Abstract
Cruciferous vegetables (Brassicaceae family) are a rich source of phytochemicals, in particular glucosinolates (GLS) and their hydrolysis products, isothiocyanates and nitriles. These phytochemicals may act as chemosensors, attracting insects, such as Pieris rapae, and stimulating oviposition. There is a lack of information on the concentrations of isothiocyanates and nitriles when an insect affects a Brassicaceae plant. In the current study, some GLS hydrolysis products were determined in healthy and Pieris rapae-infected organic cultivated broccoli plants, as well as the infesting insects’ larvae, using gas chromatography-mass spectrometry (GC-MS). This study investigated the following phytochemicals: phenethyl isothiocyanate (PEITC), erucin (ER), 3-(methylthio)propyl isothiocyanate (3MIC), and 1-cyano-4-(methylthio)butane (5MITN). All these components were quantified in the aerial and underground parts of the plants and were found in high concentrations in the roots. Among the phytochemicals studied, 5MITN presented the highest concentration in all the broccoli samples but was especially high in the stalks of the infected plants. Moreover, the analysis of a sample of Pieris rapae larvae, fed from the hosted broccoli, revealed the presence of PEITC and ER. These findings indicate that the infestation of broccoli with Pieris rapae may affect the distribution of PEITC, ER, 3MIC, and 5MITN throughout the plant. An extension of our study to conventional cultivated broccoli showed that the roots are indeed rich in GLS hydrolysis products.
1. Introduction
Broccoli (Brassica oleracea var. italica) is an economically important vegetable that is widely grown in many countries [1]. It belongs to the Brassicaceae family and is a relative of cauliflower, cabbage, Brussels sprouts, and kale. Nowadays, broccoli can be considered functional food due to the presence of phytochemicals in it, such as glucosinolates (GLS) [2], which have the potential to protect and promote human health beyond basic nutrition [3].
GLS are sulfur β-glycosides, which can be hydrolyzed via the action of the enzyme myrosinase (EC 3.2.3.1) to produce aglycon products. Depending on the pH, temperature, and concentration of Fe2+, the majority of these aglycon products are isothiocyanates (ITCs) and nitriles [4,5]. GLS are not bioactive compounds by themselves, but their hydrolysis products have been demonstrated to exhibit human and plant chemo-preventive activities [6,7,8]. Ιsothiocyanate sulforaphane (SFN, 1-isothiocyanato-4-(methylsulfinyl)-butane] (Figure 1) has attracted considerable attention in research for its bioactivities [9]. It originates from glucoraphanin and exhibits anticarcinogenic properties by inducing phase II detoxification enzymes in humans; it is also considered as a histone deacetylase inhibitor. SFN exhibits strong inhibitory effects against plant pathogenic bacteria, and antifungal activity. However, several other isothiocyanates, such as phenylethyl isothiocyanate (PEITC), erucin (ER, 4-methylthiobutyl isothiocyanate), and iberverin (3-methylthiopropyl isothiocyanate (3MIC)) are also present in broccoli [3]. ER, which is derived from GLS glucoerucin, exhibits antioxidant activity in experimental models [10], participates in the Nrf2 detoxification pathway [11], and shows protective activity against prostate cancer [12]. In addition, ER has been shown to suppress the activity of some fungi [13]. PEITC, the GLS gluconasturtiin hydrolysis product, is an inhibitor of cytochrome P450 2E1 [14] and exhibits antiobesity and antihepatosteatosis activities [15]. PEITC also suppresses the microsclerotia of Verticillium dahliae [16] and presents antifungal activity [17]. 3MIC, a homolog of ER, is the hydrolysis product of glucoiberverin, showing strong antibacterial activity against Klebsiella pneumoniae [18].
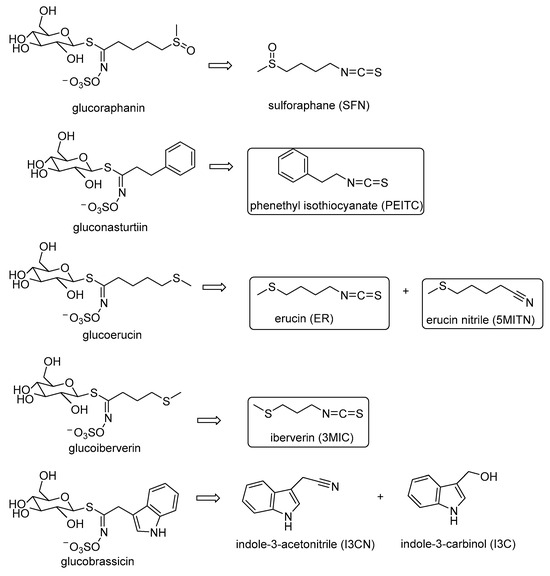
Figure 1.
Structures of main products of glucosinolates’ hydrolysis.
Contrarily, the nitriles generated from GLS hydrolysis may be harmful to human health. They could cause kidney, thyroid, and liver dysfunctions, as well as behavioral and neurological disorders. Indole-3-acetonitrile (I3CN) (Figure 1) originates from glucobrassicin, which may also produce indole-3-carbinol (I3C) (Figure 1). I3C is one of the major anticancer substances found in Brassica vegetables with chemo-preventive potential in hormone-dependent tumors [19]. Erucin nitrile (5MITN, 1-cyano-4-(methylthio)butane) may also be generated from glucoerucin, and it is responsible for the characteristic aroma observed in all specimens and especially in the roots of broccoli [20]. It also has potential activity as phytoalexin. The structures of these main GLS hydrolysis products mentioned above are shown in Figure 1.
