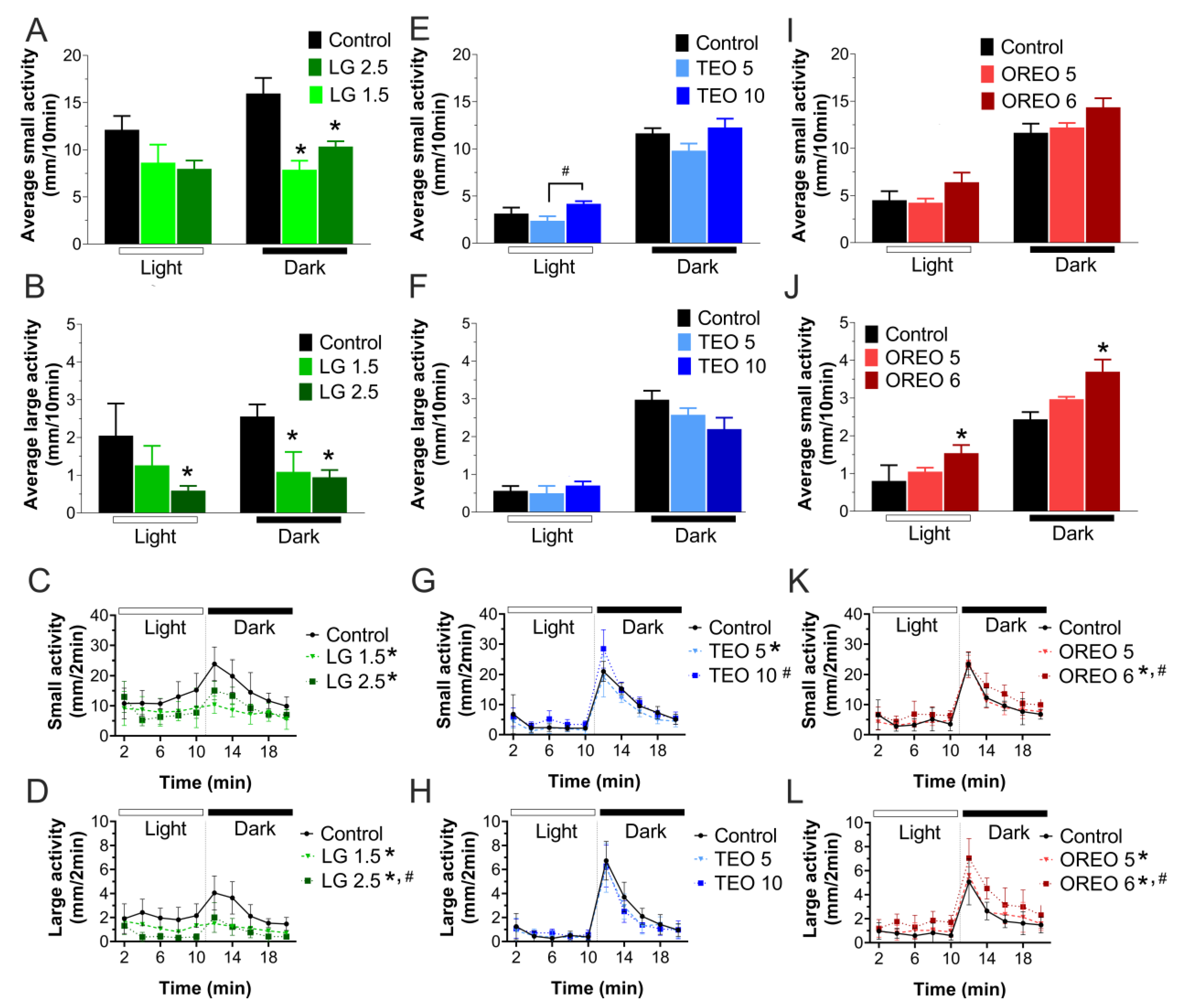
3.3. Behavior
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jayasinghe, C.D.; Jayawardena, U.A. Toxicity Assessment of Herbal Medicine Using Zebrafish Embryos: A Systematic Review. Evid.-Based Complement. Altern. Med. 2019, 2019, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Mensah, M.L.K.; Komlaga, G.; Forkuo, A.D.; Firempong, C.; Anning, A.K.; Dickson, R.A. Toxicity and Safety Implications of Herbal Medicines Used in Africa. In Herbal Medicine; IntechOpen: London, UK, 2019. [Google Scholar]
- Osaili, T.M.; Dhanasekaran, D.K.; Zeb, F.; Faris, M.E.; Naja, F.; Radwan, H.; Cheikh Ismail, L.; Hasan, H.; Hashim, M.; Obaid, R.S. A Status Review on Health-Promoting Properties and Global Regulation of Essential Oils. Molecules 2023, 28, 1809. [Google Scholar] [CrossRef] [PubMed]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Sieber, S.; Grossen, P.; Bussmann, J.; Campbell, F.; Kros, A.; Witzigmann, D.; Huwyler, J. Zebrafish as a Preclinical in Vivo Screening Model for Nanomedicines. Adv. Drug Deliv. Rev. 2019, 151–152, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Cassar, S.; Adatto, I.; Freeman, J.L.; Gamse, J.T.; Iturria, I.; Lawrence, C.; Muriana, A.; Peterson, R.T.; Van Cruchten, S.; Zon, L.I. Use of Zebrafish in Drug Discovery Toxicology. Chem. Res. Toxicol. 2020, 33, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, Y.; Rampersad, M.; Gerlai, R. Embryonic Alcohol Exposure Impairs the Dopaminergic System and Social Behavioral Responses in Adult Zebrafish. Int. J. Neuropsychopharmacol. 2015, 18, 1–8. [Google Scholar] [CrossRef]
- Basnet, R.; Zizioli, D.; Taweedet, S.; Finazzi, D.; Memo, M. Zebrafish Larvae as a Behavioral Model in Neuropharmacology. Biomedicines 2019, 7, 23. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio), 4th ed.; University of Oregon Press: Eugene, OR, USA, 2000. [Google Scholar]
- OECD-236 OECD Guidelines for the Testing of Chemicals; OECD: Paris, France, 2013.
- Thitinarongwate, W.; Mektrirat, R.; Nimlamool, W.; Khonsung, P.; Pikulkaew, S.; Okonogi, S.; Kunanusorn, P. Phytochemical and Safety Evaluations of Zingiber Ottensii Valeton Essential Oil in Zebrafish Embryos and Rats. Toxics 2021, 9, 102. [Google Scholar] [CrossRef]
