Abstract
Alzheimer’s disease (AD) is an age-associated neurodegenerative disease; it is the most common cause of senile dementia. Klotho, a single-pass transmembrane protein primarily generated in the brain and kidney, is active in a variety of metabolic pathways involved in controlling neurodegeneration and ageing. Recently, many studies have found that the upregulation of Klotho can improve pathological cognitive deficits in an AD mice model and have demonstrated that Klotho plays a role in the induction of autophagy, a major contributing factor for AD. Despite the close association between Klotho and neurodegenerative diseases, such as AD, the underlying mechanism by which Klotho contributes to AD remains poorly understood. In this paper, we will introduce the expression, location and structure of Klotho and its biological functions. Specifically, this review is devoted to the correlation of Klotho protein and the AD phenotype, such as the effect of Klotho in upregulating the amyloid-beta clearance and in inducing autophagy for the clearance of toxic proteins, by regulating the autophagy lysosomal pathway (ALP). In summary, the results of multiple studies point out that targeting Klotho would be a potential therapeutic strategy in AD treatment.
1. Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disease and the most frequent cause of dementia worldwide [1,2]. According to the World Health Organization, the prevalence of AD has been increasing. It is estimated that over 50 million people in the world are afflicted with AD and the related form of senile dementia [3,4]. AD is initially associated with memory loss, but as the disease progresses, people with AD develop cognitive dysfunction, impulsive or unpredictable behavioral problems and abnormal personality changes [5,6,7,8,9]. Neurologically, AD is characterized by neuroinflammation, extracellular amyloid-beta (Aβ) plaque, and intracellular neurofibrillary tangle (NFT) deposition which are associated with a progressive cognitive decline and neurodegeneration [10,11,12,13,14,15]. Over the past 20 years, the amyloid cascade hypothesis and tau hypothesis have been the two most popular hypotheses used to explain AD pathogenesis [16,17,18]. The amyloid cascade hypothesis proposes that the excessive generation of Aβ plaques is derived from the cleavage of the amyloid precursor protein (APP) deposit in the hippocampus and basal segment, causing neuronal damage [18,19,20]. The tau hyperphosphorylation hypothesis proposes that the deposition of intracellular NFTs formed by hyperphosphorylated tau protein causes mitochondrial dysfunction, synaptic deficits and neuronal death, eventually leading to neurodegeneration and cognitive decline [21,22,23]. Apart from these two hypotheses, researchers are also exploring new theories to explain the etiology and pathogenesis of AD. These new theories include, for example, prion transmission, gamma oscillations, lysosome dysfunction, and calcium dysregulation. It is hoped that they will be able to better explain the pathological mechanism of AD, ultimately leading to a safe, and effective therapy for AD [6,7,13].
New evidence has raised the possibility that Klotho may be an anti-AD target protein. Klotho is a protein encoded by the Klotho gene, which is highly expressed in the kidney and the choroid plexus in the brain [24,25,26,27,28,29]. Previous studies showed that Klotho protein might take part in controlling oxidative stress, ER stress, Golgi apparatus stress, cell proliferation, apoptosis and autophagy [30,31,32,33]. Klotho is reported to be involved in numerous ageing-associated pathologies [34,35], such as cardiovascular disease, chronic kidney disease, cancer, and neurodegenerative disease [36,37]. Evidence has also demonstrated that Klotho expression levels are reduced in ageing brains, as well as in the brains of patients in the early stage of AD [37,38,39]. Our understanding of the Klotho protein and its function related to the progression of AD is far from complete. However, studies with an aged amyloidogenic mice model have demonstrated that the overexpression of Klotho protein in the brain can improve AD-like pathology and cognitive impairment, and reverse neuronal damage. It can also ameliorate Aβ accumulation in a mice model by regulating the Aβ-related transporters and microglia transformation (Table 1). In the early stages of AD, degradative pathways namely autophagy, a ubiquitin-proteasome system (UPS), and chaperone-mediated autophagy (CMA) are impaired [40]. More importantly, intracellular accumulation of Aβ causes dysfunction in the lysosome and autophagy lysosomal pathway (ALP), thereby leading to neuronal loss [41]. Recent studies have demonstrated that the expression of Klotho and autophagy are related in AD pathology. Klotho over-expression can promote ALP in AD via activating the beclin1 pathway [42].

Table 1.
Recent works on Klotho protein in Alzheimer’s disease (AD).
There is further evidence that Klotho expression promotes autophagy [44]. Autophagy is an essential pathway to maintain cellular homeostasis in eukaryotes [9,16]. It helps in degrading and removing a wide variety of substrates in the cells, from the senescence-related proteins to damaged organelles and even bacteria [37,44,45,46,47,48]. Autophagy is particularly important in neuronal cells because, as they age, they tend to accumulate intracellular toxicants and damaged organelles, which must be removed by autophagy [31,49]. Defects in autophagy have been shown to contribute to the pathogenic process of different diseases, including neurodegenerative diseases [50]. Several studies have shown a close relationship between autophagy and AD [51]. Autophagy is a key regulator in Aβ homeostasis [52,53]. It triggers the clearance of APP and APP cleavage products, for example, amyloid precursor protein-cleaved C-terminal fragments (APP-CTFs) and Aβ aggregates [54]. It is known that autophagy can remove Aβ plaques in the microglia through the autophagy receptor optineurin. In addition to inhibiting Aβ formation, the autophagy-lysosome pathway (ALP) can also suppress the formation of tau-enriched neurofibrillary tangles [54,55]. During AD progression, the continuous production of Aβ causes dysfunctional ALP [49]. For instance, Aβ affects the autophagolysosome maturation by interfering with the fusion of autophagosome and lysosome [49]. At the same time, Aβ blocks the retrograde movement of autophagolysosome toward the neuronal cell-body, which contributes to the accretion of the autophagic vacuoles in the brain [56]. As far as we know now, amyloid plaques and tau neurofibrillary tangles are still the main neuropathological changes in the AD patients’ brains [21]. A recent study has illustrated the co-relation between Aβ plaques/tau protein aggregation and autophagy, in AD models [54]. Autophagy malfunction is likely to facilitate the formation of Aβ and NFTs, resulting in the progression and aggravation of AD [56,57,58]. Thus, autophagy may take part in AD, and the autophagy mechanism may be a viable therapeutic target for treating AD [54,59]. Additionally, other studies have shown that Klotho protein can stimulate autophagy induction, leading to the facilitation of Aβ and NTFs clearance [60,61,62]. Therefore, Klotho protein might be a promising therapeutic target for treating AD. That is, it could undermine the development of AD-induced neuropathies via ameliorating Aβ and tau pathologies (Figure 1), at the same time inducing autophagy to protect neurons. Herein, we review the expression, location, structure, and the biological functions of Klotho; in particular, we include studies examining Klothos’ role in regulating Aβ clearance and autophagy activation.
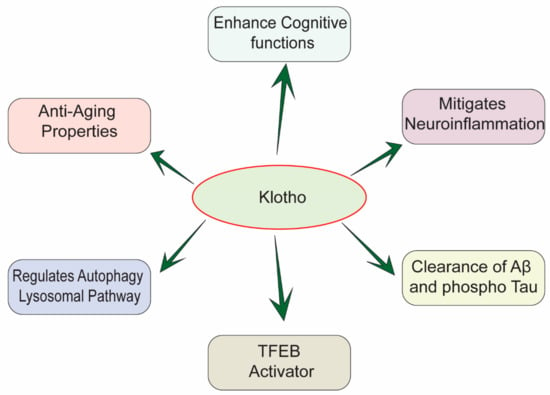
Figure 1.
Multifactorial effects of Klotho in the pathology of AD.
2. Expression, Structure and Function of Klotho Protein
The anti-ageing gene Klotho was discovered in 1997 by Kuro-o and his colleagues during their attempt to develop transgenic mice that overexpress rabbit type I sodium-proton exchanger (NHE-1). In the study, they produced transgenic mice by microinjection, and then mated the mice in order to obtain homozygous mice for examining phenotypic mutations. One of these mice exhibited an ageing-like phenotype and an overall shorter life span. This insertional mutation is now referred to as Klotho [63,64]. In Greek mythology, Klotho (Clotho) was the youngest daughter of Zeus and she spun the thread of life from her distaff onto her spindle, hence the name was given to the gene and protein, related to longevity.
The Klotho gene is located on chromosome 13q12. It is 50 kb long and consists of 5 exons and 4 introns with a molecular weight of about 130 kDa [65,66,67,68,69,70]. Both mouse and human Klotho gene produce two types of Klotho, the transmembrane form and the secreted form, through the alternative RNA splicing and ectodomain shedding of the membrane-bound Klotho by cell-surface protease (sheddases) [71,72]. Klotho gene-encoded type 1 single-pass transmembrane glycoprotein is situated in the cell membrane and Golgi apparatus. Of note, the internal domain is extremely short [72]. Lacking a functional domain, it consists of a larger extracellular domain with two internal repeats, KL1 and KL2 [29]. These repeats are cleaved by the sheddases, such as Beta-Secretase 1 (BACE1), A Disintegrin and Metalloproteinase Domain 10 (ADAM-10) and A Disintegrin and Metalloproteinase Domain 17 (ADAM-17), eventually generating a soluble form of Klotho which is released into the blood, cerebrospinal fluid and urine [65,73,74]. Loss of function in sheddases such as ADAM-17, and alpha-secretase leads to the accumulation of Aβ and a decrease in sheddases has been previously reported in AD [75,76]. Klotho is primarily found in the kidney and brain, but is also found in the skeletal muscle, lung, ovaries, urinary and testes [42,66]. Three types of Klotho protein have been distinguished: membrane, intracellular and secreted [66].