Great attention has been paid to the detection and quantification of ITCs and nitriles in the florets of Brassica plants [21,22,23] and broccoli sprouts [24,25]. The levels of sulforaphane, PEITC, and ER have been recently quantified in raw and microwaved broccoli [26]. Also, GLS and GLS hydrolysis products in radish roots have been determined in parallel with a targeted gene expression analysis of their biosynthesis [27]. However, the majority of the research on the analysis of ITCs and nitriles has mainly referred to florets and sprouts, while data on the composition of such components in roots and stalks are limited.
Insect pests may have destructive consequences for broccoli production [28]. One such pest is the cabbage white butterfly (Pieris rapae.) The insect larvae attach to the underside of the leaves, chewing on the leaves and damaging the broccoli heads. In the Brassicaceae family, GLS act as chemosensors, adopting the roles of food stimulants and mediators of oviposition for Pieris rapae and Pieris brassicae larvae [29,30]. The activity of several GLS in the stimulation of oviposition has been studied [31], and later on, a series of chemosensory ablations showed that the feeding stimulation of Pieris rapae by gluconastrutiin was mediated by one set of taste sensilla, the sensilla styloconica [32]. Furthermore, the indole and aromatic types of GLS, such as gluconasturtiin and glucobrassicin, function as spawning markers for adult females [33,34]. Currently, there is a lack of information on the concentration levels of GLS hydrolysis products, generated as a result of an insect infecting a Brassicaceae plant. Quantifying these secondary metabolites may promote a clearer understanding of the chemical strategies adopted by Brassicaceae to attract and repel insects. A study of the distribution of GLS hydrolysis products in aerial and underground parts of insect-infected broccoli plants would therefore be valuable and could shed light on the fascinating defense mechanisms of these plants.
The aim of the present study was the determination of the contents of PEITC, ER, 3MIC, and 5MITN in the aerial (florets, stalks, leaves) and underground (roots) parts of healthy and Pieris rapae-infected organic cultivated broccoli plants, using gas chromatography-mass spectrometry (GC-MS), in an effort to understand how such a pest infection may modify the metabolism of GLS. In addition, a sample of Pieris rapae larvae, fed from the hosted organic cultivated broccoli, was analyzed to understand if PEITC, ER, 3MIC, and 5MITN were present in the larvae’s body. Finally, roots collected from conventional broccoli fields were evaluated using GC-MS for their contents of PEITC, ER, 3MIC and 5MITN.
2. Materials and Methods
2.1. Reagents and Materials
ER, 5MITN, 3MIC, and PEITC were purchased from Cayman Chemical (Ann Arbor, MI, USA), Biosynth AG (Staad SG, Switzerland), Alfa Aesar (Ward Hill, MA, USA), and Acros Organics (Geel, Belgium), respectively. Dichloromethane was of analytical grade and purchased from Sigma–Aldrich Chemical Co. (St. Louis, MO, USA). A Heidolph 2 rotary evaporator (Heidolph Instruments GmbH & Co.KG, Schwabach, Germany) and Whatman filter paper grade 1 (Whatman Ltd., Maidstone, UK) were used for the preparation of extracts.
2.2. Standard Solutions
Stock solutions of PEITC, ER, 3MIC, and 5MITN (1000 mg/L) were prepared in dichloromethane and stored in dark glass containers at −20 °C.
2.3. Sampling
Organic cultivated broccoli plants, Brassica oleracea L. var. italica Plenck (Monrello), were grown in the Agricultural University of Athens field (37°59′2″ N 23°42′19″ E) until florets were ready for human consumption. Roots (R1 and R2), stalks (S1 and S2), leaves (L1 and L2), and florets (F1 and F2) from Pieris rapae-infected and healthy plants, respectively, were harvested. Roots (R3 and R5) from conventional cultivated nonfertilized plants were collected from Agrinio (38°37′47″ N 21°24′42″ E) and Vassilika (38°58′2″ N 23°21′23″ E), respectively. Roots (R4) from conventional cultivated, fertilized with nitrogen–sulfur plants were collected from a local producer field in Argos (37°39′38″ N 22°42′19″ E). All the samples were lyophilized and, after grounding to a fine homogenous powder, were stored at −20 °C until they were analyzed.
2.4. GLS Hydrolysis and Extraction Conditions
Hydrolysis of GLS was conducted according to the method previously described by Kokotou et al. [35] with some modifications. A total of 20 mL of McIIvaine buffer (0.2 M Na2HPO4 (16.47 mL), 0.1 M citric acid (3.53 mL)] (pH 7.0) was added into 1 g of dry tissue and the mixture was incubated in a water bath for 3 h at 45 ± 3 °C. After addition of 30 mL dichloromethane, the mixture was stirred for 15 min and then filtered using a Buchner funnel equipped with Whatman filter paper grade 1. The solid residue was extracted twice with 40 mL dichloromethane. The filtrates were combined into a separation funnel for the removal of the excess water and were dried with 1 g of anhydrous sodium sulfate. The solvent was then evaporated to dryness at 35 °C under vacuum, on a rotary evaporator, and dissolved in 1 mL dichloromethane for GC-MS analysis. The determination was performed in triplicates.
Pieris rapae larva was lyophilized and was then treated as described for broccoli tissues to prepare the sample for analysis.