- Cadena, P.G.; Sales Cadena, M.R.; Sarmah, S.; Marrs, J.A. Protective Effects of Quercetin, Polydatin, and Folic Acid and Their Mixtures in a Zebrafish (Danio rerio) Fetal Alcohol Spectrum Disorder Model. Neurotoxicol. Teratol. 2020, 82, 106928. [Google Scholar] [CrossRef]
- Marrs, J.A.; Clendenon, S.G.; Ratcliffe, D.R.; Fielding, S.M.; Liu, Q.; Bosron, W.F. Zebrafish Fetal Alcohol Syndrome Model: Effects of Ethanol Are Rescued by Retinoic Acid Supplement. Alcohol 2010, 44, 707–715. [Google Scholar] [CrossRef]
- Cadena, P.G.; Cadena, M.R.S.; Sarmah, S.; Marrs, J.A. Folic Acid Reduces the Ethanol-Induced Morphological and Behavioral Defects in Embryonic and Larval Zebrafish (Danio rerio) as a Model for Fetal Alcohol Spectrum Disorder (FASD). Reprod. Toxicol. 2020, 96, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Sales Cadena, M.R.; Cadena, P.G.; Watson, M.R.; Sarmah, S.; Boehm, S.L.; Marrs, J.A. Zebrafish (Danio rerio) Larvae Show Behavioral and Embryonic Development Defects When Exposed to Opioids at Embryo Stage. Neurotoxicol. Teratol. 2021, 85, 106964. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.C.G.; da Silva, J.F.; Santos, T.P.; da Silva, N.P.C.; dos Santos, A.R.; de Andrade, A.L.C.; da Souza, E.H.L.S.; Sales Cadena, M.R.; de Sá, F.B.; da Silva, V.A., Jr.; et al. The Complexation of Steroid Hormones into Cyclodextrin Alters the Toxic Effects on the Biological Parameters of Zebrafish (Danio rerio). Chemosphere 2019, 214, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, V.H.; Merrigan, P.; Dufresne, É. Down and out: Estimating the Relationship between Mental Health and Unemployment. Health Econ. 1997, 6, 397–406. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential Oils: Sources of Antimicrobials and Food Preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The Effect of Developmental and Environmental Factors on Secondary Metabolites in Medicinal Plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Majewska, E.; Kozlowska, M.; Gruczynska-Sekowska, E.; Kowalska, D.; Tarnowska, K. Lemongrass (Cymbopogon Citratus) Essential Oil: Extraction, Composition, Bioactivity and Uses for Food Preservation—A Review. Pol. J. Food Nutr. Sci. 2019, 69, 327–341. [Google Scholar] [CrossRef]
