Abstract
Biochar-based fertilizers are a new attractive alternative to P supplementation for crops, as they can gradually release the nutrient, avoiding losses and improving soil quality. In this regard, the evaluation of the P amount in biochar-based fertilizers is extremely important for their quality control. Analytical techniques that require sample solubilization are not very efficient for this task, as biochar is difficult to solubilize. Laser-induced breakdown spectroscopy (LIBS) is a promising technique to respond to this demand, as it enables a direct analysis of solid samples, avoiding the complicated process of sample solubilization. In this work, a novel method based on spark discharge (SD) coupled to LIBS was evaluated for P determination in biochar-based fertilizers prepared from three different biomasses. To overcome calibration problems in LIBS analysis, a matrix-matching procedure accomplished by the addition of eucalyptus biochar to calibration standards was used in experiments. This procedure minimized matrix effects and allowed us to achieve a satisfactory accuracy even when applied to similar but different matrices. Furthermore, the developed method is simple, fast, direct, does not generate post-analysis residues and appears appropriate for the quality control of sustainable biochar-based fertilizers and other biochar products.
1. Introduction
Phosphorus (P) is a macronutrient that plays a fundamental role in the energy metabolism of plants, whereas its deficiency causes reduced plant growth and chlorosis and necrosis in leaves [1]. The deficiency of P in agricultural soils is remediated by the application of phosphate fertilizers that are derived from the extraction, grinding and treatment of igneous or sedimentary rocks and are usually applied in high amounts, although less than 50% of P in them is taken up by plants [2,3]. The non-assimilated P is leached, causing environmental problems, economic losses and contributing to the depletion of limited terrestrial P sources. Thus, controlled-release fertilizers more effectively meet the demand of crops, avoiding the accumulation of P in the environment and minimizing the production cost by the application of a smaller amount of fertilizer [2].
Recently, the possibility of using biochar from different agricultural byproducts to produce controlled-release fertilizers was proposed [4,5]. Furthermore, biochar confers several benefits to agricultural soils, such as an increase in biodiversity and retention of water and nutrients [6,7]. In this context, the International Biochar Initiative (IBI) highlighted the importance of the analytical characterization and certification of biochar products in agriculture [8].
The Association of Official Agricultural Chemists (AOAC) recommends the vanadomolybdate yellow photometric method for P determination in fertilizers, which is based on the stable yellow-colored complex developed when an excess of a molybdate solution is added to an acidified solution of vanadate and orthophosphate [9,10]. Nowadays, faster and more efficient analytical techniques, such as inductively coupled plasma optical emission spectrometry (ICP-OES) and high-resolution continuous-source flame atomic absorption spectrometry (HR-CS FAAS), are used routinely to determine P in fertilizers and other matrices [11,12]. All these techniques, however, require P in solution; thus, solid matrices must be solubilized prior to analysis, which is not a simple task for biochars that are extremely recalcitrant materials [13]. In particular, biochar solubilization generally involves the use of highly oxidizing reagents together with high temperatures and pressures or decomposition in dry conditions using fluxing agents. These procedures increase the analytical time and sample contamination risks and generate chemical residues. Therefore, a method that enables the direct, rapid and waste-free determination of P in biochar would be more efficient to respond to actual analytical demands.
Laser-induced breakdown spectroscopy (LIBS) is a relatively novel, attractive analytical technique that reflects the technological development in analytical chemistry and is expected to be very promising for P determination in agricultural materials [14]. The analytical principle on which LIBS is based consists in the optical emission of species previously excited in a plasma obtained by a pulsed laser beam at nano-to-femtosecond intervals. During the expansion and cooling of the plasma, atoms and ions relax to fundamental energy states emitting light frequencies in the UV-visible-NIR range, which are related to their underlying electronic transitions [15]. The intensity of the emission lines treated with appropriate calibration methods can then provide quantitative information about the elements present in the plasma, i.e., in the sample [15].