2.5. Analysis of Glucosinolate Hydrolysis Products
2.5.1. GC-MS Method
PΕITC, ER, 3MIC, and 5MITN were analyzed using a DSQ II mass selective detector coupled with a THERMO GC ULTRA gas chromatograph (Thermo Scientific Inc., Waltham, MA, USA), without autoinjector. The samples (1 μL) were injected in splitless mode. A capillary column, Restek Rtx-5MS (30 m × 0.25 mm × 0.25 μm) (Restek, Bellefonte, PA, USA), was used. The program used was according to Ciska et al. [36]. Oven temperature was initially set at 35 °C for 5 min, then increased to 210 °C (8 °C/min) and maintained for 10 min. Injector and transfer line temperatures were 210 °C and 240 °C, respectively. Mass spectra were obtained via electron ionization (EI) over the range of 35–550 m/z. Ion source temperature was 230 °C, and electronic impact energy was 70 eV. The identification of compounds was based on their retention time and the EI fragmentation pattern, using standard solutions and comparison of their mass spectra, existing in libraries (Adams 07, Nist 98, Wiley 275). The data were analyzed using the program Thermo Xcalibur 2.2.44 (Thermo Scientific Inc., Waltham, MA, USA).
2.5.2. Validation, Linearity, and Detection Limits
For quantitation, the standard solutions were prepared for ER and PEITC at concentrations of 0.1–12 mg/mL (3 replicates; 10 levels (0.1, 0.3, 0.5, 0.8, 1, 2, 3, 5, 8, 12 mg/L); n = 3 × 10), for 3MIC at concentrations of 0.1–5 mg/mL (3 replicates; 8 levels (0.1, 0.3, 0.5, 0.8, 1, 2, 3, 5 mg/L); n = 3 × 8), and for 5MITN at concentrations of 10–120 mg/mL (3 replicates; 8 levels (5, 10, 20, 40, 60, 80, 100, 120 mg/L); n = 3 × 8) in dichloromethane. A reference curve for each compound was constructed The limits of detection (LOD) and quantification (LOQ) in the reference curves were calculated based on the following equations: LOD = 3.3 × Sa/b and LOQ = 10 × Sa/b, where Sa is the standard deviation, and b is the slope. Excellent linearity between the peak area of the analytes (y) and the corresponding concentration (x) was obtained. The correlation coefficient (R2), LOD, and LOQ were 0.991, 0.18 mg/L, and 0.53 mg/L for PEITC; 0.999, 0.07 mg/L, and 0.20 mg/L for ER; 0.993, 0.09 mg/L, and 0.27 mg/L for 3MIC; 0.995, 2.29 mg/L, and 6.93 mg/L for 5MITN, respectively.
3. Results
3.1. Broccoli Plants
The botanical parts of the broccoli used in the present study were the florets, stalks, leaves (aerial part), as well as roots (underground part). The broccoli plants grew 60–90 cm tall, forming upright and branching, thick green stalks carrying leathery, oblong, gray-blue to green rosette basal leaves. Florets are the dense green edible clusters of flower buds, located at the end of the central axis and the branches (stems). The parts of the plant commonly consumed by humans are the florets and the upper stem, which form the head of the broccoli. Broccoli leaves and large stalks are not usually commercially utilized, and they are considered agro-industrial by-products.
3.2. Extraction of Isothiocyanates PEITC, ER, 3MIC, and Nitrile 5MITN from Broccoli Samples, and Determination via GC-MS
Among the various solvents (methanol, methyl tert-butyl ether, dichloromethane, chloroform, and ethanol) that have been used for the extraction of ITCs and nitriles from the Brassicaceae family plants [37], dichloromethane was chosen and used, since it is a common, nonpolar solvent.
GC-MS was used for the analysis of PEITC, ER, 3MIC, and 5MITN in broccoli tissues. To date, for the vast majority of methods used for the analysis of ITCs, high-performance liquid chromatography (HPLC) has been employed. An ITC with an alkyl chain, such as ER, is a volatile organic compound and can be degraded during GC-MS analysis [38]. The majority of the researchers applying GC-MS analysis have used the split injection mode and an initial temperature exceeding 35 °C. Higher initial temperatures lead to the conversion of ITCs to thiazoles and isothiazoles [39]. For this reason, an initial temperature of 35 °C and splitless injection mode were chosen for the GC-MS program in the present study.
The concentrations of four GLS hydrolysis products were determined using GC-MS analysis. The contents of the isothiocyanates PEITC, ER, and 3MIC, and nitrile 5MITN in the broccoli florets, roots, stalks, and leaves of an infected (F1, R1, S1, and L1) and a noninfected (F2, R2, S2, and L2) plant are shown in Table 1.

Table 1.
Contents of isothiocyanates PEITC, ER, 3MIC, and nitrile 5MITN in Pieris rapae-infected and noninfected broccoli samples (μg g DW−1).
In our work, higher concentrations of the isothiocyanates PEITC, ER, and 3MIC were detected and quantified in the roots of infected (R1) and noninfected (R2) plants than in the florets, stalks, and leaves. However, 5MITN was found in much higher concentrations in the stalks (S1) of the infected plants than in the stalks (S2) of the noninfected plants and in the florets, roots, and leaves. PEITC was found in considerably higher concentrations in the roots than in the other parts of the plants. The measured concentration of ER was much higher in the roots, followed by the stalks, but it was not detected in the leaves. 3MIC was detected and quantified only in the roots of either infected (S1) or noninfected (S2) plants and not in the florets, stalks, or leaves.
Figure 2 shows a direct comparison of the concentration of isothiocyanates PEITC, ER, 3MIC, and nitrile 5MITN in the aerial and underground parts of Pieris rapae-infected and noninfected broccoli samples. The concentration of PEITC (Figure 2A) is clearly higher in the roots and stalks of the noninfected plants than in the corresponding parts of the infected plant. Similarly, higher concentrations of ER (Figure 2B) were found in noninfected roots and stalks. As mentioned above, 3MIC was found only in the roots, and its concentration (Figure 2C) followed the same trend. However, although the concentration of 5MITN was higher in the noninfected roots, its content in the stalks was clearly higher in the infected tissue in comparison to the noninfected tissues.
