Abstract
Although abundant data confirm the efficacy and safety profile of the developed vaccines against COVID-19, there are still some concerns regarding vaccination in high-risk populations. This is especially valid for patients susceptible to thrombotic or bleeding events and hesitant people due to the fear of thrombotic incidents following vaccination. This narrative review focuses on various inherited and acquired thrombotic and coagulation disorders and the possible pathophysiologic mechanisms interacting with the coagulation system during immunization in view of the currently available safety data regarding COVID-19 vaccines. Inherited blood coagulation disorders and inherited thrombotic disorders in the light of COVID-19, as well as blood coagulation and thrombotic disorders and bleeding complications following COVID-19 vaccines, along with the possible pathogenesis hypotheses, therapeutic interventions, and imaging for diagnosing are discussed in detail. Lastly, the lack of causality between the bleeding and thrombotic events and COVID-19 vaccines is debated, but still emphasizes the importance of vaccination against COVID-19, outweighing the minimal risk of potential rare adverse events associated with coagulation.
1. Introduction
The COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has affected hundreds of millions worldwide, leading to nearly 7 million deaths globally, although now declared not a worldwide concern anymore [1]. Strenuous research and analysis of various vaccine advances led to the development of multiple COVID-19 vaccines in less than a year from the pandemic’s beginning. Different types of vaccines, such as mRNA vaccines, DNA vaccines, viral vector vaccines, and inactivated virus vaccines have been approved and have shown a high degree of efficacy with variable protective levels of up to 95% (70–95% range) in vaccinated individuals against COVID-19 [2,3,4,5,6].
Despite the abundance of data revealing the safety and efficacy of the developed vaccines [5,7], there are still some concerns regarding the challenges related to vaccination in high-risk populations, especially in patients susceptible to thrombotic or bleeding events.
This narrative review focuses on various inherited thrombotic and coagulation disorders (inherited blood coagulation disorders and inherited thrombotic disorders in the light of COVID-19), and acquired thrombotic and coagulation disorders (blood coagulation and thrombotic disorders and bleeding complications following COVID-19 and COVID-19 vaccines), along with the possible pathogenesis hypotheses, therapeutic interventions, and imaging for diagnosing. We discuss possible pathophysiologic mechanisms interacting with the coagulation system during vaccination and review the currently available safety data regarding COVID-19 vaccines.
2. Search Strategy
For our narrative review, we extensively searched the scientific databases Medline (PubMed) and Scopus, along with strong supporting data from official registries. We used the following MeSH and pertinent free-text terms: (“COVID-19” OR “SARS-CoV-2” AND (“blood disorders” OR “coagulation disorders” OR “thrombotic event”) AND (“vaccine” OR “mRNA” OR “vector” OR “inactivated”). We only looked for items released before 10 July 2023. Our review structure was based on the guidelines and recommendations for writing a narrative review [8].
3. SARS-CoV-2 and COVID-19 Vaccines
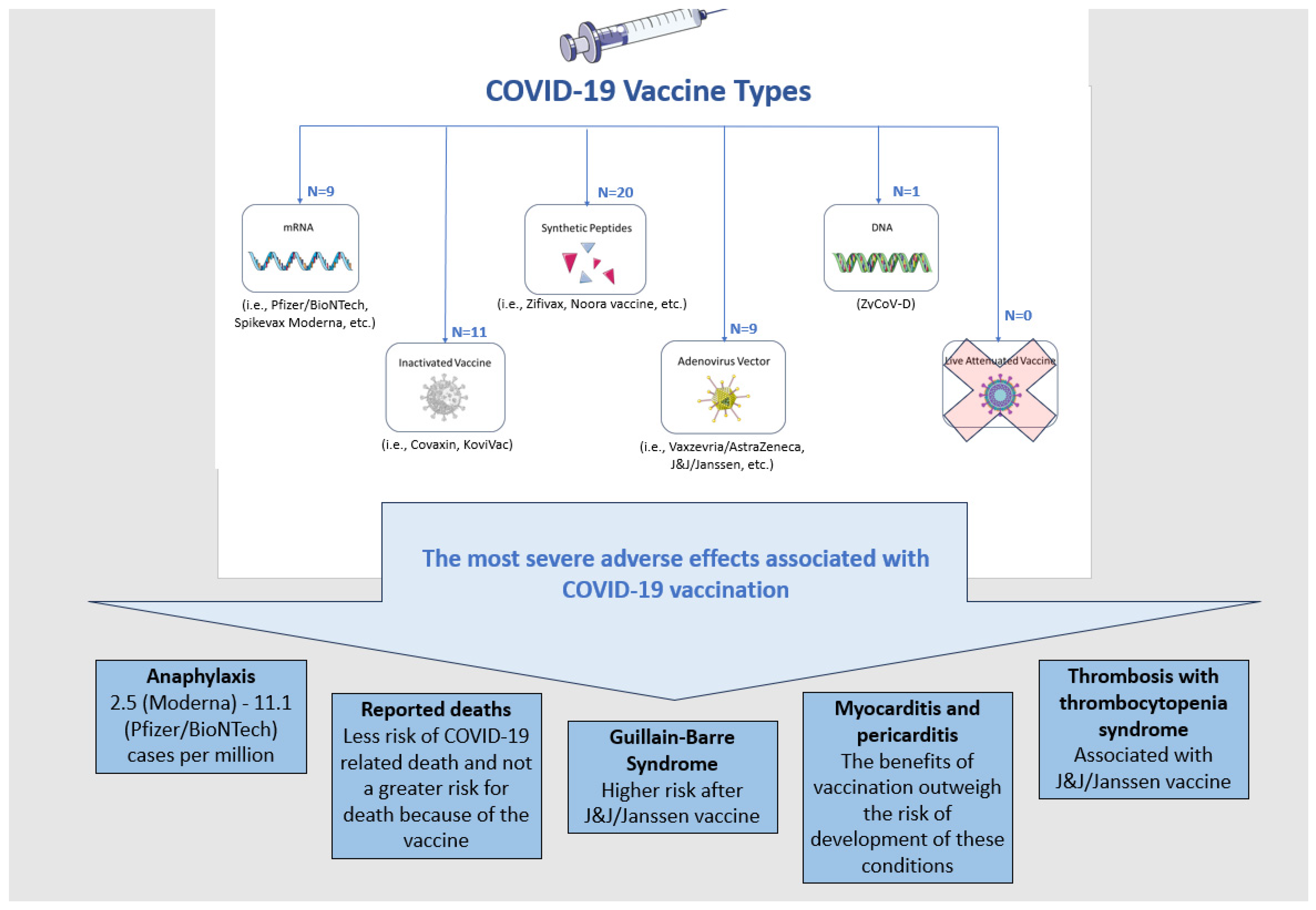
Different types of vaccines against the SARS-CoV-2 virus were developed to prevent the subsequent COVID-19. Vaccines can be divided into the following categories based on the platform used for their development: DNA, mRNA, inactivated, live attenuated, virion-like particle, viral vector, recombinant subunit and synthetic peptide (Figure 1). The figure presents the most severe conditions reported as adverse events following vaccination, with 50 already approved COVID-19 vaccines as of the end of 2022 [9].
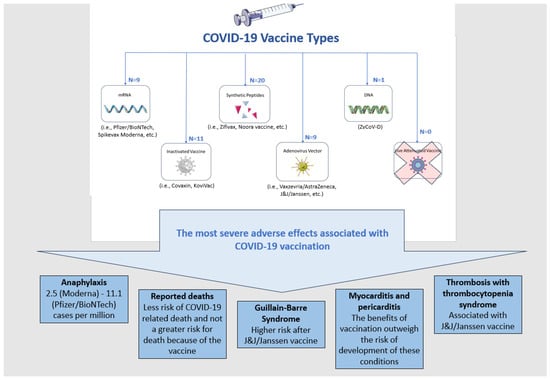
Figure 1.
The approved COVID-19 vaccines and the most severe adverse events, although very rare, associated with their use.
The data on the severe adverse effects of COVID-19 vaccines should be interpreted carefully considering the CDC, ACIP, and WHO conclusion that COVID-19 vaccination outweighs any potential risks of complications, based on the most intense safety monitoring program in the U.S. history and worldwide [10].
Some of the COVID-19 vaccines are currently available worldwide [11]. We will briefly introduce them, along with some concerns regarding their use.
One of these vaccines uses synthetic mRNA, formulated in vitro and introduced into the human body for translation into antigenic proteins by the host cells [12,13]. The bioproduct BNT162b2 (Pfizer/BioNTech) is a messenger RNA vaccine and contains a lipid nanoparticle. Nucleoside-modified RNA (modRNA) encodes the full-length spike protein of SARS-CoV-2, modified by two proline mutations to lock it into a preconfirmation [14,15,16]. mRNA-1273 is a vaccine developed by Moderna and consists of a messenger RNA that encodes the spike protein stabilized in a preconformation, or SARS-CoV-2 S(2P), formulated in lipid nanoparticles [17].
On the other hand, viral vector vaccines aim to insert a gene encoding protective exogenous antigens into a viral vector to express the target protein in the human body [12,13]. ChAdOx1-S (Oxford-AstraZeneca) is a vaccine containing the adenovirus family virus modified to include a protein-making gene from SARS-CoV-2 [18]. The adenovirus is a recombinant chimpanzee vector encoding the spike glycoprotein of SARS-CoV-2 [19]. Ad26.COV2.S (Janssen) is a bioproduct containing a recombinant, replication-incompetent human adenovirus type 26 vector encoding the spike protein of the SARS-CoV-2 virus in a conformation stabilized by pre-fusion for prevention of COVID-19 [20]. Ad5-nCoV (Convidecia™) is a vaccine containing a non-replicating viral vector adenovirus Type 5 vector, developed by CanSino Biologics/Beijing Institute of Biotechnology/Petrovax [21,22].
Sputnik V, or Gam-Covid-Vac, is a recombinant adenovirus vector vaccine by Gamaleya Research Institute [23,24]. The first dose of the bioproduct contains recombinant adenovirus rAd26, and the second dose adenovirus rAd5. Both vectors carry the gene for the full-length SARS-CoV-2 glycoprotein S [23,24].
In Turkmenistan, a synthetic peptide vaccine was approved to prevent COVID-19. It was developed by Vector Institute and named EpiVacCorona [25]. Inactivated vaccines against COVID-19 contain whole-virus cultured wild-type viruses by physical or chemical inactivation processes [12,13]. One bioproduct with an inactivated virus for the prevention of COVID-19 is BBIBP-CorVApproved, developed by the Beijing Institute of Biological Products, and approved for use in Bahrain, China, and the United Arab Emirates [26,27].
Wuhan Institute of Biological Products/Sinopharm produced another inactivated vaccine against the SARS-CoV-2 Vero cell [26]. In Russia, the inactivated CoviVac vaccine was developed by the Chumakov Center at the Russian Academy of Sciences [28]. Another type of vaccine is Bionic nanoparticle vaccines, which are bioproducts with purified recombinant proteins and bionic nanoparticles [12,13]. NVX-CoV2373 is a recombinant nanoparticle vaccine composed of trimeric full-length SARS-CoV-2 spike glycoproteins. The bioproduct contains Matrix-M1 adjuvant [29].
These approved vaccines underwent firm clinical trials and were investigated during real-life use, while minor and major adverse events associated with vaccines were strictly registered and monitored [10].
4. Inherited Blood Coagulation Disorders and COVID-19 Vaccines
Coagulopathic hemorrhagic diatheses (coagulopathies) are conditions caused by a congenital or acquired defect in the blood coagulation system. Hemorrhagic diathesis represents an increased tendency to bleed. Hereditary bleeding disorders may occur due to defective platelet function, and, depending upon the predominant functional abnormality, inherited disorders are classified into the three most common groups: defective platelet adhesion, defective platelet aggregation, and disorders of platelet release reaction [30,31].
Most inherited coagulation disorders are induced by qualitative and quantitative defects in a single coagulation factor. Two of the most common factors that are reported are the sex (X)-linked disorders—classic hemophilia or hemophilia A (factor VIII deficiency) and hemophilia B or Christmas disease (factor IX deficiency). Another common and related coagulation disorder is von Willebrand’s disease (defect of von Willebrand’s factor).
Hemophilia A is the second most common coagulation disorder, next to von Willebrand’s disease. It is inherited in an X-linked recessive pattern. Therefore, females are carriers, whereas the disease manifests clinically in males. The frequency of this type of disorder varies in different races, with the highest incidence being in British populations, particularly in royal blood descendants and some European royal families. These patients suffer from bleeding involving any organ for hours or days after injury, and the severity of bleeding correlates with plasma level factor VIII activity. Broadly, the most severe, non-lethal changes in Hemophilia A result from intra-articular hemorrhages that lead to deforming arthrosis. On the other hand, even minor traumas, such as tooth extraction, can lead to massive, life-threatening bleeding in these subsets of patients [32,33,34].
Since COVID-19 is associated with coagulation and thrombotic disorders, little was known about the infection’s impact on the clinical outcomes of patients with hemophilia. A retrospective study by Mericliler et Narayan with 1758 adult male patients reported that COVID-19 did not increase the mortality of COVID-19 in hemophilia A patients but increased the risk of bleeding and hospitalizations [35].