LIBS features several analytical advantages including simplicity, reduced sample handling, relatively short analytical times, direct and minimally destructive analysis, and no chemical waste generation. However, this technique presents some as yet unresolved problems, such as low sensitivity and matrix effects, which make it difficult to find appropriate standards for calibration. In particular, the severe matrix effects that affect LIBS measurements, especially in the case of complex matrices such as biochars, are a challenging factor in its analytical application [16]. In particular, Morais et al. (2017) found that the incorporation of Ca2+ ions into biochar to produce controlled-release fertilizer changed their original properties, and also those of the calibration standard prepared by simple physical mixing of biochar and Ca2+ ions [17]. Thus, an adequate accuracy for Ca predictions was reached by using internal standardization together with matrix matching.
In general, LIBS has high limits of detection (LOD) compared to consolidated analytical techniques such as ICP-OES [15]; thus, the configuration of a classical LIBS system did not allow the detection of P lines. Therefore, to solve this inconvenience, Viera et al. (2018) coupled LIBS to a spark discharge (SD) system to analyze P in various fertilizers [18].
In the context of the emerging demand for biochar products certification for agricultural use, the aim of this study was to attempt the use of SD-LIBS and matrix matching for P determination in different kinds of biochar-based fertilizers.
2. Materials and Methods
2.1. Samples and Calibration Standards
Samples of controlled-release biochar-based fertilizers were prepared from three substrates, i.e., eucalyptus ground leaves and branches, banana fiber and peanut shells, biomasses coming from agricultural tailings in Brazil whose biochar did not contain P level detected by SD-LIBS. To produce the biochar-based fertilizers, approximately 3.0 g of each biomass (raw material) was transferred to Erlenmeyer flasks containing 100 mL of a 2.70 g L−1 P solution that was prepared by solubilizing (NH4)2HPO4 in high-purity deionized water. The flasks were placed in an incubation chamber at 25 °C and shaken for 24 h with orbital agitation at 150 rpm. Then, the biomasses were separated by filtration and dried in an oven at 40 °C for 24 h. Successively, approximately 2.0 g of each biomass with incorporated P was transferred to porcelain crucibles that were covered with an aluminum foil to keep the atmosphere with limited oxygen, and submitted to the pyrolysis process in a muffle (under atmospheric conditions) to obtain the biochar-based fertilizer. The heating program consisted of raising the temperature to 260 °C at a rate of 10 °C min−1, and maintaining it for 60 min. Then, the muffle was allowed to cool down to a temperature close to 60 °C, and the crucibles were removed. The second stage of cooling to room temperature was conducted in a gas exhaust hood. The above procedure was repeated three times for each biomass. The samples were then homogenized using an agate mortar and pestle, and aliquots of 300 mg were transferred to a carbon steel mold to make pellets by applying a pressure of 738.8 MPa on the mold using a hydraulic press.
Calibration standards were prepared based on the concept of matrix matching by adding increasing amounts of (NH4)2HPO4 to 0.1 g of pure eucalyptus biochar obtained by the same pyrolysis process described above. Cellulose (type 101 Sigmacell) was used as a diluent to bring the standards final weight to 300 mg. The solid standards were then homogenized using an agate mortar and pestle and subjected to a pressure of 10 ton to obtain the pellets. The final content of P in the standards ranged from 0.00% to 11.7% w/w.
2.2. SD-LIBS Analysis and Validation
Spectra were acquired using a LIBS system equipped with a 1064 nm Q-switched Nd:YAG laser (Quantel, Big Sky Ultra 50, Bozeman, MT, USA) operated at 50 mJ maximum power energy with pulse duration lower than 8 ns, which provided an irradiance of about 9.7 GW cm−2 at the focal point. The system also included an optical fiber bundle and four spectrometers Ocean Optics (HR2000+, Dunedin, FL, USA) featuring an optical resolution of 0.1 nm (full width at half maximum) and covering a spectral range from 200 nm to 630 nm. The gate time and the Q-switch delay used for spectra acquisition were 1 ms and 2 μs, respectively.