The membrane-bound Klotho plays a key role as a co-receptor, creating a complex with diverse fibroblast growth factor receptors (FGFRs). It has a selective ability to bind FGFRs to endocrine FGFs and to cooperate in their biological function [28,70]. For example, Klotho forms complexes with different FGFRs (FGFR1, FGFR3 and FGFR4), and has an increased affinity towards FGF23 [77,78]. FGF23 is a bone-derived phosphaturic hormone that helps in the control of energy and mineral metabolism; for example, it regulates the secretion of phosphate and the synthesis of vitamin D [26,69,79]. Most relevant here is that FGF23 acts on the Klotho-FGFRs complex and affects ageing [78,80]. The FGF23-Klotho system governs ageing through phosphate homeostasis regulation [78,81]. Emerging evidences demonstrate that premature ageing caused by the Klotho deficiency is related to an unbalanced phosphate metabolism [60]. Mice lacking FGF23 or Klotho exhibit the same phenotype, including hyperphosphatemia, hypervitaminosis D and premature ageing [80]. The activation of Klotho-FGFR complexes by FGF23 in the kidney helps reduce the phosphate level and consequently rescues the ageing-phenotype in FGF23-deficient or Klotho-deficient mice [80]. To be specific, FGF23 promotes the lowering of the sodium-dependent phosphate-transporter expression in the proximal tubules, to limit renal phosphate reabsorption [79,82]. Higher soluble αKlotho and a lower serum concentration of FGF23 has been reported to associate in older people and dementia patients [83]. FGF23 and αKlotho regulate the AD-induced neuroinflammation via the Wnt/β-catenin pathway [84].
FGF23 also plays a critical role in calcium homeostasis in the kidney. It activates calcium reabsorption and regulates calcium homeostasis through the transient receptor potential vanilloid-5 channel (TRPV5) in the renal distal tubules [26,36,69]. It is also known that Klotho can suppress inorganic phosphate reuptake in the renal proximal tubules via inhibiting the sodium-dependent phosphate co-transporter type-IIa (NaPi-IIa). Moreover, it downregulates the expression of 25-hydroxyvitamin D3 1α-hydroxylase (CYP27B1), the main enzyme responsible for the synthesis of active calcitriol, which stimulates intestinal and renal inorganic phosphate and calcium absorption [36,69,70].
The secreted form of Klotho has anti-ageing, organ protecting, neuroprotective, and anti-fibrosis functions [85]. It is known that the expression of Klotho decreases with age in mice and humans. Studies provide evidence that Klotho deficiency stimulates ageing and shortens lifespans, while Klotho overexpression prolongs the lifespan in organisms, including Caenorhabditis elegans, Drosophila, and mice [42,86,87]. Secreted Klotho can regulate oxidative stress and inflammation through inhibiting multiple signaling pathways of cytokines and growth factors, such as insulin, insulin-like growth factor 1 (IGF-1), transforming growth factor β (TGF-β), and Interferon γ (IFNγ) [33,69,70]. In vitro studies have demonstrated that the overexpression of Klotho can suppress autophosphorylation of insulin and the IGF-1 receptor, and decreases oxidative stress. Several research findings demonstrated that suppressing insulin and IGF-1 signaling increases the lifespan in animals [88,89]. The anti-ageing function of Klotho is mainly because of its inhibitory effect on insulin/IGF-1 signaling [67,79]. Klotho has a marked effect in suppressing tyrosine phosphorylation of insulin and the IGF-1 receptor, which leads to the reduction of the activity of insulin receptor substrates (IRS) 1 and 2, and their association with phosphatidylinositol 3-kinase (PI3K), eventually suppressing the insulin/IGF-1 signaling pathways. Overall, the Klotho protein appears to induce insulin/IGF-1 resistance and inhibit the activation of the insulin/IGF-1 receptor, thereby blocking the downstream signaling cascade, and thus prolonging the lifespan [67,87,88]. Additionally, Klotho has anti-inflammatory properties. It can reduce the expression of the pro-inflammatory cytokines, IFNγ and Tumor Necrosis Factor α (TNFα). IFNγ is a cytokine with pleiotropic roles in immune and inflammatory responses, tissue homeostasis and disease immunosurveillance [90,91]. TNFα is known as a ‘master regulator’ of the production of pro-inflammatory cytokines to stimulate inflammation [92]. It is known that high levels of IFNγ and TNFα establish a pro-inflammatory environment in the cell that enhances the inflammatory response. In contrast, Klotho stimulates anti-inflammatory cytokine production. To be specific, Klotho can stimulate the production of interleukin 10 (IL-10), which is responsible for inhibiting the expression of pro-inflammatory cytokines, such as TNFα, thereby attenuating an inflammatory response [93]. According to earlier evidence, Klotho-deficient mice show an augmentation of the IFNγ and TNFα signaling pathways [26,60,65]. The secreted Klotho (sKlotho) is also an endogenous anti-fibrosis protein derived from kidneys. Earlier studies in animal models suggests that a decrease in Klotho expression can enhance/worsen fibrosis in organs, including the heart, kidney, lungs, skin, etc. Meanwhile, the overexpression of Klotho can reverse fibrosis of the heart and kidney, which is mediated by sKlotho [73,80]. Thus, Klotho may function as an anti-ageing, organ-protective, neuroprotective, and an anti-fibrosis protein through regulating signaling of different growth factors and cytokines [73].
3. Klotho Inhibits Neuroinflammation, Promotes Aβ Clearance, and Mitigates Tau Pathology in Alzheimer’s Disease
3.1. Klotho Inhibits the NLRP3/Caspase-1 Pathway
Neuroinflammatory processes have been shown to participate in AD development and progression. In the immunological context, neuroinflammation associated with AD consists of increased secretion of pro-inflammatory cytokines, a reduction of regulatory T lymphocyte (Treg) activity, and the downregulation of immunological tolerance [94]. The key event in these neuroinflammatory processes is the activation of inflammasomes [23,95]. Inflammasomes are multiprotein complexes that not only provide a platform for inflammatory caspase recruitment, cleavage and activation, but also mediate pro-inflammatory cytokine secretion and maturation [96]. NLRP3 is one of the best-characterized inflammasome components that play a vital role in AD-associated neuroinflammation [96,97]. The aggregation of Aβ stimulates cerebral inflammation by activating microglia in AD mice model [98]. Toxic Aβ peptide is a well-known NLRP3 activator. NLRP3 inflammasome is a multimeric protein made up of NLRP3, an apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and cleaved caspase-1. NLRP3 is stimulated by Aβ deposition inside the microglia, where it stimulates the cleavage and activity of a cysteine protease, caspase-1. Activated caspase-1 is known as the main IL-1β converting enzyme that stimulates the downstream pro-inflammatory cytokines IL-1β secretion [10,25,96,99]. High production of IL-1β is associated with increasing AD pathogenesis. Researchers have detected an increased level of IL-1β in the cortex and hippocampus of AD patients [98]. IL-1β is called the ‘master regulator’ in the brain inflammatory cascade because it controls the expression of other pro-inflammatory cytokines, such as TNF-α [99,100]. Induction of IL-1β expression initiates the inflammatory signaling pathway leading to cell death, neuronal injury and neuroinflammation. IL-1β regulates the production of APP and further increases the Aβ burden and plaque deposition, eventually leading to the development of AD [100]. Conversely, it has been shown that an NLRP3 deficiency can suppress brain caspase-1 and IL-1β activation, which remarkably inhibits amyloidosis and neuropathology, and ultimately enhances cognitive function in the AD mice model. These results support an important role for the NLRP3/caspase-1 pathway in the pathogenesis of AD [23,94,95]. With regard to Klotho, current findings show that Klotho overexpression can remarkably inhibit NLRP3/caspase-1 signaling pathway activation. A high concentration of NLRP3, ASC and active caspase-1, has been shown in APP/PS1 transgenic mice [35,97]. However, these increased expressions were reversed in transgenic mice that overexpress Klotho [35]. Current findings have demonstrated that, in addition to Aβ aggregation, the activation of NLRP3 inflammasome is related to tau hyperphosphorylation. Activated NLRP3 can induce tau hyperphosphorylation and aggregation [10,23,96]. An elevated Klotho level can significantly decrease tau hyperphosphorylation in AD-transgenic mice [35]. Thus, a high expression of Klotho protein can effectively suppress the activation of the NLRP3/caspase-1 pathway (Figure 2), and thereby, in turn, reduce IL-1β secretion [35,36]. These findings explain how the inhibition of inflammatory events, particularly the reduction of Aβ deposition, can prevent neuronal cell damage and cognitive decline, and possibly protect against AD development.
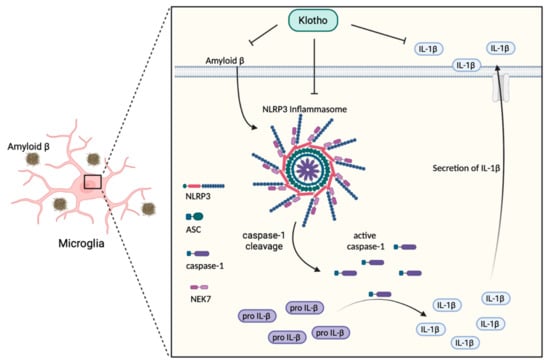
Figure 2.
Effects of Klotho on the NLRP3/caspase-1 signaling pathway in AD. Klotho inhibits the accumulation of amyloid β peptide which can affect microglia directly and stimulate the NLRP3/caspase-1 signaling in the brain. The black arrow represents the consequence of Aβ stimulation. NLRP3: Nucleotide-binding oligomerization domain leucine-rich repeat and pyrin domain-containing protein 3; ASC: Apoptosis-associated speck-like protein containing a caspase recruitment domain; pro IL-1β: inactive interleukin-1β precursor; IL-1β: interleukin-1β.
