Abstract
As the most abundant catecholamine neurotransmitter in the brain, dopamine plays an important role in the normal physiological process, and its level in urine also changes during human pathological processes. In clinic, the detection of dopamine in urine is a potential marker for the diagnosis and the treatment of endocrine-related diseases. In this work, a copper metal organic framework with catecholase-like activity was prepared via the precipitation of Cu2+ and imidazole, simulating the N-Cu coordination environment in the active site of catecholase. Cu-MOF (the copper–metal organic framework) can catalyze the oxidation of DA (dopamine) to dopaquinone using O2 in the air. The oxidation product can further react with 1,3-dihydroxynaphthalene to produce a fluorophore product. Based on the above reaction, a multimodal sensing platform with three signal outputs, including ratio-metric fluorescence, absorbance and digital information extracted from smartphone images for simple and sensitive determination of DA, was proposed, with detection limits of 0.0679, 0.3206, and 0.3718 μM, respectively. This multimodal sensing platform was able to detect DA in body fluid in a self-correcting way, as demonstrated by the successful determination of DA in normal human urine samples, and samples with a high level of interference.
1. Introduction
Dopamine (DA) is the most abundant catecholamine neurotransmitter in the brain [1], and plays an important role in the normal physiological process [2,3]. In recent years, some studies have found that abnormal changes in DA content in the body fluid can be used as an auxiliary indicator for the diagnosis of various diseases, such as hypertension, hyperthyroidism, pheochromocytoma, neuroblastoma, and other endocrine-related diseases [4,5,6,7,8]. Therefore, the specific detection of DA content in the body fluid is of great importance for the diagnosis and treatment of related diseases.
Conventional DA detection methods including electrochemical analysis (EC) [9,10,11], high-performance liquid chromatography (HPLC) [12,13], and capillary electrophoresis (CE) [14] always need relatively large instruments and professional operators. Growing demand for point-of-care testing (POCT) [15,16] used in community hospitals and among families has stimulated the development of portable and miniaturized sensing platforms, especially smartphone-based sensing approaches. Optical sensing methods such as fluorescence analysis are easy to incorporate into smartphone-based POCT sensing platforms, and have the advantages of fast response and easy operation [17]. However, most optical sensing analysis methods for DA detection use a single-signal sensing mode, which is always interfered with by background signals and varies upon environmental changes, resulting in false positive/negative results [18,19,20]. One way to solve the problem is to develop ratio-metric probes, which can achieve self-calibration by simultaneously recording the ratio of the double fluorescence/absorption signals induced by the analyte [21]. Particularly, if changes in the double fluorescence signal show the opposite trend, the ratio can be used to amplify the signal change to achieve more sensitive detection [22,23]. Alternatively, multimodal optical sensing approaches with multi-mode responses toward the same analyte can also solve the issue of single-signal output by compensating with each other to obtain more reliable and accurate detection results [24,25,26]. Until now, optical probes specific for DA with both features, i.e., ratio-metric detection and multimodal sensing, have rarely been reported [27]; research has mainly been limited by the difficulty encountered in developing such a versatile optical sensing probe.
In recent years, metal organic frameworks (MOFs) have attracted researchers’ interest due to their advantages of tunable aperture, designable structure, and various functional ligands [28]. MOFs can be designed and synthesized through various metals and abundant organic ligands for applications including the enrichment of target molecules [29], gas sorption [30] and separation [31], luminescent sensing [32], heterogeneous catalysis [33], and optical devices [34]. Recently, MOFs with enzyme-mimicking activities have attracted sufficient research interest; these have the same as or even better catalytic properties as natural enzymes, yet have the advantages of lower cost and more convenient storage than natural enzymes [35,36,37,38,39,40,41]. For instance, a highly active and stable nano-enzyme Cu-TPP(PA) MOF was prepared for the detection of dopamine via fluorescence and colorimetric dual mode [39]. By taking advantage of the peroxidase activity of Pt/NH2-MIL-101, a colorimetric detection approach for DA was developed based on the competitive consumption of •OH generated from the nano-enzyme-catalyzed decomposition of H2O2 by DA [40]. However, due to the slow progress in the research of oxidase-like nano-enzymes, more research has focused on peroxidase-like enzymes. Few papers have reported the detection of dopamine based on oxidase-like nano-enzymes. As oxidase-based sensing does not require external H2O2, as peroxidase does, it is more suitable for use as sensing probes in the development of POCT sensing platforms [42].
Catecholase is a type of natural enzyme capable of selectively catalyzing the oxidation of substrates into corresponding o-quinones [43]. Its reactive site is composed of a coupled binuclear copper metal center coordinated by six histidines [44] (Scheme 1). Inspired by this, herein, we constructed a type of Cu-MOF nano-enzyme with catecholase-like activity, using Cu2+ and imidazole to mimic the N-Cu coordination environment of the active site in catecholase. Cu-MOF catalyzed the oxidation of dopamine to dopaquinone using O2 in the air. The produced dopaquinone was further reacted with 1,3-dihydroxynaphthalene (NR), generating fluorophore product (FP) to produce both fluorescence and absorbance signals [45]. As DA can induce fluorescent changes at two wavelengths, a ratio-metric fluorescence detection mode was adopted. By using a three signal-output, i.e., ratio-metric fluorescence, absorption and the digital data extracted from the light-emitting images, the multi-mode DA sensing platform was constructed. In addition to the advantage of multi-mode detection, the sensing platform also has the following advantages. (1) Due to the higher Michael addition and intramolecular cyclization activity of dopamine compared with other catecholamines in the process of enzyme-catalyzed oxidization, this DA sensing method has high specificity towards DA over other catecholamine neurotransmitters with similar structures to dopamine. (2) Unlike other studies using peroxidase activity for DA detection, which needs the addition of H2O2, the proposed method took advantage of the oxidase activity of Cu-MOF to catalyze the oxidation of DA in the presence of O2 in the air, which simplified the sensing procedure.
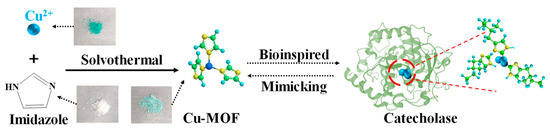
Scheme 1.
Illustration of the synthetic route of Cu-MOF. Structure of Cu-MOF and catechol oxidase (PDB code 1BT1). Color code: indigo blue (Cu), green (C), yellow (N), peacock blue (H).
2. Materials and Methods
2.1. Materials and Reagents
Imidazole, CuCl2·2H2O, dopamine hydrochloride, norepinephrine hydrochloride, epinephrine hydrochloride, D-glucose, GSH, ascorbic acid, uric acid, tannic acid, NaCl, KCl, CaCl2, L-cysteine, L-isoleucine, L-aspartic acid, L-lysine, L-tryptophan, L-glutamic acid, NaAc, L-phenylalanine and L-alanine were purchased from Aladdin Chemical Reagent Co., Ltd. (Shanghai, China). 1,3-dihydroxynaphthalene was received from Shyuanye (Shanghai, China). Methanol (analytical grade) and absolute ethanol (analytical grade) were obtained from FUYU CHEMICAL Fine Chemical Co., Ltd. (Tianjin, China). All the reagents were used as received without further purification. Milli-Q water (18.2 MΩ cm) was used to prepare all the solutions.