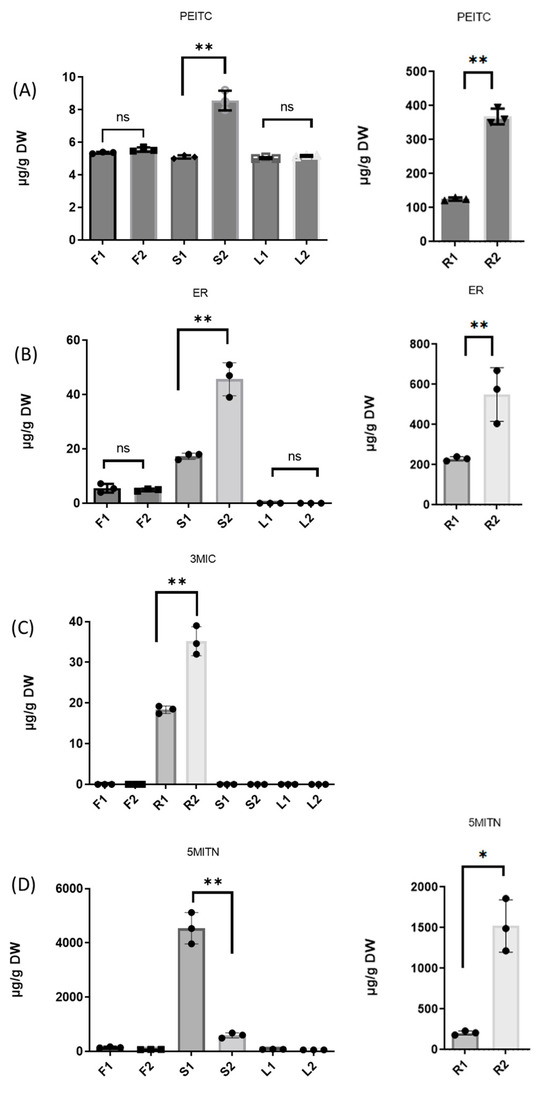
Figure 2.
Distribution of isothiocyanates PEITC (A), ER (B), and 3MIC (C), and nitrile 5MITN (D) in Pieris rapae-infected and noninfected broccoli cultivar in aerial and underground parts of the plants. Graphs were created using GraphPad Prism 9.2.0 (Dotmatics, CA, USA). One-way ANOVA statistical analysis was performed for each separate set comparing to control. ns (not significant): p > 0.05. * p < 0.05, ** p < 0.01.
The high values of ITC contents observed in the roots prompted us to extend our work to studying three additional root broccoli samples (noninfected conventional cultivated plants) grown for human consumption in different fields. Roots R3 and R5 came from nonfertilized plants, whereas root R4 originated from a nitrogen–sulfur-fertilized plant. The results are summarized in Figure 3 and Table 2.
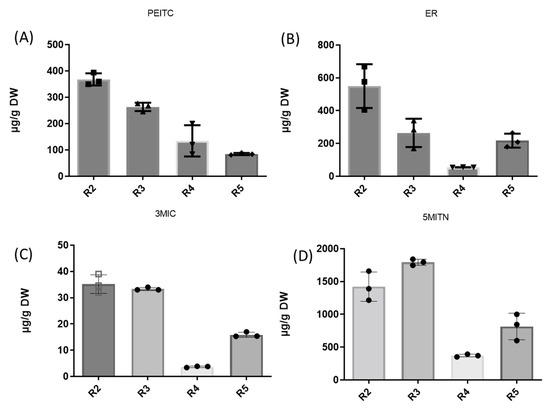
Figure 3.
Comparison of isothiocyanates PEITC (A), ER (B), 3MIC (C), and nitrile 5MITN (D) contents in roots of broccoli cultivar. Graphs were created using GraphPad Prism 9.2.0.

Table 2.
Contents of isothiocyanates PEITC, ER, 3MIC, and nitrile 5MITN in broccoli roots (μg g DW−1).
The concentrations of all ITCs and nitrile showed high variations among the four root samples. The contents of PEITC, ER, 3MIC, and 5MITN ranged from 367.6 to 86.5 μg g DW−1, 576.3 to 54.7 μg g DW−1, 34.6 to 3.9 μg g DW−1, and 1795.1 to 371.3 μg g DW−1, respectively. It should be noted that the lowest concentrations of ER, 3MIC, and 5MITN were observed in the roots of the nitrogen–sulfur-fertilized plants.
Furthermore, in an effort to gain insight into the outcome of the Pieris rapae infection, we analyzed a sample of the insect fed from the hosted broccoli to explore if GLS hydrolysis products were present in the insect. As shown in Figure 4 and Table 3, both isothiocyanates PEITC and ER were detected and quantified in Pieris rapae (triplicates). The contents of ER (1.4 ± 0.002 µg/g DW) were three times higher than those of PEITC (0.5 ±0.00 µg/g DW). However, the isothiocyanate 3MIC and nitrile 5MITN were absent.
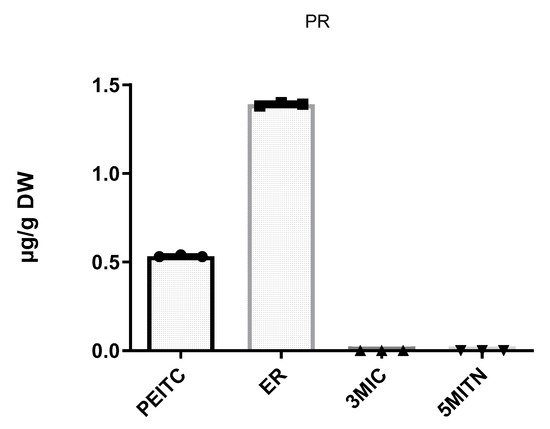
Figure 4.
