Bioanalytical Detection of Steroid Abuse in Sports Based on the Androgenic Activity Measurement
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Samples Collected
2.3. Sample Preparation
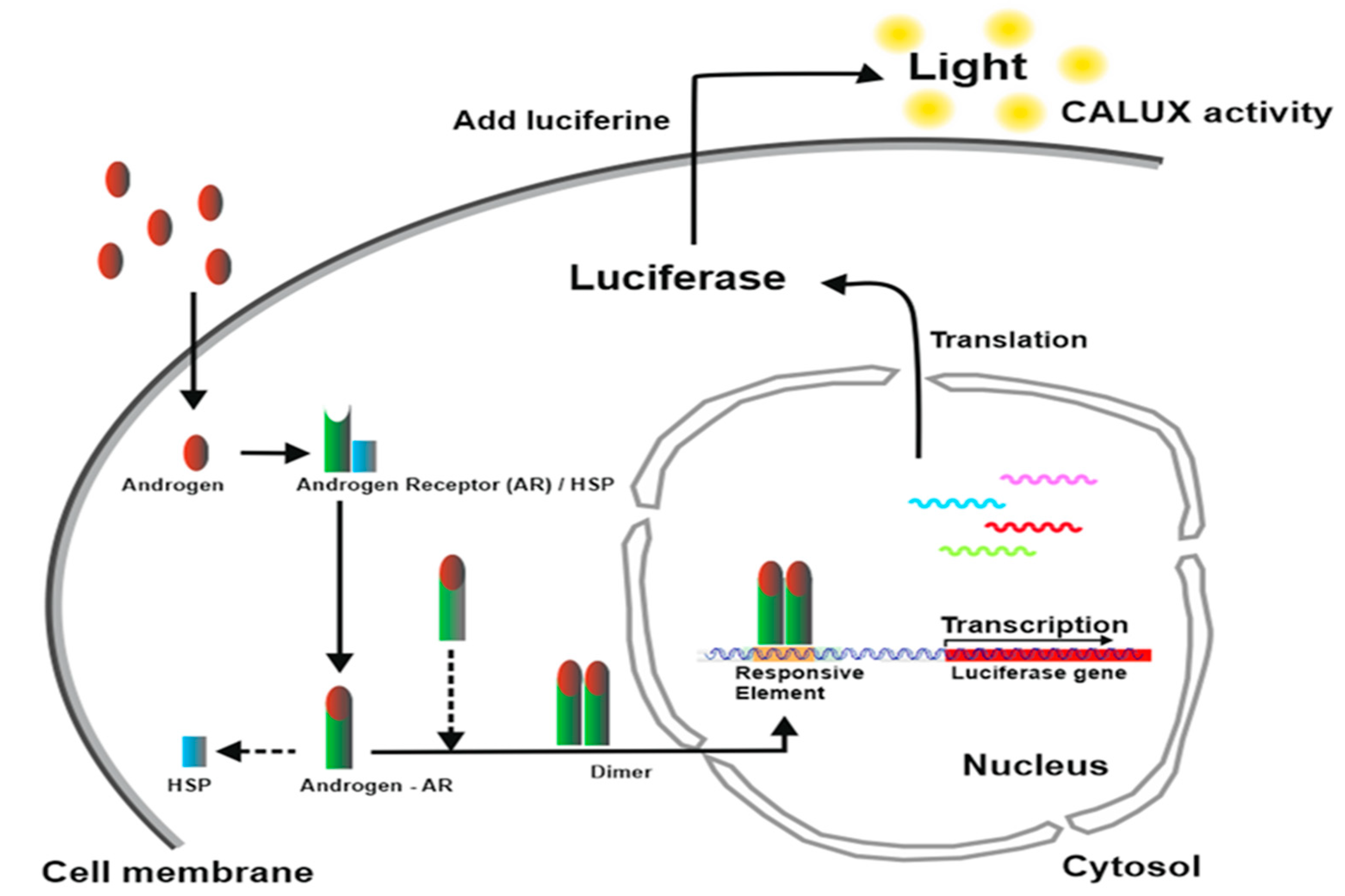
2.4. AR CALUX®
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lissavetzky, J. Química y deporte: La lucha contra el dopaje en el horizonte del siglo XXI. Arbor 2011, 187, 105–112. [Google Scholar] [CrossRef]
- Reardon, C.L.; Creado, S. Drug abuse in athletes. Subst. Abus. Rehabil. 2014, 5, 95–105. [Google Scholar] [CrossRef]
- Aguilar, M.; Muñoz-Guerra, J.; Plata, M.D.M.; Del Coso, J. Thirteen years of the fight against doping in figures. Drug Test. Anal. 2017, 9, 866–869. [Google Scholar] [CrossRef] [PubMed]
- Baron, D.; Martin, D.; Magd, S. El dopaje en el deporte y su propagación a las poblaciones en riesgo: Una revisión internacional. Rev. Of. De La Asoc. Mund. De Psiquiatr. (WPA) 2007, 5, 118–123. [Google Scholar]
- WADA. Technical Document for the Harmonization of Analysis and Reporting of 19-Norsteroids Related to Nandrolone. WADA Technical Document—TD2019NA. March 2019. Available online: https://www.wada-ama.org/en/resources/science-medicine/td2019na-0. (accessed on 17 November 2020).