2.2. Instruments and Characterization
The fluorescence spectra were collected using an F-7000 fluorescence spectrophotometer (Hitachi, Tokyo, Japan). The UV-vis absorption spectra were recorded on a U-3900 UV-vis spectrophotometer (Hitachi, Tokyo, Japan). Fourier transform infrared spectra were obtained using a VERTEX 70 FT-IR spectrophotometer (Bruker, Karlsruhe, Germany) from 400 to 4000 cm−1. Scanning electron microscope (SEM) images were obtained from an SU8010 (Hitachi, Tokyo, Japan). EDS elemental mapping was carried out using an SU8010 (Hitachi, Tokyo, Japan). The transmission electron microscope (TEM) images were obtained from a JEM-ARM200F (JEOL, Tokyo, Japan). X-ray photoelectron spectroscopy (XPS) was performed with an ESCALAB 250 surface analysis system (Thermo Scientific, Massachusetts, USA). The X-ray diffraction (XRD) pattern measurement was performed with a Maxima XRD-7000 diffractometer (Shimadzu, Kyoto, Japan). ICP-5000 (FPI, Hangzhou, China) was employed to obtain the content of Cu in Cu-MOF. Digital images were collected using a Redmi K30S smartphone under UV light at 254 nm through the “Color Grab” app, and were processed into R, G, and B parameters.
2.3. Synthesis of Cu-MOF
Cu-MOF was prepared via a previously reported method with a minor modification [46]. CuCl2·2H2O (1.25 mmol) and 200 mg of imidazole were dissolved in 5 mL of methanol, respectively, and mixed under the ultrasonication for 5 min. They were then placed in a stainless steel Teflon-lined autoclave and heated at 140 °C for 7.5 h. Afterwards, deionized water was added dropwise when the temperature dropped to 25 °C, resulting in the formation of light blue precipitates. The obtained product was washed and centrifuged thoroughly with deionized water several times. The final product Cu-MOF was dispersed in deionized water at 4 °C, or vacuum-dried at 40 °C and stored at room temperature.
2.4. Multimodal Sensing for Detection of Dopamine
A Cu-MOF stock suspension (0.2 mg/mL) was prepared by evenly suspending Cu-MOF powders in the deionized water with the assistant of ultrasonication. For the detection of DA, 1840 μL NaAc-HAc buffer (pH = 6, 0.05 M), 100 μL Cu Cu-MOF suspension, 20 μL DA solution, and 40 μL NR (0.02 M) were mixed and incubated in a 2 mL centrifuge tube for 1 h (37 °C, 200 rpm). The emission spectrum (λ ex = 320 nm) was recorded, and fluorescent intensity ratios of 490 nm and 440 nm were used as the signal output of the fluorescence mode. By recording the absorbance at 460 nm, the signal output of the colorimetric mode was also obtained. The fluorescent images were collected using a smartphone under the irradiation of a portable UV lamp (254 nm). The R, G and B parameters were obtained by app (Color Grab).
3. Results and Discussion
3.1. Preparation and Characterization of Cu-MOF
Imidazole is a classic organic ligand that shows excellent thermal stability and chemical robustness, and is widely used in zeolitic imidazolate frameworks (ZIFs) [47,48]. ZIFs are organic–inorganic hybrid frameworks made primarily from metal cations (usually Zn and Co) and organic linkers (especially imidazolate-based linkers) in solvent (usually methanol and DMF) or solvent-free methods [49,50]. In this study, we used copper as the metal core to replace zinc to mimic the active sites of catecholase. The synthetic route of Cu-MOF’s production is shown in Scheme 1. Its preparation was completed via precipitation of Cu2+ and imidazole with deionized water, simulating the N-Cu coordination environment in the active site of catecholase. As shown in the SEM image in Figure 1a, Cu-MOF exhibited a two-dimensional irregular lamellar structure and uniform distribution, with an average diameter of 200 nm, which was consistent with the transmission electron microscopy (TEM) images shown in Figure S1. As shown in the full X-ray photoelectron spectroscopy (XPS) spectrum (Figure 1b) and EDS mapping spectrum (Figure S2), C, N, O and Cu were present in Cu-MOF. C and N were originated from the imidazole ligand, and O may be derived from the solvent methanol during reactions. EDS elemental mapping showed that the distribution pattern of copper and nitrogen was consistent, suggesting that copper was well coordinated with imidazole, thus resulting in a uniform distribution. Fourier transform infrared (FT-IR) spectra were investigated to confirm the functional groups of Cu-MOF. As shown in Figure 1c, the spectrum of imidazole displayed an absorption band at 3020 cm−1, which was caused by the stretching vibrations of C-H. N–H deformation vibration at 1585 cm−1, and stretching vibrations of the C-C bond at 1490 cm−1 were also observed. The absorption at 750 cm−1 belongs to C-H torsional vibration [51,52]. All the above characteristic absorption bands of imidazole were present in the spectrum of Cu-MOF, indicating that Cu2+ and imidazole were well coordinated and did not decompose during the preparation of Cu-MOF [46]. The X-ray diffraction (XRD) pattern of Cu-MOF (Figure 1d) exhibited a strong and sharp diffraction pattern, indicating that the Cu-MOF presented a high crystallinity [48]. The interaction between Cu2+ and imidazole was carefully investigated via XPS. The fine XPS spectrum is shown in Figure S3, wherein the peak at 934.73 eV belongs to Cu 2p3/2 of Cu2+, while the peak at and 954.93 eV belongs to Cu 2p1/2 of Cu2+ [53,54]. The binding energies of Cu 2p in Cu-MOF (Figure S3b) were 951.88 eV (Cu 2p1/2) and 932.08 eV (Cu 2p3/2), with a little blue shift compared to that in CuCl2·2H2O (Figure S3a), demonstrating that Cu remained bivalent in Cu-MOF [27]. The chemical environment of the N element in Cu MOF was analyzed via N 1s XPS spectra. The XPS spectra of N 1s in imidazole (Figure S3c) can be divided into two peaks, i.e., 399.48 eV (pyrrolic N) and 398.28 eV (pyridine N), respectively [55,56]. The binding energies of N 1s in Cu-MOF were 399.53 eV and 398.68 eV (Figure S3d). Compared with the N 1s peak positions in imidazole, these peaks in Cu-MOF were all slightly red-shifted, and the N 1s XPS spectrum of imidazole confirmed that the binding energy of pyridine N and pyrrole N at 400.00 eV and 400.68 eV of Cu-MOF was attributed to their coordination with Cu (Cu-N), indicating the participation of both pyridine N and pyrrole N of imidazole in the coordination of Cu-MOF [27,46]. The content of Cu in Cu-MOF was determined, using an inductively coupled plasma optical emission spectrometer (ICP-OES), to be 31.78 ± 0.47% (Figure S4). The Brunauer–Emmett–Teller (BET) surface area of Cu-MOF was tested via a N2 adsorption isotherm at 77 K, as shown in Figure S5. The relative pressure has an obvious hysteresis loop in the P/P0 range of 0.4 to 1.0, indicating a type IV isotherm according to the classification of IUPAC [57]. The pore size of Cu-MOF is about 3.8 nm. This indicates a mesoporous structure, which further proves the successful synthesis of Cu-MOF (Figure S5).