Contents of isothiocyanates PEITC, ER, 3MIC, and nitrile 5MITN in Pieris rapae. Graphs were created using GraphPad Prism 9.2.0.

Table 3.
Contents of isothiocyanates PEITC, ER, 3MIC, and nitrile 5MITN in Pieris rapae (μg g DW−1) (triplicates).
4. Discussion
Broccoli is an annual crop, native to the countries of the eastern Mediterranean, but cultivated worldwide. According to a FAOSTAT report, it is estimated that in 1999, approximately 15.00 million tons of broccoli and cauliflower were produced worldwide, while their total production had increased to approximately 26.92 million tons in 2019 [40]. They are gaining increasing popularity in the human diet due to their rich nutritional value, as well as the presence of anticancer glucosinolates–isothiocyanates in its florets. In recent years, broccoli farming has made tremendous progress globally, with, for example, about 80,000 ha of cultivated area in China in 2019 [1].
Pieris rapae belongs to the cabbage butterflies, which may attack various plants, including broccoli and cabbage, destructively affecting crops [28]. The young larvae, which are gregarious, may skeletonize the leaves of plant or even bore into their heads. A female moth is able to lay more than one cluster of eggs on the under-surface of broccoli leaves. It has been known since the 1990s that the chemical compounds present in the leaves of broccoli or cabbage stimulate oviposition by Pieris rapae and Pieris brassicae [29,30]. GLS act as chemosensors and play the role of a “food stimulant” for Pieris rapae larvae [31,33,41]. In particular, indole- and aromatic-type GLS, such as gluconasturtiin and glucobrassicin, are spawning markers for adult females. The distribution of GLS hydrolysis products in the aerial and underground parts of insect-infected broccoli plants has attracted our attention because the measurement of such secondary metabolites may help us to better understand how Brassica adopts chemical strategies for attracting or repelling insects.
In our study, a notable variation in the content of each GLS hydrolysis product was observed in the different tissues (aerial and underground) of the healthy and Pieris rapae-infected plants. A comparison of the distribution of ITCs and nitrile studied in the different parts of the infected plants with that in the healthy plants unraveled a significant reduction in PEITC, ER, 3MIC, and 5MITN in the roots of the infected plants. There was also a significant decline in the ER content in the stalks of the infected plants. Conversely, the content of 5MITN was determined as almost seven times higher than that in the unaffected stalks. This increase in concentration of 5MITN in the stalks, accompanied by a decrease in the roots, indicated that the plant’s physiological metabolism was modified. This redistribution of contents les us to conclude that 5MITN could play the role of a phytoalexin, produced in high quantities after the activation of the glucosinolate–myrosinase defense system of the plant. Phytoalexins are generally derivatives of metabolites produced in unstressed plants, indicating that these compounds are produced by the modification of the physiological metabolism of plants [42]. Overall, the insect’s attack affects broccoli metabolism and induces a redistribution of metabolites in the tissues of the plant. In a Pieris rapae-infected broccoli plant, the concentration of 5MITN nitrile is increased, which may have deleterious effects on consumption and human health. Until now, there have been insufficient data to help us fully understand the mechanism behind the alteration in the concentrations of GLS hydrolysis products in broccoli roots after infection by Pieris rapae.
An examination of the contents of ITCs and nitrile found in Pieris rapae larvae fed infected broccoli plants was also informative. To the best of our knowledge, for the first time, ITCs were quantified in the larvae of Pieris rapae fed with organic cultivated broccoli. For female butterflies, the color, surface, and tissue of the leaves are important factors for egg-laying capacity on the host plant [43,44]. Moreover, GLS, which possess an aromatic group, can be used as spawning sites for adult females [45]. In our case, gluconasturtiin, the precursor glucosinolate of PEITC, served as the spawning site, attracting the adult female. In the present study, we show the presence of isothiocyanates PEITC and ER, in the larvae’s bodies. However, 5MITN nitrile was not detected in the larvae’s bodies.
The high values of ITCs observed in roots from organic cultivated broccoli, either infected or not, prompted us to examine the levels of these ITCs in three additional root broccoli samples from noninfected, conventional cultivated plants grown for human consumption in different fields. Isothiocyanates PEITC, ER, and 3MIC were estimated at mean concentrations of 215 ± 90.21 μg/g DW, 280.75 ± 120.54 μg/g DW, and 21.75 ± 8.76 μg/g DW, respectively. In addition, nitrile 5MITN was found at a mean concentration of 1125.75 ± 540.11 μg/g DW. These data indicate that GLS hydrolysis products are present in broccoli roots in high concentrations. Of interest is also our finding that the root (R4) of the nitrogen–sulfur-fertilized broccoli showed the lowest contents of ER, 3MIC, and 5MITN. These data are in accordance with the observations of Aires et al. [25] and Rodríguez-Hernández et al. [46] that fertilization influences the levels of ITCs. Therefore, fertilization should be a carefully considered factor when using broccoli by-products as sources of bioactive ITCs.
GLS hydrolysis products, in particular ITCs, have broad biocidal activity, including fungicidal effects [47,48,49]. Most recently, Eugui et al. demonstrated that GLS extracts from the residues of conventional and organic cultivated broccoli leaves (Brassica oleracea var. italica) may find use as potential industrially scalable biopesticides against fungi, oomycetes, and plant parasitic nematodes [50].
Broccoli may also find applications in the food industry, such as in the encapsulation of bioactive compounds and food formulation. It has been used as a carrier for the water-soluble vitamin thiamine (vitamin B1) [51], while broccoli leaves and stalks were used for the delivery of epigallocatechin gallate [52]. Thus, agro-industrial broccoli residues may find use as renewable and low-cost resources for the production of encapsulated bioactive compounds.
