Fluorescent Sensors for Detecting and Imaging Metal Ions in Biological Systems: Recent Advances and Future Perspectives
Abstract
1. Introduction
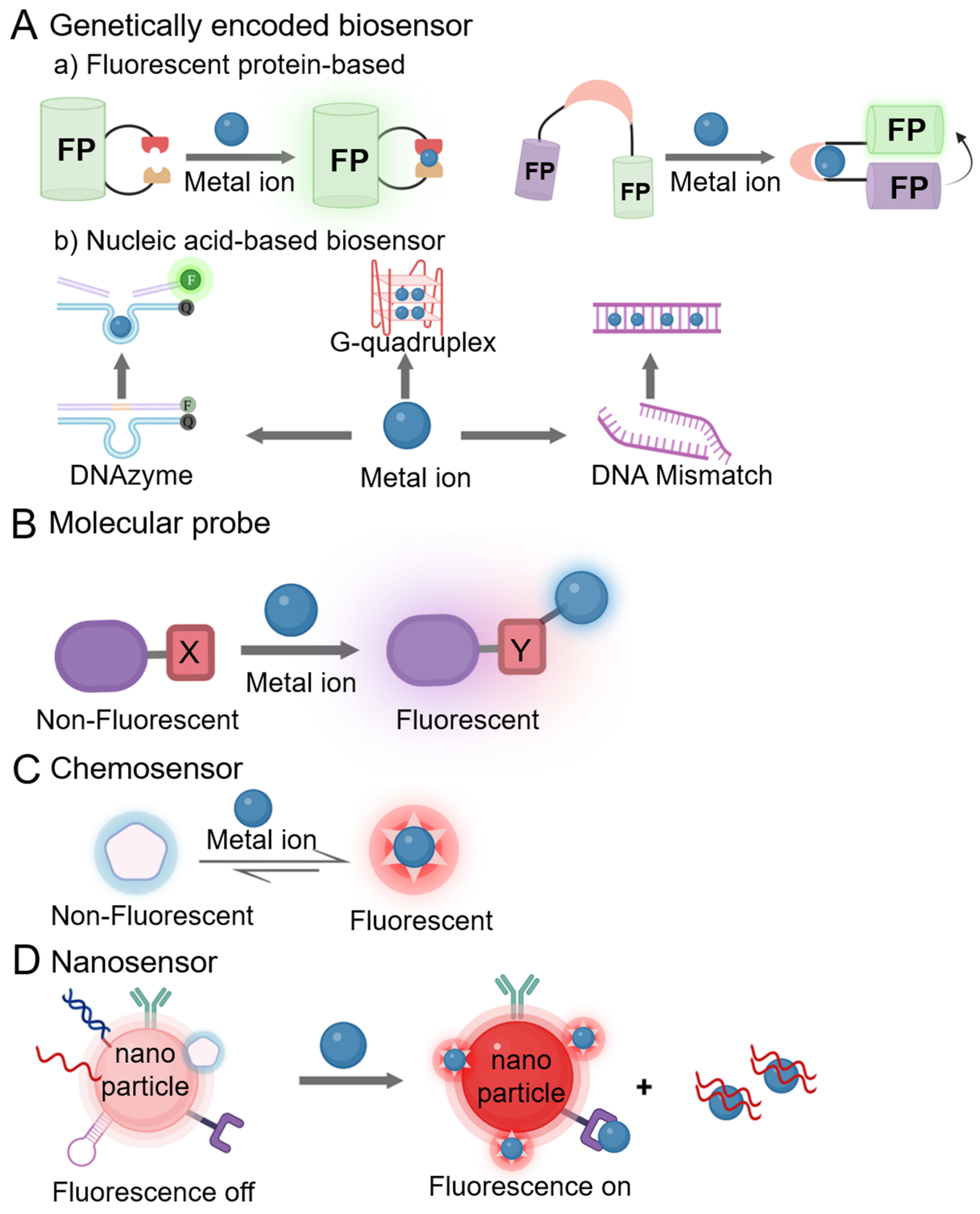
2. Categorization of Fluorescent Sensors for Metal Ions
3. In Vitro Detection of Metal Ions
3.1. Fluorescent Sensors for Essential Metal Ions
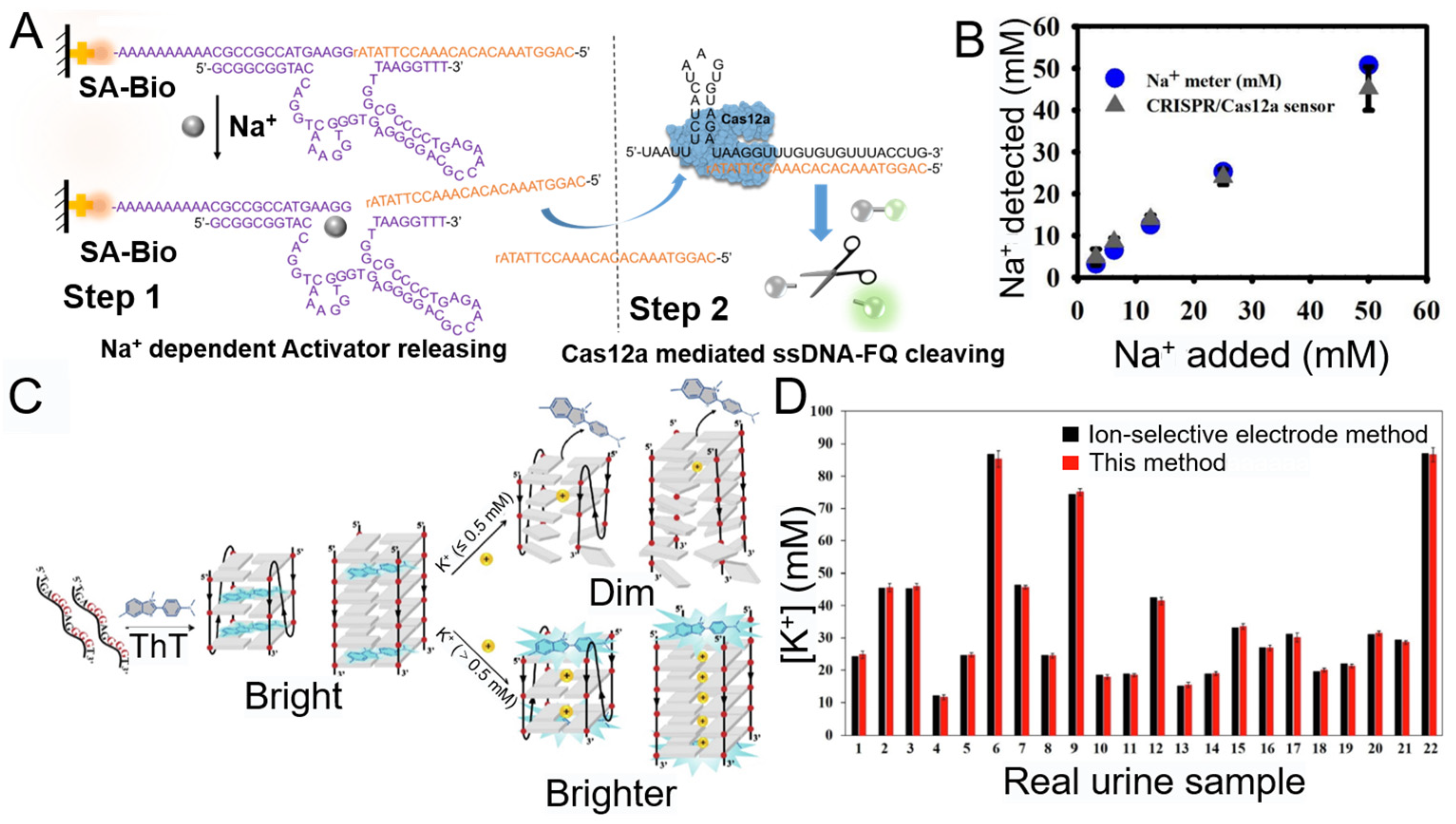
3.1.1. Na+
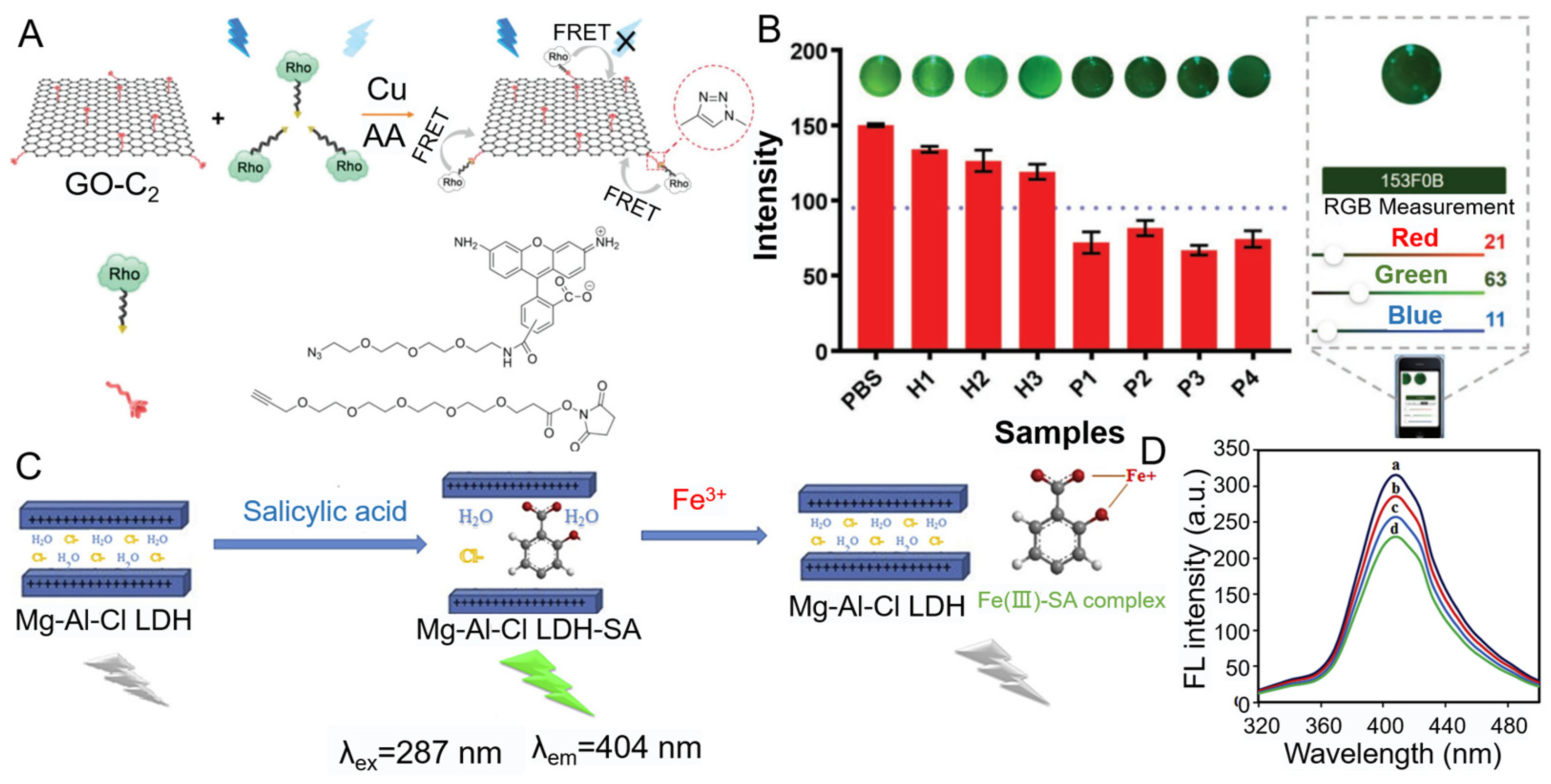
3.1.2. K+
3.1.3. Zn2+
3.1.4. Cu2+
3.1.5. Ca2+
3.1.6. Fe3+
3.2. Fluorescent Sensors for Non-Essential Metal Ions
3.2.1. Ag+
3.2.2. Pb2+

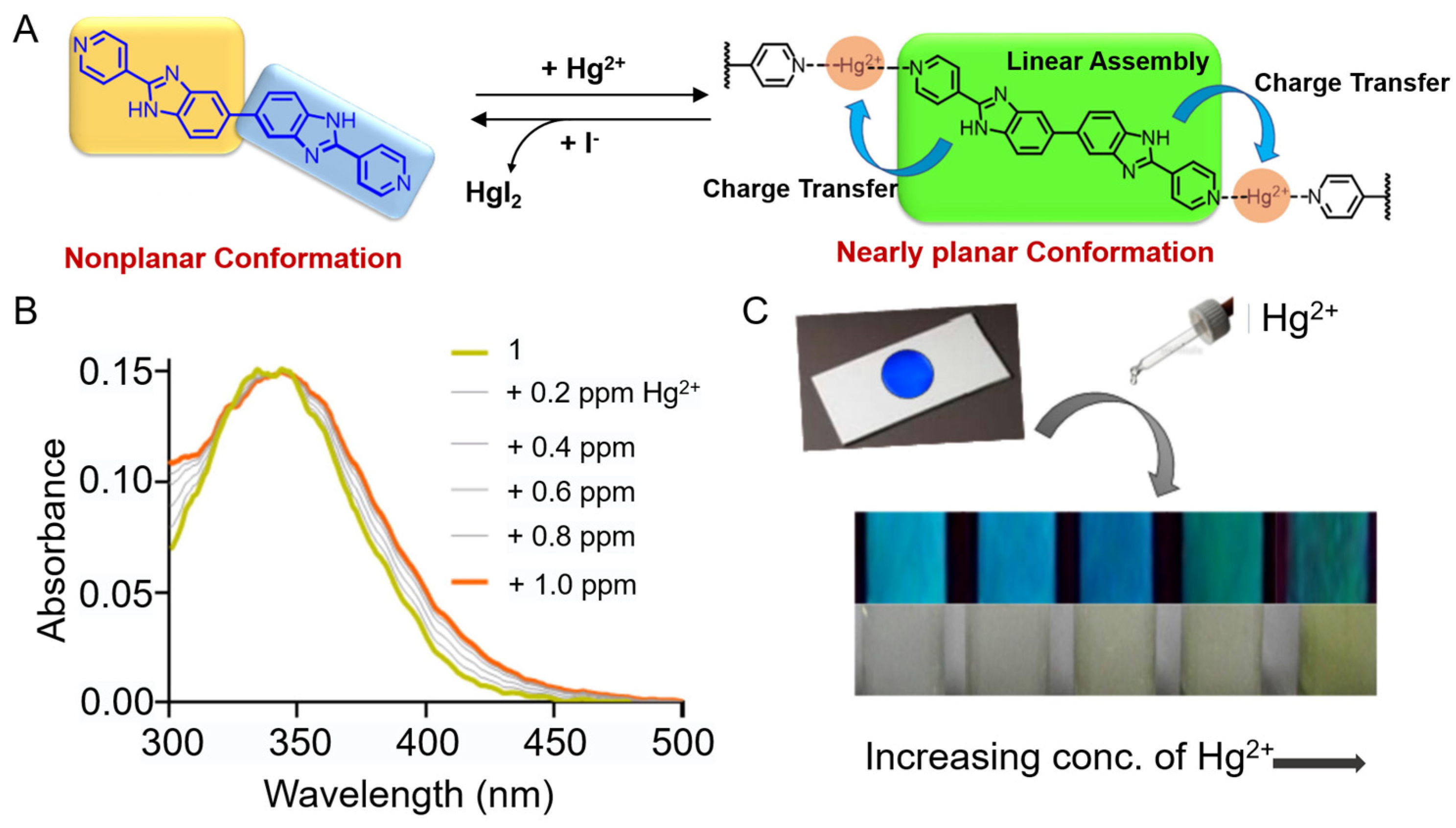
3.2.3. Hg2+
3.2.4. Al3+
3.2.5. Pt4+
4. Intracellular Imaging of Metal Ions
4.1. Fluorescent Sensors for Essential Metal Ions
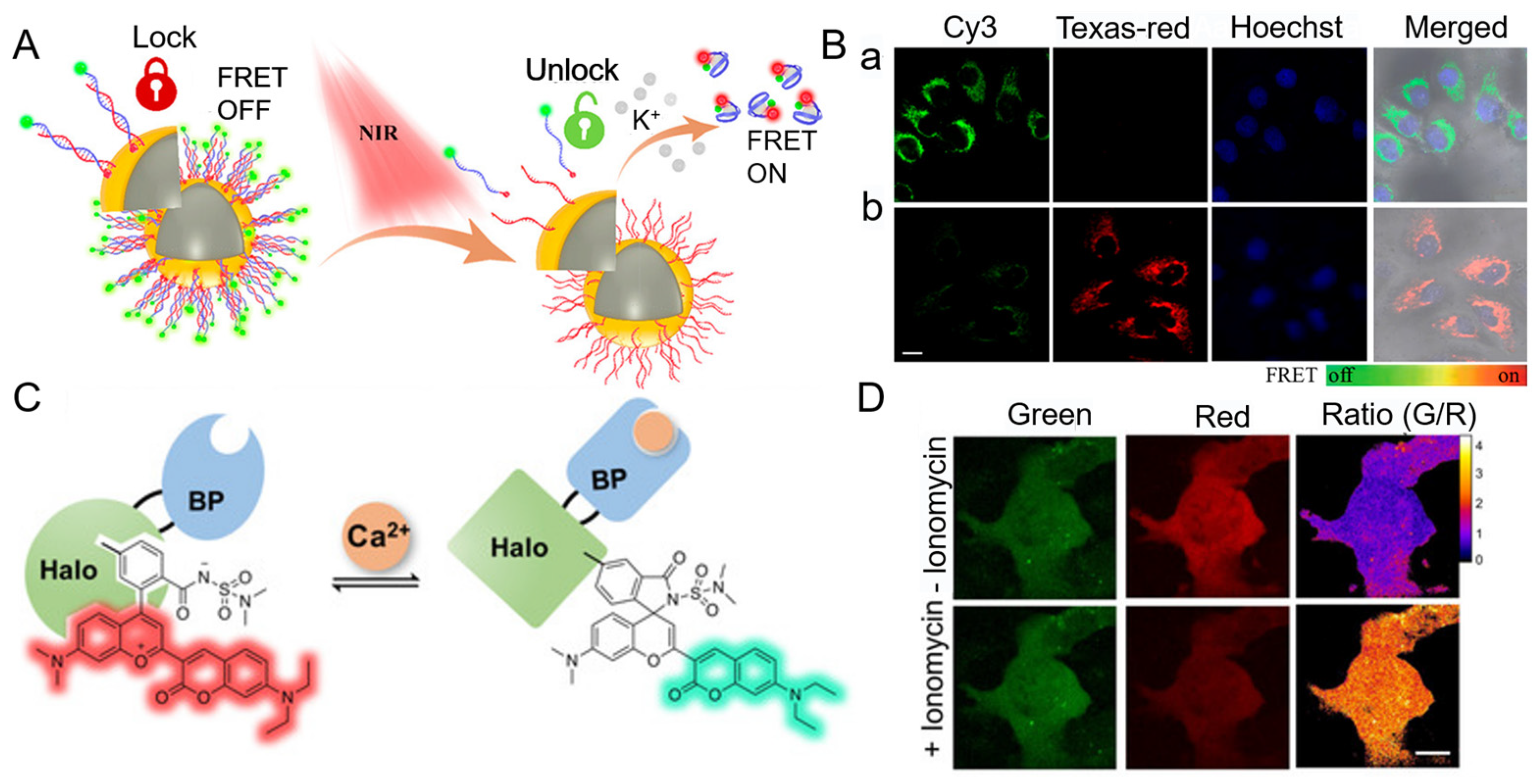
4.1.1. Na+
4.1.2. K+
4.1.3. Ca2+
4.1.4. Zn2+
4.1.5. Mg2+
4.1.6. Cu2+
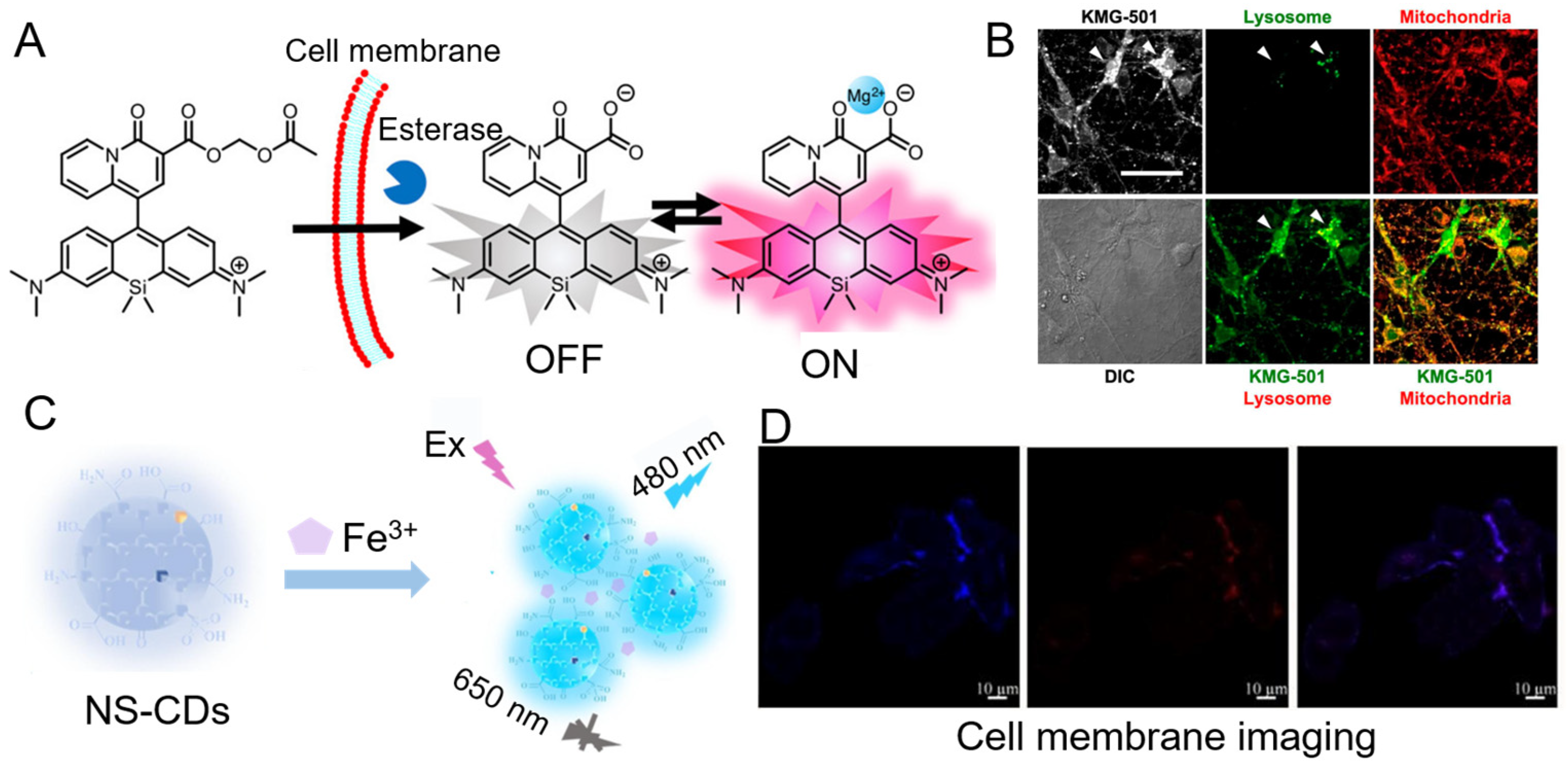
4.1.7. Fe2+/Fe3+
4.2. Fluorescent Sensors for Non-Essential Metal Ions
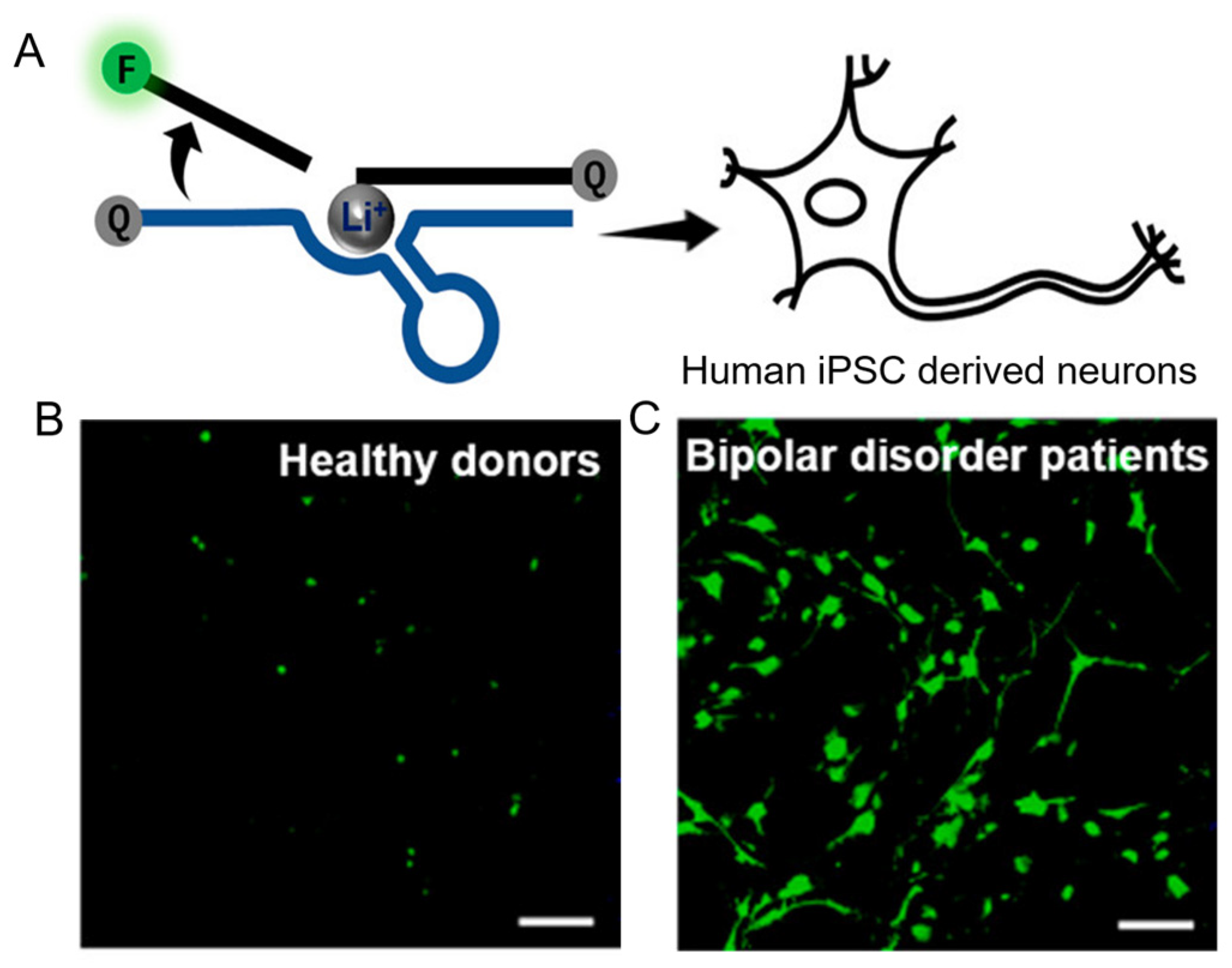
4.2.1. Li+
4.2.2. Ag+
4.2.3. Ni2+
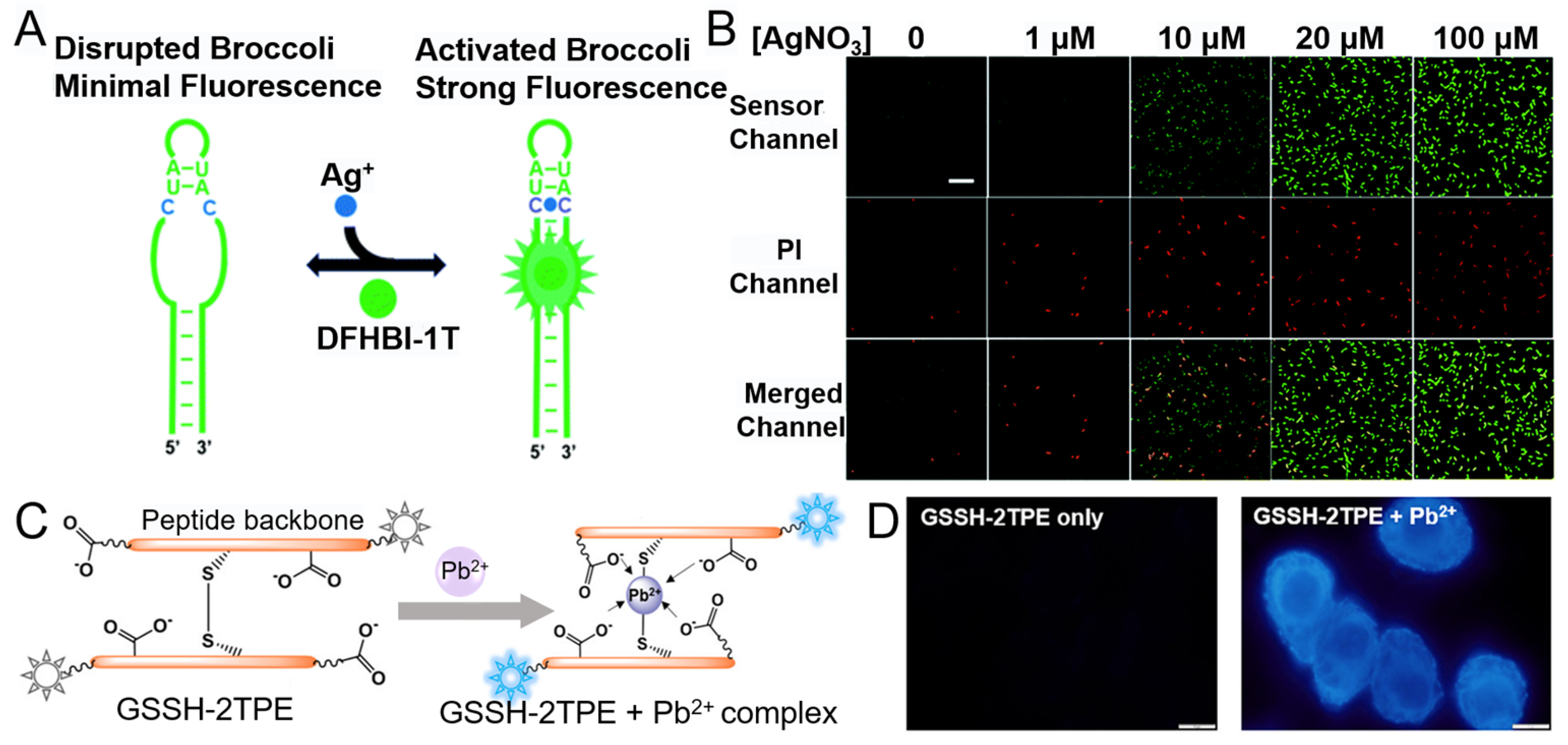
4.2.4. Pb2+
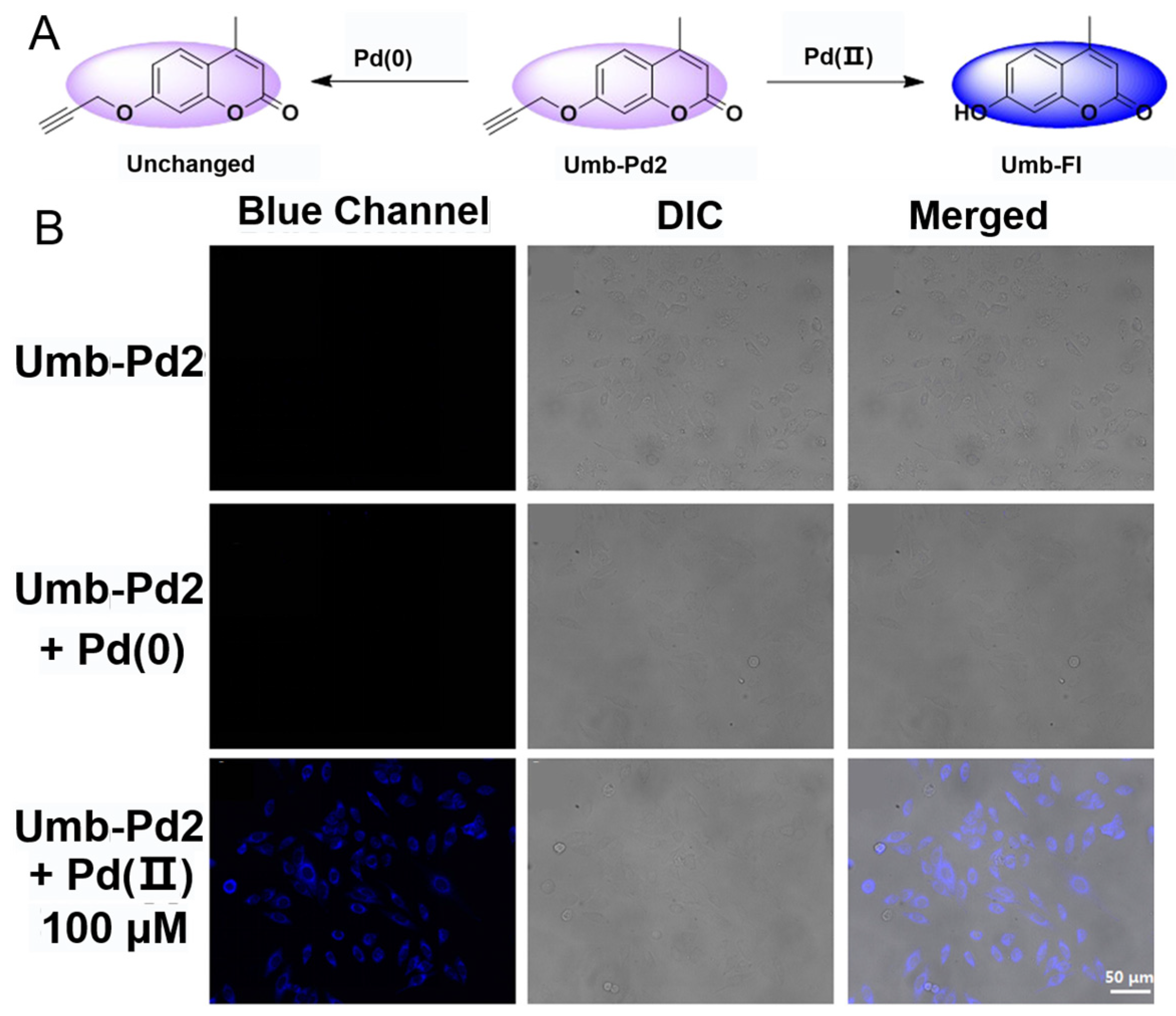
4.2.5. Pd2+
4.2.6. Hg2+
4.2.7. Cd2+
4.2.8. Au3+
4.2.9. Al3+
5. In Vivo Imaging of Metal Ions
5.1. Fluorescent Sensors for Essential Metal Ions
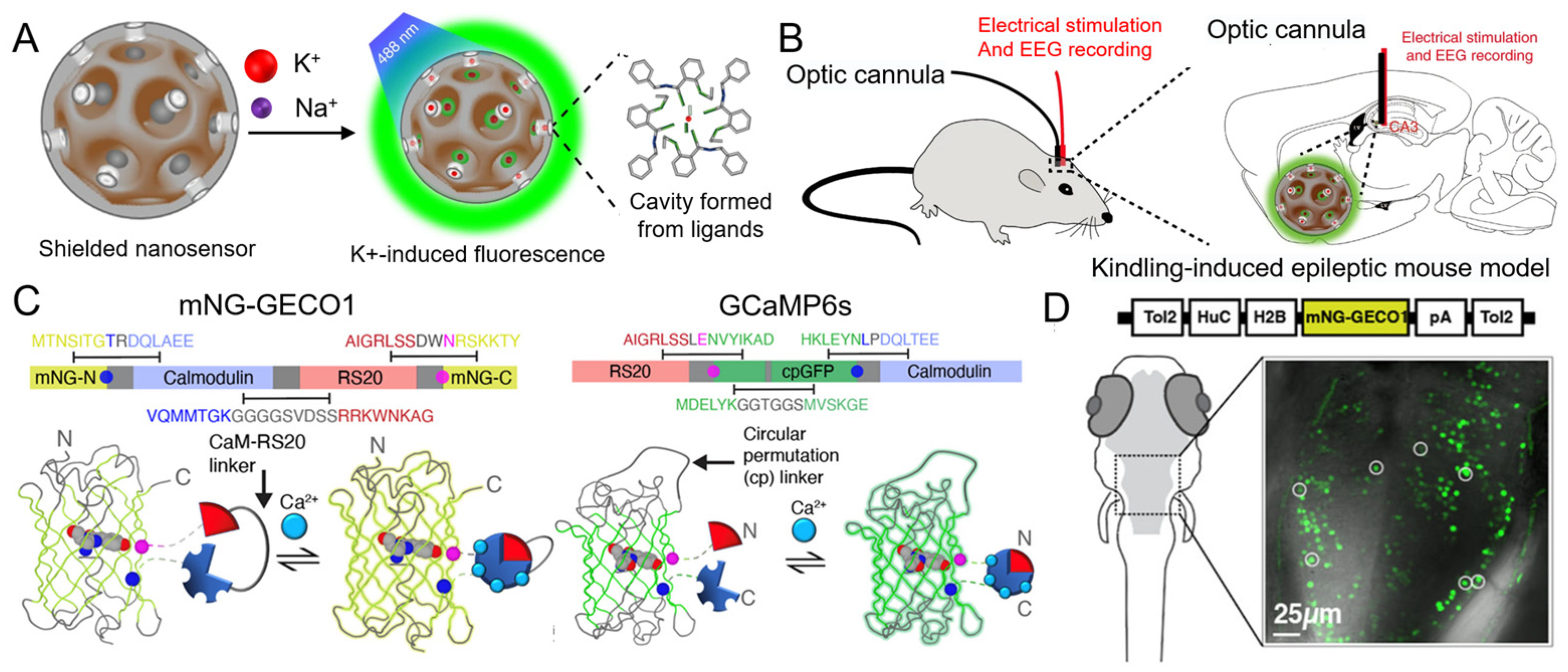
5.1.1. K+
5.1.2. Ca2+
5.1.3. Zn2+
5.1.4. Fe2+/Fe3+
5.1.5. Co2+
5.2. Fluorescent Sensors for Non-Essential Metal Ions
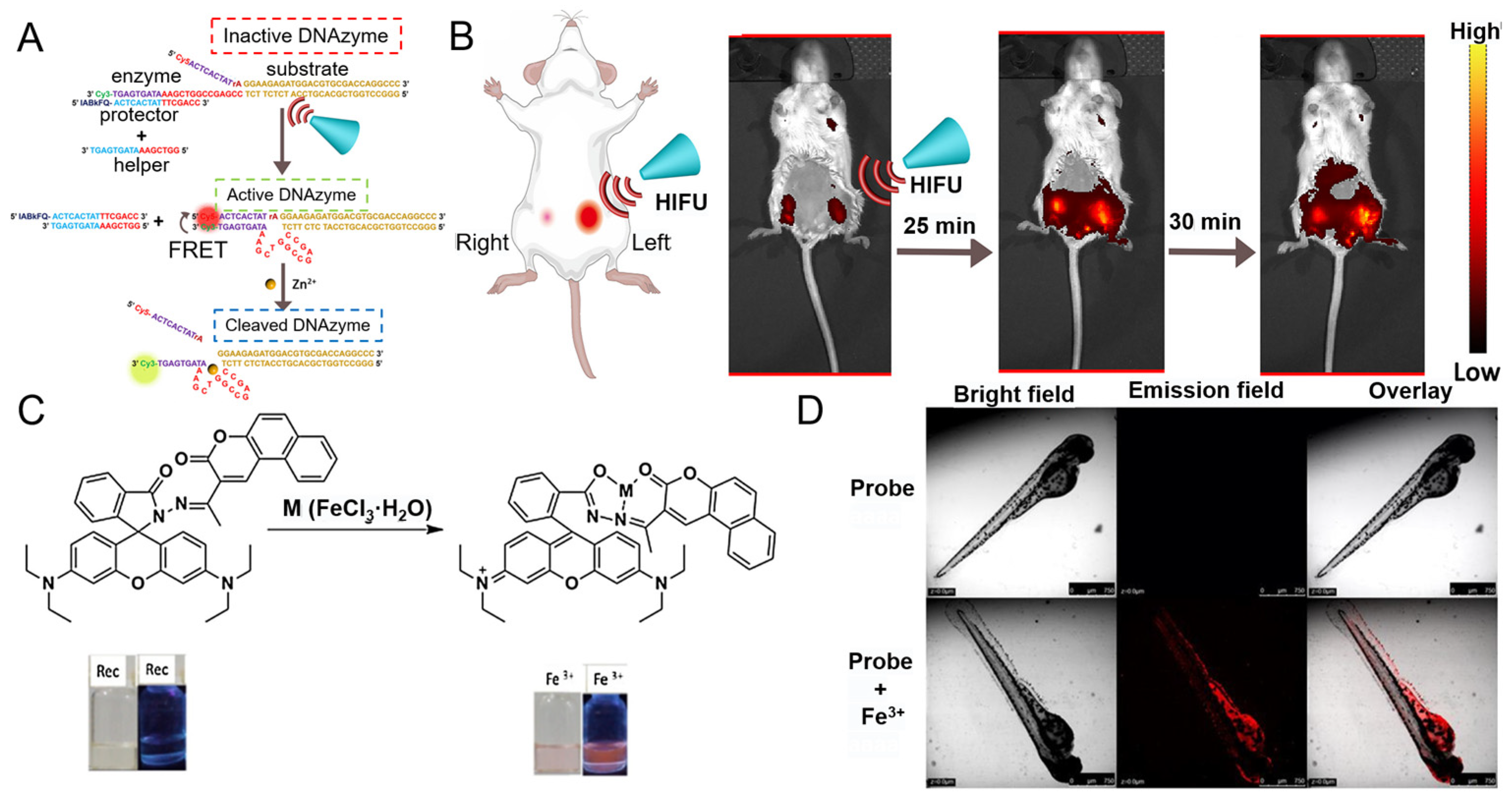
5.2.1. Li+
5.2.2. Pb2+
5.2.3. Sn2+
5.2.4. Cd2+
5.2.5. Hg2+
5.2.6. Ni2+
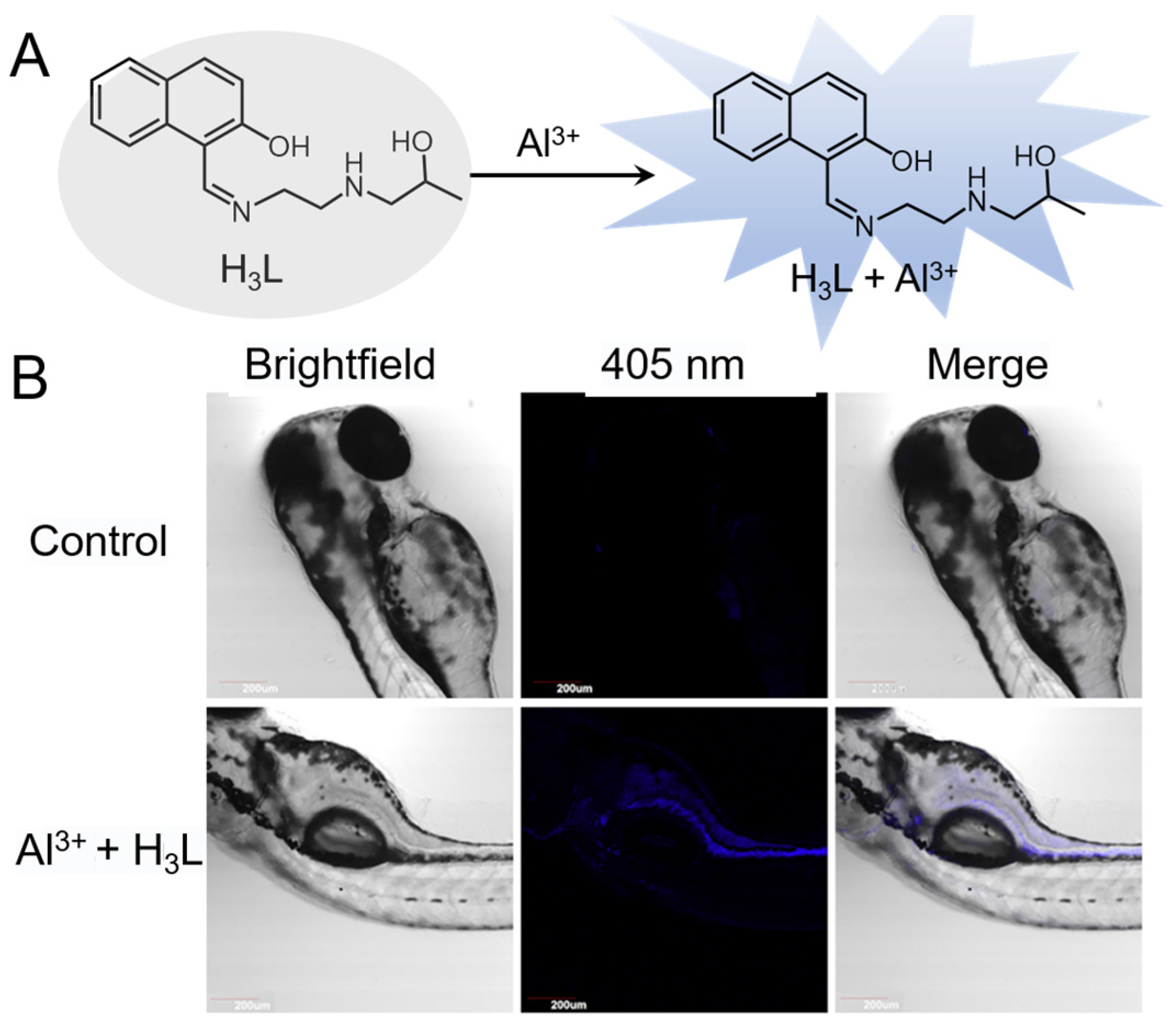
5.2.7. Al3+
6. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Acronyms | Definition |
| DNA | Deoxyribonucleic acid |
| RNA | Ribonucleic acid |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| AAS | Atomic absorption spectrophotometry |
| FAAS | Flame atomic absorption spectrometry |
| FRET | Fluorescent resonance energy transfer |
| FNA | Functional nucleic acid |
| HCR | Hybrid chain reactions |
| AIE | Aggregation-induced emission |
| BODIPY | Boron-dipyrromethene |
| GQ | G-quadruplex |
| NIR | Near-infrared |
| CD | Carbon dot |
| MOF | Metal-organic framework |
| UV | Ultraviolet |
| HSA | Human serum albumin |
| FBS | Fetal bovine serum |
| PET | Photoinduced electron transfer |
| SERS | Surface-enhanced Raman scattering |
| GFP | Green fluorescent protein |
| TP | Two-photon |
| AuNP | Gold nanoparticle |
| GSH | Glutathione |
| BD | Bipolar disorder |
| ICT | Intramolecular charge transfer |
| UNCP | Upconversion nanoparticle |
| EDTA | Ethylene diamine tetraacetic acid |
| BHQ | Black hole quencher |
References
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential metals in health and disease. Chem.-Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef] [PubMed]
- Missirlis, F. Regulation and biological function of metal ions in Drosophila. Curr. Opin. Insect Sci. 2021, 47, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Slobodian, M.R.; Petahtegoose, J.D.; Wallis, A.L.; Levesque, D.C.; Merritt, T.J.S. The Effects of Essential and Non-Essential Metal Toxicity in the Drosophila melanogaster Insect Model: A Review. Toxics 2021, 9, 269. [Google Scholar] [CrossRef]
- Lu, Y. Metal ions as matchmakers for proteins. Proc. Natl. Acad. Sci. USA 2010, 107, 1811–1812. [Google Scholar] [CrossRef]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent Sensors for Measuring Metal Ions in Living Systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef]
- Zhang, X.-B.; Kong, R.-M.; Lu, Y. Metal Ion Sensors Based on DNAzymes and Related DNA Molecules. Annu. Rev. Anal. Chem. 2011, 4, 105–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bai, Y.; Han, Z.; He, W.; Guo, Z. Photoluminescence imaging of Zn2+ in living systems. Chem. Soc. Rev. 2015, 44, 4517–4546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, Y. Biocomputing for Portable, Resettable, and Quantitative Point-of-Care Diagnostics: Making the Glucose Meter a Logic-Gate Responsive Device for Measuring Many Clinically Relevant Targets. Angew. Chem. Int. Ed. 2018, 57, 9702–9706. [Google Scholar] [CrossRef]
- Wu, Z.; Fan, H.; Satyavolu, N.S.R.; Wang, W.; Lake, R.; Jiang, J.-H.; Lu, Y. Imaging Endogenous Metal Ions in Living Cells Using a DNAzyme–Catalytic Hairpin Assembly Probe. Angew. Chem. Int. Ed. 2017, 56, 8721–8725. [Google Scholar] [CrossRef]
- Torabi, S.-F.; Wu, P.; McGhee, C.E.; Chen, L.; Hwang, K.; Zheng, N.; Cheng, J.; Lu, Y. In vitro selection of a sodium-specific DNAzyme and its application in intracellular sensing. Proc. Natl. Acad. Sci. USA 2015, 112, 5903–5908. [Google Scholar] [CrossRef]
- Hultin, S. Mosby’s Manual of Diagnostic and Laboratory Tests (4th edn). Ann. Clin. Biochem. 2012, 49, 415. [Google Scholar] [CrossRef]
- Yang, L.; Qing, Z.; Liu, C.; Tang, Q.; Li, J.; Yang, S.; Zheng, J.; Yang, R.; Tan, W. Direct Fluorescent Detection of Blood Potassium by Ion-Selective Formation of Intermolecular G-Quadruplex and Ligand Binding. Anal. Chem. 2016, 88, 9285–9292. [Google Scholar] [CrossRef] [PubMed]
- Chin, D.; Means, A.R. Calmodulin: A prototypical calcium sensor. Trends Cell Biol. 2000, 10, 322–328. [Google Scholar] [CrossRef]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef] [PubMed]
- Maity, D.; Manna, A.K.; Karthigeyan, D.; Kundu, T.K.; Pati, S.K.; Govindaraju, T. Visible–Near-Infrared and Fluorescent Copper Sensors Based on Julolidine Conjugates: Selective Detection and Fluorescence Imaging in Living Cells. Chem.-Eur. J. 2011, 17, 11152–11161. [Google Scholar] [CrossRef]
- Michielsen, C.M.S.; van Aalen, E.A.; Merkx, M. Ratiometric Bioluminescent Zinc Sensor Proteins to Quantify Serum and Intracellular Free Zn2+. ACS Chem. Biol. 2022, 17, 1567–1576. [Google Scholar] [CrossRef]
- Abdolmohammad-Zadeh, H.; Zamani-Kalajahi, M. A turn-on/off fluorescent sensor based on nano-structured Mg-Al layered double hydroxide intercalated with salicylic acid for monitoring of ferric ion in human serum samples. Anal. Chim. Acta 2019, 1061, 152–160. [Google Scholar] [CrossRef]
- Drennan-Harris, L.R.; Wongwilawan, S.; Tyson, J.F. Trace determination of total mercury in rice by conventional inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2013, 28, 259–265. [Google Scholar] [CrossRef]