3.2. Klotho Promotes the Transformation of M1 Microglia to M2 Microglia
Microglia activation and accumulation in the brain, concentrated around the Aβ plaques, are prominent characteristics of AD. This suggests an involvement of microglia in the pathogenesis of AD [101,102]. Microglia are the resident myeloid cells that participate in maintaining neuronal connectivity and regulatory processes, both of which are critical for the development of the central nervous system (CNS). Microglia also participate in regulating cognitive functions and maintaining homeostasis by eliminating dying cells, misfolded protein and cellular debris [1,101,102]. Morphologically, microglia are classified as either resting or activated. The transformation of microglia from resting to the activated state is promoted by different extracellular cytokines or factors. There is evidence that activated microglia can be hazardous to neurons. For example, they regulate the engulfment of neuronal synapses, secrete inflammatory factors and exacerbate tau pathology [101,102,103]. Activated microglia have two distinct phenotypes: pro-inflammatory (M1) and anti-inflammatory (M2). M1 microglia release pro-inflammatory cytokines and cytotoxic substances that stimulate inflammatory responses, leading to neurotoxicity, Aβ deposition and neurodegeneration (Figure 3). In contrast, M2 microglia have a neuroprotective role in the CNS. They secrete trophic factors and anti-inflammatory cytokines that induce phagocytosis, increase the uptake and clearance of extracellular Aβ, and downregulate inflammation, thereby preserving neurons and protecting against cognitive dysfunction [101,102,103,104,105]. Of note, several studies have demonstrated that Aβ aggregation activates NLRP3 inflammasome, resulting in a high level of M1 microglia, microglial production of IL-1β and pro-inflammatory cytokines, eventually exacerbating AD pathogenesis [101,103,104]. The overexpression of Klotho has been reported to suppress neuroinflammation by inhibiting the NLRP3/caspase-1 pathway in amyloidosis mouse models [35,106]. In vivo studies have shown that the overexpression of Klotho in mouse models significantly inhibits NLRP3 and decreases the production of caspase-1 and IL-1β, subsequently promoting microglial differentiation to the anti-inflammatory M2 phenotype [35,106]. This scenario is consistent with the fact that Klotho overexpression can promote microglia differentiation in the brain. Klotho suppresses NLRP3 activation and stimulates M1 microglia to differentiate into M2 microglia, eventually inducing protective microglial activities and Aβ clearance through micropinocytosis and phagocytosis. This limits AD progression [101,102,103,106]. In other words, Klotho overexpression can ameliorate Aβ deposition, synaptic loss and cognitive dysfunction through restraining the NLRP3/caspase 1 signaling pathway. This promotes the microglia transformation that clears Aβ.
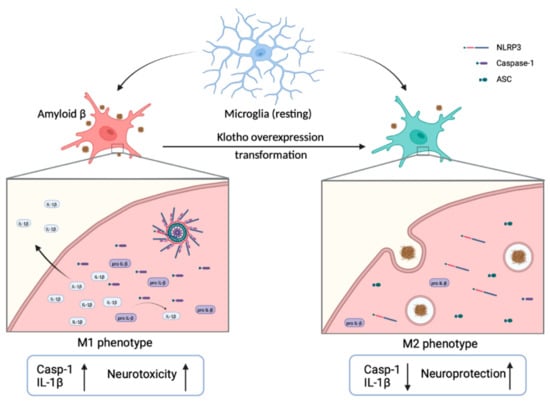
Figure 3.
Transformation of microglia with the overexpression of Klotho. Microglia are usually in a resting state in the brain. Once activated, they show either M1 or M2 phenotypes. In the activated M1 phenotype, the expression of caspase-1 and IL-1β is enhanced, which stimulate a pro-inflammatory response and cause neurotoxicity. In contrast, when Klotho is overexpressed, M1 microglia are differentiated into M2 microglia. The secretion of caspase-1 and IL-1β is decreased, thereby facilitating Aβ clearance, preserving neurons and protecting against cognitive dysfunction.
3.3. Klotho Promote Aβ Transporter-Mediated Aβ Clearance
In addition to promoting microglia mediated Aβ clearance, an excessive expression of Klotho protein can also eliminate Aβ through regulating the expression of the Aβ transporter. It is known that Aβ is central to AD pathology; the relative levels and distribution of Aβ in the brain affect the development and progression of AD [7,15,34,107]. Continuous Aβ clearance in the CNS through the blood–brain barrier (BBB) and the blood–CSF barrier is important in preventing Aβ accumulation, thereby influencing disease progression. In the interstitial fluid (ISF), the concentration of Aβ is strongly regulated by the APP. The major transport mechanism of Aβ across the BBB is through different kinds of receptors. These include the transporters that transport Aβ into the brain, such as the receptor for advanced glycation end products (RAGE) and ATP-binding cassette transporter A1 (ABCA1). They also include transporters that transport Aβ out of the brain, such as low-density lipoprotein receptor-related protein-1 (LRP1) and P-glycoprotein (P-gp) [12,48,107,108,109]. The expression of Aβ transporter in the brain is important in regulating the specific receptor-mediated transport mechanism, which controls the circulation of Aβ that enters or diffuses out of the brain. Several findings have shown that when pathogenic Aβ deposits in the AD brain, the mRNA and protein levels of RAGE and ABCA1 are highly upregulated in the cerebral vessels and the brain of the transgenic mice model of β-amyloidosis, in which Aβ is facilitated to enter the brain via the BBB. At the same time, the mRNA and protein levels of LRP1 and P-gp are downregulated in the AD brain and cerebral vessels [35,107,110]. This reduces Aβ clearance and promotes Aβ deposition in the cerebrovascular tissue. It is important to notice that according to recent research the overexpression of the Klotho protein can facilitate Aβ transporter-mediated Aβ clearance by regulating the expression of Aβ-related transporters such as LRP1 and P-gp. Moreover, Klotho upregulates soluble LRP (sLRP) that has the potential to sequester extracellular Aβ, and promote its degradation in the periphery. With Klotho upregulation, the expression of the Aβ-efflux transporter is upregulated, and the Aβ-influx transporter is significantly attenuated in the cerebral vessels [35]. Thus, Aβ is rapidly cleared across the BBB, from the brain interstitial fluid into the blood, eventually, reducing inflammation and improving the recovery of cognitive function in the transgenic AD model.
3.4. Klotho Mitigates Tau Pathology and Enhances Cognitive Function in AD
The Tau protein stabilizes and maintains the integrity and structure of microtubules in the neuronal cells [15]. In AD, the Tau protein is hyperphosphorylated and aggregated intracellularly in the neurons, leading to neuronal damage and autophagy dysfunction [14]. Therefore, the increasing symptoms associated to Tau pathology in AD has raised a lot of questions to find new therapeutic targets which can subside the Tau pathogenesis and enhance cognitive function. Klotho has been reported to have close relation with aging related abnormalities and the autophagy pathway in AD [66]. Recent studies have reported that the regulation of the activity and expression level of the Klotho protein may be a potential therapeutic target against AD and tau pathology [51,73]. Tau protein has been reported to jointly work with kinesin adaptors for the transport of cargos, such as autophagosomes and lysosomes in the neurons during the anterograde and retrograde transport of cell organelles [53]. Therefore, the disruption of autophagy and autophagosome transport may be influenced by the hyperphosphorylated Tau in AD [111]. Thus, promoting or the induction of ALP in neurons may clear the production of Tau aggregates. A recent study has illustrated that Klotho regulates the induction of autophagy and ALP in neurons which supports the regulation of the intracellular process, and the Tau protein functions [34]. Another recent study illustrated that Klotho-VS heterozygosity in AD patients showed less Tau pathology symptoms and enhanced cognitive functions [112]. This study clearly demonstrated that neuroprotective effect of Klotho by decreasing Tau-related symptoms, and the PET imaging showed less intensity of Tau symptoms compared to Aβ pathology and reduced the cognitive impairment [112]. Taken together Klotho plays a concomitant role in the regulation of the intracellular process in neurons, and mitigates Tau pathogenesis by inducing autophagy and lysosome transport functions in AD pathology.
4. Klotho Acts as an Autophagy Inducer
4.1. Klotho Stimulates the Formation of UNC51-like Kinase-1 (ULK1) Complex
Autophagy is stimulated by AMP-activated protein kinase (AMPK), which is an energy sensor responsible for regulating cellular metabolism to maintain energy homeostasis [48,68]. In contrast, autophagy is suppressed by the p38 mitogen-activated protein kinase (MAPK) and the mammalian target of rapamycin (mTOR). mTOR is known as the major inhibitory signal of autophagy [11,47,57,113,114]. Extensive works have established that yeast Autophagy Related 1 Homolog (ATG1) kinase is a key regulator in autophagy stimulation; it complexes with ATG13 and ATG17 during autophagy. These proteins are needed for autophagy induction and autophagosome generation [48,57]. Mammalian homologs of ATG1 have been identified as Unc-51 Like Autophagy Activating Kinase 1 (ULK1) and Unc-51 Like Autophagy Activating Kinase 2 (ULK2) [50,57,58]. Mammalian counterparts of ATG13 and ATG17 are reported as mATG13 and a focal adhesion kinase family-interacting protein of 200 kDa (FIP200), respectively. ULK1 is a serine/threonine-protein kinase that plays a crucial role in autophagy induction. It interacts with FIP200 and ATG13 to form the ULK1/ATG13/FIP200 complex, which integrates autophagy signals into forming autophagosome. ATG13 and FIP200 are the autophagy-essential binding partner of ULK1. These proteins help regulate ULK1 kinase activity and correct its localization in the pre-autophagosome [46,50,57,62]. Recent work has shown that Klotho protein may have a role in autophagy induction by promoting the ULK1 complex formation [62]. As described previously, the initiation of autophagy is highly regulated by the ULK1-ATG13-FIP200 complex. It integrates the signals from upstream sensors, for example, p38 MAPK and mTOR, followed by the formation and degradation of the autophagosome. Autophagy induction is inhibited when the ULK1 complex formation is blocked. However, there is evidence that, under stress conditions, Klotho protein can stimulate the ULK1 complex formation and thus induce autophagy (Figure 4). Klotho silencing increases mTOR phosphorylation and is accompanied by the inactivation of AMPK. The activated mTOR attenuate ATG13 activation, thus, prevents the formation of the ULK1 complex and also inhibits the complex activity involved in autophagosome formation [45,61,62,113]. Therefore, those cells that could not induce the initial stage of autophagy were consequently accelerated to undergo apoptotic cell death [11,48,57,62]. Klotho protein may play a role in activating the ULK1/ATG13/FIP200 complex formation by mediating mTOR signaling [109,115], by activating ATG13, and by increasing ULK1 phosphorylation by AMPK [74,116]. As a consequence, the induction of the autophagy process suppresses the development and progression of AD by eliminating the APP plaques and inhibiting the neurofibrillary tangles formation [34,39,47].