Applications of broccoli in the formulation of snacks and beverages to promote their nutraceutical potential have also been reported. Broccoli leaf powders have been used to make gluten-free sponge cakes to improve their nutraceutical potential [53] and in gluten-free bread [54]. Certainly, in vivo studies of the formulated snacks are needed to prove the health benefits of such products. The formulation of snacks might be extended to include various broccoli botanical parts, such as roots and seeds.
Future studies are needed to understand the mechanism of damage that may be caused by Pieris rapae infestation and the redistribution of GLS hydrolysis products in the various tissues of broccoli plants. To this end, detailed analytical studies for the levels of GLS hydrolysis products should be performed in all the aerial and underground parts of the broccoli plant.
Special attention should also be paid to the various underutilized broccoli by-products, such as leaves, stalks, and, in particular, the roots, which contain considerable amounts of bioactive phytochemicals, which may find applications in areas of economic interest.
Author Contributions
Conceptualization, M.G.K. and V.C.-K.; methodology, I.-D.M. and P.-K.R.; software, I.-D.M., M.G.K., and P.-K.R.; writing—review and editing, I.-D.M. and M.G.K.; supervision, M.G.K. and V.C.-K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data supporting this study are included in the article.
Acknowledgments
The authors thank the Horticultural Crops Laboratory of Agricultural University of Athens for the kind donation of broccoli plants. M.G.K. would like to thank L’Oréal-UNESCO for the award “For Women in Science 2023”.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shen, Y.; Wang, J.; Shaw, R.K.; Yu, H.; Sheng, X.; Zhao, Z.; Li, S.; Gu, H. Development of GBTS and KASP panels for genetic diversity, population structure, and fingerprinting of a large collection of broccoli (Brassica oleracea L. var. italica) in China. Front. Plant Sci. 2021, 12, 655254. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.P.T.; Stewart, J.; Lopez, M.; Ioannou, I.; Allais, F. Glucosinolates: Natural occurrence, biosynthesis, accessibility, isolation, structures, and biological activities. Molecules 2020, 25, 4537. [Google Scholar] [CrossRef]
- Li, H.; Xia, Y.; Liu, H.-Y.; Guo, H.; He, X.-Q.; Liu, Y.; Wu, D.-T.; Mai, Y.-H.; Li, H.-B.; Zou, L.; et al. Nutritional values, beneficial effects, and food applications of broccoli (Brassica oleracea var. italica Plenck). Trends Food Sci. Technol. 2022, 119, 288–308. [Google Scholar] [CrossRef]
- Wittstock, U.; Kurzbach, E.; Herfurth, A.-M.; Stauber, E. Glucosinolate breakdown. Adv. Bot. Res. 2016, 80, 125–169. [Google Scholar]
- Ilahy, R.; Tlili, I.; Pek, Z.; Montefusco, A.; Siddiqui, M.W.; Homa, F.; Hdider, C.; R’Him, T.; Lajos, H.; Lenucci, M.S. Pre- and post-harvest factors affecting glucosinolate content in broccoli. Front. Nutr. 2020, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Palliyaguru, D.L.; Yuan, J.M.; Kensler, T.W.; Fahey, J.W. Isothiocyanates: Translating the power of plants to people. Mol. Nutr. Food Res. 2018, 62, 1700965. [Google Scholar] [CrossRef] [PubMed]
- Eagles, S.K.; Gross, A.S.; McLachlan, A.J. The effects of cruciferous vegetable-enriched diets on drug metabolism: A systematic review and meta-analysis of dietary intervention trials in humans. Clin. Pharmacol. Therap. 2020, 108, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Radošević, K.; Srček, V.G.; Bubalo, M.C.; Brnčić, S.R.; Takács, K.; Redovniković, I.R. Assessment of glucosinolates, antioxidative and antiproliferative activity of broccoli and collard extracts. J. Food Comp. 2017, 61, 59–66. [Google Scholar] [CrossRef]
- Kaiser, A.E.; Baniasadi, M.; Giansiracusa, D.; Giansiracusa, M.; Garcia, M.; Fryda, Z.; Wong, T.L.; Bishayee, A. Sulforaphane: A broccoli bioactive phytocompound with cancer preventive potential. Cancers 2021, 13, 4796. [Google Scholar] [CrossRef]
- Zhang, Y.; Talalay, P.; Cho, C.G.; Posner, G.H. A major inducer of anticarcinogenic protective enzymes from broccoli: Isolation and elucidation of structure. Proc. Natl. Acad. Sci. USA 1992, 89, 2399–2403. [Google Scholar] [CrossRef]
- Ernst Insa, M.A.; Palani, K.; Esatbeyoglu, T.; Schwarz, K.; Rimbach, G. Synthesis and Nrf2-inducing activity of the isothiocyanates iberverin, iberin and cheirolin. Pharmacol. Res. 2013, 70, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Melchini, A.; Traka, M.H.; Catania, S.; Miceli, N.; Taviano, M.F.; Maimone, P.; Francisco, M.; Mithen, R.F.; Costa, C. Antiproliferative activity of the dietary isothiocyanate erucin, a bioactive compound from cruciferous vegetables, on human prostate cancer cells. Nutr. Cancer 2013, 65, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Manici, L.M.; Lazzeri, L.; Baruzzi, G.; Leoni, O.; Galletti, S.; Palmieri, S. Suppressive activity of some glucosinolate enzyme degradation products on Pythium irregulare and Rhizoctonia solani in sterile soil. Pest Manag. Sci. 2000, 56, 921–926. [Google Scholar] [CrossRef]
- Crowley, E.; Rowan, N.J.; Faller, D.; Friel, A.M. Natural and synthetic isothiocyanates possess anticancer potential against liver and prostate cancer in vitro. Anticancer Res. 2019, 39, 3469–3485. [Google Scholar] [CrossRef] [PubMed]
- Chuang, W.-T.; Yun-Ta, L.; Huang, C.-S.; Lo, C.-W.; Yao, H.-T.; Chen, H.-W.; Lii, C.-K. Benzyl isothiocyanate and phenethyl isothiocyanate inhibit adipogenesis and hepatosteatosis in mice with obesity induced by a high-fat diet. J. Agric. Food Chem. 2019, 67, 7136–7146. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, C.; Heitmann, B.; Müller, C. Biofumigation potential of Brassicaceae cultivars to Verticillium dahliae. Eur. J. Plant Pathol. 2014, 140, 341–352. [Google Scholar] [CrossRef]