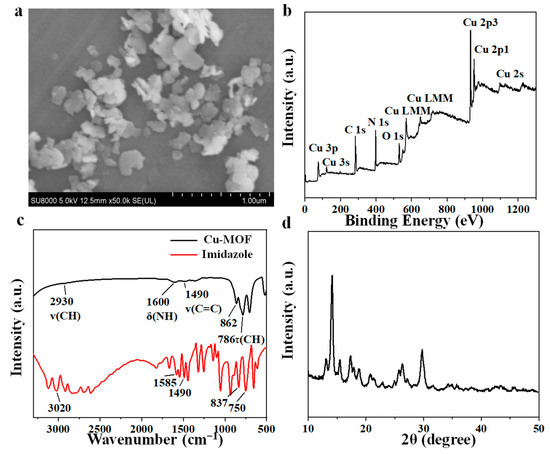
Figure 1.
(a) SEM image of Cu-MOF. (b) XPS spectrum of Cu-MOF. (c) FT-IR spectra of Cu-MOF. (d) XRD pattern of Cu-MOF.
3.2. Catalytic Performance of Cu-MOF for Catecholamine Oxidation
Catecholase can catalyze the oxidation of colorless epinephrine to red epinephrine quinone [58]. Therefore, we studied the catecholase-like activity of Cu-MOF with the help of the chromogenic reaction of epinephrine. As shown in Figure 2a,b, epinephrine alone had no significant absorbance in the visible region; however, after adding Cu-MOF, the mixture exhibited bright red with an absorption peak at 485 nm, indicating the catecholase-like activity of Cu-MOF.
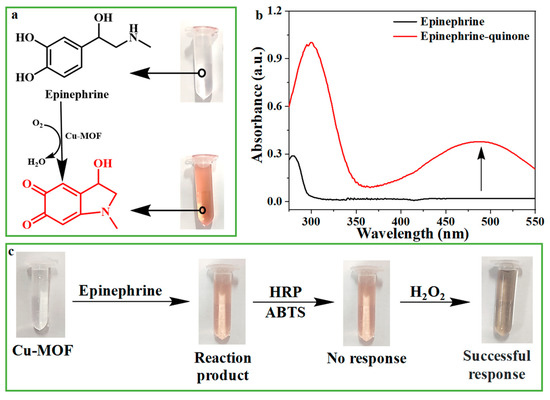
Figure 2.
(a) Cu-MOF catalyzes the reaction of epinephrine to epinephrine quinone. (b) The corresponding UV-VIS spectra of epinephrine and epinephrine quinone in Figure (a). (c) Procedure to verify whether H2O2 was produced in the process of Cu-MOF catalyzing epinephrine to produce epinephrine quinone.
Some oxidoreductases, such as glucose oxidase and uric acid oxidase, produce H2O2 by reducing O2 in the reaction. Instead, the catecholase directly converts O2 to H2O, without generating H2O2 [59]. In order to confirm the involvement of O2 in the reaction, the catalytic activity of Cu-MOF under different atmospheres (N2, air, O2) was compared, and the results are shown in Figure S6. Under other same conditions, Cu-MOF can catalyze the oxidation of epinephrine to epinephrine quinone in O2 or air, but can hardly catalyze the reaction in N2, confirming the necessity of O2 in the reaction. In order to verify the production of H2O rather than H2O2 during the reaction, after the reaction of Cu-MOF with epinephrine for a while, a mixture of HRP and ABTS, which is used for the detection of H2O2, was added. It turned out that the addition of HRP and ABTS [60] did not result in any color changes. However, the solution immediately turned dark green when additional H2O2 was added (Figure 2c). It can be concluded that no H2O2 was produced, which again verified that Cu-MOF is indeed a catecholase-like nano-enzyme, unlike other REDOX enzyme mimics [59,61]. Cu2+ alone has also been reported to catalyze the oxidation of epinephrine slowly, and to induce the color of the reaction solution to turn pale pink, showing weak catalytic activity [62]. However, under the same conditions, the catalytic activity of Cu-MOF was significantly stronger than that of equal concentration of free Cu2+ (Figure S7). In addition, the number of exposed copper sites of Cu-MOF decreased after coordination, which also confirmed the significantly increased catalytic activity of copper after coordination with imidazole.
3.3. Mechanism of the Cu-MOF Detection of DA
The Cu-MOF prepared in this study possesses catechol-like activity, which catalyzes the oxidation of dopamine by O2 in the air instead of unstable H2O2. We inferred the plausible mechanism of the Cu-MOF catalyzing the oxidation of dopamine, as indicated in Figure S8a. Cu-MOF has catechol-like activity because of the presence of a binuclear copper site in the Cu-MOF structure, which can bind to bisphenol substrates and oxidize them to quinones. At the same time, CuII is reduced to CuI, forming a coupled dual nuclear CuI site. Then, oxygen molecules bind to the double-nucleated CuI site, resulting in the binding of the double-nucleated CuI site to the oxygen ligand. Finally, oxygen is reduced to water, and CuI is oxidized to CuII to achieve catalytic cycling [63,64,65]. Briefly, Cu-MOF catalyzes the oxidation of dopamine to dopaquinone. The absorbance of dopaquinone is very weak, and there is no fluorescence signal, which is not conducive to signal output. In order to detect the changes in the reaction more sensitively, 1,3-dihydroxynaphthalene (NR) was introduced, which can react with dopaquinone to produce bright fluorescent product (FP) with strong absorbance; this is more conducive to the output of dopamine detection signals. As shown in Figure S8b, after dopamine is oxidized to dopaquinone, an intermolecular Michael addition reaction occurs between dopaquinone and NR, followed by intramolecular cyclization, and finally produces bright FP [45,66]. This plausible mechanism was verified by first mixing Cu-MOF with DA and then adding NR into the mixture. As shown in Figure S9a, NR itself showed a fluorescence emission peak at 440 nm. After the reaction, the fluorescence emission peak of NR at 440 nm decreased, whereas a new emission at 490 nm appeared, verifying the production of FP. Although other catecholamine neurotransmitters have similar structures to dopamine, their reaction with Cu-MOF and NR barely induced significant fluorescence changes, indicating the high selectivity of this sensing method (Figure S9b). The high selectivity of Cu-MOF to DA is due to the higher Michael addition and intramolecular cyclization activity of dopamine compared with other catecholamines in the enzyme-catalyzed oxidation process. According to the 3D fluorescence scan (Figure S10), there are two maximum excitation wavelengths, but the excitation wavelength of 450 nm cannot excite the fluorescence of NR, while the excitation wavelength of 320 nm can excite the fluorescence of both NR and FP at the same time, and a double-peak signal can thus be obtained. With the increase in the added DA, increasing amount of NR is consumed, accompanied by the production of FP. Correspondingly, the fluorescence emission peak at 440 nm decreased continuously, while that at 490 nm increased continuously, thus constituting the basis for ratio-metric fluorescence detection (Figure 3a). Moreover, the FP has an absorption band at 460 nm, so the corresponding absorbance at 460 nm increased with the increase in the DA concentration (Figure 3c). Similarly, after adding NR, only DA can cause intense signal changes in absorption. By collecting the ratio-metric fluorescence signal, the absorption signal, and the digital data from the light-emitting images taken by smartphone, a sensitive and selective muti-modal detection approach for DA can be developed.