- WADA. Detection of Synthetic Forms of Endogenous Anabolic Androgenic Steroids by GC/C/IRMS. 2019. Available online: https://www.wada-ama.org/sites/default/files/td2019irms_final_eng_clean.pdf. (accessed on 17 November 2020).
- WADA. Endogenous Anabolic Androgenic Steroids: Measurement and Reporting. Technical Document TD2018EAAS. 2018. Available online: https://www.wada-ama.org/sites/default/files/resources/files/td2018eaas_final_eng.pdf (accessed on 17 November 2020).
- Parr, M.K.; Schänzer, W. Detection of the misuse of steroids in doping control. J. Steroid Biochem. Mol. Biol. 2010, 121, 528–537. [Google Scholar] [CrossRef]
- Thevis, M.; Walpurgis, K.; Thomas, A. Analytical Approaches in Human Sports Drug Testing Recent Advances, Challenges, and Solutions. Anal. Chem. 2019, 92, 506–523. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.; Yazdi, T.; Masharani, U.; Tyrrell, B.; Butch, A.; Schaufele, F. Advantages and limitations of androgen receptor-based methods for detecting anabolic androgenic steroid abuse as performance enhancing drugs. PLoS ONE 2016, 11, e0151860. [Google Scholar] [CrossRef] [PubMed]
- Mareck, U.; Geyer, H.; Opfermann, G.; Thevis, M.; Schänzer, W. Factors influencing the steroid profile in doping control analysis. J. Mass Spectrom. 2008, 43, 877–891. [Google Scholar] [CrossRef]
- Baume, N.; Geyer, H.; Vouillamoz, M.; Grisdale, R.; Earl, M.; Aguilera, R.; Cowan, D.A.; Ericsson, M.; Gmeiner, G.; Kwiatkowska, D.; et al. Evaluation of longitudinal steroid profiles from male football players in UEFA competitions between 2008 and 2013. Drug Test. Anal. 2016, 8, 603–612. [Google Scholar] [CrossRef]
- Jakobsson, J.; Ekström, L.; Inotsume, N.; Garle, M.; Lorentzon, M.; Ohlsson, C.; Roh, H.-K.; Carlström, K.; Rane, A. Large differences in testosterone excretion in Korean and Swedish men are strongly associated with a UDP-glucuronosyl transferase 2B17 polymorphism. J. Clin. Endocrinol. Metab. 2006, 91, 687–693. [Google Scholar] [CrossRef]
- Ekström, L.; Cevenini, L.; Michelini, E.; Schulze, J.; Thörngren, J.-O.; Belanger, A.; Guillemette, C.; Garle, M.; Roda, A.; Rane, A. Testosterone challenge and androgen receptor activity in relation to UGT 2B17 genotypes. Eur. J. Clin. Investig. 2013, 43, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Strahm, E.; Mullen, J.E.; Ericsson, M.; Schulze, J.J.; Rane, A.; Gårevik, N.; Ekstrom, L. Dose-dependent testosterone sensitivity of the steroidal passport and GC-C-IRMS analysis in relation to the UGT2B17 deletion polymorphism. Drug Test. Anal. 2015, 7, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Martín-Escudero, P.; Muñoz-Guerra, J.; Del Prado, N.; Canales, M.G.; Ferrer, M.F.; Vargas, S.; Soldevilla, A.B.; Serrano-Garde, E.; Miguel-Tobal, F.; Casas, M.M.D.L.; et al. Impact of UGT 2B17 gene deletion on the steroid profile of an athlete. Physiol. Rep. 2015, 3, e12645. [Google Scholar] [CrossRef]