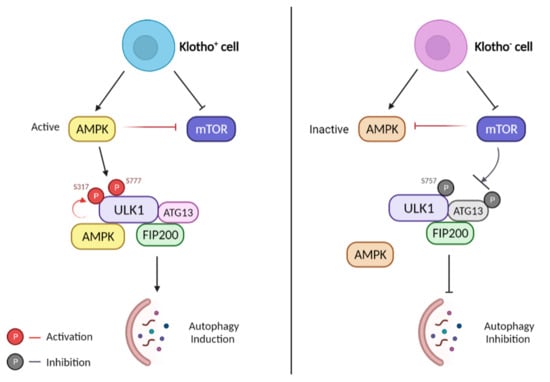
Figure 4.
Role of Klotho protein in ULK1-induced autophagy. Left: Klotho overexpression. AMPK is active and mTOR is inhibited by AMPK and Klotho protein. ULK1 is subsequently phosphorylated by active AMPK at sites Ser 317 and 777. The complex of ULK1/ATG13/FIP200 is formed and autophagy is promoted. Right: Klotho deficiency. AMPK becomes inactive while mTOR becomes active. Activated mTOR phosphorylates ULK1 on Ser757, preventing ULK1 from interacting with AMPK, suppressing the formation of the ULK1 complex, and inhibiting autophagy.
4.2. Klotho Inhibits the IGF-1/PI3K/Akt/mTOR Pathway
Recent work has indicated that autophagy induction is a valid strategy to promote and sustain the survival of neuronal cells and clear abnormal protein aggregates from the brain [56,113]. It is acknowledged that autophagy disruption has a critical role in neurodegenerative disorders. Defects in the elimination of toxic and abnormal protein promotes cellular stress, and death [34,62,113]. The progression of autophagy involves a variety of signaling pathways. The PI3K/Akt/mTOR signaling pathway is one that has been widely examined. Insulin-like growth factors (IGF-1) which are mediated by the extracellular IGF-1 receptor (IGF-1R), function to enhance the survival and proliferation of specific tissues through activating the PI3K/Akt/mTOR signaling pathway [113,117]. In addition, the PI3K/Akt/mTOR signaling pathway takes a central role in maintaining cell viability, controlling cell growth and regulating autophagy [11,117,118,119]. PI3K/Akt is one of the upstream kinases that mediates the activity of mTOR. Studies have shown that the Akt and PI3K phosphorylation causes mTOR hyperactivation in AD patients [45]. It is known that PI3K/Akt is influenced by insulin or IGF-1, which is responsible for autophagy, actin cytoskeleton, and protein synthesis through the activity of mTOR [11,33]. It was reported that the inhibition of PI3K activity can remarkably suppress the downstream signaling of Akt and mTOR pathways. Downregulated mTOR activity stimulates autophagy by activating autophagy-related proteins, thus triggering the clearance of misfolded proteins [114,118]. Klotho influences IGF-1/PI3K/Akt/mTOR signaling regulation. Several studies have illustrated that Klotho can suppress the insulin/IGF-1 signaling pathway [26,27,61,69]. In vivo and in vitro assays have shown that the upregulation of Klotho results in an inhibition of IGF-1/PI3K/Akt/mTOR signaling, which suggests that the Klotho protein may affect PI3K/Akt/mTOR signaling regulation [34,43]. At the same time, it is proved that ligustilide (LIG)-induced Klotho overexpression can inhibit the insulin/IGF-1/PI3K/Akt signaling pathway, thereby suppressing the FoxO phosphorylation. Downregulated FoxO phosphorylation then results in the transcription of FoxO, consequently decreasing oxidative stress in the brain [27]. A Klotho deficiency is associated with a significant increase in IGF-1 phosphorylation, which enhances Akt and mTOR phosphorylation. It is likely that a deficiency of the Klotho protein would induce the enhancement of mTOR signaling, in turn, suppressing autophagy [39,43]. The upregulation of the Klotho protein has an effect on the suppression of the IGF-1/PI3K/Akt/mTOR signaling pathway, thereby facilitating Aβ clearance via sustained autophagy.
4.3. Klotho Promotes Nuclear Translocation of TFEB
Transcription factor EB (TFEB) belongs to the microphthalmia/transcription factor E (MiT/TFE) family [44,120,121,122,123,124]. There is abundant evidence that TFEB inhibits toxic and abnormal protein accumulation in AD cell and mouse models, and, in turn, improves behavioral deficits, cognitive impairment, and the mitigation of neurodegeneration [53,123,125]. TFEB regulates autophagy and lysosomal biogenesis by binding to the Coordinated Lysosomal Expression and Regulation (CLEAR) motif. Moreover, TFEB activation can regulate cellular metabolism, increase the number of lysosomes and induce lysosome-mediated degradation of toxic proteins by regulating the CLEAR genes [121,122,124,126,127]. For instance, some genes take part in the initiation of autophagy (BECN1, ATG9B and WIPI1), while others take part in autophagosome elongation (ATG5 and MAP1LC3B), substrate recognition, autophagosome docking and fusion with lysosomes (UVRAG and RAB7) [44,120,121,122]. The Klotho protein has been reported to have an effect on autophagy induction by promoting TFEB nuclear translocation and increasing TFEB-mediated lysosomal gene transcription [44,123]. mTORC1 and glycogen synthase kinase 3β (GSK3β) are classified as the kinases potentially responsible for TFEB phosphorylation in most of the cell-types, thereby preventing the nuclear translocation of TFEB [120]. One report has indicated that the Klotho protein has a role in inhibiting mTOR activity, as well as suppressing the phosphorylation of TFEB [39]. When TFEB is dephosphorylated, it quickly translocates into the nucleus, and promotes the transcription of genes necessary for autophagy and lysosomal biogenesis (Figure 5). To date, studies have also shown that Klotho promotes the inactivation of GSK3β, which in-turn leads to the dephosphorylation and nucleus localization of TFEB [32]. In an experimental mouse model, it was reported that mice treated with recombinant mouse Klotho (rKlotho) had an increased level of TFEB expression when compared to the wild type [44]. This suggests that Klotho can stimulate the nuclear translocation of TFEB, and TFEB translocation then promotes the lysosomal gene transcription. Hence, the lysosomal function improved by the Klotho protein facilitates the autophagy clearance of autophagosomes in a tacrolimus-induced renal injury mouse model [32,44]. Collectively, these findings highlight the role of the Klotho protein in improving autophagy clearance and lysosomal function by promoting TFEB translocation and increasing TFEB-mediated lysosomal gene transcription through inhibiting the kinase activity of mTORC1 and GSK3β.
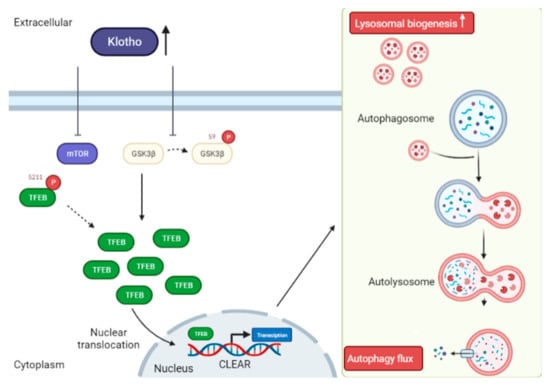
Figure 5.
Mechanism of Klotho activity in autophagy induction through TFEB nuclear translocation. Soluble Klotho (sKlotho) or recombinant mouse Klotho (rKlotho) inhibits mTOR (S211) activity, thereby preventing TFEB phosphorylation resulting in nuclear translocation and activation of the transcription factor EB (TFEB). sKlotho or rKlotho also represses glycogen synthase kinase 3β (GSK3β) (S9) phosphorylation to trigger TFEB nuclear translocation. Improved TFEB-mediated lysosomal gene transcription by sKlotho increases lysosomal biogenesis and eventually enhances autophagy flux.
5. Conclusions
Aging is a physiological change that occurs naturally, and mechanistically aging is developed by some main regulators, such as Klotho [128]. AD is developed due to the consequences of accelerated aging and some genetic mutational events with its own entity [128]. AD could be prevented or cured by targeting Klotho or can be achieved by healthy aging in a natural discipline [128]. Thus, developing therapeutic strategies to reduce cerebral Aβ deposition through repressing its generation and/or strengthening its clearance is critical for inhibiting AD progression [128]. Several lines of evidence support that Klotho may be a promising target for AD treatment. Its potential therapeutic value derives from its abilities to improve AD pathogenesis to reduce cognitive deficits and to stimulate autophagy. Klotho upregulation can improve Aβ clearance in an AD transgenic mice model, thereby alleviating cognition impairment and enhancing neuroprotection [128]. The addition of the Klotho protein can stimulate the ULK1 complex formation, TFEB nuclear translocation, as well as inhibit the IGF-1/PI3K/Akt/mTOR signaling pathway, which are all involved in autophagy activation. The mechanism of the Klotho protein involvement in AD has not been elucidated. Advancements in research on Klotho will undoubtedly bring a better understanding of its function and its mechanisms. As we have described above, accumulating evidence has shown that Klotho plays a neuroprotective function in AD. However, more deep mechanistic studies involving AD and autophagy remain elusive. The research reviewed here suggests that regulating the activity and expression level of the Klotho protein may be a potential therapeutic target against AD. Further studies are needed to explore this possibility of how Klotho is involved in autophagy processes. Moreover, other forms of autophagy and its clearance mechanisms in neurodegenerative diseases are still unknown. In future these questions need to be answered with many more research studies.