WHO and The World Federation of Hemophilia (WFH) guidelines recommend that patients with hemophilia preferably receive subcutaneous vaccination; the problem comes from the fact that the vaccine for COVID-19, as well as most vaccines, is only allowed for intramuscular administration. Bleeding following the administration of an intramuscular vaccine in hemophiliacs is challenging to control. It may require several days, even weeks, of treatment with a clotting factor [36]. These precautions are also valid for patients on anticoagulants or antiplatelets [37].
However, there are some reports of patients with acquired hemophilia A following COVID-19 vaccination [38,39,40,41,42,43,44]. Duminuco et al. described this rare coagulopathy associated with hemorrhagic complications due to the possible development of FVIII inhibitors following an immune stimulus [38].
Von Willebrand’s disease (Pseudohemophilia) has an autosomal dominant inheritance. In this disease, impaired platelet aggregation is detected. Its incidence is estimated to be 1 in 1000 of either sex. Clinically, the patients are characterized by spontaneous bleeding from mucous membranes and excessive wound bleeding [45,46].
As expected, the von Willebrand factor plays a role in COVID-19-associated coagulopathy, as its levels and activity increase in severe infection [47]. However, little to no information is available on COVID-19’s impact on patients with this disease [48].
Hemophilia B (Christmas disease) is rarer than hemophilia A, with clinical features indistinguishable from classic hemophilia [49]. Not much is known about COVID-19 in these patients. However, managing underlying hemophilia of any type requires hemostatic treatment for ambulatory patients and prevention with concentrates intensified according to the risk of complications in hospitalized patients and those on replacement therapy [50].
5. Congenital Coagulation and Thrombotic Disorders and COVID-19 Vaccines
Thrombophilia is a type of hypercoagulability that represents a pathologic state of increased clot formation without active bleeding [51].
Factor V Leiden mutation is the most common inherited thrombophilia in individuals with venous thromboembolism. The mutation leads to enhanced pro-thrombotic actions of activated factor Va by changing the cleavage site of the molecule and hindering its degradation by protein C. It is an autosomal dominant genetic condition with incomplete penetrance, so not every carrier of the mutated gene will exhibit a thrombotic event [52].
Protein C and S deficiency: Protein C is a vitamin K-dependent protease presented in low levels in human plasma. Once activated, it cleaves the activated factors V and VIII, thus inhibiting thrombin production and coagulation. The functions of protein C in inflammation and cytoprotection have been described unrelated to its role in coagulation. Protein S is a cofactor to activate protein C in the cleavage of Va and VIIIa, as well as a cofactor of the tissue factor pathway inhibitor (TFPI) [53]. Protein C and S deficiencies may be inherited and acquired. There are two types of inherited forms, most commonly with autosomal recessive inheritance. Type I is associated with decreased levels and activity of the proteins, while type II is related to normal levels but reduced anticoagulant activity. Both protein C and S deficiencies are associated with uncontrolled thrombin formation and thromboembolic events, with venous thromboembolism being more common than arterial [54].
Antithrombin III deficiency: Antithrombin III is a glycoprotein located on the vascular surface of endothelial cells that requires heparin as a cofactor with which they bind circulating thrombin, thus inhibiting coagulation. Antithrombin III deficiency is inherited in an autosomal dominant manner and presents almost exclusively with venous thrombosis. The risk of thrombotic events is higher than in protein S and C deficiencies. Antithrombin III deficiency is estimated to carry the highest venous thromboembolism risk among all hereditary thrombophilias [55].
Hyperhomocysteinemia: Hyperhomocysteinemia has been notorious for decades for causing widespread accelerated atherosclerosis and arterial thrombosis. Homocysteine is an amino acid produced in methionine metabolism that causes endothelial injury and oxidative stress when present in substantial amounts. For homocysteine to be resynthesized to methionine by the enzyme methionine synthase, a methyl group is needed in the form of methyl-tetrahydrofolate delivered by the methylenetetrahydrofolate reductase (MTHFR). Several polymorphisms of the MTHFR gene are associated with significantly elevated serum homocysteine levels, with the homozygous mutant genotype leading to the greatest homocysteine values [56].
A large observational study from the Mayo Clinic, Rochester, Minnesota, USA, identified 6067 patients with confirmed thrombophilia among almost 800,000 vaccinated patients against COVID-19. Subgroup analysis did not find any statistically significant difference in the occurrence of venous thromboembolism between patients with and without thrombophilia vaccinated against COVID-19 [57].
It has even been estimated that the absolute risk for a deep vein thrombosis while undergoing an airplane flight is 1 in 4600 people, making it 50 to 100 times more likely than thrombosis following SARS-CoV-2 vaccination [58]. A European expert consensus on Vaccine-Induced Immune Thrombotic Thrombocytopenia recommends against the systematic screening for thrombophilia; against premedication with low-molecular-weight heparin, direct oral anticoagulants, or aspirin; as well as against monitoring of changes in D-dimer [58].
6. Review of Acquired Coagulation and Thrombotic Disorders and Their Connection with COVID-19 Vaccines—Hypothesis and Therapeutic Interventions
As we previously described in detail, acquired coagulation disorders are a heterogeneous group of conditions with a complex pathophysiology. The major causes of these disorders include the development of circulating anticoagulants, impaired synthesis of clotting factors, and the development of disseminated intravascular coagulation (DIC) [59].
Circulating anticoagulants are usually autoantibodies that “attack” specific clotting factors, such as an autoantibody against factor VIII or factor V, or inhibit phospholipid-bound proteins. Patients may develop lupus anticoagulant hypoprothrombinemia or antiphospholipid syndrome. Occasionally, the latter type of autoantibody causes bleeding by binding to prothrombin–phospholipid complexes [60]. Such antibodies may develop due to autoimmune disease or be drug-induced. There are few case reports (documented patients who developed acquired hemophilia following COVID-19 vaccination.)
On the other hand, synthesis of clotting factors may be impaired either by severe liver disease (such as fulminant hepatitis, cirrhosis, acute liver failure) or by deficiency of vitamin K that is required for the synthesis of prothrombin, factor VII, factor IX, protein C, and protein S [61]. Vitamin K deficiency usually results from deficient intake or conditions causing fat malabsorption, such as coeliac disease or cystic fibrosis. Furthermore, acute liver disease may cause disproportionate fibrinolysis and bleeding due to decreased synthesis of antiplasmin. To the best of our knowledge, no known reported cases are associated with impaired clotting factor synthesis and COVID-19 vaccination.
Disseminated intravascular coagulation involves a disproportionate synthesis of thrombin and fibrin in the circulating blood, and it usually develops from exposure of tissue factor to blood, initiating the extrinsic coagulation cascade [62]. Cytokine release and impaired microvascular blood flow provoke tissue plasminogen activator (tPA) release from endothelial cells. Both tPA and plasminogen attach to fibrin, and plasmin (generated by tPA cleavage of plasminogen) divides fibrin into D-dimers and other fibrin degradation products. Therefore, excessive platelet aggregation and coagulation factor consumption occur. Therefore, DIC may cause both thrombosis and bleeding. Furthermore, administering anticoagulants may lead to bleeding events because of DIC evolution; thus, this scenario must be considered.
On the other hand, DIC that progresses briskly (hours or days) usually causes bleeding events. DIC usually presents with thrombocytopenia, increased prothrombin time and plasma D-dimer levels, and decreased fibrinogen levels. Several cases of DIC in patients have been described after adenovirus vector vaccination (ChAdOx1-nCoV-19 and Ad26.COV2.S) in the literature [63,64].
7. Blood Coagulation and Thrombotic Disorders Related to COVID-19 Disease
The variety of clinical symptoms associated with novel coronavirus infection astounded researchers. The condition can be asymptomatic, with only modest signs such as olfactory loss, overall weakness, or flu-like symptoms. However, COVID-19 infection can be severe in some patients, resulting in hypercoagulation, vascular endothelial damage, and the risk of venous and arterial thrombotic consequences [65]. Multiple research publications covering various features and signs of the disease have been published in recent months. They reported that the endothelial cells in COVID-19 play a pivotal role as mediators of the two-way communication between inflammation and coagulation [66]. On one hand, SARS-CoV-2 binding to the endothelial angiotensin-converting enzyme 2 receptor activates endothelial cells through a complicated, inflammatory response [67].
On the other hand, studies using COVID-19-infected lung tissue have also shown enhanced expression of vascular and inflammatory factors. These factors include vascular cell adhesion molecule (VCAM)-1, interleukin (IL)-8, and monocyte-chemoattractant protein (MCP)-1. Furthermore, scientific research has proven that platelet adhesion molecule vWF is upregulated in COVID-19, demonstrating endothelial involvement; however, a disintegrin and metalloproteinase with thrombospondin motifs 13 (ADAMTS13), which cleaves high-molecular-weight vWF, is downregulated, resulting in an aberrant ratio of vWF to ADAMTS-13 [68]. This disturbed ratio is at the base of the pathogenesis of COVID-19-associated coagulopathy.
In line with this, it is unsurprising that severe disease and subsequent cardiovascular consequences after SARS-CoV-2 infection are higher in patients with preexisting vascular disease, such as hypertension, diabetes, and coronary artery disease. Since the lungs are the entryway for most viruses and SARS-CoV-2, the pulmonary vasculature is the first to experience inflammation. Barrier function and vascular tone may also be indirectly affected by microvascular thrombosis. Vascular dysfunction and microvascular thrombosis contribute significantly to further developing pulmonary illness, particularly ARDS. There have been reports of increased thrombotic events such as venous thromboembolism, myocardial infarction, and stroke [69].
Although SARS-CoV-2 infection, which causes coronavirus illness (COVID-19), has been linked to thrombotic problems in adults, the prevalence of COVID-19-related thrombosis in children and adolescents is unknown. Mild disease is typical for children with acute COVID-19, but the postinfectious complication known as multisystem inflammatory syndrome in children (MIS-C) has mostly been linked to coagulopathy [70]. Evidence demonstrated that MIS-C and other post-COVID-19 complications may originate in vascular dysfunction. For example, since April 2020, previously healthy children have presented with fever, cardiovascular shock and/or Kawasaki disease symptoms, hyperinflammation, and multisystem involvement after SARS-CoV-2 infection [71]. Many of them had positive SARS-CoV-2 antibody titers but negative nasopharyngeal swabs. Thus, CDC and WHO public health advisories listed criteria for this new disease, a multisystem inflammatory syndrome in children (MIS-C) associated with coagulopathy, as a potential presenting feature [71,72,73].
Therefore, it is essential to underline that immobile patients with severe infection and those with severe inflammatory reactions have an elevated risk of VTE, a key issue for all hospitalized patients. Consequently, preventative measures, including pharmaceuticals and mechanical devices, should be employed, and early mobilization should be encouraged. Since pharmaceutical thromboprophylaxis has been demonstrated to benefit hospitalized patients with COVID-19, a higher dose of anticoagulation may be necessary to treat the severe coagulopathy associated with this virus. In an effort to strike a better balance between thrombotic and bleeding events, it was proposed early on in the pandemic that the dose of thromboprophylaxis be established to empirical therapeutic-dose anticoagulation or to intermediate-dose anticoagulation [74]. Thus, only thromboembolic problems warrant therapeutic-dose heparin in critically ill COVID-19 patients. All patients can prevent VTE with LMWH in prophylactic doses. Some ICU specialists favor unfractionated heparin infusion for VTE in renal failure (ideally guided by anti-factor Xa levels since the aPTT is inaccurate in COVID-19 patients due to excessive factor VIII levels). Heparin-induced thrombocytopenia patients could benefit from fondaparinux, argatroban, or bivalirudin [75]. However, with the growing incidence of bleeding reports associated with COVID-19, several articles recommend using anticoagulation with serious percussion and individually assessing the benefit/risk ratio because of their adverse effects (i.e., paradoxical bleeding during DIC evolution).
With respect to the prognosis, important indicators of poor outcomes in SARS-CoV-2 infections include markers of endothelial dysfunction and altered endothelial cell integrity, which are linked to pulmonary edema, intravascular thrombosis, and acute respiratory distress syndrome (ARDS). The pulmonary endothelium is critical in maintaining vascular homeostasis and permeability, regulating inflammatory responses, coagulation, and fibrinolysis [76]. Disturbances in these tightly controlled processes may directly lead to morbidity and death.
8. Thrombotic and Bleeding Events following COVID-19 Vaccination: Hypothesis for Pathogenesis and Therapeutic Interventions
With the approval of the COVID-19 vaccines, the rate of hospitalizations and severe COVID-19 disease has decreased dramatically. The clinical trials of these vaccines reported a safety profile with the reactogenicity; adverse effects such as pain at the site of inoculation, redness, fever, fatigue, headache, and allergic reactions, have been reported [5,77,78,79]. However, at the beginning of 2021, the first cases of vaccine-induced thrombotic complications after vaccination with vector vaccines ChAdOx1 nCoV-19 (AstraZeneca) and Ad26.COV2.S (Johnson & Johnson/Janssen) were observed soon after all global vaccination programs were initiated. Subsequently, the relationship between thrombocytopenia and thrombosis after administration of BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) was also reported.