- Martín-Escudero, P.; Muñoz-Guerra, J.A.; García-Tenorio, S.V.; Garde, E.S.; Soldevilla-Navarro, A.B.; Canales, M.G.; Prado, N.; Ferrer, M.E.F.; Pérez, C.F. Impact of the UGT2B17 polymorphism on the steroid profile. Results of a crossover clinical trial in athletes submitted to testosterone administration. Steroids 2018, 141, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Kuuranne, T.; Saugy, M.; Baume, N. Confounding factors, and genetic polymorphism in the evaluation of individual steroid profiling. Br. J. Sports Med. 2014, 48, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Ponzetto, F.; Baume, N.; Schweizer, C.; Saugy, M.; Kuuranne, T. Steroidal module of the athlete biological passport. Curr. Opin. Endocr. Metab. Res. 2019, 9, 14–21. [Google Scholar] [CrossRef]
- Juul, A.; Sørensen, K.; Aksglaede, L.; Garn, I.; Meyts, E.R.-D.; Hullstein, I.; Hemmersbach, P.; Ottesen, A.M. A common deletion in the uridine diphosphate glucuronyltransferase (UGT) 2B17 gene is a strong determinant of androgen excretion in healthy pubertal boys. J. Clin. Endocrinol. Metab. 2009, 94, 1005–1011. [Google Scholar] [CrossRef][Green Version]
- Sonneveld, E.; Jansen, H.J.; Riteco, J.A.C.; Brouwer, A.; Van Der Burg, B. Development of androgen-and estrogen-responsive bioassays, members of a panel of human cell line-based highly selective steroid-responsive bioassays. Toxicol. Sci. 2004, 83, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, E.; Riteco, J.A.C.; Jansen, H.J.; Pieterse, B.; Brouwer, A.; Schoonen, W.G.; Van Der Burg, B. Comparison of in vitro and in vivo screening models for androgenic and estrogenic activities. Toxicol. Sci. 2005, 89, 173–187. [Google Scholar] [CrossRef]
- Van der Burg, B.; Winter, R.; Man, H.; Vangenechten, C.; Berckmans, P.; Weimer, M.; Witters, H.; van der Lindena, S. Optimization and prevalidation of the in vitro AR CALUX® method to test androgenic and antiandrogenic activity of compounds. Reprod. Toxicol. 2010, 30, 18–24. [Google Scholar] [CrossRef]
- Cooper, E.; McGrath, K.C.Y.; Li, X.; Akram, O.; Kasz, R.; Kazlauskas, R.; McLeod, M.D.; Handelsman, D.J.; Heather, A.K. The use of tandem yeast and mammalian cell in vitro androgen bioassays to detect androgens in internet-sourced sport supplements. Drug Test. Anal. 2016, 9, 545–552. [Google Scholar] [CrossRef]
- Cooper, E.; McGrath, K.; Heather, A. In vitro androgen bioassays as a detection method for designer androgens. Sensors 2013, 13, 2148–2163. [Google Scholar] [CrossRef]
- Houtman, C.; Sterk, S.S.; van de Heijning, M.P.M.; Brouwer, A.; Stephany, R.W.; van der Burg, B.; Sonnevelda, E. Detection of anabolic androgenic steroid abuse in doping control using mammalian reporter gene bioassays. Anal. Chim. Acta 2009, 637, 247–258. [Google Scholar] [CrossRef]
- Bird, S.R.; Goebel, C.; Burke, L.M.; Greaves, R.F. Doping in sport and exercise: Anabolic, ergogenic, health and clinical issues. Ann. Clin. Biochem. 2016, 53, 196–221. [Google Scholar] [CrossRef]
- Schulze, J.J.; Lundmark, J.; Garle, M.; Skilving, I.; Ekstroöm, L.; Rane, A. Doping test results dependent on genotype of uridine diphospho-glucuronosyl transferase 2B17, the major enzyme for testosterone glucuronidation. J. Clin. Endocrinol. Metab. 2008, 93, 2500–2506. [Google Scholar] [CrossRef]