- Xiong, C.; Qin, Y.; Hu, B. On-line separation/preconcentration of V(IV)/V(V) in environmental water samples with CTAB-modified alkyl silica microcolumn and their determination by inductively coupled plasma-optical emission spectrometry. J. Hazard. Mater. 2010, 178, 164–170. [Google Scholar] [CrossRef]
- Špirić, Z.; Vučković, I.; Stafilov, T.; Kušan, V.; Frontasyeva, M. Air Pollution Study in Croatia Using Moss Biomonitoring and ICP–AES and AAS Analytical Techniques. Arch. Environ. Contam. Toxicol. 2013, 65, 33–46. [Google Scholar] [CrossRef]
- Wu, P.; He, S.; Luo, B.; Hou, X. Flame Furnace Atomic Absorption Spectrometry: A Review. Appl. Spectrosc. Rev. 2009, 44, 411–437. [Google Scholar] [CrossRef]
- Săcărescu, L.; Chibac-Scutaru, A.-L.; Roman, G.; Săcărescu, G.; Simionescu, M. Selective detection of metal ions, sulfites and glutathione with fluorescent pyrazolines: A review. Environ. Chem. Lett. 2023, 21, 561–596. [Google Scholar] [CrossRef]
- Wu, D.; Chen, L.; Lee, W.; Ko, G.; Yin, J.; Yoon, J. Recent progress in the development of organic dye based near-infrared fluorescence probes for metal ions. Coord. Chem. Rev. 2018, 354, 74–97. [Google Scholar] [CrossRef]
- Denis, M.; Pancholi, J.; Jobe, K.; Watkinson, M.; Goldup, S.M. Chelating Rotaxane Ligands as Fluorescent Sensors for Metal Ions. Angew. Chem. Int. Ed. 2018, 57, 5310–5314. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, N.; Li, M.; Chen, H.; Zhang, N.; Wang, Y.; Zheng, K. Recent progress in fluorescent probes for detection of carbonyl species: Formaldehyde, carbon monoxide and phosgene. Coord. Chem. Rev. 2020, 404, 213109. [Google Scholar] [CrossRef]
- Wu, L.; Sedgwick, A.C.; Sun, X.; Bull, S.D.; He, X.-P.; James, T.D. Reaction-Based Fluorescent Probes for the Detection and Imaging of Reactive Oxygen, Nitrogen, and Sulfur Species. Acc. Chem. Res. 2019, 52, 2582–2597. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-L.; Niu, L.-Y.; Chen, Y.-Z.; Yang, Y.; Yang, Q.-Z. A multi-emissive fluorescent probe for the discrimination of glutathione and cysteine. Biosens. Bioelectron. 2017, 90, 403–409. [Google Scholar] [CrossRef]
- Wang, S.-Q.; Wu, Q.-H.; Wang, H.-Y.; Zheng, X.-X.; Shen, S.-L.; Zhang, Y.-R.; Miao, J.-Y.; Zhao, B.-X. Novel pyrazoline-based fluorescent probe for detecting glutathione and its application in cells. Biosens. Bioelectron. 2014, 55, 386–390. [Google Scholar] [CrossRef]
- Niu, P.; Zhu, J.; Wei, L.; Liu, X. Application of Fluorescent Probes in Reactive Oxygen Species Disease Model. Crit. Rev. Anal. Chem. 2022, 31, 1–36. [Google Scholar] [CrossRef]
- Cui, W.-L.; Wang, M.-H.; Yang, Y.-H.; Wang, J.-Y.; Zhu, X.; Zhang, H.; Ji, X. Recent advances and perspectives in reaction-based fluorescent probes for imaging peroxynitrite in biological systems. Coord. Chem. Rev. 2023, 474, 214848. [Google Scholar] [CrossRef]
- Juvekar, V.; Park, S.J.; Yoon, J.; Kim, H.M. Recent progress in the two-photon fluorescent probes for metal ions. Coord. Chem. Rev. 2021, 427, 213574. [Google Scholar] [CrossRef]
- Wan, H.; Xu, Q.; Gu, P.; Li, H.; Chen, D.; Li, N.; He, J.; Lu, J. AIE-based fluorescent sensors for low concentration toxic ion detection in water. J. Hazard. Mater. 2021, 403, 123656. [Google Scholar] [CrossRef]
- Xia, P.-F.; Ling, H.; Foo, J.L.; Chang, M.W. Synthetic genetic circuits for programmable biological functionalities. Biotechnol. Adv. 2019, 37, 107393. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Da, Y.; Tian, Y. Fluorescent proteins and genetically encoded biosensors. Chem. Soc. Rev. 2023, 52, 1189–1214. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jiang, R.; Feng, Y.; Liu, Z.; Huang, J.; Ma, C.; Wang, K. Functional nucleic acid-based fluorescent probes for metal ion detection. Coord. Chem. Rev. 2022, 459, 214453. [Google Scholar] [CrossRef]
- Nagai, T.; Sawano, A.; Park, E.S.; Miyawaki, A. Circularly permuted green fluorescent proteins engineered to sense Ca2+. Proc. Natl. Acad. Sci. USA 2001, 98, 3197–3202. [Google Scholar] [CrossRef]
- Tian, L.; Hires, S.A.; Mao, T.; Huber, D.; Chiappe, M.E.; Chalasani, S.H.; Petreanu, L.; Akerboom, J.; McKinney, S.A.; Schreiter, E.R.; et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods 2009, 6, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Torabi, S.-F.; Lu, Y. Functional DNA nanomaterials for sensing and imaging in living cells. Curr. Opin. Biotechnol. 2014, 28, 88–95. [Google Scholar] [CrossRef]
- Zhou, W.; Saran, R.; Liu, J. Metal Sensing by DNA. Chem. Rev. 2017, 117, 8272–8325. [Google Scholar] [CrossRef]
- Zhao, L.; Ahmed, F.; Zeng, Y.; Xu, W.; Xiong, H. Recent Developments in G-Quadruplex Binding Ligands and Specific Beacons on Smart Fluorescent Sensor for Targeting Metal Ions and Biological Analytes. ACS Sens. 2022, 7, 2833–2856. [Google Scholar] [CrossRef]
- Lake, R.J.; Yang, Z.; Zhang, J.; Lu, Y. DNAzymes as Activity-Based Sensors for Metal Ions: Recent Applications, Demonstrated Advantages, Current Challenges, and Future Directions. Acc. Chem. Res. 2019, 52, 3275–3286. [Google Scholar] [CrossRef]
- Li, J.; Mo, L.; Lu, C.-H.; Fu, T.; Yang, H.-H.; Tan, W. Functional nucleic acid-based hydrogels for bioanalytical and biomedical applications. Chem. Soc. Rev. 2016, 45, 1410–1431. [Google Scholar] [CrossRef] [PubMed]
- Krämer, J.; Kang, R.; Grimm, L.M.; De Cola, L.; Picchetti, P.; Biedermann, F. Molecular Probes, Chemosensors, and Nanosensors for Optical Detection of Biorelevant Molecules and Ions in Aqueous Media and Biofluids. Chem. Rev. 2022, 122, 3459–3636. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, F.; Wang, Y.; Li, H.; Zhang, J.; Sun, Z.; Jiang, Y. Fluorescent Organic Small Molecule Probes for Bioimaging and Detection Applications. Molecules 2022, 27, 8421. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xing, J.; Yuan, B.; He, L.; Lu, L.; Chen, N.; Cai, P.; Wu, A.; Li, J. Organic small-molecule fluorescent probe-based detection for alkali and alkaline earth metal ions in biological systems. J. Mat. Chem. B 2023. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Li, Z.; Li, K.; Yu, X.-Q. Small molecular fluorescent probes for the detection of lead, cadmium and mercury ions. Coord. Chem. Rev. 2021, 429, 213691. [Google Scholar] [CrossRef]