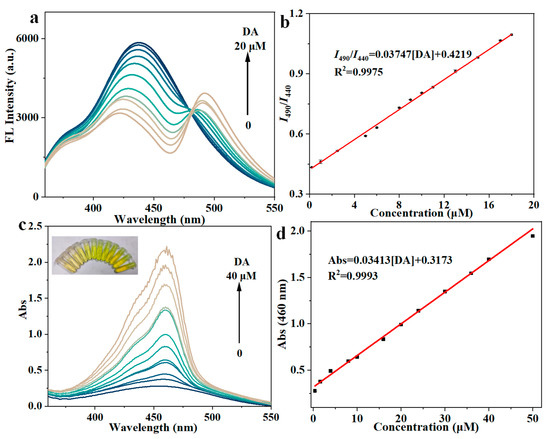
Figure 3.
(a) Fluorescence spectra of the sensing system in the presence of different concentrations of DA (λ ex = 320 nm). (b) Calibration curve of the ratio-metric fluorescence vs. DA concentration. (c) Absorption spectra of the sensing system in the presence of different concentrations of DA; the inset shows the corresponding photos of the sensing system. (d) Calibration curve of the absorbance at 460 nm vs. DA concentration. Error bars represent the standard deviation from three repeated experiments.
3.4. Analytical Performance and Practical Application
We further optimized some key experimental conditions that could affect the sensitivity of the DA sensing system, including pH, reaction time, and NR volume (Figure S11). Under optimal conditions, 20 μL of various concentrations of DA were mixed with 40 μL NR (20 mM), 100 μL Cu-MOF (0.2 mg/mL), and 1840 μL NaAc-HAc buffer (pH = 6, 0.05 M), then incubated in a 2 mL centrifuge tube for 1 h (37 °C, 200 rpm) for the detection of DA. As shown in Figure 3a,b, the ratio values of the emission intensity at 440 nm to that at 490 nm (I490/I440) showed a linear relationship with the concentration of DA in the range of 0.2–18 μM (R2 = 0.9975). The detection limit (LOD = 3σ/k, n = 11) for dopamine by the Cu-MOF/NR system was calculated to be 0.0697 μM in the ratio fluorescence mode.
Meanwhile, the colorimetric response of Cu-MOF/NR system to DA was also studied. As shown in Figure 3c,d, the absorbance value at 460 nm has a linear relationship with the change in DA concentration in the range of 1–40 μM (R2 = 0.9993). The LOD (3σ/k, n = 11) for dopamine in colorimetric mode was 0.3206 μM. We also investigated the selectivity of this sensing system towards DA by comparing the fluorescence/absorption responses of other possible interfering substances. As shown in Figure S12, other possible interferences, including norepinephrine hydrochloride, epinephrine hydrochloride, and species frequently encountered in biological fluids, such as D-glucose, GSH, ascorbic acid, uric acid, tannic acid, NaCl, KCl, Na2CO3, L-cysteine, L-isoleucine, L-aspartic acid, L-lysine, L-tryptophan, L-glutamic acid, and L-phenylalanine, were investigated, and they all had little effect on the I490/I440 value (Figure S12a). However, due to the non-negligible absorption at 460 nm (Figure S12b), some species such as uric acid indeed possessed interference to some extent in the absorption mode (Figure S13), which highlights the necessity of a multi-modal detection mode that can compensate for and correct each other.
With the increase in dopamine concentration, the Cu-MOF/NR system showed a different color intensity under the irradiation of a 254 nm UV lamp. Accordingly, a smart-phone-based DA detection platform was established (Figure 4). High-resolution photos of the Cu-MOF/NR detection system under different DA concentrations under ultraviolet light irradiation were collected, and the “Color Grab” app was used to convert RGB mode into color intensity. The calculation formula was I = 0.25R + 0.6G + 0.15B. In the range of DA concentration from 1 μM to 18 μM, there was a good linear relationship between color intensity (I) and DA concentration, and the linear correlation coefficient R2 was 0.9901(Figure 4). The detection limit of dopamine in smartphone mode was 0.3718 μM, using the Cu-MOF/NR system. The sensitivity of the Cu-MOF to DA is comparable or even better compared with previously reported optical sensors (Table 1).
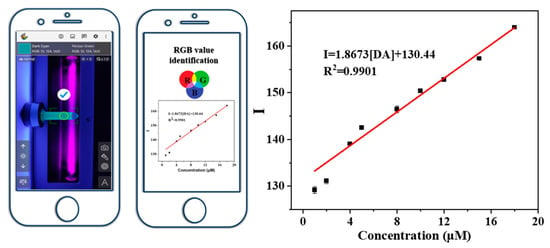
Figure 4.
Smartphone-assisted portable UV lamp visually detected DA, and a linear calibration diagram of DA concentration and color intensity obtained by converting RGB mode through mobile phone software. The error bars were obtained from three parallel experiments.

Table 1.
Analytical performance comparisons for DA detection by Cu-MOF and other reported fluorescent sensors.
In order to study the potential of the Cu-MOF/NR detection system for practical applications, DA was detected in artificial urine samples, and dopamine hydrochloride injection samples. As can be seen in Table S1, the determination of DA in artificial urine has a good recovery rate. The detection results of the DA concentration in dopamine hydrochloride injection samples were also in accordance with those of the standard values reported in medical instructions (Table S2). In addition, for the analysis of both samples, we used three detection modes, and the detection results of the same samples by the three modes were consistent, which can be mutually verified. We also verified the self-correction ability of this multimodal sensing strategy by analyzing DA–uric acid-co-spiked urine samples to simulate urine samples from patients with high level of uric acid. As shown in Table S3, the contents of DA measured using fluorescence and smartphone images were in accordance with each other, but showed significant difference from those of the absorption mode; thus, we can round off the results by deleting data obtained in absorption mode. This proved that the three modes can complement and verify each other to avoid false positive/false negative results. For resource-poor settings wherein professional instruments are limited, one can firstly utilize colorimetric mode and smartphone mode to conduct semi-quantitative detection by simply observing the color changes in the sensing system under sunlight or a UV lamp. Once the two analysis results are inconsistent with each other, the third mode, i.e., the ratio-metric fluorescence mode, can be used for arbitration. In this way, an accurate and self-calibrating method of analysis can be realized in a more cost-effective way.