Each sample pellet was placed into a sampler that could be moved in the x and y directions. The pellets’ surface was adjusted in the focal point of the waist of the laser beam (lens to sample distance, 12 cm). A previously used SD system [18] was coupled to the LIBS instrument to improve signal detectability.
Twenty emission spectra were acquired, resulting from 10 laser pulses scattered on both flat faces of each pellet, and individually pre-processed for baseline correction. The average intensities of the P atomic line at 253.55 nm were used.
To assess the accuracy of the proposed method, 100 mg of each fertilizer sample was solubilized using 3.00 mL of HNO3 70% (v/v), 2.00 mL of high-purity deionized water and 1.00 mL of H2O2 30% (v/v) in a microwave oven. The digested solutions were then diluted to 20.00 mL, homogenized and analyzed by HR-CS-FAAS. Obtained data were then compared to those achieved by SD-LIBS by applying the paired t-test.
3. Results and Discussion
The SD-LIBS spectra measured for the three samples (Figure 1) featured four P atomic lines in the spectral range from 250 to 262 nm. Similar spectra were acquired from the calibration standards (one example is shown in Figure 2 for the standard with 11.7 wt% P). The most intense line at 253.55 nm was chosen as indicator of P concentration, although it was not fully resolved from the line at 253.40 nm, that is, however, also assigned to the analyte (Figure 3).

Figure 1.
SD-LIBS spectra of the three biochar-based fertilizers with incorporated P in the range from 250 to 262 nm.
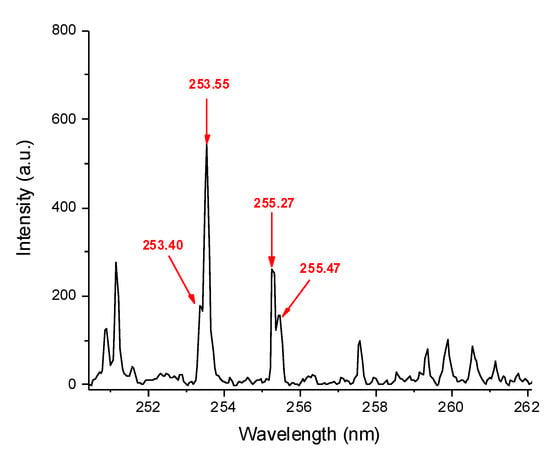
Figure 2.
SD-LIBS spectrum of the calibration standard with 11.7 P wt% in the range from 250 to 262 nm.
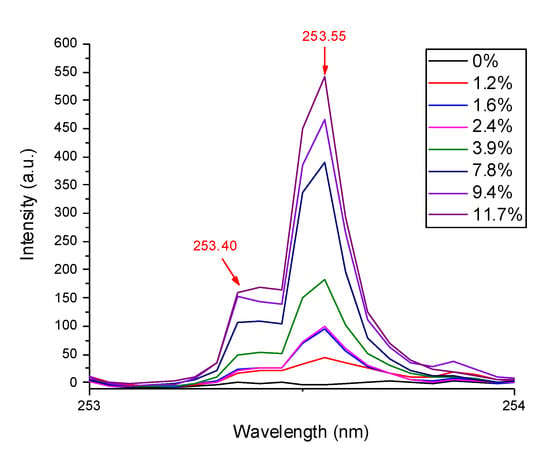
Figure 3.
P (I) emission lines at 253.40 and 253.55 nm for the calibration standards.
Although Morais et al. showed that the incorporation of Ca2+ ions into biochar changed their original properties, making the fertilizer distinct from pure biochar and also from the calibration standard prepared by physical mixing of eucalyptus biochar and Ca2+ [17], the matrix-matching procedure was evaluated here using P. The linear prediction model (analytical curve) fitted by correlating the intensity of the P (I) LIBS line at 253.55 nm and P wt% in the standards achieved a value of R2 = 0.9957 over the linear range from 0.71 to 11.73 wt% P and a sensitivity of 47.835 (Figure 4). The estimated limits of detection and quantification were 0.21 and 0.71 wt% P, respectively. Although R2 provides an estimate of the strength of the relationship between the signals and the P wt%, it does not provide a formal hypothesis test for this relationship. Thus, the analysis of variance (ANOVA) was applied to validate the correlation. The p-value for the global F test was 0.00, less than its level of significance, i.e., 0.05, which confirmed that the value of R2 was significantly different from zero and the calibration model was efficient for P wt% prediction.