- Lee, H.; Hong, K.-I.; Jang, W.-D. Design and applications of molecular probes containing porphyrin derivatives. Coord. Chem. Rev. 2018, 354, 46–73. [Google Scholar] [CrossRef]
- Chowdhury, S.; Rooj, B.; Dutta, A.; Mandal, U. Review on Recent Advances in Metal Ions Sensing Using Different Fluorescent Probes. J. Fluoresc. 2018, 28, 999–1021. [Google Scholar] [CrossRef]
- Raveendran, A.V.; Sankeerthana, P.A.; Jayaraj, A.; Chinna Ayya Swamy, P. Recent developments on BODIPY based chemosensors for the detection of group IIB metal ions. Results Chem. 2022, 4, 100297. [Google Scholar] [CrossRef]
- Ding, Y.; Zhu, W.-H.; Xie, Y. Development of Ion Chemosensors Based on Porphyrin Analogues. Chem. Rev. 2017, 117, 2203–2256. [Google Scholar] [CrossRef]
- Pooja Pandey, H.; Aggarwal, S.; Vats, M.; Rawat, V.; Pathak, S.R. Coumarin-based Chemosensors for Metal Ions Detection. Asian J. Org. Chem. 2022, 11, e202200455. [Google Scholar] [CrossRef]
- Oshchepkov, A.S.; Oshchepkov, M.S.; Oshchepkova, M.V.; Al-Hamry, A.; Kanoun, O.; Kataev, E.A. Naphthalimide-Based Fluorescent Polymers for Molecular Detection. Adv. Opt. Mater. 2021, 9, 2001913. [Google Scholar] [CrossRef]
- Hazra, A.; Roy, P. A rhodamine based dye for sensing of Group 13 metal ions. Anal. Chim. Acta 2022, 1193, 339378. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Leung, N.L.C.; Zhang, J.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. AIE-based luminescence probes for metal ion detection. Coord. Chem. Rev. 2021, 429, 213693. [Google Scholar] [CrossRef]
- Vikesland, P.J. Nanosensors for water quality monitoring. Nat. Nanotechnol. 2018, 13, 651–660. [Google Scholar] [CrossRef]
- Yaari, Z.; Yang, Y.; Apfelbaum, E.; Cupo, C.; Settle, A.H.; Cullen, Q.; Cai, W.; Roche, K.L.; Levine, D.A.; Fleisher, M.; et al. A perception-based nanosensor platform to detect cancer biomarkers. Sci. Adv. 2021, 7, eabj0852. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Guleria, P.; Mehta, S.K. Nanosensors for food quality and safety assessment. Environ. Chem. Lett. 2017, 15, 165–177. [Google Scholar] [CrossRef]
- Auffan, M.; Rose, J.; Bottero, J.-Y.; Lowry, G.V.; Jolivet, J.-P.; Wiesner, M.R. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Sears, M.E.; Kerr, K.J.; Bray, R.I. Arsenic, Cadmium, Lead, and Mercury in Sweat: A Systematic Review. J. Environ. Public Health 2012, 2012, 184745. [Google Scholar] [CrossRef]
- Zeng, Z.; Ma, R.; Liu, C.; Xu, Y.; Li, H.; Liu, F.; Sun, S. A crab-like fluorescent probe for Ga(III) detection in body fluids and biological tissues. Sens. Actuator B-Chem. 2017, 250, 267–273. [Google Scholar] [CrossRef]
- Zheng, P.; Li, M.; Jurevic, R.; Cushing, S.K.; Liu, Y.; Wu, N. A gold nanohole array based surface-enhanced Raman scattering biosensor for detection of silver(i) and mercury(ii) in human saliva. Nanoscale 2015, 7, 11005–11012. [Google Scholar] [CrossRef]
- Ding, Y.; Tang, Y.; Zhu, W.; Xie, Y. Fluorescent and colorimetric ion probes based on conjugated oligopyrroles. Chem. Soc. Rev. 2015, 44, 1101–1112. [Google Scholar] [CrossRef]
- Ma, L.; Liu, J. An in Vitro–Selected DNAzyme Mutant Highly Specific for Na+ under Slightly Acidic Conditions. ChemBioChem 2019, 20, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhang, J.; Yang, Z.; Mou, Q.; Ma, Y.; Xiong, Y.; Lu, Y. Functional DNA Regulated CRISPR-Cas12a Sensors for Point-of-Care Diagnostics of Non-Nucleic-Acid Targets. J. Am. Chem. Soc. 2020, 142, 207–213. [Google Scholar] [CrossRef]
- Kaur, G.; Kaur, N. Estimation of sodium ions using easily engineered organic nanoparticles-based turn-on fluorescent sensor: Application in biological and environmental samples. Sens. Actuator B-Chem. 2018, 265, 134–141. [Google Scholar] [CrossRef]
- Qiao, H.; Bai, J.; Zhang, S.; Li, C. A guanosine-based 2-formylphenylborate ester hydrogel with high selectivity to K+ ions. RSC Adv. 2020, 10, 28536–28540. [Google Scholar] [CrossRef]
- Cheng, Y.; Cheng, M.; Hao, J.; Jia, G.; Monchaud, D.; Li, C. The noncovalent dimerization of a G-quadruplex/hemin DNAzyme improves its biocatalytic properties. Chem. Sci. 2020, 11, 8846–8853. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cheng, M.; Hao, J.; Miao, W.; Zhou, W.; Jia, G.; Li, C. Highly Selective Detection of K+ Based on a Dimerized G-Quadruplex DNAzyme. Anal. Chem. 2021, 93, 6907–6912. [Google Scholar] [CrossRef] [PubMed]
- Chitbankluai, K.; Thavarungkul, P.; Kanatharana, P.; Kaewpet, M.; Buranachai, C. Newly found K+-Thioflavin T competitive binding to DNA G-quadruplexes and the development of a label-free fluorescent biosensor with extra low detection limit for K+ determination in urine samples. Spectroc. Acta Part A-Molec. Biomolec. Spectr. 2022, 276, 121244. [Google Scholar] [CrossRef]
- Mazumdar, D.; Nagraj, N.; Kim, H.-K.; Meng, X.; Brown, A.K.; Sun, Q.; Li, W.; Lu, Y. Activity, Folding and Z-DNA Formation of the 8-17 DNAzyme in the Presence of Monovalent Ions. J. Am. Chem. Soc. 2009, 131, 5506–5515. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Lin, Y.; Cai, L.; Basa, P.N.; Shigemoto, A.K.; Zheng, C.; Zhang, F.; Burdette, S.C.; Lu, Y. Detection and Quantification of Tightly Bound Zn2+ in Blood Serum Using a Photocaged Chelator and a DNAzyme Fluorescent Sensor. Anal. Chem. 2021, 93, 5856–5861. [Google Scholar] [CrossRef]
- Chen, F.; Xiao, F.; Zhang, W.; Lin, C.; Wu, Y. Highly Stable and NIR Luminescent Ru–LPMSN Hybrid Materials for Sensitive Detection of Cu2+ In Vivo. ACS Appl. Mater. Interfaces 2018, 10, 26964–26971. [Google Scholar] [CrossRef] [PubMed]
- He, S.-J.; Xie, Y.-W.; Chen, Q.-Y. A NIR-BODIPY derivative for sensing copper(II) in blood and mitochondrial imaging. Spectroc. Acta Part A-Molec. Biomolec. Spectr. 2018, 195, 210–214. [Google Scholar] [CrossRef]
- Zheng, W.; Li, H.; Chen, W.; Zhang, J.; Wang, N.; Guo, X.; Jiang, X. Rapid Detection of Copper in Biological Systems Using Click Chemistry. Small 2018, 14, 1703857. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Xu, Y.; Du, J.; Guan, Q.; Cai, X.; Li, F.; Wang, J.; Chen, W. A fluorescent sensor based on the cascade signal amplification strategy for ultra-sensitive detection of Cu2+. Nanoscale 2023, 15, 1806–1812. [Google Scholar] [CrossRef] [PubMed]