4. Conclusions
In summary, we have established a simple multimodal detection platform for dopamine detection, based on the Cu-MOF-catalyzed dopamine oxidation followed by the highly specific intermolecular Michael addition. As the reaction product can produce three types of signal outputs, i.e., ratio-metric fluorescence, absorption, and smartphone-assisted visualization, the detection of DA was accomplished simultaneously in three ways, with the linear ranges of DA at 0.2–18, 1–40, and 1–18 μM, and related detection limits of 0.0679, 0.3206, and 0.3718 μM, respectively. Reliable detection results with recovery ranging from 95.28–106.61% and an RSD < 3.4% confirmed the potential of this sensing system for practical applications. At the same time, the sensor platform can perform sensitive and selective detection of dopamine in urine samples with the collaboration of the three detection modes in a self-correcting way. It has potential application prospects in the rapid and sensitive diagnosis of dopamine-related diseases in clinic.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemosensors11080431/s1. Figure S1: TEM image of Cu-MOF; Figure S2: EDS mapping spectra of Cu-MOF; Figure S3: (a). Cu 2p XPS spectrum of CuCl2·2H2O. (b). Cu 2p XPS spectrum of Cu-MOF. (c). N 1s XPS spectrum of imidazole. (d). N 1s XPS spectrum of Cu-MOF; Figure S4: The mass ratio of Cu-MOF detected by ICP-OES; Figure S5: N2 sorption isotherms and pore size distribution curves of Cu-MOF; Figure S6: The catalytic activity of Cu-MOF under different gas atmospheres; Figure S7: Comparison of catalytic performance of epinephrine between copper ions and Cu-MOF at the same concentration; Figure S8: (a) The possible mechanism of the oxidation of dopamine to dopaquinone catalyzed by Cu-MOF, and (b) the formation of fluorescent products by intramolecular Michael addition of dopaquinone and 1,3-dihydroxynaphthalene; Figure S9: (a) Fluorescence spectra of DA, DA + Cu-MOF, DA + NR, and DA + NR + Cu-MOF; (b) Fluorescence response of Cu-MOF system towards different catecholamine neurotransmitters spectra. Color code: black (H2O), red (Dopamine), blue (Norepinephrine) and green (Epinephrine); Figure S10: 3D fluorescence scan of Cu-MOF+NR+DA system and corresponding fluorescence emission peak under 320 nm/450 nm excitation; Figure S11: The fluorescent responses of Cu-MOF system to DA at different (a) pH values; (b) NR volume and (c) reaction time. (d) The I490/I440 responses of Cu-MOF system to DA at different reaction times. All the error bars were calculated from three parallel experiments; Figure S12: The selectivity of the Cu-MOF platform to DA and other possible interfering substances by fluorescent mode and colorimetric mode. Detection of species and concentrations: 1. Blank; 2. DA (20 μM); 3. uric acid (200 μM); 4. Na2CO3 (20 mM); 5. KCl (20 mM); 6. NaCl (20 mM); 7. epinephrine (20 μM); 8. norepinephrine (20 μM); 9. D-glucose (200 μM); 10. L-tryptophan (200 μM); 11. L-alanine (200 μM); 12. L-isoleucine (200 μM); 13. L-lysine (200 μM); 14. L-aspartic acid (200 μM); 15. L-glutamic acid (200 μM); 16. L-phenylalanine (200 μM); 17. ascorbic acid (200 μM); 18. Tannic acid (200 μM); 19. L-cysteine (200 μM); 20. GSH (200 μM). All the error bars were calculated from three parallel experiments; Figure S13: (a) Fluorescence spectra and (b) absorption spectra of the Cu-MOF sensing system with the addition of only uric acid, DA and DA + NR + Cu-MOF in aqueous solutions. Table S1: DA Determination in Healthy Human Urine Samples by the Cu-MOF Platform through Three Modes 1; Table S2: DA Determination in dopamine hydrochloride injection sample by the Cu-MOF Platform through three modes 1; Table S3: DA Determination in Human with High Uric Acid Urine Samples with High Level of Uric Acid by the Cu-MOF Platform through Three Modes 1.
Author Contributions
Conceptualization, Y.G.; methodology, Y.G.; software, Y.G.; validation, Y.G.; investigation, Y.G.; data curation, Y.G.; writing—original draft preparation, Y.G.; writing—review and editing, M.C. and T.Y.; supervision, M.C., T.Y. and J.W.; project administration, M.C., T.Y. and J.W.; funding acquisition, J.W. and T.Y. All authors have read and agreed to the published version of the manuscript.