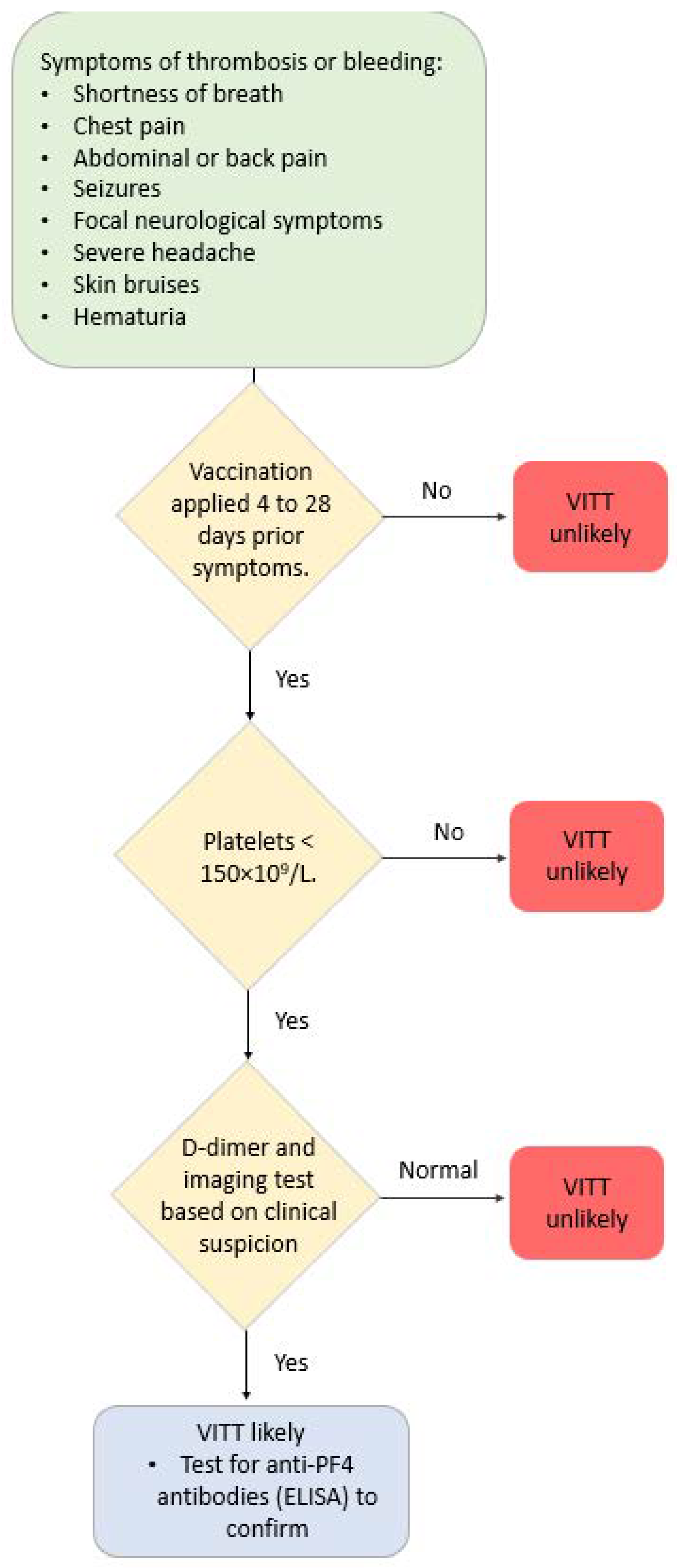
Different terms are used to refer to these conditions/complications after COVID-19 vaccination, which are predominantly localized in the venous vasculature system—vaccine-induced thrombotic thrombocytopenia (VITT), vaccine-induced pro-thrombotic immune thrombocytopenia (VIPIT), vaccine-induced immune thrombotic thrombocytopenia (VIITT) and thrombosis with thrombocytopenia syndrome (TTS) [80]. Antibodies that recognize PF4 (platelet-associated factor 4) cause these thrombotic events. The antibodies are IgG that activate platelets through specific receptors on the platelet surface. These events develop between 4 and 42 days after receiving a COVID-19 vaccine [81,82]. Figure 2 illustrates a suggested diagnostic algorithm in patients with suspected VITT.
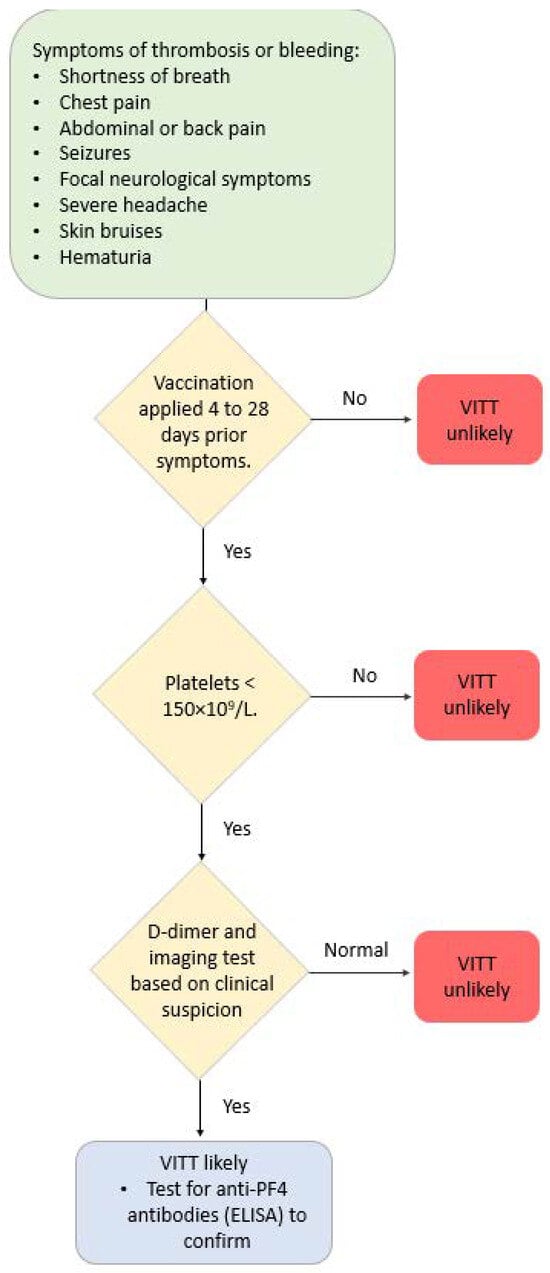
Figure 2.
Diagnostic algorithm in patients with suspected VITT.
The advent of the COVID-19 pandemic has necessitated the development of vaccines as a crucial intervention against this highly aggressive and contagious viral disease. Multiple manufacturing companies employed various techniques, including viral nucleic acids, inactivated viruses, or viral vectors, to develop vaccines to mitigate the impact of this worldwide pandemic. However, the other side of the coin is vaccine adverse reactions. Among the most serious ones is thrombocytopenia, which has been shown to be due to both COVID-19 disease and immunization. When adenoviral-based vaccines were invented and administered, reported cases of patients with thrombosis and thrombocytopenia were later found to have VITT. Venous thromboses in unexpected places, such as cerebral sinus vein thrombosis or splanchnic vein thrombosis, have been observed in COVID-19 vaccine recipients, occasionally with thrombocytopenia (thrombosis thrombocytopenia syndrome [TTS]) [83]. Vector-based COVID-19 vaccination increased TTS-related cerebral sinus vein thrombosis risks by 50-fold. These findings support a pathogenetic relationship between vector-based COVID-19 vaccinations and TTS. Vaccine antigenic complexes bind to platelet factor 4 (PF4) on platelet surfaces, causing proinflammatory responses, anti-PF4 antibody production, and pro-thrombotic cascades [84]. Similarly, VITT is characterized by the production of platelet-activating antibodies targeting PF4. This clinical manifestation bears a resemblance to autoimmune heparin-induced thrombocytopenia [85].
Non-vector-based immunization was also related to cerebral thrombosis but without TTS. Sharifian-Dorche et al. conducted a study that revealed that nearly all of the patients with VITT exhibited heightened levels of antibodies to platelet factor 4 heparin complex, which is comparable to the occurrence of “classic” heparin-induced thrombocytopenia (HIT) [86]. But opposite to “classic” HIT, autoimmune heparin-induced thrombocytopenia causes severe thrombocytopenia, increased disseminated intravascular coagulation, and atypical thrombotic events.
TTP has traditionally been characterized by microangiopathic hemolytic anemia, thrombocytopenia, neurological abnormalities, fever, and renal failure [87]. From 5% in Australia to 17–55% in the United Kingdom (UK), VITT is a potentially major adverse event with a high mortality rate [88,89,90].
By the end of September 2021, 419 cases had been documented in the UK, and 89% of those cases started after the initial dose [20]. According to our data, the global fatality rate is 51.3%, and it is higher in situations involving the central nervous system, bleeding, a low platelet count, and elevated D-dimer levels. (Table 1) A recent review found that following ChAdOx1 nCoV-19 (Oxford/AstraZeneca) immunization, VITT overall mortality was 35.9%. The occurrence of intracerebral hemorrhage (ICH), age 60 years, platelet count 25 103/L, fibrinogen 150 mg/dL, and CVST were found to be strongly linked with death and were chosen as predictors for mortality [91]. TTP incidents following the COVID-19 vaccine have been more common in women under 55 and occur 5–30 days after immunization with virus-vectored vaccines (i.e., ChAdOx1 nCoV-19, Astra-Zeneca [Cambridge, UK] and Ad26.COV2.S Janssen [Beerse, Belgium]) [92]. The proposed therapeutic strategy included plasma exchange and corticosteroid therapy with an excellent effect [93]. Concerning anticoagulants, medication selection demands serious thought. Heparin is strictly prohibited in patients with classical HIT. However, Tiede et al. reported heparin therapy on one of their TTS patients. They found no indication that heparin was promoting platelet aggregation [94]. Still, caution is needed since, even if anti-PF4 antibodies were not brought on by heparin, the polyanion heparin may increase the possibility of aggregation in some subjects. The alternative drug argatroban seems to be both secure and efficient.

Table 1.
VITT incidents following COVID-19 vaccination based on the data in the literature.
Despite rare occurrences of thrombosis with thrombocytopenia syndrome, COVID-19 vaccinations have a favorable benefit/risk profile. Table 1 presents the VITT incidents following COVID-19 vaccination based on the data in the literature [85,86,95,96,97,98,99,100].
9. Summary of Reported Literature Cases of Ultrarare VITT Complications
Table 1 summarizes the VITT reported incidents after the COVID-19 vaccine administration. Based on the data in the literature, we can conclude that most of the reported cases were following vector vaccines.
Cerebral venous thrombosis (CVT) is another complication that is more serious than VITT. It includes thrombosis in the veins of the cranial sinuses, cortical veins and deep venous structures [101]. The CVT incidence in the general population is estimated at 2 cases per 100,000 persons per year [102,103]. EMA data show that CVT after vaccination administration occurs most frequently in women under 60 [104,105].
The occurrence of potentially life-threatening complications after COVID-19 vaccination with adenoviral vector vaccines prompted temporary suspensions of these vaccines. In April 2021, vaccinations with Ad26.COV2.S were temporarily suspended first in the USA due to the reporting of more than ten cases of CVT and thrombocytopenia following administration of Ad26.COV2.S vaccine [106]. Subsequently, several more studies reported cases of VITT patients after vaccination [80,81,107]. The data showed that 48 of 52 patients tested for anti-PF4 antibody had a positive result. In addition, the studies showed that women were three times more likely than men to acquire VITT, and over 88.5% of patients were under 60 years old [108]. Other studies also have shown that these adverse effects were observed more often in women in women between 40 and 60 years [109,110,111]. The known risk factors, for now, are pregnancy, postpartum period, use of oral contraceptive pills, and hormone therapy [112,113].
A study conducted by Greinacher et al. also reported 11 patients with thrombosis or thrombocytopenia after administration of the AstraZeneca vector vaccine, ChAdOx1nCov-19 [73]. Of them, nine were women under 50 years old. Symptoms appeared between 5 and 16 days after administering the first vaccine dose. All patients had severe or moderate thrombocytopenia and the presence of anti-PF4 antibodies. In addition, except for the anticoagulant treatment of these patients, high doses of immunoglobulins (IVIG) were administered to interrupt the pro-thrombotic VITT mechanism and inhibit platelets. Many other reports of thrombotic events after the AstraZeneca vaccination have followed [63,92,111,114,115,116,117,118,119,120,121,122,123,124,125,126,127].
Another vaccine that has been associated with thrombosis is the Ad26.COV2.S of Johnson & Johnson/Janssen [106,128,129,130,131,132]. Reported cases of thrombosis related to mRNA vaccines are rare [133,134,135,136,137,138,139,140,141,142].
In 2021, a detailed EMA analysis for 213 cases of CVT after vaccination against COVID-19 was published [143]. They reported 87.8% after vaccination with ChAdOx1 nCoV-19, 12.2% after BNT162b2 vaccination, and only one case after mRNA-1273 vaccination. Immediately after EMA, many other regulatory agencies also published the data from their surveillance systems for observed thrombotic complications after vaccination against COVID-19 [96,144,145,146,147,148,149,150,151,152].