- Falcone, E.; Gonzalez, P.; Lorusso, L.; Sénèque, O.; Faller, P.; Raibaut, L. A terbium(iii) luminescent ATCUN-based peptide sensor for selective and reversible detection of copper(ii) in biological media. Chem. Commun. 2020, 56, 4797–4800. [Google Scholar] [CrossRef]
- Flora, K.; Brennan, J.D. Fluorometric Detection of Ca2+ Based on an Induced Change in the Conformation of Sol−Gel Entrapped Parvalbumin. Anal. Chem. 1998, 70, 4505–4513. [Google Scholar] [CrossRef]
- Lin, Y.; Zheng, Y.; Guo, Y.; Yang, Y.; Li, H.; Fang, Y.; Wang, C. Peptide-functionalized carbon dots for sensitive and selective Ca2+ detection. Sens. Actuator B-Chem. 2018, 273, 1654–1659. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, S.; Day, G.; Wang, X.; Yang, X.; Zhou, H.-C. Luminescent sensors based on metal-organic frameworks. Coord. Chem. Rev. 2018, 354, 28–45. [Google Scholar] [CrossRef]
- Li, W.-Y.; Yang, S.; Li, Y.-A.; Li, Q.-Y.; Guan, Q.; Dong, Y.-B. Synthesis of an MOF-based Hg2+-fluorescent probe via stepwise post-synthetic modification in a single-crystal-to-single-crystal fashion and its application in bioimaging. Dalton Trans. 2019, 48, 16502–16508. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, M.; Masoomi, M.Y. Sensitive Ratiometric Fluorescent Metal-Organic Framework Sensor for Calcium Signaling in Human Blood Ionic Concentration Media. ACS Appl. Mater. Interfaces 2020, 12, 4625–4631. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Song, Y.; Xiao, Y.; Wu, R.; Wang, L. ZnMOF-74 responsive fluorescence sensing platform for detection of Fe3+. Microchem. J. 2019, 150, 104154. [Google Scholar] [CrossRef]
- Deng, J.; Hu, J.; Zhao, J.; An, N.; Liang, K.; Wang, Q.; Zhang, Z.; Wu, R.; Zhang, F. Eco friendly synthesis of fluorescent carbon dots for the sensitive detection of ferric ions and cell imaging. Arab. J. Chem. 2021, 14, 103195. [Google Scholar] [CrossRef]
- Feng, J.; Zhao, X.; Bian, W.; Tang, X. Microwave-assisted synthesis of nitrogen-rich carbon dots as effective fluorescent probes for sensitive detection of Ag+. Mat. Chem. Front. 2019, 3, 2751–2758. [Google Scholar] [CrossRef]
- Xu, J.; Liu, M.; Zhao, W.; Wang, S.; Gui, M.; Li, H.; Yu, R. DNAzyme-based cascade signal amplification strategy for highly sensitive detection of lead ions in the environment. J. Hazard. Mater. 2022, 429, 128347. [Google Scholar] [CrossRef]
- Mathivanan, J.; Liu, H.; Gan, J.; Chandrasekaran, A.R.; Sheng, J. Fluorescent Aptaswitch for Detection of Lead Ions. ACS Appl. Bio Mater. 2022, 5, 5089–5093. [Google Scholar] [CrossRef]
- Shen, G.; Zhang, H.; Xiang, J.; Yang, F.; Wu, S.; Wang, W.; Du, N.; Zhang, J.; Sun, T.; Tang, Y. Direct detection of potassium and lead (II) ions based on assembly-disassembly of a chiral cyanine dye/TBA complex. Talanta 2019, 201, 490–495. [Google Scholar] [CrossRef]
- Yoon Lee, J.; Kumar Mehta, P.; Subedi, S.; Lee, K.-H. Development of ratiometric fluorescent probes based on peptides for sensing Pb2+ in aquatic environments and human serum. Spectroc. Acta Part A-Molec. Biomolec. Spectr. 2023, 294, 122502. [Google Scholar] [CrossRef]
- Xu, H.; Geng, F.; Jiang, X.; Shao, C.; Wang, Y.; Wang, K.; Qu, P.; Xu, M.; Ye, B.-C. Design of metal-ion-triggered assembly of label-free split G-quadruplex/duplex DNA for turn-on detection of Hg2+ in fetal calf serum. Sens. Actuator B-Chem. 2018, 255, 1024–1030. [Google Scholar] [CrossRef]
- Geng, F.; Jiang, X.; Wang, Y.; Shao, C.; Wang, K.; Qu, P.; Xu, M. DNA-based dual fluorescence signals on and ratiometric mercury sensing in fetal calf serum with simultaneous excitation. Sens. Actuator B-Chem. 2018, 260, 793–799. [Google Scholar] [CrossRef]
- Dey, N. Coordination-driven reversible supramolecular assembly formation at biological pH: Trace-level detection of Hg2+ and I− ions in real life samples. Spectroc. Acta Part A-Molec. Biomolec. Spectr. 2022, 267, 120447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, Y.; Wu, Y.; Zhao, B.; Wang, L.; Song, B.; Huang, C. Benzoindole-based bifunctional ratiometric turn-on sensor with an ICT effect for trapping of H+ and Al3+ in dual-channel cell imaging and samples. Spectroc. Acta Part A-Molec. Biomolec. Spectr. 2021, 247, 119123. [Google Scholar] [CrossRef]
- Chang, M.; Zhang, M.; Hu, H.; Liang, S. Highly selective fluorescence detection of Pt4+ over Pd2+ and Pt2+ using a polyethyleneimine-based nanosensor prepared via facile three-component reaction. Spectroc. Acta Part A-Molec. Biomolec. Spectr. 2022, 279, 121466. [Google Scholar] [CrossRef] [PubMed]
- García-Valle, F.M.; Tabernero, V.; Cuenca, T.; Mosquera, M.E.G.; Cano, J. Intramolecular C–F Activation in Schiff-Base Alkali Metal Complexes. Organometallics 2019, 38, 894–904. [Google Scholar] [CrossRef]
- Ananthan Karthick, K.; Shankar, B.; Kubendran Aravind, M.; Ashokkumar, B.; Tamilselvi, A. Small-Molecule Fluorescent Probe: Ratiometric and Selective Detection of Sodium Ions for Imaging and Solid-State Sensing Applications. ChemistrySelect 2022, 7, e202203235. [Google Scholar] [CrossRef]
- Deng, Z.; Gao, P.; Liu, H.; He, Y.; Zhong, S.; Yang, Y. Cell-Surface-Anchored DNA Sensors for Simultaneously Monitoring Extracellular Sodium and Potassium Levels. Anal. Chem. 2021, 93, 16432–16438. [Google Scholar] [CrossRef]
- Ning, J.; Liu, H.; Sun, X.; Song, G.; Shen, M.; Liao, J.; Su, F.; Tian, Y. Rational Design of a Polymer-Based Ratiometric K+ Indicator for High-Throughput Monitoring Intracellular K+ Fluctuations. ACS Appl. Bio Mater. 2021, 4, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Shen, M.; Shi, J.; Ning, J.; Su, F.; Liao, J.; Tian, Y. Intracellular potassium ion fluorescent nanoprobes for functional analysis of hERG channel via bioimaging. Sens. Actuator B-Chem. 2021, 345, 130450. [Google Scholar] [CrossRef]
- Wang, Z.; Detomasi, T.C.; Chang, C.J. A dual-fluorophore sensor approach for ratiometric fluorescence imaging of potassium in living cells. Chem. Sci. 2021, 12, 1720–1729. [Google Scholar] [CrossRef]