- Hudaib, M.; Speroni, E.; Di Pietra, A.M.; Cavrini, V. GC/MS Evaluation of Thyme (Thymus vulgaris L.) Oil Composition and Variations during the Vegetative Cycle. J. Pharm. Biomed. Anal. 2002, 29, 691–700. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. Chemical Composition and Bioactivity of Different Oregano (Origanum vulgare) Extracts and Essential Oil. J. Sci. Food Agric. 2013, 93, 2707–2714. [Google Scholar] [CrossRef]
- Piasecki, B.; Biernasiuk, A.; Skiba, A.; Skalicka-Woźniak, K.; Ludwiczuk, A. Composition, Anti-Mrsa Activity and Toxicity of Essential Oils from Cymbopogon Species. Molecules 2021, 26, 7542. [Google Scholar] [CrossRef]
- Gur, M.; Edri, T.; Moody, S.A.; Fainsod, A. Retinoic Acid Is Required for Normal Morphogenetic Movements During Gastrulation. Front. Cell Dev. Biol. 2022, 10, 857230. [Google Scholar] [CrossRef] [PubMed]
- Gibert, Y.; Gajewski, A.; Meyer, A.; Begemann, G. Induction and Prepatterning of the Zebrafish Pectoral Fin Bud Requires Axial Retinoic Acid Signaling. Development 2006, 133, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Wilburn, C.; McCarthy, R.A. Methoprene Photolytic Compounds Disrupt Zebrafish Development, Producing Phenocopies of Mutants in the Sonic Hedgehog Signaling Pathway. Mar. Biotechnol. 2003, 5, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Chen, Z. Thymol Inhibits Cell Migration and Invasion by Downregulating the Activation of PI3K/AKT and ERK Pathways in Human Colon Cancer Cells. Trop. J. Pharm. Res. 2017, 16, 2895–2901. [Google Scholar] [CrossRef]
- Li, Y.L.; Shao, M.; Shi, D.L. Rac1 Signalling Coordinates Epiboly Movement by Differential Regulation of Actin Cytoskeleton in Zebrafish. Biochem. Biophys. Res. Commun. 2017, 490, 1059–1065. [Google Scholar] [CrossRef]
- Plant, R.M.; Dinh, L.; Argo, S.; Shah, M. The Essentials of Essential Oils. Adv. Pediatr. 2019, 66, 111–122. [Google Scholar] [CrossRef]
- Polednik, K.M.; Koch, A.C.; Felzien, L.K. Effects of Essential Oil from Thymus Vulgaris on Viability and Inflammation in Zebrafish Embryos. Zebrafish 2018, 15, 361–371. [Google Scholar] [CrossRef]
- Hacke, A.C.M.; Miyoshi, E.; Marques, J.A.; Pereira, R.P. Cymbopogon Citratus (DC.) Stapf, Citral and Geraniol Exhibit Anticonvulsant and Neuroprotective Effects in Pentylenetetrazole-Induced Seizures in Zebrafish. J. Ethnopharmacol. 2021, 275, 114142. [Google Scholar] [CrossRef]
- Vieira, R.; Venâncio, C.; Félix, L. Teratogenic, Oxidative Stress and Behavioural Outcomes of Three Fungicides of Natural Origin (Equisetum arvense, Mimosa tenuiflora, Thymol) on Zebrafish (Danio rerio). Toxics 2021, 9, 8. [Google Scholar] [CrossRef]