Author Contributions
The research study was conceived and conceptualized by: M.L., A.I. and H.L.X.W.; drafting the manuscript, literature collection, methodology and editing the manuscript: T.Y.F., A.I., S.G.S., S.K., X.-J.G., Z.Z., C.-F.S., J.L., Y.K. and Y.Z. Helped organize the manuscript and provided the technical support T.Y.F., A.I., S.G.S., S.K., X.-J.G., Z.Z., C.-F.S., J.L., Y.K. and Y.Z. Reviewed and edited the paper A.I., T.Y.F., S.G.S., H.L.X.W. and M.L.; funding acquisition and resources M.L., A.I. and H.L.X.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Shenzhen Science and Technology Innovation Commission (JCYJ20180302174028790, JCYJ20180507184656626), Hong Kong Baptist University (HKBU/RC-IRCs/17-18/03, IRCMS/19-20/H02), the National Natural Science Foundation of China (81703487, and 81773926), the Hong Kong Health and Medical Research Fund (HMRF17182541, HMRF17182551), and research funds from the Hong Kong General Research Fund (GRF/HKBU12101417, GRF/HKBU12100618).
Acknowledgments
We would like to thank Carol Chu for her enormous support in ordering reagents and managing our lab needs. We would also like to thank Martha Dahlen for the English editing.
Conflicts of Interest
The authors have declared that there are no conflict of interest.
Abbreviations
Alzheimer’s disease (AD), amyloid-beta (Aβ), neurofibrillary tangle (NFT), amyloid precursor protein (APP), autophagy lysosomal pathway (ALP), Ligustilide (LIG), Forkhead-box class O (FoxO), soluble APPα (sAPPα), soluble Klotho (sKL), amyloid precursor protein-cleaved C-terminal fragment (APP-CTFs), rabbit type I sodium-proton exchanger (NHE-1), fibroblast growth factor receptors (FGFRs), transient receptor potential vanilloid-5 channel (TRPV5), sodium-dependent phosphate co-transporter type-IIa (NaPi-IIa), insulin-like growth factor 1 (IGF-1), transforming growth factor β (TGF-β), Interferon γ (IFNγ), phosphatidylinositol 3-kinase (PI3K), interleukin 10 (IL-10), secreted or soluble Klotho (sKlotho), Apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), central nervous system (CNS), pro-inflammatory (M1), anti-inflammatory (M2), blood–brain barrier (BBB), interstitial fluid (ISF), receptor for advanced glycation end products (RAGE), ATP-binding cassette transporter A1 (ABCA1), low-density lipoprotein receptor-related protein-1 (LRP1), P-glycoprotein (P-gp), soluble LRP (sLRP), UNC51-like kinase-1 (ULK1), AMP-activated protein kinase (AMPK), p38 mitogen-activated protein kinase (MAPK), mammalian target of rapamycin (mTOR), insulin-like growth factors (IGF-1), transcription factor EB (TFEB), Coordinated Lysosomal Expression and Regulation (CLEAR) motif, glycogen synthase kinase 3β (GSK3β), recombinant mouse Klotho (rKlotho).
References
- Bolos, M.; Perea, J.R.; Avila, J. Alzheimer’s disease as an inflammatory disease. Biomol. Concepts 2017, 8, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Iyaswamy, A.; Krishnamoorthi, S.K.; Song, J.X.; Yang, C.B.; Kaliyamoorthy, V.; Zhang, H.; Sreenivasmurthy, S.G.; Malampati, S.; Wang, Z.Y.; Zhu, Z.; et al. NeuroDefend, a novel Chinese medicine, attenuates amyloid-beta and tau pathology in experimental Alzheimer’s disease models. J. Food Drug Anal. 2020, 28, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Frisoni, G.B.; Altomare, D.; Thal, D.R.; Ribaldi, F.; van der Kant, R.; Ossenkoppele, R.; Blennow, K.; Cummings, J.; van Duijn, C.; Nilsson, P.M.; et al. The probabilistic model of Alzheimer disease: The amyloid hypothesis revised. Nat. Rev. Neurosci. 2022, 23, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Salloway, S.; Chalkias, S.; Barkhof, F.; Burkett, P.; Barakos, J.; Purcell, D.; Suhy, J.; Forrestal, F.; Tian, Y.; Umans, K.; et al. Amyloid-Related Imaging Abnormalities in 2 Phase 3 Studies Evaluating Aducanumab in Patients With Early Alzheimer Disease. JAMA Neurol. 2022, 79, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Liu, J.; Zhu, Z.; Su, C.F.; Sreenivasmurthy, S.G.; Iyaswamy, A.; Lu, J.H.; Chen, G.; Song, J.X.; Li, M. Traditional Chinese medicine compounds regulate autophagy for treating neurodegenerative disease: A mechanism review. Biomed. Pharmacother. 2021, 133, 110968. [Google Scholar] [CrossRef] [PubMed]
- Bondi, M.W.; Edmonds, E.C.; Salmon, D.P. Alzheimer’s Disease: Past, Present, and Future. J. Int. Neuropsychol. Soc. 2017, 23, 818–831. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Mao, C.; Hu, X.; Zhang, S.; Yang, Z.; Hu, Z.; Sun, H.; Fan, Y.; Dong, Y.; Yang, J.; et al. New Insights Into the Pathogenesis of Alzheimer’s Disease. Front. Neurol. 2019, 10, 1312. [Google Scholar] [CrossRef] [PubMed]
- Malampati, S.; Song, J.X.; Tong, B.C.-T.; Nalluri, A.; Yang, C.B.; Wang, Z.; Sreenivasmurthy, S.G.; Zhu, Z.; Liu, J.; Su, C.; et al. Targeting Aggrephagy for the Treatment of Alzheimer’s Disease. Cells 2020, 9, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Yang, C.; Iyaswamy, A.; Krishnamoorthi, S.; Sreenivasmurthy, S.G.; Liu, J.; Wang, Z.; Tong, B.C.; Song, J.; Lu, J.; et al. Balancing mTOR Signaling and Autophagy in the Treatment of Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 728. [Google Scholar] [CrossRef] [Green Version]
- Ising, C.; Venegas, C.; Zhang, S.; Scheiblich, H.; Schmidt, S.V.; Vieira-Saecker, A.; Schwartz, S.; Albasset, S.; McManus, R.M.; Tejera, D.; et al. NLRP3 inflammasome activation drives tau pathology. Nature 2019, 575, 669–673. [Google Scholar] [CrossRef]
- Jung, C.H.; Ro, S.H.; Cao, J.; Otto, N.M.; Kim, D.H. mTOR regulation of autophagy. FEBS Lett. 2010, 584, 1287–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanekiyo, T.; Cirrito, J.R.; Liu, C.C.; Shinohara, M.; Li, J.; Schuler, D.R.; Shinohara, M.; Holtzman, D.M.; Bu, G. Neuronal clearance of amyloid-beta by endocytic receptor LRP1. J. Neurosci. 2013, 33, 19276–19283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocahan, S.; Dogan, Z. Mechanisms of Alzheimer’s Disease Pathogenesis and Prevention: The Brain, Neural Pathology, N-methyl-D-aspartate Receptors, Tau Protein and Other Risk Factors. Clin. Psychopharmacol. Neurosci. 2017, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Hu, N.; Tan, M.S.; Yu, J.T.; Tan, L. Behavioral and psychological symptoms in Alzheimer’s disease. Biomed. Res. Int. 2014, 2014, 927804. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, E.; Rajmohan, V.; Raghunath, B. Neurobiology of Alzheimer’s disease. Indian J. Psychiatry 2009, 51, 55–61. [Google Scholar] [CrossRef]
- Yang, C.; Cai, C.Z.; Song, J.X.; Tan, J.Q.; Durairajan, S.S.K.; Iyaswamy, A.; Wu, M.Y.; Chen, L.L.; Yue, Z.; Li, M.; et al. NRBF2 is involved in the autophagic degradation process of APP-CTFs in Alzheimer disease models. Autophagy 2017, 13, 2028–2040. [Google Scholar] [CrossRef]
- Ng, R.C.; Jian, M.; Ma, O.K.; Bunting, M.; Kwan, J.S.; Zhou, G.J.; Senthilkumar, K.; Iyaswamy, A.; Chan, P.K.; Li, M.; et al. Chronic oral administration of adipoRon reverses cognitive impairments and ameliorates neuropathology in an Alzheimer’s disease mouse model. Mol. Psychiatry 2021, 26, 5669–5689. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Chan, H.N.; Ashok, I.; Krishnamoorthi, S.K.; Li, M.; Li, H.W.; Wong, M.S. Amyloid-beta oligomer targeted theranostic probes for in vivo NIR imaging and inhibition of self-aggregation and amyloid-beta induced ROS generation. Talanta 2021, 224, 121830. [Google Scholar] [CrossRef]
- Lehrer, S.; Rheinstein, P.H. Alignment of Alzheimer’s disease amyloid beta-peptide and klotho. World Acad. Sci. J. 2020, 2, 27. [Google Scholar] [CrossRef]
- Tang, B.L. Neuronal protein trafficking associated with Alzheimer disease: From APP and BACE1 to glutamate receptors. Cell Adhes. Migr. 2009, 3, 118–128. [Google Scholar] [CrossRef] [Green Version]
- Sreenivasmurthy, S.G.; Iyaswamy, A.; Krishnamoorthi, S.; Senapati, S.; Malampati, S.; Zhu, Z.; Su, C.F.; Liu, J.; Guan, X.J.; Tong, B.C.; et al. Protopine promotes the proteasomal degradation of pathological tau in Alzheimer’s disease models via HDAC6 inhibition. Phytomedicine 2022, 96, 153887. [Google Scholar] [CrossRef] [PubMed]