- Cui, M.-R.; Chen, L.-X.; Li, X.-L.; Xu, J.-J.; Chen, H.-Y. NIR Remote-Controlled “Lock–Unlock” Nanosystem for Imaging Potassium Ions in Living Cells. Anal. Chem. 2020, 92, 4558–4565. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jing, X.; Zhang, S.; Tian, Y. A Copper Nanocluster-Based Fluorescent Probe for Real-Time Imaging and Ratiometric Biosensing of Calcium Ions in Neurons. Anal. Chem. 2019, 91, 2488–2497. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhao, J.; Zhang, L.; Liu, R.; Huang, Y.; Lan, C.; Zhao, S. Capsicum-Derived Biomass Quantum Dots Coupled with Alizarin Red S as an Inner-Filter-Mediated Illuminant Nanosystem for Imaging of Intracellular Calcium Ions. Anal. Chem. 2018, 90, 13059–13064. [Google Scholar] [CrossRef]
- Pendin, D.; Norante, R.; De Nadai, A.; Gherardi, G.; Vajente, N.; Basso, E.; Kaludercic, N.; Mammucari, C.; Paradisi, C.; Pozzan, T.; et al. A Synthetic Fluorescent Mitochondria-Targeted Sensor for Ratiometric Imaging of Calcium in Live Cells. Angew. Chem. Int. Ed. 2019, 58, 9917–9922. [Google Scholar] [CrossRef]
- Zhou, W.; Saran, R.; Huang, P.-J.J.; Ding, J.; Liu, J. An Exceptionally Selective DNA Cooperatively Binding Two Ca2+ Ions. ChemBioChem 2017, 18, 518–522. [Google Scholar] [CrossRef]
- Li, C.; Chen, P.; Wang, Z.; Ma, X. A DNAzyme-gold nanostar probe for SERS-fluorescence dual-mode detection and imaging of calcium ions in living cells. Sens. Actuator B-Chem. 2021, 347, 130596. [Google Scholar] [CrossRef]
- Wang, L.; Hiblot, J.; Popp, C.; Xue, L.; Johnsson, K. Environmentally Sensitive Color-Shifting Fluorophores for Bioimaging. Angew. Chem. Int. Ed. 2020, 59, 21880–21884. [Google Scholar] [CrossRef]
- Zhu, W.; Takeuchi, S.; Imai, S.; Terada, T.; Ueda, T.; Nasu, Y.; Terai, T.; Campbell, R.E. Chemigenetic indicators based on synthetic chelators and green fluorescent protein. Nat. Chem. Biol. 2023, 19, 38–44. [Google Scholar] [CrossRef]
- van der Linden, F.H.; Mahlandt, E.K.; Arts, J.J.G.; Beumer, J.; Puschhof, J.; de Man, S.M.A.; Chertkova, A.O.; Ponsioen, B.; Clevers, H.; van Buul, J.D.; et al. A turquoise fluorescence lifetime-based biosensor for quantitative imaging of intracellular calcium. Nat. Commun. 2021, 12, 7159. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Hwang, K.; Lan, T.; Lu, Y. A DNAzyme-Gold Nanoparticle Probe for Uranyl Ion in Living Cells. J. Am. Chem. Soc. 2013, 135, 5254–5257. [Google Scholar] [CrossRef]
- Li, L.; Feng, J.; Fan, Y.; Tang, B. Simultaneous Imaging of Zn2+ and Cu2+ in Living Cells Based on DNAzyme Modified Gold Nanoparticle. Anal. Chem. 2015, 87, 4829–4835. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yin, X.; Huan, S.-Y.; Chen, L.; Hu, X.-X.; Xiong, M.-Y.; Chen, K.; Zhang, X.-B. Two-Photon DNAzyme–Gold Nanoparticle Probe for Imaging Intracellular Metal Ions. Anal. Chem. 2018, 90, 3118–3123. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Yang, Z.; Lake, R.J.; Li, J.; Hong, S.; Fan, H.; Zhang, X.-B.; Lu, Y. DNAzyme-Mediated Genetically Encoded Sensors for Ratiometric Imaging of Metal Ions in Living Cells. Angew. Chem. Int. Ed. 2020, 59, 1891–1896. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, Z.-L.; Chao, Q.; Li, Q.; Kong, R.; Fan, G.-C.; Luo, X. A DNAzyme-based normalized fluorescence strategy for direct quantification of endogenous zinc in living cells. Chem. Commun. 2022, 58, 577–580. [Google Scholar] [CrossRef]
- Yi, D.; Zhao, H.; Zhao, J.; Li, L. Modular Engineering of DNAzyme-Based Sensors for Spatioselective Imaging of Metal Ions in Mitochondria. J. Am. Chem. Soc. 2023, 145, 1678–1685. [Google Scholar] [CrossRef]
- Zhan, J.; Liu, Z.; Liu, R.; Zhu, J.-J.; Zhang, J. Near-Infrared-Light-Mediated DNA-Logic Nanomachine for Bioorthogonal Cascade Imaging of Endogenous Interconnected MicroRNAs and Metal Ions. Anal. Chem. 2022, 94, 16622–16631. [Google Scholar] [CrossRef]
- Jayaraj, A.; Gayathri, M.S.; Sivaraman, G.; Swamy, P.C.A. A highly potential acyclic Schiff base fluorescent turn on sensor for Zn2+ ions and colorimetric chemosensor for Zn2+, Cu2+ and Co2+ ions and its applicability in live cell imaging. J. Photochem. Photobiol. B Biol. 2022, 226, 112371. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, Y.; Peng, B.; Li, Z.; Xu, C.; Liu, Y.; Zhang, C.; Voelcker, N.H.; Li, L.; Huang, W. A fluorogenic probe based on chelation–hydrolysis-enhancement mechanism for visualizing Zn2+ in Parkinson’s disease models. J. Mat. Chem. B 2019, 7, 2252–2260. [Google Scholar] [CrossRef]
- Wang, P.; Wu, X.; Wu, J.; Liao, Y. Highly selective and sensitive peptide-based fluorescent chemosensor for detection of Zinc(II) ions in aqueous medium and living cells. J. Photochem. Photobiol. A Chem. 2019, 382, 111929. [Google Scholar] [CrossRef]
- Dischler, A.M.; Maslar, D.; Zhang, C.; Qin, Y. Development and Characterization of a Red Fluorescent Protein-Based Sensor RZnP1 for the Detection of Cytosolic Zn2+. ACS Sens. 2022, 7, 3838–3845. [Google Scholar] [CrossRef]
- Murata, O.; Shindo, Y.; Ikeda, Y.; Iwasawa, N.; Citterio, D.; Oka, K.; Hiruta, Y. Near-Infrared Fluorescent Probes for Imaging of Intracellular Mg2+ and Application to Multi-Color Imaging of Mg2+, ATP, and Mitochondrial Membrane Potential. Anal. Chem. 2020, 92, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Sadhanala, H.K.; Aryal, S.; Sharma, K.; Orpaz, Z.; Michaeli, S.; Gedanken, A. Nitrogen-doped carbon dots as a highly selective and sensitive fluorescent probe for sensing Mg2+ ions in aqueous solution, and their application in the detection and imaging of intracellular Mg2+ ions. Sens. Actuator B-Chem. 2022, 366, 131958. [Google Scholar] [CrossRef]
- Yadav, N.; Kumar, R.; Singh, A.K.; Mohiyuddin, S.; Gopinath, P. Systematic approach of chromone skeleton for detecting Mg2+ ion: Applications for sustainable cytotoxicity and cell imaging possibilities. Spectroc. Acta Part A-Molec. Biomolec. Spectr. 2020, 235, 118290. [Google Scholar] [CrossRef]
- Ranee, S.J.; Sivaraman, G.; Pushpalatha, A.M.; Muthusubramanian, S. Quinoline based sensors for bivalent copper ions in living cells. Sens. Actuator B-Chem. 2018, 255, 630–637. [Google Scholar] [CrossRef]
- Chailek, N.; Kaewnok, N.; Petdum, A.; Sirirak, J.; Chaneam, S.; Kamkaew, A.; Girdthep, S.; Wanichacheva, N. Near infrared and colorimetric fluorescence sensor for ultra-selective detection of Cu2+ level with applications in diverse water samples, brain tumor cell and flow injection analysis. J. Photochem. Photobiol. A Chem. 2021, 421, 113533. [Google Scholar] [CrossRef]