- Capatina, L.; Todirascu-Ciornea, E.; Napoli, E.M.; Ruberto, G.; Hritcu, L.; Dumitru, G. Thymus vulgaris Essential Oil Protects Zebrafish against Cognitive Dysfunction by Regulating Cholinergic and Antioxidants Systems. Antioxidants 2020, 9, 1083. [Google Scholar] [CrossRef]
| Oil | Compound | Amount (%) |
|---|---|---|
| Lemongrass (Cymbopogon flexuosus) | geranial | 46.0 |
| neral | 28.6 | |
| geraniol | 6.8 | |
| citronellol | 3.7 | |
| linalyl acetate | 1.8 | |
| α-terpineol | 1.8 | |
| 1.8-cineole | 1.6 | |
| linalool | 1.6 | |
| others | 8.1 | |
| Thyme (Thymus vulgaris) | thymol | 29.5 |
| carvacrol | 28.2 | |
| geraniol | 9.0 | |
| p-cymene | 5.6 | |
| β-caryophyllene | 4.3 | |
| γ-terpinene | 3.4 | |
| 1.8-cineole | 3.2 | |
| α-humulene | 2.1 | |
| others | 14.7 | |
| Oregano (Origanum vulgare) | thymol | 39.7 |
| β-bisabolene | 8.0 | |
| p-cymene | 7.6 | |
| γ-terpinene | 6.4 | |
| thymol methyl ether | 5.0 | |
| bicyclogermacrene | 4.0 | |
| δ-cadinene | 3.5 | |
| aromadendrene | 3.4 | |
| others | 22.4 |
| Oil | Teratogenic Effect | Oil Concentration (µg/mL) | ||||
|---|---|---|---|---|---|---|
| 1.5 | 2.5 | 3.5 | 4.5 | 6.0 | ||
| Lemongrass (Cymbopogon flexuosus) | Spine deformation (SD) | - | + | + | + | * |
| Small eyes (EY) | - | - | - | + | * | |
| Pericardial edema (PE) | - | - | - | + | * | |
| Swim-bladder inflation (SB) | - | - | - | + | * | |
| Growth retardation (GR) | - | - | - | + | * | |
| 5.0 | 10.0 | 15.0 | 20.0 | 25.0 | ||
| Thyme (Thymus vulgaris) | Spine deformation (SD) | - | - | + | + | + |
| Small eyes (EY) | - | - | + | + | + | |
| Pericardial edema (PE) | - | - | - | + | + | |
| Swim-bladder inflation (SB) | - | - | - | + | + | |
| Growth retardation (GR) | - | - | - | + | + | |
| 3.0 | 4.0 | 5.0 | 6.0 | 8.0 | ||
| Oregano (Origanum vulgare) | Spine deformation (SD) | - | - | + | + | * |
| Small eyes (EY) | - | - | + | + | * | |
| Pericardial edema (PE) | - | - | - | - | * | |
| Swim-bladder inflation (SB) | - | - | - | - | * | |
| Growth retardation (GR) | - | - | - | + | * | |
| Oil | Measurement | Experimental Group | |||
|---|---|---|---|---|---|
| Control | 1.5 µg/mL | 2.5 µg/mL | 3.5 µg/mL | ||
| Lemongrass (Cymbopogon flexuosus) | Eye length | 100.0 ± 6.5 | 99.4 ± 3.6 | 100.1 ± 7.2 | 95.2 ± 7.8 |