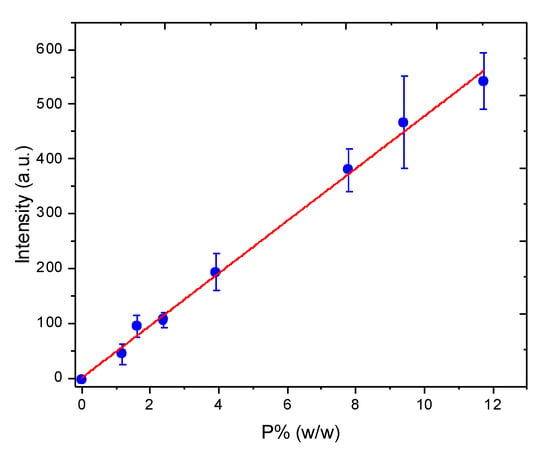
Figure 4.
Correlation between the intensities of the P (I) LIBS line at 253.55 nm and P wt% measured by HR-CS-FAAS in the calibration standards (the standard deviation is referred to the mean intensity, calculated for 20 repetitions).
The linear model so obtained was then applied to predict the P wt% level in the three biochar-based fertilizer samples. The predictive capacity of the model with respect to the reference method, and the calculated t-values are shown in Table 1. The student’s t-test applied to compare the results showed that the P wt% determined by SD-LIBS had no statistical difference from that determined by the reference method at 99% confidence level.

Table 1.
P wt% determined by SD-LIBS and the HR-CS-FAAS reference technique in biochar-based fertilizer samples (n = 3 for both methods).
Unlike the results obtained with Ca by Morais et al. [17], matrix matching for P was adequate to reach a satisfactory accuracy. These results would suggest that Ca and P interact in different ways with biochar and the process that culminates in the excitation of P in LIBS plasma, unlike Ca, was similar for the standards and the samples.
In addition to greater calibration simplicity, the proposed analytical method has built-in the characteristics of LIBS in terms of analytical simplicity, rapid analysis with no chemical waste and little sample preparation, dispensing chemical reagents and the drastic conditions of temperature and pressure, conventionally used. The method also stands out for its cost-effectiveness and for being an eco-friendly solution. However, further studies must be conducted to assess the appropriateness of the calibration strategy for biochar-based fertilizers from different sources.
4. Conclusions
A novel, simple, fast, eco-friendly and non-chemical waste-producing method based on SD-LIBS was proposed for the direct P determination in controlled-release biochar-based fertilizers. A simple procedure of matrix matching using eucalyptus biochar with P added allowed one to reach an adequate accuracy for P determination in fertilizers prepared from three different agricultural residues. Although an evaluation for new fertilizers is required, the method is promising, affording a simple solution for characterization and certification of biochar products and meeting the IBI demands in an ecologically correct and economically viable way.
Author Contributions
Conceptualization, E.C.F. and C.A.R.; Formal analysis, S.R.D.; Data curation, E.C.F.; Writing—original draft preparation, E.C.F.; Writing—review and editing, G.S.S. and J.A.G.N.; Funding acquisition, E.C.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by São Paulo Research Foundation—FAPESP, Grant number 2015/24757-9 and Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq for the research grant to E.C.F., Grant number 308200/2018-7.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Hammond, J.P.; Broadley, M.R.; White, P.J. Genetic responses to phosphorus deficiency. Ann. Bot. 2004, 94, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Rozo, G.; Bohorques, L.; Santamaría, J. Controlled release fertilizer encapsulated by a κ-carrageenan hydrogel. Polímeros 2019, 29, 1–7. [Google Scholar] [CrossRef]
- Zhang, T. Phosphorus—Recovery and Recycling, 1st ed.; IntechOpen: London, UK, 2019; p. 104. [Google Scholar]
- Quijón, M.E.G. Development of an Urea-Based Controlled-Release Nitrogen Fertilizer Using Biochar as Support Material. Ph.D. Thesis, Universidad de la Frontera, Temuco, Chile, 2013. Available online: https://doctoradorrnn.ufro.cl/wp-content/uploads/2021/01/49-maria-eugenia-gonzalez.pdf (accessed on 24 November 2021).