- Chang, D.; Shi, L.; Zhang, Y.; Zhang, G.; Zhang, C.; Dong, C.; Shuang, S. Smilax China-derived yellow-fluorescent carbon dots for temperature sensing, Cu2+ detection and cell imaging. Analyst 2020, 145, 2176–2183. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jia, N.; Zhou, X.; Han, J.; Bu, H. Cu(I)-Catalyzed Click Reaction-Triggered 3D DNA Walker for Constructing an “OFF–ON” Fluorescent Biosensor for Cu2+ Detection. ACS Appl. Bio Mater. 2021, 4, 3571–3578. [Google Scholar] [CrossRef]
- Liang, C.; Xie, X.; Shi, Q.; Feng, J.; Zhang, D.; Huang, X. Nitrogen/sulfur-doped dual-emission carbon dots with tunable fluorescence for ratiometric sensing of ferric ions and cell membrane imaging. Appl. Surf. Sci. 2022, 572, 151447. [Google Scholar] [CrossRef]
- Ge, G.; Li, L.; Chen, M.; Wu, X.; Yang, Y.; Wang, D.; Zuo, S.; Zeng, Z.; Xiong, W.; Guo, C. Green Synthesis of Nitrogen–Doped Carbon Dots from Fresh Tea Leaves for Selective Fe3+ Ions Detection and Cellular Imaging. Nanomaterials 2022, 12, 986. [Google Scholar] [CrossRef]
- Cai, H.; Xu, H.; Chu, H.; Li, J.; Zhang, D. Fabrication of multi-functional carbon dots based on “one stone, three birds” strategy and their applications for the dual-mode Fe3+ detection, effective promotion on cell proliferation and treatment on ferric toxicosis in vitro. J. Mat. Chem. B 2021, 9, 767–782. [Google Scholar] [CrossRef]
- Diao, Q.; Guo, H.; Yang, Z.; Luo, W.; Li, T.; Hou, D. A rhodamine-6G-based “turn-on” fluorescent probe for selective detection of Fe3+ in living cells. Anal. Methods 2019, 11, 794–799. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Pu, C.; Tong, Z.; Wang, M.; Wang, J. Galactose-imidazole mediated dual-targeting fluorescent probe for detecting Fe3+ in the lysosomes of hepatocytes: Design, synthesis and evaluation. Biosens. Bioelectron. 2022, 204, 114083. [Google Scholar] [CrossRef] [PubMed]
- Rattanopas, S.; Piyanuch, P.; Wisansin, K.; Charoenpanich, A.; Sirirak, J.; Phutdhawong, W.; Wanichacheva, N. Indole-based fluorescent sensors for selective sensing of Fe2+ and Fe3+ in aqueous buffer systems and their applications in living cells. J. Photochem. Photobiol. A Chem. 2019, 377, 138–148. [Google Scholar] [CrossRef]
- McGhee, C.E.; Yang, Z.; Guo, W.; Wu, Y.; Lyu, M.; De Long, C.J.; Hong, S.; Ma, Y.; McInnis, M.G.; O’Shea, K.S.; et al. DNAzyme-Based Lithium-Selective Imaging Reveals Higher Lithium Accumulation in Bipolar Disorder Patient-Derived Neurons. ACS Central Sci. 2021, 7, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Gao, Y.; Meng, Y.; Lu, W.; Liu, Y.; Han, H.; Shuang, S.; Li, L.; Dong, C. One-Step Synthesis of Label-Free Ratiometric Fluorescence Carbon Dots for the Detection of Silver Ions and Glutathione and Cellular Imaging Applications. ACS Appl. Mater. Interfaces 2019, 11, 16822–16829. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Shi, J.; Mudiyanselage, A.P.K.K.K.; Wu, R.; Zhao, B.; Zhou, M.; You, M. Genetically encoded RNA-based sensors for intracellular imaging of silver ions. Chem. Commun. 2019, 55, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.-Y.; Gao, Y.; Li, B.; Li, C.-W.; Guo, Y. Reaction-based colorimetric and ratiometric fluorescent probe for highly selective detection of silver ions. Sens. Actuator B-Chem. 2018, 270, 562–569. [Google Scholar] [CrossRef]
- Bu, F.; Zhao, B.; Kan, W.; Wang, L.; Song, B.; Wang, J.; Zhang, Z.; Deng, Q.; Yin, G. A phenanthro[9,10-d]imidazole-based AIE active fluorescence probe for sequential detection of Ag+/AgNPs and SCN− in water and saliva samples and its application in living cells. Spectroc. Acta Part A-Molec. Biomolec. Spectr. 2019, 223, 117333. [Google Scholar] [CrossRef]
- Yu, M.; Liu, B.; Guo, J.; Wu, F. A sustainable modificatory 1, 2- alternate thiacalix[4]arene for detection of silver ion. Dyes Pigment. 2023, 210, 110983. [Google Scholar] [CrossRef]
- Soleja, N.; Mohsin, M. Real time quantification of intracellular nickel using genetically encoded FRET-based nanosensor. Int. J. Biol. Macromol. 2019, 138, 648–657. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C.; Zhu, H.; Cheng, S.; Zhang, Y.; Su, M.; Rong, X.; Yu, M.; Sheng, W.; Zhu, B. An ultra-sensitive and highly selective fluorescent probe for nickel ions and its environmental and biological applications. Sens. Actuator B-Chem. 2022, 369, 132300. [Google Scholar] [CrossRef]
- Assiri, M.A.; Junaid, H.M.; Waseem, M.T.; Hamad, A.; Shah, S.H.; Iqbal, J.; Rauf, W.; Shahzad, S.A. AIEE active sensors for fluorescence enhancement based detection of Ni2+ in living cells: Mechanofluorochromic and photochromic properties with reversible sensing of acid and base. Anal. Chim. Acta. 2022, 1234, 340516. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.-Q.; Shen, W.-Y.; Zhou, Y.; Chen, S.-F.; Mi, Y.; Long, B.-F.; Young, D.J.; Hu, F.-L. A pyrazolopyrimidine based fluorescent probe for the detection of Cu2+ and Ni2+ and its application in living cells. Spectroc. Acta Part A-Molec. Biomolec. Spectr. 2019, 209, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Gui, S.; Huang, Y.; Zhu, Y.; Jin, Y.; Zhao, R. Biomimetic Sensing System for Tracing Pb2+ Distribution in Living Cells Based on the Metal–Peptide Supramolecular Assembly. ACS Appl. Mater. Interfaces 2019, 11, 5804–5811. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.K.; Jeon, J.; Ryu, K.; Park, S.-H.; Lee, K.-H. Ratiometric fluorescent detection of lead ions in aquatic environment and living cells using a fluorescent peptide-based probe. J. Hazard. Mater. 2022, 427, 128161. [Google Scholar] [CrossRef]
- Wen, J.; Lv, Y.; Xia, P.; Liu, F.; Xu, Y.; Li, H.; Chen, S.-S.; Sun, S. A water-soluble near-infrared fluorescent probe for specific Pd2+ detection. Biorg. Med. Chem. 2018, 26, 931–937. [Google Scholar] [CrossRef]
- Wang, D.; Hou, H.; Chen, W.; Wu, Y.; Peng, X.; Song, F. A turn-on fluorescent probe for palladium(II) detection with a large Stokes shift and lysosomes-targeting ability. Tetrahedron Lett. 2022, 102, 153932. [Google Scholar] [CrossRef]
- Ashwin, B.C.M.A.; Sivaraman, G.; Stalin, T.; Yuvakkumar, R.; Muthu Mareeswaran, P. Selective and sensitive fluorescent sensor for Pd2+ using coumarin 460 for real-time and biological applications. J. Photochem. Photobiol. B Biol. 2018, 183, 302–308. [Google Scholar] [CrossRef]