Regarding thrombocytopenia, the UK reported 60 cases after the ChAdOx1 nCoV-19 vaccine and 34 cases with the BNT162b2 vaccine [58]. One hundred ninety-five cases have been reported to the USA Vaccine Adverse Event Reporting System (VAERS) following the use of BioNTech/Pfizer and Moderna vaccines [152].
Understanding the possible mechanisms underlying the coagulation pathways is crucial to managing thrombotic complications after vaccination against COVID-19. Careful and long-term surveillance of the administration of vaccines is necessary. It should not be forgotten that the frequency of severe thrombotic events/complications after vaccination is relatively low. The benefits and risks of the currently approved vaccines must be evaluated, and the possibility of developing severe and long-term complications (long COVID-19) after infection with the SARS-CoV-2 virus must be considered.
Early diagnosis and treatment are crucial for VITT, including high-dose intravenous immunoglobulins and anticoagulation [153].
An international network cohort study of four countries by Markus et al. characterized the treatment options of thromboembolic events following COVID-19 vaccination. Thrombosis with thrombocytopenia syndrome (TTS) has been identified as a rare adverse event following some COVID-19 vaccines. Various guidelines have been issued on the treatment of TTS. We aimed to characterize the treatment of TTS and other thromboembolic events (venous thromboembolism (VTE) and arterial thromboembolism (ATE). Most patients with TTS received heparins, platelet aggregation inhibitors, or direct Xa inhibitors. Most VTE patients (before and after vaccination) were first treated with heparins in inpatient settings and direct Xa inhibitors in outpatient settings. In ATE patients, treatments were similar before and after vaccinations, with platelet aggregation inhibitors prescribed most frequently. Inpatient and claims data also showed substantial heparin use. Heparin use in the treatment of post-vaccine TTS suggests that most events were not identified as vaccine-induced thrombosis with thrombocytopenia by the treating clinicians [154].
The management of these events was previously covered in the WHO Guidance for clinical case management of thrombosis with thrombocytopenia syndrome (TTS) following vaccination to prevent coronavirus disease (COVID-19) in 2021 [80]. We must acknowledge that vaccine-associated thrombosis could occur in individuals with or without thrombocytopenia. However, future studies should focus on identifying alternative thrombosis-promoting components to improve the safety and efficacy of future COVID-19 vaccines.
Last but not least, there are arterial thromboembolic incidents following the COVID-19 vaccination. Such a study was provided by Wills et al. [155]. The authors reported two stroke cases due to arterial and venous thromboses within four weeks following the administration of the AstraZeneca vaccine. The patients were both young and in good health overall, with a few risk indicators for thrombosis. However, they had a swift and eventually lethal decline in cerebral function. The individuals had a notable decrease in platelet count and an abnormally high level of D-dimers, both of which are frequently documented in this particular medical condition. In all instances, quantifiable immunoglobulin G platelet factor-4 antibodies were identified using the enzyme-linked immunosorbent test, resembling the antibodies observed in cases of heparin-induced thrombocytopenia [155].
Nevertheless, instances of arterial thrombotic episodes have also been observed, as evidenced by the cases presented. Laboratory investigations commonly demonstrate a decreased platelet count, along with significantly elevated D-dimer levels and decreased fibrinogen levels. The presence of antibodies against platelet factor 4 (PF4) has been detected in affected individuals, indicating parallels to heparin-induced thrombocytopenia (HIT) even without prior heparin exposure [155]. Another cerebral incident was presented by Berlot et al. involving a patient with complete arterial occlusion of the right internal carotid artery and middle cerebral artery 9 days after the first dose of AstraZeneca (ChAdOx1 nCov-19) vaccination [156].
Going further down the road, post-COVID-19 vaccination was also associated with retinal artery occlusions, with hypercoagulability and blood thrombotic disorders being the main risk factors [157]. However, there are still no official recommendations on the use of particular types of vaccines depending on the underlying risk factors in the individuals [158]. Usually, the timing of vaccination is the decisive factor—to schedule vaccination after controlling all the risk factors and underlying conditions [159].
Furthermore, there was a case report of a patient who experienced acute myocardial infarction within 24 h after receiving the initial dose of the mRNA-1273 vaccination. The occurrence of subacute in-stent thrombosis was observed several days following percutaneous coronary intervention. VITT was suspected based on identifying antiplatelet factor 4 antibodies in the patient’s serum. We present this patient case to raise awareness among physicians regarding the potential occurrence of VITT associated with mRNA-1273. Nevertheless, it is essential to note that these findings should not, in any manner, lower the level of enthusiasm surrounding vaccination [160].
Another illustrative case report was about a male 60-year-old patient who underwent administration of the initial dosage of the AstraZeneca vaccine. Subsequently, the patient sought medical attention, reporting symptoms of discomfort and abnormal sensations in the left hand. The presence of upper limb ischemia was identified using investigative measures [161].
10. Diagnostics of Coagulopathies following COVID-19 Vaccination through Imaging
Radiology plays a crucial role in the diagnosis of vascular diseases. Different diagnostic methods depend on the localization and type of the pathology. The methods are divided into two main groups—those that use ionizing radiation and those that do not [162].
In recent years, ultrasound diagnostics has undergone serious progress and has established itself as a leading method for diagnosing vascular diseases. In addition to offering diagnosis without ionizing radiation, ultrasound machines are widely available, and the examination cost is relatively low. The method is successfully used to diagnose large blood vessels, peripheral vessels, vessels of various organs and systems, and brain vessels.
There are also various combinations of ultrasound and other methods, known as Fused Ultrasound. The method is available in combination with previously performed magnetic resonance imaging or computed tomography. Fused ultrasound is used to analyze prostate, liver, and breast diseases, and in neurological practice [163].
Computed tomography (CT) has rapidly developed in recent years with the introduction of multidetector computed tomography and dual-energy CT (DECT). A CT scan of the blood vessels is known as CT angiography (CTA) and CT venography (CTV). The method is widely available and non-invasive, and thanks to the latest software technologies, it is relatively easy to use [164]. In recent years, CTA and CTV have established themselves as the gold standard for diagnosing various blood vessel diseases—stenoses, thrombosis, aneurysms, dissections, obstructions, and bleeding, especially in emergencies. In recent years, the use of CT coronary angiography as a non-invasive alternative to classical coronary angiography has been imposed to diagnose coronary vessel diseases [165].
Recently, we documented nine case studies of anticoagulated COVID-19 patients with retroperitoneal and abdominal bleeding, emphasizing the power of CT to diagnose these complications early [166].
Magnetic resonance imaging is a modern, non-invasive method that also has a role in diagnosing blood vessels. The advantage of the method is that it can represent the vessel without using contrast material, which is particularly suitable for patients with impaired renal function, in which performing computer tomographic angiography is contraindicated. In addition to the native scan, it can also perform magnetic resonance angiography and magnetic resonance venography by applying contrast material. The contrast used in these studies is at a lower concentration than the iodine contrast used in computed tomography and can be used in patients with moderate renal function impairment. The informativeness of MPA and MPV is high, similar to that of CTA and CTV. The disadvantages of the method are the well-known disadvantages of magnetic resonance imaging in general—high cost, long-term examination, the presence of some metal implants and pacemakers, and, in most countries, no possibility of use in emergencies [167].
Thus, imaging could be critical in identifying and elucidating multiorgan complications, even in the rare case of SARS-CoV-2 vaccination [168,169]. Therefore, rapid recognition of complications following COVID-19 vaccination and appropriate treatment are appreciated.
11. Global Benefit/Risk Ratio of the Various Vaccination Types for Bleeding or Thrombotic Complications
Despite the reported cases in the literature, no clear causal relationship between vaccination and the pathology developed can be concluded with the current data. The European Medicines Agency (EMA) confirms that the overall benefit/risk ratio of COVID-19 vaccination remains positive. Due to reported menstrual bleeding after vaccination [170,171], the EMA has recommended that heavy menstrual bleeding be added to the product information as a side effect of unknown frequency for the mRNA vaccines. However, the available data reviewed involved mostly non-serious and temporary cases [172].
Our narrative review has some limitations. First, the topic of hereditary and acquired coagulation disorders is broad, and we could not cover all the disorders in vast detail. However, there is no evidence in the literature regarding some rare blood disorders and COVID-19 and COVID-19 vaccines. Second, we could not cover all the case reports covering bleeding and thrombotic events following COVID-19 vaccination, although we aimed to include the most significant. Third, there are no data on the booster doses and the risk of adverse events associated with coagulation. Therefore, we did not focus on this matter.
Along with these limitations, we believe that our paper has some strengths: this is the first review to comprehensively focus on both hereditary and acquired coagulation disorders in the light of COVID-19 and also to discuss the most-commonly described adverse effects of COVID-19 vaccines associated with bleeding or thrombotic events.
We want to emphasize that we discussed these events following COVID-19 vaccination. Still, there is no evidence of direct causality between the bleeding and thrombotic events and COVID-19 vaccines, although some hypotheses for immunological mechanisms were proposed. Based on such a hypothesis for the production of autoantibodies after vaccination, one should accept that the second and following doses will lead to immediate consequences in subjects who had already activated an abnormal immune response. However, no data support this in clinical or real-world settings [85].
Also, the prevailing data on the safety of COVID-19 vaccines confirm that the efficacy of vaccines against COVID-19 and their benefits to reduce morbidity, complications, and mortality outweigh the potential risk of rare serious adverse effects such as coagulation disorders [173,174]. The causal relationship could not be supported by analyzing data from the pharmacovigilance reports since their data are administrative and non-controlled. Thus, there is more of a statistical link than a correlation between adverse events and vaccination [175,176,177].
Regrettably, some people are still hesitant to acknowledge the risks posed by SARS-CoV-2, comparing them to earlier influenza outbreaks and ignoring the reality of mortality rates, and they hesitate to be vaccinated against COVID-19. The phenomenon of denial is significant, and reports of adverse effects, particularly thrombotic ones, have a bearing on it. A complicated phenomenon, vaccination hesitancy is influenced by people’s opinions of the effectiveness and safety of immunizations; therefore, more studies should focus on the safety and more studies should investigate vaccine fear in people [178,179,180].
Nevertheless, the incidence of thrombotic events is extremely low (1/100,000–1/1,000,000 vaccinated subjects) [107,146,181,182,183,184]. This is also valid for acquired hemophilia [185]. Furthermore, the rate of acquired hemophilia post-vaccination does not differ significantly from that of acquired hemophilia without vaccination, which has a basic prevalence of 1.5 per million, which does not allow us to estimate the hazard ratio for this condition [186].
Based on available data, Abrignani et al. [187] and Elalamy et al. [58] propose to avoid these unnecessary evaluations in the case of COVID-19 vaccination: systematic premedications with low molecular weight heparin, direct anticoagulants or aspirin, routine screening for thrombophilia, periodic assessment of PF4 antibodies after vaccination or monitoring changes in D-dimer, and systematic use of venous echo-Doppler examinations after the vaccine administration.
Also, we must consider the possibility that previous infections with SARS-CoV-2 or other viruses may increase the risk of thrombotic events or inflammatory reactions following vaccination or other interventions [188]. However, careful surveillance and long-term follow-up of the safety and effectiveness of COVID-19 vaccines are still needed, especially to gain data on vaccine-vaccine interactions and use in immunocompromised and comorbid patients [150,178].
12. Conclusions
It should be kept in mind that severe thrombotic episodes seem to occur seldom following COVID-19 vaccination. Also, the prevailing data on the safety of COVID-19 vaccines confirm that the efficacy of vaccines against COVID-19 and their benefits to reduce morbidity, complications, and mortality outweighs the potential risk of vaccines for rare serious adverse effects, such as coagulation disorders. However, careful surveillance and long-term follow-up of the safety and effectiveness of COVID-19 vaccines are still needed, especially to gain data on vaccine–vaccine interactions and use in immunocompromised and comorbid patients.
Author Contributions
Conceptualization, M.S., N.M. and T.V.; methodology, G.V.V.; software, D.M.; validation, M.G., M.P.-S. and L.C.; formal analysis, H.B.; investigation, G.H.V.; resources, L.T.; data curation, S.L.; writing—original draft preparation, M.S., N.M., G.V.V., M.P.-S., M.G., D.V. and T.V.; writing—review and editing, T.V.; visualization, N.M. and T.V.; supervision, T.V.; project administration, T.V.; funding acquisition, T.V. All authors have read and agreed to the published version of the manuscript.
Funding
This study is financed by the European Union-NextGenerationEU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project No. BG-RRP-2.004-0008.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable
Acknowledgments
This study is financed by the European Union-NextGenerationEU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project No. BG-RRP-2.004-0008.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. WHO COVID-19 Dashboard—Up to Date Data on Pandemic. WHO Heal Emerg Dashboard 2021. Available online: https://covid19.who.int/region/searo/country/id (accessed on 10 July 2023).