- Hooker, W.J.; Walker, J.C.; Smith, F.G. Toxicity of beta-phenethyl isothiocyanate to certain fungi. Am. J. Bot. 1943, 30, 632–637. [Google Scholar]
- Wilson, A.E.; Bergaentzlé, M.; Bindler, F.; Marchioni, E.; Lintz, A.; Ennahar, S. In vitro efficacies of various isothiocyanates from cruciferous vegetables as antimicrobial agents against foodborne pathogens and spoilage bacteria. Food Control 2013, 30, 318–324. [Google Scholar] [CrossRef]
- Baena, R.; Salinas, H. Cancer chemoprevention by dietary phytochemicals: Epidemiological evidence. Maturitas 2016, 94, 13–19. [Google Scholar] [CrossRef]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Food Chemistry, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Ghawi, S.K.; Methven, L.; Niranjan, K. The potential to intensify sulforaphane formation in cooked broccoli (Brassica oleracea var. italica) using mustard seeds (Sinapis alba). Food Chem. 2013, 138, 1734–1741. [Google Scholar] [CrossRef]
- Torres-Contreras, A.M.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Stability of bioactive compounds in broccoli as affected by cutting styles and storage time. Molecules 2017, 22, 636. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Pei, Y.; Qi, A.; Jingnan, R.; Gang, F.; Zelan, W.; Xixiang, L.; Siyi, P. Identification of glucosinolates and volatile odor compounds in microwaved radish (Raphanus sativus L.) seeds and the corresponding oils by UPLC-IMS-QTOF-MS and GC × GC-qMS analysis. Food Res. Int. 2023, 169, 112873. [Google Scholar]
- Hanschen, F.S.; Schreiner, M. Isothiocyanates, nitriles, and epithionitriles from glucosinolates are affected by genotype and developmental stage in Brassica oleracea varieties. Front. Plant Sci. 2017, 8, 1095. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Rosa, E.; Carvalho, R. Effect of nitrogen and sulfur fertilization on glucosinolates in the leaves and roots of broccoli sprouts (Brassica oleracea var. italica). J. Sci. Food Agric. 2006, 86, 1512–1516. [Google Scholar] [CrossRef]
- Zhang, Y.; Makaza, N.; Jiang, C.; Wu, Y.; Nishanbaev, S.Z.; Zou, L.; Sun, J.; Song, X.; Wu, Y. Supplementation of cooked broccoli with exogenous moringa myrosinase enhanced isothiocyanate formation. Food Chem. 2022, 395, 133651. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.; Lim, S.; Kim, J.; Lee, E.J. The mechanism of deterioration of the glucosinolate-myrosynase system in radish roots during cold storage after harvest. Food Chem. 2017, 233, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Krishna Moorthy, P.N.; Prasannakumar, N.R.; Mani, M.; Saroja, S.; Ranganath, H.R. Pests and their management in cruciferous vegetables (cabbage, cauliflower, knol khol, broccoli, radish, turnip, beet root). In Trends in Horticultural Entomology; Mani, M., Ed.; Spinger: Berlin/Heidelberg, Germany, 2022; pp. 997–1011. [Google Scholar]
- Renwick, J.A.A.; Radke, C.D.; Sachdev-Gupta, K.; Städler, E. Leaf surface chemicals stimulating oviposition by Pieris rapae (Lepidoptera: Pieridae) on cabbage. Chemoecology 1992, 3, 33–38. [Google Scholar] [CrossRef]
- Van Loon, J.J.A.; Blaakmeer, A.; Griepink, F.C.; Van Beek, T.A.; Schoonhoven, L.M.; De Groot, A. Leaf surface compound from Brassica oleracea (Cruciferae) induces oviposition by Pieris brassicae (Lepidoptera: Pieridae). Chemoecology 1992, 3, 39–44. [Google Scholar] [CrossRef]
- Huang, X.; Renwick, J.A.A. Relative activities of glucosinolates as oviposition stimulants for Pieris rapae and P. napi oleracea. J. Chem. Ecol. 1994, 20, 1025–1037. [Google Scholar] [CrossRef]
- Miles, C.I.; del Campo, M.L.; Renwick, J.A. Behavioral and chemosensory responses to a host recognition cue by larvae of Pieris rapae. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2005, 191, 147–155. [Google Scholar] [CrossRef]
- Stadler, E.; Renwick, J.; Radke, C.D.; Sachdev-Gupta, K. Tarsal contact chemoreceptor response to glucosinolates and cardenolides mediating oviposition in Pieris rape. Physiol. Entomol. 1995, 20, 175–187. [Google Scholar] [CrossRef]
- Badenes-Pérez, F.R. Plant glucosinolate content and host-plant preference and suitability in the small white butterfly (Lepidoptera: Pieridae) and comparison with another specialist lepidopteran. Plants 2023, 12, 2148. [Google Scholar] [CrossRef] [PubMed]
- Kokotou, M.G.; Revelou, P.K.; Pappas, C.; Constantinou-Kokotou, V. High resolution mass spectrometry studies of sulforaphane and indole-3-carbinol in broccoli. Food Chem. 2017, 237, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Ciska, E.; Drabinska, N.; Honke, J.; Narwojsz, A. Boiled Brussels sprouts: A rich source of glucosinolates and the corresponding nitriles. J. Funct Foods 2015, 19, 91–99. [Google Scholar] [CrossRef]
- Karanikolopoulou, S.; Revelou, P.-K.; Xagoraris, M.; Kokotou, M.G.; Constantinou-Kokotou, V. Current Methods for the extraction and analysis of isothiocyanates and indoles in cruciferous vegetables. Analytica 2021, 2, 93–120. [Google Scholar] [CrossRef]
- Arora, R.; Sharma, D.; Kumar, R.; Singh, B.; Vig, A.P.; Arora, S. Evaluating extraction conditions of glucosinolate hydrolytic products from seeds of Eruca sativa (Mill.) Thell. using GC-MS. J. Food Sci. 2014, 79, C1964–C1969. [Google Scholar] [CrossRef] [PubMed]
- Mukerjee, A.K.; Ashare, R. Isothiocyanates in the chemistry of heterocycles. Chem. Rev. 1991, 91, 1–24. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization Corporate Statistical Database. 2021. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 5 October 2023).