- Zhang, X.-P.; Yuan, Q.; Qi, Y.-L.; Zheng, D.-J.; Liu, Q.-X.; Wang, B.-Z.; Yang, Y.-S.; Zhu, H.-L. An umbelliferone-derivated fluorescent sensor for selective detection of palladium(II) from palladium(0) in living cells. Spectroc. Acta Part A-Molec. Biomolec. Spectr. 2019, 220, 117134. [Google Scholar] [CrossRef]
- Reagen, S.; Wu, Y.; Liu, X.; Shahni, R.; Bogenschuetz, J.; Wu, X.; Chu, Q.R.; Oncel, N.; Zhang, J.; Hou, X.; et al. Synthesis of Highly Near-Infrared Fluorescent Graphene Quantum Dots Using Biomass-Derived Materials for In Vitro Cell Imaging and Metal Ion Detection. ACS Appl. Mater. Interfaces 2021, 13, 43952–43962. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, L.; Zhou, J.; Qin, D.; Duan, H. A new phenothiazine-based fluorescence sensor for imaging Hg2+ in living cells. Appl. Organomet. Chem. 2020, 34, e5945. [Google Scholar] [CrossRef]
- Zhong, W.; Wang, L.; Qin, D.; Zhou, J.; Duan, H. Two Novel Fluorescent Probes as Systematic Sensors for Multiple Metal Ions: Focus on Detection of Hg2+. ACS Omega 2020, 5, 24285–24295. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Wu, L.; Feng, H.; Liu, Y.; Li, J.; Si, H.; Yao, X.; He, M.; He, W. The specific binding of a new 1,2,3-triazole to three blood proteins and it’s appended rhodamine complex for selective detection of Hg2+. Spectroc. Acta Part A-Molec. Biomolec. Spectr. 2020, 228, 117728. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xu, W.; Liu, Y.; Fu, N. Ultrasensitive detection and high-contrast bioimaging of Hg2+ using monothiosquaraine-based fluorescent probe via hydrogen bond promoted desulfurization. Microchem. J. 2022, 179, 107481. [Google Scholar] [CrossRef]
- Lin, W.; Xie, X.; Wang, Y.; Chen, J. A New Fluorescent Probe for Selective Cd2+ Detection and Cell Imaging. Z. Anorg. Allg. Chem. 2019, 645, 645–648. [Google Scholar] [CrossRef]
- Wang, P.; An, Y.; Liao, Y. A novel peptide-based fluorescent chemosensor for Cd(II) ions and its applications in bioimaging. Spectroc. Acta Part A-Molec. Biomolec. Spectr. 2019, 216, 61–68. [Google Scholar] [CrossRef]
- Guo, Y.; Li, T.; Xie, L.; Tong, X.; Tang, C.; Shi, S. Red pitaya peels-based carbon dots for real-time fluorometric and colorimetric assay of Au3+, cellular imaging, and antioxidant activity. Anal. Bioanal. Chem. 2021, 413, 935–943. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Makhal, S.C.; Guchhait, N. CHEF-Affected Fluorogenic Nanomolar Detection of Al3+ by an Anthranilic Acid–Naphthalene Hybrid: Cell Imaging and Crystal Structure. ACS Omega 2018, 3, 11838–11846. [Google Scholar] [CrossRef]
- Berrones-Reyes, J.; Muñoz-Flores, B.M.; Gómez-Treviño, A.; Treto-Suárez, M.A.; Páez-Hernández, D.; Schott, E.; Zarate, X.; Jiménez-Pérez, V.M. Novel fluorescent Schiff bases as Al3+ sensors with high selectivity and sensitivity, and their bioimaging applications. Mater. Chem. Phys. 2019, 233, 89–101. [Google Scholar] [CrossRef]
- Wang, C.-X.; Ai, S.-L.; Wu, B.; Huang, S.-W.; Liu, Z. Biotinylated and fluorophore-incorporated polymeric mixed micelles for tumor cell-specific turn-on fluorescence imaging of Al3+. J. Mat. Chem. B 2020, 8, 3557–3565. [Google Scholar] [CrossRef]
- Fenn, W.O. The Rôle of Potassium in Physiological Processes. Physiol. Rev. 1940, 20, 377–415. [Google Scholar] [CrossRef]
- Ning, J.; Lin, X.; Su, F.; Sun, A.; Liu, H.; Luo, J.; Wang, L.; Tian, Y. Development of a molecular K+ probe for colorimetric/fluorescent/photoacoustic detection of K+. Anal. Bioanal. Chem. 2020, 412, 6947–6957. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pan, L.; Shang, C.; Lu, B.; Wu, R.; Feng, Y.; Chen, W.; Zhang, R.; Bu, J.; Xiong, Z.; et al. A highly sensitive and selective nanosensor for near-infrared potassium imaging. Sci. Adv. 2020, 6, eaax9757. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, F.; Wang, Y.; Pan, L.; Lin, P.; Zhang, B.; Zheng, Y.; Xu, Y.; Liao, H.; Ko, G.; et al. A sensitive and specific nanosensor for monitoring extracellular potassium levels in the brain. Nat. Nanotechnol. 2020, 15, 321–330. [Google Scholar] [CrossRef]
- Akerboom, J.; Chen, T.-W.; Wardill, T.J.; Tian, L.; Marvin, J.S.; Mutlu, S.; Calderón, N.C.; Esposti, F.; Borghuis, B.G.; Sun, X.R.; et al. Optimization of a GCaMP Calcium Indicator for Neural Activity Imaging. J. Neurosci. 2012, 32, 13819. [Google Scholar] [CrossRef] [PubMed]
- Dana, H.; Sun, Y.; Mohar, B.; Hulse, B.K.; Kerlin, A.M.; Hasseman, J.P.; Tsegaye, G.; Tsang, A.; Wong, A.; Patel, R.; et al. High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat. Methods 2019, 16, 649–657. [Google Scholar] [CrossRef]
- Zarowny, L.; Aggarwal, A.; Rutten, V.M.S.; Kolb, I.; Patel, R.; Huang, H.-Y.; Chang, Y.-F.; Phan, T.; Kanyo, R.; Ahrens, M.B.; et al. Bright and High-Performance Genetically Encoded Ca2+ Indicator Based on mNeonGreen Fluorescent Protein. ACS Sens. 2020, 5, 1959–1968. [Google Scholar] [CrossRef]
- Shemetov, A.A.; Monakhov, M.V.; Zhang, Q.; Canton-Josh, J.E.; Kumar, M.; Chen, M.; Matlashov, M.E.; Li, X.; Yang, W.; Nie, L.; et al. A near-infrared genetically encoded calcium indicator for in vivo imaging. Nat. Biotechnol. 2021, 39, 368–377. [Google Scholar] [CrossRef]
- Li, M.; Xing, Y.; Zou, Y.; Chen, G.; You, J.; Yu, F. Imaging of the mutual regulation between zinc cation and nitrosyl via two-photon fluorescent probes in cells and in vivo. Sens. Actuator B-Chem. 2020, 309, 127772. [Google Scholar] [CrossRef]
- Wu, L.; Liu, Y.; Wu, X.; Li, Y.; Du, J.; Qi, S.; Yang, Q.; Xu, H.; Li, Y. A novel Near-Infrared fluorescent probe for Zn2+ and CN– double detection based on dicyanoisfluorone derivatives with highly sensitive and selective, and its application in Bioimaging. Spectroc. Acta Part A-Molec. Biomolec. Spectr. 2022, 267, 120621. [Google Scholar] [CrossRef]
- Yang, Z.; Loh, K.Y.; Chu, Y.-T.; Feng, R.; Satyavolu, N.S.R.; Xiong, M.; Nakamata Huynh, S.M.; Hwang, K.; Li, L.; Xing, H.; et al. Optical Control of Metal Ion Probes in Cells and Zebrafish Using Highly Selective DNAzymes Conjugated to Upconversion Nanoparticles. J. Am. Chem. Soc. 2018, 140, 17656–17665. [Google Scholar] [CrossRef]