- Dhama, K.; Khan, S.; Tiwari, R.; Sircar, S.; Bhat, S.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Coronavirus Disease 2019-COVID-19. Clin. Microbiol. Rev. 2020, 33, e00028-20. [Google Scholar] [CrossRef]
- Sharun, K.; Tiwari, R.; Iqbal Yatoo, M.; Patel, S.K.; Natesan, S.; Dhama, J.; Malik, Y.S.; Harapan, H.; Singh, R.K.; Dhama, K. Antibody-based immunotherapeutics and use of convalescent plasma to counter COVID-19: Advances and prospects. Expert. Opin. Biol. Ther. 2020, 20, 1033–1046. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Sharun, K.; Dhama, K. COVID-19 Vaccine Diplomacy and Equitable Access to Vaccines Amid Ongoing Pandemic. Arch. Med. Res. 2021, 52, 761–763. [Google Scholar] [CrossRef]
- Knoll, M.D.; Wonodi, C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet 2021, 397, 72–74. [Google Scholar] [CrossRef]
- Gasparyan, A.Y.; Ayvazyan, L.; Blackmore, H.; Kitas, G.D. Writing a narrative biomedical review: Considerations for authors, peer reviewers, and editors. Rheumatol. Int. 2011, 31, 1409–1417. [Google Scholar] [CrossRef]
- COVID-10 Vaccine Tracker. Data Are Accurate as of 2 December 2022. Available online: https://covid19.trackvaccines.org/vaccines/approved/#vaccine-list (accessed on 15 August 2023).
- CDC Reported Adverse Events. Safety of COVID-19 Vaccines. 13 July 2023. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html (accessed on 15 August 2023).
- Graña, C.; Ghosn, L.; Evrenoglou, T.; Jarde, A.; Minozzi, S.; Bergman, H.; Buckley, B.S.; Probyn, K.; Villanueva, G.; Henschke, N.; et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst. Rev. 2022, 12, CD015477. [Google Scholar] [CrossRef] [PubMed]
- Graham, R.L.; Donaldson, E.F.; Baric, R.S. A decade after SARS: Strategies for controlling emerging coronaviruses. Nat. Rev. Microbiol. 2013, 11, 836–848. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines–a new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261. [Google Scholar] [CrossRef]
- Pardi, N.; Tuyishime, S.; Muramatsu, H.; Kariko, K.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Weissman, D. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control Release 2015, 217, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Karikó, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Corbett, K.S.; Edwards, D.; Leist, S.R.; Abiona, O.M.; Boyoglu-Barnum, S.; Gillespie, R.A.; Himansu, S.; Schäfer, A.; Ziwawo, C.T.; DiPiazza, A.T.; et al. SARS-Co-V-2 mRNA vaccine development enabled by prototype pathogen preparedness. Nature 2020, 586, 567–571. [Google Scholar] [CrossRef]
- Vaxzevria (Previously COVID-19 Vaccine Astra Zeneca): EPAR—Product Information, Vol. 2021. European Medicines Agency, 2021. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/vaxzevria (accessed on 15 August 2023).
- Greinacher, A.; Selleng, K.; Palankar, R.; Wesche, J.; Handtke, S.; Wolff, M.; Aurich, K.; Lalk, M.; Methling, K.; Völker, U.; et al. Insights in ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. Blood 2021, 138, 2256–2268. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Xia, S.; Liu, M.; Wang, C.; Xu, W.; Lan, Q.; Feng, S.; Qi, F.; Bao, L.; Du, L.; Liu, S.; et al. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020, 30, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhong, G.; Zhang, J.; Shuai, L.; Chen, W. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 2020, 11, 4081. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Naygovzina, N.B.; Khabriev, R.U.; Krasheninnikov, A.E.; Matveev, A.V. The organizational aspects of security support of participants of clinical testing of vaccine “Gam-COVID-Vac”. Probl. Sotsialnoi Gig. Zdr. Istor. Med. 2021, 29, 5–13. (In Russian) [Google Scholar]
- Doroftei, B.; Ciobica, A.; Ilie, O.D.; Maftei, R.; Ilea, C. Mini-review discussing the reliability and efficiency of COVID-19 vaccines. Diagnostics 2021, 11, 579. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Y.; Huang, B.; Deng, W.; Yang, X. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell 2020, 182, 713–721.e9. [Google Scholar] [CrossRef] [PubMed]
- Al Kaabi, N.; Zhang, Y.; Xia, S.; Yang, Y.; Al Qahtani, M.M.; Abdulrazzaq, N.; Al Nusair, M.; Hassany, M.; Jawad, J.S.; Abdalla, J.; et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: A randomized clinical trial. JAMA 2021, 326, 35–45. [Google Scholar] [CrossRef]
- Gao, Q.; Bao, L.; Mao, H.; Wang, L.; Xu, K.; Yang, M.; Li, Y.; Zhu, L.; Wang, N.; Lv, Z.; et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020, 369, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Zhou, H.; et al. Phase 1-2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N. Engl. J. Med. 2020, 383, 2320–2332. [Google Scholar] [CrossRef]
- Andrikovics, H.; Klein, I.; Bors, A.; Nemes, L.; Marosi, A.; Varadi, A.; Tordai, A. Analysis of large structural changes of the factor VIII gene, involving intron 1 and 22, in severe hemophilia A. Haematologica 2003, 88, 778–784. [Google Scholar]
- Rossaint, R.; Afshari, A.; Bouillon, B.; Cerny, V.; Cimpoesu, D.; Curry, N.; Duranteau, J.; Filipescu, D.; Grottke, O.; Grønlykke, L.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Sixth edition. Crit. Care 2023, 27, 80. [Google Scholar] [CrossRef]
- Salen, P.; Babiker, H.M. Hemophilia A. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470265/ (accessed on 15 August 2023).
- Konkle, B.A.; Nakaya Fletcher, S. Hemophilia A. In GeneReviews® [Internet]; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Angelini, D.; Konkle, B.A.; Sood, S.L. Aging among persons with hemophilia: Contemporary concerns. Semin. Hematol. 2016, 53, 35–39. [Google Scholar] [CrossRef]
- Mericliler, M.; Narayan, G. Outcomes of COVID-19 in Adult Males with Hemophilia A: A Propensity Score-Matched Analysis. Cureus 2022, 14, e30662. [Google Scholar] [CrossRef]
- Tiede, A.; Leise, H.; Horneff, S.; Oldenburg, J.; Halimeh, S.; Heller, C.; Königs, C.; Holstein, K.; Pfrepper, C. Safety of intramuscular COVID-19 vaccination in patients with haemophilia. Haemophilia 2022, 28, 687–693. [Google Scholar] [CrossRef]
- Velikov, T.; Keremidchiev, G.; Velikova, T. How to use Safely COVID-19 Vaccines in Patients on Anticoagulants or Antiaggregants. Int. J. Preven Cardio. 2021, 1, 32–33. [Google Scholar]
- Duminuco, A.; Calagna, M.; Markovic, U.; Esposito, B.; Grasso, S.; Riccobene, C.; Di Raimondo, F.; Giuffrida, G. Acquired hemophilia A following COVID-19 vaccination—The importance of prompt diagnosis: A case report. Transfus. Apher. Sci. 2023, 62, 103577. [Google Scholar] [CrossRef]
- Al Hennawi, H.; Al Masri, M.K.; Bakir, M.; Albarazi, M.; Jazaeri, F.; Almasri, T.N.; Shoura, S.J.; Barakeh, A.R.R.; Taftafa, A.; Khan, M.K.; et al. Acquired Hemophilia A Post-COVID-19 Vaccination: A Case Report and Review. Cureus 2022, 14, e21909. [Google Scholar] [CrossRef] [PubMed]
- Happaerts, M.; Vanassche, T. Acquired hemophilia following COVID-19 vaccination: Case report and review of literature. Res. Pract. Thromb. Haemost. 2022, 6, e12785. [Google Scholar] [CrossRef]
- Emna, B.; Kmira, Z.; Hajer, B.I.; Nadia, S.; Yossra, D.; Amina, B.; Yosra, B.Y.; Haifa, R.; Abderrahim, K. Acquired hemophilia A following COVID-19 vaccine: A case report. J. Med. Case Rep. 2023, 17, 125. [Google Scholar] [CrossRef] [PubMed]
- Ai Vuen, L.; Aun Su-Yin, E.; Naila Kori, A.; Shah, T.M. Case of acquired haemophilia a in Southeast Asia following COVID-19 vaccine. BMJ Case Rep. CP 2022, 15, e246922. [Google Scholar] [CrossRef]
- Hosoi, H.; Tane, M.; Kosako, H.; Ibe, M.; Takeyama, M.; Murata, S.; Mushino, T.; Sonoki, T. Acute-type acquired hemophilia A after COVID-19 mRNA vaccine administration: A new disease entity? J. Autoimmun. 2022, 133, 102915. [Google Scholar] [CrossRef]
- Radwi, M.; Farsi, S. A case report of acquired hemophilia following COVID-19 vaccine. J. Thromb. Haemost. 2021, 19, 1515–1518. [Google Scholar] [CrossRef]
- Goodeve, A.; James, P. von Willebrand Disease. In GeneReviews® [Internet]; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Michiels, J.J.; van Vliet, H.H.; Berneman, Z.; Gadisseur, A.; van der Planken, M.; Schroyens, W.; van der Velden, A.; Budde, U. Intravenous DDAVP and factor VIII-von Willebrand factor concentrate for the treatment and prophylaxis of bleedings in patients With von Willebrand disease type 1, 2 and 3. Clin. Appl. Thromb. Hemost. 2007, 13, 14–34. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.W.; van Wijk, X.M.R.; Pham, H.P.; Marin, M.J. Role of von Willebrand Factor in COVID-19 Associated Coagulopathy. J. Appl. Lab. Med. 2021, 6, 1305–1315. [Google Scholar] [CrossRef]
- NHF President and CEO Leonard Valentino, MD, Discusses the Role of von Willebrand Factor (VWF) in Severe Cases of COVID-19. Available online: https://www.hemophilia.org/news/covid-19-and-vwf (accessed on 15 August 2023).
- Konkle, B.A.; Nakaya Fletcher, S. Hemophilia B. In GeneReviews® [Internet]; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Pipe, S.W.; Kaczmarek, R.; Srivastava, A.; Pierce, G.F.; Makris, M.; Hermans, C.; Interim Guidance; Coagulation Products Safety, Supply and Access (CPSSA) Committee of the World Federation of Hemophilia. Management of COVID-19-associated coagulopathy in persons with haemophilia. Haemophilia 2021, 27, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Dautaj, A.; Krasi, G.; Bushati, V.; Precone, V.; Gheza, M.; Fioretti, F.; Sartori, M.; Costantini, A.; Benedetti, S.; Bertelli, M. Hereditary thrombophilia. Acta Biomed. 2019, 90, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Thorelli, E.; Kaufman, R.J.; Dahlbäck, B. Cleavage of factor V at Arg 506 by activated protein C and the expression of anticoagulant activity of factor V. Blood 1999, 93, 2552–2558. [Google Scholar] [CrossRef]
- Padda, I.S.; Patel, P.; Citla Sridhar, D. Protein C and S. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557814/ (accessed on 15 August 2023).