- Iyaswamy, A.; Krishnamoorthi, S.K.; Liu, Y.W.; Song, J.X.; Kammala, A.K.; Sreenivasmurthy, S.G.; Malampati, S.; Tong, B.C.K.; Selvarasu, K.; Cheung, K.H.; et al. Yuan-Hu Zhi Tong Prescription Mitigates Tau Pathology and Alleviates Memory Deficiency in the Preclinical Models of Alzheimer’s Disease. Front. Pharmacol. 2020, 11, 584770. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.S.; Yu, J.T.; Jiang, T.; Zhu, X.C.; Tan, L. The NLRP3 inflammasome in Alzheimer’s disease. Mol. Neurobiol. 2013, 48, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Dias, G.P.; Murphy, T.; Stangl, D.; Ahmet, S.; Morisse, B.; Nix, A.; Aimone, L.J.; Aimone, J.B.; Kuro, O.M.; Gage, F.H.; et al. Intermittent fasting enhances long-term memory consolidation, adult hippocampal neurogenesis, and expression of longevity gene Klotho. Mol. Psychiatry 2021, 26, 6365–6379. [Google Scholar] [CrossRef] [PubMed]
- Halle, A.; Hornung, V.; Petzold, G.C.; Stewart, C.R.; Monks, B.G.; Reinheckel, T.; Fitzgerald, K.A.; Latz, E.; Moore, K.J.; Golenbock, D.T. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 2008, 9, 857–865. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Hwang, K.H.; Park, K.S.; Kong, I.D.; Cha, S.K. Biological Role of Anti-aging Protein Klotho. J. Lifestyle Med. 2015, 5, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Kuang, X.; Chen, Y.S.; Wang, L.F.; Li, Y.J.; Liu, K.; Zhang, M.X.; Li, L.J.; Chen, C.; He, Q.; Wang, Y.; et al. Klotho upregulation contributes to the neuroprotection of ligustilide in an Alzheimer’s disease mouse model. Neurobiol. Aging 2014, 35, 169–178. [Google Scholar] [CrossRef]
- Mytych, J.; Solek, P.; Bedzinska, A.; Rusinek, K.; Warzybok, A.; Tabecka-Lonczynska, A.; Koziorowski, M. Towards Age-Related Anti-Inflammatory Therapy: Klotho Suppresses Activation of ER and Golgi Stress Response in Senescent Monocytes. Cells 2020, 9, 261. [Google Scholar] [CrossRef] [Green Version]
- Semba, R.D.; Moghekar, A.R.; Hu, J.; Sun, K.; Turner, R.; Ferrucci, L.; O’Brien, R. Klotho in the cerebrospinal fluid of adults with and without Alzheimer’s disease. Neurosci. Lett. 2014, 558, 37–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mytych, J.; Solek, P.; Bedzinska, A.; Rusinek, K.; Warzybok, A.; Tabecka-Lonczynska, A.; Koziorowski, M. Klotho-mediated changes in the expression of Atg13 alter formation of ULK1 complex and thus initiation of ER- and Golgi-stress response mediated autophagy. Apoptosis 2020, 25, 57–72. [Google Scholar] [CrossRef]
- Mytych, J.; Solek, P.; Koziorowski, M. Klotho modulates ER-mediated signaling crosstalk between prosurvival autophagy and apoptotic cell death during LPS challenge. Apoptosis 2019, 24, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Lim, S.W.; Quan, Y.; Cui, S.; Shin, Y.J.; Ko, E.J.; Chung, B.H.; Yang, C.W. Role of Klotho in Chronic Calcineurin Inhibitor Nephropathy. Oxid. Med. Cell. Longev. 2019, 2019, 1825018. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Gurnani, P.; Nandi, A.; Kurosu, H.; Miyoshi, M.; Ogawa, Y.; Castrillon, D.H.; Rosenblatt, K.P.; et al. Regulation of oxidative stress by the anti-aging hormone klotho. J. Biol. Chem. 2005, 280, 38029–38034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, C.Y.; Yang, T.T.; Zhou, H.J.; Zhao, Y.; Kuang, X.; Duan, W.; Du, J.R. Lentiviral vector-mediated overexpression of Klotho in the brain improves Alzheimer’s disease-like pathology and cognitive deficits in mice. Neurobiol. Aging 2019, 78, 18–28. [Google Scholar] [CrossRef]
- Zhao, Y.; Zeng, C.Y.; Li, X.H.; Yang, T.T.; Kuang, X.; Du, J.R. Klotho overexpression improves amyloid-beta clearance and cognition in the APP/PS1 mouse model of Alzheimer’s disease. Aging Cell 2020, 19, e13239. [Google Scholar] [CrossRef]
- Zhu, L.; Stein, L.R.; Kim, D.; Ho, K.; Yu, G.Q.; Zhan, L.; Larsson, T.E.; Mucke, L. Klotho controls the brain-immune system interface in the choroid plexus. Proc. Natl. Acad. Sci. USA 2018, 115, E11388–E11396. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Sun, Z. Autophagy plays a critical role in Klotho gene deficiency-induced arterial stiffening and hypertension. J. Mol. Med. 2019, 97, 1615–1625. [Google Scholar] [CrossRef]
- Li, D.; Jing, D.; Liu, Z.; Chen, Y.; Huang, F.; Behnisch, T. Enhanced Expression of Secreted alpha-Klotho in the Hippocampus Alters Nesting Behavior and Memory Formation in Mice. Front. Cell. Neurosci. 2019, 13, 133. [Google Scholar] [CrossRef]
- Lin, Y.; Kuro-o, M.; Sun, Z. Genetic deficiency of anti-aging gene klotho exacerbates early nephropathy in STZ-induced diabetes in male mice. Endocrinology 2013, 154, 3855–3863. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Rodriguez, M.; Villar-Conde, S.; Astillero-Lopez, V.; Villanueva-Anguita, P.; Ubeda-Banon, I.; Flores-Cuadrado, A.; Martinez-Marcos, A.; Saiz-Sanchez, D. Neurodegeneration and Astrogliosis in the Human CA1 Hippocampal Subfield Are Related to hsp90ab1 and bag3 in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 23, 165. [Google Scholar] [CrossRef]
- Nixon, R.A. The aging lysosome: An essential catalyst for late-onset neurodegenerative diseases. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140443. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.F.; Sebti, S.; Wei, Y.; Zou, Z.; Shi, M.; McMillan, K.L.; He, C.; Ting, T.; Liu, Y.; Chiang, W.C.; et al. Disruption of the beclin 1-BCL2 autophagy regulatory complex promotes longevity in mice. Nature 2018, 558, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Zhou, H.J.; Thorne, A.H.; Chen, X.N.; Li, L.J.; Du, J.R. Neuroprotective Effect of Ligustilide through Induction of alpha-Secretase Processing of Both APP and Klotho in a Mouse Model of Alzheimer’s Disease. Front. Aging Neurosci. 2017, 9, 353. [Google Scholar] [CrossRef]
- Lim, S.W.; Shin, Y.J.; Luo, K.; Quan, Y.; Ko, E.J.; Chung, B.H.; Yang, C.W. Effect of Klotho on autophagy clearance in tacrolimus-induced renal injury. FASEB J. 2019, 33, 2694–2706. [Google Scholar] [CrossRef] [Green Version]
- de Mello, N.P.; Orellana, A.M.; Mazucanti, C.H.; de Morais Lima, G.; Scavone, C.; Kawamoto, E.M. Insulin and Autophagy in Neurodegeneration. Front. Neurosci. 2019, 13, 491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dossou, A.S.; Basu, A. The Emerging Roles of mTORC1 in Macromanaging Autophagy. Cancers 2019, 11, 1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; She, H.; Zhang, T.; Xu, H.; Cheng, L.; Yepes, M.; Zhao, Y.; Mao, Z. p38 MAPK inhibits autophagy and promotes microglial inflammatory responses by phosphorylating ULK1. J. Cell Biol. 2018, 217, 315–328. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Nixon, R.A. The role of autophagy in neurodegenerative disease. Nat. Med. 2013, 19, 983–997. [Google Scholar] [CrossRef]
- Lin, M.G.; Hurley, J.H. Structure and function of the ULK1 complex in autophagy. Curr. Opin. Cell Biol. 2016, 39, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.Z.; Zhuang, X.X.; Zhu, Q.; Wu, M.Y.; Su, H.; Wang, X.J.; Iyaswamy, A.; Yue, Z.; Wang, Q.; Zhang, B.; et al. Enhancing autophagy maturation with CCZ1-MON1A complex alleviates neuropathology and memory defects in Alzheimer disease models. Theranostics 2022, 12, 1738–1755. [Google Scholar] [CrossRef]
- Durairajan, S.S.K.; Selvarasu, K.; Bera, M.R.; Rajaram, K.; Iyaswamy, A.; Li, M. Alzheimer’s Disease and other Tauopathies: Exploring Efficacy of Medicinal Plant-Derived Compounds in Alleviating Tau-Mediated Neurodegeneration. Curr. Mol. Pharmacol. 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Iyaswamy, A.; Krishnamoorthi, S.K.; Zhang, H.; Sreenivasmurthy, S.G.; Zhu, Z.; Liu, J.; Su, C.F.; Guan, X.J.; Wang, Z.Y.; Cheung, K.H.; et al. Qingyangshen mitigates amyloid-beta and Tau aggregate defects involving PPARalpha-TFEB activation in transgenic mice of Alzheimer’s disease. Phytomedicine 2021, 91, 153648. [Google Scholar] [CrossRef] [PubMed]