- Wang, X.; Kim, G.; Chu, J.L.; Song, T.; Yang, Z.; Guo, W.; Shao, X.; Oelze, M.L.; Li, K.C.; Lu, Y. Noninvasive and Spatiotemporal Control of DNAzyme-Based Imaging of Metal Ions In Vivo Using High-Intensity Focused Ultrasound. J. Am. Chem. Soc. 2022, 144, 5812–5819. [Google Scholar] [CrossRef] [PubMed]
- Vijay, N.; Wu, S.P.; Velmathi, S. Turn on fluorescent chemosensor containing rhodamine B fluorophore for selective sensing and in vivo fluorescent imaging of Fe3+ ions in HeLa cell line and zebrafish. J. Photochem. Photobiol. A Chem. 2019, 384, 112060. [Google Scholar] [CrossRef]
- Chang, D.; Zhao, Z.; Niu, W.; Shi, L.; Yang, Y. Iron ion sensing and in vitro and in vivo imaging based on bright blue-fluorescent carbon dots. Spectroc. Acta Part A-Molec. Biomolec. Spectr. 2021, 260, 119964. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Zhao, Z.; Shi, L.; Liu, W.; Yang, Y. Lysosome-targeted carbon dots for colorimetric and fluorescent dual mode detection of iron ion, in vitro and in vivo imaging. Talanta 2021, 232, 122423. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhao, Z.; Liu, X.; Chen, P.; Fan, F.; Wu, X.; Hua, R.; Wang, Y. A novel near-infrared fluorimetric method for point-of-care monitoring of Fe2+ and its application in bioimaging. J. Hazard. Mater. 2021, 406, 124767. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Feng, S.; Gong, S.; Xia, Q.; Feng, G. In vivo imaging of Fe2+ using an easily obtained probe with a large Stokes shift and bright strong lipid droplet-targetable near-infrared fluorescence. Sens. Actuator B-Chem. 2020, 309, 127796. [Google Scholar] [CrossRef]
- Wen, X.; Wen, G.; Li, W.; Zhao, Z.; Duan, X.; Yan, W.; Trant, J.F.; Li, Y. Carbon dots for specific “off-on” sensing of Co2+ and EDTA for in vivo bioimaging. Mater. Sci. Eng. B 2021, 123, 112022. [Google Scholar] [CrossRef]
- Stubing, D.B.; Heng, S.; Abell, A.D. Crowned spiropyran fluoroionophores with a carboxyl moiety for the selective detection of lithium ions. Org. Biomol. Chem. 2016, 14, 3752–3757. [Google Scholar] [CrossRef]
- Kang, J.; Li, E.; Cui, L.; Shao, Q.; Yin, C.; Cheng, F. Lithium ion specific fluorescent reversible extraction-release based on spiropyran isomerization combining crown ether coordination and its bioimaging. Sens. Actuator B-Chem. 2021, 327, 128941. [Google Scholar] [CrossRef]
- Huang, L.; Chen, F.; Zong, X.; Lu, Q.; Wu, C.; Ni, Z.; Liu, M.; Zhang, Y. Near-infrared light excited UCNP-DNAzyme nanosensor for selective detection of Pb2+ and in vivo imaging. Talanta 2021, 227, 122156. [Google Scholar] [CrossRef] [PubMed]
- Ravichandiran, P.; Prabakaran, D.S.; Maroli, N.; Boguszewska-Czubara, A.; Masłyk, M.; Kim, A.R.; Kolandaivel, P.; Ramalingam, P.; Park, B.-H.; Han, M.-K.; et al. Mitochondria-targeted dual-channel colorimetric and fluorescence chemosensor for detection of Sn2+ ions in aqueous solution based on aggregation-induced emission and its bioimaging applications. J. Hazard. Mater. 2021, 415, 125593. [Google Scholar] [CrossRef]
- Wang, J.H.; Liu, Y.M.; Chao, J.B.; Wang, H.; Wang, Y.; Shuang, S. A simple but efficient fluorescent sensor for ratiometric sensing of Cd2+ and bio-imaging studies. Sens. Actuator B-Chem. 2020, 303, 127216. [Google Scholar] [CrossRef]
- Chen, C.-G.; Vijay, N.; Thirumalaivasan, N.; Velmathi, S.; Wu, S.-P. Coumarin-based Hg2+ fluorescent probe: Fluorescence turn-on detection for Hg2+ bioimaging in living cells and zebrafish. Spectroc. Acta Part A-Molec. Biomolec. Spectr. 2019, 219, 135–140. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, S.; Liu, C.; Zhang, Y.; Su, M.; Rong, X.; Zhu, H.; Yu, M.; Sheng, W.; Zhu, B. A novel ratiometric fluorescent probe for the detection of nickel ions in the environment and living organisms. Sci. Total Environ. 2022, 840, 156445. [Google Scholar] [CrossRef] [PubMed]
- Tai, A.W.; Lien, E.J.; Lai, M.M.C.; Khwaja, T.A. Novel N-hydroxyguanidine derivatives as anticancer and antiviral agents. J. Med. Chem. 1984, 27, 236–238. [Google Scholar] [CrossRef]
- Tian, H.; Qiao, X.; Zhang, Z.-L.; Xie, C.-Z.; Li, Q.-Z.; Xu, J.-Y. A high performance 2-hydroxynaphthalene Schiff base fluorescent chemosensor for Al3+ and its applications in imaging of living cells and zebrafish in vivo. Spectroc. Acta Part A-Molec. Biomolec. Spectr. 2019, 207, 31–38. [Google Scholar] [CrossRef]
- Lian, W.-J.; Wang, X.-T.; Xie, C.-Z.; Tian, H.; Song, X.-Q.; Pan, H.-T.; Qiao, X.; Xu, J.-Y. Mixed-ligand copper(ii) Schiff base complexes: The role of the co-ligand in DNA binding, DNA cleavage, protein binding and cytotoxicity. Dalton Trans. 2016, 45, 9073–9087. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, L.; Wang, H.; Song, J.; Ding, H.; Tang, X.-H.; Yao, H. A highly selective and sensitive turn-on fluorescent probe for the detection of Al3+ and its bioimaging. Luminescence 2017, 32, 779–785. [Google Scholar] [CrossRef] [PubMed]

| Analytes | Normal Level Range in Biological System | Reference |
|---|---|---|
| Na+ | 135–145 mM (serum) | [9,10,11] |
| K+ | 3.5–5.4 mM (serum), 19–66 mM (urea) | [12,13] |
| Ca2+ | 10−6 M (intracellular), 10−3 M (extracellular fluid) | [14] |
| Mg2+ | 0.65–1.05 mM (serum) | [15] |
| Cu2+ | 1.4–2.1 mg/kg (adult human body) | [16] |
| Zn2+ | 12–16 μM (serum) | [17] |
| Fe3+ | 14–32 μM (serum) | [18] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Zhang, W.; Xue, Y.; Zhang, J. Fluorescent Sensors for Detecting and Imaging Metal Ions in Biological Systems: Recent Advances and Future Perspectives. Chemosensors 2023, 11, 226. https://doi.org/10.3390/chemosensors11040226
Shi Y, Zhang W, Xue Y, Zhang J. Fluorescent Sensors for Detecting and Imaging Metal Ions in Biological Systems: Recent Advances and Future Perspectives. Chemosensors. 2023; 11(4):226. https://doi.org/10.3390/chemosensors11040226
Chicago/Turabian StyleShi, Yang, Wenxian Zhang, Yi Xue, and Jingjing Zhang. 2023. "Fluorescent Sensors for Detecting and Imaging Metal Ions in Biological Systems: Recent Advances and Future Perspectives" Chemosensors 11, no. 4: 226. https://doi.org/10.3390/chemosensors11040226
APA StyleShi, Y., Zhang, W., Xue, Y., & Zhang, J. (2023). Fluorescent Sensors for Detecting and Imaging Metal Ions in Biological Systems: Recent Advances and Future Perspectives. Chemosensors, 11(4), 226. https://doi.org/10.3390/chemosensors11040226