Funding
Support from the Natural Science Foundation of China (21874014, 22074011) and the Liaoning Revitalization Talents Program (XLYC2007102, XLYC1802016) is greatly appreciated. Special thanks are due to the instrumental analysis from the Analytical and Testing Center, Northeastern University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Previc, F.H. Dopamine and the origins of human intelligence. Brain Cogn. 1999, 41, 299–350. [Google Scholar] [CrossRef]
- Koopman, K.; Gaal, J.; De Krijger, R.R. Pheochromocytomas and paragangliomas: New developments with regard to classification, genetics, and cell of origin. Cancers 2019, 11, 1070. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Cai, L.X.; Lelyveld, V.S.; Hai, A.; Jasanoff, A. Molecular-level functional magnetic resonance imaging of dopaminergic signaling. Science 2014, 344, 533–535. [Google Scholar] [CrossRef]
- Tye, K.M.; Mirzabekov, J.J.; Warden, M.R.; Ferenczi, E.A.; Tsai, H.C.; Finkelstein, J.; Kim, S.-Y.; Adhikari, A.; Thompson, K.R.; Andalman, A.S.; et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 2013, 493, 537–541. [Google Scholar] [CrossRef]
- Senel, M.; Dervisevic, E.; Alhassen, S.; Dervisevic, M.; Alachkar, A.; Cadarso, V.J.; Voelcker, N.H. Microfluidic electrochemical sensor for cerebrospinal fluid and blood dopamine detection in a mouse model of Parkinson’s disease. Anal. Chem. 2020, 92, 12347–12355. [Google Scholar] [CrossRef] [PubMed]
- Li, B.-R.; Hsieh, Y.-J.; Chen, Y.-X.; Chung, Y.-T.; Pan, C.-Y.; Chen, Y.-T. An ultrasensitive nanowire-transistor biosensor for detecting dopamine release from living PC12 cells under hypoxic stimulation. J. Am. Chem. Soc. 2013, 135, 16034–16037. [Google Scholar] [CrossRef]
- Kimura, N.; Takayanagi, R.; Takizawa, N.; Itagaki, E.; Katabami, T.; Kakoi, N.; Rakugi, H.; Ikeda, Y.; Tanabe, A.; Nigawara, T.; et al. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr. Relat. Cancer 2014, 21, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liang, L.; Lin, W.; Huang, Y.; Huang, T.; Wang, W.; Ma, J.; Li, J.; Sun, L.-P.; Guan, B.-O. Optofluidic laser sensor for the detection of dopamine. Sens. Actuators B Chem. 2023, 390, 133941. [Google Scholar] [CrossRef]
- Shi, Z.; Wu, X.; Zou, Z.; Yu, L.; Hu, F.; Li, Y.; Guo, C.; Li, C.M. Screen-printed analytical strip constructed with bacteria-templated porous N-doped carbon nanorods/Au nanoparticles for sensitive electrochemical detection of dopamine molecules. Biosens. Bioelectron. 2021, 186, 113303. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Jiao, L.; Wu, N.; Xu, W.; Wu, Z.; Wu, Y.; Hu, P.; Gu, W.; Zhu, C. Defect engineering of PdMo metallene for sensitive electrochemical detection of dopamine. Chem. Eng. J. 2023, 466, 143075. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, J.; Yan, R.; Wei, L.; Lei, C. Enzymeless Electrochemical Glucose Sensors Based on Metal–Organic Framework Materials: Current Developments and Progresses. Chemosensors 2023, 11, 290. [Google Scholar]
- Syslová, K.; Rambousek, L.; Kuzma, M.; Najmanová, V.; Bubeníková-Valešová, V.; Šlamberová, R.; Kačer, P. Monitoring of dopamine and its metabolites in brain microdialysates: Method combining freeze-drying with liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 3382–3391. [Google Scholar] [CrossRef] [PubMed]
- Zorina, M.; Dotsenko, V.V.; Nesterenko, P.N.; Temerdashev, A.; Dmitrieva, E.; Feng, Y.-Q.; Atapattu, S.N. Phthalylglycyl Chloride as a Derivatization Agent for UHPLC-MS/MS Determination of Adrenaline, Dopamine and Octopamine in Urine. Molecules 2023, 28, 2900. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.-J.; Feng, J.-J.; Dong, W.-J.; Lu, Y.-H.; Li, Z.-H.; Riekkola, M.-L. Spermine-graft-dextran non-covalent copolymer as coating material in separation of basic proteins and neurotransmitters by capillary electrophoresis. J. Chromatogr. A 2010, 1217, 5130–5136. [Google Scholar] [CrossRef]
- Zhu, L.; Fu, W.; Chen, J.; Li, S.; Xie, X.; Zhang, Z.; Liu, J.; Zhou, L.; Su, B.; Chen, X. A fully integrated and handheld electrochemiluminescence device for detection of dopamine in bio-samples. Sens. Actuators B Chem. 2022, 366, 131972. [Google Scholar] [CrossRef]
- Deng, H.; Zhao, J.; Zhao, S.; Jiang, S.; Cui, G. A graphene-based electrochemical flow analysis device for simultaneous determination of dopamine, 5-hydroxytryptamine, and melatonin. Analyst 2022, 147, 1598–1610. [Google Scholar] [CrossRef] [PubMed]
- Semeniak, D.; Cruz, D.F.; Chilkoti, A.; Mikkelsen, M.H. Plasmonic Fluorescence Enhancement in Diagnostics for Clinical Tests at Point-of-Care: A Review of Recent Technologies. Adv. Mater. 2022, 2107986. [Google Scholar] [CrossRef]
- Ling, Y.; Wang, L.; Zhang, X.Y.; Wang, X.H.; Zhou, J.; Sun, Z.; Li, N.B.; Luo, H.Q. Ratiometric fluorescence detection of dopamine based on effect of ligand on the emission of Ag nanoclusters and aggregation-induced emission enhancement. Sens. Actuators B Chem. 2020, 310, 127858. [Google Scholar] [CrossRef]
- Gui, R.; An, X.; Su, H.; Shen, W.; Zhu, L.; Ma, X.; Chen, Z.; Wang, X. Rhodamine 6G conjugated-quantum dots used for highly sensitive and selective ratiometric fluorescence sensor of glutathione. Talanta 2012, 94, 295–300. [Google Scholar] [CrossRef]
- Yu, L.; Feng, L.; Xiong, L.; Li, S.; Xu, Q.; Pan, X.; Xiao, Y. Multifunctional nanoscale lanthanide metal–organic framework based ratiometric fluorescence paper microchip for visual dopamine assay. Nanoscale 2021, 13, 11188–11196. [Google Scholar] [CrossRef]
- Tyrakowski, C.M.; Snee, P.T. Ratiometric CdSe/ZnS quantum dot protein sensor. Anal. Chem. 2014, 86, 2380–2386. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-S.; Pan, C.-G.; Cao, H.-X.; Yue, M.-Z.; Wang, L.; Liang, G.-X. Highly sensitive and selective dual-emission ratiometric fluorescence detection of dopamine based on carbon dots-gold nanoclusters hybrid. Sens. Actuators B Chem. 2018, 265, 371–377. [Google Scholar] [CrossRef]