- Strano-Rossi, S.; Fiore, C.; Chiarotti, M.; Centini, F. Analytical techniques in androgen anabolic steroids (AASs) analysis for antidoping and forensic purposes. Mini Rev. Med. Chem. 2011, 11, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Peng, Y.; Sheng, S.; Lin, Q. Assessment of Urinary Total Testosterone Production by a Highly Sensitive Time-Resolved Fluorescence Immunoassay. J. Clin. Lab. Anal. 2008, 22, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Duntas, L.H.; Popovic, V. Hormones as doping in sports. Endocrine 2013, 43, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Durward-Akhurst, S.A.; Schultz, N.E.; Norton, E.M.; Rendahl, A.; McCue, M.E.; Mickelson, J.R.; Geor, R.J.; Brouwer, A.E.; Behnisch, P.A.; Besselink, H.T. Associations between endocrine disrupting chemicals and equine metabolic syndrome phenotypes. Chemosphere 2019, 218, 652–661. [Google Scholar] [CrossRef]
- Sonneveld, E.; Van der Burg, B.; Brouwer, A.; Stephany, R.; Sterk, S. The Development and application of a tight bioassay-based control system for steroids and other prohibited substances in sport doping. R. WADA 2015. Available online: https://www.wada-ama.org/en/resources/science-medicine/development-and-application-of-a-tight-bioassay-based-control-system-for (accessed on 30 November 2020).
- Cadwallader, A.B.; Lim, C.S.; Rollins, U.E.; Botrè, F. The androgen receptor and its use in biological assays: Looking toward effect-based testing and its applications. J. Anal. Toxicol. 2011, 35, 594–607. [Google Scholar] [CrossRef]
- Calabretta, M.M.; Lopreside, A.; Montali, L.; Cevenini, L.; Roda, A.; Michelini, E.A. Genetically Encoded Bioluminescence Intracellular Nanosensor for Androgen Receptor Activation Monitoring in 3D Cell Models. Sensors 2021, 21, 893. [Google Scholar] [CrossRef] [PubMed]
- Aqai, P.; Cevik, E.; Gerssen, A.; Haasnoot, W.; Nielen, M.W.F. High-throughput bioaffinity mass spectrometry for screening and identification of designer anabolic steroids in dietary supplements. Anal. Chem. 2013, 85, 3255–3262. [Google Scholar] [CrossRef] [PubMed]
- Beato, M.; Herrlich, P.; Schütz, G. Steroid hormone receptors: Many actors in search of a plot. Cell 1995, 83, 851–857. [Google Scholar] [CrossRef]
- McKenna, N.J.; O’Malley, B.W. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 2002, 108, 465–474. [Google Scholar] [CrossRef]
- Sato, T.; Matsumoto, T.; Yamada, T.; Watanabe, T.; Kawano, H.; Kato, S. Late onset of obesity in male androgen receptor-deficient (AR KO) mice. Biochem. Biophys. Res. Commun. 2003, 300, 167–171. [Google Scholar] [CrossRef]
- Ekstrom, L.; Schulze, J.J.; Guillemette, C.; Belanger, A.; Rane, A. Bioavailability of testosterone enanthate dependent on genetic variation in the phosphodiesterase 7B but not on the uridine 5′-diphospho-glucuronosyltransferase (UGT2B17) gene. Pharm. Genom. 2011, 21, 325–332. [Google Scholar] [CrossRef]


| c1 | c2 | c3 | |||
|---|---|---|---|---|---|