- Song, J.X.; Malampati, S.; Zeng, Y.; Durairajan, S.S.K.; Yang, C.B.; Tong, B.C.; Iyaswamy, A.; Shang, W.B.; Sreenivasmurthy, S.G.; Zhu, Z.; et al. A small molecule transcription factor EB activator ameliorates beta-amyloid precursor protein and Tau pathology in Alzheimer’s disease models. Aging Cell 2020, 19, e13069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.B.; Liu, J.; Tong, B.C.; Wang, Z.Y.; Zhu, Z.; Su, C.F.; Sreenivasmurthy, S.G.; Wu, J.X.; Iyaswamy, A.; Krishnamoorthi, S.; et al. TFEB, a master regulator of autophagy and biogenesis, unexpectedly promotes apoptosis in response to the cyclopentenone prostaglandin 15d-PGJ2. Acta Pharmacol. Sin. 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Stachowiak, A.; Mamun, A.A.; Tzvetkov, N.T.; Takeda, S.; Atanasov, A.G.; Bergantin, L.B.; Abdel-Daim, M.M.; Stankiewicz, A.M. Autophagy and Alzheimer’s Disease: From Molecular Mechanisms to Therapeutic Implications. Front. Aging Neurosci. 2018, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Ganley, I.G.; Lam, D.H.; Wang, J.; Ding, X.; Chen, S.; Jiang, X. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J. Biol. Chem. 2009, 284, 12297–12305. [Google Scholar] [CrossRef] [Green Version]
- Wong, P.M.; Puente, C.; Ganley, I.G.; Jiang, X. The ULK1 complex: Sensing nutrient signals for autophagy activation. Autophagy 2013, 9, 124–137. [Google Scholar] [CrossRef] [Green Version]
- Tong, B.C.; Wu, A.J.; Huang, A.S.; Dong, R.; Malampati, S.; Iyaswamy, A.; Krishnamoorthi, S.; Sreenivasmurthy, S.G.; Zhu, Z.; Su, C.; et al. Lysosomal TPCN (two pore segment channel) inhibition ameliorates beta-amyloid pathology and mitigates memory impairment in Alzheimer disease. Autophagy 2021. online ahead of print. [Google Scholar] [CrossRef]
- Abraham, C.R.; Mullen, P.C.; Tucker-Zhou, T.; Chen, C.D.; Zeldich, E. Klotho Is a Neuroprotective and Cognition-Enhancing Protein. Vitam. Horm. 2016, 101, 215–238. [Google Scholar] [CrossRef]
- Mytych, J. Klotho and neurons: Mutual crosstalk between autophagy, endoplasmic reticulum, and inflammatory response. Neural Regen. Res. 2021, 16, 1542–1543. [Google Scholar] [CrossRef] [PubMed]
- Mytych, J.; Solek, P.; Tabecka-Lonczynska, A.; Koziorowski, M. Klotho-Mediated Changes in Shelterin Complex Promote Cytotoxic Autophagy and Apoptosis in Amitriptyline-Treated Hippocampal Neuronal Cells. Mol. Neurobiol. 2019, 56, 6952–6963. [Google Scholar] [CrossRef]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Ito, M. Klotho-Related Protein KLrP: Structure and Functions. Vitam. Horm. 2016, 101, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Torbus-Paluszczak, M.; Bartman, W.; Adamczyk-Sowa, M. Klotho protein in neurodegenerative disorders. Neurol. Sci. 2018, 39, 1677–1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boksha, I.S.; Prokhorova, T.A.; Savushkina, O.K.; Tereshkina, E.B. Klotho Protein: Its Role in Aging and Central Nervous System Pathology. Biochemistry 2017, 82, 990–1005. [Google Scholar] [CrossRef]
- Dermaku-Sopjani, M.; Kolgeci, S.; Abazi, S.; Sopjani, M. Significance of the anti-aging protein Klotho. Mol. Membr. Biol. 2013, 30, 369–385. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, J.; Sun, Z. Antiaging Gene Klotho Deficiency Promoted High-Fat Diet-Induced Arterial Stiffening via Inactivation of AMP-Activated Protein Kinase. Hypertension 2016, 67, 564–573. [Google Scholar] [CrossRef] [Green Version]
- Olejnik, A.; Franczak, A.; Krzywonos-Zawadzka, A.; Kaluzna-Oleksy, M.; Bil-Lula, I. The Biological Role of Klotho Protein in the Development of Cardiovascular Diseases. Biomed. Res. Int. 2018, 2018, 5171945. [Google Scholar] [CrossRef] [Green Version]
- Paroni, G.; Panza, F.; De Cosmo, S.; Greco, A.; Seripa, D.; Mazzoccoli, G. Klotho at the Edge of Alzheimer’s Disease and Senile Depression. Mol. Neurobiol. 2019, 56, 1908–1920. [Google Scholar] [CrossRef]
- Saar-Kovrov, V.; Donners, M.; van der Vorst, E.P.C. Shedding of Klotho: Functional Implications in Chronic Kidney Disease and Associated Vascular Disease. Front. Cardiovasc. Med. 2020, 7, 617842. [Google Scholar] [CrossRef] [PubMed]
- van Loon, E.P.; Pulskens, W.P.; van der Hagen, E.A.; Lavrijsen, M.; Vervloet, M.G.; van Goor, H.; Bindels, R.J.; Hoenderop, J.G. Shedding of klotho by ADAMs in the kidney. Am. J. Physiol. Renal Physiol. 2015, 309, F359–F368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchanan, S.; Combet, E.; Stenvinkel, P.; Shiels, P.G. Klotho, Aging, and the Failing Kidney. Front. Endocrinol. 2020, 11, 560. [Google Scholar] [CrossRef]
- Shmulevich, R.; Nissim, T.B.; Wolf, I.; Merenbakh-Lamin, K.; Fishman, D.; Sekler, I.; Rubinek, T. Klotho rewires cellular metabolism of breast cancer cells through alteration of calcium shuttling and mitochondrial activity. Oncogene 2020, 39, 4636–4649. [Google Scholar] [CrossRef] [PubMed]
- Hartl, D.; May, P.; Gu, W.; Mayhaus, M.; Pichler, S.; Spaniol, C.; Glaab, E.; Bobbili, D.R.; Antony, P.; Koegelsberger, S.; et al. A rare loss-of-function variant of ADAM17 is associated with late-onset familial Alzheimer disease. Mol. Psychiatry 2020, 25, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Pietri, M.; Dakowski, C.; Hannaoui, S.; Alleaume-Butaux, A.; Hernandez-Rapp, J.; Ragagnin, A.; Mouillet-Richard, S.; Haik, S.; Bailly, Y.; Peyrin, J.M.; et al. PDK1 decreases TACE-mediated alpha-secretase activity and promotes disease progression in prion and Alzheimer’s diseases. Nat. Med. 2013, 19, 1124–1131. [Google Scholar] [CrossRef]
- Lerch, C.; Shroff, R.; Wan, M.; Rees, L.; Aitkenhead, H.; Kaplan Bulut, I.; Thurn, D.; Karabay Bayazit, A.; Niemirska, A.; Canpolat, N.; et al. Effects of nutritional vitamin D supplementation on markers of bone and mineral metabolism in children with chronic kidney disease. Nephrol. Dial. Transplant. 2018, 33, 2208–2217. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Q.; Sitara, D.; Sato, T.; Densmore, M.; Saito, H.; Schuler, C.; Erben, R.G.; Lanske, B. PTH ablation ameliorates the anomalies of Fgf23-deficient mice by suppressing the elevated vitamin D and calcium levels. Endocrinology 2011, 152, 4053–4061. [Google Scholar] [CrossRef]
- Kuro-o, M. Klotho and the aging process. Korean J. Intern. Med. 2011, 26, 113–122. [Google Scholar] [CrossRef]
- Razzaque, M.S.; Lanske, B. Hypervitaminosis D and premature aging: Lessons learned from Fgf23 and Klotho mutant mice. Trends Mol. Med. 2006, 12, 298–305. [Google Scholar] [CrossRef]
- Kolek, O.I.; Hines, E.R.; Jones, M.D.; LeSueur, L.K.; Lipko, M.A.; Kiela, P.R.; Collins, J.F.; Haussler, M.R.; Ghishan, F.K. 1alpha,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: The final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G1036–G1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.L. Regulation of ion channels by secreted Klotho: Mechanisms and implications. Kidney Int. 2010, 77, 855–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shardell, M.; Drew, D.A.; Semba, R.D.; Harris, T.B.; Cawthon, P.M.; Simonsick, E.M.; Kalyani, R.R.; Schwartz, A.V.; Kritchevsky, S.B.; Newman, A.B. Plasma Soluble alphaKlotho, Serum Fibroblast Growth Factor 23, and Mobility Disability in Community-Dwelling Older Adults. J. Endocr. Soc. 2020, 4, bvz032. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhou, M.; Peng, J.; Yang, Q.; Chu, J.; Li, R.; Jiang, Y. Mechanism of the Fibroblast Growth Factor 23/alpha-Klotho Axis in Peripheral Blood Mononuclear Cell Inflammation in Alzheimer’s Disease. Immunol. Investig. 2021. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Mencke, R.; Olauson, H.; Hillebrands, J.L. Effects of Klotho on fibrosis and cancer: A renal focus on mechanisms and therapeutic strategies. Adv. Drug Deliv. Rev. 2017, 121, 85–100. [Google Scholar] [CrossRef]
- Chateau, M.T.; Araiz, C.; Descamps, S.; Galas, S. Klotho interferes with a novel FGF-signalling pathway and insulin/Igf-like signalling to improve longevity and stress resistance in Caenorhabditis elegans. Aging 2010, 2, 567–581. [Google Scholar] [CrossRef] [Green Version]
- Bartke, A. Long-lived Klotho mice: New insights into the roles of IGF-1 and insulin in aging. Trends Endocrinol. Metab. 2006, 17, 33–35. [Google Scholar] [CrossRef]
- Kurosu, H.; Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Nandi, A.; Gurnani, P.; McGuinness, O.P.; Chikuda, H.; Yamaguchi, M.; Kawaguchi, H.; et al. Suppression of aging in mice by the hormone Klotho. Science 2005, 309, 1829–1833. [Google Scholar] [CrossRef] [Green Version]
- Altintas, O.; Park, S.; Lee, S.J. The role of insulin/IGF-1 signaling in the longevity of model invertebrates, C. elegans and D. melanogaster. BMB Rep. 2016, 49, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Ivashkiv, L.B. IFNgamma: Signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 545–558. [Google Scholar] [CrossRef]