- Kate, M.K.; van der Meer, J. Protein S deficiency: A clinical perspective. Haemophilia 2008, 14, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.O.; Rogers, H.J. Hypercoagulable states: An algorithmic approach to laboratory testing and update on monitoring of direct oral anticoagulants. Blood Res. 2014, 49, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Park, W.C.; Chang, J.H. Clinical Implications of Methylenetetrahydrofolate Reductase Mutations and Plasma Homocysteine Levels in Patients with Thromboembolic Occlusion. Vasc. Spec. Int. 2014, 30, 113–119. [Google Scholar] [CrossRef]
- Houghton, D.E.; Wysokinski, W.E.; Padrnos, L.J.; Shah, S.; Wysokinska, E.; Pruthi, R.; Ghorbanzadeh, A.; Ashrani, A.; Sridharan, M.; McBane, R.D.; et al. Venous thromboembolism after COVID-19 vaccination in patients with thrombophilia. Am. J. Hematol. 2023, 98, 566–570. [Google Scholar] [CrossRef]
- Elalamy, I.; Gerotziafas, G.; Alamowitch, S.; Laroche, J.P.; Van Dreden, P.; Ageno, W.; Beyer-Westendorf, J.; Cohen, A.T.; Jimenez, D.; Brenner, B.; et al. SARS-CoV-2 Vaccine and Thrombosis: An Expert Consensus on Vaccine-Induced Immune Thrombotic Thrombocytopenia. Thromb. Haemost. 2021, 121, 982–991. [Google Scholar] [CrossRef]
- De Lau, L.M.; Leebeek, F.W.; de Maat, M.P.; Koudstaal, P.J.; Dippel, D.W. A review of hereditary and acquired coagulation disorders in the aetiology of ischaemic stroke. Int. J. Stroke 2010, 5, 385–394. [Google Scholar] [CrossRef]
- Tripodi, A.; Mancuso, M.E.; Chantarangkul, V.; Clerici, M.; Bader, R.; Meroni, P.L.; Santagostino, E.; Mannucci, P.M. Lupus anticoagulants and their relationship with the inhibitors against coagulation factor VIII: Considerations on the differentiation between the 2 circulating anticoagulants. Clin. Chem. 2005, 51, 1883–1885. [Google Scholar] [CrossRef]
- Caldwell, S.H.; Hoffman, M.; Lisman, T.; Macik, B.G.; Northup, P.G.; Reddy, K.R.; Tripodi, A.; Sanyal, A.J.; Coagulation in Liver Disease Group. Coagulation disorders and hemostasis in liver disease: Pathophysiology and critical assessment of current management. Hepatology 2006, 44, 1039–1046. [Google Scholar] [CrossRef]
- Levi, M.; Toh, C.H.; Thachil, J.; Watson, H.G. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br. J. Haematol. 2009, 145, 24–33. [Google Scholar] [CrossRef]
- Aladdin, Y.; Algahtani, H.; Shirah, B. Vaccine-Induced Immune Thrombotic Thrombocytopenia with Disseminated Intravascular Coagulation and Death following the ChAdOx1 nCoV-19 Vaccine. J. Stroke Cerebrovasc. Dis. 2021, 30, 105938. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, V.; Caranci, F.; Negro, A.; Piscitelli, V.; Tuccillo, B.; Fasano, F.; Sirabella, G.; Marano, I.; Granata, V.; Grassi, R.; et al. A Rare Case of Cerebral Venous Thrombosis and Disseminated Intravascular Coagulation Temporally Associated to the COVID-19 Vaccine Administration. J. Pers. Med. 2021, 11, 285. [Google Scholar] [CrossRef] [PubMed]
- Bílková, S.; Hirmerová, J. Coagulopathy associated with COVID-19. Koagulopatie asociovaná s onemocněním COVID-19. Vnitr. Lek. 2020, 66, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Shaw, R.J.; Bradbury, C.; Abrams, S.T.; Wang, G.; Toh, C.H. COVID-19 and immunothrombosis: Emerging understanding and clinical management. Br. J. Haematol. 2021, 194, 518–529. [Google Scholar] [CrossRef]
- Ward, S.E.; Fogarty, H.; Karampini, E.; Lavin, M.; Schneppenheim, S.; Dittmer, R.; Morrin, H.; Glavey, S.; Ni Cheallaigh, C.; Bergin, C.; et al. ADAMTS13 regulation of VWF multimer distribution in severe COVID-19. J. Thromb. Haemost. 2021, 19, 1914–1921. [Google Scholar] [CrossRef]
- Flaumenhaft, R.; Enjyoji, K.; Schmaier, A.A. Vasculopathy in COVID-19. Blood 2022, 140, 222–235. [Google Scholar] [CrossRef]
- Whitworth, H.; Sartain, S.E.; Kumar, R.; Armstrong, K.; Ballester, L.; Betensky, M.; Cohen, C.T.; Diaz, R.; Diorio, C.; Goldenberg, N.A.; et al. Rate of thrombosis in children and adolescents hospitalized with COVID-19 or MIS-C. Blood 2021, 138, 190–198. [Google Scholar] [CrossRef]
- Riphagen, S.; Gomez, X.; Gonzalez-Martinez, C.; Wilkinson, N.; Theocharis, P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020, 395, 1607–1608. [Google Scholar] [CrossRef] [PubMed]
- Lazova, S.; Gerenska, D.; Slabakova, Y.; Velikova, T. Immunological features of the multisystem inflammatory syndrome associated with SARS-CoV-2 in children. Am. J. Clin. Exp. Immunol. 2022, 11, 64–71. [Google Scholar]
- Lazova, S.; Dimitrova, Y.; Hristova, D.; Tzotcheva, I.; Velikova, T. Cellular, Antibody and Cytokine Pathways in Children with Acute SARS-CoV-2 Infection and MIS-C-Can We Match the Puzzle? Antibodies 2022, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Flaczyk, A.; Rosovsky, R.P.; Reed, C.T.; Bankhead-Kendall, B.K.; Bittner, E.A.; Chang, M.G. Comparison of published guidelines for management of coagulopathy and thrombosis in critically ill patients with COVID 19: Implications for clinical practice and future investigations. Crit. Care 2020, 24, 559. [Google Scholar] [CrossRef]
- Cuker, A.; Tseng, E.K.; Nieuwlaat, R.; Angchaisuksiri, P.; Blair, C.; Dane, K.; Davila, J.; DeSancho, M.T.; Diuguid, D.L.; Griffin, D.O.; et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021, 5, 872–888. [Google Scholar] [CrossRef]
- Klok, F.A.; Kruip, M.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb. Res. 2020, 191, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety Efficacy of the BNT162b2 mRNACovid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Interim Results of a Phase 1-2a Trial of Ad26COV2SCovid-19 Vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef]
- WHO Guidance for Clinical Case Management of Thrombosis with Thrombocytopenia Syndome (TTS) Following Vaccination to Prevent Coronavirus Disease (COVID-19). Available online: https://apps.who.int/iris/bitstream/handle/10665/342999/WHO-2019-nCoV-TTS-2021.1-eng.pdf?sequence=1&isAllowed=y (accessed on 7 July 2023).
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.-H.; Skattør, T.H.; Tjønnfjord, G.E.; et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2124–2130. [Google Scholar] [CrossRef]
- Scully, M.; Singh, D.; Lown, R.; Poles, A.; Solomon, T.; Levi, M.; Goldblatt, D.; Kotoucek, P.; Thomas, W.; Lester, W. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Weller, S.C.; Porterfield, L.; Davis, J.; Wilkinson, G.S.; Chen, L.; Baillargeon, J. Incidence of venous thrombotic events and events of special interest in a retrospective cohort of commercially insured US patients. BMJ Open 2022, 12, e054669. [Google Scholar] [CrossRef] [PubMed]
- Palaiodimou, L.; Stefanou, M.I.; de Sousa, D.A.; Coutinho, J.M.; Papadopoulou, M.; Papaevangelou, V.; Vassilakopoulos, T.I.; Tsiodras, S.; Filippou, D.K.; Tsivgoulis, G. Cerebral venous sinus thrombosis in the setting of COVID-19 vaccination: A systematic review and meta-analysis. J. Neurol. 2022, 269, 3413–3419. [Google Scholar] [CrossRef]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
- Sharifian-Dorche, M.; Bahmanyar, M.; Sharifian-Dorche, A.; Mohammadi, P.; Nomovi, M.; Mowla, A. Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination; a systematic review. J. Neurol. Sci. 2021, 428, 117607. [Google Scholar] [CrossRef]
- Sarode, R.; Bandarenko, N.; Brecher, M.E.; Kiss, J.E.; Marques, M.B.; Szczepiorkowski, Z.M.; Winters, J.L. Thrombotic thrombocytopenic purpura: 2012 American Society for Apheresis (ASFA) consensus conference on classification, diagnosis, management, and future research. J. Clin. Apher. 2014, 29, 148–167. [Google Scholar] [CrossRef]
- Australian Government Department of Health Therapeutic Goods Administration. COVID-19 Vaccine Weekly Safety Report. Available online: https://www.tga.gov.au/news/covid-19-vaccine-safety-reports/covid-19-vaccine-weekly-safety-report-21-10-2021 (accessed on 15 August 2023).
- Therapeutic Goods Administration (TGA). Australian Government Department of Health; 2021 [citado 7 de Dezembro de 2021]. Available online: https://www.tga.gov.au/periodic/covid-19-vaccine-weekly-safety-report-14-10-2021 (accessed on 15 August 2023).
- Medicines & Healthcare Products, Regulatory Agency. Coronavirus Vaccine—Weekly Summary of Yellow Card Reporting [Internet]. GOV.UK. 2021 [citado 7 de Dezembro de 2021]. Available online: https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting (accessed on 15 August 2023).
- Hwang, J.; Park, S.H.; Lee, S.W.; Lee, S.B.; Lee, M.H.; Jeong, G.H.; Kim, M.S.; Kim, J.Y.; Koyanagi, A.; Jacob, L.; et al. Predictors of mortality in thrombotic thrombocytopenia after adenoviral COVID-19 vaccination: The FAPIC score. Eur. Heart J. 2021, 42, 4053–4063. [Google Scholar] [CrossRef]
- Thiele, T.; Weisser, K.; Schönborn, L.; Funk, M.B.; Weber, G.; Greinacher, A.; Keller-Stanislawski, B. Laboratory confirmed vaccine-induced immune thrombotic thrombocytopenia: Retrospective analysis of reported cases after vaccination with ChAdOx-1 nCoV-19 in Germany. Lancet Reg. Health Eur. 2022, 12, 100270. [Google Scholar] [CrossRef]
- Saluja, P.; Gautam, N.; Yadala, S.; Venkata, A.N. Thrombotic thrombocytopenic purpura (TTP) after COVID-19 vaccination: A systematic review of reported cases. Thromb. Res. 2022, 214, 115–121. [Google Scholar] [CrossRef]
- Tiede, A.; Sachs, U.J.; Czwalinna, A.; Werwitzke, S.; Bikker, R.; Krauss, J.K.; Donnerstag, F.; Weißenborn, K.; Höglinger, G.; Maasoumy, B.; et al. Pro-thrombotic immune thrombocytopenia after COVID-19 vaccination. Blood 2021, 138, 350–353. [Google Scholar] [CrossRef]
- Pottegård, A.; Lund, L.C.; Karlstad, Ø.; Dahl, J.; Andersen, M.; Hallas, J.; Lidegaard, Ø.; Tapia, G.; Gulseth, H.L.; Ruiz, P.L.; et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford AstraZeneca ChAdOx1-S in Denmark and Norway: Population-based cohort study. BMJ 2021, 373, n1114. [Google Scholar] [CrossRef]
- Cari, L.; Fiore, P.; Naghavi Alhosseini, M.; Sava, G.; Nocentini, G. Blood clots and bleeding events following BNT162b2 and ChAdOx1 nCoV-19 vaccine: An analysis of European data. J. Autoimmun. 2021, 122, 102685. [Google Scholar] [CrossRef]
- Palaiodimou, L.; Stefanou, M.I.; Katsanos, A.H.; Aguiar de Sousa, D.; Coutinho, J.M.; Lagiou, P.; Michopoulos, I.; Naska, A.; Giannopoulos, S.; Vadikolias, K.; et al. Cerebral Venous Sinus Thrombosis and Thrombotic Events After Vector-Based COVID-19 Vaccines: A Systematic Review and Meta-analysis. Neurology 2021, 97, e2136–e2147. [Google Scholar] [CrossRef]
- Hafeez, M.U.; Ikram, M.; Shafiq, Z.; Sarfraz, A.; Sarfraz, Z.; Jaiswal, V.; Sarfraz, M.; Chérrez-Ojeda, I. COVID-19 vaccine-associated thrombosis with thrombocytopenia syndrome (TTS): A systematic review and post hoc analysis. Clin. Appl. Thromb. Hemost. 2021, 27, 10760296211048815. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.P.; Lewis, N.; Goddard, K.; Fireman, B.; Zerbo, O.; Hanson, K.E.; Donahue, J.G.; Kharbanda, E.O.; Naleway, A.; Nelson, J.C.; et al. Surveillance for Adverse Events After COVID-19 mRNA Vaccination. JAMA 2021, 326, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Mouta Nunes de Oliveira, P.; Mendes-de-Almeida, D.P.; Bertollo Gomes Porto, V.; Crespo Cordeiro, C.; Vitiello Teixeira, G.; Saraiva Pedro, R.; Roberto Gomes Takey, P.; Kegele Lignani, L.; Reis Xavier, J.; Cardoso Doria da Gama, V.; et al. Vaccine-induced immune thrombotic thrombocytopenia after COVID-19 vaccination: Description of a series of 39 cases in Brazil. Vaccine 2022, 40, 4788–4795. [Google Scholar] [CrossRef]
- Stam, J. Thrombosis of the cerebral veins and sinuses. N. Engl. J. Med. 2005, 352, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Otite, F.O.; Patel, S.; Sharma, R.; Khandwala, P.; Desai, D.; Latorre, J.G.; Akano, E.O.; Anikpezie, N.; Izzy, S.; Malik, A.M.; et al. Trends in incidence and epidemiologic characteristics of cerebral venous thrombosis in the United States. Neurology 2020, 95, e2200–e2213. [Google Scholar] [CrossRef]
- Schulz, J.B.; Berlit, P.; Diener, H.C.; Gerloff, C.; Greinacher, A.; Klein, C.; Petzold, G.C.; Piccininni, M.; Poli, S.; Rohrig, R.; et al. COVID-19 Vaccine-Associated Cerebral Venous Thrombosis in Germany. Ann. Neurol. 2021, 90, 627–639. [Google Scholar] [CrossRef]
- European Medicines Agency. 29 March 2021 Update. COVID-19 Vaccine Safety Update VAXZEVRIA AstraZeneca AB. Available online: https://www.ema.europa.eu/en/search/search?search_api_views_fulltext=CVT+after+COVID-19+vaccination+administration (accessed on 12 July 2023).
- European Medicines Agency. COVID-19 Vaccine Janssen: EMA Finds Possible Link to Very Rare Cases of Unusual Blood Clots with Low Blood Platelets. 20 April 2021. Available online: https://www.ema.europa.eu/en/news/covid-19-vaccine-janssen-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood (accessed on 12 July 2023).