- Wittstock, U.; Agerbirk, N.; Stauber, E.J.; Olsen, C.E.; Hippler, M.; Mitchell-Olds, T.; Gershenzon, J.; Vogel, H. Successful herbivore attack due to metabolic diversion of a plant chemical defense. Proc. Natl. Acad. Sci. USA 2004, 101, 4859–4864. [Google Scholar] [CrossRef]
- Kaur, S.; Mahesh Kumar Samota, M.K.; Choudhary, M.; Choudhary, M.; Pandey, A.K.; Sharma, A.; Thakur, J. How do plants defend themselves against pathogens-Biochemical mechanisms and genetic interventions. Physiol. Mol. Biol. Plants 2022, 28, 485–504. [Google Scholar] [CrossRef]
- Itoh, Y.; Okumura, Y.; Fujii, T.; Ishikawa, Y.; Omura, H. Effects of mating on host selection by female small white butterflies Pieris rapae (Lepidoptera: Pieridae). J. Comp. Phys. A 2018, 204, 245–255. [Google Scholar] [CrossRef]
- Lund, M.; Brainard, D.C.; Szendrei, Z. Cue hierarchy for host plant selection in Pieris rapae. Entomol. Exp. Appl. 2019, 167, 330–340. [Google Scholar] [CrossRef]
- Yang, J.; Guo, H.; Jiang, N.-J.; Tang, R.; Li, G.-C.; Huang, L.-Q.; Van Loon, J.J.; Wang, C.-Z. Identification of a gustatory receptor tuned to sinigrin in the cabbage butterfly Pieris rapae. PLoS Genet. 2021, 17, e1009527. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, M.C.; Medina, S.; Gil-Izquierdo, A.; Martínez-Ballesta, C.; Moreno, D.A. Broccoli isothiocyanates content and in vitro availability according to variety and origin. Mac. J. Chem. Chem. Eng. 2013, 32, 251–264. [Google Scholar] [CrossRef]
- Brown, P.D.; Morra, M.J. Control of soil-borne plant pests using glucosinolate-containing plants. Adv. Agron. 1997, 61, 167–231. [Google Scholar]
- Rosa, E.A.S.; Heaney, R.K.; Fenwick, G.R.; Portas, C.A.M. Glucosinolates in Crop Plants; Horticultural Reviews; Wiley & Sons, Inc.: Hoboken, NJ, USA, 1997. [Google Scholar]
- Burow, M.; Losansky, A.; Muller, R.; Plock, A.; Kliebenstein, D.J.; Wittstock, U. The genetic basis of constitutive and herbivore-induced ESP-independent nitrile formation in arabidopsis. Plant Physiol. 2009, 149, 561–574. [Google Scholar] [CrossRef]
- Eugui, D.; Velasco, P.; Abril-Urías, P.; Escobar, C.; Gómez-Torres, O.; Caballero, S.; Poveda, J. Glucosinolate-extracts from residues of conventional and organic cultivated broccoli leaves (Brassica oleracea var. italica) as potential industrially-scalable efficient biopesticides against fungi, oomycetes and plant parasitic nematodes. Ind. Crops Prod. 2003, 200, 116841. [Google Scholar] [CrossRef]
- Szymandera-Buszka, K.; Piechocka, J.; Zaremba, A.; Przeor, M.; Jędrusek-Golinska, A. Pumpkin, cauliflower and broccoli as new carriers of thiamine compounds for food fortification. Foods 2021, 10, 578. [Google Scholar] [CrossRef]
- Shi, M.; Ying, D.-Y.; Hlaing, M.M.; Ye, J.-H.; Sanguansri, L.; Augustin, M.A. Development of broccoli by-products as carriers for delivering EGCG. Food Chem. 2019, 301, 125301. [Google Scholar] [CrossRef]
- Krupa-Kozak, U.; Drabinska, N.; Rosell, C.M.; Fadda, C.; Anders, A.; Jelinski, T.; Ostaszyk, A. Broccoli leaf powder as an attractive by-product ingredient: Effect on batter behaviour, technological properties and sensory quality of gluten-free mini sponge cake. Int. J. Food Sci. Technol. 2019, 54, 1121–1129. [Google Scholar] [CrossRef]
- Krupa-Kozak, U.; Drabinska, N.; Bączek, N.; Simkova, K.; Starowicz, M.; Jelinski, T. Application of broccoli leaf powder in gluten-free bread: An innovative approach to improve its bioactive potential and technological quality. Foods 2021, 10, 819. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).