- Cen, Z.; Wei, L.; Muthukumarappan, K.; Sobhan, A.; McDaniel, R. Assessment of a biochar-based controlled release nitrogen fertilizer coated with polylactic acid. J. Soil Sci. Plant Nutr. 2021, 21, 2007–2019. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Fang, Y.; Singh, B.; Singh, B.P.; Krull, E. Biochar carbon stability in four contrasting soils. Eur. J. Soil Sci. 2014, 65, 60–71. [Google Scholar] [CrossRef]
- International Biochar Initiative (IBI). Available online: http://www.biochar-international.org (accessed on 19 July 2021).
- Hoffman, W.M. AOAC Methods for the determination of phosphorus in fertilizers. J. AOAC Int. 1964, 47, 420–428. [Google Scholar] [CrossRef] [Green Version]
- Hanson, W.C. The photometric determination of phosphorus in fertilizers using the phosphovanado-molybdate complex. J. Sci. Food Agric. 1950, 1, 172–173. [Google Scholar] [CrossRef]
- Yang, W.M.; Boles, R.L.; Mawhinney, T.P. Determination of phosphorus in fertilizers by inductively coupled plasma atomic emission spectrometry. J. AOAC Int. 2002, 85, 1241–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welz, B.; Vale, M.G.R.; Pereira, E.R.; Castilho, I.N.B.; Dessuy, M.B. Continuum source atomic absorption spectrometry: Past, present and future aspects—A critical review. J. Braz. Chem. Soc. 2014, 25, 799–821. [Google Scholar] [CrossRef]
- Corazza, G.; Henn, A.S.; Mesko, M.F.; Duarte, F.; Flores, E.; Mello, P. Microwave-induced combustion of coal for further sulfur determination by inductively coupled plasma optical emission spectrometry or ion chromatography. J. Braz. Chem. Soc. 2016, 27, 1569–1576. [Google Scholar] [CrossRef]
- Yu, K.; Ren, J.; Zhao, Y. Principles, developments and applications of laser-induced breakdown spectroscopy in agriculture: A review. Artif. Intell. Agric. 2020, 4, 127–139. [Google Scholar] [CrossRef]
- Miziolek, A.W.; Palleschi, V.; Schechter, I. Laser-Induced Breakdown Spectroscopy (LIBS): Fundamentals and Applications, 1st ed.; Cambridge University Press: Cambridge, UK, 2006; pp. 1–40. [Google Scholar]
- Radziemski, L.J.; Cremers, D.A. Laser-Induced Plasmas and Applications, 1st ed.; Marcel Dekker Inc.: New York, NY, USA, 1989; p. 440. [Google Scholar]
- De Morais, C.P.; Barros, A.I.; Júnior, D.S.; Ribeiro, C.A.; Crespi, M.S.; Senesi, G.S.; Neto, J.A.G.; Ferreira, E.C. Calcium determination in biochar-based fertilizers by laser-induced breakdown spectroscopy using sodium as internal standard. Microchem. J. 2017, 134, 370–373. [Google Scholar] [CrossRef] [Green Version]
- Vieira, A.L.; Silva, T.; de Sousa, F.S.I.; Senesi, G.; Júnior, D.S.; Ferreira, E.C.; Neto, J.A.G. Determinations of phosphorus in fertilizers by spark discharge-assisted laser-induced breakdown spectroscopy. Microchem. J. 2018, 139, 322–326. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).