- See, I.; Su, J.R.; Lale, A.; Woo, E.J.; Guh, A.Y.; Shimabukuro, T.T.; Streiff, M.B.; Rao, A.K.; Wheeler, A.P.; Beavers, S.F.; et al. US Case Reports of Cerebral Venous Sinus Thrombosis with Thrombocytopenia After Ad26.COV2.S Vaccination, March 2 to April 21, 2021. JAMA 2021, 325, 2448–2456. [Google Scholar] [CrossRef]
- Franchini, M.; Testa, S.; Pezzo, M.; Glingani, C.; Caruso, B.; Terenziani, I.; Pognani, C.; Bellometti, S.A.; Castelli, G. Cerebral venous thrombosis and thrombocytopenia post-COVID-19 vaccination. Thromb. Res. 2021, 202, 182–183. [Google Scholar] [CrossRef]
- Franchini, M.; Liumbruno, G.M. Pezzo MCOVID-19 vaccine-associated immune thrombosis thrombocytopenia (VITT): Diagnostic therapeutic recommendations for a new syndrome. Eur. J. Haematol. 2021, 107, 173–180. [Google Scholar] [CrossRef]
- Rizk, J.G.; Gupta, A.; Sardar, P.; Henry, B.M.; Lewin, J.C.; Lippi, G.; Lavie, C.J. Clinical Characteristics and Pharmacological Management of COVID-19 Vaccine–Induced Immune Thrombotic Thrombocytopenia with Cerebral Venous Sinus Thrombosis. JAMA Cardiol. 2021, 6, 1451. [Google Scholar] [CrossRef]
- Sørvoll, I.H.; Horvei, K.D.; Ernstsen, S.L.; Lægreid, I.J.; Lund, S.; Grønli, R.H.; Olsen, M.K.; Jacobsen, H.K.; Eriksson, A.; Halstensen, A.M.; et al. An observational study to identify the prevalence of thrombocytopenia and anti-PF4/polyanion antibodies in Norwegian health care workers after COVID-19 vaccination. J. Thromb. Haemost. 2021, 19, 1813–1818. [Google Scholar] [CrossRef]
- Maramattom, B.V.; Moidu, F.M.; Varikkottil, S.; Syed, A.A. Cerebral venous sinus thrombosis after ChAdOx1 vaccination: The first case of definite thrombosis with thrombocytopenia syndrome from India. BMJ Case Rep. 2021, 14, e246455. [Google Scholar] [CrossRef]
- Hekmat, A.S.; Javanmardi, K. Possible Risk of Thrombotic Events following Oxford-AstraZeneca COVID-19 Vaccination in Women Receiving Estrogen. BioMed Res. Int. 2021, 2021, 7702863. [Google Scholar]
- Allas, G.D.O.; Arizala, J.D.R.; Manalo, R.V.M. COVID-19 Adenoviral Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT), COVID-19-Related Thrombosis, and the Thrombotic Thrombocytopenic Syndromes. Hematol. Rep. 2022, 14, 358–372. [Google Scholar] [CrossRef]
- Wolf, M.E.; Luz, B.; Niehaus, L.; Bhogal, P.; Bäzner, H.; Henkes, H. Thrombocytopenia and Intracranial Venous Sinus Thrombosis after “COVID-19 Vaccine AstraZeneca” Exposure. J. Clin. Med. 2021, 10, 1599. [Google Scholar] [CrossRef]
- Mehta, P.R.; Mangion, S.A.; Benger, M.; Stanton, B.R.; Czuprynska, J.; Arya, R.; Sztriha, L.K. Cerebral venous sinus thrombosis and thrombocytopenia after COVID-19 vaccination—A report of two UK cases. Brain Behav. Immun. 2021, 95, 514–517. [Google Scholar] [CrossRef]
- Guan, C.-Y.; Tsai, S.-H.; Fan, J.-S.; Lin, Y.-K.; Kao, C.-C. A rare case of a middle-age Asian male with cerebral venous thrombosis after COVID-19 AstraZeneca vaccination. Am. J. Emerg. Med. 2021, 51, 427.e3–427.e4. [Google Scholar]
- Panovska-Stavridis, I. A rare case of superior ophthalmic vein thrombosis and thrombocytopenia following ChAdOx1 nCoV-19 vaccine against SARS-CoV-2. Mediterr. J. Hematol. Infect. Dis. 2021, 13, e2021048. [Google Scholar] [CrossRef] [PubMed]
- Walter, U.; Fuchs, M.; Grossmann, A.; Walter, M.; Thiele, T.; Storch, A.; Wittstock, M. Adenovirus-Vectored COVID-19 Vaccine–Induced Immune Thrombosis of Carotid Artery. Neurology 2021, 97, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Ko, C.-A.; Sung, Y.-F.; Chen, Y.-C.; Lee, J.-T.; Lin, Y.-Q.; Lin, Y.-K. Cerebral Venous Sinus Thrombosis, Pulmonary Embolism, and Thrombocytopenia After COVID-19 Vaccination in a Taiwanese Man: A Case Report and Literature Review. Front. Neurol. 2021, 12, 738329. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Huang, L.-Y.; Chen, Y.-L.; Chan, J.-S.; Chiang, W.-F.; Lin, C.-Y.; Chen, M.-H.; Shyu, H.-Y.; Hsiao, P.-J. ChAdOx1 COVID-19 vaccine-induced thrombocytopenia syndrome. QJM Int. J. Med. 2021, 114, 733–734. [Google Scholar] [CrossRef]
- Strobel, D.; Haberkamp, S.; Zundler, S. Portal Vein Thrombosis due to Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT) after COVID Vaccination with ChAdOx1 nCoV-19. Ultraschall Med.-Eur. J. Ultrasound 2021, 42, 551–552. [Google Scholar] [CrossRef]
- Fousse, M.; Schub, D.; Merzou, F.; Fassbender, K.; Sester, M.; Kettner, M.; Lochner, P.; Schmidt, T.; Júnior, J.R.G. Case report: Cerebral sinus vein thrombosis in two patients with AstraZeneca SARS-CoV-2 vaccination. J. Neurol. 2021, 269, 583–586. [Google Scholar] [CrossRef]
- Ceschia, N.; Scheggi, V.; Gori, A.M.; Rogolino, A.A.; Cesari, F.; Giusti, B.; Cipollini, F.; Marchionni, N.; Alterini, B.; Marcucci, R. Diffuse pro-thrombotic syndrome after ChAdOx1 nCoV-19 vaccine administration: A case report. J. Med. Case Rep. 2021, 15, 496. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Chen, C.-Y.; Yu, W.-L.; Kan, W.-C.; Feng, Y.-H. Myocardial Infarction and Azygos Vein Thrombosis After ChAdOx1 nCoV-19 Vaccination in a Hemodialysis Patient. Cureus 2021, 13, 18390. [Google Scholar] [CrossRef]
- Hocking, J.; Chunilal, S.D.; Chen, V.M.; Brighton, T.; Nguyen, J.; Tan, J.; Ting, S.B.; Tran, H. The first known case of vaccine-induced thrombotic thrombocytopenia in Australia. Med. J. Aust. 2021, 215, 19. [Google Scholar] [CrossRef]
- Garnier, M.; Curado, A.; Billoir, P.; Barbay, V.; Demeyere, M.; Dacher, J.-N. Imaging of Oxford/AstraZeneca® COVID-19 vaccine-induced immune thrombotic thrombocytopenia. Diagn. Interv. Imaging 2021, 102, 649–650. [Google Scholar] [CrossRef] [PubMed]
- McKeigue, P.M.; Burgul, R.; Bishop, J.; Robertson, C.; McMenamin, J.; O’Leary, M.; McAllister, D.A.; Colhoun, H.M. Association of cerebral venous thrombosis with recent COVID-19 vaccination: Case-crossover study using ascertainment through neuroimaging in Scotland. BMC Infect. Dis. 2021, 21, 1275. [Google Scholar] [CrossRef]
- Muir, K.-L.; Kallam, A.; Koepsell, S.A.; Gundabolu, K. Thrombotic Thrombocytopenia after Ad26.COV2.S Vaccination. N. Engl. J. Med. 2021, 384, 1964–1965. [Google Scholar] [CrossRef]
- Parums, D.V. Editorial: SARS-CoV-2 mRNA Vaccines and the Possible Mechanism of Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT). Med. Sci. Monit. 2021, 27, e932899. [Google Scholar] [CrossRef] [PubMed]
- Malik, B.; Kalantary, A.; Rikabi, K.; Kunadi, A. Pulmonary embolism, transient ischaemic attack and thrombocytopenia after the Johnson & Johnson COVID-19 vaccine. BMJ Case Rep. 2021, 14, e243975. [Google Scholar] [PubMed]
- Kennedy, V.E.; Wong, C.C.; Hong, J.M.; Peng, T.A.; Brondfield, S.; Reilly, L.M.; Cornett, P.; Leavitt, A.D. VITT following Ad26.COV2.S vaccination presenting without radiographically demonstrable thrombosis. Blood Adv. 2021, 5, 4662–4665. [Google Scholar] [CrossRef]
- Curcio, R.; Gandolfo, V.; Alcidi, R.; Giacomino, L.; Campanella, T.; Casarola, G.; Rossi, R.; Chiatti, L.; D’Abbondanza, M.; Commissari, R.; et al. Vaccine-induced massive pulmonary embolism and thrombocytopenia following a single dose of Janssen Ad26.COV2.S vaccination. Int. J. Infect. Dis. 2022, 116, 154–156. [Google Scholar] [CrossRef]
- Carli, G.; Nichele, I.; Ruggeri, M.; Barra, S.; Tosetto, A. Deep vein thrombosis (DVT) occurring shortly after the second dose of mRNA SARS-CoV-2 vaccine. Intern. Emerg. Med. 2021, 16, 803–804. [Google Scholar] [CrossRef] [PubMed]
- Meo, S.A.; Bukhari, I.A.; Akram, J.; Meo, A.S.; Klonoff, D.C. COVID-19 vaccines: Comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1663–1669. [Google Scholar] [PubMed]
- Zakaria, Z.; Sapiai, N.A.; Ghani, A.R.I. Cerebral venous sinus thrombosis 2 weeks after the first dose of mRNA SARS-CoV-2 vaccine. Acta Neurochir. 2021, 163, 2359–2362. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.; Soares-Dos-Reis, R.; Meira, J.; Ferrão, D.; Soares, P.R.; Pastor, A.; Gama, G.; Fonseca, L.; Fagundes, V.; Carvalho, M. Cerebral Venous Thrombosis after BNT162b2 mRNA SARS-CoV-2 vaccine. J. Stroke Cerebrovasc. Dis. 2021, 30, 105906. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Kimihira, L.; Nagasawa, H.; Seo, K.; Wada, M. Cerebral Venous Sinus Thrombosis After BNT162b2 mRNA COVID-19 Vaccination. Cureus 2021, 13, 18775. [Google Scholar] [CrossRef]
- Al-Maqbali, J.S.; Al Rasbi, S.; Kashoub, M.S.; Al Hinaai, A.M.; Farhan, H.; Al Rawahi, B.; Al Alawi, A.M. A 59-Year-Old Woman with Extensive Deep Vein Thrombosis and Pulmonary Thromboembolism 7 Days Following a First Dose of the Pfizer-BioNTech BNT162b2 mRNA COVID-19 Vaccine. Am. J. Case Rep. 2021, 22, e932946. [Google Scholar] [CrossRef]
- Andraska, E.A.; Kulkarni, R.; Chaudhary, M.; Sachdev, U. Three cases of acute venous thromboembolism in females after vaccination for coronavirus disease 2019. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 10, 14–17. [Google Scholar] [CrossRef]
- Okada, Y.; Sakai, R.; Sato-Fitoussi, M.; Nodera, M.; Yoshinaga, S.; Shibata, A.; Kurasawa, T.; Kondo, T.; Amano, K. Potential Triggers for Thrombocytopenia and/or Hemorrhage by the BNT162b2 Vaccine, Pfizer-BioNTech. Front. Med. 2021, 8, 751598. [Google Scholar] [CrossRef]
- Finsterer, J.; Nics, S. Venous sinus thrombosis after the second jab of an mRNA-based SARS-CoV-2 vaccine. Brain Hemorrhages 2021, 3, 36–38. [Google Scholar] [CrossRef]
- Bhan, C.; Bheesham, N.; Shakuntulla, F.; Sharma, M.; Sun, C.; Weinstein, M. An unusual presentation of acute deep vein thrombosis after the Moderna COVID-19 vaccine—A case report. Ann. Transl. Med. 2021, 9, 1605. [Google Scholar] [CrossRef] [PubMed]
- Krzywicka, K.; Heldner, M.R.; Sanchez van Kammen, M.; van Haaps, T.; Hiltunen, S.; Silvis, S.M.; Levi, M.; Kremer Hovinga, J.A.; Jood, K.; Lindgren, E.; et al. Post-SARS-CoV-2-vaccination cerebral venous sinus thrombosis: An analysis of cases notified to the European Medicines Agency. Eur. J. Neurol. 2021, 28, 3656–3662. [Google Scholar] [CrossRef]
- Tobaiqy, M.; Elkout, H.; MacLure, K. Analysis of Thrombotic Adverse Reactions of COVID-19 AstraZeneca Vaccine Reported to EudraVigilance Database. Vaccines 2021, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Cari, L.; Alhosseini, M.N.; Fiore, P.; Pierno, S.; Pacor, S.; Bergamo, A.; Sava, G.; Nocentini, G. Cardiovascular, neurological, and pulmonary events following vaccination with the BNT162b2, ChAdOx1 nCoV-19, and Ad26.COV2.S vaccines: An analysis of European data. J. Autoimmun. 2021, 125, 102742. [Google Scholar] [CrossRef] [PubMed]