- Dong, X.Z.; Sun, Z.; Han, L.; Ling, Y.; Li, B.L.; Li, N.B.; Luo, H.Q. A “traffic light” signal ratiometric fluorescence sensor for highly sensitive and selective detection of dopamine. Sens. Actuators B Chem. 2022, 372, 132668. [Google Scholar] [CrossRef]
- Hou, J.; Jia, P.; Yang, K.; Bu, T.; Zhao, S.; Li, L.; Wang, L. Fluorescence and Colorimetric Dual-Mode Ratiometric Sensor Based on Zr–Tetraphenylporphyrin Tetrasulfonic Acid Hydrate Metal–Organic Frameworks for Visual Detection of Copper Ions. ACS Appl. Mater. Interfaces 2022, 14, 13848–13857. [Google Scholar] [CrossRef]
- Yu, H.; Wang, M.; Cao, J.; She, Y.; Zhu, Y.; Ye, J.; El-Aty, A.A.; Hacımüftüoğlu, A.; Wang, J.; Lao, S. Dual-mode detection of organophosphate pesticides in pear and Chinese cabbage based on fluorescence and AuNPs colorimetric assays. Food Chem. 2021, 364, 130326. [Google Scholar] [CrossRef]
- Wei, Z.; Li, H.; Liu, S.; Wang, W.; Chen, H.; Xiao, L.; Ren, C.; Chen, X. Carbon dots as fluorescent/colorimetric probes for real-time detection of hypochlorite and ascorbic acid in cells and body fluid. Anal. Chem. 2019, 91, 15477–15483. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.Y.; Song, L.P.; Wang, Y.T.; Yang, P.; Ma, Y.; Tang, B. Fluorescence/Colorimetry/Smartphone Triple-Mode Sensing of Dopamine by a COF-Based Peroxidase-Mimic Platform. Anal. Chem. 2022, 94, 14419–14425. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, R.; Newair, E.F. Electrochemical behavior of antioxidants: I. Mechanistic study on electrochemical oxidation of gallic acid in aqueous solutions at glassy-carbon electrode. J. Electroanal. Chem. 2011, 657, 107–112. [Google Scholar] [CrossRef]
- Xu, H.; Guo, J.; Li, C.; Zhao, J.; Gao, Z.; Song, Y.Y. Nanoarchitectonics of a MOF-in-Nanochannel (HKUST-1/TiO2) Membrane for Multitarget Selective Enrichment and Staged Recovery. ACS Appl. Mater. Interfaces 2022, 14, 22006–22015. [Google Scholar] [CrossRef]
- Nath, K.; Ahmed, A.; Siegel, D.J.; Matzger, A.J. Microscale Determination of Binary Gas Adsorption Isotherms in MOFs. J. Am. Chem. Soc. 2022, 144, 20939–20946. [Google Scholar] [CrossRef]
- Qian, Q.; Asinger, P.A.; Lee, M.J.; Han, G.; Mizrahi Rodriguez, K.; Lin, S.; Benedetti, F.M.; Wu, A.X.; Chi, W.S.; Smith, Z.P. MOF-based membranes for gas separations. Chem. Rev. 2020, 120, 8161–8266. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sun, M.; Zhang, L.; Song, H.; Lv, Y. A novel Ce (IV)-MOF-based cataluminescence sensor for detection of hydrogen sulfide. Sens. Actuators B Chem. 2022, 362, 131746. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, S.; Tariq, S.; Luque, R.; Verpoort, F. Self-sacrifice MOFs for heterogeneous catalysis: Synthesis mechanisms and future perspectives. Mater. Today 2022, 55, 137–169. [Google Scholar] [CrossRef]
- Fu, H.R.; Wang, N.; Wu, X.X.; Li, F.F.; Zhao, Y.; Ma, L.F.; Du, M. Circularly polarized room-temperature phosphorescence and encapsulation engineering for MOF-based fluorescent/phosphorescent white light-emitting devices. Adv. Opt. Mater. 2020, 8, 2000330. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y. Detection of tyrosine catalyzed by a Tb-MOF luminescent nanozyme. Sens. Actuators B Chem. 2022, 350, 130842. [Google Scholar] [CrossRef]
- Chao, D.; Dong, Q.; Yu, Z.; Qi, D.; Li, M.; Xu, L.; Liu, L.; Fang, Y.; Dong, S. Specific Nanodrug for Diabetic Chronic Wounds Based on Antioxidase-Mimicking MOF-818 Nanozymes. J. Am. Chem. Soc. 2022, 144, 23438–23447. [Google Scholar] [CrossRef]
- Qi, Z.; Wang, L.; You, Q.; Chen, Y. PA-Tb-Cu MOF as luminescent nanoenzyme for catalytic assay of hydrogen peroxide. Biosens. Bioelectron. 2017, 96, 227–232. [Google Scholar] [CrossRef]
- Lian, X.; Huang, Y.; Zhu, Y.; Fang, Y.; Zhao, R.; Joseph, E.; Li, J.; Pellois, J.-P.; Zhou, H.-C. Enzyme-MOF nanoreactor activates nontoxic paracetamol for cancer therapy. Angew. Chem. Int. Ed. 2018, 57, 5725–5730. [Google Scholar] [CrossRef]
- Zhang, D.; Du, P.; Chen, J.; Guo, H.; Lu, X. Pyrazolate-based porphyrinic metal-organic frameworks as catechol oxidase mimic enzyme for fluorescent and colorimetric dual-mode detection of dopamine with high sensitivity and specificity. Sens. Actuators B Chem. 2021, 341, 130000. [Google Scholar] [CrossRef]
- Li, J.; Xu, K.; Chen, Y.; Zhao, J.; Du, P.; Zhang, L.; Zhang, Z.; Lu, X. Pt Nanoparticles Anchored on NH2-MIL-101 with Efficient Peroxidase-Like Activity for Colorimetric Detection of Dopamine. Chemosensors 2021, 9, 140. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, G.; Chen, Y.; Li, X.; Wang, S.; Jiang, F.; Zhan, P.; Lu, C.; Cao, X.; Ye, Y.; et al. Electrochemical Detection of ompA Gene of C. sakazakii Based on Glucose-Oxidase-Mimicking Nanotags of Gold-Nanoparticles-Doped Copper Metal-organic Frameworks. Sensors 2023, 23, 4396. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, L.; Li, Z.; Zhou, C.; Lv, Y.; Su, X. A sensing platform for on-site detection of glutathione S-transferase using oxidized Pi@ Ce-doped Zr-based metal-organic frameworks (MOFs). Talanta 2023, 259, 124537. [Google Scholar] [CrossRef] [PubMed]
- Ünal, M.Ü. Properties of polyphenol oxidase from Anamur banana (Musa cavendishii). Food Chem. 2007, 100, 909–913. [Google Scholar] [CrossRef]
- Klabunde, T.; Eicken, C.; Sacchettini, J.C.; Krebs, B. Crystal structure of a plant catechol oxidase containing a dicopper center. Nat. Struct. Biol. 1998, 5, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, G.; Sun, J.; Wang, Q.; Li, Z.J.; Yang, X. Dual-readout tyrosinase activity assay facilitated by a chromo-fluorogenic reaction between catechols and naphthoresorcin. Anal. Chem. 2019, 92, 2316–2322. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, R.; Qi, W.; Su, R.; He, Z. Construction of biomimetic nanozyme with high laccase-and catecholase-like activity for oxidation and detection of phenolic compounds. J. Hazard. Mater. 2022, 429, 128404. [Google Scholar] [CrossRef]
- Mo, Z.; Tai, D.; Zhang, H.; Shahab, A. A comprehensive review on the adsorption of heavy metals by zeolite imidazole framework (ZIF-8) based nanocomposite in water. Chem. Eng. J. 2022, 443, 136320. [Google Scholar] [CrossRef]