| Rump length | 100.0 ± 5.0 | 101.7 ± 9.2 | 100.9 ± 3.6 | 98.9 ± 5.4 | |
| Rump anus width | 100.0 ± 3.5 | 102.6 ± 4.1 | 98.4 ± 4.3 | 98.8 ± 5.0 | |
| Standard length | 100.0 ± 3.5 | 98.1 ± 3.7 | 99.3 ± 4.3 | 90.6 ± 7.8 * | |
| Tail width | 100.0 ± 7.1 | 100.1 ± 5.1 | 98.4 ± 6.3 | 93.1 ± 5.8 * | |
| Thyme (Thymus vulgaris) | Control | 5.0 µg/mL | 10.0 µg/mL | 15.0 µg/mL | |
| Eye length | 100.0 ± 5.4 | 98.2 ± 4.2 | 99.0 ± 5.7 | 92.1 ± 7.3 * | |
| Rump length | 100.0 ± 8.6 | 99.8 ± 7.7 | 101.7 ± 4.9 | 100.7 ± 7.9 | |
| Rump anus width | 100.0 ± 3.8 | 99.4 ± 4.4 | 100.5 ± 3.4 | 101.2 ± 6.7 | |
| Standard length | 100.0 ± 5.2 | 99.4 ± 3.7 | 96.9 ± 5.5 | 95.0 ± 5.0 * | |
| Tail width | 100.0 ± 4.2 | 99.6 ± 4.6 | 96.5 ± 3.7 | 97.1 ± 5.4 | |
| Oregano (Origanum vulgare) | Control | 3.0 µg/mL | 4.0 µg/mL | 5.0 µg/mL | |
| Eye length | 100.0 ± 6.6 | 95.7 ± 4.7 | 95.6 ± 5.1 | 93.1 ± 6.9 * | |
| Rump length | 100.0 ± 6.8 | 103.5 ± 7.7 | 103.7 ± 5.2 | 101.8 ± 8.6 | |
| Rump anus width | 100.0 ± 3.9 | 99.5 ± 4.7 | 100.3 ± 4.2 | 102.0 ± 3.0 | |
| Standard length | 100.0 ± 4.9 | 99.8 ± 5.0 | 101.5 ± 6.2 | 93.1 ± 8.1 * | |
| Tail width | 100.0 ± 6.0 | 101.5 ± 6.4 | 97.7 ± 7.5 | 96.3 ± 5.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, I.I., Jr.; da Silva, N.P.C.; Marrs, J.A.; Cadena, P.G. Essential Oils Produce Developmental Toxicity in Zebrafish Embryos and Cause Behavior Changes in Zebrafish Larvae. Biomedicines 2023, 11, 2821. https://doi.org/10.3390/biomedicines11102821
da Silva II Jr., da Silva NPC, Marrs JA, Cadena PG. Essential Oils Produce Developmental Toxicity in Zebrafish Embryos and Cause Behavior Changes in Zebrafish Larvae. Biomedicines. 2023; 11(10):2821. https://doi.org/10.3390/biomedicines11102821
Chicago/Turabian Styleda Silva, Ivanildo Inacio, Jr., Niely Priscila Correia da Silva, James A. Marrs, and Pabyton Gonçalves Cadena. 2023. "Essential Oils Produce Developmental Toxicity in Zebrafish Embryos and Cause Behavior Changes in Zebrafish Larvae" Biomedicines 11, no. 10: 2821. https://doi.org/10.3390/biomedicines11102821
APA Styleda Silva, I. I., Jr., da Silva, N. P. C., Marrs, J. A., & Cadena, P. G. (2023). Essential Oils Produce Developmental Toxicity in Zebrafish Embryos and Cause Behavior Changes in Zebrafish Larvae. Biomedicines, 11(10), 2821. https://doi.org/10.3390/biomedicines11102821