- Kopitar-Jerala, N. The Role of Interferons in Inflammation and Inflammasome Activation. Front. Immunol. 2017, 8, 873. [Google Scholar] [CrossRef] [Green Version]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-alpha signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Typiak, M.; Piwkowska, A. Antiinflammatory Actions of Klotho: Implications for Therapy of Diabetic Nephropathy. Int. J. Mol. Sci. 2021, 22, 956. [Google Scholar] [CrossRef] [PubMed]
- Piancone, F.; La Rosa, F.; Marventano, I.; Saresella, M.; Clerici, M. The Role of the Inflammasome in Neurodegenerative Diseases. Molecules 2021, 26, 953. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Zhao, F.; Chojnacki, J.E.; Fulp, J.; Klein, W.L.; Zhang, S.; Zhu, X. NLRP3 Inflammasome Inhibitor Ameliorates Amyloid Pathology in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- Saresella, M.; La Rosa, F.; Piancone, F.; Zoppis, M.; Marventano, I.; Calabrese, E.; Rainone, V.; Nemni, R.; Mancuso, R.; Clerici, M. The NLRP3 and NLRP1 inflammasomes are activated in Alzheimer’s disease. Mol. Neurodegener. 2016, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Venegas, C.; Kumar, S.; Franklin, B.S.; Dierkes, T.; Brinkschulte, R.; Tejera, D.; Vieira-Saecker, A.; Schwartz, S.; Santarelli, F.; Kummer, M.P.; et al. Microglia-derived ASC specks cross-seed amyloid-beta in Alzheimer’s disease. Nature 2017, 552, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.C.; et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef]
- Mankan, A.K.; Dau, T.; Jenne, D.; Hornung, V. The NLRP3/ASC/Caspase-1 axis regulates IL-1beta processing in neutrophils. Eur. J. Immunol. 2012, 42, 710–715. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Sarlus, H.; Heneka, M.T. Microglia in Alzheimer’s disease. J. Clin. Investig. 2017, 127, 3240–3249. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, T.; Tay, T.L.; Prinz, M. Love and death: Microglia, NLRP3 and the Alzheimer’s brain. Cell Res. 2013, 23, 595–596. [Google Scholar] [CrossRef] [Green Version]
- Griffin, W.S.; Liu, L.; Li, Y.; Mrak, R.E.; Barger, S.W. Interleukin-1 mediates Alzheimer and Lewy body pathologies. J. Neuroinflammation 2006, 3, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ising, C.; Heneka, M.T. Functional and structural damage of neurons by innate immune mechanisms during neurodegeneration. Cell Death Dis. 2018, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.; Fisher, K.; Hooper, N.M. Exploiting the neuroprotective effects of alpha-klotho to tackle ageing- and neurodegeneration-related cognitive dysfunction. Neuronal Signal. 2021, 5, NS20200101. [Google Scholar] [CrossRef]
- Deane, R.; Bell, R.D.; Sagare, A.; Zlokovic, B.V. Clearance of amyloid-beta peptide across the blood-brain barrier: Implication for therapies in Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 2009, 8, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Wahrle, S.E.; Jiang, H.; Parsadanian, M.; Kim, J.; Li, A.; Knoten, A.; Jain, S.; Hirsch-Reinshagen, V.; Wellington, C.L.; Bales, K.R.; et al. Overexpression of ABCA1 reduces amyloid deposition in the PDAPP mouse model of Alzheimer disease. J. Clin. Investig. 2008, 118, 671–682. [Google Scholar] [CrossRef]
- Zhou, H.; Pu, S.; Zhou, H.; Guo, Y. Klotho as Potential Autophagy Regulator and Therapeutic Target. Front. Pharmacol. 2021, 12, 755366. [Google Scholar] [CrossRef]
- Kim, W.S.; Bhatia, S.; Elliott, D.A.; Agholme, L.; Kagedal, K.; McCann, H.; Halliday, G.M.; Barnham, K.J.; Garner, B. Increased ATP-binding cassette transporter A1 expression in Alzheimer’s disease hippocampal neurons. J. Alzheimers Dis. 2010, 21, 193–205. [Google Scholar] [CrossRef]
- Dubal, D.B.; Zhu, L.; Sanchez, P.E.; Worden, K.; Broestl, L.; Johnson, E.; Ho, K.; Yu, G.Q.; Kim, D.; Betourne, A.; et al. Life extension factor klotho prevents mortality and enhances cognition in hAPP transgenic mice. J. Neurosci. 2015, 35, 2358–2371. [Google Scholar] [CrossRef] [PubMed]
- Neitzel, J.; Franzmeier, N.; Rubinski, A.; Dichgans, M.; Brendel, M.; Alzheimer’s Disease Neuroimaging Initiative (ADNI); Malik, R.; Ewers, M. KL-VS heterozygosity is associated with lower amyloid-dependent tau accumulation and memory impairment in Alzheimer’s disease. Nat. Commun. 2021, 12, 3825. [Google Scholar] [CrossRef] [PubMed]
- Heras-Sandoval, D.; Perez-Rojas, J.M.; Hernandez-Damian, J.; Pedraza-Chaverri, J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell. Signal. 2014, 26, 2694–2701. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, H.; Wang, Z.L.; Li, W.H.; Liu, H.; Zhao, Y.X. The PI3K/AKT/mTOR pathway regulates autophagy to induce apoptosis of alveolar epithelial cells in chronic obstructive pulmonary disease caused by PM2.5 particulate matter. J. Int. Med. Res. 2020, 48, 300060520927919. [Google Scholar] [CrossRef] [PubMed]
- Mytych, J. Actions of Klotho on hippocampal neuronal cells. Vitam. Horm. 2022, 118, 223–246. [Google Scholar] [CrossRef]
- Laker, R.C.; Drake, J.C.; Wilson, R.J.; Lira, V.A.; Lewellen, B.M.; Ryall, K.A.; Fisher, C.C.; Zhang, M.; Saucerman, J.J.; Goodyear, L.J.; et al. Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat. Commun. 2017, 8, 548. [Google Scholar] [CrossRef]
- Liu, Q.; Guan, J.Z.; Sun, Y.; Le, Z.; Zhang, P.; Yu, D.; Liu, Y. Insulin-like growth factor 1 receptor-mediated cell survival in hypoxia depends on the promotion of autophagy via suppression of the PI3K/Akt/mTOR signaling pathway. Mol. Med. Rep. 2017, 15, 2136–2142. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.W.; Yuan, L.J.; Yang, Y.; Zhang, M.; Chen, W.F. IGF-1 inhibits MPTP/MPP(+)-induced autophagy on dopaminergic neurons through the IGF-1R/PI3K-Akt-mTOR pathway and GPER. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E734–E743. [Google Scholar] [CrossRef]
- Yang, L.; Wang, H.; Liu, L.; Xie, A. The Role of Insulin/IGF-1/PI3K/Akt/GSK3beta Signaling in Parkinson’s Disease Dementia. Front. Neurosci. 2018, 12, 73. [Google Scholar] [CrossRef] [Green Version]
- Di Malta, C.; Cinque, L.; Settembre, C. Transcriptional Regulation of Autophagy: Mechanisms and Diseases. Front. Cell Dev. Biol. 2019, 7, 114. [Google Scholar] [CrossRef]
- Palmieri, M.; Impey, S.; Kang, H.; di Ronza, A.; Pelz, C.; Sardiello, M.; Ballabio, A. Characterization of the CLEAR network reveals an integrated control of cellular clearance pathways. Hum. Mol. Genet. 2011, 20, 3852–3866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Arencibia, M.G.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P.; et al. TFEB links autophagy to lysosomal biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Zhang, X.Q.; Zhang, Z. Transcription factor EB agonists from natural products for treating human diseases with impaired autophagy-lysosome pathway. Chin. Med. 2020, 15, 123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, X.; Wang, S.; Chen, Y.; Liu, H. Regulation of TFEB activity and its potential as a therapeutic target against kidney diseases. Cell Death Discov. 2020, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Su, C.F.; Jiang, L.; Zhang, X.W.; Iyaswamy, A.; Li, M. Resveratrol in Rodent Models of Parkinson’s Disease: A Systematic Review of Experimental Studies. Front. Pharmacol. 2021, 12, 644219. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, J.; Yang, C. Celastrol, a TFEB (transcription factor EB) agonist, is a promising drug candidate for Alzheimer disease. Autophagy 2022. online ahead of print. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, Z.; Tong, B.C.; Iyaswamy, A.; Xie, W.J.; Zhu, Y.; Sreenivasmurthy, S.G.; Senthilkumar, K.; Cheung, K.H.; Song, J.X.; et al. A stress response p38 MAP kinase inhibitor SB202190 promoted TFEB/TFE3-dependent autophagy and lysosomal biogenesis independent of p38. Redox Biol. 2020, 32, 101445. [Google Scholar] [CrossRef]
- Nelson, P.T.; Head, E.; Schmitt, F.A.; Davis, P.R.; Neltner, J.H.; Jicha, G.A.; Abner, E.L.; Smith, C.D.; Van Eldik, L.J.; Kryscio, R.J.; et al. Alzheimer’s disease is not “brain aging”: Neuropathological, genetic, and epidemiological human studies. Acta Neuropathol. 2011, 121, 571–587. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).