| With Hydrolysis | ins/ins | n | 30 | 30 | 10 |
| median | 0.9 | 3.2 | 1.0 | ||
| IQR | 0.6–1.5 | 2.3–4.4 | 0.4–1.7 | ||
| ins/del | n | 40 | 40 | 13 | |
| median | 0.8 | 5.4 | 1.3 | ||
| IQR | 0.5–2.6 | 3.2–10 | 1.3–2.0 | ||
| del/del | n | 40 | 39 | 16 | |
| median | 0.6 | 2.1 | 0.7 | ||
| IQR | 0.4–1.5 | 1.5–2.9 | 0.4–1.0 | ||
| Without Hydrolysis | ins/ins | n | 27 | 30 | 10 |
| Median | 0.2 | 0.6 | 0.2 | ||
| IQR | 0.1–0.4 | 0.5–1.3 | 0.2–0.5 | ||
| ins/del | n | 40 | 40 | 13 | |
| Median | 0.3 | 0.8 | 0.3 | ||
| IQR | 0.1–1.7 | 0.3–1.8 | 0.1–0.8 | ||
| del/del | n | 37 | 39 | 16 | |
| Median | 0.1 | 0.4 | 0.2 | ||
| IQR | 0.1–0.2 | 0.3–0.6 | 0.1–0.3 |
| With Hydrolysis | Ratio of Means | Inter Quartile 95% Range | p | |
|---|---|---|---|---|
| (ins/del) vs. (ins/ins) | 1.37 | 0.44 | 4.28 | 0.578 |
| (del/del) vs. (ins/ins) | 0.79 | 0.26 | 2.47 | 0.690 |
| Without Hydrolysis | ||||
| (ins/del) vs. (ins/ins) | 2.21 | 0.41 | 12.03 | 0.355 |
| (del/del) vs. (ins/ins) | 0.53 | 0.10 | 2.89 | 0.464 |
| Ratio of Means | Inter Quartile Range C 95% | p | |||
|---|---|---|---|---|---|
| With Hydrolysis | ins/ins | 3.31 | 2.07 | 5.29 | <0.001 |
| ins/del | 4.15 | 3.05 | 5.67 | <0.001 | |
| del/del | 2.89 | 2.42 | 3.46 | <0.001 | |
| Without Hydrolysis | ins/ins | 4.10 | 2.96 | 5.67 | <0.001 |
| ins/del | 2.02 | 1.43 | 2.84 | <0.001 | |
| del/del | 3.95 | 3.01 | 5.19 | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Escudero, P.; Muñoz-Guerra, J.A.; García-Tenorio, S.V.; Serrano-Garde, E.; Soldevilla-Navarro, A.B.; Cortes-Carrillo, N.; Galindo-Canales, M.; del Prado, N.; Fuentes-Ferrer, M.; Fernández-Pérez, C.; et al. Bioanalytical Detection of Steroid Abuse in Sports Based on the Androgenic Activity Measurement. Chemosensors 2021, 9, 62. https://doi.org/10.3390/chemosensors9040062
Martín-Escudero P, Muñoz-Guerra JA, García-Tenorio SV, Serrano-Garde E, Soldevilla-Navarro AB, Cortes-Carrillo N, Galindo-Canales M, del Prado N, Fuentes-Ferrer M, Fernández-Pérez C, et al. Bioanalytical Detection of Steroid Abuse in Sports Based on the Androgenic Activity Measurement. Chemosensors. 2021; 9(4):62. https://doi.org/10.3390/chemosensors9040062
Chicago/Turabian StyleMartín-Escudero, Pilar, Jesus A. Muñoz-Guerra, Soledad Vargas García-Tenorio, Ester Serrano-Garde, Ana Belén Soldevilla-Navarro, Nuria Cortes-Carrillo, Mercedes Galindo-Canales, Nayade del Prado, Manuel Fuentes-Ferrer, Cristina Fernández-Pérez, and et al. 2021. "Bioanalytical Detection of Steroid Abuse in Sports Based on the Androgenic Activity Measurement" Chemosensors 9, no. 4: 62. https://doi.org/10.3390/chemosensors9040062
APA StyleMartín-Escudero, P., Muñoz-Guerra, J. A., García-Tenorio, S. V., Serrano-Garde, E., Soldevilla-Navarro, A. B., Cortes-Carrillo, N., Galindo-Canales, M., del Prado, N., Fuentes-Ferrer, M., Fernández-Pérez, C., Behnisch, P. A., & Brouwer, A. (2021). Bioanalytical Detection of Steroid Abuse in Sports Based on the Androgenic Activity Measurement. Chemosensors, 9(4), 62. https://doi.org/10.3390/chemosensors9040062