- Abbattista, M.; Martinelli, I.; Peyvandi, F. Comparison of adverse drug reactions among four COVID-19 vaccines in Europe using the EudraVigilance database: Thrombosis at unusual sites. J. Thromb. Haemost. 2021, 19, 2554–2558. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Patone, M.; Mei, X.W.; Saatci, D.; Dixon, S.; Khunti, K.; Zaccardi, F.; Watkinson, P.; Shankar-Hari, M.; Doidge, J.; et al. Risk of thrombocytopenia and thromboembolism after COVID-19 vaccination and SARS-CoV-2 positive testing: Self-controlled case series study. BMJ 2021, 374, n1931. [Google Scholar] [CrossRef]
- Gras-Champel, V.; Liabeuf, S.; Baud, M.; Albucher, J.-F.; Benkebil, M.; Boulay, C.; Bron, A.; El Kaddissi, A.; Gautier, S.; Geeraerts, T.; et al. Atypical thrombosis associated with VaxZevria® (AstraZeneca) vaccine: Data from the French Network of Regional Pharmacovigilance Centres. Therapie 2021, 76, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.J.; Stowe, J.; Ramsay, M.E.; Miller, E. Risk of venous thrombotic events and thrombocytopenia in sequential time periods after ChAdOx1 and BNT162b2 COVID-19 vaccines: A national cohort study in England. Lancet Reg. Health-Eur. 2021, 13, 100260. [Google Scholar] [CrossRef] [PubMed]
- Shay, D.K.; Gee, J.; Su, J.R.; Myers, T.R.; Marquez, P.; Liu, R. Safety monitoring of the Janssen (Johnson & Johnson) COVID-19 Vaccine—United States, March–April 2021. MMWR 2021, 70, 680–684. [Google Scholar] [PubMed]
- Smadja, D.M.; Yue, Q.-Y.; Chocron, R.; Sanchez, O.; Louet, A.L.-L. Vaccination against COVID-19: Insight from arterial and venous thrombosis occurrence using data from VigiBase. Eur. Respir. J. 2021, 58, 2100956. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-J.; Cines, D.B.; Gernsheimer, T.; Kessler, C.; Michel, M.; Tarantino, M.D.; Semple, J.W.; Arnold, D.M.; Godeau, B.; Lambert, M.P.; et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am. J. Hematol. 2021, 96, 534–537. [Google Scholar] [CrossRef]
- Guetl, K.; Raggam, R.B.; Gary, T. Thrombotic Complications after COVID-19 Vaccination: Diagnosis and Treatment Options. Biomedicines 2022, 10, 1246. [Google Scholar] [CrossRef]
- Markus, A.F.; Strauss, V.Y.; Burn, E.; Li, X.; Delmestri, A.; Reich, C.; Yin, C.; Mayer, M.A.; Ramírez-Anguita, J.M.; Marti, E.; et al. Characterising the treatment of thromboembolic events after COVID-19 vaccination in 4 European countries and the US: An international network cohort study. Front. Pharmacol. 2023, 14, 1118203. [Google Scholar] [CrossRef]
- Wills, A.; Swallow, G.; Kirkman, M.A.; Rajan, K.; Subramanian, G. Arterial and venous thrombotic stroke after ChAdOx1 nCoV-19 vaccine. Clin. Med. 2022, 22, 184–186. [Google Scholar] [CrossRef]
- Berlot, G.; Tomasini, A.; La Fata, C.; Pintacuda, S.; Rigutti, S.; Falanga, A. Widespread Arterial Thrombosis after ChAdOx1 nCov-19 Vaccination. Case Rep. Crit. Care 2022, 2022, 6804456. [Google Scholar] [CrossRef]
- Yeo, S.; Kim, H.; Lee, J.; Yi, J.; Chung, Y.R. Retinal vascular occlusions in COVID-19 infection and vaccination: A literature review. Graefe’s Arch. Clin. Exp. Ophthalmol. 2023, 261, 1793–1808. [Google Scholar] [CrossRef]
- Antonelli, M.; Penfold, R.S.; Merino, J.; Sudre, C.H.; Molteni, E.; Berry, S.; Canas, L.S.; Graham, M.S.; Klaser, K.; Modat, M.; et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: A prospective, community-based, nested, case-control study. Lancet Infect. Dis. 2022, 22, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Timing and Spacing of Immunobiologics. General Best Practice Guidelines for Immunization. Vaccine Recommendations Guidelines of the ACIP. Available online: https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/timing.html (accessed on 2 September 2023).
- Wang, C.A.; Yeh, J.S.; Hong, C.Y. Repeated Coronary Artery Thrombosis after mRNA-1273 COVID-19 Vaccination. Acta Cardiol. Sin. 2022, 38, 793–795. [Google Scholar] [CrossRef]
- Alsmady, M.M.; Al-Qaryouti, R.A.; Sultan, N.G.; Khrais, O.I.; Khrais, H. Upper Limb Ischemia Due to Arterial Thrombosis after COVID-19 Vaccination. Case Rep. Med. 2022, 2022, 4819131. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.L.; Carlsen, J.F. New Trends in Vascular Imaging. Diagnostics 2021, 11, 112. [Google Scholar] [CrossRef]
- Peycheva, M.V.; Chervenkov, L.; Harizanova, Z.; Ahmed-Popova, F.; Zahariev, Z.I. Ultrasound fusion imaging system in neurology practice. Folia Medica 2022, 64, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tang, C. Novel study and applications of CT angiography derived fractional flow reserve in China. Zhonghua Yi Xue Za Zhi 2022, 102, 2563–2566. (In Chinese) [Google Scholar] [CrossRef]
- Cademartiri, F.; Casolo, G.; Clemente, A.; Seitun, S.; Mantini, C.; Bossone, E.; Saba, L.; Sverzellati, N.; Nistri, S.; Punzo, B.; et al. Coronary CT angiography: A guide to examination, interpretation, and clinical indications. Expert. Rev. Cardiovasc. Ther. 2021, 19, 413–425. [Google Scholar] [CrossRef]
- Evrev, D.; Sekulovski, M.; Gulinac, M.; Dobrev, H.; Velikova, T.; Hadjidekov, G. Retroperitoneal and abdominal bleeding in anticoagulated COVID-19 hospitalized patients: Case series and brief literature review. World J. Clin. Cases. 2023, 11, 1528–1548. [Google Scholar] [CrossRef]
- De Leucio, A.; De Jesus, O. MR Angiogram. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK558984/ (accessed on 10 July 2023).
- Cau, R.; Mantini, C.; Monti, L.; Mannelli, L.; Di Dedda, E.; Mahammedi, A.; Nicola, R.; Roubil, J.; Suri, J.S.; Cerrone, G.; et al. Role of imaging in rare COVID-19 vaccine multiorgan complications. Insights Imaging 2022, 13, 44. [Google Scholar] [CrossRef]
- Kim, E.-J.; Yoo, S.-J. Pulmonary Embolism after Vaccination with the COVID-19 Vaccine (Pfizer, BNT162b2): A Case Report. Vaccines 2023, 11, 1075. [Google Scholar] [CrossRef]
- Issakov, G.; Tzur, Y.; Friedman, T.; Tzur, T. Abnormal Uterine Bleeding Among COVID-19 Vaccinated and Recovered Women: A National Survey. Reprod. Sci. 2023, 30, 713–721. [Google Scholar] [CrossRef]
- Ljung, R.; Xu, Y.; Sundström, A.; Leach, S.; Hallberg, E.; Bygdell, M.; Larsson, M.; Arthurson, V.; Gisslén, M.; Gedeborg, R.; et al. Association between SARS-CoV-2 vaccination and healthcare contacts for menstrual disturbance and bleeding in women before and after menopause: Nationwide, register-based cohort study. BMJ 2023, 381, e074778. [Google Scholar] [CrossRef]
- Mahase, E. COVID-19: Unusual blood clots are “very rare side effect” of Janssen vaccine, says EMA. BMJ 2021, 373, n1046. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.F.; Calina, D.; Poulas, K.; Docea, A.O.; Tsatsakis, A.M. Safety of COVID-19 vaccines administered in the EU: Should we be concerned? Toxicol. Rep. 2021, 8, 871–879. [Google Scholar] [CrossRef]
- MacIntyre, C.R.; Veness, B.; Berger, D.; Hamad, N.; Bari, N. Thrombosis with Thrombocytopenia Syndrome (TTS) following AstraZeneca ChAdOx1 nCoV-19 (AZD1222) COVID-19 vaccination—A risk–benefit analysis for people <60 years in Australia. Vaccine 2021, 39, 4784–4787. [Google Scholar] [CrossRef]
- Dyer, O. COVID-19: EMA defends AstraZeneca vaccine as Germany and Canada halt rollouts. BMJ 2021, 373, n883. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, H.G.; Hadler, S.C.; Moulia, D. Use of COVID-19 vaccines after reports of adverse events among adult recipients of Janssen (Johnson & Johnson) and mRNA COVID-19 Vaccines (Pfizer-BioNTech and Moderna): Update from the Advisory Committee on Immunization Practices—United States, July 2021. MMWR 2021, 70, 1094–1099. [Google Scholar]
- Oliver, S.E.; Wallace, M.; See, I.; Mbaeyi, S.; Godfrey, M.; Hadler, S.C.; Jatlaoui, T.C.; Twentyman, E.; Hughes, M.M.; Rao, A.K.; et al. Use of the Janssen (Johnson & Johnson) COVID-19 Vaccine: Updated Interim Recommendations from the Advisory Committee on Immunization Practices—United States, December 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 90–95. [Google Scholar] [CrossRef]
- Kantarcioglu, B.; Patel, K.; Lewis, J.; Iqbal, O.; Siddiqui, F.; Jabeen, N.; Laddu, A.R.; Carter, C.A.; Fareed, J. Public Perceptions of Current COVID-19 Vaccinations. Results of a Pilot Survey. Clin. Appl. Thromb. 2021, 27. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, G.; Kabakchieva, P.; Miteva, D.; Batselova, H.; Velikova, T. Effectiveness and safety of COVID-19 vaccines in patients with diabetes as a factor for vaccine hesitancy. World J. Diabetes. 2022, 13, 738–751. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Naveen, R.; Houshmand, N.; Moghadam Kia, S.; Joshi, M.; Saha, S.; Jagtap, K.; Agarwal, V.; Nune, A.; Nikiphorou, E.; et al. Vaccine hesitancy decreases, long term concerns remain in myositis, rheumatic disease patients: A comparative analysis of the COVAD surveys. Rheumatology 2023, kead057, Epub ahead of print. [Google Scholar] [CrossRef]
- McMurry, R.; Lenehan, P.; Awasthi, S.; Silvert, E.; Puranik, A.; Pawlowski, C.; Venkatakrishnan, A.; Anand, P.; Agarwal, V.; O’Horo, J.C.; et al. Real-time analysis of a mass vaccination effort confirms the safety of FDA-authorized mRNA COVID-19 vaccines. Med 2021, 2, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNACovid-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef]
- Bikdeli, B.; Chatterjee, S.; Arora, S.; Monreal, M.; Jimenez, D.; Krumholz, H.M.; Goldhaber, S.Z.; Elkind, M.S.; Piazza, G. Cerebral Venous Sinus Thrombosis in the U.S. Population, After Adenovirus-Based SARS-CoV-2 Vaccination, and After COVID-19. J. Am. Coll. Cardiol. 2021, 78, 408–411. [Google Scholar] [CrossRef]
- Huh, K.; Na, Y.; Kim, Y.-E.; Radnaabaatar, M.; Peck, K.R.; Jung, J. Predicted and Observed Incidence of Thromboembolic Events among Koreans Vaccinated with ChAdOx1 nCoV-19 Vaccine. J. Korean Med. Sci. 2021, 36, e197. [Google Scholar] [CrossRef]
- Soliman, D.S.; Al Battah, A.; Al Faridi, D.; Ibrahim, F. Acquired Hemophilia A Developed Post COVID-19 Vaccine: An Extremely Rare Complication. J. Med. Cases. 2022, 13, 1–4. [Google Scholar] [CrossRef]
- Nowak, K.M.; Carpinteiro, A.; Szalai, C.; Saner, F.H. Acquired Hemophilia A: A Permanent Challenge for All Physicians. Medicines 2022, 9, 21. [Google Scholar] [CrossRef]
- Abrignani, M.G.; Murrone, A.; De Luca, L.; Roncon, L.; Di Lenarda, A.; Valente, S.; Caldarola, P.; Riccio, C.; Oliva, F.; Gulizia, M.M.; et al. COVID-19, Vaccines, and Thrombotic Events: A Narrative Review. J. Clin. Med. 2022, 11, 948. [Google Scholar] [CrossRef]
- Angeli, F.; Spanevello, A.; Reboldi, G.; Visca, D. Verdecchia PSARS-CoV-2 vaccines: Lights shadows. Eur. J. Intern. Med. 2021, 88, 1–8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).