- Fu, Z.; Guo, F.; Qiu, J.; Zhang, R.; Wang, M.; Wang, L. Extension of the alkyl chain length to adjust the properties of lac-case-mimicking MOFs for phenolic detection and discrimination. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2022, 281, 121606. [Google Scholar] [CrossRef]
- Xiao, Y.; Hong, A.N.; Hu, D.; Wang, Y.; Bu, X.; Feng, P. Solvent-free synthesis of zeolitic imidazolate frameworks and the catalytic properties of their carbon materials. Chem. A Eur. J. 2019, 25, 16358–16365. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Jang, M.S.; Kwon, H.J.; Ahn, W.S. Zeolitic imidazolate frameworks: Synthesis, functionalization, and catalytic/adsorption applications. Catal. Surv. Asia 2014, 18, 101–127. [Google Scholar] [CrossRef]
- Monim-Ul-Mehboob, M.; Shaheen, M.A.; Sarwar, M.; Nawaz, S.; Ahmad, T.; Tahir, M.N.; Javaid, H.M.; Saleem, M.; Ahmad, S. Crystal structure and antimicrobial properties of tetrakis (imidazole) copper (II) triiodide, [Cu (imidazole) 4](I3) 2. Inorg. Nano-Met. Chem. 2017, 47, 37–40. [Google Scholar]
- Li, F.; Hu, D.; Yuan, Y.; Luo, B.; Song, Y.; Xiao, S.; Chen, G.; Fang, Y.; Lu, F. Zeolite Y encapsulated Cu (II) and Zn (II)-imidazole-salen catalysts for benzyl alcohol oxidation. Mol. Catal. 2018, 452, 75–82. [Google Scholar] [CrossRef]
- Lu, W.; Shen, J.; Zhang, P.; Zhong, Y.; Hu, Y.; Lou, X.W. Construction of CoO/Co-Cu-S hierarchical tubular heterostructures for hybrid supercapacitors. Angew. Chem. 2019, 131, 15587–15593. [Google Scholar] [CrossRef]
- Zhao, X.; Deng, M.; Rao, G.; Yan, Y.; Wu, C.; Jiao, Y.; Deng, A.; Yan, C.; Huang, J.; Wu, S.; et al. High-performance SERS substrate based on hierarchical 3D Cu nanocrystals with efficient morphology control. Small 2018, 14, 1802477. [Google Scholar] [CrossRef]
- Li, D.-M.; Li, S.-Q.; Huang, J.-Y.; Yan, Y.-L.; Zhang, S.-Y.; Tang, X.-H.; Fan, J.; Zheng, S.-R.; Zhang, W.-G.; Cai, S.-L. A recyclable bipyridine-containing covalent organic framework-based QCM sensor for detection of Hg (II) ion in aqueous solution. J. Solid. State Chem. 2021, 302, 122421. [Google Scholar] [CrossRef]
- Mahmudunnabi, R.G.; Umer, M.; Seo, K.D.; Park, D.S.; Chung, J.H.; Shiddiky, M.J.; Shim, Y.B. Exosomal microRNAs array sensor with a bioconjugate composed of p53 protein and hydrazine for the specific lung cancer detection. Biosens. Bioelectron. 2022, 207, 114149. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Ma, H.; Zheng, N.; Chen, Y.; Jiang, L. Laccase-like catalytic activity of Cu-tannic acid nanohybrids and their application for epinephrine detection. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126105. [Google Scholar] [CrossRef]
- Li, M.; Chen, J.; Wu, W.; Fang, Y.; Dong, S. Oxidase-like MOF-818 nanozyme with high specificity for catalysis of catechol oxidation. J. Am. Chem. Soc. 2020, 142, 15569–15574. [Google Scholar] [CrossRef]
- Fang, X.; Wu, X.-M.; Hu, X.-L.; Li, Z.-J.; Wang, G.-L. Native carbon nanodots as a fluorescent probe for assays based on the use of glucose oxidase or horseradish peroxidase. Microchim. Acta 2016, 183, 2761–2770. [Google Scholar] [CrossRef]
- Liang, H.; Lin, F.; Zhang, Z.; Liu, B.; Jiang, S.; Yuan, Q.; Liu, J. Multicopper laccase mimicking nanozymes with nucleotides as ligands. ACS Appl. Mater. Interfaces 2017, 9, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Yang, X.; Song, Y.; Huang, H.; Li, Y. Current research progress on laccase-like nanomaterials. New J. Chem. 2022, 46, 3541–3550. [Google Scholar] [CrossRef]
- Solomon, E.I.; Sundaram, U.M.; Machonkin, T.E. Multicopper oxidases and oxygenases. Chem. Rev. 1996, 96, 2563–2606. [Google Scholar] [CrossRef] [PubMed]
- Augustine, A.J.; Kjaergaard, C.; Qayyum, M.; Ziegler, L.; Kosman, D.J.; Hodgson, K.O.; Hedman, B.; Solomon, E.I. Systematic perturbation of the trinuclear copper cluster in the multicopper oxidases: The role of active site asymmetry in its reduction of O2 to H2O. J. Am. Chem. Soc. 2010, 132, 6057–6067. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.M.; Solomon, E.I. Electron transfer and reaction mechanism of laccases. Cell. Mol. Life Sci. 2015, 72, 869–883. [Google Scholar] [CrossRef]
- Zhao, J.-H.; Liu, G.-Y.; Wang, S.; Lu, S.-S.; Sun, J.; Yang, X.R. In Situ Specific Chromogenic and Fluorogenic Reaction for Straight forward and Dual-Modal Dopamine Detection. Chin. J. Anal. Chem. 2020, 48, e20081–e20088. [Google Scholar] [CrossRef]
- Sun, Y.; Song, Z.; Ni, X.; Dramou, P.; He, H. A boric acid-functionalized lanthanide metal-organic gel: A ratiometric fluorescence probe with rapid and sensitive detection of dopamine. Microchem. J. 2021, 169, 106579. [Google Scholar] [CrossRef]
- Nejad, M.a.F.; Hormozi-Nezhad, M.R. Design of a ratiometric fluorescent probe for naked eye detection of dopamine. Anal. Methods 2017, 9, 3505–3512. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, W.; Fan, Y.Z.; Dong, J.X.; Zhang, H.; Luo, H.Q.; Li, N.B. Label-free fluorescent discrimination and detection of epinephrine and dopamine based on bioinspired in situ copolymers and excitation wavelength switch. Anal. Chim. Acta 2019, 1054, 167–175. [Google Scholar] [CrossRef]
- Jana, J.; Chung, J.S.; Hur, S.H. ZnO-associated carbon dot-based fluorescent assay for sensitive and selective dopamine detection. Acs Omega 2019, 4, 17031–17038. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, Y.; Li, Q.; Yang, D.; Yang, Y. Fluorescence detection of dopamine based on the peroxidase-like activity of Fe3O4-MWCNTs@ Hemin. Microchim. Acta 2023, 190, 259. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-Y.; Chen, M.; Huang, N.-H.; Li, R.-T.; Pan, W.-L.; Zhang, W.-H.; Chen, W.-H.; Chen, J.-X. Facile and recyclable dopamine sensing by a label-free terbium (III) metal− organic framework. Talanta 2021, 221, 121399. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, R.; Tuccitto, N.; Pappalardo, A.; Sfrazzetto, G.T. Smartphone-based dopamine detection by fluorescent supramolecular sensor. Molecules 2022, 27, 7503. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).